Background: MPK38, a member of the AMPK family, may be functionally linked to the p53 signaling pathway.
Results: MPK38 stimulates p53 signaling through Ser15 phosphorylation.
Conclusion: MPK38 functions as a novel regulator promoting p53 activity and function.
Significance: This study contributes to the clarification of the role of MPK38 as an activator of p53 signaling, helping to address the uncertain physiological functions of MPK38.
Keywords: Apoptosis, Cell Cycle, p53, Protein Phosphorylation, Protein-Protein Interactions, Serine/Threonine Protein Kinase, Signal Transduction, MPK38
Abstract
Murine protein serine-threonine kinase 38 (MPK38) is a member of the AMP-activated protein kinase-related serine/threonine kinase family. In this study, we show that MPK38 physically associates with p53 via the carboxyl-terminal domain of MPK38 and the central DNA-binding domain of p53. This interaction is increased by 5-fluorouracil or doxorubicin treatment and is responsible for Ser15 phosphorylation of p53. Ectopic expression of wild-type Mpk38, but not kinase-dead Mpk38, stimulates p53-mediated transcription in a dose-dependent manner and up-regulates p53 targets, including p53, p21, MDM2, and BAX. Consistently, knockdown of MPK38 shows an opposite trend, inhibiting p53-mediated transcription. MPK38 functionally enhances p53-mediated apoptosis and cell cycle arrest in a kinase-dependent manner by stimulating p53 nuclear translocation. We also demonstrate that MPK38-mediated p53 activation is induced by removing MDM2, a negative regulator of p53, from the p53-MDM2 complex as well as by stabilization of interaction between p53 and its positive regulators, including NM23-H1, serine/threonine kinase receptor-associated protein, and 14-3-3. This leads to the enhancement of p53 stability. Together, these results suggest that MPK38 may act as a novel regulator for promoting p53 activity through direct phosphorylation of p53 at Ser15.
Introduction
The p53 tumor suppressor plays an important role in cellular processes such as growth arrest, senescence, and apoptosis, in response to a broad array of cellular damage (1, 2). It is clear that the triggering of p53 activation must be tightly regulated because the activation of p53 is a central event in response to different types of stress. Post-translational modifications play an important role in both the stabilization and activation of p53 (3). p53 phosphorylation has been widely investigated and is associated with its stabilization. The phosphorylation of three amino-terminal sites, Ser15, Thr18, and Ser20, impairs the interaction between p53 and MDM2 and leads to accumulation of p53 (4). Ataxia telangiectasia-mutated and ataxia telangiectasia and Rad3-related kinases have been shown to phosphorylate p53 at Ser15 after γ-irradiation and UV radiation, respectively (5–7), whereas Ser20 was phosphorylated by cell cycle checkpoint kinase 1 (CHK1) and cell cycle checkpoint kinase 2 (CHK2) (8–10). Notably, Ser15 phosphorylation is known to be critical for p53-dependent transactivation (11). To date, although many kinases and their phosphorylation sites have been characterized (12), the physiological role of p53 phosphorylation still requires clarification. Based on this, the identification of additional modulators of p53 phosphorylation will provide greater insight into the regulation of p53 activity.
Murine protein serine-threonine kinase 38 (MPK38), also known as maternal embryonic leucine zipper kinase (MELK), is a member of the AMP-activated protein kinase (AMPK)2-related serine/threonine kinase family (13, 14) and is localized in the cell cortex, cytoplasm, or nucleus in a cell cycle-dependent manner (15, 16). In human U2OS cells, the ectopic expression of Mpk38 induced an accumulation of cells in the G2 phase of the cell cycle, suggesting that MPK38 was involved in the negative regulation of G2/M progression (17). This result strongly indicates that MPK38 is a potential cell cycle regulator. In addition, a recent report suggested that cyclin-dependent kinase 1 (CDK1) and mitogen-activated protein kinase (MAPK) might be involved in MPK38 phosphorylation during M phase in Xenopus embryos (18). However, the physiological functions and molecular mechanism(s) underlying MPK38 activity remain unclear, despite implications of its involvement in various cellular processes, including cell cycle, spliceosome assembly, carcinogenesis, embryonic hematopoiesis, self-renewal of stem cells, and apoptosis (19–21).
To explore the functional link between MPK38 and p53 signaling pathways, we investigated the effect of MPK38 on p53 and its downstream targets. This study provides evidence that MPK38 physically interacts with p53 in vivo and in vitro and phosphorylates Ser15 within the amino-terminal transactivation domain of p53, which subsequently leads to the stimulation of p53 activity. This interaction apparently contributes to the enhancement of p53-dependent apoptosis and cell cycle arrest by modulating the stability of p53.
MATERIALS AND METHODS
Cell Culture, Plasmids, Cell Lines, Antibodies, MPK38-specific siRNA, and p53 Substitution Mutants
HEK293, MCF7, H1299, HCT116, and p53-null HCT116 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Invitrogen). The p53-luciferase reporter plasmid and kinase-dead (K40R) MPK38 construct have been described previously (20, 23). HCT116 cells (MPK38(KD)) stably expressing Mpk38-specific shRNA were generated using Mpk38-specific oligonucleotides in the presence of 1.5 μg/ml puromycin (Sigma) as described previously (20). The anti-p53(DO-1), anti-FLAG(M2), anti-His, anti-β-actin, anti-p21, anti-BAX, anti-MDM2, anti-histone(H2B), and anti-NM23-H1 antibodies used in immunoprecipitation and immunoblot analyses have been described previously (20, 25, 26), and the anti-phospho-p53(S15) antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-MPK38, anti-glutathione S-transferase (GST), and anti-STRAP antibodies have been described previously (20, 24). The Alexa Fluor-594 anti-mouse secondary antibody and Alexa Fluor-488 anti-rabbit secondary antibody have also been described previously (26). The Mpk38-specific siRNA oligonucleotide has also been described previously (20). The p53 substitution mutants used for the in vitro kinase assays were generated by PCR using wild-type p53 as the template, as described previously (20, 21). The following mutant and wild-type p53 primers were used: p53(S15A) forward, 5′-GCCCCCTCTGGCTCAGGAAACAT-3′, and reverse, 5′-ATGTTTCCTGAGCCAGAGGGGGC-3′; p53(T118A) forward, 5′-CATTCTGGGGCGGCCAAGTCT-3′, and reverse, 5′-AGACTTGGCCGCCCCAGAATG-3′; p53(S260A) forward, 5′-CTGGAAGACGCGAGTGGTAAT-3′, and reverse, 5′-ATTACCACTCGCGTCTTCCAG-3′; and wild-type p53 forward, 5′-GCGAATTCATGGAGGAGCCGCAGTCA-3′ containing an EcoRI site (underlined), and reverse, 5′-GCCTCGAGTCAGTCTGAGTCAGGCCC-3′ containing an XhoI site (underlined). The amplified PCR products were cloned into pGEX4T-1 (Amersham Biosciences) cut with EcoRI and XhoI.
Transfection and Binding Assays
Cells were transfected with the plasmids described above using WelFect-ExTM Plus (WelGENE, Daegu, Korea), according to the manufacturer's instructions. In vivo and in vitro binding assays were performed as described previously (24, 25).
MPK38 Kinase Assay
In vitro kinase assays using recombinant MPK38 proteins were performed as described previously (21). For in vitro kinase assays using MPK38 proteins purified from HEK293 cells expressing GST-MPK38, cells were transiently transfected with pEBG-Mpk38 (a GST tagged MPK38-encoding expression vector) using WelFect-ExTM Plus and solubilized with lysis buffer (20 mm HEPES, pH 7.9, 10 mm EDTA, 0.1 m KCl, and 0.3 m NaCl). The cleared lysates were then precipitated by glutathione-Sepharose beads. The GST precipitates were washed three times with lysis buffer and twice with kinase buffer (50 mm HEPES, pH 7.4, 1 mm DTT, and 10 mm MgCl2) and were then subjected to an in vitro kinase assay, as described previously (20, 21), using recombinant p53 as a substrate in the presence of 5 μCi of [γ-32P]ATP, followed by SDS-PAGE and autoradiography.
Preparation of Nuclear and Cytoplasmic Fractions
Each fraction was prepared as described previously (25). For immunoblot analyses, MCF7 cells (∼4 × 105 cells per 60 mm dish) transfected with the expression vectors described above or an Mpk38-specific siRNA were used for the preparation of nuclear and cytoplasmic fractions.
Ubiquitination and Luciferase Reporter Assay
The ubiquitination assay was performed using p53-null HCT116 cells transfected with expression plasmids encoding p53, wild-type and kinase-dead MPK38, and HA-tagged ubiquitin, as described previously (26). For the luciferase reporter assay, MCF7 or p53-null HCT116 cells were transfected using the WelFect-ExTM Plus with the p53-luciferase reporter plasmid, along with each expression vector as indicated in the presence of wild-type p53 or its mutant p53(S15A). Luciferase activity was assessed using a luciferase assay kit (Promega) following the manufacturer's instructions, as described previously (24). The experiments were independently repeated at least three times.
Immunofluorescence Staining
The coverslips for MCF7 cells transfected with the appropriate plasmids as indicated were prepared as described previously (26). The mouse anti-p53 antibody (diluted 1000-fold in PBS) or rabbit anti-MPK38 antibody (diluted 200-fold in PBS) were applied to the coverslips for 2 h at 37 °C and then incubated with a 1000-fold dilution of Alexa Fluor-594 anti-mouse secondary antibody or Alexa Fluor-488 anti-rabbit secondary antibody at 37 °C for 1 h. Cell nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI). Confocal laser scanning microscopy observations were done on a Leica Dmire2 laser (Germany).
Assays for Apoptosis and Cell Cycle Arrest
A green fluorescent protein (GFP)-based cell death assay was performed using MCF7 or p53-null HCT116 cells grown on sterile coverslips transfected with pEGFP (a GFP-encoding expression vector) together with the expression vectors described above, as indicated and as described previously (20, 26). The DAPI-stained nuclei of GFP-positive cells were analyzed for apoptotic morphology by fluorescence microscopy. The percentage of apoptotic cells was calculated as the number of GFP-positive cells with apoptotic nuclei divided by the total number of GFP-positive cells. The terminal deoxynucleotide transferase-mediated dUTP nick end-labeling (TUNEL)-based apoptosis analysis was performed as described previously (20). Fluorescence-activated cell sorting (FACS) analysis for evaluating cell cycle arrest was performed as described previously (23). Briefly, the transfectants of MCF7 cells expressing the indicated proteins were treated with doxorubicin (100 ng/ml) for 24 h and trypsinized and then washed twice with ice-cold PBS. The cells were then incubated at 37 °C for 30 min with a solution (1 mm Tris-HCl, pH 7.5) of 50 μg/ml propidium iodide and 1 mg/ml RNase A. Flow cytometry was performed on a FACSCalibur-S system (BD Biosciences).
Statistical Analysis
All experiments were repeated at least three times, and results are expressed as the means ± S.E. A p value relative to the control of p < 0.05, as calculated by Student's t test, was considered statistically significant.
RESULTS
MPK38 Interacts Physically with p53
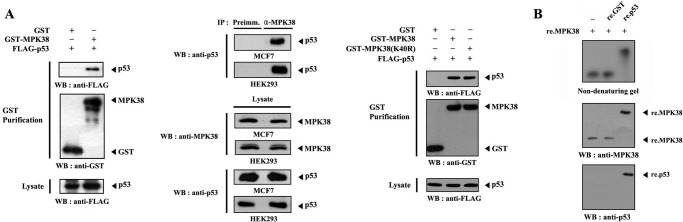
AMPK has been shown to activate p53 function through the phosphorylation at Ser15, although there is no evidence to show direct phosphorylation (27, 28). MPK38 is a member of the AMPK family that is largely associated with cell survival under conditions of environmental challenge, such as nutrient starvation (29–31). Thus, it is possible that MPK38 is functionally linked to the p53 signaling pathway. To test this hypothesis, we first performed cotransfection experiments using GST-MPK38 and FLAG-p53 expression vectors to determine whether MPK38 can interact with p53 in cells. The binding of FLAG-p53 with GST-MPK38 was analyzed by immunoblotting with an anti-FLAG antibody. p53 was present in the coprecipitate only when coexpressed with GST-MPK38 but not with the control GST alone (Fig. 1A, left panel). Furthermore, we verified the in vivo binding of MPK38 and p53 using coimmunoprecipitation experiments in MCF7 and HEK293 cells. Endogenous MPK38 was immunoprecipitated with anti-MPK38 antibody from cell lysates, and the binding of endogenous p53 was subsequently analyzed by immunoblotting with an anti-p53 antibody. The p53 was present in the MPK38 immunoprecipitate but not in the control normal rabbit serum immunoprecipitate (Fig. 1A, middle panel). These results indicate that the physical association of MPK38 with p53 can occur in cells. To investigate the effect of MPK38 kinase activity on the association between MPK38 and p53, we also analyzed the effect of kinase-dead (K40R) MPK38 on the association of these proteins using an in vivo binding assay. Constructs expressing kinase-dead MPK38 did not show any difference in complex formation compared with the control expressing wild-type MPK38 (Fig. 1A, right panel), suggesting that MPK38 kinase activity seems not to be required for complex formation. We next analyzed the in vitro association of purified recombinant MPK38 and p53 using nondenaturing PAGE. Purified recombinant MPK38 was subjected to autophosphorylation using [γ-32P]ATP and was then incubated with unlabeled recombinant p53. A shift in the mobility of autophosphorylated MPK38 was clearly detected when incubated with p53 but not with the control GST alone (Fig. 1B). Together, these results demonstrate that MPK38 directly associates with p53 without relying on additional proteins in mammalian cells.
FIGURE 1.
MPK38 interacts with p53 in mammalian cells. A, in vivo association of MPK38 with p53. HEK293 cells were transfected with the indicated expression vectors. GST precipitates were analyzed for the presence of p53 by anti-FLAG antibody immunoblot (left panel). Cell lysates from MCF7 or HEK293 cells were subjected to immunoprecipitation using either rabbit preimmune serum (Preimm.) or anti-MPK38 antibody (α-MPK38), followed by immunoblot analysis using an anti-p53 antibody to determine complex formation between endogenous MPK38 and p53 (middle panel). To examine the effect of MPK38 kinase activity on complex formation between MPK38 and p53, HEK293 cells were transfected with the indicated expression plasmids. GST fusion proteins were purified on glutathione-Sepharose beads (GST purification), and MPK38-p53 complex formation was determined by anti-FLAG antibody immunoblot (right panel). B, in vitro association of MPK38 with p53. For native PAGE (∼8%) of the MPK38-p53 complex, purified recombinant MPK38 was autophosphorylated in the presence of 5 μCi of [γ-32P]ATP. Autophosphorylated MPK38 (∼2 μg) was incubated with unlabeled recombinant GST, as a nonspecific control, or p53 (3 μg each) at room temperature for 1 h. The same blot was stripped and re-probed with anti-MPK38 and anti-p53 antibodies to confirm the presence of MPK38 and p53 on the radioactive band shifts. IP, immunoprecipitation; WB, Western blot; re., recombinant.
Identification of Binding Domains Required for MPK38-p53 Complex Formation
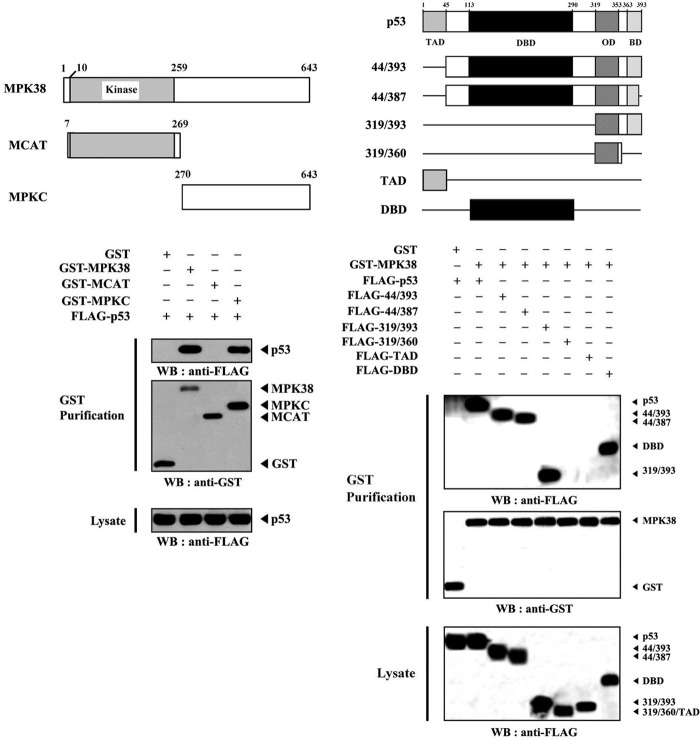
Because MPK38 physically interacted with p53, we next carried out in vivo binding assays with two MPK38 deletion constructs to determine which region of MPK38 was involved in p53 binding. Results showed that the carboxyl-terminal regulatory domain (MPKC; amino acids 270–643) of MPK38 was responsible for p53 binding (Fig. 2, left panel). However, the amino-terminal kinase domain (MCAT; amino acids 7–269) was unable to bind with p53. This result indicates that the carboxyl-terminal regulatory domain of MPK38 is required for p53 binding. To identify specific interaction domains of p53 for MPK38 binding, we also performed in vivo binding assays using a set of six p53 deletion mutants in HEK293 cells. MPK38 interacted with wild-type p53, p53(44/393), p53(44/387), p53(319/393), and the DNA-binding domain (DBD) of p53 but not with p53(319/360) and the transactivation domain of p53 (Fig. 2, right panel). These results indicate that the interaction of MPK38 with p53 may be mediated by the DBD of p53. In addition, the basic domain within residues 363–393 served as a secondary interaction domain. It has been shown previously that NM23-H1 and STRAP directly associate with p53 through cysteine residues within the central DBD of p53 (26). Based on this, we tested if complex formation between MPK38 and p53 also requires cysteine residues present in the DBD domain of p53 using in vivo binding assays with a set of five cysteine to serine substitution mutants (C135S, C176S, C238S, C242S, and C277S) of the p53 DBD. None of the substitution mutants affected the interaction of MPK38 with p53, indicating that this interaction is not mediated by cysteine residues present in the DBD domain (data not shown). Together, these results demonstrated that the physical association between MPK38 and p53 is mediated by the carboxyl-terminal regulatory domain of MPK38 and the DBD domain (or basic domain) of p53 but not by cysteine residues present in either of these two proteins.
FIGURE 2.
Identification of the MPK38 and p53 interaction domains. Schematic representation of wild-type and deletion constructs of Mpk38 (left panel) and p53 (right panel) is indicated. Numbers indicate the amino acid residues. HEK293 cells transfected with the indicated expression vectors were lysed and then precipitated using glutathione-Sepharose beads (GST purification), and protein complexes were analyzed by immunoblot analysis using an anti-FLAG antibody. The same blot was re-probed with an anti-GST antibody to confirm the expression of GST fusion proteins in the coprecipitates, and the expression level of p53 in total cell lysates was determined by anti-FLAG antibody immunoblot. WB, Western blot; TAD, transactivation domain; OD, oligomerization domain; BD, basic domain.
p53 Signals Influence Complex Formation between MPK38 and p53
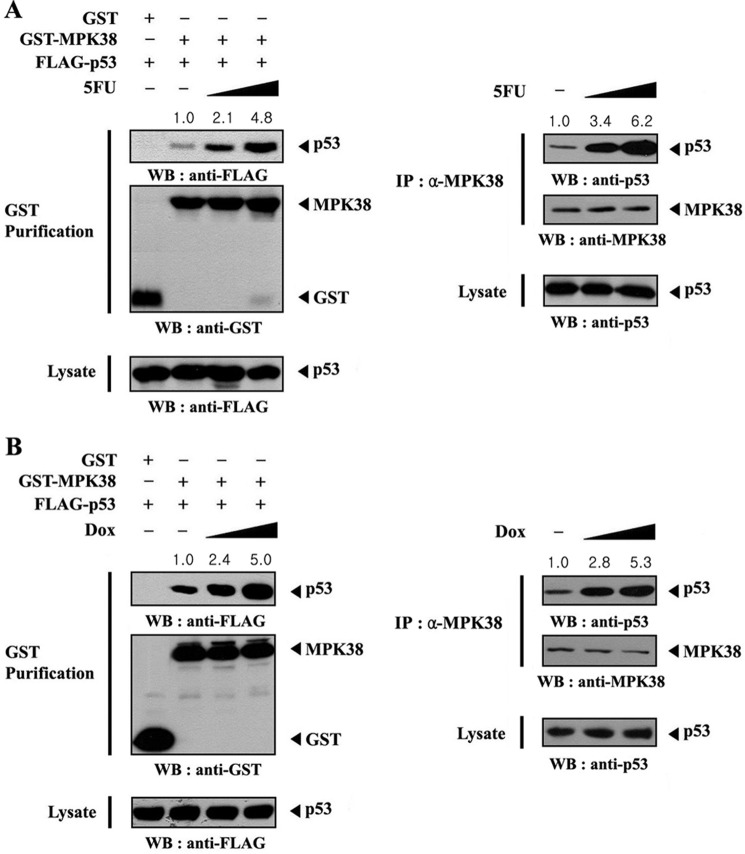
To investigate the role of MPK38 in the regulation of p53 activity, we next assessed whether p53 stimuli, such as 5-fluorouracil (5FU) and doxorubicin, can influence MPK38-p53 complex formation in HEK293 cells transfected with plasmid vectors expressing GST-MPK38 and FLAG-p53. After 48 h of transfection, cells were incubated in media with or without 5FU. MPK38 was precipitated, and the coprecipitation of p53 was determined by anti-FLAG immunoblot. MPK38-p53 association was dose-dependently increased by 5FU treatment, compared with the untreated control (Fig. 3A, left panel). We also determined the effect of 5FU on the endogenous interaction between MPK38 and p53. Exposure of the cells to 5FU resulted in a dose-dependent increase in endogenous MPK38-p53 complex formation (Fig. 3A, right panel), implying that MPK38 may be involved in the regulation of p53 signaling. Similarly, doxorubicin treatment resulted in a dose-dependent increase in the association between exogenous MPK38 and p53 (Fig. 3B, left panel) and endogenous MPK38 and p53 (Fig. 3B, right panel). These results indicate that the interaction between MPK38 and p53 appears to be dependent on p53 signals.
FIGURE 3.
Modulation of the association between MPK38 and p53 by p53 signals. HEK293 cells transfected with the indicated expression vectors were incubated with or without increasing amounts of 5-FU (0.38 and 0.57 mm, 30 h) or doxorubicin (Dox) (100 and 150 ng/ml, 24 h) as described previously (23). Cell lysates were purified on glutathione-Sepharose beads (GST purification), followed by immunoblot analysis using an anti-FLAG antibody to determine the complex formation between MPK38 and p53 (A and B, left panels). The endogenous level of MPK38-p53 complex in the presence or absence of 5FU (or doxorubicin) was also analyzed by immunoblotting with an anti-p53 antibody (A and B, right panels). The relative level of MPK38-p53 complex formation was quantitated by densitometric analysis, and the fold increase relative to untreated control cells expressing MPK38 and p53 (A and B, left panels) or untreated HEK293 cells (A and B, right panels) was calculated. IP, immunoprecipitation; WB, Western blot.
MPK38 Phosphorylates p53 at Ser15
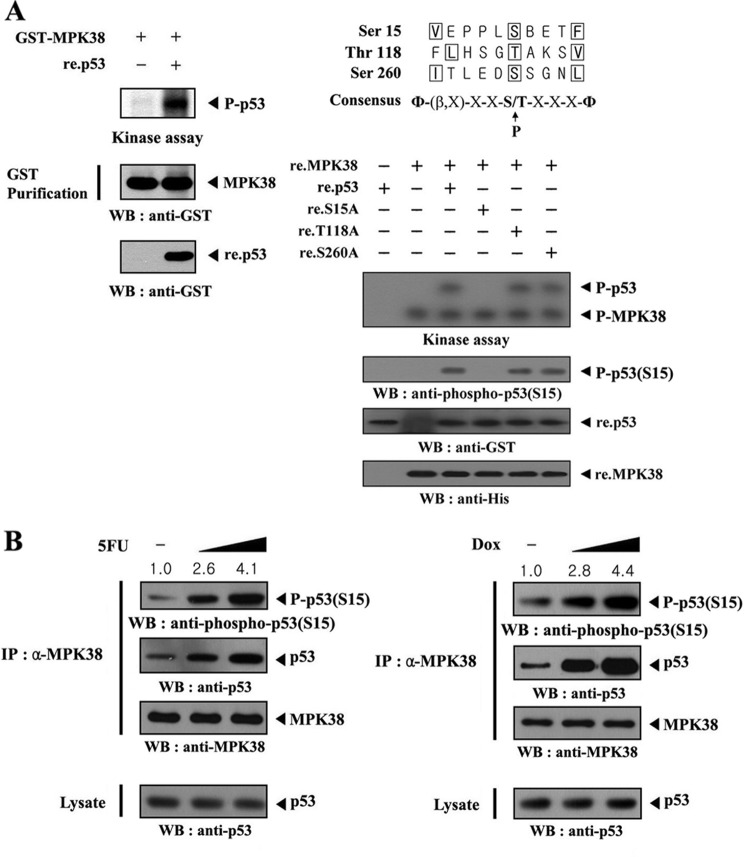
Given that p53 physically interacts with MPK38, we performed in vitro kinase assays to examine whether p53 is a substrate for MPK38. MPK38 proteins precipitated from HEK293 cells expressing GST-MPK38 were incubated with [γ-32P]ATP to allow phosphorylation of the recombinant p53. p53 phosphorylation was clearly detected in the presence of MPK38 (Fig. 4A, left panel), indicating that p53 may be a substrate for MPK38. Next, we tried to identify potential phosphorylation sites of MPK38 on p53 using in vitro kinase assays, based on alignment analysis with the MPK38/AMPK consensus motif Φ-(X, basic)-XX(S/T)XXX-Φ, where Φ is a hydrophobic residue (21, 22). Three putative phosphorylation sites (Ser15, Thr118, and Ser260) on MPK38 were selected and used for the MPK38 kinase assay. Mutation of p53 Ser15 to Ala15 (S15A), but not other p53 mutants (T118A and S260A), completely abolished MPK38-dependent phosphorylation (Fig. 4A, right panel), indicating that the Ser15 within the amino-terminal region of p53 represents a potential phosphorylation site for MPK38. This was also confirmed by immunoblot analysis using an anti-phospho-p53(S15) antibody (Fig. 4, A and B). These results suggest that MPK38 directly phosphorylates p53 at Ser15 through direct interaction.
FIGURE 4.
Identification of MPK38 phosphorylation sites on p53. A, in vitro phosphorylation of p53 at Ser15 by MPK38. Recombinant p53 proteins (∼3 μg) were mixed with 10 μm ATP, 5 μCi of [γ-32P]ATP, and 10 mm MgCl2 in 20 μl of kinase buffer and incubated with precipitated MPK38 from HEK293 cell extracts containing GST-MPK38 for 15 min at 37 °C with frequent gentle mixing. Expression levels of MPK38 and p53 were monitored by immunoblotting with an anti-GST antibody (left panel). Alignment of the MPK38/AMPK consensus motif with putative MPK38 phosphorylation sites on p53 is indicated (right, upper panel). Approximately 3 μg of recombinant p53 or one of its substitution mutants (S15A, T118A, and S260A) were mixed with 10 μm ATP, 5 μCi of [γ-32P]ATP, and 10 mm MgCl2 in 20 μl of kinase buffer and incubated with recombinant wild-type MPK38 (∼4 μg) for 15 min at 37 °C (right, lower). Ser15 phosphorylation of p53 and expression of recombinant p53 and MPK38 were determined by immunoblotting with anti-phospho-p53(S15), anti-GST, and anti-His antibodies, respectively (right lower, 2nd to bottom panels). B, in vivo phosphorylation of p53 at Ser15 by MPK38. The endogenous level of p53 phosphorylation at Ser15 in the presence or absence of 5-fluorouracil (or doxorubicin (Dox)) was also analyzed by immunoblotting with an anti-phospho-p53(S15) antibody. The relative level of p53 phosphorylation at Ser15 was quantitated by densitometric analysis, and the fold increase relative to untreated HEK293 cells was calculated. P-p53 and P-p53(S15) indicate the phosphorylated p53 and p53 at Ser15, respectively. Φ, hydrophobic residue; β, basic residue; IP, immunoprecipitation; WB, Western blot.
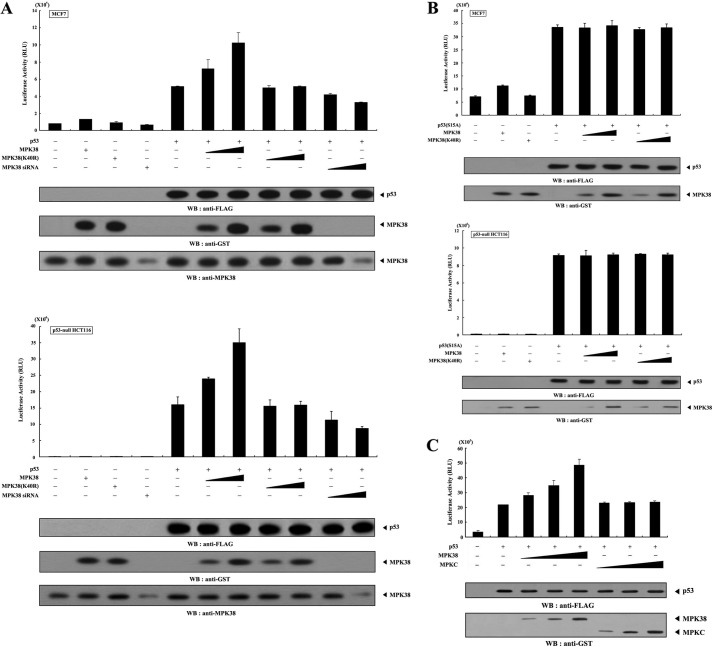
MPK38 Enhances p53-induced Transcription in a Kinase-dependent Manner
To investigate the role of MPK38-p53 complex formation, we analyzed the effect of MPK38 on p53-induced transcription. A p53-luciferase reporter was cotransfected with increasing amounts of wild-type and kinase-dead Mpk38 into MCF7 and p53-null HCT116 cells in the presence or absence of p53. The addition of wild-type MPK38 markedly enhanced p53-induced transcription in a dose-dependent manner (Fig. 5A, lane 5 versus lanes 6 and 7), suggesting that MPK38 may act as a positive regulator of p53. In contrast, expression of MPK38 itself had little effect on p53-induced transcription (Fig. 5A, 2nd lane). We also examined the effect of Mpk38-specific siRNA on p53-induced transcription. As expected, transfection with Mpk38-specific siRNA resulted in a dose-dependent decrease in p53-induced transcription (Fig. 5A, 5th lane versus 10th and 11th lanes). In addition, the expression of kinase-dead MPK38 had no effect on p53-induced transcription compared with the control expressing p53 alone (Fig. 5A, 5th lane versus 8th and 9th lanes). Similar results were also observed in the presence of 5FU as a p53 signal (data not shown). These results suggest that the kinase activity of MPK38 is required for the regulation of p53-induced transcription.
FIGURE 5.
Enhancement of p53-mediated transcription by MPK38. A and B, MCF7 and p53-null HCT116 cells were transiently transfected with 0.2 μg of p53 luciferase plasmid, 0.1 μg of an expression plasmid for β-galactosidase as an internal control, and increasing amounts of wild-type and kinase-dead (K40R) forms of Mpk38 (0.5 and 1.5 μg) and Mpk38 siRNA (100 and 200 nm) as indicated in the presence of p53 or p53(S15A) (0.3 μg each). Luciferase activity was measured 48 h after transfection and normalized to β-galactosidase activity. C, MCF7 cells were transfected with 0.2 μg of p53 luciferase plasmid and increasing amounts (0.4, 0.8, and 1.2 μg) of wild-type Mpk38 or Mpkc, as indicated, in the presence or absence of p53 (0.3 μg). The expression level of p53, MPK38, and MPKC was determined by immunoblotting with the indicated antibodies. Data shown are means (± S.E.) of three independent experiments. p < 0.05 relative to control indicates significance as calculated by Student's t test. WB, Western blot.
Given that MPK38 phosphorylates Ser15 of p53, which is critical for p53 activation, we next examined whether MPK38 affects p53-induced transcription in the presence of the p53(S15A) mutant, which is defective in MPK38-mediated phosphorylation (see Fig. 4A). Both MCF7 and p53-null HCT116 cells were transfected with the indicated expression vectors encoding wild-type and kinase-dead MPK38, together with the p53(S15A) mutant. Neither wild-type nor kinase-dead MPK38 had an effect on p53(S15A)-induced transcription in either cell line (Fig. 5B). This result indicates that the phosphorylation of p53 at Ser15 by MPK38 plays a crucial role in MPK38-mediated regulation of p53-induced transcription.
To further analyze the direct role of MPK38-mediated phosphorylation of p53 in the regulation of p53-induced transcription, we also performed a luciferase reporter assay using MPKC, which is able to physically associate with MPK38 but is defective in kinase activity (see Fig. 2). Expression of wild-type MPK38 resulted in a dose-dependent increase in p53 transcriptional activity, whereas MPKC had no effect (Fig. 5C). A similar trend was also observed in cells treated with 5FU instead of p53 (data not shown). These results strongly suggest that direct phosphorylation of p53 by MPK38, rather than physical interaction with p53, plays a key role in MPK38-mediated regulation of p53-induced transcription.
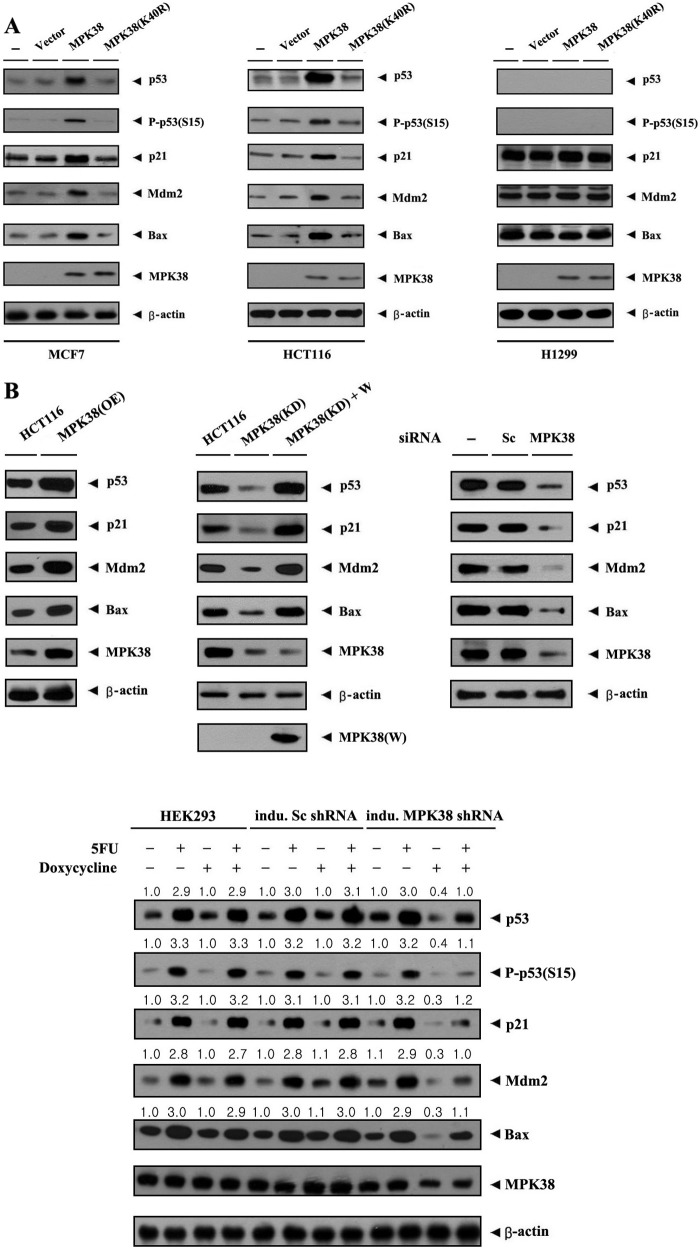
MPK38 Up-regulates the Expression of p53 Targets in a Kinase-dependent Manner
To analyze the role of MPK38 in p53 signaling, we also carried out transfection experiments using wild-type and kinase-dead Mpk38 in MCF7, HCT116, and p53-null H1299 cells. Overexpression of wild-type MPK38 markedly increased the expression of p53 target genes, including p53, p21, MDM2, and BAX, which are normally up-regulated by p53 signals, in both MCF7 and HCT116 cells (Fig. 6A, left and middle panels). In contrast, overexpression of kinase-dead MPK38 did not show such an effect. However, the MPK38-mediated stimulatory effect on p53 targets was not detected in p53-null H1299 cells (Fig. 6A, right panel), indicating that this stimulatory effect is truly p53-dependent. To further confirm the positive role of MPK38 in p53 signaling, we also generated HCT116 cells stably overexpressing MPK38 (MPK38(OE)) and analyzed them for p53-induced gene expression. Overexpression of MPK38 remarkably increased the expression of p53, p21, MDM2, and BAX compared with the control parental HCT116 cells (Fig. 6B, upper left panel). Consistently, MPK38 knockdown cells decreased the expression of p53 target genes (Fig. 6B, upper middle panel, 2nd lane). The effects induced by MPK38 knockdown were sufficiently reversed by expressing a wobble mutant of MPK38 (Fig. 6B, upper middle panel, 3rd lane), suggesting that the increased expression of p53 target genes by MPK38 is specific. In addition, similar results were also observed in analyses with HCT116 cells transfected with an Mpk38-specific siRNA (Fig. 6B, upper right panel, 3rd lane) or an inducible Mpk38 shRNA HEK293 cell line (Fig. 6B, lower panel). Collectively, these results suggest that MPK38 interacts with p53 in vivo and physiologically activates p53 signaling.
FIGURE 6.
Up-regulation of p53 targets by MPK38. A, effect of MPK38 on the expression of p53 target genes. MCF7, HCT116, and p53-null H1299 cells transfected with the indicated plasmid vectors expressing an empty vector (Vector), wild-type MPK38, and kinase-dead (K40R) MPK38 were lysed and subjected to immunoblot analysis using anti-p53, anti-phospho-p53(S15), anti-p21, anti-MDM2, anti-BAX, anti-MPK38, and anti-β-actin antibodies. B, modulation of p53 target genes by overexpression and knockdown of MPK38. The following cells were used for immunoblot analyses: parental HCT116 cells; HCT116 cells stably overexpressing MPK38 (MPK38(OE)); HCT116 cells stably expressing Mpk38-specific shRNA (MPK38(KD)); MPK38(KD) cells (MPK38(KD)+W) transfected with ∼3 μg of GST-Mpk38 wobble mutant (20); HCT116 cells transfected with 200 nm of control scrambled siRNA (Sc) or Mpk38-specific siRNA (MPK38); and inducible Mpk38 shRNA HEK293 cells (21). The cells were lysed and subjected to immunoblot analysis using anti-p53, anti-phospho-p53(S15), anti-p21, anti-MDM2, anti-BAX, anti-MPK38, anti-β-actin, and anti-GST antibodies. The endogenous expression level of MPK38 was determined by anti-MPK38 immunoblotting. The presence of equal amounts of protein in each lane was verified by anti-β-actin immunoblotting. Sc, scrambled; indu., inducible.
MPK38 Enhances p53 Stability by Modulating the Regulators of p53
It is possible that MPK38 binding modulates the interaction between p53 and MDM2, a negative regulator of p53, as p53 has been shown to form an inactive complex with MDM2 (1, 33). Based on this, we investigated whether MPK38 modulates the MDM2 binding to p53 in HEK293 cells. Results showed that the coexpression of wild-type MPK38 considerably decreased complex formation between p53 and MDM2 compared with the expression in the absence of MPK38 (Fig. 7A, left panel). The endogenous interaction between p53 and MDM2 was similarly decreased by wild-type MPK38, but this effect was not observed in the presence of kinase-dead MPK38 (Fig. 7A, middle panel, 1st lane versus 2nd and 3rd lanes). Consistently, the transfection of Mpk38 siRNA resulted in a dose-dependent increase in p53-MDM2 complex (Fig. 7A, middle panel, 1st lane versus 4th and 5th lanes). Meanwhile, the analysis with the p53(S15A) mutant lacking in MPK38-mediated phosphorylation (see Fig. 4A) clearly indicated a crucial role for p53 phosphorylation at Ser15 in the MPK38-mediated regulation of p53 signaling, because no difference was observed in the modulation of p53-MDM2 complex formation in the presence of mutant p53(S15A) (Fig. 7A, right panel, 3rd and 4th lanes). We extended our analysis to determine whether MPK38 contributes to the interaction between p53 and its known activators, including NM23-H1 (26), STRAP (26), and 14-3-3 (34). HEK293 cells transfected with vectors expressing FLAG-NM23-H1, FLAG-STRAP, FLAG-14-3-3, and p53 in the presence or absence of wild-type and kinase-dead Mpk38 were subjected to immunoprecipitation using an anti-p53 antibody, followed by immunoblot analysis with an anti-FLAG antibody. There was a remarkable increase in complex formation between p53 and NM23-H1, p53 and STRAP, or p53 and 14-3-3 in cells expressing wild-type MPK38, compared with expression in the absence of MPK38 (Fig. 7B, left panels). In contrast, kinase-dead MPK38 had no effect on the association between p53 and its activators. Consistently, knockdown of endogenous MPK38 dose-dependently decreased the complex formation between p53 and NM23-H1, p53 and STRAP, or p53 and 14-3-3 (Fig. 7B, middle panels). This effect was not detected in the p53(S15A) mutant (Fig. 7B, right panels), a similar result to the effect of p53(S15A) on p53-MDM2 complex formation described above. Furthermore, to confirm the positive role of MPK38 in the regulation of p53 activity, we also measured the stability of p53 with an immunoblot analysis in HCT116 and MCF7 cells. Expression of wild-type MPK38 considerably increased the stability of p53 compared with the control, an empty vector, but this effect was not observed in the presence of kinase-dead MPK38 (Fig. 7C, upper panel). In a physiological context, the knockdown of endogenous MPK38 by an inducible Mpk38 shRNA system apparently showed a decreasing effect on the stability of p53 (Fig. 7C, lower panel). These observations led us to examine p53 ubiquitination using an in vivo ubiquitination assay in the presence of wild-type and kinase-dead MPK38. In agreement with the above results obtained from the analysis of p53 stability, the coexpression of wild-type MPK38 dose-dependently prevented p53 ubiquitination compared with the control, which expressed p53 alone (Fig. 7D). In contrast, kinase-dead MPK38 had no effect on the ubiquitination of p53. Together, these results suggest that MPK38-mediated phosphorylation of p53 at Ser15 contributes to the dissociation of MDM2 from the p53-MDM2 complex as well as to the stabilization of interactions between p53 and its activators, including NM23-H1, STRAP, and 14-3-3, leading to the enhancement of p53 activity.
FIGURE 7.
Enhancement of p53 stability by MPK38. A and B, modulation of p53-MDM2, p53-NM23-H1, p53-STRAP, and p53–14-3-3 complexes by MPK38. HEK293 cells transfected with the indicated combinations of expression vectors for p53, p53(S15A), MDM2, NM23-H1, STRAP, 14-3-3, and wild-type or kinase-dead (K40R) MPK38, plus increasing amounts (100 and 200 nm) of Mpk38 siRNA were lysed. The lysates were then immunoprecipitated with an anti-p53 antibody (IP) and immunoblotted with the indicated antibodies to determine the level of p53-MDM2 (A), p53-NM23-H1 (B, top panel), p53-STRAP (B, middle panel), and p53–14-3-3 (B, bottom panel) complexes. C, measurement of p53 stability by anti-p53 immunoblotting. HCT116 (or MCF7) cells transiently transfected with an empty vector (Vector) or increasing amounts of wild-type or kinase-dead (K40R) Mpk38, as indicated, or inducible Mpk38 shRNA HEK293 cells (indu. MPK38 shRNA) were used for immunoblot analysis. Inducible silencing of endogenous MPK38 expression by doxycycline (1 μg/ml, 72 h) was determined by immunoblotting with an anti-MPK38 antibody. Time intervals indicate the number of minutes after cycloheximide (CHX) treatment (20 μg/ml). β-Actin was used as a loading control. D, effect of MPK38 on p53 ubiquitination. p53-null HCT116 cells (23) were transfected with vectors expressing HA-tagged ubiquitin (Ub), p53, and wild-type (WT) and kinase-dead (K40R) MPK38, as indicated. Cell lysates were subjected to immunoprecipitation using an anti-p53 antibody (IP), followed by immunoblot analysis using an anti-HA antibody to determine the level of p53 ubiquitination. NS indicates nonspecific proteins; IP, immunoprecipitation; WB, Western blot.
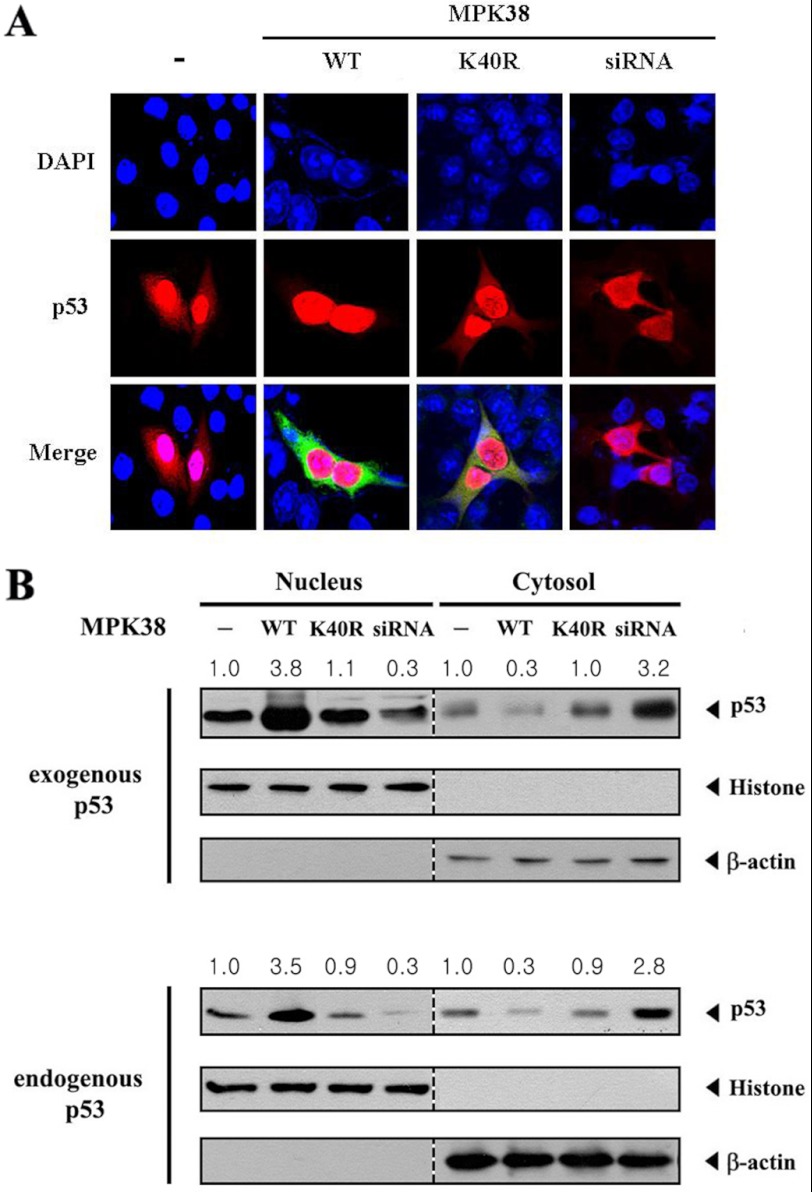
MPK38 Stimulates p53 Nuclear Translocation
We examined whether MPK38 can modify the nuclear translocation of p53 because MPK38 enhanced p53-induced transcription in a dose-dependent manner (see Fig. 5). MCF7 cells were transiently transfected with the indicated expression vectors encoding wild-type and kinase-dead MPK38 or an Mpk38-specific siRNA, together with p53. Cells expressing p53 in the absence of MPK38 exhibited predominantly nuclear staining with some cytoplasmic staining (Fig. 8A, 1st panel), but the coexpression of wild-type MPK38 markedly stimulated the nuclear translocation of p53 (Fig. 8A, 2nd panel). However, such a nuclear translocation of p53 was not detected in the presence of kinase-dead MPK38 (Fig. 8A, 3rd panel). Consistently, knockdown of endogenous MPK38 by an Mpk38-specific siRNA showed a reverse effect on the nuclear translocation of p53 compared with cells expressing wild-type MPK38 (Fig. 8A, 1st panel versus 2nd and 4th panels). We also examined whether MPK38 affects the p53 nuclear translocation using an immunoblot analysis of cytoplasmic and nuclear fractions of cell extracts transfected with the indicated expression vectors or an Mpk38-specific siRNA. The accumulation of p53 in the nuclear fraction was markedly increased in cells expressing wild-type MPK38 compared with the control untransfected cells (Fig. 8B, Nucleus, 1st lane versus 2nd lane), whereas the cytoplasmic accumulation of p53 was concurrently decreased (Fig. 8B, Cytosol, 1st lane versus 2nd lane). In contrast, such a change in p53 localization was not observed in cells expressing kinase-dead MPK38 (Fig. 8B, Nucleus and Cytosol, 1st lane versus 3rd lane), consistent with the above confocal microscopy data. To verify that MPK38 is responsible for regulating the nuclear translocation of endogenous p53, we also performed an immunoblot analysis in the absence of exogenous p53 under the same conditions. Similar results were also observed for the translocation of endogenous p53 (Fig. 8B, endogenous p53). Together, these results suggest that MPK38 stimulates the nuclear accumulation of p53 by enhancing the stabilization of p53.
FIGURE 8.
Stimulation of p53 nuclear translocation by MPK38. A, modulation of p53 nuclear translocation by MPK38. MCF7 cells were transiently transfected with the indicated expression vectors for wild-type (WT) and kinase-dead (K40R) MPK38 in the presence of p53. Cells were immunostained with anti-p53 or anti-MPK38 antibodies, followed by an Alexa Fluor-594 anti-mouse secondary antibody (for p53, red) or Alexa Fluor-488 anti-rabbit secondary antibody (for MPK38, green), and analyzed by confocal microscopy. Nuclei were visualized with DAPI. B, analysis of p53 nuclear translocation by immunoblot. Cytoplasmic and nuclear fractions of MCF7 cells under the same conditions as described in A were analyzed by immunoblotting with anti-p53, anti-histone(H2B), and anti-β-actin antibodies. Nucleus and cytosol indicate the nuclear and cytoplasmic fractions, respectively. The localization of endogenous p53 was also verified by immunoblot analysis (endogenous p53). The relative level of p53 nuclear (or cytoplasmic) translocation was quantified by densitometry. The data are representative of at least three independent experiments.
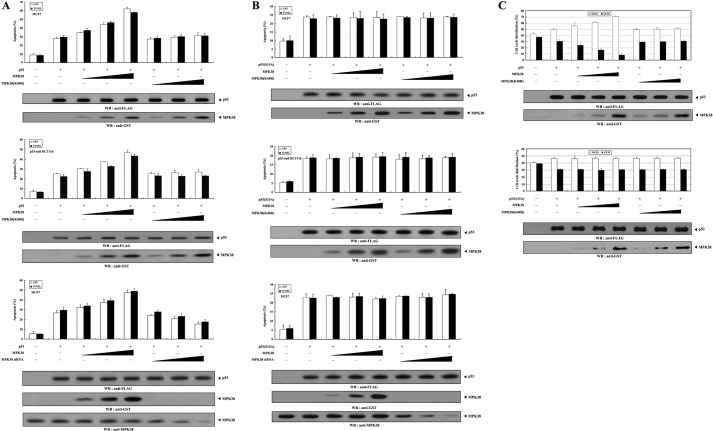
MPK38 Stimulates p53-induced Apoptosis and Cell Cycle Arrest
We next investigated whether the expression of MPK38 affects p53 functions such as apoptosis and cell cycle arrest, because MPK38 interacted with p53 and enhanced p53-induced transcriptional activity (see Figs. 1 and 5). Toward this end, we first examined the effect of MPK38 on p53-induced apoptosis in MCF7 and p53-null HCT116 cells using a GFP-based cell death assay (24) and TUNEL staining (20). Expression of wild-type MPK38 resulted in an increase in p53-induced apoptosis that was proportional to the amount of MPK38 added in the presence of p53 (Fig. 9A, top and middle panels, 2nd lane versus 3rd to 5th lanes). However, the kinase-dead MPK38 had no such effect on p53-induced apoptosis in both cells (Fig. 9A, top and middle panels, 2nd lane versus 6th to 8th lanes), indicating a crucial role for the kinase activity of MPK38 in the regulation of p53-induced apoptosis. Consistently, an opposing trend was observed in the presence of Mpk38-specific siRNA (Fig. 9A, bottom panel, 2nd lane versus 6th to 8th lanes). Similarly, the stimulatory effect of MPK38 on p53-induced apoptosis was also detected in the presence of 5FU, which induces p53-mediated apoptosis (data not shown). Because the dose-dependent stimulation of p53-induced transcription by MPK38 was completely abrogated in the presence of mutant p53(S15A) (see Fig. 5B), we further investigated whether Ser15 phosphorylation of p53 by MPK38 plays an important role in the regulation of p53-induced apoptosis. No difference was observed in p53-induced apoptosis in the presence of p53(S15A) (Fig. 9B), implying that MPK38-mediated phosphorylation of p53 at Ser15 plays a key role in the MPK38-mediated regulation of p53-induced apoptosis. To further confirm the positive role of MPK38 in p53 functions, we also performed flow cytometry analysis to analyze cell cycle arrest in MCF7 cells treated with doxorubicin (23). Coexpression with wild-type MPK38 dose-dependently enhanced the G0/G1 arrest compared with the control p53 alone, whereas kinase-dead MPK38 did not affect p53-mediated cell cycle arrest (Fig. 9C, upper panels). As expected, such a change was not detected in the presence of mutant p53(S15A) (Fig. 9C, lower panels). These results indicate that MPK38 binds directly to p53 and stimulates p53-mediated signaling through p53 phosphorylation at Ser15.
FIGURE 9.
Stimulation of p53-mediated apoptosis and cell cycle arrest by MPK38. A and B, effect of MPK38 on p53-mediated apoptosis. MCF7 and p53-null HCT116 cells were transiently transfected with increasing amounts of Mpk38 (wild-type and kinase-dead (K40R): 0.4, 0.8, and 1.2 μg) and Mpk38-specific siRNA (50, 100, and 200 nm) as indicated, with or without 3 μg of GFP, in the presence or absence of p53 (A) or p53(S15A) (B). Apoptotic cell death was determined using a GFP-based cell death assay or terminal deoxynucleotide transferase-mediated dUTP nick end-labeling. C, effect of MPK38 on p53-mediated cell cycle arrest. MCF7 cells under the same conditions as described in A and B were treated with 100 ng/ml doxorubicin for 24 h, and G0/G1 populations were analyzed by FACScan. The indicated percentages represent the G0/G1 (white bars) and G2/M (black bars) arrest. The expression level of p53 and MPK38 was determined by immunoblotting with the indicated antibodies. Data shown are the means (± S.E.) of at least three independent experiments. p < 0.05 relative to control indicated significance as calculated by Student's t test. WB, Western blot.
DISCUSSION
Recent reports have implicated MPK38, also known as Melk, in various cellular processes, including cell cycle, spliceosome assembly, carcinogenesis, self-renewal of stem cells, and apoptosis (19, 20). In an attempt to determine the molecular mechanism of MPK38 action, MPK38-interacting proteins have been previously identified. To date, MPK38 has been shown to physically interact with several cellular proteins, including a zinc finger protein, ZPR9 (35), a transcription and splicing factor, NIPP1 (36), a protein-tyrosine phosphatase, CDC25B (17), a transcriptional modulator, Smads (21), and a MAP kinase kinase kinase, ASK1 (20). In addition, recent studies have shown that MPK38 may be involved in tumorigenesis, because its expression is highly elevated in tumor-derived progenitor cells (37) and cancers of nondifferentiated cells (38), as well as in multiple human tumor samples and cell lines (39). Meanwhile, the most common genetic change associated with human cancer is mutation or deletion of the gene for p53 tumor suppressor protein, and p53 signaling is closely associated with other signalings that may play important roles in carcinogenesis (40). Given these observations, we hypothesized that the functional association between MPK38 and p53 may occur in vivo.
In this study, we have shown that MPK38 physically interacts with p53 and stimulates p53 signaling. Our data provide evidence that the kinase activity of MPK38 is required for its ability to stimulate p53-induced transcription (Fig. 5) and p53-mediated biological functions, such as apoptosis and cell cycle arrest (Fig. 9). In addition, wild-type MPK38, but not kinase-dead MPK38, stimulates the nuclear translocation of p53 proteins (Fig. 8), supporting the possibility that MPK38 may act as a positive regulator of p53 signaling. However, our present results do not support the possibility that the stimulation of p53 signaling by MPK38 is due only to direct interaction between MPK38 and p53 because the kinase-dead MPK38 did not contribute to the stimulation of p53 signaling, although it could associate with p53 at levels similar to wild-type MPK38 (see Fig. 1A). Consistent with this, MPKC, which is able to physically associate with MPK38 but lacks kinase activity (see Fig. 2), had no effect on p53-induced transcription (Fig. 5C).
p53 is tightly regulated through a complex series of events, including translational regulation, interaction with regulatory proteins such as MDM2 and 14-3-3, and a series of post-translational modifications, for example, phosphorylation and acetylation at multiple sites (2, 41, 42). Among these, the association of p53 phosphorylation with protein stabilization has been widely investigated. Human p53 has 23 different phosphorylation sites (12). Multiple sites on p53 are phosphorylated by many different kinases in response to various stresses (32). In particular, the amino-terminal phosphorylation sites, including Ser15, Thr18, Ser20, Ser33, and Ser37, are interesting because when phosphorylated the physical interaction between p53 and its negative regulator MDM2 is significantly decreased, although the binding of the acetyltransferase p300/CBP/PCAF is promoted, thereby increasing the level and stability of p53. Ser15 is phosphorylated by ataxia telangiectasia-mutated or ataxia telangiectasia and Rad3-related kinase after the exposure of cells to infrared (IR) or ultraviolet (UV) light (5–7). These stresses also lead to Ser20 phosphorylation through CHK1 and CHK2 (3, 8). In fact, besides IR and UV, many stresses have been shown to induce Ser15 phosphorylation, which is thought to nucleate a series of subsequent p53 post-translational modifications (43). In addition, a recent study showed that the activation of AMPK can induce Ser15 phosphorylation, which is critical for proliferation control when glucose levels are low (27). Inspection of MPK38 phosphorylation sites using the MPK38/AMPK consensus motif indicates the presence of three potential phosphorylation sites (Ser15, Thr118, and Ser260) on p53. To gain insight into the mechanism(s) by which MPK38 activates p53 function, we examined whether Ser15, Thr118, or Ser260 represents a potential phosphorylation site for MPK38 kinase using an in vitro kinase assay. MPK38 was shown to phosphorylate Ser15 of p53 but not Thr118 and Ser260 (Fig. 4A), suggesting that MPK38 may act as a positive regulator of p53 via p53 phosphorylation at Ser15. The stimulation of p53 activity by MPK38, in addition to its direct interaction with p53, prompted us to investigate whether MPK38 affects p53 stability through MDM2, a known negative regulator of p53, or through positive regulators of p53, including NM23-H1, STRAP, and 14-3-3. In vivo binding assays were performed in HEK293 cells transfected with wild-type or kinase-dead Mpk38. Wild-type MPK38 destabilized the association between p53 and MDM2 in a dose-dependent manner. However, when kinase-dead MPK38 was expressed, it did not alter the level of this association (Fig. 7A). In addition, MPK38 inhibited p53 ubiquitination as expected in an in vivo ubiquitination assay (Fig. 7D). These data suggest that MPK38 stimulates p53 function through the destabilization of the p53-MDM2 complex. It is possible that the positive regulators of p53 are also involved in the MPK38-mediated regulation of p53 signaling because MPK38 potentiated the physical association between p53 and its positive regulators, including NM23-H1, STRAP, and 14-3-3, leading to the stimulation of p53 activity and function (Fig. 7B).
In summary, the results presented here suggest that MPK38 stimulates p53 signaling through direct interaction with and Ser15 phosphorylation of p53 and that MPK38 may exert a p53-activating effect, in addition to the proposed oncogenic action judged by its elevated expression in tumors relative to normal counterparts. The in vivo interaction of MPK38 with p53 may provide a molecular basis for the proposed MPK38 functions. In particular, the role of MPK38 as an activator of p53 signaling apparently suggests its function as a critical component of the cell cycle, contributing to the characterization of uncertain physiological functions of MPK38.
Acknowledgments
We gratefully thank Dr. Haiyoung Jung (Cell Therapy Research Center, Korea Research Institute of Bioscience and Biotechnology) for technical assistance on the MPK38 kinase assay and construction of p53 substitution mutants.
This work was supported by National Research Foundation of Korea Grant R0A-2007-000-20006-0.
- AMPK
- AMP-activated protein kinase
- STRAP
- serine/threonine kinase receptor-associated protein
- 5FU
- 5-fluorouracil
- DBD
- DNA-binding domain.
REFERENCES
- 1. Levine A. J. (1997) p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 [DOI] [PubMed] [Google Scholar]
- 2. Prives C., Hall P. A. (1999) The p53 pathway. J. Pathol. 187, 112–126 [DOI] [PubMed] [Google Scholar]
- 3. Appella E., Anderson C. W. (2001) Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268, 2764–2772 [DOI] [PubMed] [Google Scholar]
- 4. Shieh S. Y., Ikeda M., Taya Y., Prives C. (1997) DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91, 325–334 [DOI] [PubMed] [Google Scholar]
- 5. Banin S., Moyal L., Shieh S., Taya Y., Anderson C. W., Chessa L., Smorodinsky N. I., Prives C., Reiss Y., Shiloh Y., Ziv Y. (1998) Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674–1677 [DOI] [PubMed] [Google Scholar]
- 6. Canman C. E., Lim D. S., Cimprich K. A., Taya Y., Tamai K., Sakaguchi K., Appella E., Kastan M. B., Siliciano J. D. (1998) Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281, 1677–1679 [DOI] [PubMed] [Google Scholar]
- 7. Tibbetts R. S., Brumbaugh K. M., Williams J. M., Sarkaria J. N., Cliby W. A., Shieh S. Y., Taya Y., Prives C., Abraham R. T. (1999) A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13, 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chehab N. H., Malikzay A., Appel M., Halazonetis T. D. (2000) Chk2/hCds1 functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev. 14, 278–288 [PMC free article] [PubMed] [Google Scholar]
- 9. Shieh S. Y., Ahn J., Tamai K., Taya Y., Prives C. (2000) The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14, 289–300 [PMC free article] [PubMed] [Google Scholar]
- 10. Hirao A., Kong Y. Y., Matsuoka S., Wakeham A., Ruland J., Yoshida H., Liu D., Elledge S. J., Mak T. W. (2000) DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287, 1824–1827 [DOI] [PubMed] [Google Scholar]
- 11. Dumaz N., Meek D. W. (1999) Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J. 18, 7002–7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toledo F., Wahl G. M. (2006) Regulating the p53 pathway. In vitro hypotheses, in vivo veritas. Nat. Rev. Cancer 6, 909–923 [DOI] [PubMed] [Google Scholar]
- 13. Gil M., Yang Y., Lee Y., Choi I., Ha H. (1997) Cloning and expression of a cDNA encoding a novel protein serine-threonine kinase predominantly expressed in hematopoietic cells. Gene 195, 295–301 [DOI] [PubMed] [Google Scholar]
- 14. Heyer B. S., Warsowe J., Solter D., Knowles B. B., Ackerman S. L. (1997) New member of the Snf1/AMPK kinase family, Melk, is expressed in the mouse egg and preimplantation embryo. Mol. Reprod. Dev. 47, 148–156 [DOI] [PubMed] [Google Scholar]
- 15. Blot J., Chartrain I., Roghi C., Philippe M., Tassan J. P. (2002) Cell cycle regulation of pEg3, a new Xenopus protein kinase of the KIN1/PAR-1/MARK family. Dev. Biol. 241, 327–338 [DOI] [PubMed] [Google Scholar]
- 16. Chartrain I., Couturier A., Tassan J. P. (2006) Cell cycle-dependent cortical localization of pEg3 protein kinase in Xenopus and human cells. Biol. Cell 98, 253–263 [DOI] [PubMed] [Google Scholar]
- 17. Davezac N., Baldin V., Blot J., Ducommun B., Tassan J. P. (2002) Human pEg3 kinase associates with and phosphorylates CDC25B phosphatase. A potential role for pEg3 in cell cycle regulation. Oncogene 21, 7630–7641 [DOI] [PubMed] [Google Scholar]
- 18. Badouel C., Körner R., Frank-Vaillant M., Couturier A., Nigg E. A., Tassan J. P. (2006) M phase MELK activity is regulated by MPF and MAPK. Cell Cycle 5, 883–889 [DOI] [PubMed] [Google Scholar]
- 19. Beullens M., Vancauwenbergh S., Morrice N., Derua R., Ceulemans H., Waelkens E., Bollen M. (2005) Substrate specificity and activity regulation of protein kinase MELK. J. Biol. Chem. 280, 40003–40011 [DOI] [PubMed] [Google Scholar]
- 20. Jung H., Seong H. A., Ha H. (2008) Murine protein serine-threonine kinase 38 activates apoptosis signal-regulating kinase 1 via Thr838 phosphorylation. J. Biol. Chem. 283, 34541–34553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seong H. A., Jung H., Ha H. (2010) Murine protein serine-threonine kinase 38 stimulates TGF-β signaling in a kinase-dependent manner via direct phosphorylation of Smad proteins. J. Biol. Chem. 285, 30959–30970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jakobsen S. N., Hardie D. G., Morrice N., Tornqvist H. E. (2001) 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J. Biol. Chem. 276, 46912–46916 [DOI] [PubMed] [Google Scholar]
- 23. Jung H., Seong H. A., Ha H. (2008) Critical role of cysteine residue 81 of macrophage migration inhibitory factor (MIF) in MIF-induced inhibition of p53 activity. J. Biol. Chem. 283, 20383–20396 [DOI] [PubMed] [Google Scholar]
- 24. Seong H. A., Jung H., Choi H. S., Kim K. T, Ha H. (2005) Regulation of transforming growth factor-β signaling and PDK1 kinase activity by physical interaction between PDK1 and serine-threonine kinase receptor-associated protein. J. Biol. Chem. 280, 42897–42908 [DOI] [PubMed] [Google Scholar]
- 25. Seong H. A., Jung H., Ha H. (2007) NM23-H1 tumor suppressor physically interacts with serine-threonine kinase receptor-associated protein, a transforming growth factor-β (TGF-β) receptor-interacting protein, and negatively regulates TGF-β signaling. J. Biol. Chem. 282, 12075–12096 [DOI] [PubMed] [Google Scholar]
- 26. Jung H., Seong H. A., Ha H. (2007) NM23-H1 tumor suppressor and its interacting partner STRAP activate p53 function. J. Biol. Chem. 282, 35293–35307 [DOI] [PubMed] [Google Scholar]
- 27. Jones R. G., Plas D. R., Kubek S., Buzzai M., Mu J., Xu Y., Birnbaum M. J., Thompson C. B. (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 [DOI] [PubMed] [Google Scholar]
- 28. Okoshi R., Ozaki T., Yamamoto H., Ando K., Koida N., Ono S., Koda T., Kamijo T., Nakagawara A., Kizaki H. (2008) Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J. Biol. Chem. 283, 3979–3987 [DOI] [PubMed] [Google Scholar]
- 29. Kato K., Ogura T., Kishimoto A., Minegishi Y., Nakajima N., Miyazaki M., Esumi H. (2002) Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 21, 6082–6090 [DOI] [PubMed] [Google Scholar]
- 30. Suzuki A., Kusakai G., Kishimoto A., Lu J., Ogura T., Esumi H. (2003) ARK5 suppresses the cell death induced by nutrient starvation and death receptors via inhibition of caspase 8 activation but not by chemotherapeutic agents or UV irradiation. Oncogene 22, 6177–6182 [DOI] [PubMed] [Google Scholar]
- 31. Suzuki A., Kusakai G., Kishimoto A., Lu J., Ogura T., Lavin M. F., Esumi H. (2003) Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J. Biol. Chem. 278, 48–53 [DOI] [PubMed] [Google Scholar]
- 32. Bode A. M., Dong Z. (2004) Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 4, 793–805 [DOI] [PubMed] [Google Scholar]
- 33. Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. (1992) The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69, 1237–1245 [DOI] [PubMed] [Google Scholar]
- 34. Stavridi E. S., Chehab N. H., Malikzay A., Halazonetis T. D. (2001) Substitutions that compromise the ionizing radiation-induced association of p53 with 14-3-3 proteins also compromise the ability of p53 to induce cell cycle arrest. Cancer Res. 61, 7030–7033 [PubMed] [Google Scholar]
- 35. Seong H. A., Gil M., Kim K. T., Kim S. J., Ha H. (2002) Phosphorylation of a novel zinc-finger-like protein, ZPR9, by murine protein serine-threonine kinase 38 (MPK38). Biochem. J. 361, 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vulsteke V., Beullens M., Boudrez A., Keppens S., Van Eynde A., Rider M. H., Stalmans W., Bollen M. (2004) Inhibition of spliceosome assembly by the cell cycle-regulated protein kinase MELK and involvement of splicing factor NIPP1. J. Biol. Chem. 279, 8642–8647 [DOI] [PubMed] [Google Scholar]
- 37. Hemmati H. D., Nakano I., Lazareff J. A., Masterman-Smith M., Geschwind D. H., Bronner-Fraser M., Kornblum H. I. (2003) Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. U.S.A. 100, 15178–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rhodes D. R., Yu J., Shanker K., Deshpande N., Varambally R., Ghosh D., Barrette T., Pandey A., Chinnaiyan A. M. (2004) Large scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc. Natl. Acad. Sci. U.S.A. 101, 9309–9314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gray D., Jubb A. M., Hogue D., Dowd P., Kljavin N., Yi S., Bai W., Frantz G., Zhang Z., Koeppen H., de Sauvage F. J., Davis D. P. (2005) Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer Res. 65, 9751–9761 [DOI] [PubMed] [Google Scholar]
- 40. Vousden K. H., Lu X. (2002) Live or let die. The cell's response to p53. Nat. Rev. Cancer 2, 594–604 [DOI] [PubMed] [Google Scholar]
- 41. Giaccia A. J., Kastan M. B. (1998) The complexity of p53 modulation. emerging patterns from divergent signals. Genes Dev. 12, 2973–2983 [DOI] [PubMed] [Google Scholar]
- 42. Meek D. W. (1999) Mechanisms of switching on p53. A role for covalent modification? Oncogene 18, 7666–7675 [DOI] [PubMed] [Google Scholar]
- 43. Meek D. W. (2004) The p53 response to DNA damage. DNA Repair 3, 1049–1056 [DOI] [PubMed] [Google Scholar]