Background: Protein quality control proteases degrade damaged proteins and protein fragments.
Results: The human serine protease HTRA1 degrades tau aggregates and is induced by its substrates.
Conclusion: A member of the widely conserved HtrA family is involved in protein quality control in mammalian cells.
Significance: HTRA1 might function as a tau protease in vivo.
Keywords: Calpain, Caspase, Protease, Protein Degradation, Serine Protease, HTRA1, Protein Quality Control
Abstract
Protective proteases are key elements of protein quality control pathways that are up-regulated, for example, under various protein folding stresses. These proteases are employed to prevent the accumulation and aggregation of misfolded proteins that can impose severe damage to cells. The high temperature requirement A (HtrA) family of serine proteases has evolved to perform important aspects of ATP-independent protein quality control. So far, however, no HtrA protease is known that degrades protein aggregates. We show here that human HTRA1 degrades aggregated and fibrillar tau, a protein that is critically involved in various neurological disorders. Neuronal cells and patient brains accumulate less tau, neurofibrillary tangles, and neuritic plaques, respectively, when HTRA1 is expressed at elevated levels. Furthermore, HTRA1 mRNA and HTRA1 activity are up-regulated in response to elevated tau concentrations. These data suggest that HTRA1 is performing regulated proteolysis during protein quality control, the implications of which are discussed.
Introduction
Human HTRA1 belongs to the widely conserved high temperature requirement A (HtrA)4 family of homo-oligomeric and ATP-independent serine proteases (1, 2). HtrAs of pro- and eukaryotes are implicated in protein quality control. They can act as key stress sensors and regulators of unfolded protein response signaling pathways and can mediate the repair and assembly or the removal of damaged, fragmented, and mislocalized proteins (3–9). Defining features of HtrA proteases are their homo-oligomeric architecture and the presence of C-terminal PDZ domains that can be involved in substrate processing, sensing of misfolded proteins, mediating allosteric and cooperative regulation of the proteolytic activity, and in the switch between various oligomeric states (2).
Among the four human HTRAs, HTRA1–4, the ubiquitously expressed HTRA1 consists of a signal sequence for secretion, partial insulin-like growth factor-binding protein-7 domain, serine protease domain resembling classic serine proteases such as trypsin and one C-terminal PDZ domain. Like Escherichia coli DegP, a prototypic HtrA protease involved in protein quality control, HTRA1 is activated by oligomerization. Substrate binding triggers the switch between the resting and the active conformations and between various oligomeric states (10, 11).
HTRA1 has at least three cellular localizations and a multitude of functions. Extracytoplasmic HTRA1 is involved in the homeostasis of the extracellular matrix, and elastin, fibulin 5, nidogen 2, fibronectin, fibromodulin, aggrecan, and decorin have been identified as substrates (12–17). Intracellular HTRA1 was localized to microtubules and to the nucleus (18, 19). Cytoplasmic HTRA1 has been implicated in the degradation of tuberin thereby modulating cell growth and proliferation (20). Furthermore, microtubule-associated HTRA1 degrades tubulins thereby inhibiting cell migration (18, 21). Consequently, HTRA1 has been implicated in several severe pathologies including cancer, age-related macular degeneration, Alzheimer disease (AD), arthritis, and familial ischemic cerebral small vessel disease (12, 13, 22–26). In many of these diseases, protein fragments or aggregates are either causative for disease or are disease-modifying factors that are produced or degraded by HTRA1.
Recent studies suggest that substrate specificity and processing of individual HtrA proteases can differ significantly. Whereas bacterial DegS is a regulatory protease that cleaves its single substrate at one defined position, other HtrAs such as E. coli DegP digest a great many of un- or misfolded proteins into small peptides (8, 9, 27, 28). However, these and other studies suggested that HtrA proteases do not degrade protein aggregates. This model was supported, for example, by the precise understanding of the proteolytic mechanism of DegP, requiring concurrent binding of substrates to its PDZ domain 1 and the active site for both activation and proteolysis (28–30). However, the recent elucidation of crystal structures of HTRA1 and complementing mechanistic studies indicating that proteolysis and activation of HTRA1 occur in a PDZ domain-independent manner prompted us to address the question of whether human HTRA1 is able to use protein aggregates as substrates (10).
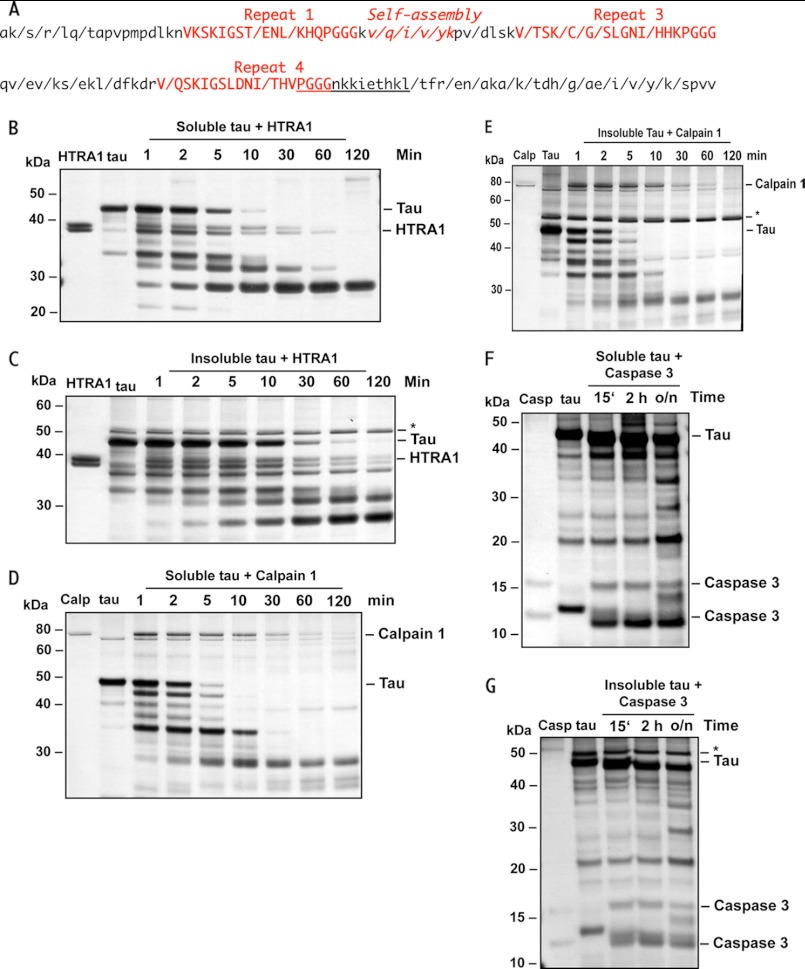
To test whether HTRA1 degrades protein aggregates we used tau as a model substrate because tau is, like HTRA1, associated with microtubules. Furthermore, tau is of important clinical relevance, and its aggregation is widely studied. It is therefore well established that the tau protein can aggregate into intracellular neurofibrillary tangles that are specific pathological features of AD and other tauopathies. Normal tau, which is abundant in axons, is thought to regulate microtubule dynamics. Interaction of tau with microtubules is mediated by its microtubule binding domain consisting of three or four repeats sharing the consensus sequence VXSKXGSXXN(L/I)XHXPGGG. Posttranslational modifications, such as phosphorylation, can decrease the affinity of tau to microtubules leading to dissociation and polymerization into straight or paired helical filaments, ribbons, and other conformations. Progressive intermolecular interactions of fibrils composed of paired helical filaments of tau lead to the formation of neurofibrillary tangles. A core domain of paired helical filaments consists of three or four repeats of tau involved in stable tau-tau interactions (Fig. 1A). Within this core domain, one short stretch, the self- assembly region VQI(I/V)XK, seems of critical importance. Proteolytic processing of tau stimulates the assembly of tau into fibrils. Such assembly can capture further full-length tau proteins for processing leading to progressive growth of the toxic protein aggregates eventually causing cell death (for review see Refs. 31, 32).
FIGURE 1.
Proteolysis of tau by HTRA1, calpain 1, and caspase 3. A, HTRA1 cleaves tau at multiple sites in its core domain which is relevant for aggregation. WT-, PHP-, and Ala-tau were completely digested by HTRA1. Proteolytic products were identified by mass spectrometry. Forward slashes indicate cleavage sites that were identical in the three tau variants analyzed. For simplicity, we show cleavage sites detected in tau Ala239–Val399, the region that is implicated in aggregation. The sequence of the synthetic substrate PGGGNKKIETHKL-pNA is underlined. B and C, HTRA1 degrades tau. Purified soluble (B) and insoluble (C) tau derivatives were incubated with or without HTRA1. Samples were removed at the time points indicated and subjected to SDS-PAGE and visualized by silver staining. D–G, degradation of tau by calpain 1 (D and E) and caspase 3 (F and G) was carried out as described in B and C (see “Experimental Procedures”), respectively. The band marked by an asterisk present in all insoluble fractions of tau (C, E, and G) is a contaminant and therefore not degraded by either protease.
EXPERIMENTAL PROCEDURES
Plasmids
Bacterial expression vectors of human taus and the GFP-tau construct pEGFP-C3–3Rtau were described earlier (33, 34). Expression plasmid pHTRA1 was generated by PCR and subcloning of HTRA1 into pBABE (35). HTRA1-mCherry constructs were derivatives of pmCherry-N1 (Clontech). Expression of WT-tau, Ala-tau, and pseudohyperphosphorylated (PHP)-tau was done as described (36).
Purification of HTRA1, HTRA1ΔPDZ Domain, WT-Tau, Ala-Tau, and PHP-Tau
For all in vitro protease assays, HTRA1 and HTRA1ΔPDZ domain were purified and used as described (24) except that an additional hydroxyapatite column (Bio-Rad) was added. Recombinant tau variants were isolated by boiling of cleared bacterial cell extracts (lysis buffer: 33 mm Tris-HCl, pH 8, 100 mm KCl) in the water bath for 30 min. Tau remains soluble, whereas the precipitated bacterial proteins were cleared from the lysate by centrifugation (35,000 × g, 40 min). For further purification of the 3R tau variants (0N3R, 352 amino acids), the supernatant was incubated with ammonium sulfate at 30% saturation for 30 min, 4 °C, followed by centrifugation at 20,000 × g, 30 min. Tau was precipitated from the supernatant with 40% saturated ammonium sulfate. Purified tau was suspended in 80 mm PIPES/KOH, pH 6.8, 1 mm EGTA, 1 mm MgCl2. For the isolation of insoluble and soluble fractions of tau, protein reconstituted from ammonium sulfate pellets was incubated at 25 °C for 20 min to ensure complete dissolving of precipitated tau. Ultracentrifugation (100,000 × g, for 1 h) was used to separate insoluble pellet (3RP) and soluble fractions (3RS). The insoluble protein was resuspended from the resulting pellet and the protein concentration determined by Bradford quantitation.
Purification of 441-Amino Acid WT-Tau Protein
The 4R WT isoform of human tau (2N4R, 441 amino acids) used for heparin-induced fibrillization was purified in the same way as the 3R variants described above except that instead of ammonium sulfate precipitation the boiled lysate was subjected to hydroxyapatite chromatography (Bio-Rad). 4R tau was eluted with an NaCl gradient in 100 mm HEPES, 10 mm KPO4, 2 mm DTT, pH 7.6. Subsequently, monomeric and dimeric 4R tau was isolated using size exclusion chromatography (Superdex HiLoad 200 26/60; GE Healthcare).
Heparin-induced 4R Tau Fibrillization, Degradation of 4R Tau Fibrils
The in vitro formation of PHF-like tau filaments was performed as described (37). Briefly, 20 μm 4R tau was incubated at 55 °C, 10 min in aggregation buffer (100 mm sodium acetate, pH 7.0, 2 mm DTT) before addition of 50 μm heparin (Sigma-Aldrich) and incubation at 37 °C, 1,000 rpm, for the time points indicated. Proteolytic digests of the tau aggregates were performed as described above except for the following modifications. A 5-fold molar excess of tau over the protease was used based on the molecular mass of monomeric tau, 5 mm reducing agent Tris(2-carboxyethyl)phosphine was added to the reactions and 50 mm NaH2PO4, pH 8, was used for proteolysis by HTRA1.
Atomic Force Microscopy (AFM)
Tau protein samples (1 μm tau in PBS, pH 7.5) were deposited on a freshly cleaved mica surface (Plano GmbH) without any previous treatment and adsorbed for 3 min at room temperature. After addition of 15 μl of 1× TAEM (40 mm Tris, 20 mm acetic acid, 2 mm EDTA, 12.5 mm Mg acetate, pH 8.0), the sample was scanned in tapping mode using a MultiModeTM microscope (Veeco Metrology, Santa Barbara, CA) equipped with a Nanoscope IV controller. 0.58 N/m force constant cantilevers with sharpened pyramidal tips (SNL-10 tips, Veeco Metrology) were used for scanning. After engagement, the tapping amplitude set point was typically less than 1 volt, and the scan rates ranged between 1 and 2 Hz. Multiple AFM images were recorded from different locations of the mica surface to ensure reproducibility of the results. All images were analyzed with the Nanoscope 6.14R1 and ImageJ software (38).
Thioflavin T (ThT) Fluorescence
To characterize the aggregation state of tau, 10-μl duplicates of the samples at a tau concentration of 20 μm were added to 90 μl of 12 μm ThT (Sigma-Aldrich) in 50 mm glycine, pH 8.5, using black clear-bottom 96-well microtiter plates. After incubation at 37 °C, 900 rpm, the fluorescence was measured with a SpectraMax M5 Microplate Reader (Molecular Devices). For emission spectra, the excitation wavelength was kept constant at 440 nm with emission wavelengths ranging from 450 to 520 nm in 5-nm intervals. Single measurements were performed at an excitation and emission wavelength of 440 and 480 nm, respectively. The cutoff filter was set to 450 nm in all cases.
Protease Assays and Mass Spectrometry
Protease assays were performed at 37 °C in 50 mm Tris-HCl, pH 8.0. For mass spectrometry, 1 μg of HTRA1 was incubated with 10 μg of WT-tau, Ala-tau, or PHP-tau in a final volume of 300 μl in 50 mm Tris-HCl, pH 8.0. After 4 h at 37 °C, samples were centrifuged for 30 min, and the supernatants containing tau fragments were precipitated with acetone. MALDI-TOF mass spectra were obtained as described (24). Samples were loaded onto 10% SDS-PAGE and analyzed by Western blotting using a specific antibody against the tau396 phosphorylation site.
HTRA1 Assay
The proteolytic activity of human recombinant HTRA1s was measured using PGGGNKKIETHKL-p-nitroaniline (pNA) (tau fragment (Pro363–Leu376)) and VFNTLPMMGKASPV-pNA as substrates that were synthesized following standard procedures (10, 39, 40). 1.35 μm HTRA1s were incubated with 500 μm respective substrate and the concentrations of WT- or PHP-tau indicated in 50 mm Tris-HCl, pH 8, at 37 °C. The release of nitroaniline was monitored continuously by measuring the absorption at λ = 405 nm every minute for 90 min. The reactions were performed in 96-well microplates using a SpectraMax M5 Microplate Reader. For calculation of the specific activity, a time segment of linear increase in absorption was used to quantify the turnover of the substrate by employing the specific molar absorption coefficient of nitroaniline, 8,800 m−1× cm1.
Caspase 3 Assay
The proteolytic activity of human recombinant caspase 3 was assayed using 200 μm Ac-DEVD-pNA (AAT Bioquest) as a substrate and 50 mm HEPES, pH 7.4, 100 mm NaCl, 0.1% CHAPS, 1 mm EDTA, 10% glycerol, 10 mm DTT, as the reaction buffer.
Fluorometric Calpain Assay
Human calpain 1 (Calbiochem) activity was determined at 23 °C using Suc-LLVY-AMC (Sigma) as a substrate in 50 mm Tris-HCl, 100 mm NaCl, 2 mm CaCl2, 1 mm DTT, pH 7.5. Substrate and calpain 1 concentrations were 200 μm and 0.52 μm, respectively. Fluorescence was monitored continuously by performing single measurements every minute for 90 min with an excitation wavelength of 380 nm and an emission wavelength of 450 nm using a SpectraMax M5 Microplate Reader, and the cutoff filter was set to 435 nm. From the initial linear increase in fluorescence, the concentration of free AMC was quantified using an AMC standard curve generated. The specific activity of calpain 1 was calculated as the ratio of substrate turnover and amount of enzyme.
Proteolysis of Insoluble and Soluble Tau Fractions
Purified WT-3R tau samples were centrifuged at 100,000 × g, 4 °C, for 1 h to separate the soluble and insoluble fractions using an Optima MAX-XP Benchtop Ultracentrifuge (Beckman Coulter). The pellets containing the insoluble tau protein were resuspended with 50 mm Tris-HCl, pH 8. The protein samples were diluted to the assay concentration with the respective assay buffers, which were 50 mm Tris-HCl, pH 8, for HTRA1, 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 2 mm CaCl2, 1 mm DTT for calpain 1 and 50 mm HEPES, pH 7.4, 100 mm NaCl, 0.1% CHAPS, 1 mm EDTA, 10% glycerol, 10 mm DTT for caspase 3. Before addition of the protease, tau was diluted in assay buffer to the final concentration, which was for calpain 1 and HTRA1 20 ng/μl and for caspase 3 1.8 ng/μl. After incubation of the tau solutions at the assay temperature for 2 min, protease was added at the molar ratio of protease to substrate of 1:10. The samples were incubated at 37 °C (HTRA1 and caspase 3) or 23 °C (calpain 1) with agitation, aliquots were taken at the time points indicated. Aliquots were mixed with SDS loading dye and a final concentration of 40 mm reducing agent TCEP and immediately frozen with liquid N2. Prior to SDS-PAGE using Novex NuPage 10% Bis-Tris gels (Invitrogen) and MES running buffer, the samples were heat-treated at 75 °C for 10 min. Protein bands were visualized by silver staining.
Transfection
Stable transfection and transduction of PC12 cells with tau constructs or HTRA1 were carried out as described (36, 41).
Microscopy
To analyze the localization of HTRA1 in U373 cells, cells were transiently transfected. Cells were plated in a 35-mm poly-d-lysine-coated glass-bottom dish (MatTek) at a density of 105 cells/plate. 24 h after plating, cells were transfected by Jet-Pei (Polyplus) either with one plasmid or for co-localization studies with two plasmids. 24 h after transfection cells were analyzed in a Leica TCS SL (SP5) laser confocal microscope, and Leica Confocal Software was used for imaging. Images were taken using the HCX PL APO ×63 oil objective lens.
Quantitative RT-PCR (qRT-PCR)
qRT-PCR was carried out as described (42) using the following primers: ratHtrA1 forward, CCTTTTTGATGACATCACTGACATC and ratHtrA1 reverse, GATGTAATCTCCGGAGCATATATC; rat β-actin forward, GATTACTGCTCTGGCTCCTAG and rat β-actin reverse, ACTCATCGTACTCCTGCTTGC; human tau forward, CCATGCCAGACCTGAAGAAT and tau reverse, TGCTCAGGTCAACTGGTTTG. The housekeeping gene β-actin was used to normalize the results.
Human Brain Tissue
Brain tissue was obtained from the German Brain Bank “Brain-Net” and collected at the Institute of Neuropathology, University Hospital Muenster. Prior to autopsies, consent from patients' families was obtained to use samples for research. In all cases (AD brain and controls) staging of AD-related neurofibrillary pathology according to Braak was performed (43, 44). All control cases have Braak stages I or II (absence of neurofibrillary tangles in the frontal cortex); all AD cases have Braak stages V or VI (abundant neurofibrillary tangles in the frontal cortex). 100 mg of tissue was taken from frozen postmortem samples of the frontal gray matter of 29 AD cases (mean age 76.2 years; range 63–88; mean postmortem time 23.8 h, range 5–48 h) and 24 sex-matched controls (mean age 71.3 years; range 59–92; mean postmortem time 19.3 h, range 5–43 h).
Human Brain Homogenates
Tissue was homogenized in 250 mm sucrose, 20 mm Tris, 1 mm EDTA, and 0.1 mm EGTA by sonication. Protein extraction was performed by formic acid treatment as described (45).
Quantitative Analysis of Neuritic Plaques and Neurofibrillary Tangles
Quantitative analysis of neuritic plaques and neurofibrillary tangles was performed using sections from the medial frontal gyrus (46). Briefly, immunophenotypes of AD brains were analyzed quantitatively by determining the number of Aβ/tau-positive neuritic plaques and the number of tau-positive neurofibrillary tangles (anti-Aβ, 6F/3D, 1:1,000; anti-tau, AT8, 1:2,000). Eight consecutive representative fields from severely affected tissue areas were evaluated by computer-based image analysis.
HTRA1, Tau, and TauP396 ELISA
HTRA1 in human brain samples was quantified by HTRA1-specifc ELISA as described (14). Tau and tauP396 levels in human brain samples were determined by tau and tauP396-specific ELISAs (Invitrogen).
Statistical Analyses
HTRA1 mRNA levels in PC12 cell lines were analyzed by ANOVA, data are presented as means. The HTRA1 and tau protein levels as well as numbers of neuritic plaques and tangles in patient brains were analyzed by two-tailed p values with correlation coefficient using Prism (GraphPad) and SPSS version 16.0 (SPSS) statistical analysis software. A Pearson r value within 0 to −1 indicates that one variable increases as the other decreases.
Antibodies
Rabbit polyclonal antibodies against HTRA1 were generated using the purified recombinant PDZ domain of HTRA1 (residues 377–480). Production of mouse monoclonal antibody is described elsewhere (24). Antibodies against tubulin, WT-tau, phosphorylated tau396, tau repeat, β-actin, Aβ were from Invitrogen, Innogenetics, MP Biomedicals, IBL, and DAKO, respectively.
RESULTS
HTRA1 Digests Tau in Vitro
First, we examined whether soluble tau is a substrate of HTRA1 in vitro. We used purified normal human tau (WT), PHP human tau in which the phosphorylated Ser/Thr residues were mutated to Glu residues, and a corresponding Ala mutant (Ala-tau) in which Ser/Thr residues were mutated to Ala residues (36). Tau substrates were incubated with purified HTRA1, and the proteolytic products were identified by mass spectrometry. HTRA1 cleaved each tau substrate into 45 fragments. 22 cleavage sites that are located in the microtubule binding and self-assembly regions were identical in WT-tau, PHP-tau, and Ala-tau (Fig. 1A). The produced fragments varied in length between 8 and 38 residues with the majority of products ranging from 9 to 22 residues (supplemental Fig. S1). Sequence analysis of the products revealed a preference for Leu, Val, and Ile at the P1 position but little further specificities (supplemental Fig. S2). These results are in agreement with recent data of complete digests of citrate synthase and malate dehydrogenase (10). For convenient determination of enzymatic parameters of tau processing, we synthesized peptide substrates derived from the produced tau fragments by introducing a C-terminal pNA group, i.e. KHQPGGGKV-pNA, VYKPVDLSKV-pNA, and PGGGNKKIETHKL-pNA. Whereas KHQPGGGKV-pNA was not processed by HTRA1, the specific activities for VYKPVDLSKV-pNA and PGGGNKKIETHKL-pNA were 0.9 and 2.2 nmol × mg−1× min−1, respectively. We therefore chose PGGGNKKIETHKL-pNA for further experiments. PGGGNKKIETHKL corresponds to residues 364–376 of tau and contains the C terminus of the fourth repeat region (Fig. 1A, UniProt accession no. P10636). Processing of PGGGNKKIETHKL-pNA followed Michaelis-Menten kinetics with a Km of 1.9 mm and Vmax of 64 nmol/mg of protease per min. The fact that a 13-mer peptide is a substrate suggests that HTRA1 in addition to full-length tau could also degrade tau fragments that are produced for example by other proteases.
We subsequently tested whether HTRA1 digests tau aggregates by incubating two purified tau samples. One, termed 3RS, was the supernatant fraction following ultracentrifugation, and the insoluble form, termed 3RP, corresponded to the pellet fraction following ultracentrifugation. The two fractions were subsequently characterized further with respect to their β-sheet content and the size of the tau particles present in both samples. ThT, a common amyloid-specific fluorescent dye (47), showed characteristic fluorescence emission at λ = 490 nm, suggesting a high β-sheet content (supplemental Fig. S3A). The ThT signal of soluble tau is assumed to be caused by soluble oligomers rich in β-sheet structures. Dynamic light scattering suggested the enrichment of larger particles, probably aggregated tau protein, in the insoluble fraction as indicated by increased hydrodynamic radii and total scattering intensities (supplemental Fig. S3, B and C, and supplemental Experimental Procedures). AFM was employed to assess further the tau species found in the soluble and insoluble fractions. Whereas soluble tau is present as small particles, such as monomeric and oligomeric tau species, the sizes of the insoluble material obtained from ultracentrifugation showed a much broader distribution with a major fraction representing large aggregated material (supplemental Fig. S3, D–F), which was of amorphous structure. At a molar substrate:protease ratio of 10:1, HTRA1 digested soluble and insoluble tau within 30 and 120 min, respectively (Fig. 1, B and C). We concluded that in addition to soluble proteins, HTRA1 is able to digest insoluble aggregated tau protein. To compare the activity of HTRA1 with two other proteases known to cleave tau we incubated both tau samples with human calpain 1 and recombinant human caspase 3 (48, 49). Calpain digested soluble and insoluble tau about twice and 10 times as fast as HTRA1, respectively (Fig. 1, D and E). Furthermore, and in contrast to HTRA1 and calpain 1, caspase 3 digested tau not completely, but generated large tau fragments (Fig. 1, F and G), which can be explained by the pronounced substrate specificity of caspases (50). It should be noted that in the insoluble tau fractions, an additional band of unknown identity migrates slightly above the tau monomer. This protein is not digested by either protease.
As AFM images of the tau aggregates described above did not show the characteristic, well ordered fibrillar structure of tau aggregates typically found in tauopathies, we produced tau fibrils by incubation of the 441 amino acid isoform of tau (4R) with heparin for 7 days (37). The formation of amyloid aggregates was detected by an increase in ThT fluorescence over time (Fig. 2A). The resulting aggregates detected by AFM revealed fibrillar structures of varying lengths (100 nm up to >2 μm) and a characteristic width of approximately 20–25 nm (Fig. 2B) (51). These highly ordered tau filaments were also subjected to proteolytic cleavage and were digested by both HTRA1 and calpain 1 (Fig. 2, C and D), albeit with lower efficiency compared with the soluble and insoluble material. Only after overnight incubation was almost all of the tau protein proteolyzed, which can be explained by the tight packing of tau monomers within amyloid-like fibers rendering them more protease-resistant. These results suggest the ability of HTRA1 to degrade not only amorphous tau aggregates, but also PHF-like tau filaments characterized by tight intermolecular interactions giving rise to the highly structured, cross-β-sheet-rich assembly typical for amyloid (52, 53).
FIGURE 2.
Proteolytic degradation of tau fibrils by HTRA1 and calpain 1. Fibrillization of 4R-tau (20 μm) was induced by incubation the anionic co-factor heparin (50 μm) for 7 days. A, the extent of fibril formation was monitored by ThT fluorescence at an excitation and emission wavelength of 440 and 485 nm, respectively. 10 μl of the aggregation samples was incubated with 12 μm ThT in duplicate at the indicated time points. B, AFM analysis revealed the typical fibrillar morphology of in vitro PHF-like tau aggregates, whereas the control (small image) does not show any fibrillar structures when heparin is not used. C and D, 4R-tau fibrils were subjected to proteolytic cleavage by HTRA1 (C) and calpain 1 (D), performed as described in Fig. 1.
HTRA1 Is Activated by Tau
Because HTRA1 function can be activated by its substrates (10), we tested whether tau was able to act as an inducer of the proteolytic activity of HTRA1, using calpain 1 and caspase 3 as a control. To quantify HTRA1 activity we used two synthetic substrates PGGGNKKIETHKL-pNA and VFNTLPMMGKASPV-pNA. The addition of tau and PHP-tau caused a 4–6-fold activation of HTRA1 activity, whereas calpain 1 and caspase 3 were only slightly or not at all activated under these conditions (Table 1). To confirm that in contrast to other HtrA proteases, HTRA1 does not employ an allosteric mechanism involving its PDZ domain, we repeated these assays with an HTRA1 construct lacking its PDZ domain. Here, the addition of tau and PHP-tau caused an activation of the proteolytic activity by a factor of about 10 and 20 for VFNTLPMMGKASPV-pNA and PGGGNKKIETHKL-pNA, respectively. The increase in activation compared with HTRA1 and the differences between the two substrates used were in part due to the differences in basal activities, i.e. the activities without tau.
TABLE 1.
Proteolytic activities of HTRA1, calpain 1 and caspase 3 in the presence and absence of tau and PHP-tau
PGGGNKKIETHKL-pNA is derived from tau, VFNTLPMMGKASPV-pNA is a previously introduced synthetic HTRA1 substrate (10). The values shown represent means of four to nine independent experiments for each protease with an S.E. of < 10%.
| Protease | Substrate | Fold activation by |
||||||
|---|---|---|---|---|---|---|---|---|
| 0a | WT tau |
PHP tau |
||||||
| 0.5a | 1a | 2a | 0.5a | 1a | 2a | |||
| HTRA1 | PGGGNKKIETHKL-pNA | 1b | 5.6 | 5.7 | 5.5 | 5.9 | 5.0 | 5.0 |
| HTRA1 | VFNTLPMMGKASPV-pNA | 1c | 4.5 | 4.4 | 3.9 | 4.3 | 4.2 | 3.9 |
| HtrA1 ΔPDZ | PGGGNKKIETHKL-pNA | 1d | 22.4 | 23.0 | 21.7 | 21.2 | 20.5 | 21.1 |
| HtrA1 ΔPDZ | VFNTLPMMGKASPV-pNA | 1e | 10.2 | 9.6 | 8.8 | 10.6 | 10.1 | 9.2 |
| Calpain 1 | Suc-LLVY-AMC | 1f | 1.2 | 1.3 | 1.3 | 1.2 | 1.3 | 1.4 |
| Caspase 3 | Ac-DEVD-pNA | 1g | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
a Molar ratio tau: protease. Specific activities of the proteases indicated in the absence of added tau proteins. These specific activities were set to 1 for the calculation of the activation factors.
b 2.7 nmol × mg−1 × min−1.
c 6.5 nmol × mg−1 × min−1.
d 1.2 nmol × mg−1 × min−1.
e 4.8 μmol × mg−1 × min−1.
f 2.9 nmol × mg−1 × min−1.
g 12.6 μmol × mg−1 × min−1.
Tau Levels and Regulation of HTRA1 Expression in Cell Culture
Having established tau and tau aggregates as HTRA1 substrates, we wished to address the question of whether tau might be a physiological substrate of HTRA1. Initially, we asked whether a modulation of HTRA1 levels affects tau levels in cells. Therefore, PC12 rat pheochromocytoma cells stably overexpressing tau or PHP-tau were transduced with an HTRA1 plasmid. Western blots of cell extracts revealed on average a 5-fold decrease in WT-tau and PHP-tau levels in cells expressing increased amounts of HTRA1 (Fig. 3A), suggesting that HTRA1 degrades WT- and PHP-tau in these cells. An alternative explanation of the observed reduction of tau levels upon overexpression of HTRA1 could be reduced transcription of the tau genes. However, this model is unlikely because RNA levels of WT-tau and PHP-tau, as determined by qRT-PCR, were only slightly reduced when HTRA1 was overproduced (supplemental Fig. S4). As shRNA-mediated HTRA1 knockdown was toxic to cells, it was not possible to study tau levels in cells that do not express HTRA1.
FIGURE 3.
Regulation and cellular localization of HTRA1. A, levels of tau in PC12 cells expressing various amounts of HTRA1. PC12 cells stably overexpressing human WT-tau and PHP-tau transduced with human HTRA1 (hHTRA1). Cell lysates were subjected to Western blotting using antibodies against tau, HTRA1, and β-actin (loading control). rHtrA1 is rat HtrA1 that is produced by PC12 cells. B, induction of HTRA1 expression by overexpression of tau and PHP-tau. HTRA1 mRNA levels and measured by qRT-PCR following overexpressing of WT- and PHP-tau in PC12 cells. Control is PC12 cells not overexpressing WT- and PHP-tau. C, laser confocal microscopy of HTRA1-mCherry (red) and tau-GFP (green). Astrocytoma U373 cells were transiently transfected with plasmids expressing proteolytically inactive HTRA1-mCherry lacking the signal sequence and tau-GFP. D, confocal microscopy of HTRA1-mCherry (red) and stained tubulin (green). U373 cells were transfected with plasmids expressing HTRA1-mCherry. Tubulin was stained using a specific antibody. Nuclei were stained blue with DAPI. Cells were analyzed by laser confocal microscopy.
Because HTRAs are protein folding stress proteases, we reasoned that elevated levels of PHP-tau might trigger an increased production of HTRA1. To initially test this hypothesis, WT-tau and PHP-tau were overexpressed at similar levels in PC12 cells (Fig. 3A). Subsequently, HTRA1 mRNA levels were determined by qRT-PCR. These results showed that HTRA1 mRNA levels were moderately up-regulated by a factor of 2.5 following overexpression of WT-tau and by a factor of 6.5 following overexpression of PHP-tau (Fig. 3B).
The model that tau might be a physiological substrate of HTRA1 would require a co-localization of protease and substrate. We therefore transfected astrocytoma U373 cells with HTRA1-mCherry and tau-GFP constructs and performed confocal microscopy (Fig. 3C). For these experiments, a proteolytically inactive S306A mutant of HTRA1 lacking a signal sequence was used. These data show a co-localization of HTRA1 and tau at the cytoskeletal network. In addition, co-localization of HtrA1-mCherry and microtubules was shown in U373 cells lacking exogenous tau (Fig. 3D). Our data support previous studies showing co-localization of HTRA1 with tangles and senile plaques as determined by immunohistochemistry of AD brains (24).
Correlation of HTRA1 and Tau Levels in Patient Samples
To obtain further evidence for the correlation of HTRA1 and tau levels, 29 AD patient and 24 control brains were analyzed by ELISAs measuring HTRA1, total tau, and phosphorylated tauP396 levels (Fig. 4, A–D). As expected, the total tau levels were similar in patient and control brains whereas the levels of phosphorylated tau were significantly increased in AD versus control brains. Interestingly, total tau and phosphorylated tau levels correlate inversely with HTRA1 levels in AD but not in control brains in statistically relevant manner (p = 0.018–0.026). To confirm our findings, we used contralateral frontal cortex to determine the content of neurofibrillary tangles and neuritic plaques that are the pathological hallmarks of AD (Fig. 4, E and F). Samples from 25 of the 29 AD patients were used. The remaining four samples resisted immunohistochemical analyses. As with tau protein levels, the number of neurofibrillary tangles (Fig. 4E) and neuritic plaques (Fig. 4F) also correlate inversely with HTRA1 levels (p = 0.012–0.018). In control brains (normal brains) the content of phosphorylated tau (Fig. 4F) as well as neurofibrillary tangles and neuritic plaques (data not shown) was low, and no correlation with HTRA1 level was found.
FIGURE 4.
HTRA1 levels correlate with tau levels and neuritic AD pathology in patient brains. A–D, protein was extracted from homogenized cortex of 29 AD patients (A and C) and 24 normal brain samples (B and D). Subsequently, WT-tau (A and B), tauP396 (C and D), and HTRA1 levels were measured by ELISA. E and F, neurofibrillary tangles (NFT) (E) and neuritic plaques (F) determined by immunohistochemistry (number/mm2) correlated with HTRA1 protein levels in frontal cortex sections of 25 AD brains.
DISCUSSION
Whereas the involvements of bacterial HtrA proteases in all aspects of protein quality control have been widely studied and convincingly demonstrated (2), there is limited experimental evidence that human HTRAs are involved in similar processes. A recent study showed the association of HTRA1 with amyloid deposits in the human cornea (54). Accordingly, the observations that HTRA1 degrades aggregated tau as well as Aβ peptides (24), its up-regulation on the transcriptional level and the activation of its proteolytic activity in response to the presence of elevated tau concentrations, respectively, suggest that the protein quality control function of HtrA proteases is conserved. The results presented in this work provide direct biochemical evidence for the association of HTRA1 with amyloid aggregates. Even though HTRA1 performs its tasks in an ATP-independent manner, aggregated and fibrillar forms of tau are degraded. The products of the proteolytic reaction are typically between 8 and 22 residues long, and sequence specificity analysis suggests a preference for Leu, Val, and Ile at the P1 position but little further specificities. This feature is similar to the bacterial HtrA family member DegP, which degrades unfolded substrates with little specificity into fragments of a mean length of 13–15 residues (28). However, DegP does not degrade aggregates.
There are at least two possibilities why HTRA1 is able to degrade tau aggregates. First, the active site of HTRA1 is surface-accessible (10); and second, the PDZ domain might be instrumental in this process because it might be involved in binding and thus extracting tau monomers from aggregates. In addition, it is unlikely that HTRA2 and HTRA3 degrade tau in cells because they are localized to mitochondria (55, 56), and there are still insufficient data on HTRA4 available to speculate about a possible functional redundancy with HTRA1 (22).
Digests of two synthetic substrates revealed that HTRA1 is activated by its native substrate tau. That both HTRA1 and HTRA1ΔPDZ domain are activated by substrate indicates that HTRA1 employs an induced-fit mechanism where substrate bound to the active site directly induces a rearrangement of regulatory elements within the protease domain (10) rather than an allosteric mechanism that was previously identified for all other HtrA proteases studied so far (2). In contrast, the mechanism of transcriptional up-regulation of HTRA1 in neuronal cells upon overexpression of tau and PHP-tau remains elusive. Even though there are numerous reports in the literature describing the up-regulation of HTRA1 expression, for example during endometriosis (57), age-related macular degeneration (58), neuronal development (59), following chemotherapy (60, 61), and during arthritis (15, 62), very little is known about the regulation of the HTRA1 gene on the transcriptional level. Therefore, the future identification of the signaling cascades and relevant transcription factors mediating the up-regulation of the HTRA1 promoter in response to the presence of increased levels of intracellular tau protein might be of general importance as these pathways might also play a role in the pathologies mentioned above.
Both the transcriptional and posttranscriptional regulatory mechanisms that contribute to elevated HTRA1 activity are expected to contribute to the marked reduction of tau levels upon overexpression of HTRA1 in neuronal PC12 cells. Furthermore, a significant inverse correlation between HTRA1 and tau levels is observed in AD patient brains. These data suggest that HTRA1 might function as a tau protease in vivo.
Unfolded protein response systems may represent one strategy of how organisms prevent early onset of protein folding diseases. Because these diseases are more prevalent in aged individuals it is conceivable that the various stress response systems are overwhelmed by additional tasks. In case of AD, additional substrates produced by aged tissues could compete with tau or Aβ for clearance by stress response factors leading to the accumulation and ultimately aggregation of toxic protein fragments. The serine protease HTRA1 represents a viable candidate for a disease-modifying factor that might contribute, perhaps in concert with other proteases such as calpain, to maintaining Aβ and tau levels low. This notion is supported by previous work suggesting that HTRA1 is involved in the β-amyloid pathway by performing alternative processing of various amyloid precursor protein fragments, i.e. Cys99, Aβ42, and Aβ40. In line with this hypothesis, accumulation of Aβ was observed in astrocytoma cell culture supernatants following chemical inhibition of HTRA1 and by co-localization of HTRA1 with β-amyloid deposits in human brain samples (24). The recently reported association of HTRA1 with corneal amyloid deposits of TGFBI (transforming growth factor β-induced gene), however, raises both the possibility of the generation of amyloidogenic fragments and the clearance of amyloid aggregates through proteolysis by HTRA1 (54). Moreover, recent evidence from studying adult macular degeneration suggests that it is the increased activity of HTRA1, resulting from its overexpression that causes disease symptoms (12, 13). These data illustrate conflicting findings with regard to the toxicity of protein fragments, their aggregation, as well as the functional role of proteolytic processing by protein quality control factors. Addressing these contrasting models in future studies is likely an important step for our general understanding of the underlying mechanisms of protein folding diseases.
Brain tissue was obtained from the German Brain Bank “Brain-Net,” which is supported by the Federal Ministry of Education and Research.

This article contains supplemental Figs. S1–S4, Experimental Procedures, and additional references.
- HtrA
- high temperature requirement A
- AD
- Alzheimer disease
- AFM
- atomic force microscopy
- Bis-Tris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- PHP
- pseudohyperphosphorylated
- pNA
- p-nitroaniline
- qRT-PCR
- quantitative RT-PCR
- ThT
- thioflavin T.
REFERENCES
- 1. Clausen T., Southan C., Ehrmann M. (2002) The HtrA family of proteases: implications for protein composition and cell fate. Mol. Cell 10, 443–455 [DOI] [PubMed] [Google Scholar]
- 2. Clausen T., Kaiser M., Huber R., Ehrmann M. (2011) HTRA proteases: regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 12, 152–162 [DOI] [PubMed] [Google Scholar]
- 3. Kley J., Schmidt B., Boyanov B., Stolt-Bergner P. C., Kirk R., Ehrmann M., Knopf R. R., Naveh L., Adam Z., Clausen T. (2011) Structural adaptation of the plant protease Deg1 to repair photosystem II during light exposure. Nat. Struct. Mol. Biol. 18, 728–731 [DOI] [PubMed] [Google Scholar]
- 4. Sawa J., Malet H., Krojer T., Canellas F., Ehrmann M., Clausen T. (2011) Molecular adaptation of the DegQ protease to exert protein quality control in the bacterial cell envelope. J. Biol. Chem. 286, 30680–30690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merdanovic M., Clausen T., Kaiser M., Huber R., Ehrmann M. (2011) Protein quality control in the bacterial periplasm. Annu. Rev. Microbiol. 65, 149–168 [DOI] [PubMed] [Google Scholar]
- 6. Isaac D. D., Pinkner J. S., Hultgren S. J., Silhavy T. J. (2005) The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc. Natl. Acad. Sci. U.S.A. 102, 17775–17779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ehrmann M., Clausen T. (2004) Proteolysis as a regulatory mechanism. Annu. Rev. Genet. 38, 709–724 [DOI] [PubMed] [Google Scholar]
- 8. Wilken C., Kitzing K., Kurzbauer R., Ehrmann M., Clausen T. (2004) Crystal structure of the DegS stress sensor: how a PDZ domain recognizes misfolded protein and activates a protease. Cell 117, 483–494 [DOI] [PubMed] [Google Scholar]
- 9. Spiess C., Beil A., Ehrmann M. (1999) A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97, 339–347 [DOI] [PubMed] [Google Scholar]
- 10. Truebestein L., Tennstaedt A., Mönig T., Krojer T., Canellas F., Kaiser M., Clausen T., Ehrmann M. (2011) Substrate-induced remodeling of the active site regulates human HTRA1 activity. Nat. Struct. Mol. Biol. 18, 386–388 [DOI] [PubMed] [Google Scholar]
- 11. Krojer T., Sawa J., Schäfer E., Saibil H. R., Ehrmann M., Clausen T. (2008) Structural basis for the regulated protease and chaperone function of DegP. Nature 453, 885–890 [DOI] [PubMed] [Google Scholar]
- 12. Jones A., Kumar S., Zhang N., Tong Z., Yang J. H., Watt C., Anderson J., Amrita, Fillerup H., McCloskey M., Luo L., Yang Z., Ambati B., Marc R., Oka C., Zhang K., Fu Y. (2011) Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proc. Natl. Acad. Sci. U.S.A. 108, 14578–14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vierkotten S., Muether P. S., Fauser S. (2011) Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch's membrane via cleavage of extracellular matrix components. PLoS ONE 6, e22959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grau S., Richards P. J., Kerr B., Hughes C., Caterson B., Williams A. S., Junker U., Jones S. A., Clausen T., Ehrmann M. (2006) The role of human HtrA1 in arthritic disease. J. Biol. Chem. 281, 6124–6129 [DOI] [PubMed] [Google Scholar]
- 15. Tsuchiya A., Yano M., Tocharus J., Kojima H., Fukumoto M., Kawaichi M., Oka C. (2005) Expression of mouse HtrA1 serine protease in normal bone and cartilage and its up-regulation in joint cartilage damaged by experimental arthritis. Bone 37, 323–336 [DOI] [PubMed] [Google Scholar]
- 16. Chamberland A., Wang E., Jones A. R., Collins-Racie L. A., LaVallie E. R., Huang Y., Liu L., Morris E. A., Flannery C. R., Yang Z. (2009) Identification of a novel HtrA1-susceptible cleavage site in human aggrecan: evidence for the involvement of HtrA1 in aggrecan proteolysis in vivo. J. Biol. Chem. 284, 27352–27359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hadfield K. D., Rock C. F., Inkson C. A., Dallas S. L., Sudre L., Wallis G. A., Boot-Handford R. P., Canfield A. E. (2008) HtrA1 inhibits mineral deposition by osteoblasts: requirement for the protease and PDZ domains. J. Biol. Chem. 283, 5928–5938 [DOI] [PubMed] [Google Scholar]
- 18. Chien J., Ota T., Aletti G., Shridhar R., Boccellino M., Quagliuolo L., Baldi A., Shridhar V. (2009) Serine protease HtrA1 associates with microtubules and inhibits cell migration. Mol. Cell. Biol. 29, 4177–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clawson G. A., Bui V., Xin P., Wang N., Pan W. (2008) Intracellular localization of the tumor suppressor HtrA1/Prss11 and its association with HPV16 E6 and E7 proteins. J. Cell. Biochem. 105, 81–88 [DOI] [PubMed] [Google Scholar]
- 20. Campioni M., Severino A., Manente L., Tuduce I. L., Toldo S., Caraglia M., Crispi S., Ehrmann M., He X., Maguire J., De Falco M., De Luca A., Shridhar V., Baldi A. (2010) The serine protease HtrA1 specifically interacts and degrades the tuberous sclerosis complex 2 protein. Mol. Cancer Res. 8, 1248–1260 [DOI] [PubMed] [Google Scholar]
- 21. Chien J., He X., Shridhar V. (2009) Identification of tubulins as substrates of serine protease HtrA1 by mixture-based oriented peptide library screening. J. Cell. Biochem. 107, 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chien J., Campioni M., Shridhar V., Baldi A. (2009) HtrA serine proteases as potential therapeutic targets in cancer. Curr. Cancer Drug Targets 9, 451–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Z., Camp N. J., Sun H., Tong Z., Gibbs D., Cameron D. J., Chen H., Zhao Y., Pearson E., Li X., Chien J., Dewan A., Harmon J., Bernstein P. S., Shridhar V., Zabriskie N. A., Hoh J., Howes K., Zhang K. (2006) A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 314, 992–993 [DOI] [PubMed] [Google Scholar]
- 24. Grau S., Baldi A., Bussani R., Tian X., Stefanescu R., Przybylski M., Richards P., Jones S. A., Shridhar V., Clausen T., Ehrmann M. (2005) Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. U.S.A. 102, 6021–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milner J. M., Patel A., Rowan A. D. (2008) Emerging roles of serine proteinases in tissue turnover in arthritis. Arthritis Rheum. 58, 3644–3656 [DOI] [PubMed] [Google Scholar]
- 26. Hara K., Shiga A., Fukutake T., Nozaki H., Miyashita A., Yokoseki A., Kawata H., Koyama A., Arima K., Takahashi T., Ikeda M., Shiota H., Tamura M., Shimoe Y., Hirayama M., Arisato T., Yanagawa S., Tanaka A., Nakano I., Ikeda S., Yoshida Y., Yamamoto T., Ikeuchi T., Kuwano R., Nishizawa M., Tsuji S., Onodera O. (2009) Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N. Engl. J. Med. 360, 1729–1739 [DOI] [PubMed] [Google Scholar]
- 27. Hasenbein S., Meltzer M., Hauske P., Kaiser M., Huber R., Clausen T., Ehrmann M. (2010) Conversion of a regulatory into a degradative protease. J. Mol. Biol. 397, 957–966 [DOI] [PubMed] [Google Scholar]
- 28. Krojer T., Pangerl K., Kurt J., Sawa J., Stingl C., Mechtler K., Huber R., Ehrmann M., Clausen T. (2008) Interplay of PDZ and protease domain of DegP ensures efficient elimination of misfolded proteins. Proc. Natl. Acad. Sci. U.S.A. 105, 7702–7707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krojer T., Sawa J., Huber R., Clausen T. (2010) HtrA proteases have a conserved activation mechanism that can be triggered by distinct molecular cues. Nat. Struct. Mol. Biol. 17, 844–852 [DOI] [PubMed] [Google Scholar]
- 30. Merdanovic M., Mamant N., Meltzer M., Poepsel S., Auckenthaler A., Melgaard R., Hauske P., Nagel-Steger L., Clarke A. R., Kaiser M., Huber R., Ehrmann M. (2010) Determinants of structural and functional plasticity of a widely conserved protease chaperone complex. Nat. Struct. Mol. Biol. 17, 837–843 [DOI] [PubMed] [Google Scholar]
- 31. Morris M., Maeda S., Vossel K., Mucke L. (2011) The many faces of tau. Neuron 70, 410–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brandt R., Hundelt M., Shahani N. (2005) Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim. Biophys. Acta 1739, 331–354 [DOI] [PubMed] [Google Scholar]
- 33. Eidenmüller J., Fath T., Hellwig A., Reed J., Sontag E., Brandt R. (2000) Structural and functional implications of tau hyperphosphorylation: information from phosphorylation-mimicking mutated tau proteins. Biochemistry 39, 13166–13175 [DOI] [PubMed] [Google Scholar]
- 34. Roger B., Al-Bassam J., Dehmelt L., Milligan R. A., Halpain S. (2004) MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr. Biol. 14, 363–371 [DOI] [PubMed] [Google Scholar]
- 35. Morgenstern J. P., Land H. (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18, 3587–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fath T., Eidenmüller J., Brandt R. (2002) Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer's disease. J. Neurosci. 22, 9733–9741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li W., Lee V. M. (2006) Characterization of two VQIXXK motifs for tau fibrillization in vitro. Biochemistry 45, 15692–15701 [DOI] [PubMed] [Google Scholar]
- 38. Abramoff M. D., Magalhaes P. J., Ram S. J. (2004) Image Processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 39. Hauske P., Meltzer M., Ottmann C., Krojer T., Clausen T., Ehrmann M., Kaiser M. (2009) Selectivity profiling of DegP substrates and inhibitors. Bioorg. Med. Chem. 17, 2920–2924 [DOI] [PubMed] [Google Scholar]
- 40. Meltzer M., Hasenbein S., Hauske P., Kucz N., Merdanovic M., Grau S., Beil A., Jones D., Krojer T., Clausen T., Ehrmann M., Kaiser M. (2008) Allosteric activation of HtrA protease DegP by stress signals during bacterial protein quality control. Angew. Chem. Int. Ed. Engl. 47, 1332–1334 [DOI] [PubMed] [Google Scholar]
- 41. Xia S. J., Rajput P., Strzelecki D. M., Barr F. G. (2007) Analysis of genetic events that modulate the oncogenic and growth suppressive activities of the PAX3-FKHR fusion oncoprotein. Lab. Invest. 87, 318–325 [DOI] [PubMed] [Google Scholar]
- 42. Severino A., Campioni M., Straino S., Salloum F. N., Schmidt N., Herbrand U., Frede S., Toietta G., Di Rocco G., Bussani R., Silvestri F., Piro M., Liuzzo G., Biasucci L. M., Mellone P., Feroce F., Capogrossi M., Baldi F., Fandrey J., Ehrmann M., Crea F., Abbate A., Baldi A. (2007) Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 50, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 43. Mirra S. S., Heyman A., McKeel D., Sumi S. M., Crain B. J., Brownlee L. M., Vogel F. S., Hughes J. P., van Belle G., Berg L. (1991) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 41, 479–486 [DOI] [PubMed] [Google Scholar]
- 44. Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K. (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang L. S., Gordon-Krajcer W., Ksiezak-Reding H. (1997) Tau released from paired helical filaments with formic acid or guanidine is susceptible to calpain-mediated proteolysis. J. Neurochem. 69, 1548–1558 [DOI] [PubMed] [Google Scholar]
- 46. Egensperger R., Kösel S., von Eitzen U., Graeber M. B. (1998) Microglial activation in Alzheimer disease: association with APOE genotype. Brain Pathol. 8, 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Friedhoff P., Schneider A., Mandelkow E. M., Mandelkow E. (1998) Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry 37, 10223–10230 [DOI] [PubMed] [Google Scholar]
- 48. Rissman R. A., Poon W. W., Blurton-Jones M., Oddo S., Torp R., Vitek M. P., LaFerla F. M., Rohn T. T., Cotman C. W. (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Invest. 114, 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mercken M., Grynspan F., Nixon R. A. (1995) Differential sensitivity to proteolysis by brain calpain of adult human tau, fetal human tau and PHF-tau. FEBS Lett. 368, 10–14 [DOI] [PubMed] [Google Scholar]
- 50. Talanian R. V., Quinlan C., Trautz S., Hackett M. C., Mankovich J. A., Banach D., Ghayur T., Brady K. D., Wong W. W. (1997) Substrate specificities of caspase family proteases. J. Biol. Chem. 272, 9677–9682 [DOI] [PubMed] [Google Scholar]
- 51. Crowther R. A. (1991) Straight and paired helical filaments in Alzheimer disease have a common structural unit. Proc. Natl. Acad. Sci. U.S.A. 88, 2288–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berriman J., Serpell L. C., Oberg K. A., Fink A. L., Goedert M., Crowther R. A. (2003) Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-β structure. Proc. Natl. Acad. Sci. U.S.A. 100, 9034–9038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barghorn S., Davies P., Mandelkow E. (2004) Tau paired helical filaments from Alzheimer's disease brain and assembled in vitro are based on β-structure in the core domain. Biochemistry 43, 1694–1703 [DOI] [PubMed] [Google Scholar]
- 54. Karring H., Runager K., Thøgersen I. B., Klintworth G. K., Højrup P., Enghild J. J. (2012) Composition and proteolytic processing of corneal deposits associated with mutations in the TGFBI gene. Exp. Eye Res. 96, 163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suzuki Y., Imai Y., Nakayama H., Takahashi K., Takio K., Takahashi R. (2001) A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 8, 613–621 [DOI] [PubMed] [Google Scholar]
- 56. Beleford D., Rattan R., Chien J., Shridhar V. (2010) High temperature requirement A3 (HtrA3) promotes etoposide- and cisplatin-induced cytotoxicity in lung cancer cell lines. J. Biol. Chem. 285, 12011–12027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dentillo D. B., Meola J., Rosa e Silva J. C., Giuliatti S., Silva W. A., Jr., Ferriani R. A., Martelli L. (2010) Deregulation of LOXL1 and HTRA1 gene expression in endometriosis. Reprod. Sci. 17, 1016–1023 [DOI] [PubMed] [Google Scholar]
- 58. Yang Z., Tong Z., Chen Y., Zeng J., Lu F., Sun X., Zhao C., Wang K., Davey L., Chen H., London N., Muramatsu D., Salasar F., Carmona R., Kasuga D., Wang X., Bedell M., Dixie M., Zhao P., Yang R., Gibbs D., Liu X., Li Y., Li C., Campochiaro B., Constantine R., Zack D. J., Campochiaro P., Fu Y., Li D. Y., Katsanis N., Zhang K. (2010) Genetic and functional dissection of HTRA1 and LOC387715 in age-related macular degeneration. PLoS Genet. 6, e1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Launay S., Maubert E., Lebeurrier N., Tennstaedt A., Campioni M., Docagne F., Gabriel C., Dauphinot L., Potier M. C., Ehrmann M., Baldi A., Vivien D. (2008) HtrA1-dependent proteolysis of TGF-β controls both neuronal maturation and developmental survival. Cell Death Differ. 15, 1408–1416 [DOI] [PubMed] [Google Scholar]
- 60. Chien J., Aletti G., Baldi A., Catalano V., Muretto P., Keeney G. L., Kalli K. R., Staub J., Ehrmann M., Cliby W. A., Lee Y. K., Bible K. C., Hartmann L. C., Kaufmann S. H., Shridhar V. (2006) Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J. Clin. Invest. 116, 1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Spugnini E. P., Cardillo I., Verdina A., Crispi S., Saviozzi S., Calogero R., Nebbioso A., Altucci L., Cortese G., Galati R., Chien J., Shridhar V., Vincenzi B., Citro G., Cognetti F., Sacchi A., Baldi A. (2006) Piroxicam and cisplatin in a mouse model of peritoneal mesothelioma. Clin. Cancer Res. 12, 6133–6143 [DOI] [PubMed] [Google Scholar]
- 62. Polur I., Lee P. L., Servais J. M., Xu L., Li Y. (2010) Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histol. Histopathol. 25, 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]