Background: This study was done to elucidate the biochemical mechanisms underlying phosphorylation-dependent regulation of PDK1.
Results: MPK38 inactivates PDK1 activity and function by phosphorylating PDK1 at Thr354.
Conclusion: MPK38 acts as a putative protein kinase to negatively regulate PDK1.
Significance: This study defines a novel mechanism in which MPK38 directly interacts with and phosphorylates Thr354 of PDK1, thereby inhibiting PDK1 activity.
Keywords: Phosphatidylinositol-dependent Kinase-1 (PDK1), Protein Phosphorylation, Protein-Protein Interactions, Signal Transduction, Transforming Growth Factor beta (TGFbeta), AMP-activated Protein Kinase, ASK1, MPK38
Abstract
Murine protein serine-threonine kinase 38 (MPK38) is a member of the AMP-activated protein kinase-related serine/threonine kinase family, which acts as cellular energy sensors. In this study, MPK38-induced PDK1 phosphorylation was examined to elucidate the biochemical mechanisms underlying phosphorylation-dependent regulation of 3-phosphoinositide-dependent protein kinase-1 (PDK1) activity. The results showed that MPK38 interacted with and inhibited PDK1 activity via Thr354 phosphorylation. MPK38-PDK1 complex formation was mediated by the amino-terminal catalytic kinase domain of MPK38 and the pleckstrin homology domain of PDK1. This activity was dependent on insulin, a PI3K/PDK1 stimulator, as well as various apoptotic stimuli, including TNF-α, H2O2, thapsigargin, and ionomycin. MPK38 inhibited PDK1 activity in a kinase-dependent manner and alleviated PDK1-mediated suppression of TGF-β (or ASK1) signaling, probably via the phosphorylation of PDK1 at Thr354. In addition, MPK38-mediated inhibition of PDK1 activity was accompanied by the modulation of PDK1 binding to its positive and negative regulators, serine/threonine kinase receptor-associated protein and 14-3-3, respectively. Together, these findings suggest an important role for MPK38-mediated phosphorylation of PDK1 in the negative regulation of PDK1 activity.
Introduction
The 3-phosphoinositide-dependent protein kinase-1 (PDK1) is a member of the protein kinase A, G, and C subfamily of protein kinases. PDK1 acts via a PH3 domain and binds phosphoinositides such as phosphatidylinositol 3,4-P2 and phosphatidylinositol 3,4,5-P3 to phosphorylate Thr308 of PKB/Akt. Phosphorylation of both Thr308 and Ser473 is required for maximal activation of PKB/Akt (1, 2). These residues are phosphorylated independently and are dephosphorylated by PDK1/protein phosphatase 2A (PP2A) (for Thr308) and mammalian target of rapamycin complex 2 (mTORC2)/PH domain leucine-rich repeat protein phosphatases (for Ser473), respectively (3–6). In addition, analysis of cellular proteins that interact with PDK1 shows that the activity and function of PDK1 is regulated by PDK1-interacting proteins within cells (7–10). Although an important role for PDK1 in cell survival signaling is well characterized, the mechanism by which PDK1 activity is regulated through phosphorylation remains largely unknown. It was previously thought that PDK1 is constitutively active because it shows a high level of basal activity in unstimulated cells and cannot be further activated by growth factor stimulation (11). However, recent studies strongly suggest that PDK1 activity is regulated in a phosphorylation-dependent manner. Multiple phosphorylation sites on PDK1 (Ser25, Ser241, Ser393, Ser396, and Ser410) have been identified in unstimulated HEK293 cells, but only the phosphorylation of Ser241 (Ser244 in mouse PDK1) within the activation loop is responsible for PDK1 activity (12). Phosphorylation of Ser396 is necessary for nuclear shuttling of PDK1 (13). Phosphorylation of mouse PDK1 at Ser163 is also involved in fine-tuning PDK1 activity (14). In addition, PDK1 in HEK293 cells treated with pervanadate, a tyrosine phosphatase inhibitor, undergoes tyrosine phosphorylation at Tyr9, Tyr373, and Tyr376, leading to its activation (15). RET/PTC, a thyroid-specific oncogenic kinase (16), stimulates PDK1 activity by phosphorylating Tyr9 (17). Protein kinase Cθ (PKCθ), a kinase implicated in hyperlipidemia-induced insulin resistance, negatively regulates PDK1 activity via phosphorylation at Ser504 and Ser532 (18). Reduced PDK1 phosphorylation at Ser244, which is stimulated by insulin, occurs in the liver of obese/obese mice (19). We recently demonstrated that PDK1 undergoes apoptosis signal-regulating kinase 1 (ASK1)-dependent phosphorylation at Ser394 and Ser398, which suppresses its activity (20). These findings suggest that the phosphorylation of PDK1 plays an important role in regulating PDK1 activity and function.
Murine protein serine-threonine kinase 38 (MPK38)/maternal embryonic leucine zipper kinase (MELK) is a member of the AMP-activated protein kinase-related serine/threonine kinase family (21, 22) and is activated by apoptotic cellular stresses, such as H2O2 and transforming growth factor-β (TGF-β), suggesting functional cross-talk between MPK38 and the ASK1 or TGF-β signaling pathways (23, 24). MPK38 is thought to play a role in various cellular processes, including the cell cycle, embryonic development, spliceosome assembly, gene expression, cell proliferation, hematopoiesis, oncogenesis, and apoptosis; however, its precise function is unclear (23–28).
Therefore, to explore the phosphorylation-dependent regulation of PDK1, we investigated whether MPK38 contributes to the phosphorylation of PDK1 and whether it plays a regulatory role in the PDK1 activity. We showed that MPK38 physically interacts with and phosphorylates PDK1 at Thr354, thereby inhibiting its activity and function. Our work also suggests that MPK38, like PKCθ and ASK1 (18, 20), acts as a putative protein kinase to negatively regulate PDK1 in cells.
MATERIALS AND METHODS
Antibodies and Cell Culture
Anti-PDK1, anti-phospho-AKT(T308), anti-phospho-BAD(S136), anti-MPK38, anti-GST, anti-His, anti-FLAG (M2), and anti-β-actin antibodies have been described previously (24, 29). The anti-Myc antibody was purchased from Santa Cruz Biotechnology. The anti-rabbit phospho-PDK1(T354) antibody was raised against a synthetic phosphopeptide antigen LHQQTPPKL, where T represents phosphothreonine (Young In Frontier, Seoul, Korea). HEK293, 293T, NIH 3T3, R1.1, and HaCaT cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Invitrogen) at 37 °C in 5% CO2.
Plasmid Constructs and Inducible MPK38 shRNA Cell Line
Wild-type (WT) and kinase-dead (KD) PDK1, PDK1(PH), PDK1(CA), wild-type, and kinase-dead (K40R) Mpk38, Mcat, Mpkc, ASK1, activator protein 1 (AP-1)-Luc reporter, and c-fos have been described previously (10, 24). An inducible Mpk38 shRNA HEK293 cell line was generated as described previously (23).
PDK1 Mutants and RNA Interference
PDK1 mutants (T354A, S394A/S398A, and S394A/S398A/T354A) were generated by PCR as described previously (20). In brief, wild-type PDK1 was used as the template for amplification with either PDK1 forward 5′-GCGAATTCATGGCCAGGACCACCAGCCAG-3′ (EcoRI site underlined) or reverse 5′-GCCAGCTGTCACTGCACAGCGGCGTCCGG-3′ (SalI site underlined) primers, in conjunction with one of the following mutant primers containing alterations in the nucleotide sequence of wild-type PDK1: for T354A, sense 5′-CTGCACCAGCAGGCGCCTCCGAAGCTC-3′ and antisense 5′-GAGCTTCGGAGGCGCCTGCTGGTGCAG-3′, and for S394A/S398A, sense 5′-TCCTCCTCCGCACACTCCCTGGCAGCCTCCGAC-3′ and antisense 5′-GTCGGAGGCTGCCAGGGAGTGTGCGGAGGAGGA-3′. A FLAG-tagged S394A/S398A double mutant of PDK1 was used as the template to generate the S394A/S398A/T354A triple mutant of PDK1, and the T354A forward and reverse primers were used for PCR amplification. The amplified PCR products were cut with EcoRI/XbaI and subsequently cloned into pBluescript SK (Stratagene). The ClaI/NotI fragments of the resulting plasmids were then cloned into the pEBG vector to yield GST-tagged PDK1 mutants (T354A, S394A/S398A, and S394A/S398A/T354A). The Mpk38 siRNA (1, 5′-CAGGCAGACAAUGGAGGAUTT-3′; 2, 5′-AACCCAAGGGUAACAAGGATT-3′) corresponding to coding regions (1, amino acids 297–303; 2, amino acids 156–162) of Mpk38 (GenBankTM accession number NM010790) and a nonspecific control siRNA (5′-GCGCGGGGCACGUUGGUGUTT-3′) were used for RNA interference experiments (24, 29).
Assays for in Vivo and in Vitro Protein Interactions
Preparation of Recombinant Proteins and the PDK1 Kinase Assay
Recombinant glutathione S-transferase (GST) or His-tagged wild-type and deletion constructs of PDK1 and MPK38 were purified by affinity chromatography on glutathione-Sepharose 4B or His columns (Amersham Biosciences). The PDK1 kinase assay was performed as described previously (29) using immunoprecipitated or recombinant PDK1 proteins. Approximately 500 ng of recombinant SGK (Upstate) was used as the substrate.
Luciferase Reporter Assay
Assays were carried out in 293T cells as described previously (24). Luciferase activity was assayed using the Dual-Luciferase assay system (Promega) according to the manufacturer's instructions and normalized to β-galactosidase activity.
GFP-based Cell Death Assay
Green fluorescent protein (GFP)-based cell death assays were carried out in HEK293, 293T, and HaCaT cells as described previously (23, 24). The nuclei of GFP-positive cells were stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) and analyzed for apoptotic morphology under a fluorescence microscope. The percentage of apoptotic cells was calculated as the number of GFP-positive cells with apoptotic nuclei divided by the total number of GFP-positive cells.
Cell Cycle Analysis
Assays were performed in HaCaT cells as described previously (23). Cells were transfected with the indicated plasmid combinations using WelFect-ExTM Plus (WelGENE, Daegu, Korea). The cell fraction at each stage of the cell cycle was analyzed after treatment with 10% serum for 24 h in the presence or absence of 2 ng/ml porcine TGF-β1 (R & D Systems). Flow cytometry analysis was performed using the FACSCalibur-S system (BD Biosciences).
Statistical Analysis
Values represent the means ± S.E. A p value <0.05 calculated using the Student's t test was considered statistically significant.
RESULTS
MPK38 Interacts with PDK1 Both in Vitro and in Vivo
We previously showed that PDK1 inhibits Smad-mediated signaling via direct interaction with Smad proteins (29). In addition, MPK38 physically interacts with and phosphorylates Smad proteins, resulting in the stimulation of TGF-β signaling (23). Therefore, we speculated that there may be a direct or indirect functional link between MPK38 and PDK1 signaling pathways in cells.
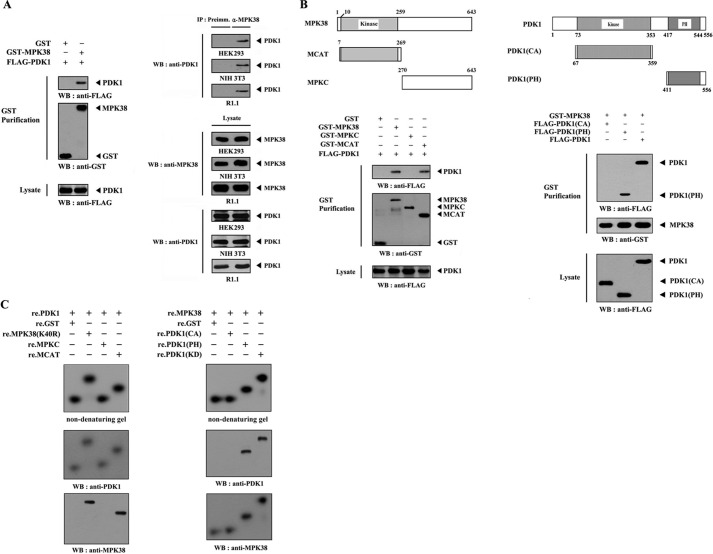
To test this hypothesis, we examined whether MPK38 physically interacts with PDK1 in cells using cotransfection experiments incorporating HEK293 cells expressing GST-MPK38 and FLAG-PDK1. The interaction between MPK38 and PDK1 was analyzed by immunoblotting with an anti-FLAG antibody. The presence of PDK1 was detected in the coprecipitate only when coexpressed with GST-MPK38 but not with GST alone (control) (Fig. 1A, left panel). The endogenous interaction between MPK38 and PDK1 was subsequently determined by coimmunoprecipitation experiments in HEK293, NIH 3T3, and R1.1 cells. Immunoprecipitation of endogenous MPK38 by the anti-MPK38 antibody followed by immunoblotting with an anti-PDK1 antibody identified a physical interaction between the two endogenous proteins (Fig. 1A, right panel). These observations prompted us to map the interaction domain of MPK38, which is involved in binding to PDK1. We performed in vivo binding assays using HEK293 cells expressing wild-type MPK38 and two deletion constructs of MPK38 as follows: MCAT harboring the catalytic kinase domain (amino acids 7–269), and MPKC comprising the carboxyl-terminal regulatory domain (amino acids 270–643). Wild-type MPK38 and MCAT were able to bind PDK1, but no binding of MPKC to PDK1 was detected (Fig. 1B, left panel). This indicates that the amino-terminal kinase domain of MPK38 is responsible for PDK1 binding. Using the same approach, we determined which domain of PDK1 contributes to MPK38 binding. The carboxyl-terminal PH domain (amino acids 411–556) of PDK1 was found to be required for MPK38 binding, whereas the catalytic domain (amino acids 67–359) was unable to bind MPK38 (Fig. 1B, right panel), suggesting the carboxyl-terminal PH domain of PDK1 as a potential site for MPK38 binding.
FIGURE 1.
MPK38 interacts with PDK1. A, in vivo association of MPK38 with PDK1. GST alone or GST-MPK38 was cotransfected into HEK293 cells along with FLAG-PDK1. GST fusion proteins were purified on glutathione-Sepharose beads (GST Purification), and complex formation was analyzed by immunoblotting with an anti-FLAG antibody (left panel). Equal amounts of cell lysate from HEK293, NIH 3T3, and R1.1 cells were immunoprecipitated with either rabbit preimmune serum (Preimm.) or anti-MPK38 antibody (α-MPK38) followed by immunoblot analysis with an anti-PDK1 antibody to determine endogenous binding (right top panels). B, mapping of the MPK38 and PDK1 binding domains. The schematic structures of wild-type and deletion constructs of Mpk38 (left panel) and PDK1 (right panel) are shown. HEK293 cells transfected with the indicated expression vectors were lysed, precipitated using glutathione-Sepharose beads, and then immunoblotted with an anti-FLAG antibody to determine the level of MPK38-PDK1 binding. C, in vitro binding of MPK38 and PDK1. For native PAGE of the MPK38-PDK1 complex, autophosphorylated His-tagged PDK1 or MPK38 (each 2–3 μg), prepared in the presence of the respective kinase buffers (24, 29), were incubated with unlabeled recombinant GST-tagged kinase-dead MPK38 (or PDK1) and its deletion constructs (for MPK38, MPKC, and MCAT; for PDK1, CA, and PH) (each 5 μg), together with the nonspecific control GST at room temperature for 1 h. The same blot was stripped and re-probed with anti-PDK1 and anti-MPK38 antibodies to confirm the presence of PDK1 and MPK38 on the radioactive band shifts (middle and bottom panels). The purity of recombinant PDK1 and MPK38 proteins used for this experiment was analyzed by Coomassie Blue staining (see supplemental Fig. 4A). IP, immunoprecipitation; re., recombinant; WB, Western blot; MPK38(K40R), kinase-dead MPK38; Preimm., preimmune serum.
To confirm this, we analyzed the in vitro association of purified recombinant PDK1 with MPK38 using nondenaturing PAGE. Autophosphorylated recombinant PDK1 was incubated with an unlabeled recombinant kinase-dead (K40R) MPK38 with one of its deletion mutants (MPKC and MCAT) or with GST as a nonspecific control. A shift in the mobility of 32P-labeled PDK1 was clearly detected upon incubation with kinase-dead MPK38 or MCAT, but no shift was observed upon incubation with GST alone or MPKC (Fig. 1C, left panel). Similarly, a shift in the mobility of 32P-labeled MPK38 was clearly evident upon incubation with kinase-dead (KD) PDK1 and its deletion mutant PDK1(PH), but it was undetectable in the presence of GST alone or PDK1(CA) (Fig. 1C, right panel). Cumulatively, these results demonstrate that the physical interaction between MPK38 and PDK1 is mediated by the amino-terminal kinase domain of MPK38 and the carboxyl-terminal PH domain of PDK1.
Modulation of MPK38-PDK1 Complex Formation by Insulin and Apoptotic Stimuli
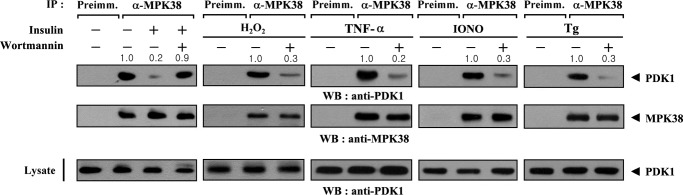
Because MPK38 and PDK1 interact with each other (see Fig. 1), and MPK38 stimulates apoptosis signal-regulating kinase 1 (ASK1) signaling (24), we assessed whether a PI3K/PDK1 stimulator (insulin) or MPK38/ASK1 stimulators (H2O2, TNF-α, thapsigargin, and ionomycin) had an effect on MPK38-PDK1 complex formation in HEK293 cells. As shown in Fig. 2, the exposure of the cells to insulin resulted in a considerable decrease in endogenous MPK38-PDK1 complex formation, but this effect was alleviated by treatment with wortmannin (a PI3K inhibitor). In addition, the endogenous interaction between MPK38 and PDK1 appeared to be decreased by treatment with agents that stimulate MPK38/ASK1. These results suggest that MPK38 may be associated with the PI3K/PDK1 signaling pathway.
FIGURE 2.
MPK38/ASK1 and PDK1 signaling decreases MPK38-PDK1 binding. HEK293 cells were incubated with or without wortmannin (100 nm, 30 min) and then treated with insulin (100 nm, 20 min). The cell lysates were then immunoprecipitated (IP) with either rabbit preimmune serum (Preimm.) or an anti-MPK38 antibody (α-MPK38) followed by immunoblotting with an anti-PDK1 antibody to determine endogenous MPK38-PDK1 binding (1st panel). Equal amounts of cell lysate were treated with or without the following stimuli (20): H2O2 (2 mm, 30 min), TNF-α (500 ng/ml, 30 min), thapsigargin (Tg) (20 μm, 30 min), or ionomycin (IONO) (1 μm, 24 h). The cell lysates were then immunoprecipitated with the indicated rabbit preimmune serum or anti-MPK38 antibody (α-MPK38) and immunoblotted with an anti-PDK1 antibody to assess endogenous MPK38-PDK1 binding (2nd to 4th panels). WB, Western blot.
MPK38 Inhibits PDK1 Activity via Thr354 Phosphorylation
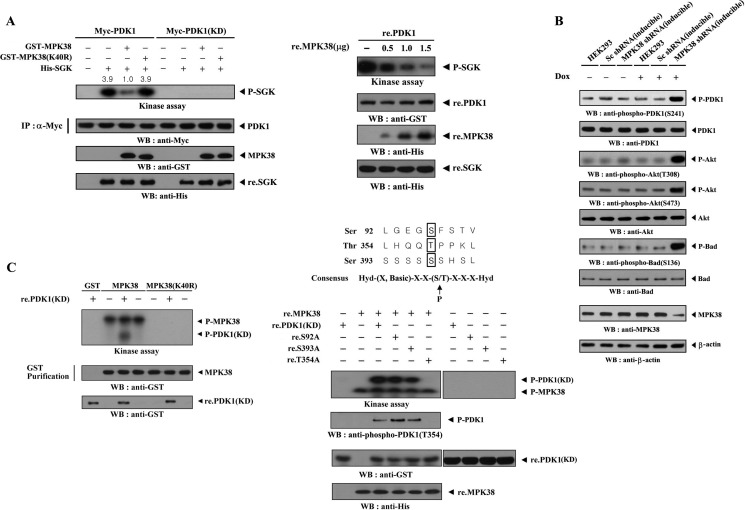
To determine whether MPK38 affected PDK1 kinase activity, HEK293 cells were transfected with either PDK1 alone or with both PDK1 and Mpk38. PDK1 kinase activity decreased markedly when PDK1 was coexpressed with wild-type MPK38, whereas coexpression of kinase-dead (K40R) MPK38 had no effect on the PDK1 kinase activity compared with the control expressing wild-type PDK1 alone (Fig. 3A, left panel). However, this effect was not observed under the same conditions in the presence of kinase-dead PDK1, suggesting that PDK1-mediated phosphorylation of the SGK substrate occurred without relying on additional proteins coimmunoprecipitated with PDK1. This result indicates that MPK38 inhibited PDK1 kinase activity in a kinase-dependent manner. Another approach using recombinant PDK1 and MPK38 proteins showed that recombinant wild-type MPK38 directly inhibited PDK1 kinase activity in a dose-dependent manner (Fig. 3A, right panel).
FIGURE 3.
MPK38 inhibits PDK1 kinase activity. A, HEK293 cells were transiently transfected with Myc-tagged wild-type or kinase-dead PDK1 in the presence or absence of GST-tagged wild-type and kinase-dead Mpk38. Cell lysates were subjected to immunoprecipitation (IP) with an anti-Myc antibody, and the Myc immunoprecipitates were analyzed for PDK1 kinase activity in an in vitro kinase assay using recombinant SGK as the substrate (left panel). Purified recombinant PDK1 proteins (re.PDK1) were incubated at room temperature for 1 h with the indicated amount of recombinant MPK38 (re.MPK38) in 50 μl of 50 mm HEPES buffer, pH 7.4, and then analyzed for PDK1 kinase activity in an in vitro kinase assay (right panel). P-SGK indicates phosphorylated SGK. B, effect of MPK38 on PDK1 downstream signaling. HEK293 cells harboring the stably integrated pSingle-tTS-shRNA vector containing scrambled shRNA (inducible Sc shRNA) or the pSingle-tTS-shRNA vector containing Mpk38-specific shRNA (inducible MPK38 shRNA), or parental HEK293 cells, were cultured in the presence or absence of 1 μg/ml doxycycline (Dox) for 72 h, and PDK1 downstream signaling was determined by immunoblot analysis. Inducible silencing of endogenous MPK38 by doxycycline was assessed by immunoblotting using an anti-MPK38 antibody. β-Actin was used as a loading control. C, identification of MPK38 phosphorylation sites on PDK1. After 48 h of transfection with GST-tagged wild-type or kinase-dead Mpk38, HEK293 cell lysates were subjected to precipitation with glutathione-Sepharose beads (GST purification) and analyzed in an in vitro kinase assay with recombinant kinase-dead PDK1 as the substrate (left panel). The in vitro kinase assay was performed with ∼3 μg of recombinant GST-tagged kinase-dead PDK1 or one of its substitution mutants (S92A, T354A, or S393A) in the presence or absence of recombinant His-tagged wild-type MPK38 (∼6 μg) (right panel). The purity of recombinant MPK38 and PDK1 proteins used for this experiment was confirmed by Coomassie Blue staining (see supplemental Fig. 4B). P-MPK38 and P-PDK1(KD) indicate autophosphorylated MPK38 and phosphorylated kinase-dead PDK1, respectively.
To assess whether the MPK38-mediated inhibition of PDK1 kinase activity influenced PDK1-mediated signaling, we developed a stable system for the tetracycline-inducible expression of Mpk38 shRNA in HEK293 cells (inducible MPK38 shRNA). Parental HEK293 cells, HEK293 cells expressing a scrambled shRNA (inducible Sc shRNA), or inducible MPK38 shRNA cells were either untreated or treated with doxycycline to induce the knockdown of endogenous MPK38. Anti-phospho-specific antibodies for PDK1 Ser241, AKT Thr308, AKT Ser473, and BAD Ser136 were then used for immunoblot analysis to assess PDK1 downstream signaling. As shown in Fig. 3B, knockdown of endogenous MPK38 markedly stimulated PDK1 downstream signaling. These findings point to the inhibition of PDK1-mediated signaling upon MPK38 binding and suggest that MPK38 is a potential negative regulator of PDK1 activity.
We next determined whether PDK1 acts as a substrate for MPK38 using the recombinant nonphosphorylated form of the kinase-dead (KD) PDK1 protein as a substrate in the MPK38 kinase assay. Wild-type and kinase-dead MPK38 proteins, purified from GST-MPK38-expressing HEK293 cell lysates using glutathione-Sepharose beads, were incubated with [γ-32P]ATP to allow phosphorylation of recombinant PDK1(KD). Phosphorylation of recombinant PDK1(KD) was clearly detected in the presence of wild-type MPK38 but not in the presence of GST alone or kinase-dead MPK38 (Fig. 3C, left panel), indicating that PDK1 may be a substrate for MPK38. Next, to identify the MPK38 phosphorylation sites on PDK1, we performed an alignment analysis using the AMP-activated protein kinase/MPK38 consensus sequence (23, 30); three potential MPK38 phosphorylation sites (Ser92, Thr354, and Ser393) on PDK1 were selected. In vitro kinase assays using recombinant MPK38 and PDK1(KD) substitution mutants (S92A, T354A, and S393A) showed that MPK38 phosphorylated two PDK1(KD) mutants, S92A and S393A, but not the T354A mutant (Fig. 3C, right panel). This was also confirmed by immunoblot analysis using an anti-phospho-PDK1(T354) antibody. These results suggest that MPK38 physically interacts with and phosphorylates PDK1 at Thr354.
MPK38 Alleviates PDK1-mediated Suppression of Apoptosis in a Kinase-dependent Manner
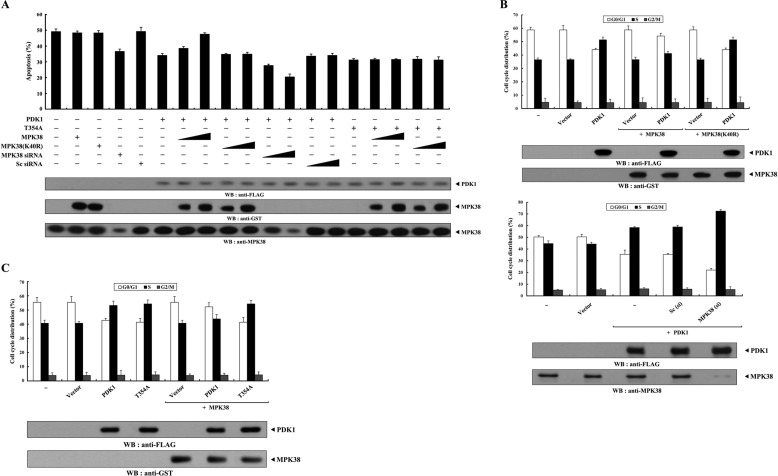
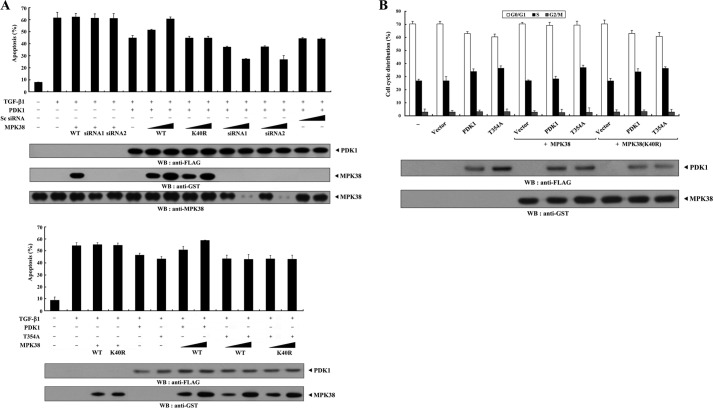
To investigate whether MPK38 regulates PDK1-mediated suppression of apoptosis, we analyzed the effect of MPK38 on PDK1-mediated suppression of TNF-α-induced apoptosis using the GFP system (10, 29). Expression of PDK1 in the presence of TNF-α resulted in a considerable decrease in apoptotic cell death compared with that in control cells expressing an empty vector. However, wild-type MPK38, but not kinase-dead (K40R) MPK38, alleviated this suppression in a dose-dependent manner (Fig. 4A, compare 6th lane with 7th to 10th lanes). This result implies that MPK38 contributes to the negative regulation of the PDK1-mediated survival signaling pathway through direct interaction and phosphorylation. To confirm this, we examined the effect of Mpk38 siRNA on TNF-α-induced apoptosis. As expected, knockdown of endogenous MPK38 decreased TNF-α-induced apoptosis in a dose-dependent manner compared with control cells expressing PDK1 alone (Fig. 4A, compare 6th lane with 11th to 14th lanes). To determine the role of MPK38-mediated phosphorylation of PDK1 at Thr354 in the regulation of PDK1 signaling, we also assessed the effect of MPK38 on PDK1(T354A)-mediated signaling because the T354A mutant was found to be defective in MPK38-mediated phosphorylation (see Fig. 3C). Neither wild-type nor kinase-dead MPK38 had an effect on PDK1(T354A)-mediated suppression of apoptosis compared with that in a control expressing PDK1(T354A) alone (Fig. 4A, compare 15th lane with 16th to 19th lanes). These results indicate that MPK38 negatively regulates PDK1 activity by phosphorylating it on Thr354. We then extended our analysis to examine whether MPK38 regulated serum-induced cell growth induced by PDK1 activation. Flow cytometry analysis using HaCaT cells showed that coexpression of wild-type MPK38 considerably decreased the percentage of cells in S phase compared with that in control cells expressing PDK1 alone (Fig. 4B, upper panel, 3rd lane versus 5th lane, ∼51 versus ∼41%; see supplemental Fig. 1A), although kinase-dead MPK38 had no effect (Fig. 4B, upper panel, 3rd lane versus 7th lane, ∼51 versus ∼51%; see supplemental Fig. 1A). These results suggest that the decrease in the number of S phase cells observed in the presence of MPK38 was due to MPK38-mediated phosphorylation of PDK1, because transfected Mpk38 (wild-type or kinase-dead) itself did not influence the percentage of cells in S phase (Fig. 4B, upper panel, 2nd lane versus 4th and 6th lanes, ∼37 versus ∼37%; see supplemental Fig. 1A). To further confirm the negative role of MPK38 in PDK1-mediated cell growth, we performed flow cytometry analysis using HaCaT cells transfected with Mpk38-specific siRNA. Knockdown of endogenous MPK38 resulted in a marked increase in the percentage of cells in S phase cells compared with that in control cells expressing PDK1 alone (Fig. 4B, lower panel, 3rd lane versus 5th lane, ∼58 versus ∼72%; see supplemental Fig. 1A). However, we did not observe any change in the percentage of cells in S phase in the presence of control scrambled siRNA (Fig. 4B, lower panel, 3rd lane versus 4th lane, ∼58 versus ∼59%; see supplemental Fig. 1A). With regard to PDK1 phosphorylation at Thr354, MPK38 had no effect on the accumulation of S phase cells in the presence of the T354A mutant (Fig. 4C, 4th lane versus 7th lane, ∼54 versus ∼54%; see supplemental Fig. 1B). These results suggest that MPK38-mediated phosphorylation of PDK1 at Thr354 plays an important role in the negative regulation of PDK1-mediated cell growth.
FIGURE 4.
MPK38 inhibits PDK1 activity toward TNF-α-induced apoptosis and serum-induced cell growth. A, effect of MPK38 on PDK1-mediated suppression of TNF-α-induced apoptosis. 293T cells were transiently transfected with the indicated plasmid vectors (PDK1 and T354A, 2 μg each; wild-type and kinase-dead Mpk38, 0.4 and 0.8 μg), Mpk38-specific siRNA1 (50 and 200 nm), and scrambled siRNA (50 and 200 nm). After 24 h, cells were treated with TNF-α (20 ng/ml) and cycloheximide (10 μg/ml) for 14 h to induce apoptosis. Apoptotic cell death was then determined using a GFP-based cell death assay (24). B and C, effect of MPK38 on PDK1-mediated cell cycle progression. HaCaT cells transfected with the indicated combinations of plasmid vectors (PDK1, 0.5 μg; wild-type and kinase-dead Mpk38, 0.4 μg each), together with Mpk38-specific siRNA1 and scrambled siRNA (200 nm each), were synchronized in G0/G1 by hydroxyurea treatment (2 mm, 20 h), as described (33). Cells were collected after treatment with 10% serum for 24 h and the number of cells in G0/G1, S, and G2/M were assessed by flow cytometry. Data represent the mean (±S.E.) of at least three independent experiments. WB, Western blot.
MPK38 Alleviates PDK1-mediated Suppression of TGF-β Signaling in a Kinase-dependent Manner
PDK1 inhibits TGF-β signaling (29); therefore, to investigate whether MPK38 is involved in the regulation of PDK1-mediated TGF-β signaling, we examined the effect of MPK38 on PDK1-mediated suppression of TGF-β-induced apoptosis. Wild-type MPK38, but not kinase-dead MPK38, increased apoptotic cell death in a dose-dependent manner compared with that in control cells expressing PDK1 alone (Fig. 5A, upper panel, 6th lane versus 7th to 10th lanes). However, neither wild-type nor kinase-dead MPK38 had an effect on PDK1(T354A)-mediated suppression of TGF-β-induced apoptosis (Fig. 5A, lower panel, 6th lane versus 9th to 12th lanes). This result implies that MPK38 contributes to the regulation of PDK1-mediated TGF-β signaling by phosphorylating PDK1 at Thr354. We also performed flow cytometry analysis using HaCaT cells to determine whether MPK38 had a similar effect on PDK1-mediated suppression of TGF-β-induced cell cycle arrest. Coexpression of wild-type MPK38 decreased the accumulation of cells in S phase after 24 h of serum stimulation in the presence of TGF-β1 compared with that in control cells expressing PDK1 alone (Fig. 5B, 3rd lane versus 6th lane, ∼34 versus ∼28%; see supplemental Fig. 2). However, kinase-dead MPK38 had no effect on the accumulation of S phase cells (Fig. 5B, 3rd lane versus 9th lane, ∼34 versus ∼34%; see supplemental Fig. 2). These results are consistent with those obtained from the analysis of MPK38 involvement in PDK1-mediated suppression of TNF-α-induced apoptosis and cell cycle arrest (Fig. 4). Furthermore, the effect of MPK38 on PDK1-mediated TGF-β signaling was due to the phosphorylation of PDK1 at Thr354 (induced by MPK38 itself) because wild-type MPK38 had no effect on the accumulation of S phase cells in the presence of the T354A mutant (Fig. 5B, 4th lane versus 7th lane, ∼36 versus ∼37%; see supplemental Fig. 2). Taken together, these results suggest that MPK38 alleviates PDK1-mediated suppression of TGF-β signaling by phosphorylating PDK1 at Thr354.
FIGURE 5.
MPK38 inhibits PDK1 activity toward TGF-β-induced apoptosis and cell cycle arrest. A, effect of MPK38 on PDK1-mediated suppression of TGF-β-induced apoptosis. HaCaT cells were transiently transfected with increasing amounts of wild-type and kinase-dead Mpk38 (0.5 and 1.5 μg) and Mpk38 siRNAs (50 and 200 nm) as indicated, together with an expression vector encoding GFP (1 μg) in the presence or absence of the wild-type and mutant form (T354A) of PDK1 (2 μg each). After treatment of the transfected cells with TGF-β1 (2 ng/ml, 20 h), apoptotic cell death was determined using a GFP-based cell death assay. B, effect of MPK38 on PDK1-mediated suppression of TGF-β-induced cell cycle arrest. HaCaT cells (∼2 × 105/dish) transfected with wild-type and mutant form (T354A) of PDK1 (0.5 μg each) in the presence or absence of wild-type and kinase-dead Mpk38 (0.4 μg each) were synchronized in G0/G1 by hydroxyurea treatment (2 mm, 20 h). Cells were collected after treatment with 10% serum for 24 h in the presence of TGF-β1, and the percentage of cells in the G0/G1, S, or G2/M phases was analyzed by flow cytometry. Data are representative of at least three independent experiments performed in duplicate. MPK38(K40R), kinase-dead MPK38. WB, Western blot.
MPK38 Alleviates PDK1-mediated Suppression of ASK1 Signaling in a Kinase-dependent Manner
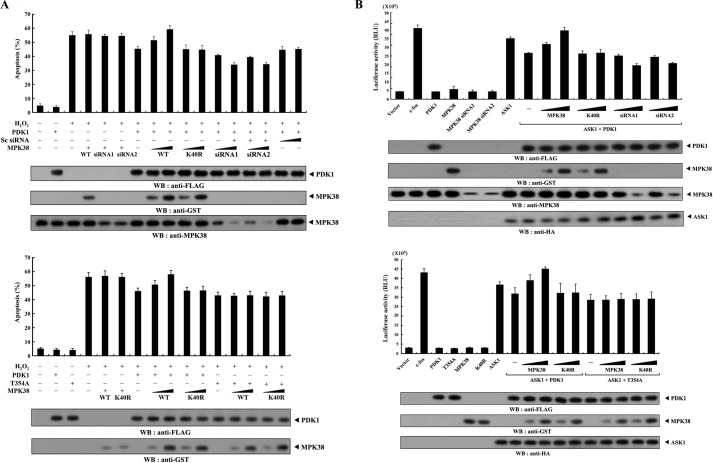
Because PDK1 interacts with ASK1 and inhibits ASK1-mediated signaling (20), we also examined whether MPK38 had an effect on PDK1-mediated suppression of H2O2-induced cell death. Wild-type MPK38, but not kinase-dead MPK38, alleviated PDK1-mediated suppression of H2O2-induced apoptosis in a dose-dependent manner (Fig. 6A, upper panel, 7th lane versus 8th to 11th lanes). To further confirm this observation, we performed knockdown experiments using Mpk38 siRNA. Transfection of Mpk38 siRNA into HEK293 cells potentiated the PDK1-mediated suppression of H2O2-induced apoptosis in a dose-dependent manner (Fig. 6A, upper panel, 7th lane versus 12th to 17th lanes). These results suggest that MPK38 contributes to the alleviation of PDK1-mediated suppression of ASK1 signaling by inhibiting PDK1 activity via direct interaction and phosphorylation. To verify this, we compared the effect of MPK38 on PDK1-mediated suppression of H2O2-induced apoptosis in the presence of wild-type PDK1 with its effect in the presence of the T354A mutant, which is defective in MPK38-mediated phosphorylation. In contrast with the negative effect of wild-type MPK38 on PDK1-mediated suppression of H2O2-induced apoptosis (Fig. 6A, lower panel, 7 to 11 lanes), neither wild-type nor kinase-dead MPK38 affected PDK1(T354A)-mediated suppression of H2O2-induced apoptosis (Fig. 6A, lower panel, 12th to 16th lanes), indicating a crucial role for PDK1 phosphorylation at Thr354 in the MPK38-mediated regulation of H2O2-induced apoptosis suppressed by PDK1.
FIGURE 6.
MPK38 inhibits PDK1 activity toward H2O2-induced apoptosis and JNK-mediated transcription. A, effect of MPK38 on PDK1-mediated suppression of H2O2-induced apoptosis. HEK293 cells were transiently transfected with increasing amounts of wild-type and kinase-dead Mpk38 (0.4 and 0.8 μg) or Mpk38 siRNAs (100 and 200 nm) in the presence of the wild-type or mutant form (T354A) of PDK1 (1.5 μg each). Apoptotic cell death was determined in the GFP-based cell death assay. Cells exposed to 1 mm H2O2 for 9 h were used as a positive control. B, effect of MPK38 on PDK1-mediated suppression of JNK-mediated transcription. 293T cells were transfected with PDK1 (wild-type and mutant form (T354A), 1 μg each), Mpk38 (wild-type and kinase-dead (K40R), 0.6 and 1.2 μg), ASK1 (0.8 μg), and Mpk38 siRNAs (50 and 200 nm), together with 0.2 μg of AP-1 luciferase plasmid and β-galactosidase plasmid (0.2 μg) as an internal control in the presence or absence of c-fos (0.7 μg) as indicated. Luciferase activity was measured 48 h after transfection and normalized to β-galactosidase activity. Data represent the mean (±S.E.) of three independent experiments. RLU, relative light unit; WB, Western blot.
Because PDK1 suppresses ASK1-induced AP-1 transcriptional activity (20), it is possible that MPK38 also enhances AP-1 transcriptional activity. To test this hypothesis, we performed an AP-1-responsive luciferase reporter assay to determine whether MPK38 affected the transcriptional activity of AP-1. As expected, wild-type MPK38, but not kinase-dead MPK38, increased AP-1-dependent luciferase activity in the presence of ASK1 and PDK1 in a dose-dependent manner (Fig. 6B, upper panel, 8th to 12th lanes). However, this effect was not observed in the presence of ASK1 and T354A mutants (Fig. 6B, lower panel, 13th to 17th lanes), suggesting that MPK38-mediated phosphorylation of PDK1 at Thr354 is also required for the alleviation of PDK1-mediated suppression of AP-1 transcriptional activity.
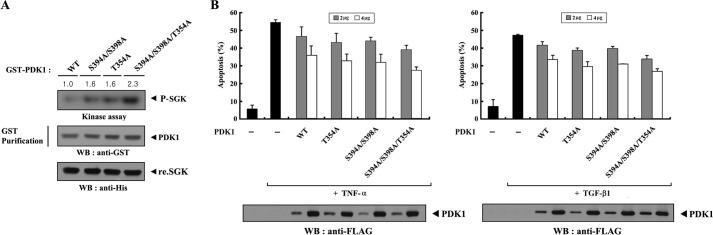
Phosphorylation of PDK1 at Thr354, Ser394, and Ser398 Functions Cooperatively to Inhibit PDK1 Activity
ASK1-mediated phosphorylation of PDK1 at Ser394 and Ser398 contributes to the inhibition of PDK1 activity (20). Therefore, to establish whether the phosphorylation of PDK1 at Thr354 induced by MPK38 has a similar effect on the regulation of PDK1 activity, we first analyzed the kinase activity of the T354A mutant using an in vitro kinase assay. The results showed that the T354A mutant induced PDK1 kinase activity at a level comparable with that of the S394A/S398A double mutant, which is defective in ASK1-mediated phosphorylation (Fig. 7A, 1st to 3rd lanes). Next, to determine the cooperative effects of PDK1 phosphorylation at Thr354, Ser394, and Ser398 in the negative regulation of PDK1 activity, we measured the kinase activity of the PDK1 S394A/S398A/T354A triple mutant and compared it with that of two other PDK1 mutants, T354A and S394A/S398A. The S394A/S398A/T354A triple mutant showed higher phosphorylation of SGK than the T354A single mutant or the S394A/S398A double mutant (Fig. 7A, 1st lane versus 2nd to 4th lanes). These results provide evidence that the phosphorylation of PDK1 at Thr354, Ser394, and Ser398 is cooperatively involved in the negative regulation of PDK1 activity. If this is the case, the increased kinase activity of the PDK1 mutants defective in MPK38- and/or ASK1-mediated phosphorylation may influence PDK1-mediated cell survival functions. To verify this, we analyzed the effect of three PDK1 mutants (T354A, S394A/S398A, and S394A/S398A/T354A) on PDK1-mediated suppression of TNF-α-induced apoptosis. As expected, the suppressive effect of the S394A/S398A/T354A triple mutant was stronger than that of the T354A or S394A/S398A mutants (Fig. 7B, left panel). A similar result was also obtained when we analyzed the effect of the three PDK1 mutants on PDK1-mediated suppression of TGF-β-induced apoptosis (Fig. 7B, right panel). Together, these results indicate that the phosphorylation of PDK1 at Thr354, Ser394, and Ser398 by MPK38 and ASK1 has a cooperative effect on the negative regulation of PDK1 activity.
FIGURE 7.
Cooperative effect of PDK1 phosphorylation at Thr354, Ser394, and Ser398 on the negative regulation of PDK1 activity. A, effect of mutations of Thr354, Ser394, and Ser398 to Ala on PDK1 kinase activity. After 48 h of transfection with GST-PDK1 or one of its substitution mutants (S394A/S398A, T354A, or S394A/S398A/T354A), HEK293 cell lysates were subjected to precipitation with glutathione-Sepharose beads (GST purification) and analyzed in an in vitro kinase assay with recombinant SGK as the substrate to assess the kinase activity of PDK1. The relative level of kinase activity was quantitated by densitometric analysis, and the fold increase relative to the control expressing wild-type PDK1 was calculated. B, effect of mutating Thr354, Ser394, and Ser398 to Ala on PDK1-mediated suppression of TNF-α (or TGF-β)-induced apoptosis. 293T (left panel) or HEK293 (right panel) cells were transfected with the indicated plasmid vectors. Cells were treated with TNF-α (20 ng/ml) and cycloheximide (10 μg/ml) for 14 h or TGF-β1 (2 ng/ml) for 20 h to induce apoptosis. The data shown are representative of at least three independent experiments. WB, Western blot.
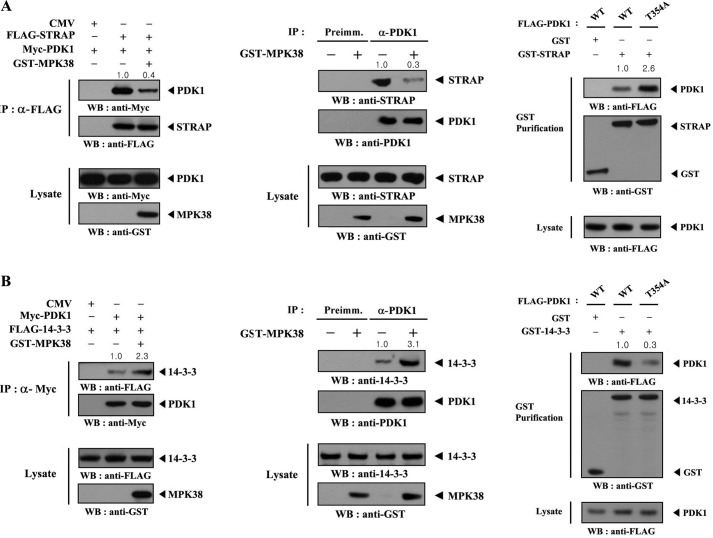
MPK38 Modulates Complex Formation between PDK1 and Its Regulators STRAP and 14-3-3 Protein
Because STRAP, originally identified as a TGF-β receptor-interacting protein (32), positively regulates PDK1 activity (10), we thought that MPK38 may have an effect on the binding of PDK1 to STRAP. To test this, HEK293 cells transfected with FLAG-STRAP and Myc-PDK1 in the presence or absence of Mpk38 were subjected to immunoprecipitation using an anti-FLAG antibody followed by immunoblot analysis with an anti-Myc antibody. There was a considerable decrease in complex formation between PDK1 and STRAP in cells coexpressing MPK38 (Fig. 8A, left panel). Concordantly, coexpression of MPK38 decreased endogenous complex formation between PDK1 and STRAP (Fig. 8A, middle panel). Because PDK1 forms an inactive complex with 14-3-3 (8), we also investigated the effect of MPK38 on PDK1-14-3-3 complex formation. The results showed that MPK38 markedly increased the interaction between PDK1 and 14-3-3 (Fig. 8B, left and middle panels). These results suggest that PDK1 inactivation by MPK38 modulates complex formation between PDK1 and its positive and negative regulators, STRAP and 14-3-3, respectively.
FIGURE 8.
MPK38 induces the dissociation of STRAP from PDK1 and association of 14-3-3 with PDK1. HEK293 cells, transfected with the indicated plasmid vectors, were lysed, immunoprecipitated (IP), and then immunoblotted with the indicated antibodies to assess the levels of PDK1-STRAP (A) or PDK1–14-3-3 (B) complex formation (left panels). Cell lysates from HEK293 cells transfected with or without GST-MPK38 were subjected to immunoprecipitation using either rabbit preimmune serum (Preimm.) or an anti-PDK1 antibody (α-PDK1) followed by immunoblotting using an anti-STRAP antibody or anti-14-3-3 antibody to determine endogenous complex formation between PDK1 and STRAP or PDK1 and 14-3-3 (A and B, middle panels). To determine the effect of PDK1 phosphorylation at Thr354 on endogenous association between PDK1 and STRAP or PDK1 and 14-3-3, HEK293 cells were transfected with the indicated plasmid vectors and lysed and purified on glutathione-Sepharose beads (GST Purification). The amount of complex formation was evaluated by immunoblotting with an anti-FLAG antibody (A and B, right panels). WB, Western blot.
We next examined the effect of MPK38-mediated phosphorylation of PDK1 at Thr354 on complex formation between PDK1 and its regulators, STRAP and 14-3-3, using in vivo binding assays. The results showed that MPK38-mediated phosphorylation of PDK1 at Thr354 stabilized complex formation between PDK1 and its negative regulator 14-3-3, but destabilized complex formation between PDK1 and its positive regulator STRAP, thereby inhibiting PDK1 activity (Fig. 8, A and B, right panels). This finding suggests that the phosphorylation of PDK1 at Thr354 induced by MPK38 has an important impact on the interaction of PDK1 with its regulators (STRAP and 14-3-3) and plays a key role in the progress of PDK1-mediated signaling.
DISCUSSION
In this study, we show that MPK38 inhibits PDK1, a master kinase for regulating multiple signaling pathways (34, 35), through direct interaction and phosphorylation. MPK38 inactivates PDK1 by phosphorylating PDK1 at Thr354. This suggests that the MPK38-dependent phosphorylation of PDK1 at Thr354, like Ser394/Ser398 phosphorylation by ASK1 and Ser504/Ser532 phosphorylation by PKCθ (18, 20), plays a negative role in the regulation of PDK1 activity and function.
We investigated whether MPK38 directly phosphorylates PDK1 using an in vitro kinase assay because MPK38 physically interacted with PDK1 both in vivo and in vitro (Fig. 1). We found that MPK38-mediated phosphorylation of PDK1 occurred exclusively at Thr354, leading to the inhibition of PDK1 activity and function (Figs. 4–7). These findings indicate that the Thr354 of PDK1 is a MPK38 phosphorylation site for the negative regulation of PDK1.
Our results also showed that the T354A mutant itself had a comparatively minor effect on PDK1 function when compared with the control wild-type PDK1 (Figs. 4–7), suggesting the possibility of other potential phosphorylation sites on PDK1 that regulate its activity. Indeed, a similar trend was observed in our previous study showing that ASK1-dependent phosphorylation of PDK1 at Ser394 and Ser398 plays a negative regulatory role in the PDK1 function (20). This observation, together with that of PKCθ (18), strongly indicates that the phosphorylation of PDK1 plays an important role in the regulation of PDK1 activity and function. To examine whether the phosphorylation sites (Thr354, Ser394, and Ser398) on PDK1, which are known to inhibit the PDK1 activity (20), had a cooperative effect on the negative regulation of PDK1 activity and function, we analyzed the kinase activity and apoptotic suppression of a PDK1 triple mutant (S394A/S398A/T354A), together with two other PDK1 mutants, T354A and S394A/S398A, which are defective in MPK38- and ASK1-mediated phosphorylation, respectively. The results showed that the PDK1 triple mutant (S394A/S398A/T354A) had a stronger effect on the kinase activity and apoptotic suppressive function of PDK1 than T354A or S394A/S398A (Fig. 7), indicating that PDK1 phosphorylation at Thr354, Ser394, and Ser398 has a cooperative effect on the negative regulation of PDK1 signaling. Recent studies suggest the involvement of docking interactions between protein kinases and their substrates for achieving substrate specificity and regulation of protein kinase activities (36). Based on this, one may raise the argument that the regulation of PDK1 activity by MPK38 is due to the direct docking interaction between PDK1 and MPK38. However, MPK38, unlike other protein family kinases A, G, and C interacting with PDK1, was found to interact with PDK1 via the amino-terminal kinase domain of MPK38 (see Fig. 1B). In addition, as in the case of SGK (Fig. 7A), similar results (data not shown) were obtained when we analyzed the effect of the three PDK1 mutants on PDK1 kinase activity using other PDK1 substrates that do not possess docking sites, including Smads (Smad2, -3, -4, and -7) (29), STRAP (10), and ZPR9 (see Ref. 31 and data not shown). In this context, it seems that the most likely mechanism by which MPK38 may inhibit the PDK1 activity would be through the change of PDK1 intrinsic activity, probably via direct phosphorylation of PDK1 at Thr354 by MPK38, rather than docking interaction-mediated regulation of PDK1 activity.
Modulating the association between ASK1 and its regulators, such as TRX and 14-3-3, has been proposed as a potential mechanism for the MPK38-mediated stimulation of ASK1 activity (24). Therefore, we speculated that MPK38 may inhibit PDK1 signaling, possibly by influencing the association between PDK1 and its regulators, STRAP (10) and 14-3-3 (8). To test this hypothesis, we examined the effect of MPK38 on STRAP and 14-3-3 binding to PDK1 using in vivo binding assays. Coexpression of MPK38 markedly decreased the association between PDK1 and its positive regulator STRAP and increased the association between PDK1 and its negative regulator 14-3-3 (Fig. 8). These results indicate that the MPK38-mediated inhibition of PDK1 signaling is accompanied by the modulation of PDK1 binding to its regulators, STRAP and 14-3-3, similar to the MPK38-mediated stimulation of ASK1 signaling reported previously (24). As PDK1 phosphorylation at Thr354 plays an important role in the negative regulation of PDK1 activity and function, it is likely that Thr354 phosphorylation of PDK1 may influence binding between PDK1 and its regulators (STRAP and 14-3-3). We found that complex formation between PDK1 and its positive regulator STRAP increased in the presence of the T354A mutant compared with the control expressing wild-type PDK1, whereas complex formation between PDK1 and its negative regulator 14-3-3 decreased (Fig. 8, A and B, right panels). These results suggest that MPK38-mediated phosphorylation of PDK1 at Thr354 modulates the interaction between PDK1 and its regulators (STRAP and 14-3-3), which is crucial for determining the manner of PDK1 signaling and eventually inhibits PDK1 activity and function.
The fact that PDK1 physically interacts with both ASK1 and MPK38 led us to hypothesize that a ternary complex consisting of PDK1, MPK38, and ASK1 occurs within cells (supplemental Fig. 3). In fact, the PDK1-MPK38-ASK1 complex was detected in unstressed cells; however, treatment with H2O2 disrupted this ternary complex and led to the formation of a binary complex between MPK38 and ASK1, which allowed the stimulation of ASK1 signaling, probably by stabilizing the MPK38-ASK1 complex in the presence of H2O2. In addition to the ternary complex, it is possible that the binary PDK1-ASK1 complex exists in unstressed cells because the interaction domains of ASK1 responsible for PDK1 and MPK38 binding, as well as the interaction domains of PDK1 responsible for ASK1 and MPK38 binding, are equivalent (20, 24). Treatment with H2O2 also disrupted the binary complex between PDK1 and ASK1 (20), promoting complex formation between MPK38 and ASK1 (24) and subsequently leading to ASK1 activation.
Collectively, the results of this study define a novel mechanism in which MPK38 directly interacts with and phosphorylates Thr354 of PDK1, thereby inhibiting PDK1 activity. The results also provide evidence that Thr354 of PDK1, like Ser394 and Ser398 of ASK1 (20) and Ser504 and Ser532 of PKCθ (18), represents a potential phosphorylation site for the negative regulation of PDK1 activity. Furthermore, the finding that MPK38 phosphorylates PDK1 on Thr354, thereby negatively regulating PDK1 activity, will contribute to a better understanding of the regulatory mechanism(s) involved in PDK1 activity and function.
This work was supported by National Research Foundation of Korea Grant R0A-2007-000-20006-0 funded by the Korean Government.

This article contains supplemental Figs. 1–4.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) NM010790.
- PH
- pleckstrin homology
- STRAP
- serine/threonine kinase receptor-associated protein
- SGK
- serum/glucocorticoid regulated kinase
- KD
- kinase-dead
- CA
- catalytic domain.
REFERENCES
- 1. Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 2. Downward J. (1998) Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10, 262–267 [DOI] [PubMed] [Google Scholar]
- 3. Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase that phosphorylates and activates protein kinase Bα. Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 4. Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G. F., Holmes A. B., Gaffney P. R., Reese C. B., McCormick F., Tempst P., Coadwell J., Hawkins P. T. (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279, 710–714 [DOI] [PubMed] [Google Scholar]
- 5. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 6. Bayascas J. R., Alessi D. R. (2005) Regulation of Akt/PKB Ser473 phosphorylation. Mol. Cell 18, 143–145 [DOI] [PubMed] [Google Scholar]
- 7. Fujita N., Sato S., Ishida A., Tsuruo T. (2002) Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 277, 10346–10353 [DOI] [PubMed] [Google Scholar]
- 8. Sato S., Fujita N., Tsuruo T. (2002) Regulation of kinase activity of 3-phosphoinositide-dependent protein kinase-1 by binding to 14-3-3. J. Biol. Chem. 277, 39360–39367 [DOI] [PubMed] [Google Scholar]
- 9. Balendran A., Casamayor A., Deak M., Paterson A., Gaffney P., Currie R., Downes C. P., Alessi D. R. (1999) PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol. 9, 393–404 [DOI] [PubMed] [Google Scholar]
- 10. Seong H. A., Jung H., Choi H. S., Kim K. T., Ha H. (2005) Regulation of transforming growth factor-β signaling and PDK1 kinase activity by physical interaction between PDK1 and serine-threonine kinase receptor-associated protein. J. Biol. Chem. 280, 42897–42908 [DOI] [PubMed] [Google Scholar]
- 11. Toker A., Newton A. C. (2000) Cellular signaling. Pivoting around PDK-1. Cell 103, 185–188 [DOI] [PubMed] [Google Scholar]
- 12. Casamayor A., Morrice N. A., Alessi D. R. (1999) Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1. Identification of five sites of phosphorylation in vivo. Biochem. J. 342, 287–292 [PMC free article] [PubMed] [Google Scholar]
- 13. Scheid M. P., Parsons M., Woodgett J. R. (2005) Phosphoinositide-dependent phosphorylation of PDK1 regulates nuclear translocation. Mol. Cell. Biol. 25, 2347–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riojas R. A., Kikani C. K., Wang C., Mao X., Zhou L., Langlais P. R., Hu D., Roberts J. L., Dong L. Q., Liu F. (2006) Fine-tuning PDK1 activity by phosphorylation at Ser163. J. Biol. Chem. 281, 21588–21593 [DOI] [PubMed] [Google Scholar]
- 15. Yang K. J., Shin S., Piao L., Shin E., Li Y., Park K. A., Byun H. S., Won M., Hong J., Kweon G. R., Hur G. M., Seok J. H., Chun T., Brazil D. P., Hemmings B. A., Park J. (2008) Regulation of 3-phosphoinositide-dependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding. J. Biol. Chem. 283, 1480–1491 [DOI] [PubMed] [Google Scholar]
- 16. Ito T., Seyama T., Iwamoto K. S., Hayashi T., Mizuno T., Tsuyama N., Dohi K., Nakamura N., Akiyama M. (1993) In vitro irradiation is able to cause RET oncogene rearrangement. Cancer Res. 53, 2940–2943 [PubMed] [Google Scholar]
- 17. Kim D. W., Hwang J. H., Suh J. M., Kim H., Song J. H., Hwang E. S., Hwang I. Y., Park K. C., Chung H. K., Kim J. M., Park J., Hemmings B. A., Shong M. (2003) RET/PTC (rearranged in transformation/papillary thyroid carcinomas) tyrosine kinase phosphorylates and activates phosphoinositide-dependent kinase 1 (PDK1). An alternative phosphatidylinositol 3-kinase-independent pathway to activate PDK1. Mol. Endocrinol. 17, 1382–1394 [DOI] [PubMed] [Google Scholar]
- 18. Wang C., Liu M., Riojas R. A., Xin X., Gao Z., Zeng R., Wu J., Dong L. Q., Liu F. (2009) Protein kinase Cθ (PKCθ)-dependent phosphorylation of PDK1 at Ser504 and Ser532 contributes to palmitate-induced insulin resistance. J. Biol. Chem. 284, 2038–2044 [DOI] [PubMed] [Google Scholar]
- 19. Kondo T., Kahn C. R. (2004) Altered insulin signaling in retinal tissue in diabetic states. J. Biol. Chem. 279, 37997–38006 [DOI] [PubMed] [Google Scholar]
- 20. Seong H. A., Jung H., Ichijo H., Ha H. (2010) Reciprocal negative regulation of PDK1 and ASK1 signaling by direct interaction and phosphorylation. J. Biol. Chem. 285, 2397–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gil M., Yang Y., Lee Y., Choi I., Ha H. (1997) Cloning and expression of a cDNA encoding a novel protein serine/threonine kinase predominantly expressed in hematopoietic cells. Gene 195, 295–301 [DOI] [PubMed] [Google Scholar]
- 22. Heyer B. S., Warsowe J., Solter D., Knowles B. B., Ackerman S. L. (1997) New member of the Snf1/AMPK kinase family, Melk, is expressed in the mouse egg and preimplantation embryo. Mol. Reprod. Dev. 47, 148–156 [DOI] [PubMed] [Google Scholar]
- 23. Seong H. A., Jung H., Ha H. (2010) Murine protein serine-threonine kinase 38 stimulates TGF-β signaling in a kinase-dependent manner via direct phosphorylation of Smad proteins. J. Biol. Chem. 285, 30959–30970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung H., Seong H. A., Ha H. (2008) Murine protein serine-threonine kinase 38 activates apoptosis signal-regulating kinase 1 via Thr838 phosphorylation. J. Biol. Chem. 283, 34541–34553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saito R., Tabata Y., Muto A., Arai K., Watanabe S. (2005) Melk-like kinase plays a role in hematopoiesis in the zebrafish. Mol. Cell. Biol. 25, 6682–6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beullens M., Vancauwenbergh S., Morrice N., Derua R., Ceulemans H., Waelkens E., Bollen M. (2005) Substrate specificity and activity regulation of protein kinase MELK. J. Biol. Chem. 280, 40003–40011 [DOI] [PubMed] [Google Scholar]
- 27. Gray D., Jubb A. M., Hogue D., Dowd P., Kljavin N., Yi S., Bai W., Frantz G., Zhang Z., Koeppen H., de Sauvage F. J., Davis D. P. (2005) Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer Res. 65, 9751–9761 [DOI] [PubMed] [Google Scholar]
- 28. Vulsteke V., Beullens M., Boudrez A., Keppens S., Van Eynde A., Rider M. H., Stalmans W., Bollen M. (2004) Inhibition of spliceosome assembly by the cell cycle-regulated protein kinase MELK and involvement of splicing factor NIPP1. J. Biol. Chem. 279, 8642–8647 [DOI] [PubMed] [Google Scholar]
- 29. Seong H. A., Jung H., Kim K. T., Ha H. (2007) 3-Phosphoinositide-dependent PDK1 negatively regulates transforming growth factor-β-induced signaling in a kinase-dependent manner through physical interaction with Smad proteins. J. Biol. Chem. 282, 12272–12289 [DOI] [PubMed] [Google Scholar]
- 30. Jakobsen S. N., Hardie D. G., Morrice N., Tornqvist H. E. (2001) 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J. Biol. Chem. 276, 46912–46916 [DOI] [PubMed] [Google Scholar]
- 31. Seong H. A., Jung H., Manoharan R., Ha H. (2011) Positive regulation of apoptosis signal-regulating kinase 1 signaling by ZPR9 protein, a zinc finger protein. J. Biol. Chem. 286, 31123–31135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Datta P. K., Chytil A., Gorska A. E., Moses H. L. (1998) Identification of STRAP, a novel WD domain protein in transforming growth factor-β signaling. J. Biol. Chem. 273, 34671–34674 [DOI] [PubMed] [Google Scholar]
- 33. Jung H., Seong H. A., Ha H. (2007) NM23-H1 tumor suppressor and its interacting partner STRAP activate p53 function. J. Biol. Chem. 282, 35293–35307 [DOI] [PubMed] [Google Scholar]
- 34. Kikani C. K., Dong L. Q., Liu F. (2005) “New”-clear functions of PDK1. Beyond a master kinase in the cytosol? J. Cell Biochem. 96, 1157–1162 [DOI] [PubMed] [Google Scholar]
- 35. Fayard E., Tintignac L. A., Baudry A., Hemmings B. A. (2005) Protein kinase B/Akt at a glance. J. Cell Sci. 118, 5675–5678 [DOI] [PubMed] [Google Scholar]
- 36. Biondi R. M., Nebreda A. R. (2003) Signaling specificity of Ser/Thr protein kinases through docking site-mediated interactions. Biochem. J. 372, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]