Background: Stabilin-2 is expressed in the liver endothelium and serves as the primary heparin clearance receptor in mammals.
Results: Increased sulfation and length of heparin increase affinity for Stabilin binding/endocytosis.
Conclusion: The data demonstrate that 3-O-sulfation is not required, but greatly enhances binding to the Stabilin receptors.
Significance: Customized heparin may have therapeutic applications for obtaining the optimal balance between anticoagulation and clearance.
Keywords: Endocytosis, Endothelial Cell, Heparan Sulfate, Heparin, Receptors, Arixtra, Stabilin, Glycosaminoglycan, Liver
Abstract
As one of the most widely used drugs worldwide, heparin is an essential anticoagulant required for surgery, dialysis, treatment of thrombosis, cancer, and general circulatory management. Stabilin-2 is a scavenger clearance receptor with high expression in the sinusoidal endothelium of liver. It is believed that Stabilin-2 is the primary receptor for the clearance of unfractionated and low molecular weight heparins in the liver. Here, we identify the modifications and length of the heparin polymer that are required for binding and endocytosis by both human Stabilin receptors: Stabilin-2 and its homolog Stabilin-1 (also found in liver endothelium). Using enzymatically synthesized 35S-labeled heparan sulfate oligomers, we identified that sulfation of the 3-OH position of N-sulfated glucosamine (GlcNS) is the most beneficial modification for binding and endocytosis via both Stabilin receptors. In addition, our data suggest that a decasaccharide is the minimal size for binding to the Stabilin receptors. These findings define the physical parameters of the heparin structure required for efficient clearance from blood circulation. These results will also aid in the design of synthetic heparins with desired clearance rates.
Introduction
Heparin is an anticoagulant drug that is widely prescribed for procedures in which blood is handled, including surgery, blood/plasma donation, coronary and peripheral vascular procedures, thrombosis, dialysis, and in the washing and preparation of medical equipment (1). The current supply of heparin is derived from the pulp of porcine small intestine and is harvested as unfractionated heparin (UFH)3 with polymeric chain lengths ranging from 3,000 to 30,000 Da. UFH is depolymerized into smaller sizes with chain lengths ranging from 2000 to 8000 Da to yield the products known as low molecular weight heparin (LMWH). Generally, both UFH and LMWH are fast-acting coagulants with different clearance rates. UFH has a circulatory half-life of 30–60 min, in contrast to LMWH, which has a half-life of 2–6 h (2, 3).
The clearance of heparin is primarily mediated by liver sinusoidal endothelial cells (4). The primary receptor responsible for the systemic clearance of heparin is Stabilin-2/HARE (Stab2)(HARE, hyaluronic acid receptor for endocytosis) (5, 6). Thus, the binding affinity of heparin to Stab2 likely relates to the clearance rate of heparin. Stab2 is presented on the cell surface as two isoforms: 315- and 190-kDa type I receptors. A subset of the 315-kDa receptor undergoes proteolytic cleavage to produce the smaller 190-kDa receptor. Both 315-kDa and 190-kDa Stab2 receptors bind to and participate in systemic clearance of heparin (7, 8). The binding affinity of Stab2 to UFH is higher than LMWH, suggesting that interactions between Stab2 and heparin are dependent on the length of the polymer. Stab2 receptors are also responsible for clearing other glycosaminoglycans, including hyaluronic acid and chondroitin sulfates. It is known that the Stab2 binding domain(s) for heparin are distinct from the Link domain for binding hyaluronic acid and chondroitin sulfates (9).
Stabilin-1 (Stab1) is a Stab2 homologous protein expressed in humans, which has not been reported to bind to heparin previously. The topological organization of Stab1 is similar to Stab2 in that they share 21 EGF/EGF-like domains, seven Fasciclin-1 domains, and one X-link domain with an overall 41% amino acid identity and 56% similarity. Stab1, like Stab2, is expressed in the endothelium of liver, lymph node, and spleen, but is also uniquely expressed in activated macrophages and continuous endothelial vasculature (10, 11), suggesting that this receptor is immune-responsive. Both Stab1 and Stab2 bind an array of ligands. Stab1 binds to SPARC (secreted protein acidic and rich in cysteine) (12) and lactogen (13); Stab2 binds to collagen propeptides (6) in addition to glycosaminoglycans. The common ligands for both receptors are acetylated LDL (5, 14), GDF-15 (15), phosphatidylserine (16–18), and advanced glycation end products (19).
Heparan sulfate (HS) is a glycosaminoglycan consisting of repeating disaccharide units of glucuronic acid (GlcA) or iduronic acid (IdoA) and glucosamine, with each of the saccharide units capable of carrying sulfo groups. The positions of the sulfo groups, the location of the IdoA residues, and the size of the HS chain determine its biological functions. Heparin is a special form of HS, carrying higher levels of sulfation and IdoA. HS is a highly heterogeneous complex mixture. Its biosynthesis involves the preparation of a polysaccharide backbone and a series of sulfations and epimerization modifications. The enzymes that carry out these modification reactions have been expressed and purified (20). Utilizing recombinant HS biosynthetic enzymes, we demonstrated the synthesis of HS polysaccharides with specific sulfation patterns (21, 22) and HS oligosaccharides with defined structures (23, 24). The synthesized polysaccharides and oligosaccharides act as valuable reagents to probe the structural selectivity of HS in a given biological or biochemical experiment (20, 24).
In this study, we used synthetic HS polysaccharides and oligosaccharides to probe the structural requirements for binding to the Stabilin receptors. We report that 3-O-sulfation has a significant impact on binding/endocytosis. The size of polymer also plays a role in endocytosis. We describe the minimal size and sulfations required for endocytosis in recombinant cell lines and in murine liver endothelium. For the first time, we demonstrate that Stab1 is also responsible for the internalization of heparin in cell culture and in binding assays performed in vitro. Our findings advance the understanding of the mechanism for clearing HS and heparin. Our results also provide a molecular basis for designing heparin anticoagulant drugs with different clearance rates.
EXPERIMENTAL PROCEDURES
Materials, Solutions, and Buffers
Heparin (or unfractionated heparin) was from Sigma. Fondaparinux (Arixtra®) was purchased from a local pharmacy. Flp-In 293 cells, serum, high glucose Dulbecco's modified Eagle's medium (DMEM), Hygromycin B, Zeocin, and glutamine were from Invitrogen. Western blot analysis was completed by either colorimetric or chemiluminescence detection of blotted protein. Anti-V5 antibodies and resins were from Bethyl Laboratories (Montgomery, TX). Other materials, reagents, and kits were obtained as described recently (9). Tris-buffered saline with Tween 20 (TBST) contains 20 mm Tris-HCl, pH 7.0, 150 mm NaCl, and 0.1% Tween 20. TBST/BSA is TBST with 1.0% (w/v) bovine serum albumin (BSA). Phosphate-buffered saline (PBS) contains 137 mm NaCl, 8 mm Na2HPO4, 1.5 mm KH2PO4, 2.7 mm KCl, pH 7.2. Hanks' buffered saline solution contains 5 mm KCl, 0.4 mm KH2PO4, 0.8 mm MgSO4, 137 mm NaCl, 0.3 mm Na2HPO4, 5.5 mm glucose, 1.26 mm CaCl2, 0.5 mm MgCl2, and 28 μm phenol red; at the time of use, 3.5 g/100 ml of NaHCO3 was added, and the pH was adjusted to 7.2 with HCl. Endocytosis Medium contains DMEM supplemented with 0.05% BSA.
Preparation of 35S-labeled HS Constructs
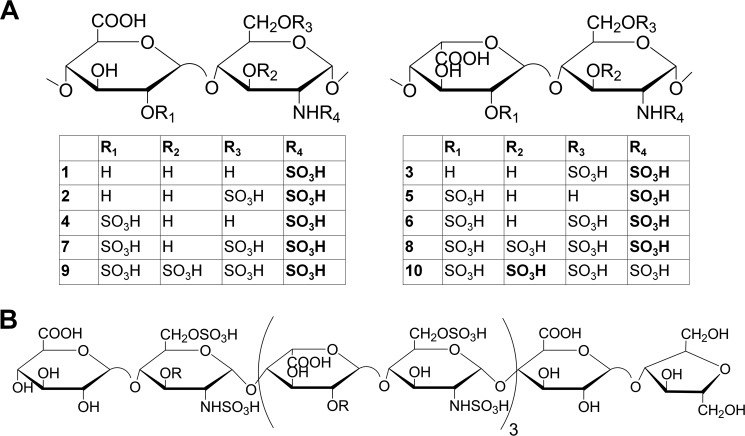
A total of 27 HS constructs were prepared for this study using a chemoenzymatic approach published previously (Fig. 1 and Table 1) (22, 24). Constructs 1 through 10 (Fig. 1A) are polysaccharide constructs differing in sulfation types and IdoA content, whereas constructs 11 through 23 are oligosaccharide constructs ranging from hepta- to nonadecasaccharides. A representative structure of a decasaccharide (15b) is shown in Fig. 1B. For the synthesis of polysaccharide constructs (1 through 10), N-sulfo heparosan was used as a starting material and incubated with the appropriate enzymes and the sulfo donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) (22). The polysaccharide products were analyzed by disaccharide analysis to confirm the anticipated sulfations (25). To prepare the oligosaccharide constructs (11 through 23), both elongation and modification steps were involved. During the elongation step, a disaccharide starting material (GlcA-AnMan) was first elongated to the desirable size with KfiA (N-acetyl glucosaminyl transferase of Escherichia coli K5 strain) and pmHS2 (Pasteurella multocida heparosan synthase 2) in the presence of UDP-GlcA and UDP-GlcNAc or UDP-GlcNTFA. The elongated products were confirmed by electrospray ionization mass spectrometry (ESI-MS). The oligosaccharides were then converted to N-sulfo oligosaccharides by treating with triethylamine followed by N-sulfotransferase modification. The products were demonstrated to have the anticipated molecular size and purity by ESI-MS. The ESI-MS spectra of the octa- to decasaccharides are shown in supplemental Fig. S1. For those oligosaccharides larger than dodecasaccharides, the ESI-MS spectra are shown in separate publications (23).4
FIGURE 1.
Chemical structures of different HS constructs. A, the general structure of all 10 different constructs. The top left structure shows the structures of those constructs without iduronic acid residues. The top right structure shows the structures of those constructs with iduronic acid residues. Each individual polysaccharide construct is shown in the table underneath those two generic structures. B, the structure of a decasaccharide (10-mer, 15b) that displays full binding ability to the Stabilin receptors as described under “Results.” To maintain clarity, the structures of other oligosaccharide constructs are not shown. The abbreviated structures are listed in Table 1.
TABLE 1.
Summary of the polysaccharide and oligosaccharide constructs
| Constructs | Anticipated disaccharide repeating unit | Size of the construct | Modification steps involved | 35S labeling site |
|---|---|---|---|---|
| 1 | (-GlcA-GlcNS-)n | Polysaccharide, >6,000 Da | NST | N-[35S]sulfation |
| 2 | (-GlcA-GlcNS6S-)n | Polysaccharide, >6,000 Da | NST and 6-OST | N-[35S]sulfation |
| 3 | (-IdoA-GlcNS6S-)n | Polysaccharide, >6,000 Da | NST, C5-epi, and 6-OST | N-[35S]sulfation |
| 4 | (-GlcA2S-GlcNS-)n | Polysaccharide, >6,000 Da | NST and 2-OST | N-[35S]sulfation |
| 5 | (-IdoA2S-GlcNS-)n | Polysaccharide, >6,000 Da | NST, C5-epi, and 2-OST | N-[35S]sulfation |
| 6 | (-IdoA2S-GlcNS6S-)n | Polysaccharide, >6,000 Da | NST, C5-epi, 2-OST, and 6-OST | N-[35S]sulfation |
| 7 | (-GlcA2S-GlcNS6S-)n | Polysaccharide, >6,000 Da | NST, 2-OST, and 6-OST | N-[35S]sulfation |
| 8 | (-GlcA-GlcNS ± 6S ± 3S-)n and (-IdoA2S-GlcNS6S-)m | Polysaccharide, >6,000 Da | NST, C5-epi, 2-OST, 6-OST, and 3-OST-1 | N-[35S]sulfation |
| 9 | (-GlcA2S-GlcNS6S-)n and (-GlcA-GlcNS ± 6S ± 3S-)m | Polysaccharide, >6,000 Da | NST, 2-OST, 6-OST, and 3-OST-1 | N-[35S]sulfation |
| 10 | Heparin, (-IdoA2S-GlcNS6S-)n and (-GlcA-GlcNS ± 6S ± 3S-)m | Polysaccharide, >6,000 Da | 3-OST-5 | 3-O-[35S]sulfation |
| 11 | GlcNAc6S-GlcA-GlcNS6S3S-IdoA2S-GlcNS6S-GlcA-AnMan | Heptasaccharide (7-mer) | Synthesis started from pure N-sulfo, N-acetylated heptasaccharide | 3-O-[35S]sulfation |
| 12a | GlcA-GlcNS6S- (IdoA ± 2S-GlcNS6S)2-GlcA-AnMan | Octasaccharide (8-mer no 3-O-sulfation) | Synthesis started from pure N-sulfo octasaccharide | N-[35S]sulfation |
| 12b | GlcA-GlcNS6S ± 3S- (IdoA ± 2S-GlcNS6S)2-GlcA-AnMan | Octasaccharide (8-mer with 3-O-sulfation) | Synthesis started from pure N-sulfo octasaccharide | N-[35S]sulfation |
| 13a | GlcNS6S-GlcA-GlcNS6S- (IdoA ± 2S-GlcNS6S)2-GlcA-AnMan | Nonasaccharide (9-mer no 3-O-sulfation) | Synthesis started from pure N-sulfo nonasaccharide | N-[35S]sulfation |
| 13b | GlcNS6S-GlcA-GlcNS6S ± 3S- (IdoA ± 2S-GlcNS6S)2-GlcA-AnMan | Nonasaccharide (9-mer with 3-O-sulfation) | Synthesis started from pure N-sulfo nonasaccharide | N-[35S]sulfation |
| 14 | GlcA-GlcNS6S ± 3S- (GlcA ± 2S-GlcNS6S)3-GlcA-AnMan | Decasaccharide (10-mer no IdoA) | Synthesis started from pure N-sulfo decasaccharide | 6-O-[35S]sulfation |
| 15a | GlcA-GlcNS6S- (IdoA ± 2S-GlcNS6S)3-GlcA-AnMan | Decasaccharide (10-mer no 3-O-sulfation) | Synthesis started from pure N-sulfo decasaccharide | 6-O-[35S]sulfation |
| 15b | GlcA-GlcNS6S ± 3S- (IdoA ± 2S-GlcNS6S)3-GlcA-AnMan | Decasaccharide (10-mer with 3-O-sulfation) | Synthesis started from pure N-sulfo decasaccharide | 6-O-[35S]sulfation |
| 16 | GlcA-GlcNS6S ± 3S- (GlcA ± 2S-GlcNS6S)4-GlcA-AnMan | Dodecasaccharide (12-mer no IdoA) | Synthesis started from pure N-sulfo dodecasaccharide | 6-O-[35S]sulfation |
| 17a | GlcA-GlcNS6S- (IdoA ± 2S-GlcNS6S)4-GlcA-AnMan | Dodecasaccharide (12-mer no 3-O-sulfation) | Synthesis started from pure N-sulfo decasaccharide | 6-O-[35S]sulfation |
| 17b | GlcA-GlcNS6S ± 3S- (IdoA ± 2S-GlcNS6S)4-GlcA-AnMan | Dodecasaccharide (12-mer with 3-O-sulfation) | Synthesis started from pure N-sulfo decasaccharide | 6-O-[35S]sulfation |
| 18 | GlcNS6S- (GlcA ± 2S-GlcNS6S ± 3S)3- (GlcA-GlcNAc6S)2-GlcA-GlcNS6S-GlcA-AnMan | Pentadecasaccharide (15-mer no IdoA) | Synthesis started from pure N-sulfo, N-acetylated pentadecasaccharide | 6-O-[35S]sulfation |
| 19 | GlcNS6S- (IdoA ± 2S-GlcNS6S ± 3S)3- (GlcA-GlcNAc6S)2-GlcA-GlcNS6S-GlcA-AnMan | Pentadecasaccharide (15-mer) | Synthesis started from pure N-sulfo, N-acetylated pentadecasaccharide | 6-O-[35S]sulfation |
| 20 | GlcNAc6S- (GlcA ± 2S-GlcNS6S ± 3S)4- (GlcA-GlcNAc6S)2-GlcA-GlcNS6S-GlcA-AnMan | Heptadecasaccharide (17-mer no IdoA) | Synthesis started from pure N-sulfo, N-acetylated heptadecasaccharide | 6-O-[35S]sulfation |
| 21 | GlcNAc6S- (IdoA2S-GlcNS6S ± 3S)4- (GlcA-GlcNAc6S)2-GlcA-GlcNS6S-GlcA-AnMan | Heptadecasaccharide (17-mer) | Synthesis started from pure N-sulfo, N-acetylated heptadecasaccharide | 6-O-[35S]sulfation |
| 22 | GlcNS6S- (IdoA2S-GlcNS6S ± 3S)5- (GlcA-GlcNAc6S)2-GlcA-GlcNS6S-GlcA-AnMan | Nonadecasaccharide (19-mer no IdoA) | Synthesis started from pure N-sulfo, N-acetylated nonadecasaccharide | 6-O-[35S]sulfation |
| 23 | GlcNS6S- (IdoA2S-GlcNS6S ± 3S)5- (GlcA-GlcNAc6S)2-GlcA-GlcNS6S-GlcA-AnMan | Nonadecasaccharide (19-mer) | Synthesis started from pure N-sulfo, N-acetylated nonadecasaccharide | 6-O-[35S]sulfation |
The oligosaccharides were then modified by C5-epimerase, 2-O-sulfotransferase (2-OST), 6-O-sulfotransferase 1 and 3 (6-OST-1 and 6-OST-3), and 3-O-sulfotransferase 1 (3-OST-1). After the modifications, a mixture of oligosaccharides with different levels of sulfation was obtained as determined by DEAE (diethylaminoethyl)-HPLC (supplemental Fig. S2). To introduce a 35S label to the polysaccharides or oligosaccharides, [35S]PAPS replaced unlabeled PAPS. 35S-labeled 3-O-sulfated heparin was prepared by incubating heparin with 3-OST-5 enzyme and [35S]PAPS, and the product was purified by DEAE chromatography.
The procedures for preparing the N-sulfo heparosan, PAPS and [35S]PAPS, UDP-GlcNTFA, and disaccharide (GlcA-AnMan) starting materials are described elsewhere (22, 23, 27). The enzymes used for the synthesis, including KfiA, pmHS2, NST, C5-epimerase, 2-OST, 6-OST-1, 6-OST-3, and 3-OST-1, were expressed in E. coli as described previously (24).
Expression Plasmids
The cDNA for human Stab1 (a kind gift of J. Kzhyshkowska, University of Heidelberg) and Stab2/315-HARE were ligated into the multi-cloning site of the pcDNA5/FRT/V5-6×HIS-TOPO vector. The Stab2/190-HARE cDNA encoding the C-terminal 1416 amino acids is cloned in pSecTag/FRT/V5–6×HIS-TOPO, which provides a secretion signal for the 190-HARE protein (8). Plasmids encoding the secreted ectodomain were generated by single primer deletion mutagenesis (7) in which the transmembrane and cytoplasmic domain encoding regions were deleted, and the resulting plasmids were then used to create stable cells that secreted properly folded and functional ectodomains in the medium. The ectodomains comprised amino acids M1-P2475 for Stab1, M1-T2458 for Stabilin-2/315-HARE, and S1136-V2453 for Stab2/190-HARE.
Endocytosis Assays
Stably transfected cells expressing Stab1 or Stab2 receptors or only Hygromycin B-resistant (empty vector) were plated in 24-well dishes and grown in DMEM with 8% FBS and 50 μg/ml Hygromycin B for at least 2 days prior to the experiments. The cells were incubated at 37 °C for 3 h with fresh Endocytosis Medium supplemented with labeled [35S]HS constructs (2.0 × 104 cpm/ml). For those experiments utilizing antithrombin III (ATIII), the [35S]HS constructs were preincubated with ATIII (0.2 mg/ml) for 30 min prior to diluting 10-fold in endocytosis medium. Specific binding or endocytosis was assessed in the presence of excess unlabeled heparin (0.1 mg/ml) to determine background count per minute (cpm) values. These values were subtracted from all data points to determine the specific [35S]HS endocytosis. At the termination times, cells were washed three times with ice-cold Hanks' buffered saline solution and lysed in 0.3 n NaOH, and radioactivity and protein content were determined and expressed as cpm/μg of protein ± S.D. 35S radioactivity of all samples was measured by a Beckman-Coulter LS6500 scintillation counter. Nonspecific binding/background radiation levels were consistently between 16 and 20 cpm for all experiments.
Direct Binding Assays
To assess direct protein-HS binding, ectodomains of each receptor were expressed and secreted in stable cell lines. The ectodomains were immunoprecipitated with a goat anti-V5 resin (Bethyl Laboratories), washed with TBS, and then incubated with 4.0 × 105 cpm of each [35S]HS construct for 1.5 h under rotation. The resin was centrifuged, washed three times with TBS, and then placed in scintillation fluid and quantified by a Beckman-Coulter LS6500 scintillation counter. The amount of protein on the resin was quantified by separation with 5% SDS-PAGE, blotted, and probed with rabbit anti-V5 antibody (Bethyl Laboratories), and images were captured on film.
Assessment of Liver Clearance
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska under the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines. BALB/c mice were placed under general anesthesia (isoflurane) on a warming platform during the entire procedure. Once unconsciousness was confirmed, the mice were injected via the lateral tail vein using a 27-gauge × 1/2 inch needle with 0.053 μCi of 35S-labeled HS construct. The radiolabeled material was allowed to circulate for 10 min followed by abdominal exposure and severance of the descending aorta abdominalis for bleed out. The liver was collected, briefly washed to get rid of residual blood, cut into smaller (∼0.1 g) pieces, weighed, and then homogenized with a PowerGen 125 (Fisher) tissue homogenizer in 0.75 ml of 1% Nonidet P-40. Homogenized tissue was then spun at 12,000 × g for 2 min to clear out insoluble material, and supernatants were mixed with 4 ml of scintillation fluid and counted.
RESULTS
35S-labeled Heparin Binds Stabilin-1 and Stabilin-2 Receptors
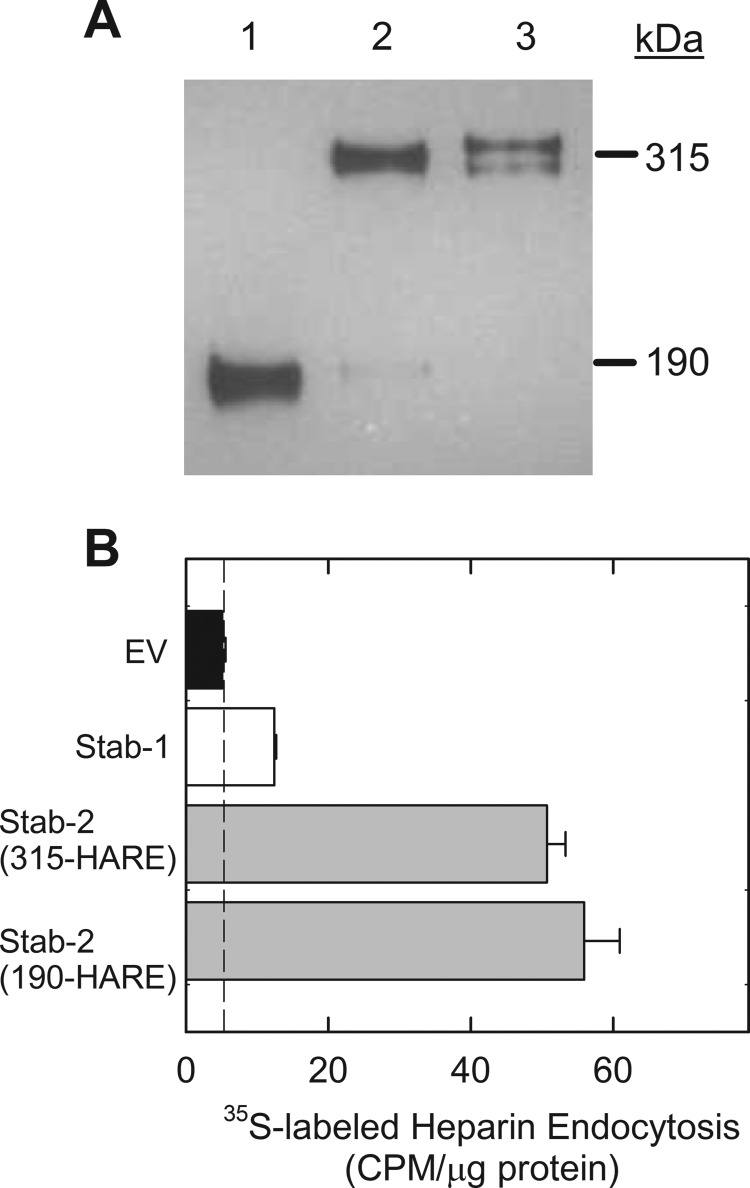
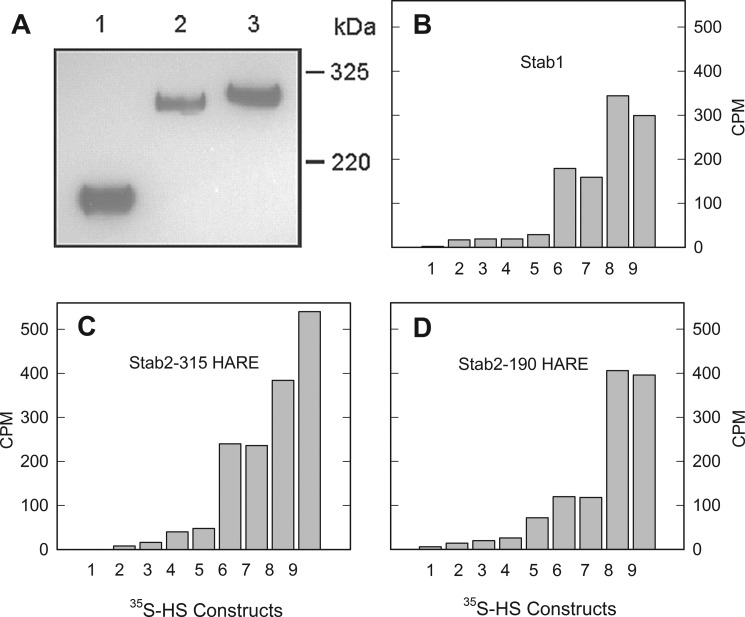
Cell lines stably expressing Stab2 and empty vector were created as described previously (7, 8). The cell line stably expressing human Stab1 cDNA was also constructed with identical methodology. The expressions of Stab1 and Stab2 are shown by a representative Western blot of the cell lysates probed with the anti-V5 antibody (Fig. 2A). The expression of the recombinant receptors is similar to the native receptors in that a percentage of the full-length Stab2 is processed to the smaller 190-kDa isoform and Stab1 is expressed as a tight doublet at around 323 kDa. The level of expression for 315-HARE Stab2 is less than that of 190-HARE Stab2 in the recombinant cell lines; thus, the HS binding values for the 315-HARE cell line are typically lower.
FIGURE 2.
Internalization of 3-O-sulfated heparin via Stab1 and Stab2. A, cell lysates (20 μg) were separated by 5% SDS-PAGE, blotted to nitrocellulose, and probed with anti-V5 antibody. Lane 1, Stab2/190-HARE; lane 2, Stab2/315-HARE; lane 3, Stab1. B, stable cell lines expressing Stab1 (white bar), both Stab2 isoforms (gray bars), and empty vector (EV, black bar) were incubated with 35S-labeled 3-O-sulfated heparin for 3 h. The dotted line represents nonspecific binding values and is subtracted from the data to determine receptor-specific endocytosis. Endocytosis was evaluated by cpm/μg of cell lysate protein for each cell line, mean ± S.D., n = 3.
35S-labeled heparin was used as a positive control to assess binding and endocytosis throughout the study. The 35S-labeled heparin (or 35S-labeled 3-O-sulfated heparin) was specifically labeled at the 3-OH position by incubating heparin with 3-OST-5 enzyme and [35S]PAPS. As shown in Fig. 2B, we observed an elevated level of endocytosis for 35S-labeled heparin in cells expressing Stab receptors when compared with cells expressing the empty vector. Stab1, the weakest receptor for heparin, bound heparin at a level slightly above that of the negative control (empty vector cells). Two Stab2 isoforms (190-HARE and 315-HARE) displayed significantly higher levels of internalization of heparin than the negative control (Fig. 2B).
Structural Requirement for HS Endocytosis
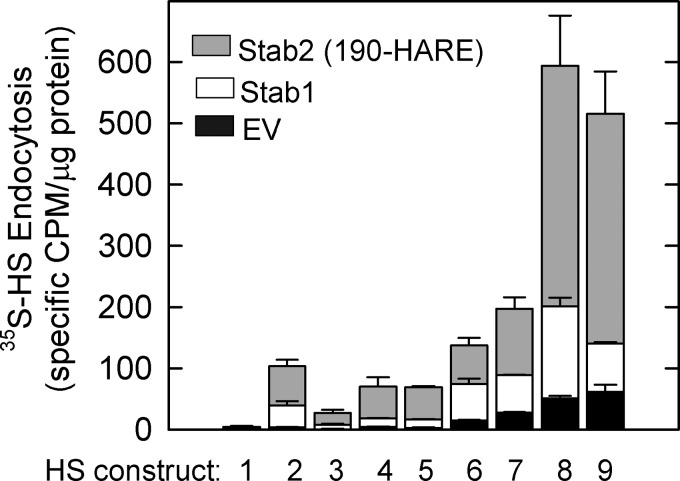
HS contains various types of sulfations and GlcA versus IdoA residues. To understand the structural selectivity for binding to the Stabilin receptors, we chemoenzymatically produced a series of HS polysaccharides with specific modifications ranging from N-sulfation alone on the glucosamine residue (construct 1) to the much more complex sulfation patterns commonly found in heparin and HS (constructs 8 and 9) (Table 1 and Fig. 1). We observed that the polysaccharides carrying N-sulfation only or a combination of N-sulfation and a single O-sulfation type display low internalization via the Stabilin receptors (Fig. 3, constructs 1-5). Increasing the O-sulfation types (or combining N-sulfation with 2-O- and 6-O-sulfation) elevated the internalization via the Stabilin receptors (Fig. 3, constructs 6 and 7). Interestingly, the addition of 3-O-sulfation showed the highest internalization rate (Fig. 3, constructs 8 and 9). Given the fact that 3-O-sulfation represents a rare modification in HS (28), our results suggest that Stabilin receptors recognize unique sulfation patterns independent of the charge density. This conclusion was further strengthened by the competitive inhibition using antithrombin (see Fig. 5) as well as by employing the oligosaccharide substrates as described below (see Fig. 7). Constructs 6 and 8 (with IdoA residues) and 7 and 9 (without IdoA residues) exhibited similar binding to the receptors, suggesting that the presence of IdoA has no significant effect on endocytosis.
FIGURE 3.
Modifications of heparosan determine internalization rates. The empty vector (EV, black bars), Stab1 (white bars), and Stab2/190-HARE (gray bars) cell lines were incubated with equal amounts of each modified HS construct for 3 h. The cells were then washed, and the lysates were counted by scintillation autoradiography. The amounts of [35S]HS constructs were determined by specific cpm/μg of protein cell lysate, mean ± S.D., n = 3.
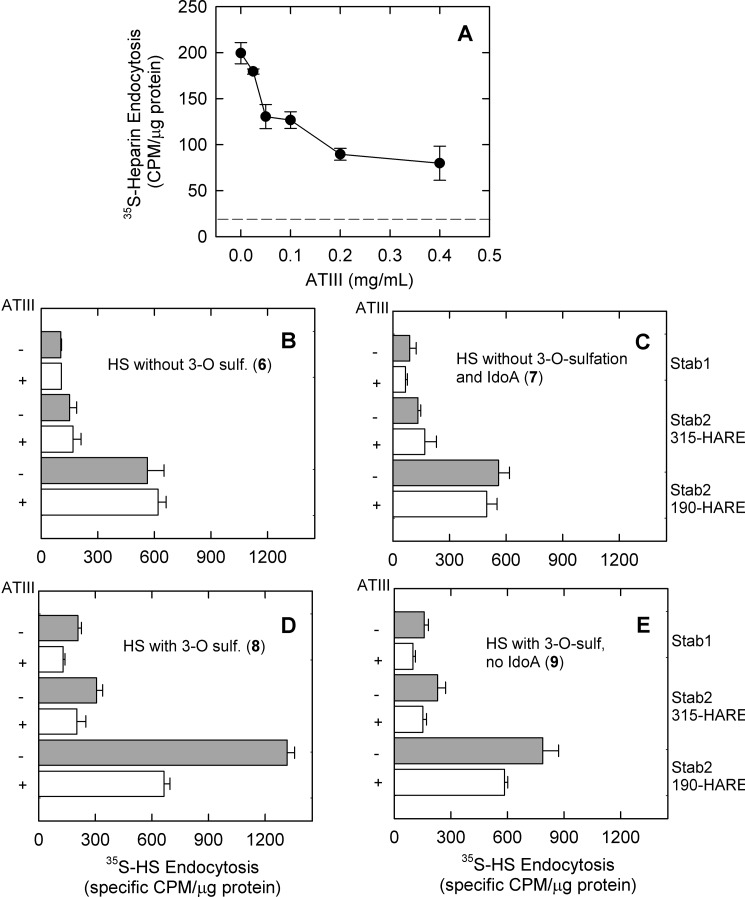
FIGURE 5.
ATIII significantly inhibits Stabilin-mediated heparin endocytosis. A, a dose-response curve for ATIII against heparin was generated by incubating Stab1 cells with labeled heparin and varying amounts of ATIII. At each concentration, cells were processed as described under “Experimental Procedures.” The dotted line represents the threshold for nonspecific binding. B–E, an equal amount of each construct (6-9) was preincubated with endocytosis medium (gray bars) or endocytosis medium supplemented with 0.2 mg/ml ATIII (white bars) and then added to each indicated cell line. After 3 h, cells were washed, and radioactivity and protein contents were determined as specific cpm/μg of protein, mean ± S.D., n = 4. For constructs 8 and 9 (D and E), the statistical differences in internalization in the presence or absence of ATIII were p ≤ 0.05 for all cell lines in contrast to constructs 6 and 7 (B and C), which showed no significant difference. 3-O sulf., 3-O-sulfation.
FIGURE 7.
The 3-O-sulfated decasaccharide is the minimal size and modification required for binding to the Stabilin receptors. Both Stab1 (A) and Stab2/315-HARE (B) were incubated with heparin (positive control, black bar) and ultralow molecular weight heparin (7-mer, negative control), 3-O-sulfated oligosaccharides (12b, 13b, 15b, 17b; dark gray bars), and non-3-O-sulfated oligosaccharides (12a, 13a, 15a, 17a; white bars) for 3 h. Endocytosis of each oligonucleotide was confirmed by washing the cells with Hanks' buffered saline solution and determining radioactivity and protein levels, mean ± S.D., n = 3.
We also examined the direct binding between the polysaccharide constructs and different Stabilin receptor ectodomains (Fig. 4). The Stabilin receptors were captured from the medium of cells secreting the corresponding receptor (Fig. 4A) using anti-V5 antibody coupled to Sepharose resin and exposed to 35S-labeled HS constructs. As anticipated, a similar trend was observed for direct binding, namely that higher sulfation levels for a given construct lead to greater binding to the Stabilin receptors (Fig. 4, B–D). In these experiments, the empty vector negative control was used as a baseline for nonspecific polysaccharide binding due to the inherent “stickiness” of the high charge on the HS to the antibody resin. Again, the polysaccharides carrying 3-O-sulfation (constructs 8 and 9) exhibited the highest binding toward the Stabilin receptors. We also observed that the binding levels of 8 and 9 to the 190-HARE and Stab1 receptors were comparable, suggesting that the receptor makes no distinction between IdoA and GlcA (Fig. 4, B and D). Although slightly higher binding was observed for construct 9 with 315-HARE (Fig. 4C), we cannot conclude that IdoA decreases the binding of HS to the receptor.
FIGURE 4.
Direct binding of HS oligosaccharides with Stab1 and Stab2 ectodomains. Ectodomains/secreted versions of each receptor were immunopurified by V5 resin and were either subject to 5% SDS-PAGE, blotted, and probed with anti-V5 antibody (Lane 1, s190-HARE/Stab2; lane 2, s315-HARE/Stab2; lane 3, sStab1) (A) or incubated with an equal amount of each HS construct for 1.5 h under rotation (B–D). The resin was washed with Tris-buffered saline three times and then mixed with scintillation mixture, and cpm was determined. Counts per minute were normalized to the amount of protein on the resin.
Antithrombin III Competes with Stabilins for HS Binding
ATIII is a serine protease inhibitor that regulates blood coagulation by targeting thrombin and factor Xa (29). Inhibition of these targets occurs via the formation of a 1:1 complex between ATIII and HS that contains a specific heparin pentasaccharide sequence for ATIII binding (30, 31). The 3-O-sulfated glucosamine residue within the pentasaccharide is the last modification step during heparin biosynthesis and is carried out by 3-OST-1 (32). Because the 3-O-sulfated polysaccharides exhibited increased Stabilin-mediated endocytosis, we asked whether Stabilin and ATIII bind to the same site in HS. First, we demonstrated the inhibition of [35S]heparin endocytosis with Stab1 cells in the presence of increasing concentrations of ATIII (Fig. 5A). We utilized both ATIII-binding polysaccharide constructs, constructs 8 and 9 containing the 3-O-sulfation, and ATIII-nonbinding polysaccharide constructs, constructs 6 and 7. As expected, constructs 6-9 displayed distinct Stabilin-mediated internalization effects in the presence and absence of ATIII. ATIII did not interfere with the endocytosis of constructs 6 and 7 in all three cell lines (Fig. 5, B and C). In contrast, endocytosis of constructs 8 and 9 was significantly decreased, but not entirely inhibited, in all three cell lines (Fig. 5, D and E). It is also interesting to note that there is a significant amount of internalization of 35S-polysaccharide after ATIII inhibition (constructs 8 and 9). The internalization levels that could not be inhibited by ATIII for constructs 8 and 9 were similar to the ones observed for constructs 6 and 7. These data suggest that ATIII and membrane-bound Stabilin receptors bind to the same site in HS for internalization. Our data also suggest that Stabs bind to HS in two modes. One mode is that it binds to the site that also interacts with ATIII, which may be the more significant interaction site; another mode is that it binds to highly negatively charged polysaccharides without structural selectivity.
The Size of the Saccharide Versus Endocytosis in Stab1 and Stab2 Cell Lines
We next investigated the length of heparin required for endocytosis. First, we used heparin oligosaccharides of different lengths ranging from a 10-mer to a 19-mer (constructs 14-23). None of these oligosaccharides were taken up by the empty vector cells including those without IdoA residues (data not shown). Next, we tested all of the constructs 14-23 with both Stab1 and Stab2 (315-HARE) cell lines. As summarized in Fig. 6B, both the 7-mer (11) and 8-mer (12) exhibit very little endocytosis, but endocytosis is slightly increased in the 9-mer (13b), especially for the Stab1 cells. The biggest jump in the incidence of endocytosis occurs when one additional sugar is added to make a 10-mer (15b). The amount of internalized oligosaccharides remained steady up to a nonadecasaccharide (19-mer, 23). From these data, the minimal size for significant endocytosis is a 10-mer for both Stab1 and Stab2 receptors. Similar results were observed for the 190-HARE receptor.
FIGURE 6.
Size of oligosaccharide affects endocytic rates. A, Both Stab1 and Stab2/315-HAREcell lines were incubated with the 7-mer (11), 8-mer (12b), 9-mer (13b), 10-mer (15b), 15-mer (19), and 19-mer (23) for 3 h. All cell lines were washed with Hanks' buffered saline solution followed by determination of cpm by scintillation counting and protein by the Bradford assay; mean ± S.D., n = 5. B, both radiolabeled 7-mer and 19-mer oligosaccharides were incubated for 3 h with Stab1 cells (white bars) and Stab2/315-HARE cells (gray bars). To confirm that the short synthetic pentasaccharide does not bind with any of the Stabilin receptors, we added a 50-fold excess clinical grade Arixtra (Ax) to the endocytosis medium to both the 19-mer (black bars) and the 7-mer (not shown). After a 3-h incubation for all samples, the cells were washed and lysed, and radioactivity and protein were determined, mean ± S.D., n = 3. The Stab2/190-HARE cell lines showed similar results (data not shown).
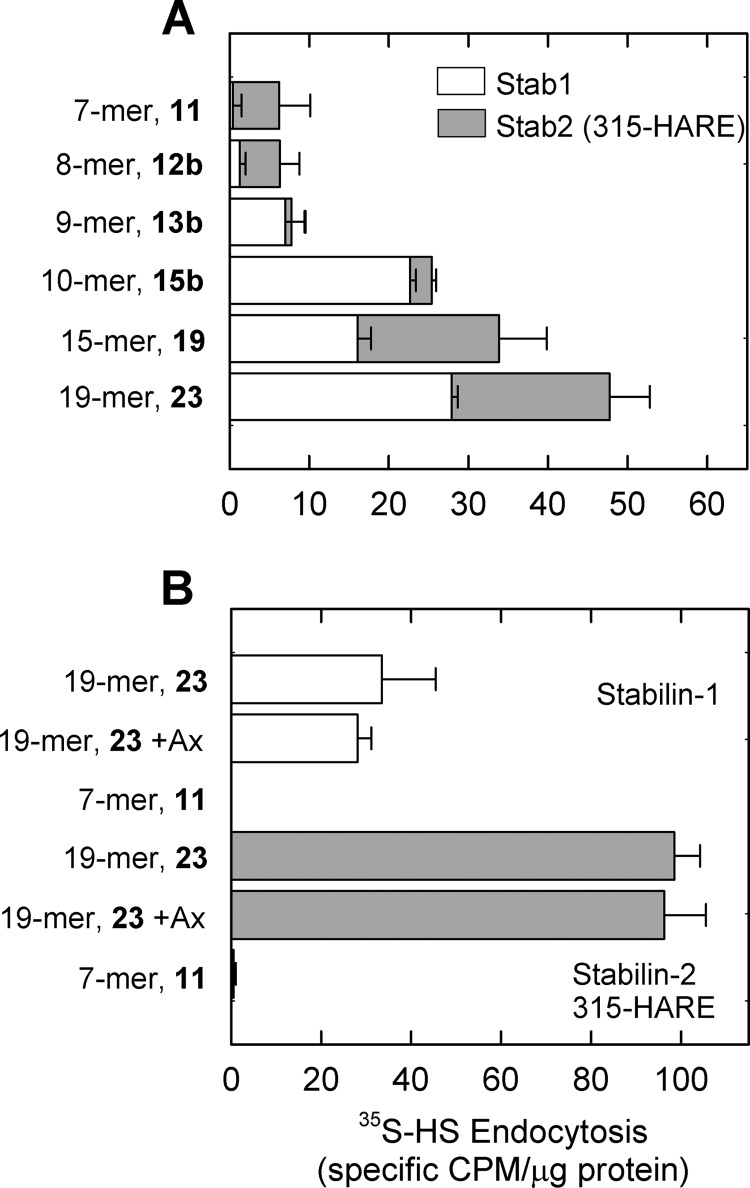
We next compared the internalization of the 7-mer (11) and 19-mer (23) for internalization in full-length Stab1 and Stab2 cell lines. We did not observe any internalization of the 7-mer in cells expressing either Stab1 (white bars) or Stab2/315-HARE (gray bars) (Fig. 6B). Furthermore, we did not observe a significant decrease in the internalization of the 19-mer (23) by competition with the synthetic pentasaccharide, Arixtra, at 50-fold molar excess. These results demonstrate that the minimal length of a heparin oligosaccharide cleared by these cell lines is at least 10 sugars in length and that the synthetic pentasaccharide is not cleared by cells expressing the Stabilin receptors.
3-O-Sulfation Is Required for Decasaccharides to Bind to Stab Receptors and for Endocytosis
To further establish the role of 3-O-sulfation and the size requirement for the binding to Stab receptors, we utilized a series of oligosaccharides with and without 3-O-sulfation. These experiments also utilized labeled heparin as a positive control (black bars) and a structurally defined heptasaccharide (7-mer, 11) (24) as a negative control. Both the Stab1 (Fig. 7A) and the Stab2 (Fig. 7B) cells show robust endocytosis with heparin and no endocytosis with 11. The oligosaccharides composed of eight or nine sugars without 3-O-sulfation (12a and 13a) did not show endocytosis in contrast to their 3-O-sulfated counterparts that exhibited marginal to very low endocytosis (12b and 13b, Fig. 7, gray bars). This is in contrast with the 3-O-sulfated decasaccharide (15b) and dodecasaccharide (17b), which exhibit robust endocytosis with the dodecasaccharide that is almost comparable with the heparin control (Fig. 7, A and B). Furthermore, the deca- and dodecasaccharide without 3-O-sulfation (15a and 17a, white bars) were not internalized near the extent as their sulfated counterparts (gray bars). Taken together, our data are consistent with the conclusions that 3-O-sulfation plays a significant role in binding to Stab receptors in the endocytosis of heparin and that the minimum size for binding to the Stab receptors is a decasaccharide.
3-O-Sulfated Decasaccharide Retention in Liver Is Similar to Heparin
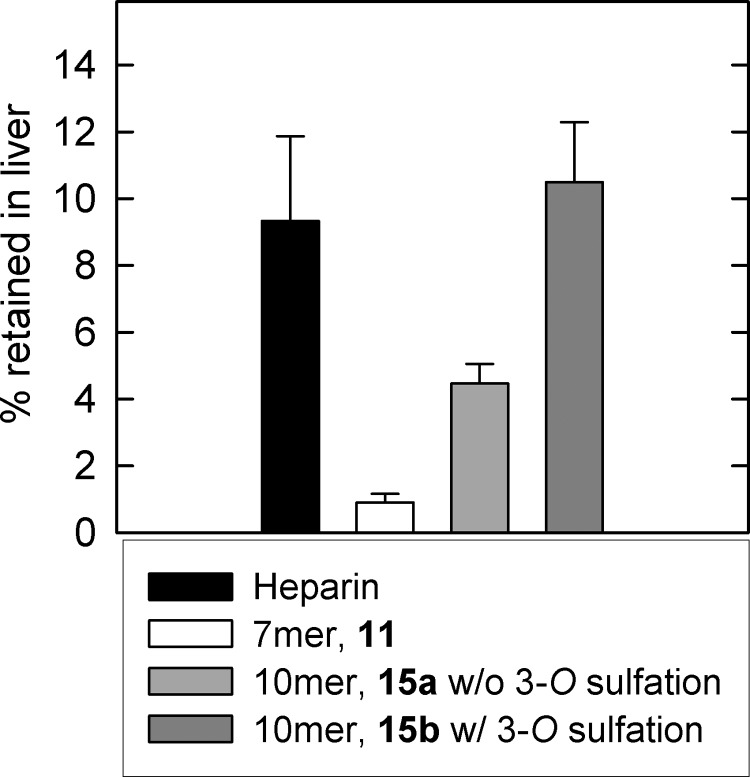
It is known that UFH is retained by liver to display a fast clearance rate, whereas Fondaparinux (a pentasaccharide) is not and displays a slow clearance rate in vivo (33). Therefore, these size and sulfation parameters should hold true if the Stabilin receptors are responsible for the bulk clearance of systemic heparin. To test this, we individually injected mice via the tail vein with four 35S-labeled constructs: heparin (positive control); 7-mer (21, negative control); the decasaccharide without 3-O-sulfation (12a); and the decasaccharide with 3-O-sulfation (12b) (Fig. 8). Mice under general anesthesia were injected with equal amounts of radioactive material that was allowed to circulate; the mice were then bled out, and the livers were collected and processed for scintillation counting. We found that the 7-mer (21, white bar) was not retained in the liver. The decasaccharide with 3-O-sulfation (12b, dark gray bar) was retained in the liver to a similar degree as heparin (black bar). In contrast, the retention of the decasaccharide without 3-O-sulfation (12a) in the liver was significantly reduced. To this end, our data suggest that the 3-O-sulfation contributed to the retention in liver in vivo.
FIGURE 8.
3-O-sulfation is required for efficient liver retention. Mice were injected via the tail vein with equal amounts of labeled heparin (black bar), 7-mer (11, white bar), decasaccharide without 3-O-sulfation (15a, light gray bar), or decasaccharide with 3-O-sulfation (15b, dark gray bar). After a short incubation time, mice were bled out, and livers were processed for scintillation counting. The data were measured by (cpm/mass of liver) of at least three liver samples from each mouse divided by the total cpm injected. Three mice were used for each construct (n = 3 ± S.E.).
DISCUSSION
Understanding the clearance rate of heparin has significant clinical implications for improving the safety of heparin-based drugs. For surgical applications, a fast-clearing heparin drug is preferred, allowing the anticoagulant effect to rapidly disappear after the operation and thus reducing the risk for bleeding side effects. In contrast, a slow-clearing heparin is more desirable for patients with ongoing use, avoiding repetitive dosing. Indeed, UFH is widely used in surgical procedures and kidney dialysis, whereas LMWH and Fondaparinux are more commonly used as prophylactic agents among high risk patients prone to thrombosis (34, 35). Although it is known that the size of the heparin chain plays a role in the rate of clearance in patients, the precise structural requirements for regulating clearance remain uncharacterized. Previous studies have demonstrated that a liver endothelial cell receptor, known as Stab, is primarily responsible for heparin clearance (5). By taking advantage of our recent success in synthesizing heparins (22, 24), we investigated the contribution of the sulfation and the size of heparin to the binding to Stab proteins. Our results provide a molecular basis for designing synthetic heparins with desired clearance rates for unique clinical applications.
Our study reveals two binding modes between heparin and Stab proteins. In one binding mode, the heparin polysaccharide binds to Stab1/2 nonspecifically; such binding requires a long polysaccharide chain. Another binding mode is specific, in which Stab proteins recognize a saccharide domain containing a 3-O-sulfo group. In the specific binding mode, a much shorter oligosaccharide is sufficient to display high affinity to Stabs. We also demonstrate that a 3-O-sulfated decasaccharide displays higher retention in the liver in mice when compared with its counterpart without 3-O-sulfation, suggesting that Stab binding affinity correlates to the clearance in vivo.
Our data also suggest that Stab proteins recognize a unique saccharide sequence. Although we do not know the precise saccharide structure recognized by the Stabilin receptors at the present time, some overlaps between the Stab-binding sequence and ATIII-binding sequence exist. We know that 3-O-sulfation is a critical modification for displaying high binding affinity to ATIII and for carrying anticoagulant activity (36). Further, competitive binding of the Stab receptors and ATIII to heparins was observed in this study. The mechanistic effect of 3-O-sulfation on the binding to the Stabilin receptors is currently unknown. We postulate that this effect is unlikely to be purely attributed to an increase in charge density because 3-O-sulfation is a rare modification in HS (29). A recent study suggests that 3-O-sulfation may affect the conformation of neighboring IdoA2S residues to rearrange the positioning of sulfo groups (37). In addition, previous studies have shown that one natural highly sulfated chondroitin sulfate (CS-D) did not compete with heparin in contrast to other similar chondroitin sulfates (CS-B, CS-E) that demonstrated some degree of competition, providing evidence that charge is not the only determinant for heparin binding to the Stabilins (9).
This is the first study demonstrating the structural selectivity of heparin binding to Stab1. The Stab1 receptor is expressed in the liver sinusoidal endothelium as well as in alternatively activated macrophages (11) and other physiological niches (38) and may play a role in both systemic and localized clearance of heparinoid molecules. Activated macrophages may modulate the amount of dermatan sulfate in wound fluids that promote FGF-2 activity (39, 40). It is possible that Stab 1 plays a role in regulating the amount of anticoagulant HS in wound fluid.
It should be noted that the rate of endocytosis also depends on the concentration of Stab receptors on the cell surface. The 190-HARE Stab2 isoform always showed the highest increase in endocytosis because these cells produce more receptor per microgram of cell lysate than the other two cell lines. Not surprisingly, the liver endothelial cells exhibit a much higher ratio of 190/315-HARE Stab2 than the recombinant cell lines, which may account for the rapid uptake of heparin within the liver (41). The consistently lower internalization rate of heparin in the Stab1 cells may be reflective of the amount of total surface receptor available to bind and internalize heparin, a lower binding affinity, or the unavailability to bind ligand due to the very short transient time on the cell surface. Others have reported the very short transient time of Stab1 on the cell surface (11, 42). In these experiments, we noticed a discrepancy in binding versus endocytosis. Our in vitro binding assays by immunoprecipitation of the ectodomain of Stab1 revealed that the binding of the HS oligomers was qualitatively about as high as that of the Stab2 ectodomains. This contrasts with the cell-based assays and reveals that a combination of surface availability and rapid turnover, not binding affinity, may be responsible for lower endocytic rates in Stab1 cells. In addition, the expression of Stab receptors can be regulated by other cellular mechanisms. A genetic screen in which human umbilical vein endothelial cells activated with VEGF revealed an increase of Stab1 expression reveals that growth factors and cytokines are able to alter endocytosis profiles of tissues (26).
This work was supported, in whole or in part, by National Institutes of Health Grant 1R01HL094463 (to J. L.). This work was also supported by funds from the University of Nebraska (to E. N. H.).

This article contains supplemental Figs. S1 and S2.
Y. Xu, E. H. Pempe, and J. Liu, submitted for publication.
- UFH
- unfractionated heparin
- HARE
- hyaluronic acid receptor for endocytosis
- HS
- heparan sulfate
- CS
- chondroitin sulfate
- Stab1
- Stabilin-1
- Stab2
- Stabilin-2
- LMWH
- low molecular weight heparin
- IdoA
- iduronic acid
- PAPS
- 3′-phosphoadenosine 5′-phosphosulfate
- OST
- sulfotransferase
- ATIII
- antithrombin III
- ESI
- electrospray ionization
- AnMan
- 2,5-Anhydromannitol
- GlcNTFA
- N-trifluoroacetylated glucosamine
- NST
- N-sulfotransferase.
REFERENCES
- 1. Jacobs L. G. (2003) Prophylactic anticoagulation for venous thromboembolic disease in geriatric patients. J. Am. Geriatr. Soc. 51, 1472–1478 [DOI] [PubMed] [Google Scholar]
- 2. Verhaeghe R. (1998) The use of low molecular weight heparins in cardiovascular disease. Acta Cardiol. 53, 15–21 [PubMed] [Google Scholar]
- 3. Dinwoodey D. L., Ansell J. E. (2006) Heparins, low molecular weight heparins, and pentasaccharides. Clin. Geriatr. Med. 22, 1–15 [DOI] [PubMed] [Google Scholar]
- 4. Oie C. I., Olsen R., Smedsrød B., Hansen J. B. (2008) Liver sinusoidal endothelial cells are the principal site for elimination of unfractionated heparin from the circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G520–G528 [DOI] [PubMed] [Google Scholar]
- 5. Harris E. N., Weigel J. A., Weigel P. H. (2008) The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J. Biol. Chem. 283, 17341–17350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansen B., Longati P., Elvevold K., Nedredal G. I., Schledzewski K., Olsen R., Falkowski M., Kzhyshkowska J., Carlsson F., Johansson S., Smedsrød B., Goerdt S., Johansson S., McCourt P. (2005) Stabilin-1 and stabilin-2 are both directed into the early endocytic pathway in hepatic sinusoidal endothelium via interactions with clathrin/AP-2, independent of ligand binding. Exp. Cell Res. 303, 160–173 [DOI] [PubMed] [Google Scholar]
- 7. Harris E. N., Kyosseva S. V., Weigel J. A., Weigel P. H. (2007) Expression, processing, and glycosaminoglycan binding activity of the recombinant human 315-kDa hyaluronic acid receptor for endocytosis (HARE). J. Biol. Chem. 282, 2785–2797 [DOI] [PubMed] [Google Scholar]
- 8. Harris E. N., Weigel J. A., Weigel P. H. (2004) Endocytic function, glycosaminoglycan specificity, and antibody sensitivity of the recombinant human 190-kDa hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 279, 36201–36209 [DOI] [PubMed] [Google Scholar]
- 9. Harris E. N., Weigel P. H. (2008) The ligand-binding profile of HARE: hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low density lipoprotein, dermatan sulfate, and CS-E. Glycobiology 18, 638–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goerdt S., Bhardwaj R., Sorg C. (1993) Inducible expression of MS-1 high molecular weight protein by endothelial cells of continuous origin and by dendritic cells/macrophages in vivo and in vitro. Am. J. Pathol. 142, 1409–1422 [PMC free article] [PubMed] [Google Scholar]
- 11. Kzhyshkowska J., Gratchev A., Goerdt S. (2006) Stabilin-1, a homeostatic scavenger receptor with multiple functions. J. Cell Mol. Med. 10, 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kzhyshkowska J., Workman G., Cardó-Vila M., Arap W., Pasqualini R., Gratchev A., Krusell L., Goerdt S., Sage E. H. (2006) Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J. Immunol. 176, 5825–5832 [DOI] [PubMed] [Google Scholar]
- 13. Kzhyshkowska J., Gratchev A., Schmuttermaier C., Brundiers H., Krusell L., Mamidi S., Zhang J., Workman G., Sage E. H., Anderle C., Sedlmayr P., Goerdt S. (2008) Alternatively activated macrophages regulate extracellular levels of the hormone placental lactogen via receptor-mediated uptake and transcytosis. J. Immunol. 180, 3028–3037 [DOI] [PubMed] [Google Scholar]
- 14. Kzhyshkowska J., Gratchev A., Brundiers H., Mamidi S., Krusell L., Goerdt S. (2005) Phosphatidylinositide 3-kinase activity is required for stabilin-1-mediated endosomal transport of acLDL. Immunobiology 210, 161–173 [DOI] [PubMed] [Google Scholar]
- 15. Schledzewski K., Géraud C., Arnold B., Wang S., Gröne H. J., Kempf T., Wollert K. C., Straub B. K., Schirmacher P., Demory A., Schönhaber H., Gratchev A., Dietz L., Thierse H. J., Kzhyshkowska J., Goerdt S. (2011) Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J. Clin. Invest. 121, 703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park S. Y., Jung M. Y., Kim H. J., Lee S. J., Kim S. Y., Lee B. H., Kwon T. H., Park R. W., Kim I. S. (2008) Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 15, 192–201 [DOI] [PubMed] [Google Scholar]
- 17. Lee S. J., Park S. Y., Jung M. Y., Bae S. M., Kim I. S. (2011) Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood 117, 5215–5223 [DOI] [PubMed] [Google Scholar]
- 18. Park S. Y., Jung M. Y., Lee S. J., Kang K. B., Gratchev A., Riabov V., Kzhyshkowska J., Kim I. S. (2009) Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J. Cell Sci. 122, 3365–3373 [DOI] [PubMed] [Google Scholar]
- 19. Tamura Y., Adachi H., Osuga J., Ohashi K., Yahagi N., Sekiya M., Okazaki H., Tomita S., Iizuka Y., Shimano H., Nagai R., Kimura S., Tsujimoto M., Ishibashi S. (2003) FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J. Biol. Chem. 278, 12613–12617 [DOI] [PubMed] [Google Scholar]
- 20. Peterson S., Frick A., Liu J. (2009) Design of biologically active heparan sulfate and heparin using an enzyme-based approach. Nat. Prod. Rep. 26, 610–627 [DOI] [PubMed] [Google Scholar]
- 21. Chen J., Avci F. Y., Muñoz E. M., McDowell L. M., Chen M., Pedersen L. C., Zhang L., Linhardt R. J., Liu J. (2005) Enzymatic redesigning of biologically active heparan sulfate. J. Biol. Chem. 280, 42817–42825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen J., Jones C. L., Liu J. (2007) Using an enzymatic combinatorial approach to identify anticoagulant heparan sulfate structures. Chem. Biol. 14, 986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu R., Xu Y., Chen M., Weïwer M., Zhou X., Bridges A. S., DeAngelis P. L., Zhang Q., Linhardt R. J., Liu J. (2010) Chemoenzymatic design of heparan sulfate oligosaccharides. J. Biol. Chem. 285, 34240–34249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu Y., Masuko S., Takieddin M., Xu H., Liu R., Jing J., Mousa S. A., Linhardt R. J., Liu J. (2011) Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins. Science 334, 498–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xia G., Chen J., Tiwari V., Ju W., Li J. P., Malmstrom A., Shukla D., Liu J. (2002) Heparan sulfate 3-O-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for herpes simplex virus, type 1. J. Biol. Chem. 277, 37912–37919 [DOI] [PubMed] [Google Scholar]
- 26. Wary K. K., Thakker G. D., Humtsoe J. O., Yang J. (2003) Analysis of VEGF-responsive genes involved in the activation of endothelial cells. Mol. Cancer 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou X., Chandarajoti K., Pham T. Q., Liu R., Liu J. (2011) Expression of heparan sulfate sulfotransferases in Kluyveromyces lactis and preparation of 3′-phosphoadenosine-5′-phosphosulfate. Glycobiology 21, 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J., Pedersen L. C. (2007) Anticoagulant heparan sulfate: structural specificity and biosynthesis. Appl. Microbiol. Biotechnol. 74, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberg R. D., Shworak N. W., Liu J., Schwartz J. J., Zhang L. (1997) Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge, but how is synthesis regulated? J. Clin. Invest. 99, 2062–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olson S. T., Chuang Y. J. (2002) Heparin activates antithrombin anticoagulant function by generating new interaction sites (exosites) for blood clotting proteinases. Trends Cardiovasc. Med. 12, 331–338 [DOI] [PubMed] [Google Scholar]
- 31. Richard B., Swanson R., Olson S. T. (2009) The signature 3-O-sulfo group of the anticoagulant heparin sequence is critical for heparin binding to antithrombin but is not required for allosteric activation. J. Biol. Chem. 284, 27054–27064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J., Shworak N. W., Fritze L. M., Edelberg J. M., Rosenberg R. D. (1996) Purification of heparan sulfate d-glucosaminyl 3-O-sulfotransferase. J. Biol. Chem. 271, 27072–27082 [DOI] [PubMed] [Google Scholar]
- 33. Hirsh J., O'Donnell M., Eikelboom J. W. (2007) Beyond unfractionated heparin and warfarin: current and future advances. Circulation 116, 552–560 [DOI] [PubMed] [Google Scholar]
- 34. Tobu M., Iqbal O., Hoppensteadt D., Neville B., Messmore H. L., Fareed J. (2004) Anti-Xa and anti-IIa drugs alter international normalized ratio measurements: potential problems in the monitoring of oral anticoagulants. Clin. Appl. Thromb. Hemost. 10, 301–309 [DOI] [PubMed] [Google Scholar]
- 35. Weitz J. I. (2010) Potential of new anticoagulants in patients with cancer. Thromb. Res. 125, Suppl. 2, S30–S35 [DOI] [PubMed] [Google Scholar]
- 36. Atha D. H., Lormeau J. C., Petitou M., Rosenberg R. D., Choay J. (1985) Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry 24, 6723–6729 [DOI] [PubMed] [Google Scholar]
- 37. Moon A. F., Xu Y., Woody S. M., Krahn J. M., Linhardt R. J., Liu J., Pedersen L. C. (2012) Dissecting the substrate recognition of 3-O-sulfotransferase for the biosynthesis of anticoagulant heparin. Proc. Natl. Acad. Sci. U.S.A. 109, 5265–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Falkowski M., Schledzewski K., Hansen B., Goerdt S. (2003) Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, in avascular tissues, and at solid/liquid interfaces. Histochem. Cell Biol. 120, 361–369 [DOI] [PubMed] [Google Scholar]
- 39. Penc S. F., Pomahac B., Winkler T., Dorschner R. A., Eriksson E., Herndon M., Gallo R. L. (1998) Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J. Biol. Chem. 273, 28116–28121 [DOI] [PubMed] [Google Scholar]
- 40. Taylor K. R., Rudisill J. A., Gallo R. L. (2005) Structural and sequence motifs in dermatan sulfate for promoting fibroblast growth factor-2 (FGF-2) and FGF-7 activity. J. Biol. Chem. 280, 5300–5306 [DOI] [PubMed] [Google Scholar]
- 41. Zhou B., Weigel J. A., Fauss L., Weigel P. H. (2000) Identification of the hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 275, 37733–37741 [DOI] [PubMed] [Google Scholar]
- 42. Prevo R., Banerji S., Ni J., Jackson D. G. (2004) Rapid plasma membrane-endosomal trafficking of the lymph node sinus and high endothelial venule scavenger receptor/homing receptor stabilin-1 (FEEL-1/CLEVER-1). J. Biol. Chem. 279, 52580–52592 [DOI] [PubMed] [Google Scholar]