Background: 8-Oxo-7,8-dihydroguanine (8-oxoG) is an abundant DNA base lesion repaired by 8-oxoguanine glycosylase (OGG1) via the base excision repair pathway.
Results: OGG1 binds to its repair product 8-oxoG and activates canonical Ras family GTPases, causing gene activation via MAPK signaling.
Conclusion: OGG1 complexed with 8-oxoG has guanine nucleotide exchange factor activity.
Significance: OGG1 modulates cellular signaling via its DNA repair-independent function.
Keywords: Base Excision Repair, DNA Damage, DNA Damage Response, GTPase, Reactive Oxygen Species (ROS), 8-Oxoguanine, 8-Oxoguanine DNA Glycosylase
Abstract
8-Oxo-7,8-dihydroguanine (8-oxoG), arguably the most abundant base lesion induced in mammalian genomes by reactive oxygen species, is repaired via the base excision repair pathway that is initiated with the excision of 8-oxoG by OGG1. Here we show that OGG1 binds the 8-oxoG base with high affinity and that the complex then interacts with canonical Ras family GTPases to catalyze replacement of GDP with GTP, thus serving as a guanine nuclear exchange factor. OGG1-mediated activation of Ras leads to phosphorylation of the mitogen-activated kinases MEK1,2/ERK1,2 and increasing downstream gene expression. These studies document for the first time that in addition to its role in repairing oxidized purines, OGG1 has an independent guanine nuclear exchange factor activity when bound to 8-oxoG.
Introduction
Reactive oxygen species, generated both endogenously during respiration and by various oxidases and environmental insults, induce multiple types of damage in the genome, including strand breaks and several types of oxidized bases (1). The highly mutagenic 8-oxoG5 is a predominant base lesion (2, 3) that does not block replication or transcription (4, 5). In the genome, 8-oxoG is predominantly repaired via the base excision repair pathway, in which OGG1 recognizes and excises this lesion (1, 6). Unrepaired 8-oxoG in the genome has been linked to various pathological states, including cancer and aging processes (7, 8); however, Ogg1-null mice have a normal phenotype and longevity (9, 10), suggesting that alternative, compensatory enzymes could also repair 8-oxoG, at least in the active genes. On the other hand, Ogg1-deficient mice are resistant to inflammation, implicating OGG1 in proinflammatory signaling (11, 12). We thus hypothesized a DNA repair-independent function of OGG1 that depends on the free 8-oxoG base. We report here that the OGG1·8-oxoG complex activates canonical Ras family GTPases, cellular signaling, and gene expression. We thus document for the first time a distinct, cell-signaling function of a DNA repair enzyme.
EXPERIMENTAL PROCEDURES
Reagents
8-oxoG was from Cayman Chemicals (Ann Arbor, MI); 7,8-dihydro-8-oxoadenine (8-oxoA) was from BioLog Life Science Institute, Axxora, LLC (San Diego, CA); and guanine and 8-oxo-deoxyguanosine (8-oxodG) were from Sigma-Aldrich. 2,6-Diamino-4-hydroxy-5-formo-midopyrimidine (FapyG) was a kind gift of Dr. Miral Dizdaroglu (National Institute of Standards and Technology (NIST), Gaithersburg, MD). GTP, GDP, and GTPγS were from Cytoskeleton (Denver, CO); H-Ras, N-Ras, and K-Ras proteins6 were from Novus Biologicals (Littleton, CO); Pan-Ras antibody was from Millipore; nickel-nitrilotriacetic acid-agarose beads were from Qiagen (Valencia, CA); K-Ras and N-Ras antibodies were from Santa Cruz Biotechnology; antibodies to ERK1/2, MEK1/2, phospo-ERK1/2, -MEK1/2 were from Cell Signaling; and FITC- and Alexa Fluor 488-conjugated antibodies were from Invitrogen. Active Ras pulldown assay kit was from Pierce Biotechnology (Thermo Fisher Scientific); and siRNAs for Ras and OGG1 depletion were from Dharmacon (Thermo Fisher Scientific Inc.).
Cellular Studies
Human diploid fibroblast (MRC5) and HeLa-S cells were maintained in Earle's minimum essential and Dulbecco's modified Eagle's low glucose medium, respectively. All media were supplemented with 10% fetal bovine serum, glutamine, penicillin, and streptomycin; cells were grown at 37 °C in a 5% CO2. 8-oxoG base (0.01–30 μm) was added to cells in serum-free media where indicated, and cell extracts were made at 0, 5, 10, 15, 20, and 30 min after 8-oxoG addition.
Animals and Treatments
Animal experiments were performed according to the National Institutes of Health Guidelines for Use of Experimental Animals and approved by the University of Texas Animal Care and Use Committee (Protocol number: 0807044A). Eight-week-old female BALB/c mice (The Jackson Laboratory) were challenged intranasally with 60 μl of 8-oxoG (1 μm) in saline (or with control saline) under mild anesthesia (13). The animals were sacrificed after 15 min, and lung extracts were prepared for measuring the Ras-GTP levels.
Assessment of Ras-GTP
Ras-GTP levels were quantified with the Active Ras pulldown assay kits. Briefly, the cells were lysed in 25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 60 mm MgCl2, 1% Nonidet P-40, and 5% glycerol, and Ras-GTP in 250-μg extracts was captured by the Ras-binding domain of Raf1 immobilized to glutathione resin (14, 15). After washing with binding buffer, the activated Ras was eluted with Laemmli buffer (0.125 m Tris-HCl, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, pH 6.8) and quantified by Western blotting and densitometry.
Protein Interaction Assays
The interaction of OGG1 with H-, N-, or K-Ras was analyzed as described previously (16, 17). Briefly, individual His-Ras proteins were immobilized on nickel-nitrilotriacetic acid-agarose beads in interaction buffer (50 mm NaH2PO4, 300 mm NaCl, 20 mm imidazole, 0.05% Tween 20, pH 7.5) and incubated for 30 min at 4 °C. After three washes in interaction buffer, untagged OGG1 ± 8-oxoG was added in the presence or absence of GTP or GDP. The samples were incubated for 30 min at 4 °C and washed twice with interaction buffer, and the proteins eluted with Laemmli buffer were analyzed by Western blotting.
Immunodetection of Proteins
Cell extracts fractionated by SDS-PAGE (4–20% polyacrylamide) were transferred onto nitrocellulose membranes that were then incubated with primary antibodies and washed in Tris-buffered saline with Tween 20, and antibody binding was detected with HRP-conjugated secondary antibody and visualization by enhanced chemiluminescence detection (13). Subcellular localization of proteins was visualized in a Nikon Eclipse Ti microscope (60×) (13).
Fluorescence Spectroscopy
The binding of 8-oxoG to OGG1 was assessed by monitoring the decrease in intrinsic tryptophan fluorescence (18). Briefly, 0.5 μm OGG1 (100 μl) was incubated with increasing concentrations of 8-oxoG base (or 8-oxodG and FapyG as controls; 0.0, 0.0001, 0.0005, 0.001, 0.02, 004, 0.8, 1.2, 1.6, or 2.0 μm) for 10 min at 24 °C in 25 mm Tris-HCl (pH 7.6) containing 1 mm DTT. The tryptophan fluorescence at λem = 290–400 nm (λex = 280 nm) was analyzed in a SPEX FluoroMax spectrofluorometer (Horiba Jobin Yvon Inc., Edison, NJ). The binding constant Kd was calculated by plotting ΔF (change in fluorescence emission maximum, 336 nm) versus ligand concentration according to the equation ΔF = ΔFmax [ligand]/Kd + [ligand] (18).
Guanine Nucleotide Exchange Assay
Nucleotide-free H-Ras (6 pmol) was loaded with an equimolar amount of GDP or GTP in a buffer containing 20 mm Tris (pH 7.5), 150 mm NaCl, 3 μm MgCl2, 1 mm dithiothreitol, and 50 μg of bovine serum albumin at 24 °C (16, 19). Guanine nucleotide exchange assays were initiated by the addition of OGG1 ± 8-oxoG in the presence of a 10-fold excess of GTPγS or GDP. The molecular ratio of Ras and OGG1·8-oxoG was 1:1 or 10:1. After 0, 0.5, 1, 2, 4, 8, 16, or 32 min, nucleotide exchange reactions were terminated by adding 60 mm MgCl2. Ras-GTP levels were determined using Active Ras pulldown assays. Changes in Ras levels were analyzed by Western blotting.
Down-regulation of Gene Expression
Cells were transfected with control siRNA (siGENOME nontargeting siRNA) or target-specific siRNAs for Harvey (H)-Ras (catalog number M-004142), Kirsten (K)-Ras (catalog number M-005069), neuroblastoma RAS viral oncogene homolog (N)-Ras (catalog number M-003919), and/or OGG1 (catalog number M-010957) (siGENOME SMARTpools, Thermo Scientific) using INTERFERinTM reagent (Polyplus Transfection Inc.), and incubated in growth medium for 72 h.
Statistical Analysis
Results were analyzed for significant differences using analysis of variance and Student's t test. Differences were considered significant at p < 0.05. Data are expressed as the mean ± S.D.
RESULTS AND DISCUSSION
The free 8-oxoG base is generated exclusively during the repair of 8-oxoG in DNA, initiated by OGG1 (1). To mimic a transient increase in its intracellular level, we added 8-oxoG base to OGG1-proficient cells (MRC5) and analyzed the impact on the transcriptome using Affymetrix GeneChip. Ingenuity Pathways Analysis of microarray gene expression data (National Center for Biotechnology Information (NCBI), GEO accession number GSE26813) showed that 8 of the top 10 pathways that responded to 8-oxoG involved the small G protein Ras (supplemental Fig. S1). Ras GTPases activate a variety of cellular signaling pathways (20, 21).
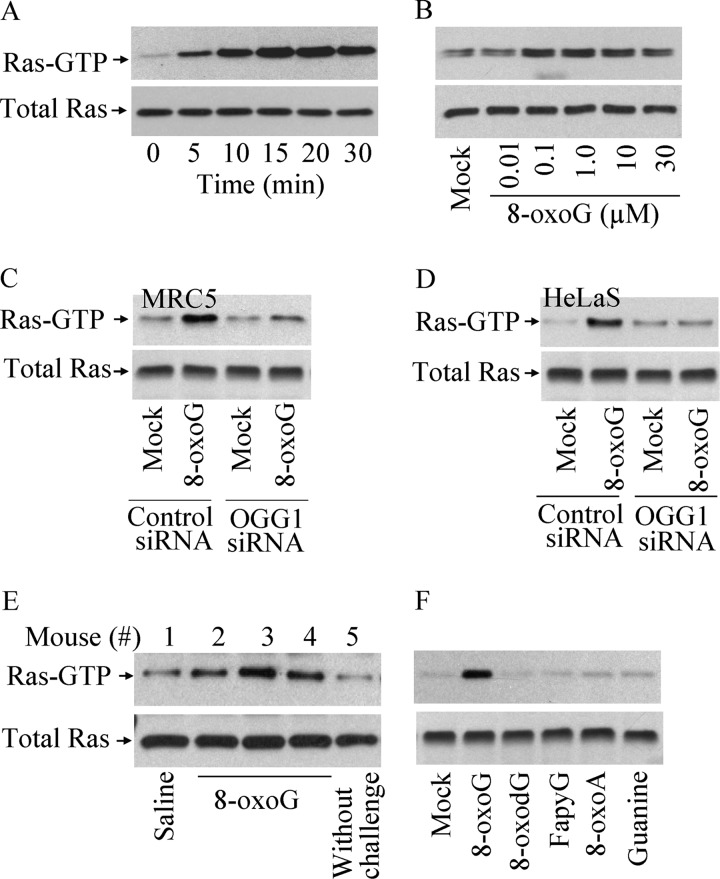
To confirm these observations, we showed that the addition of 8-oxoG increased GTP-bound Ras levels in a time- (Fig. 1A) and dose-dependent (Fig. 1B) manner. The lowest dose of 8-oxoG base that increased Ras-GTP to a detectable level was 100 nm in MRC5 cells (Fig. 1B). The time course of Ras activation was consistent with rapid cellular uptake of 8-oxoG base. For example, at 1 min after the addition, ∼70% of 8-oxoG was taken up by the cells, as shown by GS/MS analysis (supplemental Fig. S2A). Importantly, after intranasal challenge of mice (13) with 8-oxoG (60 μl of 1 μm 8-oxoG), we observed increased Ras-GTP levels in the lungs (Fig. 1E).
FIGURE 1.
Activation of Ras GTPases in OGG1-expressing cells by 8-oxoG base. A–F, parallel cell cultures were exposed to 1 μm (A, C, D, and F) or increasing concentration of 8-oxoG (B). Cell extracts were made at the times points indicated (in A) or 15 min after challenge (B, C, D, E, and F). Ras-GTP levels were determined by GST pulldown assays (15, 16). A and B, activation of Ras GTPases in a time-dependent (A) and dose-dependent manner (B) in MRC5 cells upon 8-oxoG exposure. C and D, siRNA-mediated OGG1 depletion causes decreased Ras activation. C and D, MRC5 (C) and HeLa-S (D) cells were transfected with siRNA to OGG1 (see “Experimental Procedures”) or control siRNA using two cycles of transfection and cell culture and then exposed to 8-oxoG for 15 min. Ras-GTP levels were determined as above. E, increase in Ras-GTP levels in murine lungs. BALB/c mice were challenged intranasally with 60 μl of 1 μm 8-oxoG (13), and the lungs were excised after 15 min. Ras-GTP levels in individual mouse lung extracts were determined as above. F, 8-oxoG base, but not 8-oxodG, FapyG, 8-oxoA, or guanine base increased Ras-GTP levels. 250 and 25 μg of cell extract per assay were used to determine Ras-GTP and Ras levels, respectively; n = 3–8.
We hypothesized that 8-oxoG bound to OGG1 mediates guanine nucleotide exchange in Ras. In support of this idea, adding 8-oxoG to OGG1-depleted MRC5 (Fig. 1C; supplemental Fig. S2, B and D) or HeLa-S cells (Fig. 1D; supplemental Fig. S2, C and D) did not cause an increase in Ras-GTP levels. Free 8-oxoG was unique in increasing the Ras-GTP level as neither 8-oxodG nor other oxidized bases (FapyG or 8-oxoA) nor the original guanine base displayed this activity (Fig. 1F).
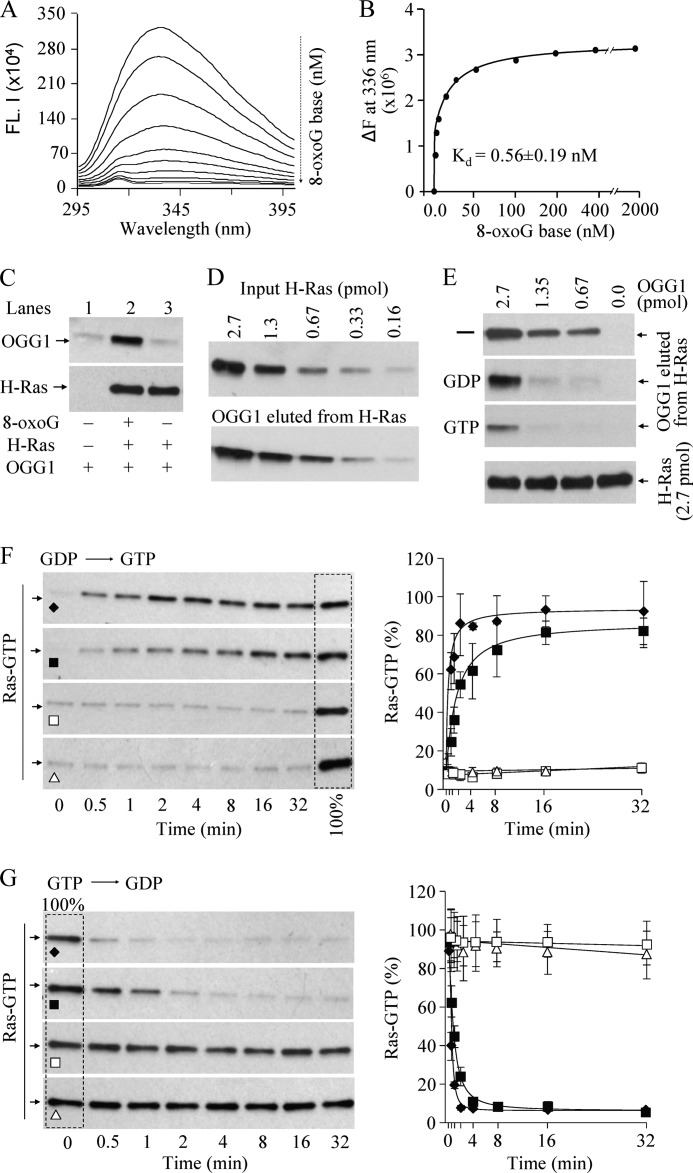
The binding of free 8-oxoG base to OGG1 was analyzed by changes in the intrinsic Trp fluorescence of OGG1 (18). A concentration-dependent decrease in Trp fluorescence (Fig. 2A) indicated the OGG1 conformational change as a result of the interaction. The binding constant (Kd) 0.56 ± 0.19 nm calculated from the binding isotherms (Fig. 2B) indicates its high affinity for 8-oxoG, which was unexpected, and predicted that the product binding would inhibit the enzyme. However, we observed the exact opposite in that 8-oxoG stimulated the activity of OGG1 in a concentration-dependent manner (supplemental Fig. S3A). This suggests that 8-oxoG serves as a cofactor for OGG1 by binding to an independent site and not to the active site pocket in OGG1. This was further supported by our observation that the free FapyG base, with abundance similar to that of 8-oxoG in oxidatively damaged DNA, and which is an equally good OGG1 substrate (22, 23), did not affect the fluorescence of OGG1. Furthermore, OGG1 did not bind 8-oxodG (supplemental Fig. S3, B and C), demonstrating the specificity of the binding of OGG1 to free 8-oxoG base.
FIGURE 2.
OGG1 complexed with 8-oxoG has guanine nucleotide exchange factor activity. A, fluorescent spectroscopic analysis of 8-oxoG binding to OGG1. FL indicates fluorescence. B, the Kd was calculated from the binding isotherms shown in A (18). C, interaction of OGG1 with Ras protein requires 8-oxoG base. H-Ras (2.7 pmol) was bound to nickel-agarose beads (see “Experimental Procedures”), and OGG1 ± 8-oxoG was added. After washing three times, the amount of OGG1 bound was analyzed by immunoblotting. D, OGG1 physically interacts with Ras protein. Increasing amounts (0.16–2.7 pmol) of His-H-Ras were immobilized on nickel-agarose beads before incubation with OGG1 (2.7 pmol) plus 8-oxoG (2.7 pmol) for 1 h at 4 °C. The proteins were stripped from the beads for immunoblotting. Upper panel, His-H-Ras bound to nickel-agarose beads; lower panel, OGG1 protein eluted from H-Ras. E, guanine nucleotides decrease the OGG1 interaction with Ras. His-H-Ras (2.7 pmol) bound to agarose beads was incubated with untagged OGG1 (2.7, 1.3, 0.67, and 0 pmol) and equimolar 8-oxoG for 30 min, and a 10-fold molar excess of GTP or GDP was then added for 30 min at 24 °C. Levels of eluted proteins were determined by Western blotting. F, exchange of Ras-bound GDP for GTP. H-Ras protein (6 pmol) was loaded with GDP (6 pmol), and nucleotide exchange was initiated by adding OGG1 (6 pmol) plus 8-oxoG (♦) or 0.6 pmol of OGG1 plus 8-oxoG (■), OGG1 (□), or 8-oxoG (▴) alone, together with GTPγS (60 pmol). G, GTP-GDP exchange by OGG1. H-Ras protein (6 pmol) was loaded with GTP, and guanine nucleotide exchange was initiated by adding 6 pmol of OGG1 plus 8-oxoG (♦) or 0.6 pmol of OGG1 plus 8-oxoG (■), OGG1 (□), or 8-oxoG (▴) alone, together with 10-fold excess GDP. Ras-GTP was quantified using pulldown immunoblot assays as in Fig. 1. G and F, right panels, bands in left panels were quantitated by densitometry (ImageJ), and the time course of nucleotide exchange is depicted graphically. n = 3–4.
Activation of GTPases involves displacement of GDP by GTP, a process mediated by GEFs (24). GEFs accelerate the exchange of GDP for GTP in Ras-GTPase, and active GTP-bound Ras releases GEF (24, 25). We then explored a possible interaction between OGG1 and Ras (16, 17, 26) and observed that in the presence of 8-oxoG, OGG1 specifically was bound to H-Ras (Fig. 2C, lane 2). However, OGG1 alone did not interact with H-Ras under identical conditions (Fig. 2C, lane 1), suggesting that an 8-oxoG-induced conformational change in OGG1 (Fig. 2A) allows its binding to Ras. Quantitation of eluted OGG1 and comparison with input Ras indicated a nearly equimolar binding of OGG1 to H-Ras (Fig. 2D). Furthermore, GTP was more effective than GDP in inhibiting the interaction between Ras and OGG1·8-oxoG (Fig. 2E). These data strongly suggest that the conformation of nucleotide-free Ras allows the most stable interaction with OGG1·8-oxoG, which is weakened in the presence of guanine nucleotides. Similar interactions of K-Ras and N-Ras with OGG1·8-oxoG were also observed, and guanine nucleotides, especially GTP, decreased these interactions (supplemental Fig. S4, A and B). Our observations are consistent with those showing high affinity binding between nucleotide-free Ras and GEF (e.g. CDC25), which is decreased to an undetectable level by guanine nucleotides, especially GTP, due to nucleotide-induced conformational changes in the Ras protein (16, 27). In controls, other oxidized base-specific DNA glycosylases (NEIL1 and NEIL2) did not interact with H-Ras (supplemental Fig. S4, C and D).
Increases in the Ras-GTP level upon exposure of cells to 8-oxoG (Fig. 1) and physical interaction between Ras and OGG1 (Fig. 2, C, D, and E) could cause guanine nucleotide exchange. Indeed, in the presence of 8-oxoG, OGG1 caused replacement of GDP-bound to Ras with GTP (Fig. 2F) at equimolar or higher molar ratios of H-Ras:OGG1. We subsequently showed that OGG1 also catalyzed the release of H-Ras-bound GTP replacement with GDP (Fig. 2G). OGG1 or 8-oxoG alone did not induce guanine nucleotide exchange (Fig. 2, F and G). Densitometric analysis of the bands in Fig. 2, F and G (left panels) shows striking similarities between the kinetics of GDP-GTP and GTP-GDP exchange on Ras (Fig. 2, F and G, right panels), suggesting that OGG1 indiscriminately releases the nucleotide in vitro and allows rebinding; thus its activity is similar to that of other Ras-GEFs (16, 27). In the intracellular environment, due to the high GTP:GDP ratio (∼10-fold higher GTP than GDP; (25)), the released GDP is exchanged for GTP in the Ras protein. OGG1·8-oxoG induced similar guanine nucleotide exchange with N-Ras and K-Ras (supplemental Fig. S5, A–D).
Ras-GTP binds to the Ras-binding domain (RBD) of the Raf1 serine/threonine kinase (14), and its subsequent phosphorylation is necessary, but not sufficient, for mediating the mitogen-activated protein kinase (MAPK) activity of Raf1 as phosphorylated Raf1 requires additional protein-protein and membrane-lipid interactions (28). Increasing the cellular 8-oxoG level in MRC5 cells induced rapid phosphorylation of the MAPK kinase (MEK1/2) and extracellular signal-regulated kinase (ERK1/2) and the nuclear translocation of the latter (Fig. 3, A and B). To verify that ERK1/2 phosphorylation is Ras-dependent, H-, K-, and N-Ras were depleted with siRNA (Fig. 3C, upper panel). After 8-oxoG addition, ERK1/2 phosphorylation was significantly decreased in N-Ras-ablated MRC5 cells (Fig. 3C, middle panel, last lane).
FIGURE 3.
Activated Ras-mediated phosphorylation of MAPKs in 8-oxoG-treated cells. A, MRC5 cells were exposed to 1 μm 8-oxoG and lysed at the times indicated, and phosphorylated MEK 1/2 (p-MEK1/2) and -ERK1/2 (p-ERK1/2) levels were determined (upper panels) by Western blotting. Total MEK1/2 and ERK1/2 levels served as loading controls (lower panels). B, nuclear accumulation of phosphorylated ERK1/2 in MRC5 cells in response to 8-oxoG. Right panels, DAPI-stained nuclei. Scale bars: 10 μm. C, N-Ras-dependent ERK1/2 phosphorylation in MRC5 cells. Upper panel, levels of total Ras after depletion by siRNA to H-, K-, and N-Ras, shown by Western blotting. siRNA-transfected cells were treated with 1 μm 8-oxoG for 15 min, and phosphorylated ERK1/2 and ERK1/2 levels were assessed by Western blot analysis (middle and lower panels). D, isotype-specific activation of Ras in 8-oxoG-exposed MRC5 cells. Upper panel, activation of Ras isoforms after 8-oxoG addition (15 min). Ras-GTP levels were determined in 250 μg of cell extracts by pulldown assay (15, 16). Middle panel, H-, K-, and N-Ras levels in mock- and 8-oxoG-treated cells. Lower panel, total Ras in cell extracts (25 μg/lane) is shown by pan-Ras antibody. n = 3–6.
Immunoblotting analysis showed abundant expression of N-Ras and K-Ras in MRC5 cells, whereas H-Ras was barely detectable (Fig. 3D, middle panel). Importantly, the addition of 8-oxoG to these cells resulted almost exclusively in N-Ras activation (Fig. 3D, upper panels), demonstrating selectivity to the activation process. These data are consistent with those showing that siRNA to N-Ras decreased ERK1/2 phosphorylation, whereas only a marginal effect of K-Ras depletion was seen (Fig. 3C, middle panel). To test whether 8-oxoG activates only N-Ras or other isoforms as well, we examined HeLa-S cells, which express H-Ras, K-Ras, and N-Ras (supplemental Fig. 6A). After 8-oxoG addition, H-, K-, and N-Ras were all activated (supplemental Fig. 6B), which implies that the 8-oxoG base activates Ras isoforms in a cell type-specific manner in OGG1-proficient cells.
In conclusion, we document for the first time that OGG1 binds the free 8-oxoG base at a nonsubstrate site with high affinity and that this complex interacts with the canonical Ras family GTPases to increase their GTP-bound forms by facilitating guanine nucleotide exchange. OGG1-mediated Ras activation initiates signal transduction for transcriptional activation of downstream genes. We propose that 8-oxoG released from DNA by OGG1 binds back to the enzyme to activate signaling pathways including those that may modulate expression of enzymes that inhibit 8-oxoG-induced mutagenesis by activating or preventing 8-oxoG incorporation into DNA from the nucleotide pool. We thus tested whether OGG1·8-oxoG-induced signaling affects the expression of MutT homolog-1 (MTH1) and MTH2 isoforms, the human homologs of Escherichia coli MutT, which hydrolyzes 8-oxodGTP (28). MTH1 but not the MTH2 gene was activated in MRC5 cells at 6 h after treatment with 8-oxoG (supplemental Fig. S7). Whether 8-oxoG similarly activates DNA repair in vivo has not yet been investigated. In any case, our results so far suggest an unusual, complex signaling network activated by small GTPases and triggered by the generation and repair of 8-oxoG in the genome.
Acknowledgments
We thank Dr. David Konkel (Biochemistry and Molecular Biology) for careful editing of the manuscript. We thank Drs. Miral Dizdaroglu and Pawel Jaruga (Chemical Science and Technology Laboratory, National Institute of Standards and Technology, Gaithersburg, MD) for assessment of 8-oxoG base levels. We also thank the anonymous reviewer for suggesting examination of the effect of 8-oxoG addition on MTH expression.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 ES018948 (to I. B.) from the NIEHS, 021830 (to I. B.) from the NIA/AG, and NIAID/AI062885-01 (to I. B.) from the NIAID and the Grants N01HV00245 (to Dr. A. Kurosky) and R01CA81063 (to S. M.) from the NHLBI Proteomic Center.

This article contains supplemental Figs. S1–S7.
H-Ras and K-Ras indicate mammalian homologs of Harvey and Kirsten sarcoma virus oncogene, respectively, and N-Ras indicates neuroblastoma RAS viral oncogene homolog.
- 8-oxoG
- 8-oxo-7,8-dihydroguanine
- 8-oxoA
- 7,8-dihydro-8-oxoadenine
- 8-oxodG
- 8-oxoguanine deoxynucleoside
- GEF
- guanine nucleotide exchange factor
- OGG1
- 8-oxoguanine DNA glycosylase 1
- MRC5
- human diploid lung fibroblast
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- FapyG
- 2,6-diamino-4-hydroxy-5-formo-midopyrimidine
- MTH
- MutT homolog.
REFERENCES
- 1. Mitra S., Hazra T. K., Roy R., Ikeda S., Biswas T., Lock J., Boldogh I., Izumi T. (1997) Complexities of DNA base excision repair in mammalian cells. Mol. Cells 7, 305–312 [PubMed] [Google Scholar]
- 2. Shibutani S., Takeshita M., Grollman A. P. (1991) Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 349, 431–434 [DOI] [PubMed] [Google Scholar]
- 3. Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. (1992) 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G → T and A → C substitutions. J. Biol. Chem. 267, 166–172 [PubMed] [Google Scholar]
- 4. Maga G., Villani G., Crespan E., Wimmer U., Ferrari E., Bertocci B., Hübscher U. (2007) 8-Oxo-guanine bypass by human DNA polymerases in the presence of auxiliary proteins. Nature 447, 606–608 [DOI] [PubMed] [Google Scholar]
- 5. Haracska L., Yu S. L., Johnson R. E., Prakash L., Prakash S. (2000) Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase η. Nat. Genet. 25, 458–461 [DOI] [PubMed] [Google Scholar]
- 6. Rosenquist T. A., Zharkov D. O., Grollman A. P. (1997) Cloning and characterization of a mammalian 8-oxoguanine DNA glycosylase. Proc. Natl. Acad. Sci. U.S.A. 94, 7429–7434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shigenaga M. K., Hagen T. M., Ames B. N. (1994) Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. U.S.A. 91, 10771–10778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. David S. S., O'Shea V. L., Kundu S. (2007) Base excision repair of oxidative DNA damage. Nature 447, 941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klungland A., Rosewell I., Hollenbach S., Larsen E., Daly G., Epe B., Seeberg E., Lindahl T., Barnes D. E. (1999) Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl. Acad. Sci. U.S.A. 96, 13300–13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minowa O., Arai T., Hirano M., Monden Y., Nakai S., Fukuda M., Itoh M., Takano H., Hippou Y., Aburatani H., Masumura K., Nohmi T., Nishimura S., Noda T. (2000) Mmh/Ogg1 gene inactivation results in accumulation of 8-hydroxyguanine in mice. Proc. Natl. Acad. Sci. U.S.A. 97, 4156–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Touati E., Michel V., Thiberge J. M., Avé P., Huerre M., Bourgade F., Klungland A., Labigne A. (2006) Deficiency in OGG1 protects against inflammation and mutagenic effects associated with H. pylori infection in mouse. Helicobacter 11, 494–505 [DOI] [PubMed] [Google Scholar]
- 12. Mabley J. G., Pacher P., Deb A., Wallace R., Elder R. H., Szabó C. (2005) Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J. 19, 290–292 [DOI] [PubMed] [Google Scholar]
- 13. Boldogh I., Bacsi A., Choudhury B. K., Dharajiya N., Alam R., Hazra T. K., Mitra S., Goldblum R. M., Sur S. (2005) ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Invest. 115, 2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Block C., Janknecht R., Herrmann C., Nassar N., Wittinghofer A. (1996) Quantitative structure-activity analysis correlating Ras/Raf interaction in vitro to Raf activation in vivo. Nat. Struct. Biol. 3, 244–251 [DOI] [PubMed] [Google Scholar]
- 15. Taylor S. J., Resnick R. J., Shalloway D. (2001) Nonradioactive determination of Ras-GTP levels using activated Ras interaction assay. Methods Enzymol. 333, 333–342 [DOI] [PubMed] [Google Scholar]
- 16. Lai C. C., Boguski M., Broek D., Powers S. (1993) Influence of guanine nucleotides on complex formation between Ras and CDC25 proteins. Mol. Cell. Biol. 13, 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chataway T. K., Barritt G. J. (1995) Purification of histidine-tagged Ras and its use in the detection of Ras-binding proteins. Mol. Cell. Biochem. 144, 167–173 [DOI] [PubMed] [Google Scholar]
- 18. Hegde M. L., Theriot C. A., Das A., Hegde P. M., Guo Z., Gary R. K., Hazra T. K., Shen B., Mitra S. (2008) Physical and functional interaction between human oxidized base-specific DNA glycosylase NEIL1 and flap endonuclease 1. J. Biol. Chem. 283, 27028–27037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Field J., Broek D., Kataoka T., Wigler M. (1987) Guanine nucleotide activation of, and competition between, RAS proteins from Saccharomyces cerevisiae. Mol. Cell. Biol. 7, 2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vetter I. R., Wittinghofer A. (2001) The guanine nucleotide-binding switch in three dimensions. Science 294, 1299–1304 [DOI] [PubMed] [Google Scholar]
- 21. Wittinghofer F. (1998) Ras signaling: caught in the act of the switch-on. Nature 394, 337–343 [DOI] [PubMed] [Google Scholar]
- 22. Hu J., de Souza-Pinto N. C., Haraguchi K., Hogue B. A., Jaruga P., Greenberg M. M., Dizdaroglu M., Bohr V. A. (2005) Repair of formamidopyrimidines in DNA involves different glycosylases: role of the OGG1, NTH1, and NEIL1 enzymes. J. Biol. Chem. 280, 40544–40551 [DOI] [PubMed] [Google Scholar]
- 23. Bourne H. R., Sanders D. A., McCormick F. (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132 [DOI] [PubMed] [Google Scholar]
- 24. Boriack-Sjodin P. A., Margarit S. M., Bar-Sagi D., Kuriyan J. (1998) The structural basis of the activation of Ras by Sos. Nature 394, 337–343 [DOI] [PubMed] [Google Scholar]
- 25. Meller N., Irani-Tehrani M., Kiosses W. B., Del Pozo M. A., Schwartz M. A. (2002) Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nat. Cell Biol. 4, 639–647 [DOI] [PubMed] [Google Scholar]
- 26. Mosteller R. D., Han J., Broek D. (1994) Identification of residues of the H-ras protein critical for functional interaction with guanine nucleotide exchange factors. Mol. Cell. Biol. 14, 1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. (1992) Raf-1 activates MAP kinase-kinase. Nature 358, 417–421 [DOI] [PubMed] [Google Scholar]
- 28. Nakabeppu Y., Oka S., Sheng Z., Tsuchimoto D., Sakumi K. (2010) Programmed cell death triggered by nucleotide pool damage and its prevention by MTH1 with oxidized purine nucleoside triphosphatase. Mutat. Res. 703, 51–58 [DOI] [PubMed] [Google Scholar]