Background: The mechanism of melanopsin chromophore regeneration is poorly understood.
Results: In situ melanopsin was partially bleached by high-intensity UV light or hydroxylamine and could be restored by cis-retinal only.
Conclusion: Melanopsin resistance to light and UV bleaching is consistent with a bistable mechanism of chromophore regeneration.
Significance: Further evidence suggests that melanopsin behaves as a bistable pigment in situ.
Keywords: 7-Helix Receptor, Circadian Rhythms, Phototransduction, Physiology, Vision, Melanopsin
Abstract
Melanopsin is the photopigment of mammalian intrinsically photosensitive retinal ganglion cells, where it contributes to light entrainment of circadian rhythms, and to the pupillary light response. Previous work has shown that the melanopsin photocycle is independent of that used by rhodopsin (Tu, D. C., Owens, L. A., Anderson, L., Golczak, M., Doyle, S. E., McCall, M., Menaker, M., Palczewski, K., and Van Gelder, R. N. (2006) Inner retinal photoreception independent of the visual retinoid cycle. Proc. Natl. Acad. Sci. U.S.A. 103, 10426–10431). Here we determined the ability of apo-melanopsin, formed by ex vivo UV light bleaching, to use selected chromophores. We found that 9-cis-retinal, but not all-trans-retinal or 9-cis-retinol, is able to restore light-dependent ipRGC activity after bleaching. Melanopsin was highly resistant to both visible-spectrum photic bleaching and chemical bleaching with hydroxylamine under conditions that fully bleach rod and cone photoreceptor cells. These results suggest that the melanopsin photocycle can function independently of both rod and cone photocycles, and that apo-melanopsin has a strong preference for binding cis-retinal to generate functional pigment. The data support a model in which retinal is continuously covalently bound to melanopsin and may function through a reversible, bistable mechanism.
Introduction
In addition to rods and cones, mammalian retinas contain a population of intrinsically photosensitive retinal ganglion cells (ipRGCs)3 (2–4). These photoreceptive cells are found in a small subpopulation of ganglion cell neurons that primarily project to areas of the brain involved in circadian rhythm orchestration and pupillary light responses (5). Unlike rods and cones, which employ rhodopsin and cone pigments for phototransduction, ipRGCs use melanopsin (6–8). All opsins utilize cis-to-trans isomerization of the retinaldehyde chromophore as the primary photoreceptive event and must regenerate the cis chromophore to regain photosensitivity. Although photocycles for chromophore regeneration of rhodopsin and, to a lesser extent, cone opsins are well understood (9), the mechanisms of melanopsin pigment regeneration are less well studied (10–12). Previous work demonstrated that melanopsin can function independently of the retinal pigmented epithelium-based photocycle that forms the basis for all rod and most cone pigment regeneration (1). The bleaching characteristics of melanopsin in situ have not been systemically studied to date.
EXPERIMENTAL PROCEDURES
Tissue Preparation
C57Bl/6 mice (The Jackson Laboratories, Bar Harbor, ME) were used as the wild-type strain. Melanopsin knockout mice (Opn4−/−) were bred in a mixed 129/C57/Bl6 background (13). For analysis of ipRGC responses, multielectrode array recordings were obtained from wild-type animals at ages P8-P10 or P13–16 (7). Opn4−/− P13–16 mice were used to record rod- and cone-driven responses. Mice were maintained in a 12:12-h light-dark cycle. All experiments were performed in accordance with Association for Research in Vision and Ophthalmology guidelines for animal studies under an approved animal study protocol at the University of Washington.
All procedures were performed under dim red light illumination. Mice were euthanized by CO2 narcosis and cervical dislocation. Isolated mouse retinas were cut into squares and positioned with the vitreal face in contact with a multielectrode array (MEA) (Multi Channel Systems, Reutlingen, Germany) and superperfused (2 ml/min) with a bicarbonate-buffered physiologic solution (125 mm NaCl, 2.5 mm KCl, 1 mm MgCl2, 1.25 mm NaH2PO4, 20 mm glucose, 26 mm NaHCO3, 2 mm CaCl2, 500 mm glutamine) oxygenated with 95% O2/5% CO2 to obtain a pH of 7.4. The temperature of both the perfusate and the tissue chamber was maintained at 33.0 °C. For ipRGC recordings from wild-type P8–10 mouse retinas, spontaneous retinal waves (14) (as well as any input from rod and cone photoreceptors) were suppressed by using glutamatergic (50 mm d(2)-2-amino-5-phosphonopentanoic acid, 20 mm d(−)-2-amino-4-phosphonobutyric acid, 10 mm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and cholinergic (5 nm epibatidine) inhibitors (Tocris Biosciences, Ellisville, MO). For ipRGC recordings from wild-type P13–16 mouse retinas, only glutamatergic blockade was used (100 mm d(2)-2-amino-5-phosphonopentanoic acid, 50 mm d(−)-2-amino-4-phosphonobutyric acid, 40 mm CNQX). Signals from Opn4−/− mouse retinas were recorded without pharmacological intervention.
Multielectrode Recordings
Electrophysiologic recordings were performed using planar arrays of 60 electrodes (30 μm diameter, 200 μm interelectrode spacing, Multi Channel Systems). Raw electrical signals were amplified, filtered, and digitized through an analog-to-digital card (National Instruments, Austin, TX) written to disk, and analyzed off-line, as described previously (15).
Retinas were stimulated with a xenon light source (Sutter Instruments, Novato, CA) fed through a liquid light guide and diffusing filter (Thorlabs Inc., Newton, NJ). Intensities and wavelengths of light were adjusted via neutral density and narrow band-pass 480-nm interference filters (full width, half maximal, 10 nm),(Thorlabs, Inc., Newton, NJ) and calibrated with a radiometer (Advanced Photonics International, Fairfield, CT). Light stimuli were delivered and monitored by a computer-controlled shutter (Vincent Associates, Rochester, NY). UV light was provided by an Optimax 365 UV flashlight (Spectronics Corp.) mounted 10 cm above the tissue. The Optimax contains a single high-output UV light-emitting diode with a narrow spectrum centered at 365 nm. At 10 cm, the device delivered 9.19 × 1015 photons/cm/s (IR 16) as measured by radiometry.
Retinoids
Both 9-cis-retinal and all-trans-retinal were purchased from Sigma. Retinals were dissolved in acetonitrile, aliquoted, and dried under argon. 9-cis-retinol was generated by reduction of 9-cis-retinal (16). Briefly, 2 mg of 9-cis-retinal was suspended in acetonitrile and incubated with 20 mg sodium borohydride for 15 min. Reaction products were extracted and analyzed by UV spectrophotometry and HPLC and dried under argon. All retinoids were resuspended in ethanol for determination of concentration and experimental use.
UV Retinal Depletion and Retinoid Treatment
Irradiance response curves were determined for each wild-type P8–10 mouse retina by using 480-nm light at IR 13.6, 12.6, and 11.6 (4.0 × 1013, 4.0 × 1012, and 4.0 × 1011 photons/cm/s, respectively). Retinas were exposed to 365-nm UV light for 15 min, allowed to recover for 10 min, and then tested regularly at IR 13.6 over 2 h post-UV light treatment. Empirically, we found that retinas retaining more than 2% of pre-UV treatment light activity had a high probability of spontaneous recovery of more than 50% of baseline activity over the course of 2 h. Retinas with responses recorded immediately after irradiation that were not at or below 2% of pre-UV treatment were excluded from analysis. All retinas were assayed for KCl-induced activity at the end of each experiment. Retinas with no KCl-inducible activity were assumed to have died and were also excluded from analysis. Approximately 33% of treated retinas met both criteria and were utilized in these experiments. Retinas used for retinoid replenishing experiments were treated in the same manner up to the 10-min post-UV light test. Thereafter, retinas were superperfused for 30 min with a mixture of the test retinoid in a non-carbogenated HEPES/bicarbonate-buffered physiologic solution (10 mm HEPES (pH 7.4), 125 mm NaCl, 2.5 mm KCl, 1 mm MgCl2, 1.25 mm NaH2PO4, 12 mm glucose, 26 mm NaHCO3, 2 mm CaCl2, 500 mm glutamine) with 1% ethanol as a carrier mixed with carbogen-bubbled bicarbonate-buffered physiologic solution at a 1:1 ratio just prior to tissue delivery. Retinas were returned to the bicarbonate-buffered physiologic solution and tested for light-evoked activity (480-nm light at IR 13.6) 60 min and 90 min post-UV light treatment.
HPLC Analysis of Retinoids
To validate the UV depletion protocol, eight whole retinas from P8–10 wild-type mice were treated with UV light and snap-frozen on dry ice. Whole control retinas from the other eye of the same animals were removed and immediately frozen on dry ice. Samples were suspended in 1 ml of 12 mm phosphate buffer, 137 mm NaCl, 2.7 mm KCl, 20 mm NH2OH, and 50% methanol (v/v) and homogenized in a glass-glass Dounce homogenizer. After 20 min of incubation at room temperature, homogenates were extracted with 4 ml of hexane. Organic phases were collected, dried down by vacuum centrifugation, and redissolved in 0.2 ml of hexane. Retinoids were separated on a normal phase HPLC column (5-μm ZORBAX SIL, 4.6 × 250 mm, Agilent Technology) by a step gradient of ethyl acetate in hexane (0.5% for 15 min and 6% from 15 to 60 min at a 1.4 ml/min flow rate). Retinoids were detected at 325 and 360 nm by using a diode array detector and identified on the basis of their elution times and characteristic UV/Vis spectra. Retinylaldehydes and retinol were quantified on the basis of standard curves that correlated integrated peak areas calculated from chromatograms with known amounts of synthetic standards injected onto the column.
Hydroxylamine Treatments
Hydroxylamine, obtained from Sigma, was dissolved in a bicarbonate-buffered physiologic solution and neutralized with Tris base to pH 7.4. Retinas were superperfused with neutralized hydroxylamine bubbled with carbogen with or without 480-nm light exposure (IR 13.6). Concentrations for hydroxylamine-only treatments were 10 mm and 30 mm, whereas those for hydroxylamine-light cotreatment were 10 mm, 30 mm, and 60 mm. Retinas were returned to bicarbonate-buffered physiologic solution and tested with IR 13.6 at 10 and 60 min post-hydroxylamine treatment.
RESULTS
Melanopsin Is Resistant to Long-term Bleaching by Visible Light
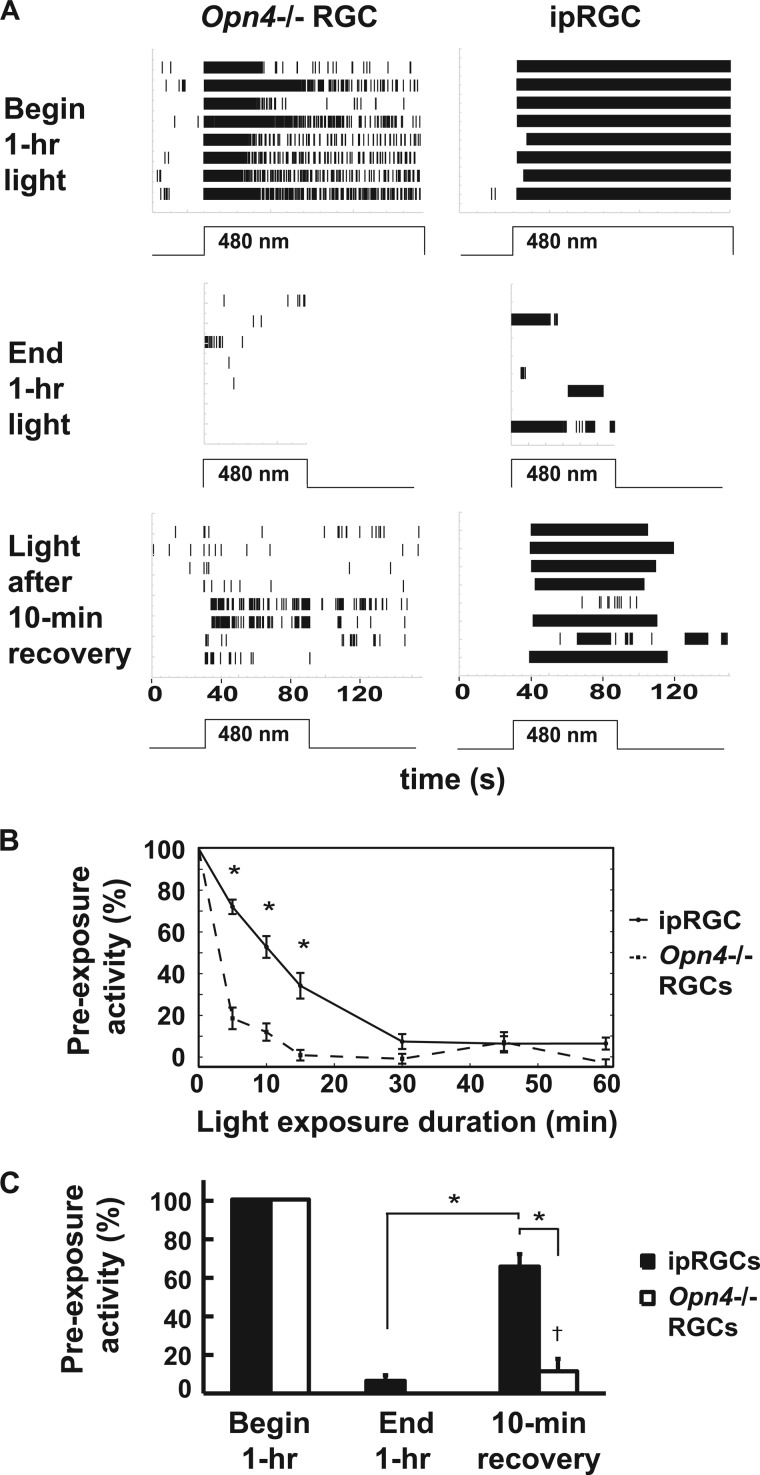
To determine whether melanopsin can be photobleached by visible light in situ, we exposed retinas from P8–10 wild-type mice, under glutamatergic and cholinergic blockade, to saturating light (480 nm, IR 13.6) for 1 h. Retinal ganglion cell activity was measured by multielectrode array recording (7). As rod and cone signaling is not established by P10, only melanopsin-dependent ipRGC cell firing is observed under these conditions (7). During dissection, the retinal pigmented epithelium remains firmly attached to the sclera and is therefore absent from the retinal preparation. As controls, retinas from slightly older P13–16 melanopsin-deficient mice (Opn4−/−), which demonstrate only rod- and cone-dependent retinal ganglion cell firing, were exposed to the identical lighting regimen. Following the 1-hour light exposure, light-dependent ipRGC firing activity was still present but had decreased to 7% of its initial activity (Figs. 1, A and B). In the Opn4−/− retina, all light-dependent ganglion cell firing activity was abolished at the end of the 1-hour light exposure. However, the difference in sensitivity between the two was not statistically significant. This diminution in ganglion cell activity could represent either bleaching of the chromophore, adaptation of ganglion cell firing, or both. To distinguish these possibilities, we analyzed the sensitivity of ipRGCs and rod- and cone-driven RGCs. Following a 10-min dark recovery period, a marked and statistically significant recovery (p < 0.05, repeated measures one-way ANOVA, Bonferroni-corrected), of 65% of baseline light-dependent ipRGC activity was restored in P8–10 wild-type retinas, whereas minimal activity not statistically different from zero (one-sample Student's t test) was detected in rod- and cone-driven ganglion cells of the Opn4−/− retina (Fig. 1B). This result demonstrates that the melanopsin chromophore is substantially, spontaneously regenerated under conditions that largely bleached the rod and cone chromophore for extended periods. This is consistent with the finding that ipRGCs function independently from the RPE (1), as no RPE is present in the experimental preparation. Interestingly, when rods and cones are bleached by 99.9% in intact animals, the time for recovery of sensitivity is comparable with what we observe for ipRGCs (17, 18).
FIGURE 1.
ipRGC activity is resistant to light bleaching. A, raster plots of light-induced firing activity in 8 Opn4−/− RGCs from two retinas and eight wild-type ipRGCs from one retina at the beginning and end of a continuous 1-h, 480-nm light exposure (IR 13.6) followed by a 10-min dark recovery period and a 1-min light stimulus. B, percent light-induced activity (defined as percent initial activity) from Opn4−/− RGCs (n = 8 cells from 2 retinas) and ipRGCs (n = 16 cells from 2 retinas) during 1-min recording periods taken at various times over the course of the same continuous 1-h, 480-nm light exposure (IR 13.6) as in A. C, firing activity of Opn4−/− RGCs (n = 8 cells from 2 retinas) and ipRGCs (n = 16 cells from 2 retinas) from the time points in A. *, p < 0.05, statistically significant difference in firing rate between ipRGCs and Opn4−/− RGCs (repeated measures one-way ANOVA, Bonferroni-corrected); †, difference from zero not statistically significant (one-sample Student's t test). Error bars are mean ± S.E.
Melanopsin Is Bleached for Extended Periods by Intense 365-nm UV Light
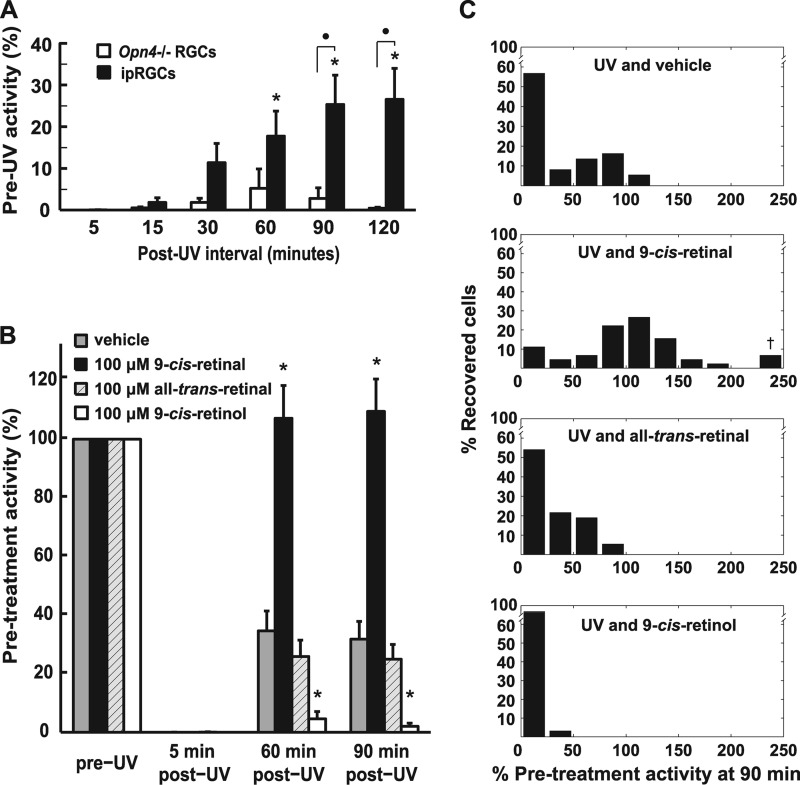
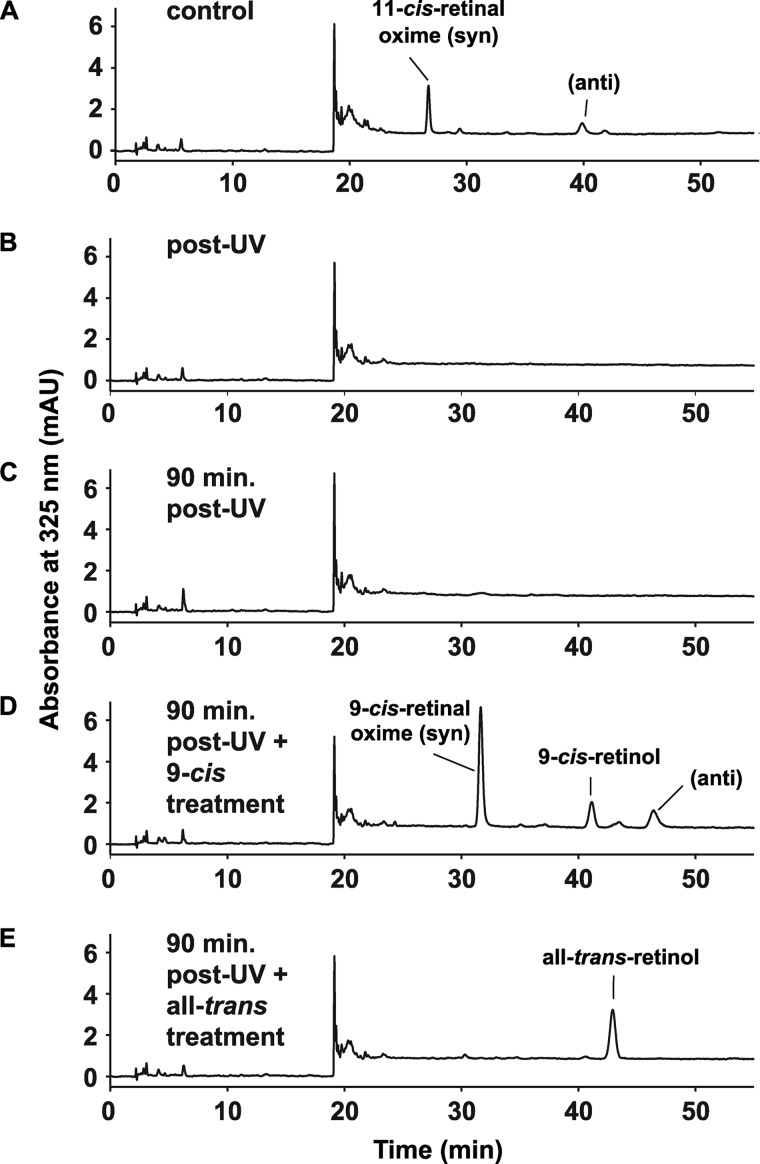
As bright visible light did not fully bleach melanopsin, we used intense UV light at 365 nm instead to degrade endogenous retinoids (19). Treatment of retinas with 15 min of UV light reduced light-dependent ipRGC firing to < 2% of maximal pre-UV light-evoked spiking 10 min after exposure in approximately one-third of retinas. This was followed by recovery from < 2% to a statistically significant stable sensitivity level of ∼25% maximal pre-UV light-induced activity over the course of 1 h in the dark (p < 0.05, repeated measures one-way ANOVA, Bonferroni-corrected). Non-ipRGC activity from Opn4−/− retinas exposed to the same UV treatment did not show any significant recovery (Fig. 2A). HPLC analysis of retinoid composition extracted from eight whole, untreated retinas revealed the presence of 80 pmols of 11-cis-retinal per retina (∼5 pmol retinoids per mg of protein), whereas immediately after UV treatment, retinoid levels were below the HPLC-detectible limit of ∼2 pmols per retina (Figs. 3, A and B), demonstrating a more than 5-fold reduction in retinoid content of the treated retina. Retinoids could not be detected at the 90-min recovery time point (Fig. 3C).
FIGURE 2.
9-cis-retinal restores ipRGC activity after UV depletion. A, retinas were treated for 15-min with UV to deplete endogenous retinoids. Rod- and cone-driven RGC (Opn4−/−, n = 10 cells from 4 retinas) and ipRGC (wild-type, n = 23 cells from 4 retinas) activity in response to a periodic 1-min, 480-nm test stimulus (IR 13.6) was monitored for 2 h following UV treatment. *, p < 0.05, statistically significant difference in firing rate between ipRGCs and Opn4−/− RGCs; ●, ipRGC firing rate increase from zero statistically significant (repeated measures one-way ANOVA, Bonferroni-corrected). B, ipRGC activity is restored after 15-min of UV treatment by the addition of 100 μm 9-cis-retinal (n = 45 cells from 5 retinas). No activity exceeding that of vehicle control (n = 37 cells from 5 retinas) is seen with the addition of 100 μm all-trans-retinal (n = 37 cells from 6 retinas) or 100 μm 9-cis-retinol (n = 32 cells from 4 retinas). *, p < 0.05, 9-cis-retinal and 9-cis-retinol treatments resulted in a statistically significant difference from vehicle control and all other retinoid treatments (two-way ANOVA, Bonferroni-corrected). C, normalized histograms of ipRGC activity at the 90-min time point from B demonstrate that only 9-cis-retinal increases the activity of most cells above vehicle control. The y axis is the percentage of treated cells responding at the percent of their pre-UV light-induced activity level; †, three cells with activity of 234%, 330%, and 403%, respectively. Error bars are mean ± S.E.
FIGURE 3.
UV treatment decreases endogenous retinoids to below HPLC-detectable levels, whereas exogenously added retinoids are confirmed to penetrate tissue. A, one retina was taken from each of eight P8-P10 animals, frozen, and later assayed by HPLC for endogenous retinoids levels. B, the contralateral retina from each animal in A was exposed to 15 min of UV light, frozen, and assayed by HPLC. No retinoids were detected after UV treatment. The remaining chromatograms show retinoid levels from retinas taken after UV exposure and subsequent treatment with vehicle (n = 3) (C), 100 μm 9-cis-retinal (n = 4) (D), and 100 μm all-trans-retinal (n = 7) (E).
UV-bleached Melanopsin Reconstitutes with 9-cis-retinal but Not All-trans-retinal
To test the in situ preference of bleached melanopsin for cis-retinal versus all-trans-retinal, we UV-bleached retinas using the method described above, followed by treatment with cis or trans-retinaldehyde. Treatment of UV light-exposed retinas with 100 μm 9-cis-retinal reconstituted retinal light-induced spiking activity to 110% of pre-UV levels, well above the vehicle control (Fig. 2B) (p < 0.05, two-way ANOVA, Bonferroni-corrected), whereas 20 μm 9-cis-retinal failed to reconstitute light-dependent signaling over levels observed with the vehicle control (supplemental Fig. S1A). Treatment with 100 μm all-trans-retinal failed to increase activity above control levels (Fig. 2B), as did both higher (200 μm) and lower (20 μm) concentrations (supplemental Fig. S1B). Indeed, 200 μm all-trans-retinal treatment decreased activity levels to well below the vehicle control. This is most likely caused by toxic effects of all-trans-retinal. Evidence for toxic effects such oxidative phosphorylation decoupling has been documented with similarly high retinoid concentrations in cell culture (20). Exposure of all-trans-retinal-supplemented retinas to high-intensity white light (to facilitate possible photoconversion of trans- to cis-retinal) also failed to reconstitute subsequent photosensitivity above levels achieved by vehicle alone (data not shown).
Fig. 2C shows the distribution of rescued individual cell firing activity in response to light 90-min post-UV light exposure following treatment with vehicle and 100 μm retinoids. These histograms demonstrate that only 9-cis-retinal shifted the distribution of cell responses away from the depleted post-UV state (seen with vehicle treatment) back toward the baseline “pre-UV” distribution centered near a 100% response. Interestingly, a few cells in the 9-cis-retinal treatment had a dramatically increased response to light compared with prebleach levels, as was noted in a patch clamp study of ipRGC by Do et al. (21).
HPLC analysis of retinoid-treated retinas showed adequate penetration of exogenously applied retinoids (Fig. 3, D and E) reaching or exceeding pretreatment levels at ∼44 pmol/mg/retina (n = 4 retinas) for 9-cis-retinal treatment and ∼10 pmol retinoid/mg/retina for all-trans-retinal (n = 7 retinas). Binding with 200 μm all-trans-retinal treatment was ∼6 pmol/mg (n = 3 retinas). No retinoids were detected after the 20 μm cis- or trans-retinoid treatments.
ipRGCs Are Unable to Utilize 9-cis-retinol in Their Photoresponse
A second photocycle present in the retina is found in Müller glial cells, which produce 11-cis-retinol. Cones, but not rods, can use the cis-retinol product of this photocycle (22). To test if ipRGCs can utilize this photocycle, UV-bleached retinas were treated with several concentrations of 9-cis-retinol. Photosensitivity was not reconstituted following 100 μm or several lower concentrations of this retinoid (Fig. 2B and supplemental Fig. S1C). Moreover, 100 μm 9-cis-retinol treatment also appeared to decrease significantly the ability of cells to respond to light below that of cells treated with vehicle only (Fig. 2C), whereas lower concentrations of 9-cis-retinol lessened this effect (Fig. S1C). In contrast to cis- and trans-retinaldehyde, uptake of 9-cis-retinol was not detected by HPLC analysis (data not shown).
Melanopsin in Situ Resists Hydroxylamine Bleach
Hydroxylamine is used to chemically bleach opsins (23). This small molecule interferes with formation of the protonated Schiff base between retinaldehyde and the lysine side chain of the opsin-binding site by substituting for the lysine side chain. The products of this reaction are apo-opsin and retinyloxime. Cone opsins are bleached by exposure to hydroxylamine in the dark, presumably because the chromophore is exposed to the external milieu in the opsin ground state (23, 24). In the case of rhodopsin in rods, light is required for activation to metarhodopsin II before hydroxylamine accesses and attacks the Schiff base, as 11-cis-retinal is protected from the local environment by the rhodopsin molecule ground state (25–27). Hydroxylamine can also prevent regeneration of the opsin-retinal molecule by sequestering cis-retinal and all-trans-retinal as retinal oximes after light bleaching (28). Compared with rod and cone opsins, bistable pigments of invertebrate rhabdomeric photoreceptors are highly resistant to hydroxylamine bleaching, even after light exposure (29).
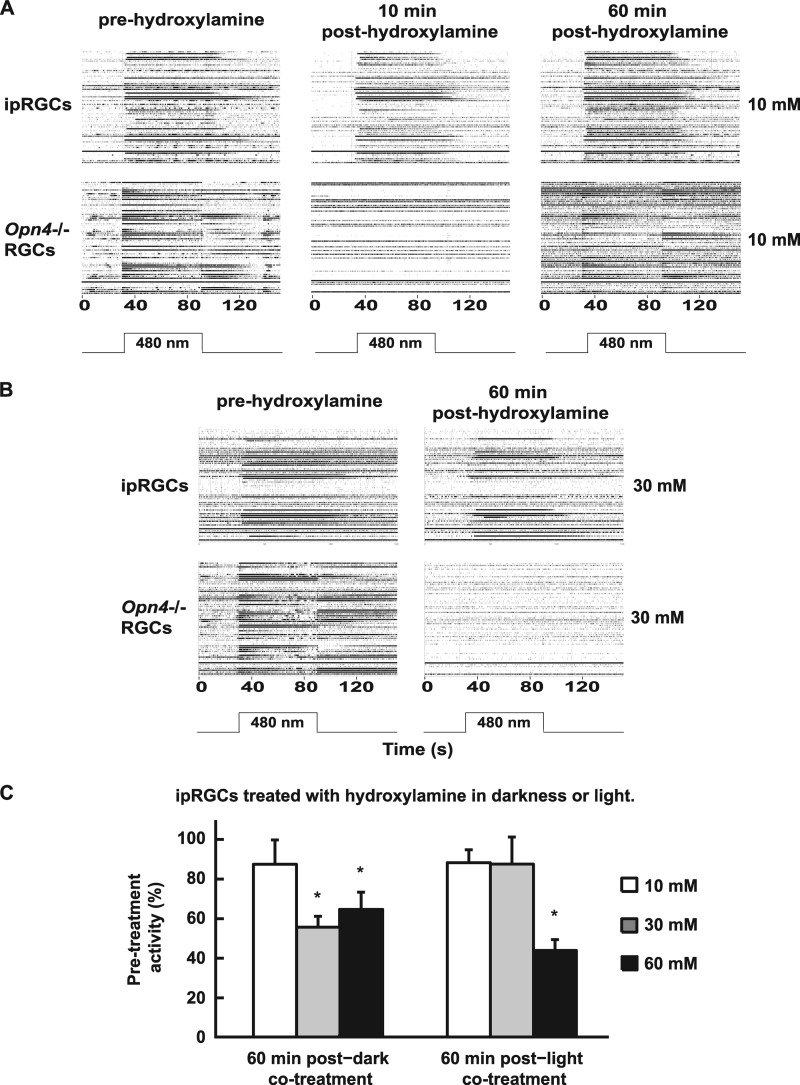
To test whether melanopsin is susceptible to hydroxylamine bleaching in the dark, wild-type P13–16 retinas under glutamatergic blockade (i.e. ipRGCs only) were treated for 15 min with hydroxylamine concentrations used previously to bleach cones (30, 31) without light exposure. For direct comparison with cone opsin-based activity, P13–16 Opn4−/− retinas (i.e. with RGC firing from rods and cones only) were treated identically but without glutamatergic blockade. Following 10 mm hydroxylamine treatment, non-ipRGC spiking activity in Opn4−/− retinas was transiently abolished at 10 min post-treatment but recovered by 60 min, whereas ipRGC activity was unaltered at both time points (Fig. 4A). With 30 mm hydroxylamine treatment, activity in Opn4−/− retinas was abolished at 60 min, whereas ipRGC activity in wild-type retinas was reduced only to ∼50% of pretreatment levels (Fig. 4B).
FIGURE 4.
ipRGCs are resistant to hydroxylamine bleaching. RGC firing raster plots of retinas (one retina per row) from P13–16 wild-type mice under glutamatergic blockade to record ipRGCs and Opn4−/− mice not under blockade to record rod- and cone-driven RGCs treated for 15 min with hydroxylamine in the perfusate at 10 mm (A) or 30 mm (B) and then tested with a 1-min, 480-nm light stimulus (IR 13.6) 60 min post-treatment. ipRGCs were resistant to hydroxylamine at concentrations that bleach cones. C, cells from P8–10 wild-type mouse retinas treated with 10 mm, 30 mm, and 60 mm hydroxylamine in either darkness or 480 nm light (IR 13.6) were resistant to hydroxylamine bleaching under conditions that bleach rods and cones. Cell numbers for the treatments were 36 cells from four retinas for 10 mm/dark, 33 cells from three retinas for 10 mm/light, 35 cells from four retinas for 30 mm/dark, 50 cells from three retinas for 30 mm/light, 48 cells from six retinas for 60 mm/dark, and 62 cells from six retinas for 60 mm/light. *, p < 0.05, significantly different from pretreatment defined 100% activity level (one-sample Student's t test, Bonferroni-corrected). Error bars are mean ± S.E.
To test whether melanopsin susceptibility to hydroxylamine is increased by light exposure, wild-type P8–10 retinas under glutamatergic blockade were treated for 15 min with hydroxylamine at similar concentrations as those used previously, along with concurrent 480 nm light, at a level that saturates ipRGC firing (IR 13.6). For comparison, similar retinas were treated with hydroxylamine in the dark. At 60 min following 10 mm/light cotreatment, ipRGC activity decreased only mildly, as also occurred in the dark (Fig. 4C). However, following 30 mm/light cotreatment, activity again decreased slightly but less than in darkness alone. Similar moderate levels of activity were seen following 60 mm hydroxylamine treatment in either light or dark, both being statistically different from pretreatment levels (p < 0.05, one-sample Student's t test, Bonferroni-corrected for multiple comparisons). These results suggest that light increases the apparent susceptibility of melanopsin to hydroxylamine only slightly at higher concentrations. Of note, 10 min following 30 and 60 mm hydroxylamine treatment with or without light, the ability of all ganglion cells to fire in response to elevated KCl was reduced or eliminated. However, firing ability recovered to normal levels by 60 min (data not shown) with a concurrent return of light-induced activity in ipRGCs. This indicates that hydroxylamine exerts some nonspecific effects. Despite this caveat, our data indicate that the melanopsin chromophore is substantially reconstituted under several conditions that lead to bleaching of rod and cone pigments.
DISCUSSION
Melanopsin shares greater sequence similarity to opsins commonly found in invertebrate rhabdomeric photoreceptors than to vertebrate ciliary opsins, displaying only 30% sequence similarity with other mammalian ciliary (rod and cone) opsins, but about 40% sequence similarity with rhabdomeric cephalopod rhodopsin (32, 33). This suggests that melanopsin functions more like rhabdomeric opsins, which have long-lasting meta states and regenerate the chromophore via sequential photon absorption. When ciliary opsins absorb a photon of light, 11-cis-retinal is isomerized into all-trans-retinal and released from the opsin protein (34). A separate isomerase then reconverts esterified trans-retinal to cis-retinol (34, 35). In rhabdomeric opsins, all-trans-retinal is not released from the opsin, but instead absorbs a second photon and is re-isomerized to 11-cis-retinal. This is possible because rhabdomeric pigments have stable meta-states that last for seconds to minutes, whereas vertebrate meta states last only milliseconds (36, 37). Rhabdomeric opsins are thus considered bistable molecules.
Suggestions of melanopsin bistability have been supported by heterologous expression experiments. Panda et al. (38) found that expressing mouse melanopsin in Xenopus oocytes conferred white light sensitivity that was dependent upon the addition of 9-cis-retinal to these cells. Addition of all-trans-retinal had only 10% of the effect of 9-cis-retinal, suggesting that melanopsin has a preference for the cis-chromophore. Coexpressing arrestin with melanopsin increased the activity of the latter activity 12-fold after 9-cis-retinal incubation. Depending upon the arrestin coexpressed, the all-trans-retinal-dependent light activity of melanopsin increased 10- to 30-fold, approaching that conferred by 9-cis-retinal, perhaps through arrestin stabilizing the meta states. Melyan et al. (39) found that human melanopsin expressed in Neuro2A cells also conferred an intensity-dependent light response to these cells. Again, this response required preincubation with 9-cis-retinal. Incubation with all-trans-retinal produced a response about 25% of the magnitude observed with 9-cis-retinal. When cells were incubated with all-trans-retinal and illuminated with 540 nm light for 10 min, the photoresponse increased 3-fold over that following all-trans-retinal incubation alone. Taken together, these results suggest that melanopsin is capable of bistable functioning.
Most recently Davies et al. (40) have shown that of the five zebrafish melanopsin homologues (40, 41), a subset of three can produce small light responses when expressed in heterologous tissue culture cells in the presence of all-trans-retinal. This suggests that not all forms of melanopsin have equivalent chromophore requirements. As in previous studies, severalfold higher concentrations of all-trans-retinal over 9-cis-retinal are needed for any light response to occur with the expressed zebrafish melanopsin. This is comparable with our results indicating a strong preference of melanopsin for cis chromophore. Additionally, the Davies et al. studies (40, 41) were performed in heterologous cell culture where access to the chromophore and presence of chromophore processing components may differ markedly from the in vivo preparations in this experiment.
Melanopsin Photosensitivity Is Reconstituted by the cis-retinal Chromophore after UV Bleaching in Situ
In this study, prolonged 480-nm light exposure reduced light-activated ipRGC activity. This could result from either adaptation of melanopsin signaling (42) or bleaching of pigment. However, light-driven ipRGC activity recovered substantially within minutes (Fig. 1), suggesting either rapid chromophore regeneration or reversal of adaptation. To obtain a more definitive bleaching paradigm, high-intensity UV light was employed to bleach melanopsin by degrading endogenous retinoids. UV light exposure for 15 min lowered ipRGC firing to a stable 25% of maximal prebleach activity and reduced endogenous retinoids to below our HPLC-detectable limits of 0.1 pmol (43). Exposure to more than 15 min of UV light led to substantial tissue mortality (data not shown). Residual ipRGC activity found after treatment likely represents a very small quantity of cis-retinal resistant to destruction by UV light, suggesting either that melanopsin has very high affinity for trace amounts of the remaining intact chromophore or that it may itself help to shield the cis-chromophore bound to it from UV-mediated destruction. This possibility suggests that melanopsin may not readily release its chromophore upon activation. It is remarkable that any activity remained with such low retinoid levels, as no rod- and cone-driven activity remained following the same bleaching conditions (Fig. 2).
Following bleaching, reconstitution of ipRGC activity by addition of exogenous retinoid was successful only with 100 μm 9-cis-retinal. No restoration of light-dependent activity above the base line was seen following any tested concentration of all-trans-retinal. HPLC-measured levels of post-reconstitution retinoids were higher following 9-cis-retinal rescue than after all-trans-retinal, likely because of cis-specific binding mechanisms in rod and cone photoreceptors. Despite this lower level of all-trans-retinal, the amount found in retinas after exogenous supplementation was equivalent to prebleached levels of total endogenous retinoids. Therefore, adequate penetration of the retina by passive diffusion of retinoids was likely achieved, suggesting that melanopsin in ipRGCs in situ has a functional preference for the cis over the trans conformation of retinal. This preference presumably extends from 9-cis-retinal to 11-cis-retinal. (9-cis-retinal was used in these experiments because of the large quantities needed and its stability during long periods of constant perfusion of retinas on MEAs that made use of 11-cis-retinal practically unfeasible.)
ipRGCs Function Independently of the Müller Glial-based Photocycle
Melanopsin has been shown previously to function independently of the retinal pigment epithelium-based photocycle (1). The only other known photocycle present in the retina is found in Müller glial cells. But instead of producing 11-cis-retinal, the Müller glial cell-based photocycle converts all-trans-retinol produced by photoreceptors into 11-cis-retinol that is then transported to cones, which have the capacity to oxidize the alcohol to the 11-cis-retinal required to bind cone opsin (for a review, see Ref. 22). Experiments demonstrating that cones are capable of using 11-cis-retinol showed that although bleaching of cones followed by 11-cis-retinol exposure led to a restoration in cone sensitivity, the same treatment of rods led to a further decrease of sensitivity by several log units beyond that caused by bleaching alone (43). As we were unable to restore light-activated activity to ipRGCs after the exogenous addition of 9-cis-retinol at several concentrations but, in fact, saw a decreased sensitivity to light in a similar fashion to that seen in rods (supplemental Fig. S1C), we conclude that ipRGCs do not utilize cis-retinol and, therefore, function independently of the cone-specific photocycle. Thus, there would appear to be at least three functional photocycles in the murine retina. Neither the RPE-based rod/cone nor the Müller-based cone photocycle is needed to regenerate chromophore in ipRGC.
Melanopsin Displays Bistable-like Pigment Behavior in Response to in Situ Hydroxylamine Treatment
Hydroxylamine treatment constitutes a classic method for photospectrometric analysis of opsins. Here we used it in live tissue to chemically bleach native photopigments in situ (25–27). We observed only minimal to moderate bleaching of melanopsin by low concentrations hydroxylamine, either in the dark or with concurrent light exposure. This contrasts with the complete transient or sustained rod and cone opsin bleaching following hydroxylamine treatment under the same conditions. Although 60 mm hydroxylamine treatment led to a consistently moderate to large loss of light sensitivity in ipRGCs and 30 mm hydroxylamine showed inconsistently sized losses, we believe these changes were mostly due to off-target effects as they coincided with decreased RGC firing in response to high K+ concentrations. Above 50 mm, hydroxylamine has been shown to cause significant disruptions in G-protein-coupled receptor phosphorylation and Gqα palmitoylation-mediated membrane localization (44, 45). These and potentially other nonspecific effects of hydroxylamine could lead to the apparent loss of light sensitivity seen here as well as the inability of all ganglion cells to fire in response to an increased KCl concentration. We do not know the reason for the difference in ipRGC responses following light or dark treatment and 30 mm hydroxylamine treatment. Presumably, this result reflects differences in access of hydroxylamine to the chromophore in light versus dark conditions, but a full mechanistic explanation will likely require some structural insight into melanopsin chromophore binding.
Because hydroxylamine does not abolish ipRGC activity in the dark as it does cone activity, or in the light as it does rod activity our results suggest that the chromophore of melanopsin is relatively protected from the external milieu and not readily available for hydroxylamine binding in either its ground state or its signaling meta state. This is a characteristic of bistable pigments, which do not easily release chromophore (i.e. octopus rhodopsin (29)). This finding may also explain the remarkable preservation of ipRGC signaling in animals that have been severely depleted of vitamin A (46).
Davies et al. (40) showed that in vitro some melanopsin forms have bistable-like behavior and some do not. Our hydroxylamine data suggests that mouse melanopsin does exhibit bistable-like behavior. We suggest that in vivo, at concentrations not toxic to retina, all-trans-retinal does not form functional pigment with apo-melanopsin. In vivo, only the cis conformation can bind melanopsin to form pigment. Therefore, in vivo, cis-retinal is the required chromophore for pigment reconstitution. However, our data are consistent with a model in which chromophore, once bound to melanopsin, can remain in a long-lived meta state in the trans conformation, which might be reset to cis by sequential photon absorption (the hallmark of a bistable pigment). It is not a requirement for apo-opsin to bind both cis and trans chromophore equivalently for the pigment to function in a bistable manner.
All bistable pigments have been shown to “photoreverse” at a different wavelength (usually red-shifted) from the activation wavelength. This reversal reisomerizes all-trans-retinal to 11-cis-retinal. In vitro work by Melyan et al. (39) showed that melanopsin expressed in cell culture appeared to convert exogenously added all-trans to 11-cis-retinal after prolonged exposure to bright light above 540 nm (39). This reversal was assessed by an increase in the light-evoked current above what could be evoked with all-trans-retinal in the dark. Similarly, Mure et al. (47) showed that animals preexposed to 630-nm light had potentiated firing of suprachiasmatic neurons in vivo in response to 480-nm light, presumably through increased ipRGC activity. However, Mawad and Van Gelder (48) found no potentiation of ipRGCs in whole retina studied ex vivo by using multielectrode array recording under the identical lighting protocol, suggesting the potentiation seen by Mure et al. (48) relied on non-ipRGC- or non-melanopsin-based mechanisms. Thus, it is unclear whether ipRGCs in situ have a distinct red-shifted photoreversal mechanism.
Heterologously expressed melanopsin from the protochordate Amphioxus does appear to have a red-shifted photoreversal mechanism (49, 50). Difference spectra of recombinant Amphioxus melanopsin purified from HEK293 cells and exposed to alternating blue and orange light indicate a reversal wavelength near 515 nm. This constitutes a red shift of 30 nm from the forward activation wavelength. Red shift magnitudes vary greatly among rhabdomeric opsins from different species, ranging from 7 nm in squid to 100 nm in fly (29, 51). Even opsins from quite closely related species such as squid and octopus do not have similar red shift magnitudes, (i.e. 7 nm and 35 nm, respectively (29, 52)). It is possible that murine melanopsin may function as a bistable pigment but with a reversal wavelength spectrum that overlaps so closely with the forward activation spectrum that the two cannot be functionally separated (Fig. 5).
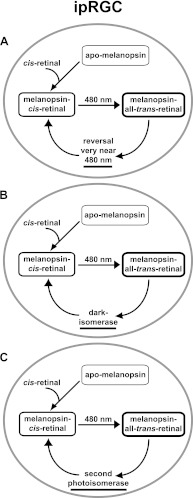
FIGURE 5.
Three models for the ipRGC photocycle. In all models of the ipRGC photocycle above, apo-melanopsin can only bind the cis conformation of retinal, but recycling of all-trans-retinal differs. The models are not mutually exclusive. A, after binding, melanopsin may have a bistable photocycle with a reversal wavelength so close to the forward wavelength that the two are nearly indistinguishable under normal circumstances. B, melanopsin may also possess dark isomerase activity that fully resets melanopsin activity to dark-adapted levels. C, there may also exist a photoisomerase that reisomerizes all-trans-retinal into 11-cis-retinal similar to the retinochrome in squid.
However, photoreversal, even if operative, does not fully explain the behavior of melanopsin. As shown in Fig. 1, melanopsin photosensitivity is reconstituted substantially in the dark. Thus, a mechanism must exist whereby all-trans-retinal in the dark is converted back to cis-retinal. Restoration of activity in the dark is not consistent with a purely bistable pigment that does not release its chromophore. Such a mechanism is suggested by the work of Terakita et al. (50). Using purified, recombinant Amphioxus melanopsin, this group showed a blue shift of the activated melanopsin spectrum toward the preexposure spectrum after a 5-min dark incubation. It is possible that melanopsin acts as a dark isomerase itself by using stored energy in this protein to convert the trans- to cis-chromophore. Such thermal relaxation has been established as a second, but slower mechanism of chromophore regeneration to photoreversal in bacterial and archeabacterial opsins (53). Alternatively, the melanopsin photocycle could mirror the cephalopod photocycle, in which a second photoisomerase, similar to retinochrome (54), recycles all-trans back into 11-cis-retinal in a light-dependent manner.
In conclusion, our results suggest that 1) melanopsin is remarkably resistant to bleaching by light, UV radiation, and hydroxylamine; 2) apo-melanopsin has a preference for cis over trans-retinal; and 3) melanopsin does not appear to release chromophore easily to allow bleaching by hydroxylamine. Definitive photocycle assessment will require single cell measurements in vivo and purified melanopsin. Closest to achieving this objective is Walker et al. (12), who determined that immunopanned ipRGCs maintained in the dark contained only 11-cis-retinal, whereas cells exposed to light after panning predominantly contained all-trans-retinal. This is consistent with the requirement for the cis confirmation of retinal for the light response found here. In addition, we found that melanopsin is not readily bleached by hydroxylamine, which suggests that it does not easily release its chromophore after light activation and its isomerization to all-trans-retinal. The latter is a characteristic of bistable pigments. If melanopsin is a bistable pigment, it is possible that the in vivo apo-form may require the cis conformation for chromophore binding.
Acknowledgments
We thank Angela Sandt for technical assistance, Dr. Jack Saari for help with retinol preparation, and Dan Possin for help with figure presentation.
This research was supported, in whole or in part, by National Institutes of Health Grants EY009339, P30EY001730, P30EY07031, and T32EY007031. This work was also supported by unrestricted support from Research to Prevent Blindness and the Burroughs-Wellcome Clinical Scientist Award in Translational Research (to R. N. V. G.).

This article contains supplemental Fig. 1.
- ipRGC
- intrinsically photosensitive retinal ganglion cell
- P8
- postnatal day 8
- MEA
- multielectrode array
- ANOVA
- analysis of variance
- IR
- log10 irradiance.
REFERENCES
- 1. Tu D. C., Owens L. A., Anderson L., Golczak M., Doyle S. E., McCall M., Menaker M., Palczewski K., Van Gelder R. N. (2006) Inner retinal photoreception independent of the visual retinoid cycle. Proc. Natl. Acad. Sci. U.S.A. 103, 10426–10431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Güler A. D., Altimus C. M., Ecker J. L., Hattar S. (2007) Multiple photoreceptors contribute to nonimage-forming visual functions predominantly through melanopsin-containing retinal ganglion cells. Cold Spring Harbor Symp. Quant. Biol. 72, 509–515 [DOI] [PubMed] [Google Scholar]
- 3. Peirson S., Foster R. G. (2006) Melanopsin. Another way of signaling light. Neuron 49, 331–339 [DOI] [PubMed] [Google Scholar]
- 4. Do M. T., Yau K. W. (2010) Intrinsically photosensitive retinal ganglion cells. Physiol. Rev. 90, 1547–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen S. K., Badea T. C., Hattar S. (2011) Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 476, 92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hattar S., Lucas R. J., Mrosovsky N., Thompson S., Douglas R. H., Hankins M. W., Lem J., Biel M., Hofmann F., Foster R. G., Yau K. W. (2003) Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tu D. C., Zhang D., Demas J., Slutsky E. B., Provencio I., Holy T. E., Van Gelder R. N. (2005) Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron 48, 987–999 [DOI] [PubMed] [Google Scholar]
- 8. Panda S., Provencio I., Tu D. C., Pires S. S., Rollag M. D., Castrucci A. M., Pletcher M. T., Sato T. K., Wiltshire T., Andahazy M., Kay S. A., Van Gelder R. N., Hogenesch J. B. (2003) Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527 [DOI] [PubMed] [Google Scholar]
- 9. Kuksa V., Imanishi Y., Batten M., Palczewski K., Moise A. R. (2003) Retinoid cycle in the vertebrate retina. Experimental approaches and mechanisms of isomerization. Vision Res. 43, 2959–2981 [DOI] [PubMed] [Google Scholar]
- 10. Nickle B., Robinson P. R. (2007) The opsins of the vertebrate retina. Insights from structural, biochemical, and evolutionary studies. Cell Mol. Life Sci. 64, 2917–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu Y., Zhong H., Wang M. H., Luo D. G., Liao H. W., Maeda H., Hattar S., Frishman L. J., Yau K. W. (2005) Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc. Natl. Acad. Sci. U.S.A. 102, 10339–10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walker M. T., Brown R. L., Cronin T. W., Robinson P. R. (2008) Photochemistry of retinal chromophore in mouse melanopsin. Proc. Natl. Acad. Sci. U.S.A. 105, 8861–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panda S., Sato T. K., Castrucci A. M., Rollag M. D., DeGrip W. J., Hogenesch J. B., Provencio I., Kay S. A. (2002) Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216 [DOI] [PubMed] [Google Scholar]
- 14. Wong W. T., Myhr K. L., Miller E. D., Wong R. O. (2000) Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. J. Neurosci. 20, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holy T. E., Dulac C., Meister M. (2000) Responses of vomeronasal neurons to natural stimuli. Science 289, 569–572 [DOI] [PubMed] [Google Scholar]
- 16. Garwin G. G., Saari J. C. (2000) High-performance liquid chromatography analysis of visual cycle retinoids. Methods Enzymol. 316, 313–324 [DOI] [PubMed] [Google Scholar]
- 17. Saari J. C., Nawrot M., Kennedy B. N., Garwin G. G., Hurley J. B., Huang J., Possin D. E., Crabb J. W. (2001) Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron 29, 739–748 [DOI] [PubMed] [Google Scholar]
- 18. Saari J. C., Nawrot M., Garwin G. G., Kennedy M. J., Hurley J. B., Ghyselinck N. B., Chambon P. (2002) Analysis of the visual cycle in cellular retinol-binding protein type I (CRBPI) knockout mice. Invest. Ophthalmol. Vis. Sci. 43, 1730–1735 [PubMed] [Google Scholar]
- 19. Tu D. C., Batten M. L., Palczewski K., Van Gelder R. N. (2004) Nonvisual photoreception in the chick iris. Science 306, 129–131 [DOI] [PubMed] [Google Scholar]
- 20. Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C. L., Matsuyama S., Palczewski K. (2009) Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 284, 15173–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Do M. T., Kang S. H., Xue T., Zhong H., Liao H. W., Bergles D. E., Yau K. W. (2009) Photon capture and signalling by melanopsin retinal ganglion cells. Nature 457, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J. S., Kefalov V. J. (2011) The cone-specific visual cycle. Prog. Retin. Eye Res. 30, 115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wald G., Brown P. K., Smith P. H. (1955) Iodopsin. J. Gen. Physiol. 38, 623–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abrahamson E. W., Fager R. S., Mason W. T. (1974) Comparative properties of vertebrate and invertebrate photoreceptors. Exp. Eye Res. 18, 51–67 [DOI] [PubMed] [Google Scholar]
- 25. Dartnall H. J. (1968) The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 8, 339–358 [DOI] [PubMed] [Google Scholar]
- 26. Leibrock C. S., Lamb T. D. (1997) Effect of hydroxylamine on photon-like events during dark adaptation in toad rod photoreceptors. J. Physiol. 501, 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brin K. P., Ripps H. (1977) Rhodopsin photoproducts and rod sensitivity in the skate retina. J. Gen. Physiol. 69, 97–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wald G., Brown P. K. (1950) The synthesis of rhodopsin from Retinene(1). Proc. Natl. Acad. Sci. U.S.A. 36, 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hubbard R., St. George R. C. (1958) The rhodopsin system of the squid. J. Gen. Physiol. 41, 501–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowmaker J. K., Loew E. R. (1976) The action of hydroxylamine on visual pigments in the intact retina of the frog (Rana temporaria). Vision Res. 16, 811–818 [DOI] [PubMed] [Google Scholar]
- 31. Nemargut J. P., Wang G. Y. (2009) Inhibition of nitric oxide synthase desensitizes retinal ganglion cells to light by diminishing their excitatory synaptic currents under light adaptation. Vision Res. 49, 2936–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Provencio I., Jiang G., De Grip W. J., Hayes W. P., Rollag M. D. (1998) Melanopsin. An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. U.S.A. 95, 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellingham J., Whitmore D., Philp A. R., Wells D. J., Foster R. G. (2002) Zebrafish melanopsin. Isolation, tissue localisation and phylogenetic position. Brain Res. Mol. Brain Res. 107, 128–136 [DOI] [PubMed] [Google Scholar]
- 34. Palczewski K. (2006) G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kiser P. D., Golczak M., Maeda A., Palczewski K. (2011) Key enzymes of the retinoid (visual) cycle in the vertebrate retina. Biochim. Biophys. Acta 1821, 137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hardie R. C., Postma M. (2007) in The Senses: A Comprehensive Review (Bushnell M., Smith D., Beauchamp G., Firestein S., Dallos P., Oertel D., Masland R., Albright T., Kaas J., Gardner E., Basbaum A., eds) Vol. 1, pp. 77–130, Academic Press, Waltham, MA [Google Scholar]
- 37. Hillman P., Hochstein S., Minke B. (1983) Transduction in invertebrate photoreceptors. Role of pigment bistability. Physiol. Rev. 63, 668–772 [DOI] [PubMed] [Google Scholar]
- 38. Panda S., Nayak S. K., Campo B., Walker J. R., Hogenesch J. B., Jegla T. (2005) Illumination of the melanopsin signaling pathway. Science 307, 600–604 [DOI] [PubMed] [Google Scholar]
- 39. Melyan Z., Tarttelin E. E., Bellingham J., Lucas R. J., Hankins M. W. (2005) Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433, 741–745 [DOI] [PubMed] [Google Scholar]
- 40. Davies W. I., Zheng L., Hughes S., Tamai T. K., Turton M., Halford S., Foster R. G., Whitmore D., Hankins M. W. (2011) Functional diversity of melanopsins and their global expression in the teleost retina. Cell Mol. Life Sci. 68, 4115–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matos-Cruz V., Blasic J., Nickle B., Robinson P. R., Hattar S., Halpern M. E. (2011) Unexpected diversity and photoperiod dependence of the zebrafish melanopsin system. PLoS ONE 6, e25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong K. Y., Dunn F. A., Berson D. M. (2005) Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron 48, 1001–1010 [DOI] [PubMed] [Google Scholar]
- 43. Stecher H., Palczewski K. (2000) Multienzyme analysis of visual cycle. Methods Enzymol. 316, 330–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pepperberg D. (1992) Hydroxylamine-dependent inhibition of rhodopsin phosphorylation in the isolated retina. Exp. Eye Res. 54, 369–376 [DOI] [PubMed] [Google Scholar]
- 45. Pepperberg D. R., Morrison D. F., O'Brien P. J. (1995) Depalmitoylation of rhodopsin with hydroxylamine. Methods Enzymol. 250, 348–361 [DOI] [PubMed] [Google Scholar]
- 46. Thompson C. L., Selby C. P., Van Gelder R. N., Blaner W. S., Lee J., Quadro L., Lai K., Gottesman M. E., Sancar A. (2004) Effect of vitamin A depletion on nonvisual phototransduction pathways in cryptochromeless mice. J. Biol. Rhythms 19, 504–517 [DOI] [PubMed] [Google Scholar]
- 47. Mure L. S., Rieux C., Hattar S., Cooper H. M. (2007) Melanopsin-dependent nonvisual responses. Evidence for photopigment bistability in vivo. J. Biol. Rhythms 22, 411–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mawad K., Van Gelder R. N. (2008) Absence of long-wavelength photic potentiation of murine intrinsically photosensitive retinal ganglion cell firing in vitro. J. Biol. Rhythms 23, 387–391 [DOI] [PubMed] [Google Scholar]
- 49. Koyanagi M., Kubokawa K., Tsukamoto H., Shichida Y., Terakita A. (2005) Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr. Biol. 15, 1065–1069 [DOI] [PubMed] [Google Scholar]
- 50. Terakita A., Tsukamoto H., Koyanagi M., Sugahara M., Yamashita T., Shichida Y. (2008) Expression and comparative characterization of Gq-coupled invertebrate visual pigments and melanopsin. J. Neurochem. 105, 883–890 [DOI] [PubMed] [Google Scholar]
- 51. Vought B. W., Salcedo E., Chadwell L. V., Britt S. G., Birge R. R., Knox B. E. (2000) Characterization of the primary photointermediates of Drosophila rhodopsin. Biochemistry 39, 14128–14137 [DOI] [PubMed] [Google Scholar]
- 52. Koutalos Y., Ebrey T. G., Tsuda M., Odashima K., Lien T., Park M. H., Shimizu N., Derguini F., Nakanishi K., Gilson H. R. (1989) Regeneration of bovine and octopus opsins in situ with natural and artificial retinals. Biochemistry 28, 2732–2739 [DOI] [PubMed] [Google Scholar]
- 53. Chow B. Y., Han X., Bernstein J. G., Monahan P. E., Boyden E. S. (2011) in Neuromethods (Walz W., ed) Vol. 55, pp. 99–132, Humana Press, New York [Google Scholar]
- 54. Ozaki K., Hara R., Hara T., Kakitani T. (1983) Squid retinochrome. Configurational changes of the retinal chromophore. Biophys. J. 44, 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]