Background: The mechanisms by which NF-κB signaling is up-regulated in dystrophic muscles are unclear.
Results: [Ca2+]rest is elevated in mdx myotubes as a result of both sarcolemmal Ca2+ entry and SR release, resulting in NF-κB-induced iNOS expression.
Conclusion: Ca2+ alterations at rest modulate NF-κB transcriptional activity and pro-inflammatory gene expression.
Significance: This allows for understanding the mechanism that relates elevated resting calcium and altered gene expression in muscular dystrophy.
Keywords: Calcium Intracellular Release, Dystrophin, Muscular Dystrophy, NF-κB Transcription Factor, Nitric-oxide Synthase, Inositol trisphosphate Receptors, Resting Calcium, Ryanodine Receptors
Abstract
Duchenne muscular dystrophy (DMD) is a genetic disorder caused by dystrophin mutations, characterized by chronic inflammation and severe muscle wasting. Dystrophic muscles exhibit activated immune cell infiltrates, up-regulated inflammatory gene expression, and increased NF-κB activity, but the contribution of the skeletal muscle cell to this process has been unclear. The aim of this work was to study the pathways that contribute to the increased resting calcium ([Ca2+]rest) observed in mdx myotubes and its possible link with up-regulation of NF-κB and pro-inflammatory gene expression in dystrophic muscle cells. [Ca2+]rest was higher in mdx than in WT myotubes (308 ± 6 versus 113 ± 2 nm, p < 0.001). In mdx myotubes, both the inhibition of Ca2+ entry (low Ca2+ solution, Ca2+-free solution, and Gd3+) and blockade of either ryanodine receptors or inositol 1,4,5-trisphosphate receptors reduced [Ca2+]rest. Basal activity of NF-κB was significantly up-regulated in mdx versus WT myotubes. There was an increased transcriptional activity and p65 nuclear localization, which could be reversed when [Ca2+]rest was reduced. Levels of mRNA for TNFα, IL-1β, and IL-6 were similar in WT and mdx myotubes, whereas inducible nitric-oxide synthase (iNOS) expression was increased 5-fold. Reducing [Ca2+]rest using different strategies reduced iNOS gene expression presumably as a result of decreased activation of NF-κB. We propose that NF-κB, modulated by increased [Ca2+]rest, is constitutively active in mdx myotubes, and this mechanism can account for iNOS overexpression and the increase in reactive nitrogen species that promote damage in dystrophic skeletal muscle cells.
Introduction
Duchenne muscular dystrophy (DMD)2 is a lethal human X-linked genetic disorder caused by mutations in the dystrophin gene (1). DMD is a progressive muscle-wasting disease characterized by loss of the ability to walk between 6 and 12 years of age and death, caused by respiratory failure and cardiac dysfunction in their twenties (2). Like humans with DMD, mdx mice lack dystrophin due to an X-linked mutation providing an accepted model to study the human disease (1). In normal skeletal muscle, dystrophin is associated with a complex of glycoproteins known as dystrophin-glycoprotein complex, providing a linkage between the extracellular matrix and cytoskeleton (3). Dystrophin has an important role in stabilizing the sarcolemma, so in muscle fibers that lack this protein, membrane damage is recurrent (4, 5). However, although membrane fragility is an important factor, it does not fully explain the onset and progression of DMD.
The microenvironment of dystrophic muscle consists of activated immune cell infiltrates and up-regulated inflammatory gene expression (6). Nuclear factor-κB (NF-κB) consists of a family of transcription factors that play critical roles in inflammation, immunity, cell proliferation, differentiation, and survival (7). The NF-κB transcription factor family in mammals consists of five proteins, p65 (RelA), RelB, c-Rel, p105/p50 (NF-κB1), and p100/52 (NF-κB2), that form homo- and heterodimeric complex (7). NF-κB has been implicated in mdx pathology, because blockade of this pathway through pharmacological or genetic approaches improves muscle histology, reduces pro-inflammatory gene expression, and ameliorates damage (8–12). NF-κB activity is increased in muscles from mdx mice and DMD patients (10, 13–15). The p65/p50 heterodimer is the predominant form of NF-κB in most cells and controls the expression of a wide array of genes critical in the immune response and inflammation (16). IkBα retains the p65/p50 heterodimer in the cytoplasm. Upon stimulation, IkBα is quickly phosphorylated by the IKK complex, ubiquinated, and degraded, thus allowing the translocation to the nucleus of the NF-κB complex (7).
IKKα/β or p65 gene ablation in transgenic animals or by adeno-associated virus improves pathology in mdx mouse muscles (8–12). Acharyya et al. (10) have shown that NF-κB activity can be seen in both muscle and immune cells and that mdx muscle pathology was improved in mdx/p65+/− but not mdx/p50+/− mice.
NF-κB gene targets, such as the pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and iNOS are up-regulated in muscles from Duchenne patients and mdx mice (10, 17–20). In mdx mice, injections of the nonspecific NF-κB inhibitor curcumin have been shown to reduce NF-κB activation and TNF-α, IL-1β, and iNOS expression (6, 9).
iNOS or NOS2, originally discovered in cytokine-induced macrophages, is a largely calcium-independent NOS, which is expressed at highest levels in immunologically activated cells and is normally absent in resting cells (21). iNOS expression is increased in muscles from mdx mice and can be reversed by curcumin (9, 22, 23). High levels of nitric oxide (NO) production lead to the formation of peroxynitrite, a highly reactive species contributing to muscle oxidative damage (21, 24). In addition, iNOS expression has been associated with S-nitrosylation of type 1 ryanodine receptor (RyR1), calcium dysregulation, and muscle pathology in mdx mice (22).
Although there are many examples in the literature indicating that resting intracellular free Ca2+ concentration ([Ca2+]rest) is higher in skeletal muscle cells from mdx mice and DMD patients compared with normal cells (25–30), others authors have not (31, 32). The mechanism that has been proposed for causing this elevation in [Ca2+]rest assumes recurrent membrane damage due the failure of dystrophin function to stabilize the sarcolemma (5, 33), allowing Ca2+ leak into the cell through the damaged membrane. An alternative explanation is an increased Ca2+ entry through transient receptor potential channel 1 (TRPC1) and hyperactive store-operated calcium entry (SOCE) in mdx muscle fibers (25, 34–38).
Several studies have shown that NF-κB activity can be modulated by intracellular Ca2+ levels (39–42). In skeletal muscle cells, depolarization with high K+ or electrical stimulation activates NF-κB through Ca2+ signals elicited by the ryanodine (RyR) and inositol 1,4,5-triphosphate (IP3R) receptors (40).
In dystrophic muscle cells, increased [Ca2+]rest, has been mainly thought to cause necrosis through calpain activation and mitochondrial permeability transition pore (29, 43, 44). Here, we have revisited the issue of elevated [Ca2+]rest in dystrophic mdx skeletal muscle cells showing that it is a complex process that involves sarcolemmal Ca2+ entry as well as SR Ca2+ leak, both through RyRs and IP3Rs. In addition, we demonstrate that the level of [Ca2+]rest modulates the transcription factor NF-κB activity and iNOS expression in mdx myotubes.
MATERIALS AND METHODS
Cell Culture
All procedures for animal use were in accordance with guidelines approved by the Bioethical Committee at the Facultad de Medicina, Universidad de Chile. Primary myotubes from wild type C57BL/6 and mdx mice were isolated according to the method of Rando and Blau (45). The myoblasts were grown and differentiated as described previously (46).
Determination of [Ca2+]rest by Ca2+-selective Microelectrodes
Double-barreled Ca2+-selective microelectrodes were prepared and calibrated as described previously (47). Only those electrodes with a linear relationship between pCa3 and pCa8 (Nernstian response, 28.5 mV per pCa unit at 24 °C) were used experimentally. To better mimic the intracellular ionic conditions, all calibration solutions were supplemented with 1 mm Mg2+. All electrodes were then re-calibrated after making measurements of [Ca2+]rest, and if the two calibration curves did not agree within 3 mV from pCa7 to pCa8, the data from that microelectrode was discarded. Myotubes were impaled with the double-barreled microelectrode, and potentials were recorded via high impedance amplifier (WPI Duo-773). The potential from the 3 m KCl barrel (Vm) was subtracted electronically from VCaE to produce a differential Ca2+-specific potential (VCa) that represents the [Ca2+]rest. Vm and VCa were filtered (30–50 KHz) to improve the signal-to-noise ratio and stored in a computer for further analysis. The experiments were performed in Krebs-Ringer solution (in mm: 125 NaCl, 5 KCl, 2 CaCl2, 1.2 MgSO4, 6 glucose, and 25 Hepes/Tris, pH 7.4). The low Ca2+ solution was prepared replacing the CaCl2 with MgCl2 (≈7 μm Ca2+). Ca2+-free solution was prepared by omitting Ca2+ and adding Mg2+ (2 mm) and EGTA (2 mm). We avoided measurements of [Ca2+]rest after long incubations in both solutions (more than 5 min) because, despite the fact that the solution was supplemented with 2 mm Mg2+, all myotubes began to show a significant depolarization (>8 mV) after this interval.
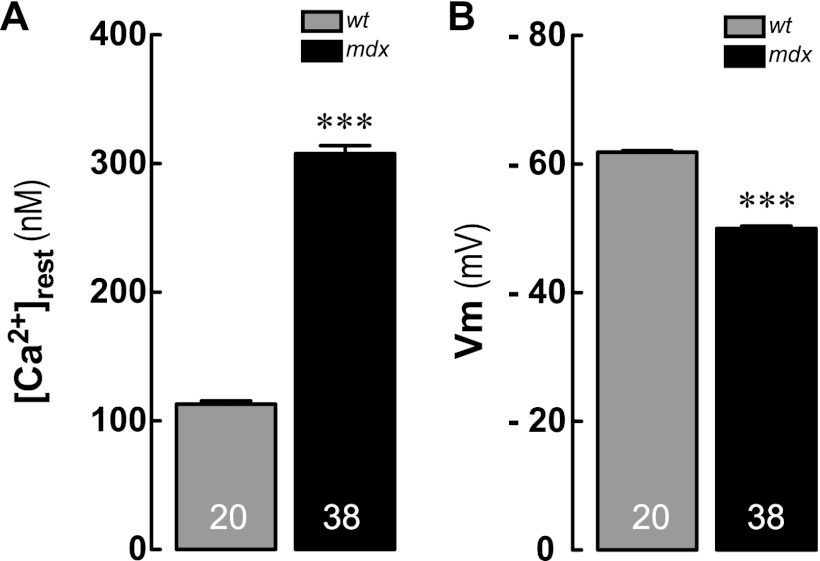
In every experiment, we determined the [Ca2+]rest in control conditions in both WT and mdx myotubes, and data were expressed as the total average for basal [Ca2+]rest for WT and mdx myotubes (Fig. 1).
FIGURE 1.
A, resting intracellular Ca2+ concentrations ([Ca2+]rest); B, resting membrane potentials (Vm) measured by double-barreled microelectrodes in WT and mdx primary myotubes. Data are expressed as means ± S.E., n = 20 for WT and n = 38 for mdx, indicated inside the bars. ***, p < 0.001 versus WT.
Sarcoplasmic Reticulum Ca2+ Content
To estimate the total amount of Ca2+ stored in the intracellular compartments, primarily from the SR stores, myotubes were loaded with 5 μm Fluo-4-AM or Fluo-5N-AM for 30 min at 37 °C. Cells were placed on the stage of an inverted microscope equipped with epifluorescence illumination (XCite® Series 120 or Lambda DG4) equipped with a CCD cooled camera (Retiga 2000R or Stanford Photonics 12 bit digital). The cell-containing coverslips or μ-clear 96-well/plates (Greiner Bio-one) were placed in the microscope for fluorescence measurements after excitation with a 488-nm wavelength filter system (Lambda 10–2 or DG4, Sutter Instruments). The emission signal was acquired at a frequency of 10 frames/s. The amount of SR Ca2+ was estimated by taking the area under the curve of the signal induced by 5 μm ionomycin in Ca2+-free solution to minimize Ca2+ entry. Fluorescence data (F) was analyzed by normalizing with respect to basal fluorescence (F0) and expressed as (F − F0)/F0.
Resting Ca2+ Entry
Myotubes were loaded with Fura2-AM (5 μm) for 30 min at 37 °C and the cells were perfused with low Ca2+ solution for 1 min; then the perfusion system was switched to Mn2+-containing solution (in mm: 125 NaCl, 5 KCl, 0.5 MnCl2, 2.7 MgSO4, 6 glucose, and 25 Hepes/Tris, pH 7.4) for 1 min. During the quench, the perfusion system was switched to Mn2+ containing solution with gadolinium tri-chloride for an additional 1 min (Gd3+, 20 μm), to study the effect of the later on Ca2+ entry. To calculate the fluorescence quench rate, the stable part of the signal was fitted to a linear regression. The derived slope was expressed as fluorescence arbitrary units/s. The excitation wavelength used to measure Mn2+ quench of Fura-2 was monitored using a 357/7-nm excitation and 510/80-nm emission filter.
Immunofluorescence and Confocal Microscopy
For immunofluorescence localization of the NF-κB p65 subunit, differentiated myotubes were fixed in 4% paraformaldehyde for 10 min at RT. Cells were rinsed with PBS, then blocked with PBS, 1% BSA for 1 h at room temperature, and incubated overnight with p65 antibody at a 1:200 dilution at 4 °C. Cells were washed and then incubated for 1 h with Alexa Fluor-488 anti-rabbit antibody (Invitrogen). Hoechst was used for nuclear visualization. Immunofluorescence was observed in a confocal microscope (Carl Zeiss, Axiovert 200, LSM 5-Pascal) and images were deconvolved using Iterative Deconvolution software of ImageJ. To determine the nuclear localization of the NF-κB p65 subunit, the fluorescence intensity of nuclear and cytosolic region of interest was calculated for at least 10 different myotubes in three different experiments and averaged to calculate the ratio of nuclear over cytoplasmic intensity using the ImageJ program. z-stack images were reconstructed using the Interactive 3D Surface Plot ImageJ plugin (rsb.info.nih.gov) that translates the luminance of an image as height for the plot. DAF-FM diacetate (Molecular Probes) fluorescence was detected according to the manufacturer's instructions by confocal microscopy with an excitation 488 nm wavelength argon laser.
Western Blot
Total protein lysates were prepared from differentiated myotubes by homogenizing them in a lysis buffer containing 20 mm Tris-HCl, pH 7.5, 1% Triton X-100, 1 mm EDTA, 1 mm EGTA, 20 mm NaF, 1 mm Na2P2O7, 10% glycerol, 150 mm NaCl, 10 mm Na3VO4, 1 mm PMSF, and protease inhibitors (CompleteTM, Roche Applied Science). Proteins were separated using SDS-PAGE and transferred to PVDF membranes. The following primary antibodies and their dilutions were used: NF-κB p65 subunit (1:1000; Cell Signaling); iNOS (1:2000; Santa Cruz Biotechnology); GAPDH (1:2000, Santa Cruz Biotechnology); and β-actin (1:10,000, Sigma). The protein bands in the blots were visualized using an ECL detection kit (Pierce), and the intensity of the bands was determined with ImageJ densitometric analysis.
siRNA Transfection
NF-κB p65 and scramble siRNA were purchased from Santa Cruz Biotechnology. NF-κB p65 siRNA is a pool of four target-specific double-stranded siRNAs. Myoblasts at 50–70% confluence were transfected with both siRNAs (50 nm) with DharmaFECT Duo (Dharmacon) for 3 h at 37 °C and 5% CO2 in 35-mm culture plates in Opti-MEM (Invitrogen). Following transfection, myoblasts were differentiated for 48 h and lysed for protein detection by Western blot.
NF-κB Luciferase Reporter Activity Determinations
A plasmid containing five tandems repeats of NF-κB-binding sites cloned upstream of a luciferase reporter gene (pNF-κB-Luc) was obtained from Agilent Technologies and subcloned in a lentiviral vector with neomycin resistance, and lentiviral particles were produced by transient transfection of HEK 293T cells as described (48). Supernatants were collected, and myoblast cultures were transduced immediately after isolation at a multiplicity of infection of 1:500 in the presence of 6 μg/ml protamine sulfate for 3 h. Cells were allowed to recover for 48 h and then selected with neomycin (400 μg/ml) for 9 days. After infection and selection, myoblasts were completely normal and differentiated into myotubes similarly to uninfected cells. To minimize clonal variations, we pooled together more than 100 G418-resistant clones from each transduction to perform the experiments. Luciferase activity was determined using a Dual-Luciferase reporter assay system (Promega) according to the manufacturer's instructions, and light detection was carried out in a Berthold F12 luminometer. Results were normalized with total protein, and the relation “luciferase mg−1 protein” was shown. We studied the response to lipopolysaccharide (LPS), a strong activator of NF-κB as a control (data not shown).
Real Time PCR
Total RNA from myotubes cultures was obtained using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. cDNA was prepared by reverse transcription of 1 μg of total RNA, using SuperScript II (Invitrogen). Real time PCR was performed using a Stratagene Mx3000P as follows. Primers were used at a final concentration of 400 nm. Briefly, 1–4 μl of cDNA reaction together with the appropriate primers was added to 10 μl of Brilliant III UltraFast SYBR Green QPCR master mix (Agilent Technologies) to a total volume of 20 μl. No-template control reactions were also prepared for each gene. The cycling parameters for all genes were as follows: 95 °C for 3 min, then 50 cycles of 95 °C for 20 s, and 60 °C for 20 s. Expression values were normalized to GAPDH and are reported in units of 2−ΔΔCt ± S.E (49). PCR products were verified by melting-curve analysis, resolved by electrophoresis on 2% agarose gel, and stained with ethidium bromide.
The TNF-α, IL-1β, IL-6, iNOS, and GAPDH mRNA transcripts were quantified using oligonucleotide primers designed based on sequences published in NCBI GenBankTM with the open-source PerlPrimer software (50). The forward and reverse primers sequences used in this study are shown in Table 1.
TABLE 1.
Oligonucleotides used for detection of mRNA levels by real time PCR in myotubes derived from WT and mdx mice primary culture
| mRNA | Accession no. | Forward primer (5′ → 3′) | Reverse primer (5′ →3 ′) | Amplicon | Source or Ref. |
|---|---|---|---|---|---|
| bp | |||||
| GAPDH | NM_008084.2 | CTCATGACCACAGTCCATGC | TTCAGCTCTGGGATGACCTT | 155 | This study |
| iNOS | NM_010927.3 | CAGCTCAAGAGCCAGAAACG | TTACTCAGTGCCAGAAGCTG | 141 | This study |
| IL-1β | NM_008361.3 | CTTTGAAGAAGAGCCCATCC | TTTGTCGTTGCTTGGTTCTC | 229 | This study |
| IL-6 | NM_031168.1 | CCAATTTCCAATGCTCTCCT | ACCACAGTGAGGAATGTCCA | 182 | (83) |
| TNF-α | NM_013693.2 | TCACACTCAGATCATCTTCTC | ATGAGATAGCAAATCGGCTG | 261 | This study |
Statistics
All values are expressed as mean ± S.E. from at least three different determinations. Results of luciferase activity, p65 immunofluorescence, DAF-FM fluorescence, and Western blot were transformed with the WT basal average (y = y′/(WT basal average)) to normalize to 1 with S.E. Statistical analysis was performed using an unpaired two-tailed t test or analysis of variance-Bonferroni to determine significance (p < 0.05).
RESULTS
[Ca2+]rest Is Increased in Dystrophic mdx Myotubes
Resting membrane potentials (Vm) and [Ca2+]rest were measured in differentiated WT and mdx myotubes with Ca2+-selective microelectrodes. The [Ca2+]rest observed in mdx myotubes was significantly higher than that observed in WT myotubes (308 ± 6 nm, n = 38 versus 113 ± 2 nm, n = 20, p < 0.001) (Fig. 1A). Vm was significantly increased in mdx myotubes compared with the WT counterpart (−50 ± 0.2 mV, n = 38 versus −62 ± 0.2 mV, n = 20, p < 0.001) (Fig. 1B).
Blockade of Ca2+ Entry in mdx Myotubes Reduced but Did Not Normalize [Ca2+]rest
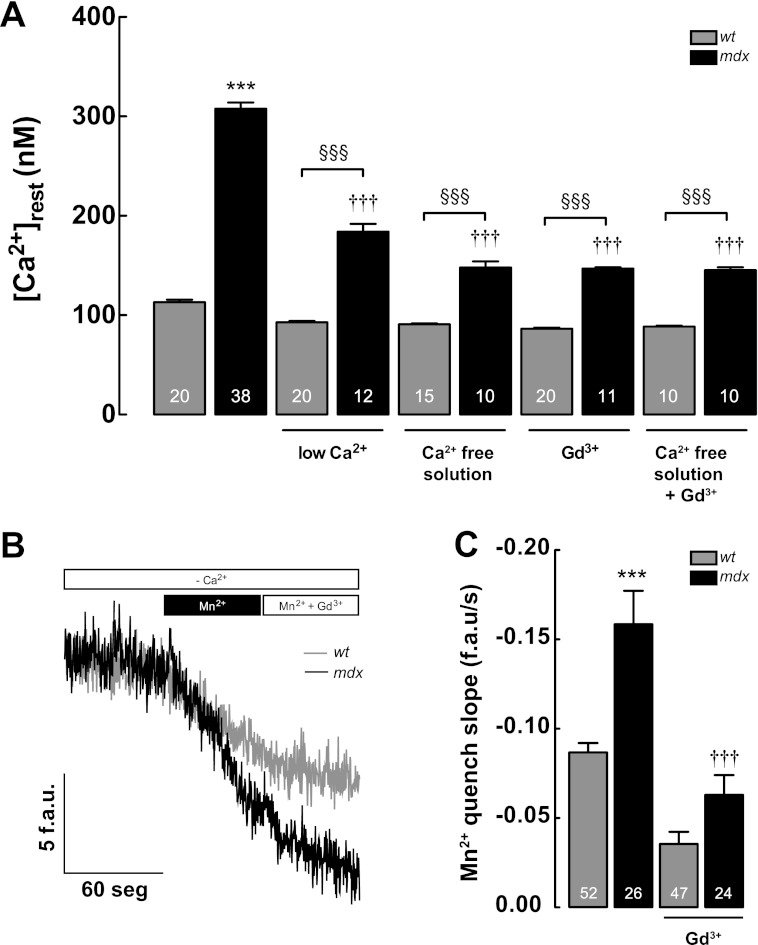
Several studies have suggested that [Ca2+]rest is increased in mdx skeletal muscle cells, due to an increased Ca2+ entry from extracellular space through TRPC1 and/or SOCE channels (25, 34–38). To explore the contribution of extracellular Ca2+ to [Ca2+]rest in WT and mdx myotubes, we used four different strategies as follows: low Ca2+ solution, Ca2+-free solution, Krebs-Ringer solution supplemented with gadolinium trichloride (Gd3+, 20 μm), and Ca2+-free solution with Gd3+(see under “Materials and Methods”). Cells were incubated for 2 min before [Ca2+]rest determinations were made. We observed a nonsignificant reduction in [Ca2+]rest in WT myotubes after the addition of low Ca2+ solution, Ca2+-free solution, and Gd3+ solution (92.9 ± 1 nm, n = 20, 91.0 ± 1 nm, n = 15, and 86.3 ± 1 nm, n = 20, all p > 0.05 compared with the WT basal value) (Fig. 2A). In mdx myotubes, there was a significant decrease in [Ca2+]rest in all conditions (184 ± 8 nm, n = 12, with low Ca2+, 148 ± 6 nm in Ca2+ free solution, n = 10, 147 ± 1 nm, n = 11, after Gd3+, all of them p < 0.001 compared with the mdx basal value) (Fig. 2A). The addition of Gd3+ to the Ca2+-free solution did not cause a further reduction of [Ca2+]rest, suggesting that Gd3+ by itself was able to block the active Ca2+-entry pathway. In addition, we estimated Ca2+ entry by Mn2+ quench of Fura-2 fluorescence. Rates of Mn2+ quench were significantly higher in mdx myotubes (Fig. 2, B and C), and this was completely blocked by the addition of Gd3+ (20 μm). Although inhibition of Ca2+ entry by either Gd3+ or removal of extracellular Ca2+ reduced [Ca2+]rest in mdx myotubes, it did not return it to WT levels, suggesting an additional mechanism(s) causing [Ca2+]rest dysregulation in mdx myotubes.
FIGURE 2.
Ca2+ entry contribution to [Ca2+]rest measured in WT and mdx myotubes. A, effects of removal of extracellular Ca2+ (low Ca2+ solution and Ca2+-free solution) and Gd3+ treatment on [Ca2+]rest. B and C, measurements of resting Ca2+ entry using Mn2+ quench in WT and mdx myotubes. B, representative traces of Fura-2 fluorescence quench by Mn2+ measured under resting conditions. C, quantification of the rate of Mn2+ quench, between WT and mdx myotubes. Gd3+ (20 μm) was added during the experimental determination of Mn2+ quench as shown in figure. Data are expressed as mean ± S.E. ***, p < 0.001 versus WT basal value; †††, p < 0.001 versus mdx basal value. §§§, p < 0.001 is indicated in the figure. f.a.u./s, fluorescence arbitrary units/s.
Inhibition of RyRs and IP3Rs Reduced [Ca2+]rest in mdx Myotubes
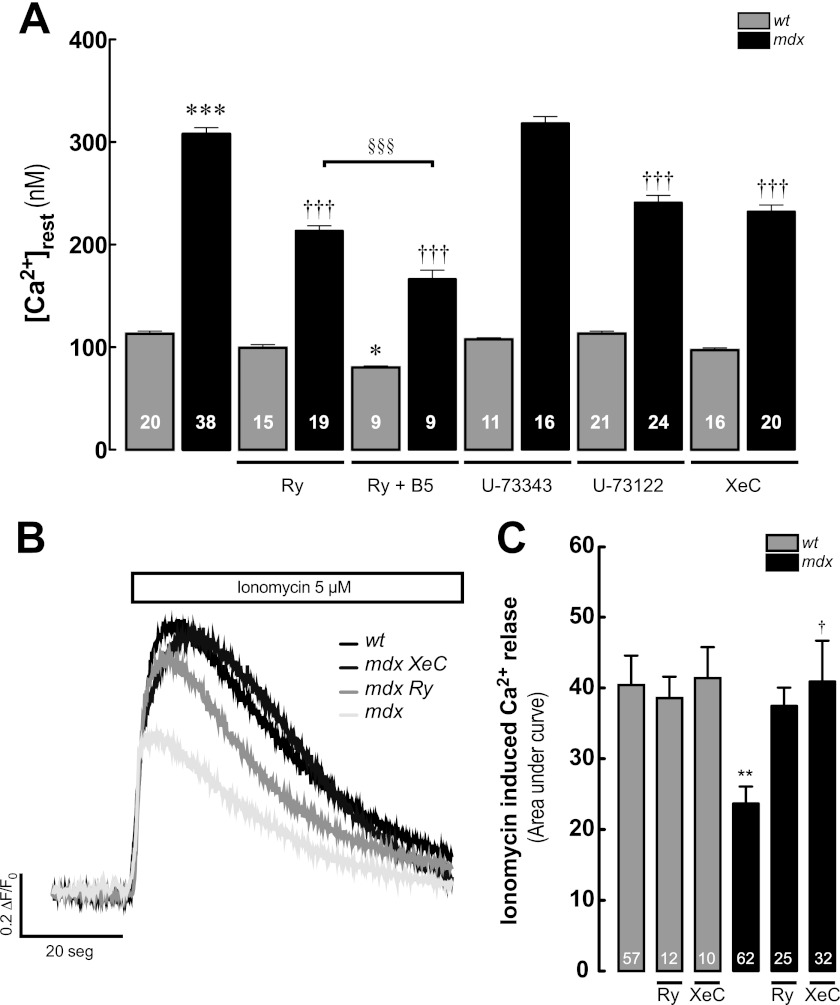
We have previously reported that [Ca2+]rest depends largely on a Ry-insensitive leak by RyR1 (“RyR1 leak”) and was unaffected by Ry treatment (47). Bastadin 5 (B5), a brominated macrocyclic derivative of dityrosine, isolated from the marine sponge Icanthellabasta (51), interacts with RyR1, modulating RyR1 gating behavior in an FKBP12-dependent manner. B5 can be used as a pharmacological tool to convert RyR1 from its leak conformation into a gating conformation, restoring the Ry sensitivity (47). We studied the contribution of RyR1 to the [Ca2+]rest in mdx myotubes (Fig. 3A). Ry treatment alone did not modify [Ca2+]rest in WT myotubes but did cause a significant reduction of [Ca2+]rest in mdx myotubes (99 ± 3 nm, n = 15, p > 0.05, 213 ± 5 nm, n = 19, p < 0.001 compared with WT and mdx basal values, respectively). Addition of B5 in the presence of Ry significantly diminished [Ca2+]rest in both WT and mdx myotubes (80 ± 1 nm, n = 9, p < 0.05, 166 ± 9 nm, n = 9, p < 0.001 compared with WT and mdx basal values, respectively) but also did not reduce mdx basal values to those seen in WT.
FIGURE 3.
RyR and IP3R participation in [Ca2+]rest in WT and mdx myotubes. A, effect of Ry (30 μm), Ry + B5 (Ry, 30 μm, and B5, 10 μm), U-73343 (5 μm), U-73122 (5 μm), and XeC (5 μm) on [Ca2+]rest (treatments for 3 h). B, representative traces of Fluo-4 fluorescence signals after the addition of 5 μm ionomycin, in the absence of extracellular Ca2+ (Ca2+-free solution) in WT, mdx, and Ry- and XeC-treated mdx myotubes. C, average area under the curve of the ionomycin-induced Ca2+ transients. Data are expressed as means ± S.E. ***, p < 0.001; **, p < 0.01; *, p < 0.05 versus WT basal value, †, p < 0.05; †††, p < 0.001 versus mdx basal value. §§§, p < 0.001 is indicated in the figure.
We have previously demonstrated that the expression of IP3Rs, as well as the total mass of inositol 1,4,5-trisphosphate, is increased in both mdx and human DMD-derived cell lines compared with normal cells (52). U-73122 (a PLC inhibitor) and Xestospongin C (an IP3R blocker) significantly reduced the [Ca2+]rest only in mdx myotubes (241 ± 7 nm, n = 24, and 232 ± 6 nm, n = 20, respectively, both p < 0.001 compared with the mdx basal value), without any significant effect in WT myotubes (113 ± 2 nm, n = 21, and 97 ± 2 nm, n = 16, respectively, p > 0.05 compared with WT basal values) (Fig. 3A), whereas U-73343 (an inactive PLC inhibitor) did not modify [Ca2+]rest in either WT or mdx myotubes.
To quantify the level of the SR Ca2+ store, we exposed WT and mdx myotubes loaded with Fluo-4-AM to 5 μm ionomycin in Ca2+-free solution. Under these conditions, the total Ca2+ released was significantly smaller in mdx myotubes compared with WT myotubes (area under curve = 23.7 ± 2.4 versus 40.4 ± 4.2, p < 0.01) (Fig. 3, B and C, and representative fluorescence images in supplemental Fig. S1). Similar results were obtained in Fluo-5N-loaded myotubes (supplemental Fig. S2). Moreover, treatment (3 h) with either Ry (30 μm) or XeC (5 μm), partially restored the SR Ca2+ content in mdx myotubes (Fig. 3C), suggesting that the reduction in the SR Ca2+ levels is due to a Ca2+ leak from the SR through both RyRs and IP3Rs that modulates [Ca2+]rest.
NF-κB Activity Is Up-regulated in Dystrophic Myotubes and Can Be Reversed with Inhibitors That Reduce [Ca2+]rest
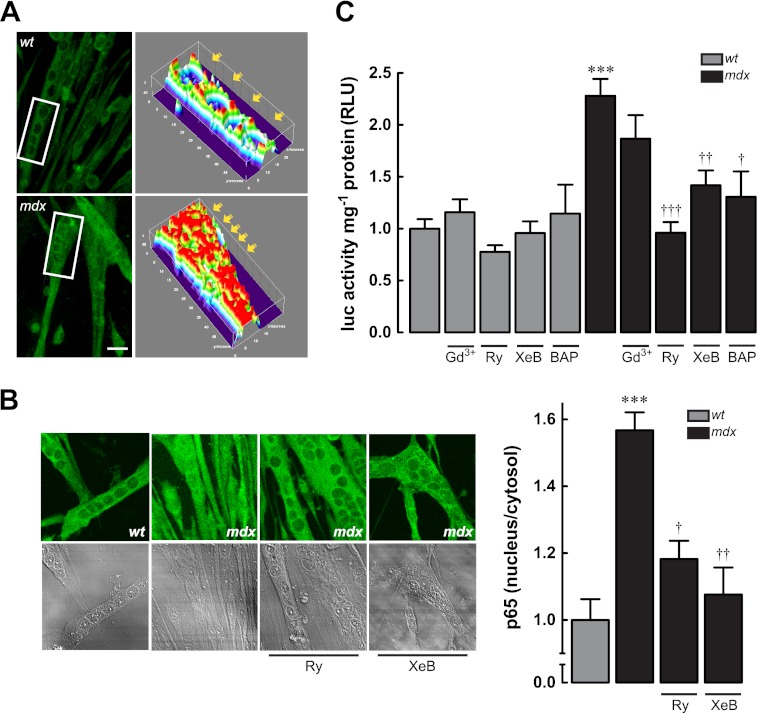
We studied the subcellular distribution of the p65 subunit of NF-κB. Fig. 4A shows an increased nuclear localization of p65 in mdx myotubes compared with WT myotubes, measured by immunofluorescence and confocal microscopy. Three-dimensional reconstruction of z-stack images shows that p65 is located primarily in the cytosol, but in mdx myotubes, the distribution is diffuse with both cytoplasmic and nuclear localization. Basal fluorescence ratio of p65 between nucleus and cytosol was increased about 50% in dystrophic myotubes compared with normal myotubes (Fig. 4B). We assessed the transcriptional activity of NF-κB using a reporter that contains five tandems repeats of NF-κB-binding sites cloned upstream of a luciferase gene (see “Materials and Methods”). Luciferase activity was increased 2.5-fold in mdx myotubes compared with WT myotubes (Fig. 4C). To establish a correlation between [Ca2+]rest and NF-κB transcriptional activity, we treated myotubes for 6 h with Gd3+, Ry, and XeB (53, 54) as described previously at the same concentrations. None of these drugs caused a significant change in the luciferase reporter activity in WT myotubes (p > 0.05) (Fig. 4C). However, blockade of sarcolemmal Ca2+ entry with Gd3+ reduced the luciferase reporter activity by 19% (p > 0.05), and pretreatment with Ry or XeB reduced it by 58 and 38%, in mdx myotubes, respectively (p < 0.001 and p < 0.01, respectively, compared with the mdx basal value). Furthermore, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA AM, 50 μm) treatment reduced NF-κB transcriptional activity in mdx myotubes by 43% (p < 0.05) without any significant effect in WT myotubes. To confirm the contribution of Ca2+ released by SR, we measured the subcellular distribution of p65 in the presence of Ry or XeB. Both inhibitors reduced nuclear/cytosol p65 fluorescence by mdx myotubes (Fig. 4B).
FIGURE 4.
NF-κB activity in WT and mdx myotubes. A, left panel, representative z-stack immunofluorescence images obtained by confocal microscopy; right panel, three-dimensional reconstructions made with the ImageJ (National Institutes of Health) plugin Interactive 3D Surface Plot. B, effect of SR Ca2+ release inhibition in p65 subcellular distribution. C, NF-κB luciferase reporter activity in WT and mdx myotubes treated with Ca2+ inhibitors. Myotubes were incubated for 6 h and then lysed for luciferase activity determination. Data are expressed as mean ± S.E. from at least three different determinations, ***, p < 0.001 versus WT basal value, †††, p < 0.001; ††, p < 0.01 and †, p < 0.05 versus mdx basal value.
TNF-α, IL-1β, and IL-6 Gene Expression Was Similar in WT and mdx Myotubes
To identify gene targets that can be modulated by [Ca2+]rest-dependent NF-κB up-regulation, we studied the levels of mRNA for TNF-α, IL-1β, and IL-6 in both myotube models. We did not observe any significant difference in the mRNA levels of these cytokines between WT and mdx myotubes under resting conditions (supplemental Fig. S3).
iNOS Is Overexpressed in mdx Myotubes and Is Dependent on [Ca2+]rest and NF-κB Activity
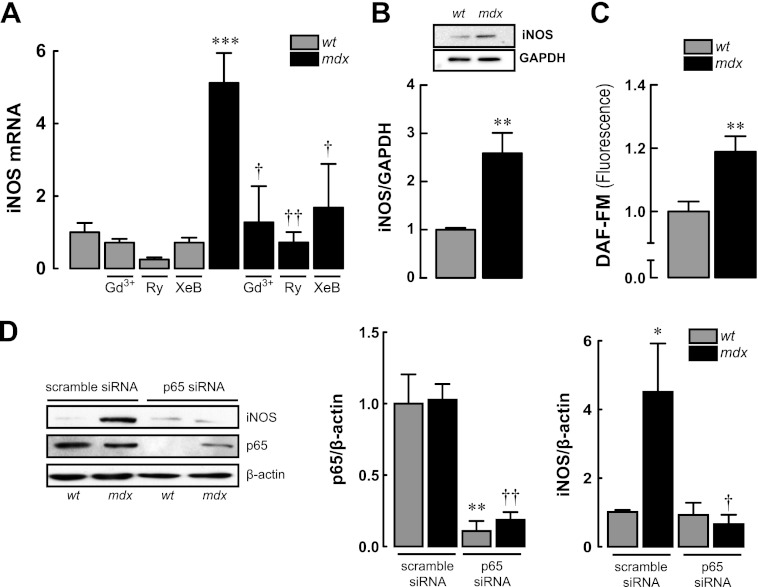
We observed increased iNOS mRNA levels and protein expression in mdx myotubes (p < 0.001 and p < 0.01, respectively, compared with WT myotubes) (Fig. 5, A and B). Moreover, nitric oxide (NO) production, assessed by DAF-FM fluorescence, was ≈20% higher in mdx compared with WT myotubes (Fig. 5C). In myotubes transfected with p65 siRNA, the expression of p65 protein, after 48 h, was reduced by 89 and 82% in WT and mdx myotubes, respectively (Fig. 5D). p65 knockdown in mdx myotubes normalized iNOS protein levels to WT values showing that the latter is regulated by the activity level of the former (Fig. 5D). Moreover, treatment with compounds that lower [Ca2+]rest for 6 h significantly reduced iNOS mRNA levels in mdx myotubes (75% Gd3+, 86% Ry, and 66% XeB), but it had no significant effect in WT myotubes (Fig. 5A).
FIGURE 5.
iNOS expression in WT and mdx myotubes. A, iNOS mRNA levels assessed by real time PCR showing effects of [Ca2+]rest reduction on iNOS mRNA expression. B, iNOS protein expression determined by Western blot. C, levels of nitric oxide (NO) was determined with DAF-FM fluorescence probe with confocal microscopy. D, effects of p65 knockdown by siRNA in the levels of p65 and iNOS proteins expression determined by Western blot. Myoblasts were transfected and then differentiated to myotubes for 48 h before the protein determination. Data are expressed as means ± S.E. from at least three different determinations. ***, p < 0.001; **, p < 0.01 and *, p < 0.05 versus WT basal value, ††, p < 0.01 and †, p < 0.05 versus mdx basal value.
p38 MAPK Is Involved in NF-κB Up-regulation in mdx Myotubes
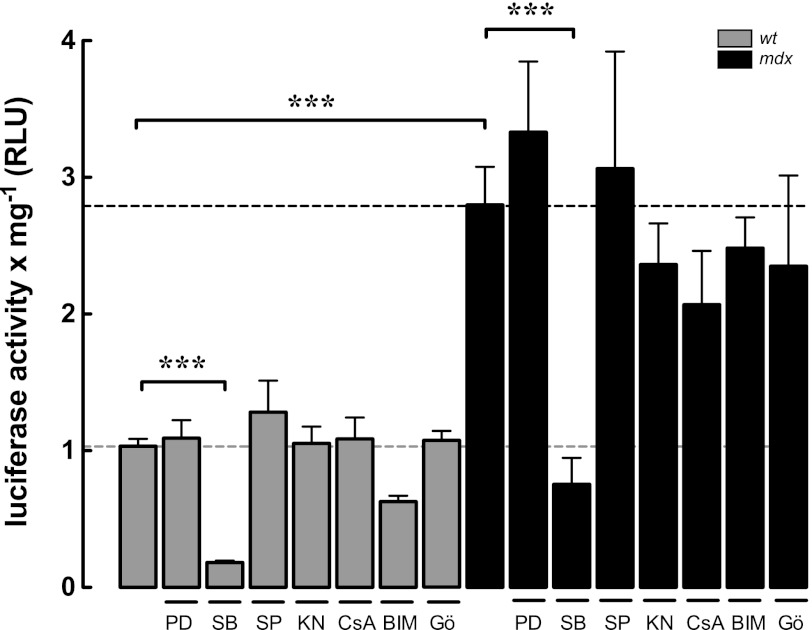
Several Ca2+-sensitive pathways can modulate the activity of the NF-κB signaling pathway (41, 55). To determine the signal transduction pathways involved in the [Ca2+]rest-dependent NF-κB up-regulation, we used specific pharmacological blockers for ERK1/2, JNK, p38 MAPKs, Ca2+/calmodulin-dependent kinase II, calcineurin A, and protein kinase C (PKC) (Fig. 6). Only p38 MAPK inhibition with SB-203580 (10 μm) significantly reduced the NF-κB luciferase reporter activity in both WT and mdx myotubes by 82 and 73%, respectively (p < 0.001).
FIGURE 6.
NF-κB transcriptional activity is modulated by p38 MAPK activity in both WT and mdx myotubes. Myotubes were treated with PD-98059 (PD) (ERK1/2 inhibitor, 10 μm), SB-203580 (SB) (p38 inhibitor, 10 μm), SP-600125 (SP) (JNK inhibitor, 10 μm), KN-93 (KN) Ca2+/calmodulin-dependent kinase II (CaMKII, 10 μm), cyclosporin A (CsA) (10 μm), bisindolylmaleimide I (BIM-I) (PKC inhibitor, 2.5 μm), and Gö-6976 (Gö) ( specific inhibitor of calcium-responsive PKCs, 10 μm) for 6 h and then lysed for luciferase activity determination. Data are expressed as mean ± S.E. from at least three different determinations ***, p < 0.001.
DISCUSSION
In dystrophic skeletal muscle cells, increased [Ca2+]rest has been mainly related with calpain activation and mitochondrial permeability transition pore aperture as factors that induce death in skeletal muscle fibers. Here, we show the first evidence that elevated [Ca2+]rest in dystrophic myotubes causes altered function of the transcription factor NF-κB leading to iNOS expression. In addition, our data show that the increased [Ca2+]rest in mdx myotubes is multifactorial, involving both Ca2+ entry and Ca2+ SR leak, through RyRs and IP3Rs.
We have previously shown that the [Ca2+]rest in DMD muscle fibers was ∼370 nm, although in normal muscle fibers it was ∼100 nm (27). Other authors have shown similar calcium concentrations in adult mdx fibers compared with the WT fibers (25, 28). A possible explanation to why some authors did not find elevated [Ca2+]rest in dystrophic muscle cells may be due, in part, to methodological differences in fluorescent dye calibration, the previous contractile activity, and age of the fibers. Moreover, fluorescent dyes, as 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid derivatives, are Ca2+ chelators and can artificially reduce [Ca2+]rest. Thus in most cases, the [Ca2+]rest values that have been reported in muscle cells using this method are significantly lower (range 20–80 nm) than those reported using Ca2+-selective microelectrodes (100–120 nm).
TRPC1-dependent Ca2+ entry is increased in mdx muscle fibers (25, 34, 36). Both GsMTx4 and streptomycin reduced [Ca2+]rest and prevented the rise of the [Ca2+]rest following eccentric contractions improving the muscle function and increasing myofiber regeneration in mdx mice (25, 28, 36). In addition to stretch channel activation, SOCE has emerged as another contributor in increased resting Ca2+ entry in mdx fibers (35, 37, 38). We have found that Gd3+, an unspecific blocker of Ca2+-entry through SOCE and transient receptor potential channels (56), reduced [Ca2+]rest by 52% in mdx myotubes and that long term treatment with Gd3+ was associated with a reduction in NF-κB activity and iNOS expression. However, blocking Ca2+ entry did not completely normalize [Ca2+]rest, suggesting a possible intracellular contribution.
In primary mdx myotubes, treatment with Ry reduced the [Ca2+]rest by 31% and adding B5 decreased it further to 46%. We have previously shown that [Ca2+]rest depends largely on Ry-insensitive leak of RyR1 channels (RyR1 leak) that can be blocked by Ry + B5 treatment in normal myotubes (47). Bellinger et al. (22) have shown that RyR1 isolated from mdx skeletal muscle shows an age-dependent increase in S-nitrosylation coincident with muscle pathology, which depleted the channel complex of FKBP12, resulting in “leaky channels.” Depletion of FKBP12 from RyR1 channel to nitrosative stress may render it sensitive to Ca2+-mediated activation (22).
Both IP3R blockade with XeC (IP3R blocker) and U-73122 (PLC inhibitor) treatment resulted in 25 and 22% reduction in the [Ca2+]rest in mdx myotubes, respectively. These combined data strongly demonstrate that the SR plays an important role in the dysregulation [Ca2+]rest observed in dystrophic myotubes.
There is a controversy concerning the SR Ca2+ levels in mdx skeletal muscle cells. Robert et al. (57) demonstrated an increased SR Ca2+ loading capacity after depletion in mdx compared with WT. However, other authors have shown a reduced expression of calsequestrin-like proteins, lower SR Ca2+ loading (58), and reduced sarco/endoplasmic reticulum Ca2+-ATPase activity in mdx muscles (59). Recently, Robin et al. (60) demonstrated an elevated passive SR Ca2+ leak in mdx fibers, using fibers voltage-clamped at −80 mV and exposed to cyclopiazonic acid. Our results have shown that Ry- or XeC-treated mdx myotubes have an increase in SR Ca2+ store content suggesting that SR leak occurs through these Ca2+ channels. SERCA1a overexpression in mdx diaphragm muscle by adeno-associated virus gene transfer resulted in a reduction of centrally located nuclei and reduced susceptibility to eccentric contraction-induced damage (61). More recently, δ-sarcoglycan-null and mdx mice animals that overexpress SERCA1 through transgenesis show an improvement in muscle damage and excitation-contraction coupling and restore the [Ca2+]rest and [Ca2+]SR in both dystrophic models (62). Together, this suggests that the filling state of the SR contributes significantly to the dysregulation [Ca2+]rest observed in mdx muscles.
Several reports indicate that resting membrane potentials are more positive in mdx muscles fibers than WT (27, 63–65). We have found that mdx myotubes showed a partial membrane depolarization compared with WT. None of the drugs used in this study, all of which have a major effect on [Ca2+]rest, induced a significant repolarization in the mdx myotubes. These are not surprising results because none of them have any effect on ion permeability or ion-translocating enzymes involved in maintaining the resting membrane potential value.
Numerous facts indicate that the dystrophic skeletal muscle cells have impaired excitation-contraction coupling. Comparisons of the cytosolic Ca2+ transients evoked by a single action potential have shown that the Ca2+ transients are reduced in mdx and mdx;utr−/− fibers compared with WT fibers (66–68). Muscle weakness observed in isolated fibers from mdx mice and DMD patients has not been fully explained. The reduction in the Ca2+ transient evoked by single action potential, increased Vm, increased [Ca2+]rest, and a reduced Ca2+ loading capacity of the SR could provide a mechanism for contractile dysfunction and impaired force production in DMD patients.
Several studies have shown that NF-κB activity is increased in mdx skeletal muscles (8–15), but the mechanisms causing this abnormality have not been previously unveiled. Acharyya et al. (10) reported an increased NF-κB DNA binding activity and IKK activation, without any change in IκBα expression and phosphorylation and normal levels of p65 with an increased phosphorylation. The authors proposed direct p65 activation by IKKβ (10). On the contrary, Singh et al. (15) have found an increase in the expression of both p65 and IκBα and increased IκBα phosphorylation, indicating that NF-κB activation in mdx muscles is due to a complex mechanism and not only IKK activation. Both examined activation of NF-κB in whole muscle extracts. Because dystrophic muscles are associated with a large amount of activated immune cell infiltrates, which have increased NF-κB activity (7, 10), it is possible that this increase was not due to changes in muscle cells. Here, we used the myotube model to determine whether NF-κB can be activated in dystrophic skeletal muscle cells without contribution from the immune system. We observed that NF-κB transcriptional activity, measured by a luciferase reporter, was increased in mdx myotubes, and we observed a significant increase in p65 nucleus/cytosol fluorescence. Both luciferase activity and p65 nuclear localization could be reduced by agents that modulate [Ca2+]rest in mdx myotubes but were not changed by these drugs in WT myotubes.
We do not know the exact mechanism that accounts for [Ca2+]rest-dependent activation of NF-κB in muscle cells. Several Ca2+-sensitive pathways can modulate the activity of NF-κB (41). We have previously shown that membrane depolarization activates NF-κB through increases in intracellular Ca2+ through RyR and IP3R. This Ca2+-dependent modulation has been attributed to calcineurin A, PKC, and ERK1/2 pathway activation in normal myotubes (40). We have not found any significant effect in the luciferase activity when we preincubated with specific blockers of these signaling pathways in WT and mdx myotubes. Similar results were obtained with Ca2+/calmodulin-dependent kinase II and JNK inhibitors. Surprisingly, p38 inhibition by SB-203580 dramatically reduced the luciferase activity of the NF-κB reporter. The p38 MAPK is activated by various stimuli, including exercise, contraction, insulin, environmental stress, and pro-inflammatory cytokines (69). SB-203580 is a specific blocker of the p38 MAPK that inhibits the catalytic activity of this protein (70).
Badger et al. (71) has shown that SB-203580 blocks IL-1-induced p38 kinase activity, NO production, and iNOS expression in chondrocytes. In addition, in C6 glioma cells, the stimulation with LPS increases iNOS mRNA expression, NO production, phosphorylation of p38, and the activation of NF-κB. Treatment with SB-203580 reduced iNOS expression and NO production; however, it did not modify the NF-κB DNA binding activity (72).
Nakamura et al. (73) have shown that calcineurin A, JNK1, and p38 signaling pathways were constantly activated in dystrophic mdx;utr−/− hearts, associated with an increased p38 phosphorylation. However, in skeletal muscle, a reduction in p38 phosphorylation has been shown but was accompanied by an increase in p38 protein expression in whole lysates from mdx tibialis anterior muscles (74). Several reports have shown that calcium activates p38 MAPK, but the mechanisms by which it does so are poorly understood. In cerebellar granular cells, glutamate stimulates the activity of p38 through Ca2+ entry from extracellular space and Rho GTPase activation (75, 76). In myotubes, caffeine increases p38 phosphorylation via Ca2+/calmodulin-dependent kinase II activation and participates in the expression of PGC-1α and mitochondrial biogenesis (77). Further studies will be required to clarify this issue in mdx skeletal muscle cells and the precise mechanism involved in the NF-κB activation.
Finally, we observed that iNOS expression could also be modulated by [Ca2+]rest through NF-κB under resting conditions. p65 knockdown normalized the iNOS protein levels in mdx myotubes to WT levels, similar to the effect of the agents that lowered [Ca2+]rest had on iNOS mRNA expression; iNOS overexpression by this mechanism could be responsible for the oxidative and nitrosative stress observed in mdx muscles (26) and can provide a positive loop for Ca2+ deregulation in dystrophic skeletal muscle cells.
Overexpression of TRPC3 (skeletal muscle-specific transgenic mice) and the associated increase in calcium influx resulted in a phenotype of muscular dystrophy (78). The authors have shown an increase in central nucleation of fibers, increased numbers of smaller myofibers, fibrosis, and infiltration of inflammatory cells. Moreover, sarco/endoplasmic reticulum Ca2+-ATPase overexpression in Sgcg−/−, mdx, and in TRPC3 transgenic mice mitigated biochemical and histological features of muscular dystrophy improving the altered intracellular Ca2+ handling (62). As described above, S-nitrosylation of RyR induces Ca2+ alterations related with an augmented spontaneous Ca2+ spark frequency (22). In addition, transient receptor potential channels elicited robust elevation of Ca2+ in response to the NO donor S-nitroso-N-acetyl-dl-penicillamine, especially TRPC5 (79). TRPC5, TRPA1, and TRPM1 channels were increased in mdx skeletal muscle at certain stages (80). These modifications induced by NO could exacerbate the pathology in mdx muscles.
We did not find any difference in cytokine expression in mdx myotubes (supplemental Fig. S3). Because macrophages and lymphocytes are specialized immune cells (infiltrated in dystrophic muscle), we think that they may be responsible for the secretion of these cytokines. This hypothesis is reinforced by IKKβ (upstream activator of NF-κB) deletion in myeloid cells from mdx mice, a procedure that reduced inflammation and concomitantly TNF-α and IL-1β expression (10). In addition, production of pro-inflammatory cytokines is probably a complex process that requires simultaneous activation of pathways other than NF-κB. iNOS promoter has two bona fide NF-κB-binding sites (reviewed in Ref. 81). TNF-α is often described as one of the classical NF-κB-dependent cytokines. However, there are numerous contradictory data for a role for NF-κB as a classic activator of TNF-α, and it seems that expression of this cytokine requires nuclear factor of activated T-cells activation, as well as other co-activators (reviewed in Ref. 82).
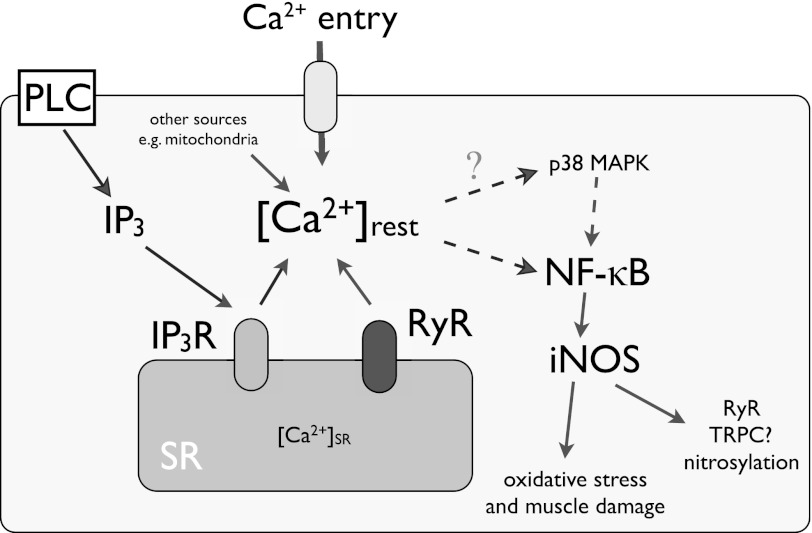
In summary, we have found that increased [Ca2+]rest is modulated by Ca2+ entry as a result of SR unloading caused by Ca2+ leak through RyR and IP3R in dystrophic myotubes and that this alteration increases NF-κB activity and iNOS expression, likely through p38 activation. These mechanisms can provide several potential therapy targets to improve muscle degeneration observed in DMD patients and explain the progressive damage observed in this pathology (Fig. 7).
FIGURE 7.
Proposed model for [Ca2+]rest deregulation, NF-κB up-regulation, and iNOS expression in mdx myotubes. In addition to the Ca2+ entry through reported TRPC1 and SOCE (Gd3+ sensitive), [Ca2+]rest deregulation in mdx myotubes is a complex event that involves Ca2+ entry and SR Ca2+ leak through RyR ad IP3R. The data collected in this work suggest that increased [Ca2+]rest, increases NF-κB and iNOS expression in dystrophic myotubes. PLC, phospholipase C.
Acknowledgments
We thank Dr. Claudio F. Perez and Dr. Karen Westerman for their help in designing our experimental procedures. We also acknowledge Dr. Peter Schupp for the gift of Ianthella basta.
This work was supported, in whole or in part, by National Institutes of Health Grants AR43140 and AR052354 (to P. D. A. and J. R. L.). This work was also supported by Grants Fondo Nacional de Investigación Cientifica y Tecnológica 1110467, Fondo de Financiamiento de Centros de Excelencia en Investigación 15010006, and Asotiation Francaise Contre les Myopathies 14562 (to E. J.), Grant AT-24100066 from Comisión Nacional de Investigación Cientifica y Tecnológica, Vicerrectoría de Asuntos Académicos, Universidad de Chile, and Programa de Mejoramiento de lo Calidad y Equidad de la Educación travel support UCH 0714, Universidad de Chile (to F. A.).

This article contains supplemental Figs. S1–S3.
- DMD
- Duchenne muscular dystrophy
- iNOS
- inducible nitric-oxide synthase
- IP3
- inositol 1,4,5-trisphosphate receptor
- Ry
- ryanodine
- RyR
- ryanodine receptor
- SR
- sarcoplasmic reticulum
- XeC
- xestospongin C
- SOCE
- store-operated calcium entry
- B5
- bastadin 5
- IKK
- IκBα kinase
- DAF-FM diacetate
- 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate.
REFERENCES
- 1. Blake D. J., Weir A., Newey S. E., Davies K. E. (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 82, 291–329 [DOI] [PubMed] [Google Scholar]
- 2. Emery A. E. (2002) The muscular dystrophies. Lancet 359, 687–695 [DOI] [PubMed] [Google Scholar]
- 3. Henry M. D., Campbell K. P. (1996) Dystroglycan. An extracellular matrix receptor linked to the cytoskeleton. Curr. Opin. Cell Biol. 8, 625–631 [DOI] [PubMed] [Google Scholar]
- 4. Pasternak C., Wong S., Elson E. L. (1995) Mechanical function of dystrophin in muscle cells. J. Cell Biol. 128, 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petrof B. J., Shrager J. B., Stedman H. H., Kelly A. M., Sweeney H. L. (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. U.S.A. 90, 3710–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans N. P., Misyak S. A., Robertson J. L., Bassaganya-Riera J., Grange R. W. (2009) Immune-mediated mechanisms potentially regulate the disease time course of Duchenne muscular dystrophy and provide targets for therapeutic intervention. PM R 1, 755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oeckinghaus A., Ghosh S. (2009) The NF-κB family of transcription factors and its regulation. Cold Spring Harbor Perspect. Biol. 1, a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siegel A. L., Bledsoe C., Lavin J., Gatti F., Berge J., Millman G., Turin E., Winders W. T., Rutter J., Palmeiri B., Carlson C. G. (2009) Treatment with inhibitors of the NF-κB pathway improves whole body tension development in the mdx mouse. Neuromuscul. Disord. 19, 131–139 [DOI] [PubMed] [Google Scholar]
- 9. Pan Y., Chen C., Shen Y., Zhu C. H., Wang G., Wang X. C., Chen H. Q., Zhu M. S. (2008) Curcumin alleviates dystrophic muscle pathology in mdx mice. Mol. Cells 25, 531–537 [PubMed] [Google Scholar]
- 10. Acharyya S., Villalta S. A., Bakkar N., Bupha-Intr T., Janssen P. M., Carathers M., Li Z. W., Beg A. A., Ghosh S., Sahenk Z., Weinstein M., Gardner K. L., Rafael-Fortney J. A., Karin M., Tidball J. G., Baldwin A. S., Guttridge D. C. (2007) Interplay of IKK/NF-κB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Invest. 117, 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang Y., Reay D. P., Salay M. N., Mi M. Y., Clemens P. R., Guttridge D. C., Robbins P. D., Huard J., Wang B. (2010) Inhibition of the IKK/NF-κB pathway by AAV gene transfer improves muscle regeneration in older mdx mice. Gene Ther. 17, 1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Messina S., Bitto A., Aguennouz M., Minutoli L., Monici M. C., Altavilla D., Squadrito F., Vita G. (2006) Nuclear factor-κB blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp. Neurol. 198, 234–241 [DOI] [PubMed] [Google Scholar]
- 13. Dogra C., Srivastava D. S., Kumar A. (2008) Protein-DNA array-based identification of transcription factor activities differentially regulated in skeletal muscle of normal and dystrophin-deficient mdx mice. Mol. Cell. Biochem. 312, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Durham W. J., Arbogast S., Gerken E., Li Y. P., Reid M. B. (2006) Progressive nuclear factor-κB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve 34, 298–303 [DOI] [PubMed] [Google Scholar]
- 15. Singh R., Millman G., Turin E., Polisiakeiwicz L., Lee B., Gatti F., Berge J., Smith E., Rutter J., Sumski C., Winders W. T., Samadi A., Carlson C. G. (2009) Increases in nuclear p65 activation in dystrophic skeletal muscle are secondary to increases in the cellular expression of p65 and are not solely produced by increases in IκB-α kinase activity. J. Neurol. Sci. 285, 159–171 [DOI] [PubMed] [Google Scholar]
- 16. Tak P. P., Firestein G. S. (2001) NF-κB. A key role in inflammatory diseases. J. Clin. Invest. 107, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hnia K., Gayraud J., Hugon G., Ramonatxo M., De La Porte S., Matecki S., Mornet D. (2008) l-Arginine decreases inflammation and modulates the nuclear factor-κB/matrix metalloproteinase cascade in mdx muscle fibers. Am. J. Pathol. 172, 1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar A., Boriek A. M. (2003) Mechanical stress activates the nuclear factor-κB pathway in skeletal muscle fibers. A possible role in Duchenne muscular dystrophy. FASEB J. 17, 386–396 [DOI] [PubMed] [Google Scholar]
- 19. Porreca E., Guglielmi M. D., Uncini A., Di Gregorio P., Angelini A., Di Febbo C., Pierdomenico S. D., Baccante G., Cuccurullo F. (1999) Hemostatic abnormalities, cardiac involvement, and serum tumor necrosis factor levels in X-linked dystrophic patients. Thromb. Haemost. 81, 543–546 [PubMed] [Google Scholar]
- 20. Porter J. D., Khanna S., Kaminski H. J., Rao J. S., Merriam A. P., Richmonds C. R., Leahy P., Li J., Guo W., Andrade F. H. (2002) A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum. Mol. Genet. 11, 263–272 [DOI] [PubMed] [Google Scholar]
- 21. Ji L. L. (2008) Modulation of skeletal muscle antioxidant defense by exercise. Role of redox signaling. Free Radic. Biol. Med. 44, 142–152 [DOI] [PubMed] [Google Scholar]
- 22. Bellinger A. M., Reiken S., Carlson C., Mongillo M., Liu X., Rothman L., Matecki S., Lacampagne A., Marks A. R. (2009) Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 15, 325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Louboutin J. P., Rouger K., Tinsley J. M., Halldorson J., Wilson J. M. (2001) iNOS expression in dystrophinopathies can be reduced by somatic gene transfer of dystrophin or utrophin. Mol. Med. 7, 355–364 [PMC free article] [PubMed] [Google Scholar]
- 24. Pacher P., Beckman J. S., Liaudet L. (2007) Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeung E. W., Whitehead N. P., Suchyna T. M., Gottlieb P. A., Sachs F., Allen D. G. (2005) Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J. Physiol. 562, 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whitehead N. P., Yeung E. W., Allen D. G. (2006) Muscle damage in mdx (dystrophic) mice. Role of calcium and reactive oxygen species. Clin. Exp. Pharmacol. Physiol. 33, 657–662 [DOI] [PubMed] [Google Scholar]
- 27. López J. R., Briceño L. E., Sánchez V., Horvart D. (1987) Myoplasmic [Ca2+] in Duchenne muscular dystrophy patients. Acta Cient. Venez. 38, 503–504 [PubMed] [Google Scholar]
- 28. Allen D. G., Gervasio O. L., Yeung E. W., Whitehead N. P. (2010) Calcium and the damage pathways in muscular dystrophy. Can. J. Physiol. Pharmacol. 88, 83–91 [DOI] [PubMed] [Google Scholar]
- 29. Turner P. R., Westwood T., Regen C. M., Steinhardt R. A. (1988) Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335, 735–738 [DOI] [PubMed] [Google Scholar]
- 30. Bakker A. J., Head S. I., Williams D. A., Stephenson D. G. (1993) Ca2+ levels in myotubes grown from the skeletal muscle of dystrophic (mdx) and normal mice. J. Physiol. 460, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gailly P., Boland B., Himpens B., Casteels R., Gillis J. M. (1993) Critical evaluation of cytosolic calcium determination in resting muscle fibers from normal and dystrophic (mdx) mice. Cell Calcium 14, 473–483 [DOI] [PubMed] [Google Scholar]
- 32. Pressmar J., Brinkmeier H., Seewald M. J., Naumann T., Rüdel R. (1994) Intracellular Ca2+ concentrations are not elevated in resting cultured muscle from Duchenne (DMD) patients and in MDX mouse muscle fibers. Pflugers Arch. 426, 499–505 [DOI] [PubMed] [Google Scholar]
- 33. Mokri B., Engel A. G. (1998) Duchenne dystrophy. Electron microscopic findings point to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology 51, 1–10 [PubMed] [Google Scholar]
- 34. Vandebrouck C., Martin D., Colson-Van Schoor M., Debaix H., Gailly P. (2002) Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J. Cell Biol. 158, 1089–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vandebrouck A., Ducret T., Basset O., Sebille S., Raymond G., Ruegg U., Gailly P., Cognard C., Constantin B. (2006) Regulation of store-operated calcium entries and mitochondrial uptake by minidystrophin expression in cultured myotubes. FASEB J. 20, 136–138 [DOI] [PubMed] [Google Scholar]
- 36. Gervásio O. L., Whitehead N. P., Yeung E. W., Phillips W. D., Allen D. G. (2008) TRPC1 binds to caveolin-3 and is regulated by Src kinase. Role in Duchenne muscular dystrophy. J. Cell Sci. 121, 2246–2255 [DOI] [PubMed] [Google Scholar]
- 37. Edwards J. N., Friedrich O., Cully T. R., von Wegner F., Murphy R. M., Launikonis B. S. (2010) Up-regulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am. J. Physiol. Cell Physiol 299, C42–C50 [DOI] [PubMed] [Google Scholar]
- 38. Boittin F. X., Petermann O., Hirn C., Mittaud P., Dorchies O. M., Roulet E., Ruegg U. T. (2006) Ca2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J. Cell Sci. 119, 3733–3742 [DOI] [PubMed] [Google Scholar]
- 39. Riquelme D., Alvarez A., Leal N., Adasme T., Espinoza I., Valdés J. A., Troncoso N., Hartel S., Hidalgo J., Hidalgo C., Carrasco M. A. (2011) High frequency field stimulation of primary neurons enhances ryanodine receptor-mediated Ca2+ release and generates hydrogen peroxide, which jointly stimulate NF-κB activity. Antioxid. Redox. Signal. 14, 1245–1259 [DOI] [PubMed] [Google Scholar]
- 40. Valdés J. A., Hidalgo J., Galaz J. L., Puentes N., Silva M., Jaimovich E., Carrasco M. A. (2007) NF-κB activation by depolarization of skeletal muscle cells depends on ryanodine and IP3 receptor-mediated calcium signals. Am. J. Physiol. Cell Physiol. 292, C1960–C1970 [DOI] [PubMed] [Google Scholar]
- 41. Lilienbaum A., Israël A. (2003) From calcium to NF-κB signaling pathways in neurons. Mol. Cell. Biol. 23, 2680–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sée V., Rajala N. K., Spiller D. G., White M. R. (2004) Calcium-dependent regulation of the cell cycle via a novel MAPK-NF-κB pathway in Swiss 3T3 cells. J. Cell Biol. 166, 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spencer M. J., Croall D. E., Tidball J. G. (1995) Calpains are activated in necrotic fibers from mdx dystrophic mice. J. Biol. Chem. 270, 10909–10914 [DOI] [PubMed] [Google Scholar]
- 44. Millay D. P., Sargent M. A., Osinska H., Baines C. P., Barton E. R., Vuagniaux G., Sweeney H. L., Robbins J., Molkentin J. D. (2008) Genetic and pharmacologic inhibition of mitochondria dependent necrosis attenuates muscular dystrophy. Nat. Med. 14, 442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rando T. A., Blau H. M. (1994) Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Casas M., Altamirano F., Jaimovich E. (2012) Measurement of calcium release due to inositol trisphosphate receptors in skeletal muscle. Methods Mol. Biol. 798, 383–393 [DOI] [PubMed] [Google Scholar]
- 47. Eltit J. M., Yang T., Li H., Molinski T. F., Pessah I. N., Allen P. D., Lopez J. R. (2010) RyR1-mediated Ca2+ leak and Ca2+ entry determine resting intracellular Ca2+ in skeletal myotubes. J. Biol. Chem. 285, 13781–13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Westerman K. A., Penvose A., Yang Z., Allen P. D., Vacanti C. A. (2010) Adult muscle “stem” cells can be sustained in culture as free-floating myospheres. Exp. Cell Res. 316, 1966–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2(−ΔΔCT) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 50. Marshall O. J. (2004) PerlPrimer. Cross-platform, graphical primer design for standard, bisulfite, and real time PCR. Bioinformatics 20, 2471–2472 [DOI] [PubMed] [Google Scholar]
- 51. Mack M. M., Molinski T. F., Buck E. D., Pessah I. N. (1994) Novel modulators of skeletal muscle FKBP12/calcium channel complex from Ianthella basta. Role of FKBP12 in channel gating. J. Biol. Chem. 269, 23236–23249 [PubMed] [Google Scholar]
- 52. Liberona J. L., Powell J. A., Shenoi S., Petherbridge L., Caviedes R., Jaimovich E. (1998) Differences in both inositol 1,4,5-trisphosphate mass and inositol 1,4,5-trisphosphate receptors between normal and dystrophic skeletal muscle cell lines. Muscle Nerve 21, 902–909 [DOI] [PubMed] [Google Scholar]
- 53. Casas M., Figueroa R., Jorquera G., Escobar M., Molgó J., Jaimovich E. (2010) IP3-dependent post-tetanic calcium transients induced by electrostimulation of adult skeletal muscle fibers. J. Gen. Physiol. 136, 455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jaimovich E., Mattei C., Liberona J. L., Cardenas C., Estrada M., Barbier J., Debitus C., Laurent D., Molgó J. (2005) Xestospongin B, a competitive inhibitor of IP3-mediated Ca2+ signaling in cultured rat myotubes, isolated myonuclei, and neuroblastoma (NG108-15) cells. FEBS Lett. 579, 2051–2057 [DOI] [PubMed] [Google Scholar]
- 55. Ho R. C., Hirshman M. F., Li Y., Cai D., Farmer J. R., Aschenbach W. G., Witczak C. A., Shoelson S. E., Goodyear L. J. (2005) Regulation of IκB kinase and NF-κB in contracting adult rat skeletal muscle. Am. J. Physiol. Cell Physiol. 289, C794–C801 [DOI] [PubMed] [Google Scholar]
- 56. Várnai P., Hunyady L., Balla T. (2009) STIM and Orai. The long-awaited constituents of store-operated calcium entry. Trends Pharmacol. Sci. 30, 118–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Robert V., Massimino M. L., Tosello V., Marsault R., Cantini M., Sorrentino V., Pozzan T. (2001) Alteration in calcium handling at the subcellular level in mdx myotubes. J. Biol. Chem. 276, 4647–4651 [DOI] [PubMed] [Google Scholar]
- 58. Culligan K., Banville N., Dowling P., Ohlendieck K. (2002) Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle. J. Appl. Physiol. 92, 435–445 [DOI] [PubMed] [Google Scholar]
- 59. Kargacin M. E., Kargacin G. J. (1996) The sarcoplasmic reticulum calcium pump is functionally altered in dystrophic muscle. Biochim. Biophys. Acta 1290, 4–8 [DOI] [PubMed] [Google Scholar]
- 60. Robin G., Berthier C., Allard B. (2012) Sarcoplasmic reticulum Ca2+ permeation explored from the lumen side in mdx muscle fibers under voltage control. J. Gen. Physiol. 139, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morine K. J., Sleeper M. M., Barton E. R., Sweeney H. L. (2010) Overexpression of SERCA1a in the mdx diaphragm reduces susceptibility to contraction-induced damage. Hum. Gene Ther. 21, 1735–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goonasekera S. A., Lam C. K., Millay D. P., Sargent M. A., Hajjar R. J., Kranias E. G., Molkentin J. D. (2011) Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J. Clin. Invest. 121, 1044–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carlson C. G., Samadi A., Siegel A. (2005) Chronic treatment with agents that stabilize cytosolic IκB-α enhances survival and improves resting membrane potential in MDX muscle fibers subjected to chronic passive stretch. Neurobiol. Dis. 20, 719–730 [DOI] [PubMed] [Google Scholar]
- 64. Miles M. T., Cottey E., Cottey A., Stefanski C., Carlson C. G. (2011) Reduced resting potentials in dystrophic (mdx) muscle fibers are secondary to NF-κB-dependent negative modulation of ouabain-sensitive Na+-K+ pump activity. J. Neurol. Sci. 303, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Carlson C. G., Roshek D. M. (2001) Adult dystrophic (mdx) end plates exhibit reduced quantal size and enhanced quantal variation. Pflugers Arch. 442, 369–375 [DOI] [PubMed] [Google Scholar]
- 66. Woods C. E., Novo D., DiFranco M., Vergara J. L. (2004) The action potential evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibers. J. Physiol. 557, 59–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hollingworth S., Zeiger U., Baylor S. M. (2008) Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibers of mdx and normal mice. J. Physiol. 586, 5063–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Capote J., DiFranco M., Vergara J. L. (2010) Excitation-contraction coupling alterations in mdx and utrophin/dystrophin double knockout mice. A comparative study. Am. J. Physiol. Cell Physiol. 298, C1077–C1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Röckl K. S., Witczak C. A., Goodyear L. J. (2008) Signaling mechanisms in skeletal muscle. Acute responses and chronic adaptations to exercise. IUBMB Life 60, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kumar S., Jiang M. S., Adams J. L., Lee J. C. (1999) Pyridinylimidazole compound SB-203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 263, 825–831 [DOI] [PubMed] [Google Scholar]
- 71. Badger A. M., Cook M. N., Lark M. W., Newman-Tarr T. M., Swift B. A., Nelson A. H., Barone F. C., Kumar S. (1998) SB-203580 inhibits p38 mitogen-activated protein kinase, nitric oxide production, and inducible nitric oxide synthase in bovine cartilage-derived chondrocytes. J. Immunol. 161, 467–473 [PubMed] [Google Scholar]
- 72. Won J. S., Lee J. K., Suh H. W. (2001) Forskolin inhibits expression of inducible nitric-oxide synthase mRNA via inhibiting the mitogen-activated protein kinase in C6 cells. Brain Res. Mol. Brain Res. 89, 1–10 [DOI] [PubMed] [Google Scholar]
- 73. Nakamura A., Harrod G. V., Davies K. E. (2001) Activation of calcineurin and stress-activated protein kinase/p38-mitogen-activated protein kinase in hearts of utrophin-dystrophin knockout mice. Neuromuscul. Disord. 11, 251–259 [DOI] [PubMed] [Google Scholar]
- 74. Ljubicic V., Khogali S., Renaud J. M., Jasmin B. J. (2012) Chronic AMPK stimulation attenuates adaptive signaling in dystrophic skeletal muscle. Am. J. Physiol. Cell Physiol. 302, C110–C121 [DOI] [PubMed] [Google Scholar]
- 75. Kawasaki H., Morooka T., Shimohama S., Kimura J., Hirano T., Gotoh Y., Nishida E. (1997) Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J. Biol. Chem. 272, 18518–18521 [DOI] [PubMed] [Google Scholar]
- 76. Semenova M. M., Mäki-Hokkonen A. M., Cao J., Komarovski V., Forsberg K. M., Koistinaho M., Coffey E. T., Courtney M. J. (2007) Rho mediates calcium-dependent activation of p38α and subsequent excitotoxic cell death. Nat. Neurosci. 10, 436–443 [DOI] [PubMed] [Google Scholar]
- 77. Wright D. C., Geiger P. C., Han D. H., Jones T. E., Holloszy J. O. (2007) Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J. Biol. Chem. 282, 18793–18799 [DOI] [PubMed] [Google Scholar]
- 78. Millay D. P., Goonasekera S. A., Sargent M. A., Maillet M., Aronow B. J., Molkentin J. D. (2009) Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 106, 19023–19028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yoshida T., Inoue R., Morii T., Takahashi N., Yamamoto S., Hara Y., Tominaga M., Shimizu S., Sato Y., Mori Y. (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2, 596–607 [DOI] [PubMed] [Google Scholar]
- 80. Krüger J., Kunert-Keil C., Bisping F., Brinkmeier H. (2008) Transient receptor potential cation channels in normal and dystrophic mdx muscle. Neuromuscul. Disord. 18, 501–513 [DOI] [PubMed] [Google Scholar]
- 81. Kleinert H., Schwarz P. M., Förstermann U. (2003) Regulation of the expression of inducible nitric-oxide synthase. Biol. Chem. 384, 1343–1364 [DOI] [PubMed] [Google Scholar]
- 82. Falvo J. V., Tsytsykova A. V., Goldfeld A. E. (2010) Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 11, 27–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Buvinic S., Almarza G., Bustamante M., Casas M., López J., Riquelme M., Sáez J. C., Huidobro-Toro J. P., Jaimovich E. (2009) ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 284, 34490–34505 [DOI] [PMC free article] [PubMed] [Google Scholar]