Background: The functions of taurine in brain development are largely unknown.
Results: Taurine inhibits K+-Cl− cotransporter 2 (KCC2) activity via the with-no-lysine (WNK) protein kinase signaling pathway.
Conclusion: Regulation of KCC2 by taurine may play an important role in developmental Cl− homeostasis and GABA action.
Significance: Our results shed new light on the involvement of taurine during brain developmental at the molecular level.
Keywords: Radial Migration, Neurobiology, Neurochemistry, Neurodevelopment, Transporters, GABA, KCC2, WNK1, Taurine
Abstract
GABA inhibits mature neurons and conversely excites immature neurons due to lower K+-Cl− cotransporter 2 (KCC2) expression. We observed that ectopically expressed KCC2 in embryonic cerebral cortices was not active; however, KCC2 functioned in newborns. In vitro studies revealed that taurine increased KCC2 inactivation in a phosphorylation-dependent manner. When Thr-906 and Thr-1007 residues in KCC2 were substituted with Ala (KCC2T906A/T1007A), KCC2 activity was facilitated, and the inhibitory effect of taurine was not observed. Exogenous taurine activated the with-no-lysine protein kinase 1 (WNK1) and downstream STE20/SPS1-related proline/alanine-rich kinase (SPAK)/oxidative stress response 1 (OSR1), and overexpression of active WNK1 resulted in KCC2 inhibition in the absence of taurine. Phosphorylation of SPAK was consistently higher in embryonic brains compared with that of neonatal brains and down-regulated by a taurine transporter inhibitor in vivo. Furthermore, cerebral radial migration was perturbed by a taurine-insensitive form of KCC2, KCC2T906A/T1007A, which may be regulated by WNK-SPAK/OSR1 signaling. Thus, taurine and WNK-SPAK/OSR1 signaling may contribute to embryonic neuronal Cl− homeostasis, which is required for normal brain development.
Introduction
GABA is an inhibitory neurotransmitter in the central nervous system; however, in immature neurons, GABA is excitatory. This functional switch in action of GABA is due to a decrease in intracellular Cl− concentration ([Cl−]i). GABA-induced depolarization is induced by Cl− effluxes due to higher [Cl−]i in immature neurons, whereas hyperpolarization is induced by Cl− influxes due to lower [Cl−]i in mature neurons (1–3).
The developmental [Cl−]i decrease in neurons is predominantly caused by KCC2 up-regulation and down-regulation of the Na+-K+-2Cl− cotransporter, NKCC1 (2–7). KCC2 transports K+ and Cl− across the plasma membrane in accordance with the electrochemical driving force, whereas NKCC1 takes up Na+, K+, and Cl− (8–10). KCC2 is modulated at the transcriptional level by the downstream effects of brain-derived neurotrophic factor (11–13) and GABA (14). Furthermore, KCC2 activity is regulated at the post-translational level by phosphorylation (15–18) and other mechanisms (19–22).
Because GABAA receptor Cl− channels contribute to radial migration of cortical neurons (23–25) and intrinsic KCC2 expression is comparatively low in immature cortical neurons (26–28), we hypothesize that high [Cl−]i and subsequent GABAA receptor-mediated depolarization may be critical for GABA actions during early development. Although an excitatory GABA action is essential for postnatal maturation of cortical neurons, radial migration is not affected by ectopically overexpressed KCC2 that was functionally active at postnatal day 0–1 (P0-P1)3 (29). Therefore, we investigated whether KCC2 may be inhibited by undefined embryonic factors. Taurine is a candidate due to enrichment in immature neurons during cerebral cortex development (30, 31). In addition, taurine-deficient kittens show abnormal neuronal migration in the cerebral cortex and cerebellum (32, 33). We observed that ectopically expressed KCC2 is suppressed by taurine in the embryonic brain and becomes functional in postnatal brains. This inhibitory effect of taurine on KCC2 activity may be mediated by the with-no-lysine (WNK) protein kinase signaling pathway.
EXPERIMENTAL PROCEDURES
Animals
Wistar rats were purchased from SLC (Hamamatsu, Japan). Experiments were conducted in accordance with the Guidelines for Use of Laboratory Animals of the Hamamatsu University School of Medicine.
Plasmid Construction
pCAGGS-eGFP that carries enhanced GFP downstream of a CAG promoter (34, 35) was kindly provided by Dr. J. Miyazaki (Okasa University, Osaka, Japan). For KCC2 expression, cDNA encoding FLAG-tagged KCC2 on pCMV-KCC2 (21, 36) was inserted into pCAGGS-eGFP. Each KCC2 mutant was generated by a standard two-step megaprimer PCR with mutated oligonucleotides (37). The first step included two PCRs that span the mutation site. Final PCR products were cloned into the above vectors, and mutations were verified by DNA sequencing. pGBE-WNK1S382E and pGBE-WNK1S382A were kindly provided by Dr. D. R. Alessi (University of Dundee, Dundee, Scotland, UK) and described elsewhere (38).
In Utero Electroporation (IUE)
Pregnant rats were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally) at embryonic day 14.5 (E14.5) or E15.5, and the uterine horns were exposed. Plasmid DNA was dissolved in PBS at a final concentration of 0.5–1.5 μg/μl with Fast Green (final concentration 0.05% (v/v)). For cotransfection, a molar ratio of 1 (GFP expression plasmid):5 (KCC2- and WNK1-derivative expression plasmids) was used. Plasmids (∼1 μl) were injected into the lateral ventricle using a glass micropipette (outer diameter 1.0; World Precision Instruments Inc., Sarasota, FL). Electric pulses were produced by an electroporator, CUY21EDIT (Nepa Gene, Ichikawa, Japan), and delivered by an electrode with a round plate forceps type with a 5-mm diameter. Electric pulses (43–46 V, 50 ms) were applied five times at intervals of 950 ms. Uterine horns were then returned to the abdomen.
Guanidinoethanesulfonic Acid (GES) Treatment and in Vivo Implantation of Poly(dl-lactide-co-glycolide) (PLGA)-absorbed GES
PLGA has high bioaffinity and biodegradability and is therefore a useful material for sustained drug release (39). GES is an analog of taurine that competes with taurine for the taurine transporter (TauT) (40–42). GES treatment and in vivo implantation of PLGA-absorbed GES was performed by dissolving 20 mg of PLGA (Wako, Osaka, Japan) into 100 μl of N-methyl-2-pyrrolidone (Wako) (43) with 10 μl of 10 mm GES or water (control). Pregnant rats were maintained from gestational day 11.5 on tap water in the absence (control) or presence of 1% (w/v) GES. At gestational day 15.5, uterine horns were exposed under anesthesia, and the PLGA mixture (∼0.5 μl) was injected with or without GES into the embryonic lateral ventricles. Embryonic cerebral cortices were obtained at E18.5, followed by sample preparation for immunoblotting (see below).
Brain Slice Preparation
After animals (pregnant, P1, or P7 rats) were deeply anesthetized, blocks of brain were quickly removed. Neocortical coronal slices (350 μm) were cut using a vibratome (VT-100, Leica (Wetzlar, Germany)) and placed in oxygenated modified artificial cerebrospinal fluid (ACSF) at 4 °C. Slices were allowed to recover for 60 min on nylon meshes (1-mm pores) in standard ACSF. Modified ACSF contained 220 mm sucrose, 2.5 mm KCl, 1.25 mm NaH2PO4, 12 mm MgSO4, 0.5 mm CaCl2, 26 mm NaHCO3, and 30 mm glucose. Standard ACSF contained 126 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 2 mm MgSO4, 2 mm CaCl2, 26 mm NaHCO3, and 20 mm glucose.
Primary Neocortical Neuron Culture
For primary cortical neuron culture, cerebral cortices were dissected out of E15.5 brains that underwent IUE with the indicated plasmids at E14.5. After removal of the meninges, cortices were cut into small pieces (<1 mm3) in PBS. Tissue was sedimented and washed with Dulbecco's minimum essential medium and then incubated at 37 °C for 10 min in an enzyme solution containing 2.5 mg/ml trypsin and 0.01% (v/v) DNase I (Sigma). After enzymatic digestion and mechanical trituration in serum-free medium, cells were suspended in medium and counted. Isolated cells were then plated in poly-l-lysine-coated culture dishes (35-mm diameter) or 24-well plates at 1.5 × 106 cells/dish or 5 × 104 cells/well, respectively, and maintained in serum-free medium. Serum-free culture medium consisted of Neurobasal medium (Invitrogen) with 2% (v/v) B27 supplement (Invitrogen), 0.5 mm l-glutamine, and 100 units/ml penicillin/streptomycin (Invitrogen). Half medium volumes were exchanged every other day. As for reagent application, blockers of GABAA and glycine receptors (bicuculline methiodide (BMI) and strychnine (Str), respectively) and taurine were added 5–6 h after plating cells for experimentation. Kinase and phosphatase inhibitors were applied after starting electrophysiological recordings. Neurons were used for experimentation after culture for 3–4 days.
Electrophysiology
Brain slices or primary cortical neurons on coverslips were placed in a recording chamber for observation under a microscope (BX51WI, Olympus (Tokyo, Japan)). Specimens were continuously perfused with oxygenated standard ACSF containing 0.3 μm tetrodotoxin (Wako), 50 μm d-(−)-2-amino-5-phosphonovaleric acid (Tocris Cookson, Bristol, UK), 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (Tocris Cookson), and 3 μm CGP55384 (Tocris Cookson) at a flow rate of 2–3 ml/min at room temperature. Cells were imaged using a ×40 water immersion objective lens (numerical aperture = 0.80; Olympus). To visualize cells expressing GFP and monomeric red fluorescent protein (mRFP) as positive transfectant markers, fluorescence was monitored using U-MWIB2 (BP460–490, BA510IF) and U-MWIG2 (BP520–550, BA580IF) filter sets, respectively. Fluorescence was captured by a cooled charge-coupled device camera (ImagEM C9100-13, Hamamatsu Photonics (Hamamatsu, Japan)). The system was controlled by AQUACOSMOS 2.5 software (Hamamatsu Photonics).
Cells were viewed under transillumination on a monitor via an infrared differential interference contrast filter to observe GFP or mRFP fluorescence. Patch electrodes were fabricated from 1.5-mm diameter borosilicate capillary tubing (GD-1.5, Narishige (Tokyo, Japan)) using a P97 horizontal puller (Sutter Instruments, Novato, CA). The electrode resistance ranged from 6 to 10 megaohms. For whole-cell patch clamp recordings (44), the pipette solution contained 130 mm potassium methanesulfonic acid, 10 mm KCl, 2 mm MgCl2, 0.1 mm EGTA, and 10 mm HEPES (pH 7.3 with KOH). For the gramicidin-perforated patch clamp recordings (21, 45) in Fig. 2, B and D, the pipette solution contained 140 mm KCl, 0.1 mm CaCl2, 0.05 mm EGTA, and 10 mm HEPES (pH 7.3 with KOH). Gramicidin (Sigma) was dissolved in dimethyl sulfoxide (50 mg/ml) and then diluted in the pipette-filling solution to a final concentration of 5–10 μg/ml just before the experiment. Although gramicidin-perforated patch clamp recordings more accurately measure [Cl−]i (6, 45), whole-cell patch clamp recordings were used to evaluate the reversal potential of GABA (EGABA) due to sparse and fragile GFP/mRFP-labeled neurons in brain slices. Although whole-cell patch clamp recordings may underestimate [Cl−]i differences, the method has been widely used (20, 46, 47) and demonstrated obvious postnatal differences, as shown in Fig. 1.
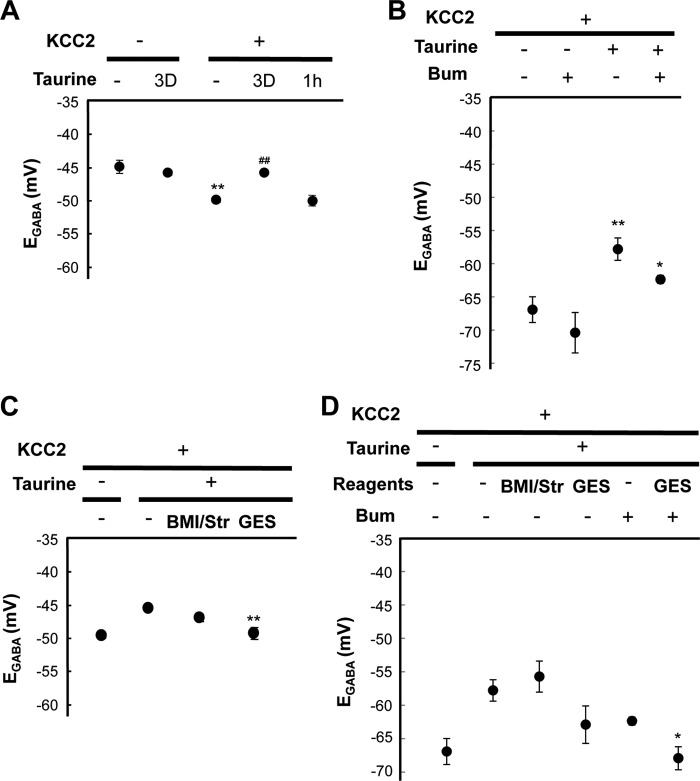
FIGURE 2.
Intracellular taurine inhibits KCC2 activity. KCC2 was transfected with mRFP by means of IUE at E14.5, and embryonic brains were dissected at E15.5. Neurons were then cultured with the indicated reagents (taurine, BMI/Str, and GES) for 3–4 days, followed by patch clamp recordings. When cells were treated with an NKCC inhibitor (100 μm bumetanide; Bum), EGABA values of cells were evaluated after more than 10 min of treatment. A, whole-cell patch clamp recordings were carried out, and EGABA values of KCC2-transfected and taurine-untreated cells were significantly more negative compared with that of cells treated with taurine for 3 days (3D) but not 1 h (n = 6–36; **, p < 0.01 versus KCC2-negative; ##, p < 0.01 versus taurine-untreated, unpaired t test). B, gramicidin-perforated patch clamp recordings were performed at DIV 3–4. Taurine-treated cell EGABA was significantly more positive compared with that of untreated cells (n = 4–11; *, p < 0.05; **, p < 0.01 versus taurine-untreated, unpaired t test). C, KCC2 activity was significantly disinhibited by a taurine transporter inhibitor (500 μm GES) but not blockers of GABAA and glycine receptors (10 μm BMI, 1 μm Str; BMI/Str) (n = 5–6, left two lanes identical to Fig. 2A are shown as references; **, p < 0.01 versus without channel/transporter blockers, unpaired t test). D, gramicidin-perforated patch clamp recordings were performed at DIV 3–4. GES-treated cell EGABA values were more negative compared with that of untreated cells (n = 3–11, lanes 1, 2, and 5 from the left identical to Fig. 2B are shown as references; *, p < 0.05 versus GES-untreated, unpaired t test). Error bars, S.E.
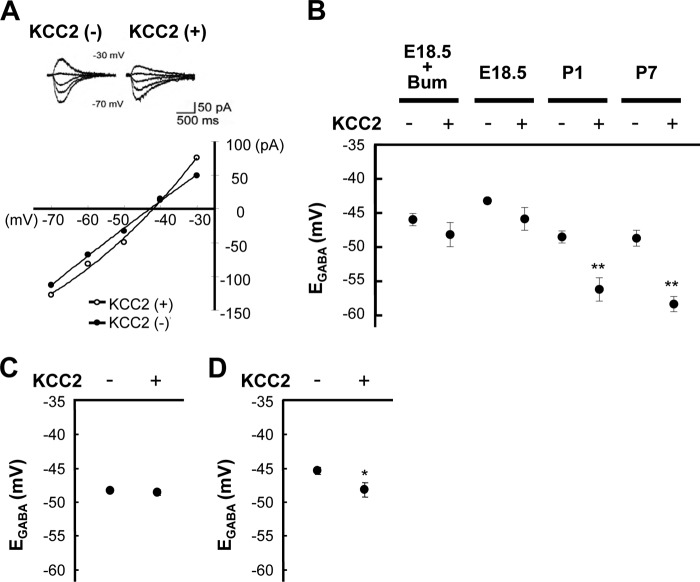
FIGURE 1.
KCC2 activity is inhibited in embryonic brains but not in postnatal brains and cultured neurons. A, GFP was transfected with or without KCC2 by IUE in rat embryos at E15.5, and brain slices were prepared for electrophysiological experiments at E18.5. Typical currents activated by GABA in transfected cells were recorded by a whole-cell patch clamp technique. The graph represents the current-voltage relationship for GABA-activated responses shown in the traces. B, GFP was transfected as described in A, and the samples were prepared at E18.5, P1, and P7. Control cell EGABA is significantly different with or without ectopic KCC2 at P1 and P7 but not at E18.5. (n = 5–22; **, p < 0.01 versus KCC2-negative, unpaired t test). When cells were treated with an NKCC inhibitor (100 μm bumetanide; Bum), EGABA of cells were evaluated after more than 10 min of treatment. C, mRFP was transfected with or without KCC2 by IUE in rat embryos at E14.5, and brain slices were used for electrophysiological experiments at E19.5. Control cell EGABA is not significantly different with or without ectopic KCC2 at E19.5. (n = 7–9; p > 0.05 versus KCC2-negative, unpaired t test). D, to obtain in vitro samples, IUE was performed at E14.5, embryonic brains were dissected at E15.5, and neurons were then cultured for an additional 4 days. KCC2-expressing cell EGABA at DIV 4 is significantly more negative compared with that of KCC2-negative cells (n = 8–10; *, p < 0.05 versus KCC2-negative, unpaired t test). Error bars, S.E.
Recordings were performed after confirming that GABA-induced current had been stable for at least 10 min. Membrane currents were recorded using an Axopatch 200B amplifier and digitized at 2 kHz by a Digidata 1332A data acquisition system and pClamp 9 software (Axon Instruments, Union City, CA). To measure EGABA, voltage steps were used, and at each membrane potential, GABA (50 or 100 μm) was added for 50–300 ms through another pipette to the recorded cell soma. Peak current responses for each voltage were plotted, and the data were fitted using Clampfit 9 software (Axon Instruments).
Immunostaining
Under deep anesthesia, postnatal pups were perfused with 4% (w/v) paraformaldehyde in 0.1 m phosphate buffer (pH 7.4), and brains were removed by decapitation and postfixed with paraformaldehyde for 3–4 h. For embryos, brains were removed by decapitation without perfusion and fixed with paraformaldehyde for 3–4 h. Brains were then placed in 30% (w/v) sucrose in 0.1 m phosphate buffer for 3 days at 4 °C, followed by freezing in powdered dry ice. Frontal sections (30 μm) were cut using a microtome (HM400R, Microm (Walldorf, Germany)). Sections were immersed in a blocking buffer (10% (v/v) horse serum, 0.05% (w/v) NaN3, and 0.2% (v/v) Triton X-100 in phosphate buffer) for 1 h at room temperature and then overnight at 4 °C with anti-KCC2 (Millipore, Billerica, MA), anti-NKCC1 (T4, Developmental Studies Hybridoma Bank (Iowa City, IA)), anti-GFP (Aves Labs, Tigard, OR), anti-Tbr1 (Abcam, Tokyo, Japan), and anti-Cux1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) antibodies. They were then incubated with secondary antibodies (Invitrogen or Jackson Laboratory (Bar Harbor, ME)) and mounted. Cells were visualized by fluorescence microscopy (BZ-9000, Kyence (Osaka, Japan)) or confocal microscopy (FV1000, Olympus), and GFP-positive cell percentages were calculated in each region. Based on cytoarchitecture with DAPI counterstaining, regions with abundant cells were considered the subventricular zone/ventricular zone (VZ/SVZ) and cortical plate. The intermediate zone was defined as the region between these areas (48).
Immunoblotting
Under deep anesthesia, neocortices were removed, homogenized, and lysed in lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% (v/v) Triton X-100, 12 mm β-glycerophosphate, 1 mm sodium orthovanadate, and protease inhibitor mixture (Sigma)). After centrifugation at 12,000 × g for 30 min at 4 °C, supernatants were collected. Protein concentration was estimated using the Bradford reagent (Sigma). Aliquots of 30 μg of protein were mixed with Laemmli sample buffer and boiled at 95 °C for 10 min or 37 °C for 1 h. For cultured cell immunoblotting, cells were washed with cold PBS and then treated with lysis buffer followed by the procedure described above. Samples were resolved by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Blots were probed with antibodies to WNK1 phospho-Ser-382, total WNK1, SPS1-related proline/alanine-rich kinase (SPAK) phospho-Ser-373, total SPAK, total oxidative stress response 1 (OSR1) (kindly provided by D. R Alessi (38)), and actin (Sigma) and then detected using HRP-conjugated secondary antibodies (Millipore or Cell Signaling (Danvers, MA)) and an ECL kit (Amersham Biosciences).
Statistical Analysis
Data are the means ± S.E. Data groups were compared using one-way analysis of variance, paired Student's t test, unpaired Student's t test, or Mann-Whitney U test as appropriate. Dunnett's test was used to compensate for multiple experimental procedures. p < 0.05 was regarded as statistically significant.
RESULTS
KCC2 Activity Is Inhibited in Embryonic Brain but Not in Neurons in Vitro
Because radial migration of cortical neurons is attributable to channel-type GABA receptors (23–25), we had questioned whether high [Cl−]i and resultant GABA receptor-mediated depolarization are critical to make GABA work properly at early developmental stages. Interestingly, Cancedda et al. (29) showed that ectopic KCC2 is functional without affecting developmental radial migration. Similar to their results, KCC2-expressing cells showed significantly greater negative EGABA compared with that of KCC2-negative cells in postnatal (P1 and P7) brain slices (KCC2(−), −48.5 ± 0.9 mV; KCC2(+), −56.2 ± 1.7 mV, n = 6–8, p = 0.004 at P1; KCC2(−), −48.7 ± 1.2 mV; KCC2(+), −58.3 ± 1.1 mV (n = 5–22, p < 0.001 at P7)) (Fig. 1B) when KCC2 was introduced by IUE at E15.5, and then whole-cell patch clamp recordings with a low Cl− pipette solution at various developmental days were carried out. Unexpectedly, however, there was no significant difference in EGABA between KCC2-expressing and KCC2-negative cells in E18.5 brain slices (KCC2(−), −43.0 ± 0.5 mV; KCC2(+), −45.8 ± 1.7 mV (n = 6–13, p = 0.13)) (Fig. 1, A and B). Moreover, the NKCC1 inhibitor bumetanide did not elicit a significant difference between either cell preparation (KCC2(−), −46.0 ± 0.9 mV; KCC2(+), −48.2 ± 1.8 mV (p = 0.27, n = 5–6)) (Fig. 1B), whereas it tended to shift EGABA to the negative direction. This result rules out the possibility of compensation by NKCC1. Consistently, relatively higher levels of ectopic KCC2 protein were detectable at all ages examined, whereas compensatory NKCC1 expression was not observed (supplemental Fig. 1, A and B). Thus, ectopic KCC2 protein was functional in postnatal brains, which is consistent with a previous study (29). However, KCC2 was not observed to be functional in embryonic brains.
These data suggest that circumferential differences before and after parturition may be important for KCC2 activity. To examine this possibility, we compared KCC2 activity between in vitro cultured neurons and in vivo neurons. Cells were transfected with mRFP with or without KCC2 by IUE at E14.5, and subsequent analysis of brain slices at E19.5 revealed no differences in EGABA (KCC2(−), −48.3 ± 0.2 mV; KCC2(+), −48.7 ± 0.4 mV (n = 7–9, p = 0.24)) (Fig. 1C). Next, cells were transfected as described, and brains were dissected at E15.5 for an additional 4 days of primary culture to obtain neurons in vitro. Interestingly, EGABA was significantly more negative in KCC2-positive cells compared with that of KCC2-negative cells (KCC2(−), −45.5 ± 0.5 mV; KCC2(+), −48.5 ± 1.0 mV (n = 8–10, p = 0.02)) (Fig. 1D). Thus, the dysfunction of KCC2 shown in vivo was not observed in vitro.
Intracellular Taurine Inhibits KCC2 Activity
We considered the possibility that an endogenous factor not present in culture medium may inhibit KCC2 activity in vivo. Taurine was a candidate due to enrichment in the fetal brain, which decreases following birth (49). Neuron culture in medium supplemented with taurine for 3 days resulted in significantly greater positive EGABA when KCC2 was transfected (taurine(−), −49.5 ± 0.4 mV; taurine(+), −45.3 ± 0.3 mV (n = 34–36, p < 0.001)) (Fig. 2A). To represent more reliable [Cl−]i values, gramicidin-perforated patch clamp recordings were also carried out. Gramicidin forms pores that are permeable to monovalent cations and to small uncharged molecules but not to Cl−, permitting reliable recordings of the GABA-activated current (21, 45). Consistently, taurine treatment resulted in more depolarized EGABA (taurine(−), −66.9 ± 1.9 mV; taurine(+), −57.8 ± 1.7 mV (n = 6–11, p = 0.004)) (Fig. 2B), and similar results were still obtained when bumetanide was applied (taurine(−), −70.4 ± 3.0 mV; taurine(+), −62.4 ± 0.6 mV (n = 4, p = 0.04)) (Fig. 2B).
Taurine is considered to play versatile intracellular and extracellular roles in organisms. For example, taurine is an agonist of GABAA and glycine receptors (50, 51) and acts as an osmolyte and a membrane-stabilizing factor (49, 52). To elucidate the mechanism of the taurine effect on KCC2 modification, pharmacological experiments were performed. BMI and Str block GABAA and glycine receptors, respectively, and were applied simultaneously. A change was not observed in EGABA (Fig. 2, C and D), suggesting that the effect of taurine is not mediated by GABAA or glycine receptors. To block taurine uptake into cells, TauT was inhibited by GES (53). As shown in Fig. 2C, TauT inhibition restored KCC2 activity affected by taurine (−49.1 ± 0.9 mV, n = 5, p < 0.001 versus control). Gramicidin-perforated patch clamp recordings also provided a comparable difference in the presence of bumetanide (GES(−), −62.4 ± 0.6 mV; GES(+), −67.9 ± 1.7 mV (n = 3–4, p = 0.02)) (Fig. 2D). These reagents were not cytotoxic and did not affect cell proliferation (supplemental Fig. 2). Thus, this result indicates that KCC2 activity is affected by intracellular but not extracellular taurine.
Taurine Inhibits KCC2 Activity via Serine/Threonine Phosphorylation
Because KCC2 is known to be regulated by kinases (15, 17, 54–56), phosphorylation-related reagents were used to evaluate the effect on KCC2 activity. The tyrosine kinase inhibitor AG18 and tyrosine phosphatase inhibitor vanadate did not affect EGABA (supplemental Table 1A). In contrast, the broad spectrum kinase inhibitor staurosporine (Staur) shifted EGABA toward the negative in 15–20 min in the presence of taurine (control, −45.2 ± 0.3 mV; Staur, −47.6 ± 0.5 mV, n = 5, p = 0.002 (supplemental Fig. 3A and Table 1A). Considering that 1 h of taurine treatment did not have an effect on EGABA (Fig. 2A), these results suggest that chronic but not acute taurine treatment inhibited KCC2 activity in a serine/threonine phosphorylation-dependent manner. Moreover, staurosporine also shifted KCC2-positive cell EGABA significantly toward the negative in embryonic brain slices at E18.5 but was less effective in postnatal brain slices at P7 (control, −46.5 ± 0.8 mV; Staur, −51.0 ± 1.1 mV, n = 6, p = 0.007 at E18.5; control, −57.6 ± 1.7 mV; Staur, −59.1 ± 1.6 mV (n = 6, p = 0.06 at P7)) (supplemental Fig. 3B). In contrast, vanadate did not affect EGABA at either age (supplemental Table 1B).
Thr-906 and Thr-1007 Mutation in KCC2 Affects Activity and Sensitivity to Taurine
A recent report by Rinehart et al. (54) revealed developmental changes in phosphorylation of KCC2. The study demonstrated that Thr-906 and Thr-1007 in KCC2 are highly phosphorylated perinatally and decrease during postnatal development. In addition, phosphorylation may inhibit KCC2 activity because substitution of Thr with Ala stimulates KCC2 function in vitro (54). Therefore, mutant KCC2 derivatives (T906A, T1007A, and T906A/T1007A) were constructed and used for experimentation. Cells were transfected with either wild-type KCC2 (KCC2wt) or KCC2 derivatives by IUE at E14.5 and dissected at E15.5 for a further 3 days of primary culture. As shown in Fig. 3A, KCC2T906A/T1007A-transfected cell EGABA was more negative compared with that of KCC2wt-transfected cells (KCC2wt, −49.5 ± 0.4 mV; KCC2T906A/T1007A, −56.3 ± 3.0 mV, n = 8–34, p < 0.001). Moreover, the EGABA of KCC2T906A/T1007A-transfected cells that were incubated with taurine for 3 days was significantly more negative compared with that of untreated cells (−65.8 ± 3.0 mV, n = 8, p = 0.02 versus untreated). Both single mutants facilitated KCC2 activity but did not alter activity as drastically as the double mutant (KCC2T906A, −52.7 ± 2.2 mV (n = 11, p = 0.22); T1007A, −54.0 ± 1.8 mV (n = 13, p = 0.03)) (Fig. 3A). The single mutants were less susceptible to taurine (Fig. 3A). In addition, replacement of Thr with Glu (T906E/T1007E), which mimics phosphothreonine, eliminated sensitivity to taurine (taurine(−), −48.1 ± 1.4 mV; taurine(+), −46.1 ± 0.6 mV, n = 6–11, p = 0.32) (Fig. 3A). These results suggest that taurine induces Thr-906 and/or Thr-1007 phosphorylation, resulting in inhibition of KCC2 activity.
FIGURE 3.
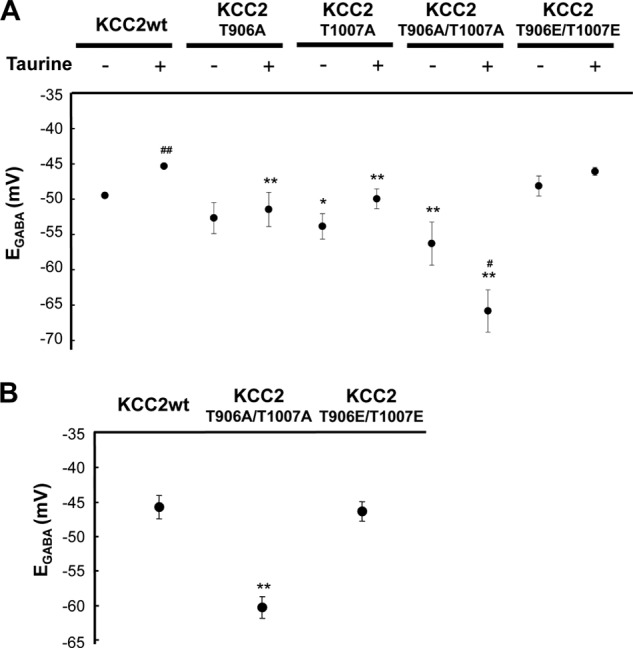
KCC2T906A/T1007A is not inhibited by taurine. A, KCC2wt or KCC2 derivatives were cotransfected with mRFP at E14.5, embryonic brains were dissected at E15.5, and neurons were cultured with or without 3 mm taurine for 3 days. KCC2T906A and KCC2T1007A cell EGABA values were more negative compared with that of KCC2wt-transfected cells but were not as intensively negative when compared with KCC2T906A/T1007A. Furthermore, taurine shifted the KCC2T906A/T1007A-transfected cell EGABA to the negative. KCC2T1007E/T1007E cell EGABA was similar to that of KCC2wt but was less sensitive to taurine (n = 6–36, two left lanes are identical to Fig. 2A; *, p < 0.05; **, p < 0.01 versus KCC2wt, analysis of variance followed by Dunnett's test; #, p < 0.05; ##, p < 0.01 versus taurine-untreated, unpaired t test). B, IUE with KCC2wt or KCC2 derivatives was performed at E15.5, and brain slices were used for electrophysiological experiments at E18.5. KCC2T906A/T1007A-transfected cell EGABA was significantly more negative compared with that of KCC2wt-transfected cells, whereas that of KCC2T906E/T1007E was not (n = 4–13, left lane is identical to Fig. 1B; **, p < 0.01 versus KCC2wt, analysis of variance followed by Dunnett's test). Error bars, S.E.
To examine the effect of mutant KCC2 in vivo, cells were transfected with either KCC2wt or KCC2 derivatives by IUE at E15.5, and EGABA of brain slices at E18.5 was analyzed. In parallel with in vitro results, KCC2T906A/T1007A-transfected cell EGABA was significantly more negative compared with that of KCC2wt- and KCC2T906E/T1007E-transfected cells (KCC2wt, −45.9 ± 1.7 mV; KCC2T906A/T1007A, −60.3 ± 1.6 mV (p < 0.001); KCC2T906E/T1007E, −44.3 ± 1.6 mV (p = 0.82, n = 4–13)) (Fig. 3B).
Taurine Activates WNK- STE20/SPAK/OSR1 Pathway
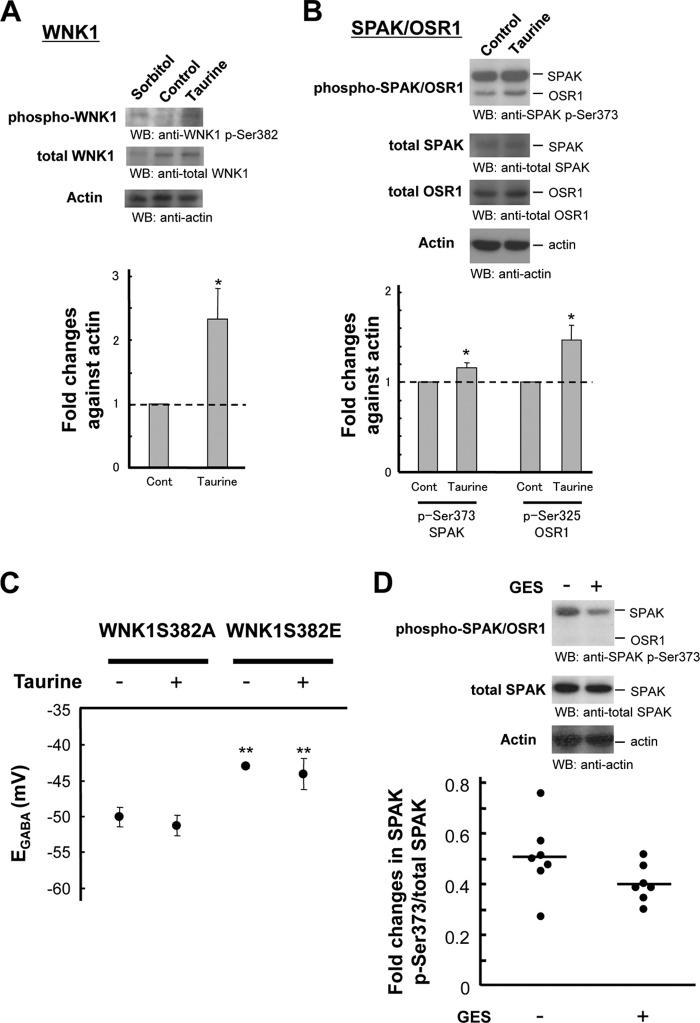
Previous reports suggest that the WNK-SPAK/OSR1 pathway is involved in regulation and phosphorylative activation of NKCC1 (57–60). Lifton and colleagues (54, 61, 62) propose that the WNK signaling pathway also activates KCCs, including KCC2. WNKs are activated by osmotic stress; however, the upstream signaling remains unknown (38, 59, 63). We hypothesized that taurine may stimulate the WNK pathway. To explore this possibility, cultured neurons were treated with or without taurine for 3 days, followed by evaluation of WNK1 activation. Ser-382 phosphorylation in WNK1 is known to enhance activity (38) and was phosphorylated to a greater extent in taurine-treated cells compared with that in untreated cells (Fig. 4A). Moreover, phosphorylation of Ser-373 in SPAK and Ser-325 in OSR1, both downstream of the WNK pathway and potential kinases of KCCs (57, 64–67), were increased in parallel (Fig. 4B).
FIGURE 4.
WNK-SPAK/OSR1 pathway activated by taurine facilitates KCC2 inhibition. A and B, embryonic brains were dissected at E15.5, and neurons were cultured with or without 3 mm taurine for 3 days. Cell lysates were analyzed by immunoblotting (WB) with the indicated antibodies. The first lanes on the left in A show cells incubated with 0.5 m sorbitol for 30 min at DIV 3 as a positive control. Taurine increased phosphorylation of WNK1, SPAK, and OSR1. Protein loading was monitored by immunoblotting for actin. Bar graphs show that phosphorylated levels of Ser-382 of WNK1, Ser-373 of SPAK, and Ser-325 of OSR1 were significantly greater in taurine-treated cells (n = 3; *, p < 0.05 versus control, unpaired t test). C, KCC2 was cotransfected by IUE with the indicated WNK1 derivatives at E14.5, embryonic brains were dissected at E15.5 and neurons were cultured with or without taurine for 3 days. EGABA was more positive in WNK1S382E-transfected cells, and the effects of WNK1 derivatives were not affected by taurine. (n = 4–6; **, p < 0.01 versus WNK1S382A, unpaired t test). D, GES (a taurine transporter inhibitor)-treated and -untreated cerebral cortices were homogenized and lysed as described under “Experimental Procedures.” Aliquots of 30 μg of protein were loaded, and the phosphorylation status of SPAK was determined by immunoblotting using an antibody for phospho-Ser-373 in SPAK. The graph shows that the phosphorylated level of Ser-373 in SPAK decreased in GES-treated brains (n = 7; p = 0.099 versus control, Mann-Whitney U test). Error bars, S.E.
To assess the fundamental correlation between the WNK pathway and KCC2 activity, inactivated (Ser-382 to Ala; S382A) or activated WNK1 (Ser-382 to Glu; S382E) (38) was cotransfected with KCC2 into cells that were cultured with or without taurine. EGABA was then evaluated after 3 days in culture. Cells with WNK1S382E showed significantly greater positive EGABA compared with that of WNK1S382A (S382A, −50.0 ± 1.4 mV; S382E, −43.1 ± 0.2 mV (n = 5–6, p < 0.001)) (Fig. 4C). Moreover, the effects of WNK1 derivatives were not affected by taurine (Fig. 4C). Thus, WNK1 activation sufficiently inhibits KCC2 activity, suggesting that KCC2 inhibition by taurine is mediated by the WNK pathway. As WNK-SPAK/OSR1 activated NKCC1, taurine indeed tended to shift EGABA to the positive direction in a bumetanide-sensitive manner in the absence of ectopic KCC2 (supplemental Fig. 4).
WNK-SPAK/OSR1 Signaling Is Activated by Taurine in Vivo
To examine whether taurine affects WNK-SPAK/OSR1 signaling in vivo, the biocompatible polymer PLGA was used for sustained reagent release in the brain (43, 68). GES-containing water was provided to pregnant rats from gestational day 11.5, and embryonic brains received GES-containing PLGA at E15.5 to reduce the cellular taurine concentration. SPAK Ser-373 phosphorylation was decreased in GES-treated cerebral cortices compared with that of the control at E18.5 (n = 7, p = 0.099 versus control) (Fig. 4D). This suggests that WNK-SPAK/OSR1 signaling is activated by taurine in vivo.
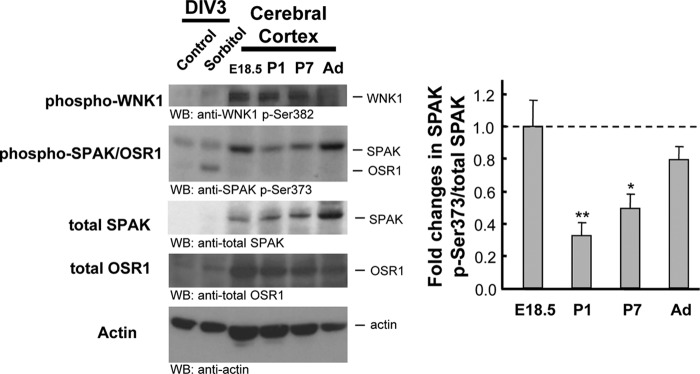
Phosphorylation of SPAK Significantly Decreases Postnatally
Although taurine concentrations in the brain increases toward parturition and decreases postnatally, taurine remains relatively high for more than a week following birth compared with that of adult rodents (69, 70). The small postnatal reduction in taurine concentration cannot explain the appearance of KCC2 activity soon after birth at P1. Therefore, we observed developmental changes in WNK-SPAK/OSR1 signaling. Phosphorylation of WNK1 was not changeable; however, SPAK Ser-373 was phosphorylated at E18.5, which significantly decreased at P1 (p = 0.006 at P1, p = 0.03 at P7, p = 0.45 at adult versus E18.5, n = 3) (Fig. 5). Interestingly, phosphorylated and non-phosphorylated (total) SPAK increased during postnatal development. These results suggest that KCC2 activity, regulated by WNK-SPAK/OSR1 signaling, is not only affected by intracellular taurine but also developmentally modulated.
FIGURE 5.
WNK-SPAK/OSR1 signaling activity is developmentally regulated. Cerebral cortices were homogenized and lysed as described. Aliquots of 30 μg of protein were loaded, and the phosphorylation status of SPAK was determined by immunoblotting (WB) using an antibody for phospho-Ser-373 in SPAK. The bar graph shows that the phosphorylation level of Ser-373 in SPAK was significantly decreased at P1 and P7 compared with that of E18.5 and increased upon adulthood. Non-phosphorylated SPAK also increased, whereas the proportion of phosphorylated SPAK was relatively unchanged (n = 3; **, p < 0.01; *, p < 0.05 versus E18.5, analysis of variance followed by Dunnett's test). Error bars, S.E.
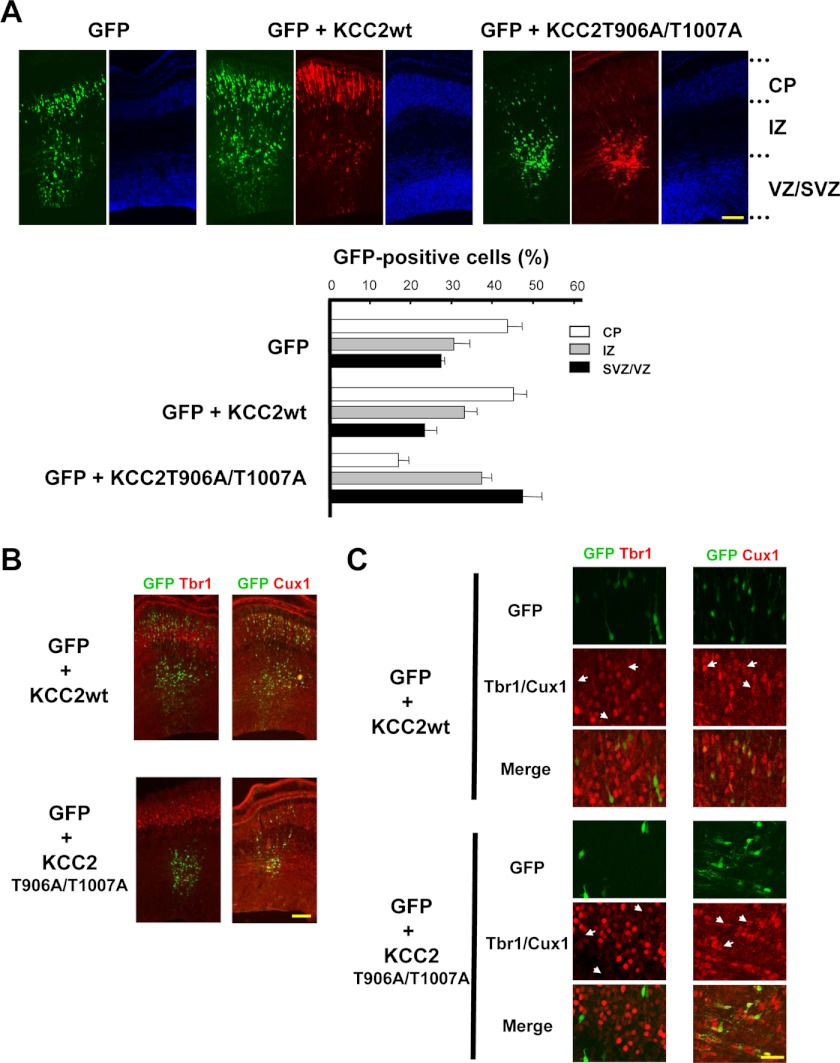
KCC2T906A/T1007A Inhibits Radial Migration in Developing Brain
The WNK-SPAK/OSR1 pathway regulates NKCC1, resulting in enhanced cellular Cl− influx. This suggests that a physiological role of taurine-WNK-SPAK/OSR1 signaling may be contributing to embryonic Cl− homeostasis. Cancedda et al. (29) reported that KCC2 overexpression does not affect radial migration despite a presumed decrease in [Cl−]i (29). In the previous study, lowered [Cl−]i elicited by ectopic KCC2 was observed following birth; however, our results indicate that [Cl−]i remained high because KCC2 does not function in embryonic brains and does function postnatally. To examine the effect of [Cl−]i on radial migration, taurine-insensitive KCC2T906A/T1007A was transfected by IUE at E15.5, and radial migration at E18.5 was observed. KCC2wt-positive (GFP + KCC2) cells migrated toward the pial surface, as did the control (GFP); however, most KCC2T906A/T1007A-positive cells remained at the VZ/SVZ border (Fig. 6A). These GFP signals colocalized with Cux1, which is known to be expressed in several cortical surface layers (II/III and IV after laminar organization) (71, 72), but not with Tbr1, a marker of deep cortical layers (Fig. 6, B and C) (72, 73). Thus, low [Cl−]i perturbs neuronal radial migration in the developing cerebral cortex. Taken together, WNK-SPAK/OSR1 signaling that is partially stimulated by taurine may play an important role in embryonic Cl− homeostasis and neuronal migration.
FIGURE 6.
Lowering [Cl−]i perturbs neuronal radial migration. A, GFP was transfected with or without the indicated KCC2 derivatives by IUE at E15.5, and immunohistochemical staining was performed at E18.5. GFP (green), anti-KCC2 (red), and DAPI (blue) are shown. Scale bar, 100 μm. The bar graph shows the estimation of neuronal migration in cerebral cortices transfected with the indicated KCC2 derivatives. Although there was no obvious difference with or without KCC2wt, a greater proportion of KCC2T906A/T1007A-positive cells remained around the VZ/SVZ compared with that of KCC2wt. CP, cortical plate; IZ, intermediate zone. n = 3–5. Upper and lower areas with abundant cells are regarded as cortical plate and VZ/SVZ, respectively, whereas the intermediate zone is in the interim (see “Experimental Procedures”). B, GFP was transfected with or without the indicated KCC2 derivatives by IUE at E15.5, and immunohistochemical staining was performed at E18.5. GFP (green) and anti-Tbr1 or anti-Cux1 (red) are shown. Scale bar, 100 μm. C, photographs of the same sections in B are shown at greater magnification. Most of the GFP labeling colocalized with Cux1 but not with Tbr1. The white arrows indicate typical GFP-positive cells. Scale bar, 25 μm. Error bars, S.E.
DISCUSSION
Taurine is present in the embryonic brain by transportation from maternal blood via placental TauT (74). In addition, fetuses ingest taurine-rich amniotic fluid. Although fetal taurine decreases postnatally (49, 75), infants receive taurine via breast milk, which contains a high taurine concentration (75). Osmoregulation and neuronal modulation via GABAA and glycine receptors are considered to involve taurine (50–52). However, the involvement of taurine in brain development remains largely unknown. KCC2-transfected cell EGABA shows a positive shift with taurine, which is rescued by GES (Fig. 2, C and D). TauT takes up taurine in a Na+- and Cl−-dependent manner and regulates intracellular taurine, suggesting an important role for intracellular taurine (40). In addition, BMI/Str and 1-h taurine treatments had no effect on EGABA, which may negate the possibility of extracellular taurine-mediated signal activation. Taurine is involved in various biological roles, such as tRNA stability (76) and retinal development (77, 78). However, the signaling pathways that use intracellular taurine are unknown.
We observed that taurine is implicated in WNK activity. WNK signaling is activated by stimuli, such as osmotic stress; however, the precise pathway leading to activation is unknown (38, 59). Our results indicate that taurine uptake is crucial for WNK activation, and only intracellular taurine activates WNKs, which are also involved in osmoregulation (52). There are no significant osmolarity differences with or without 3 mm taurine (without taurine, 215 ± 2 mosm versus with taurine, 216 ± 4 mosm (n = 4–5, p = 0.41)). In addition, 3 mm GABA did not affect phosphorylation of SPAK/OSR1 (data not shown), which indicates a specific action of taurine.
KCC2 gene up-regulation is essential for Cl− homeostasis during development, and phosphorylation of KCC2 is another important factor (5, 12, 15, 18, 55, 56). Ser-940 phosphorylation regulates KCC2 function by modulating cell surface KCC2 expression (56). Tyr-1087 phosphorylation affects oligomerization, which plays a pivotal role in KCC2 activity without affecting cell surface expression (20, 55). Rinehart et al. (54) indicated that Thr-906 and Thr-1007 phosphorylation does not affect cell surface KCC2 expression. In our study, oligomerization and plasmalemmal localization were not affected by taurine (data not shown), suggesting that phosphorylation of these sites may provide another mechanism of KCC2 activity modulation.
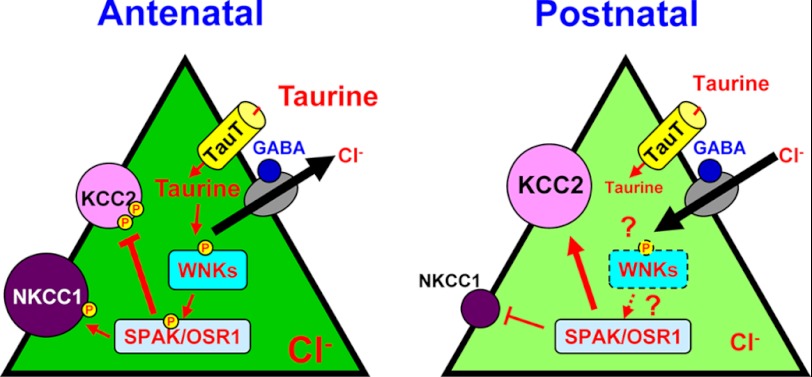
We also found in rats that WNK-SPAK/OSR1 signaling is regulated during development by observing an activation decrease soon after parturition. Conversely, Thr-907 and Thr-1007 phosphorylation in KCC2 gradually decreases upon adulthood in mice (54). This observation may be due to non-phosphorylated SPAK competing with the phosphorylated form for target sites because total SPAK protein also increases, which results in less phosphorylated KCC2. Alternatively, a difference between species may exist. Nevertheless, potential taurine regulation of KCC2 activity would be attributable to the WNK-SPAK/OSR1 pathway because WNK1S382E inhibits KCC2 activity in the absence of taurine, as shown in Fig. 4C. Although taurine activates phosphorylation of WNK1 in vitro and WNK-SPAK/OSR1 signaling in vivo, as shown in Fig. 4, other WNK members, such as WNK2 and WNK3, may also induce activation (59, 79, 80). A hypothetical model of the cellular mechanism that demonstrates taurine inactivation of KCC2 is shown in Fig. 7.
FIGURE 7.
Hypothetical model of Cl− homeostasis regulated by taurine and WNK-SPAK/OSR1 signaling during perinatal periods. To control the excitatory/inhibitory balance mediated by GABA, [Cl−]i is regulated by activation of the WNK-SPAK/OSR1 signaling pathway via KCC2 inhibition and possibly NKCC1 activation (54, 58, 59). In immature neurons, taurine is taken up into cells through TauT and activates WNK-SPAK/OSR1 signaling (left). Red arrows and T-shaped bars indicate activation and inactivation, respectively. Later (possibly a while after birth), this activation pathway induced by taurine diminishes, resulting in release of KCC/NKCC activity (right), whereas SPAK/OSR1 signaling recovers somewhat upon adulthood. Interestingly, in contrast to kinase signaling leading to KCC2 inhibition, other kinases are also known to facilitate KCC2 activity (see “Discussion”).
Importantly, taurine-induced WNK-SPAK/OSR1 signaling may play a crucial role in brain development. Although the signaling pathway affects various molecules, regulation of [Cl−]i related to GABA action may be very important during development. Although [Cl−]i has been suggested to have no effect on radial migration by IUE with KCC2 (29), this observation may be inconclusive (81). Indeed, our results show that ectopically expressed KCC2 is functionally inactive during embryogenesis; however, it becomes active postnatally. Moreover, aberrant [Cl−]i mediated by the KCC2 mutant shown in Fig. 6 disrupts normal radial migration. Because taurine inhibits KCC2 activation, our results using a taurine-insensitive KCC2 mutant may support previous studies showing that taurine-deficient kittens exhibit abnormalities in neuronal migration in the visual cortex and the external granule cell layer of the cerebellum (32, 33).
A number of neuron types are generated relatively early during embryonic development, such as Cajal-Retzius and subplate cells in the cerebral cortex, which play regulatory roles in migration. Several reports have shown that these early generated neurons in the marginal zone and subplate are activated by GABA and glycine (82–85). These early generated neurons can express KCC2 as early as the embryonic and neonatal stages (86). In addition, taurine is enriched in these brain areas (data not shown). Therefore, the present results suggest that KCC2 is not functional due to the distribution of taurine, which affects WNK-SPAK/OSR1 signaling and preserves GABAergic excitation. This signaling cascade may have broader important roles in brain development than previously reported.
Acknowledgments
We thank Dr. D. R. Alessi (University of Dundee, Dundee, Scotland, UK), Dr. J. A. Payne (University of California, Davis, CA), and Dr. J. Miyazaki (Osaka University, Osaka, Japan) for providing reagents.
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas Elucidation of Neural Network Function in the Brain 2002195 (to A. F.) and Molecular Interaction and Modal Shift of Cellular Sensor 21026013 (to A. F.) and Grant-in-Aid for Scientific Research on Innovative Area Neural Diversity and Neocortical Organization 23123505 (to K. I.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and Grants-in-Aid from the Japan Society for the Promotion of Science 19390058 (to A. F.) and 23500387 (to K. I.), Salt Science Research Foundation Grant 1125 (to K. I.), and a grant from the Kanzawa Medical Research Foundation (to K. I.).

This article contains supplemental Table 1 and Figs. 1–4.
- Pn
- postnatal day n
- En
- embryonic day n
- WNK
- with-no-lysine kinase
- KCC2wt
- wild-type KCC2
- IUE
- in utero electroporation
- GES
- guanidinoethanesulfonic acid
- PLGA
- poly(dl-lactide-co-glycolide)
- TauT
- taurine transporter
- ACSF
- artificial cerebrospinal fluid
- BMI
- bicuculline methiodide
- Str
- strychnine
- EGABA
- reversal potential of GABA
- VZ
- ventricular zone
- SVZ
- subventricular zone
- mRFP
- monomeric red fluorescent protein
- DIV
- day(s) in vitro
- Staur
- staurosporine
- SPAK
- STE20/SPS1-related proline/alanine-rich kinase.
REFERENCES
- 1. Ben-Ari Y. (2002) Excitatory actions of GABA during development. The nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 [DOI] [PubMed] [Google Scholar]
- 2. Dzhala V. I., Talos D. M., Sdrulla D. A., Brumback A. C., Mathews G. C., Benke T. A., Delpire E., Jensen F. E., Staley K. J. (2005) NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 11, 1205–1213 [DOI] [PubMed] [Google Scholar]
- 3. Blaesse P., Airaksinen M. S., Rivera C., Kaila K. (2009) Cation-chloride cotransporters and neuronal function. Neuron 61, 820–838 [DOI] [PubMed] [Google Scholar]
- 4. Lu J., Karadsheh M., Delpire E. (1999) Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J. Neurobiol. 39, 558–568 [PubMed] [Google Scholar]
- 5. Rivera C., Voipio J., Payne J. A., Ruusuvuori E., Lahtinen H., Lamsa K., Pirvola U., Saarma M., Kaila K. (1999) The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255 [DOI] [PubMed] [Google Scholar]
- 6. Yamada J., Okabe A., Toyoda H., Kilb W., Luhmann H. J., Fukuda A. (2004) Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurons is mediated by NKCC1. J. Physiol. 557, 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukuda A. (2005) Diuretic soothes seizures in newborns. Nat. Med. 11, 1153–1154 [DOI] [PubMed] [Google Scholar]
- 8. Payne J. A., Rivera C., Voipio J., Kaila K. (2003) Cation-chloride co-transporters in neuronal communication, development, and trauma. Trends Neurosci. 26, 199–206 [DOI] [PubMed] [Google Scholar]
- 9. Payne J. A., Stevenson T. J., Donaldson L. F. (1996) Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J. Biol. Chem. 271, 16245–16252 [DOI] [PubMed] [Google Scholar]
- 10. Payne J. A. (1997) Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am. J. Physiol. 273, C1516–C1525 [DOI] [PubMed] [Google Scholar]
- 11. Coull J. A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M. W., De Koninck Y. (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 [DOI] [PubMed] [Google Scholar]
- 12. Rivera C., Voipio J., Thomas-Crusells J., Li H., Emri Z., Sipilä S., Payne J. A., Minichiello L., Saarma M., Kaila K. (2004) Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 24, 4683–4691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aguado F., Carmona M. A., Pozas E., Aguiló A., Martínez-Guijarro F. J., Alcantara S., Borrell V., Yuste R., Ibañez C. F., Soriano E. (2003) BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development 130, 1267–1280 [DOI] [PubMed] [Google Scholar]
- 14. Ganguly K., Schinder A. F., Wong S. T., Poo M. (2001) GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 105, 521–532 [DOI] [PubMed] [Google Scholar]
- 15. Wake H., Watanabe M., Moorhouse A. J., Kanematsu T., Horibe S., Matsukawa N., Asai K., Ojika K., Hirata M., Nabekura J. (2007) Early changes in KCC2 phosphorylation in response to neuronal stress result in functional down-regulation. J. Neurosci. 27, 1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khirug S., Huttu K., Ludwig A., Smirnov S., Voipio J., Rivera C., Kaila K., Khiroug L. (2005) Distinct properties of functional KCC2 expression in immature mouse hippocampal neurons in culture and in acute slices. Eur. J. Neurosci. 21, 899–904 [DOI] [PubMed] [Google Scholar]
- 17. Kelsch W., Hormuzdi S., Straube E., Lewen A., Monyer H., Misgeld U. (2001) Insulin-like growth factor 1 and a cytosolic tyrosine kinase activate chloride outward transport during maturation of hippocampal neurons. J. Neurosci. 21, 8339–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiumelli H., Cancedda L., Poo M. M. (2005) Modulation of GABAergic transmission by activity via postsynaptic Ca2+-dependent regulation of KCC2 function. Neuron 48, 773–786 [DOI] [PubMed] [Google Scholar]
- 19. Ikeda K., Onimaru H., Yamada J., Inoue K., Ueno S., Onaka T., Toyoda H., Arata A., Ishikawa T. O., Taketo M. M., Fukuda A., Kawakami K. (2004) Malfunction of respiratory-related neuronal activity in Na+,K+-ATPase α2 subunit-deficient mice is attributable to abnormal Cl− homeostasis in brainstem neurons. J. Neurosci. 24, 10693–10701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blaesse P., Guillemin I., Schindler J., Schweizer M., Delpire E., Khiroug L., Friauf E., Nothwang H. G. (2006) Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J. Neurosci. 26, 10407–10419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inoue K., Yamada J., Ueno S., Fukuda A. (2006) Brain-type creatine kinase activates neuron-specific K+-Cl− co-transporter KCC2. J. Neurochem. 96, 598–608 [DOI] [PubMed] [Google Scholar]
- 22. Balakrishnan V., Becker M., Löhrke S., Nothwang H. G., Güresir E., Friauf E. (2003) Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J. Neurosci. 23, 4134–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heck N., Kilb W., Reiprich P., Kubota H., Furukawa T., Fukuda A., Luhmann H. J. (2007) GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb. Cortex 17, 138–148 [DOI] [PubMed] [Google Scholar]
- 24. Behar T. N., Schaffner A. E., Scott C. A., Greene C. L., Barker J. L. (2000) GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb. Cortex 10, 899–909 [DOI] [PubMed] [Google Scholar]
- 25. Behar T. N., Schaffner A. E., Scott C. A., O'Connell C., Barker J. L. (1998) Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J. Neurosci. 18, 6378–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang C., Shimizu-Okabe C., Watanabe K., Okabe A., Matsuzaki H., Ogawa T., Mori N., Fukuda A., Sato K. (2002) Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res. Dev. Brain Res. 139, 59–66 [DOI] [PubMed] [Google Scholar]
- 27. Li H., Tornberg J., Kaila K., Airaksinen M. S., Rivera C. (2002) Patterns of cation-chloride cotransporter expression during embryonic rodent CNS development. Eur. J. Neurosci. 16, 2358–2370 [DOI] [PubMed] [Google Scholar]
- 28. Shimizu-Okabe C., Yokokura M., Okabe A., Ikeda M., Sato K., Kilb W., Luhmann H. J., Fukuda A. (2002) Layer-specific expression of Cl− transporters and differential [Cl−]i in newborn rat cortex. Neuroreport 13, 2433–2437 [DOI] [PubMed] [Google Scholar]
- 29. Cancedda L., Fiumelli H., Chen K., Poo M. M. (2007) Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J. Neurosci. 27, 5224–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimada M., Shimono R., Watanabe M., Imahayashi T., Ozaki H. S., Kihara T., Yamaguchi K., Niizeki S. (1984) Distribution of 35S-taurine in rat neonates and adults. A whole-body autoradiographic study. Histochemistry 80, 225–230 [DOI] [PubMed] [Google Scholar]
- 31. Flint A. C., Liu X., Kriegstein A. R. (1998) Nonsynaptic glycine receptor activation during early neocortical development. Neuron 20, 43–53 [DOI] [PubMed] [Google Scholar]
- 32. Sturman J. A., Moretz R. C., French J. H., Wisniewski H. M. (1985) Taurine deficiency in the developing cat. Persistence of the cerebellar external granule cell layer. J. Neurosci. Res. 13, 405–416 [DOI] [PubMed] [Google Scholar]
- 33. Palackal T., Moretz R., Wisniewski H., Sturman J. (1986) Abnormal visual cortex development in the kitten associated with maternal dietary taurine deprivation. J. Neurosci. Res. 15, 223–239 [DOI] [PubMed] [Google Scholar]
- 34. Niwa H., Yamamura K., Miyazaki J. (1991) Efficient selection for high expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 [DOI] [PubMed] [Google Scholar]
- 35. Inoue K., Branigan D., Xiong Z. G. (2010) Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J. Biol. Chem. 285, 7430–7439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inoue K., Ueno S., Fukuda A. (2004) Interaction of neuron-specific K+-Cl− cotransporter, KCC2, with brain-type creatine kinase. FEBS Lett. 564, 131–135 [DOI] [PubMed] [Google Scholar]
- 37. Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 38. Zagórska A., Pozo-Guisado E., Boudeau J., Vitari A. C., Rafiqi F. H., Thastrup J., Deak M., Campbell D. G., Morrice N. A., Prescott A. R., Alessi D. R. (2007) Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J. Cell Biol. 176, 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller R. A., Brady J. M., Cutright D. E. (1977) Degradation rates of oral resorbable implants (polylactates and polyglycolates). Rate modification with changes in PLA/PGA copolymer ratios. J. Biomed. Mater. Res. 11, 711–719 [DOI] [PubMed] [Google Scholar]
- 40. Liu Q. R., López-Corcuera B., Nelson H., Mandiyan S., Nelson N. (1992) Cloning and expression of a cDNA encoding the transporter of taurine and β-alanine in mouse brain. Proc. Natl. Acad. Sci. U.S.A. 89, 12145–12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dominy J., Jr., Thinschmidt J. S., Peris J., Dawson R., Jr., Papke R. L. (2004) Taurine-induced long-lasting potentiation in the rat hippocampus shows a partial dissociation from total hippocampal taurine content and independence from activation of known taurine transporters. J. Neurochem. 89, 1195–1205 [DOI] [PubMed] [Google Scholar]
- 42. Janeke G., Siefken W., Carstensen S., Springmann G., Bleck O., Steinhart H., Höger P., Wittern K. P., Wenck H., Stäb F., Sauermann G., Schreiner V., Doering T. (2003) Role of taurine accumulation in keratinocyte hydration. J. Invest. Dermatol. 121, 354–361 [DOI] [PubMed] [Google Scholar]
- 43. Kranz H., Brazeau G. A., Napaporn J., Martin R. L., Millard W., Bodmeier R. (2001) Myotoxicity studies of injectable biodegradable in situ forming drug delivery systems. Int. J. Pharm. 212, 11–18 [DOI] [PubMed] [Google Scholar]
- 44. Inoue K., Ueno S., Yamada J., Fukuda A. (2005) Characterization of newly cloned variant of rat glycine receptor α1 subunit. Biochem. Biophys. Res. Commun. 327, 300–305 [DOI] [PubMed] [Google Scholar]
- 45. Ebihara S., Shirato K., Harata N., Akaike N. (1995) J. Physiol. 484 77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jarolimek W., Lewen A., Misgeld U. (1999) A furosemide-sensitive K+-Cl− cotransporter counteracts intracellular Cl− accumulation and depletion in cultured rat midbrain neurons. J. Neurosci. 19, 4695–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. DeFazio R. A., Keros S., Quick M. W., Hablitz J. J. (2000) Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J. Neurosci. 20, 8069–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carić D., Gooday D., Hill R. E., McConnell S. K., Price D. J. (1997) Determination of the migratory capacity of embryonic cortical cells lacking the transcription factor Pax-6. Development 124, 5087–5096 [DOI] [PubMed] [Google Scholar]
- 49. Sturman J. A. (1993) Taurine in development. Physiol. Rev. 73, 119–147 [DOI] [PubMed] [Google Scholar]
- 50. Schmieden V., Kuhse J., Betz H. (1992) Agonist pharmacology of neonatal and adult glycine receptor alpha subunits. Identification of amino acid residues involved in taurine activation. EMBO J. 11, 2025–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. del Olmo N., Bustamante J., del Río R. M., Solís J. M. (2000) Taurine activates GABAA but not GABAB receptors in rat hippocampal CA1 area. Brain Res. 864, 298–307 [DOI] [PubMed] [Google Scholar]
- 52. Lambert I. H. (2004) Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem. Res. 29, 27–63 [DOI] [PubMed] [Google Scholar]
- 53. Barakat L., Wang D., Bordey A. (2002) Carrier-mediated uptake and release of taurine from Bergmann glia in rat cerebellar slices. J. Physiol. 541, 753–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rinehart J., Maksimova Y. D., Tanis J. E., Stone K. L., Hodson C. A., Zhang J., Risinger M., Pan W., Wu D., Colangelo C. M., Forbush B., Joiner C. H., Gulcicek E. E., Gallagher P. G., Lifton R. P. (2009) Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell 138, 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watanabe M., Wake H., Moorhouse A. J., Nabekura J. (2009) Clustering of neuronal K+-Cl− cotransporters in lipid rafts by tyrosine phosphorylation. J. Biol. Chem. 284, 27980–27988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee H. H., Walker J. A., Williams J. R., Goodier R. J., Payne J. A., Moss S. J. (2007) Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J. Biol. Chem. 282, 29777–29784 [DOI] [PubMed] [Google Scholar]
- 57. Dowd B. F., Forbush B. (2003) PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J. Biol. Chem. 278, 27347–27353 [DOI] [PubMed] [Google Scholar]
- 58. Kahle K. T., Rinehart J., Lifton R. P. (2010) Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochim. Biophys. Acta 1802, 1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Richardson C., Alessi D. R. (2008) The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signaling pathway. J. Cell Sci. 121, 3293–3304 [DOI] [PubMed] [Google Scholar]
- 60. Xu B., English J. M., Wilsbacher J. L., Stippec S., Goldsmith E. J., Cobb M. H. (2000) WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 275, 16795–16801 [DOI] [PubMed] [Google Scholar]
- 61. Garzón-Muvdi T., Pacheco-Alvarez D., Gagnon K. B., Vázquez N., Ponce-Coria J., Moreno E., Delpire E., Gamba G. (2007) WNK4 kinase is a negative regulator of K+-Cl− cotransporters. Am. J. Physiol. Renal Physiol. 292, F1197–F1207 [DOI] [PubMed] [Google Scholar]
- 62. de Los Heros P., Kahle K. T., Rinehart J., Bobadilla N. A., Vázquez N., San Cristobal P., Mount D. B., Lifton R. P., Hebert S. C., Gamba G. (2006) WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc. Natl. Acad. Sci. U.S.A. 103, 1976–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Anselmo A. N., Earnest S., Chen W., Juang Y. C., Kim S. C., Zhao Y., Cobb M. H. (2006) WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc. Natl. Acad. Sci. U.S.A. 103, 10883–10888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moriguchi T., Urushiyama S., Hisamoto N., Iemura S., Uchida S., Natsume T., Matsumoto K., Shibuya H. (2005) WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 280, 42685–42693 [DOI] [PubMed] [Google Scholar]
- 65. Vitari A. C., Thastrup J., Rafiqi F. H., Deak M., Morrice N. A., Karlsson H. K., Alessi D. R. (2006) Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem. J. 397, 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Piechotta K., Lu J., Delpire E. (2002) Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J. Biol. Chem. 277, 50812–50819 [DOI] [PubMed] [Google Scholar]
- 67. Vitari A. C., Deak M., Morrice N. A., Alessi D. R. (2005) The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem. J. 391, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Veziers J., Lesourd M., Jollivet C., Montero-Menei C., Benoit J. P., Menei P. (2001) Analysis of brain biocompatibility of drug-releasing biodegradable microspheres by scanning and transmission electron microscopy. J. Neurosurg. 95, 489–494 [DOI] [PubMed] [Google Scholar]
- 69. Benítez-Diaz P., Miranda-Contreras L., Mendoza-Briceño R. V., Peña-Contreras Z., Palacios-Prü E. (2003) Prenatal and postnatal contents of amino acid neurotransmitters in mouse parietal cortex. Dev. Neurosci. 25, 366–374 [DOI] [PubMed] [Google Scholar]
- 70. Akahori S., Ejiri K., Sekiba K. (1986) Taurine concentrations in fetal, neonatal, and pregnant rats. Acta Med. Okayama 40, 93–101 [DOI] [PubMed] [Google Scholar]
- 71. Nieto M., Monuki E. S., Tang H., Imitola J., Haubst N., Khoury S. J., Cunningham J., Gotz M., Walsh C. A. (2004) Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J. Comp. Neurol. 479, 168–180 [DOI] [PubMed] [Google Scholar]
- 72. Georgala P. A., Manuel M., Price D. J. (2011) The generation of superficial cortical layers is regulated by levels of the transcription factor Pax6. Cereb. Cortex 21, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hevner R. F., Shi L., Justice N., Hsueh Y., Sheng M., Smiga S., Bulfone A., Goffinet A. M., Campagnoni A. T., Rubenstein J. L. (2001) Tbr1 regulates differentiation of the preplate and layer 6. Neuron 29, 353–366 [DOI] [PubMed] [Google Scholar]
- 74. Ramamoorthy S., Leibach F. H., Mahesh V. B., Han H., Yang-Feng T., Blakely R. D., Ganapathy V. (1994) Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem. J. 300, 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gaull G. E. (1989) Taurine in pediatric nutrition. Review and update. Pediatrics 83, 433–442 [PubMed] [Google Scholar]
- 76. Suzuki T., Suzuki T., Wada T., Saigo K., Watanabe K. (2002) Taurine as a constituent of mitochondrial tRNAs. New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 21, 6581–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Osakada F., Ikeda H., Mandai M., Wataya T., Watanabe K., Yoshimura N., Akaike A., Sasai Y., Takahashi M. (2008) Toward the generation of rod and cone photoreceptors from mouse, monkey, and human embryonic stem cells. Nat. Biotechnol. 26, 215–224 [DOI] [PubMed] [Google Scholar]
- 78. Warskulat U., Borsch E., Reinehr R., Heller-Stilb B., Roth C., Witt M., Häussinger D. (2007) Taurine deficiency and apoptosis. Findings from the taurine transporter knockout mouse. Arch. Biochem. Biophys. 462, 202–209 [DOI] [PubMed] [Google Scholar]
- 79. Kahle K. T., Rinehart J., de Los Heros P., Louvi A., Meade P., Vazquez N., Hebert S. C., Gamba G., Gimenez I., Lifton R. P. (2005) WNK3 modulates transport of Cl− in and out of cells. Implications for control of cell volume and neuronal excitability. Proc. Natl. Acad. Sci. U.S.A. 102, 16783–16788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gamba G., Rinehart J., Vázquez N., Kahle K. T., Hodson C. A., Ring A. M., Gulcicek E. E., Louvi A., Bobadilla N. A., Lifton R. P. (2011) WNK2 kinase is a novel regulator of essential neuronal cation-chloride cotransporters. J. Biol. Chem. 286, 30171–30180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang D. D., Kriegstein A. R. (2009) Defining the role of GABA in cortical development. J. Physiol. 587, 1873–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hanganu I. L., Okabe A., Lessmann V., Luhmann H. J. (2009) Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb. Cortex 19, 89–105 [DOI] [PubMed] [Google Scholar]
- 83. Kilb W., Hanganu I. L., Okabe A., Sava B. A., Shimizu-Okabe C., Fukuda A., Luhmann H. J. (2008) Glycine receptors mediate excitation of subplate neurons in neonatal rat cerebral cortex. J. Neurophysiol. 100, 698–707 [DOI] [PubMed] [Google Scholar]
- 84. Kilb W., Ikeda M., Uchida K., Okabe A., Fukuda A., Luhmann H. J. (2002) Depolarizing glycine responses in Cajal-Retzius cells of neonatal rat cerebral cortex. Neuroscience 112, 299–307 [DOI] [PubMed] [Google Scholar]
- 85. Mienville J. M. (1998) Persistent depolarizing action of GABA in rat Cajal-Retzius cells. J. Physiol. 512, 809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Achilles K., Okabe A., Ikeda M., Shimizu-Okabe C., Yamada J., Fukuda A., Luhmann H. J., Kilb W. (2007) Kinetic properties of Cl uptake mediated by Na+-dependent K+-2Cl cotransport in immature rat neocortical neurons. J. Neurosci. 27, 8616–8627 [DOI] [PMC free article] [PubMed] [Google Scholar]