Background: Proper glycosylation of α-dystroglycan is a complex process critical for function.
Results: Pre-existing O-mannose sites can regulate O-GalNAc addition by the ppGalNAc-Ts.
Conclusion: O-Mannosylation has a significant impact on the pattern of another form of glycosylation (O-GalNAc) in α-dystroglycan.
Significance: Contributions to disease phenotypes associated with α-dystroglycan O-mannosylation defects may arise from its impact on other types of glycosylation.
Keywords: Glycoprotein, Glycosylation, Glycosyltransferases, Mass Spectrometry (MS), Muscular Dystrophy, Post-translational Modification, UDP-GalNAc Polypeptide N-Acetylgalactosaminyltransferase, α-Dystroglycan
Abstract
O-Linked glycosylation is a functionally and structurally diverse type of protein modification present in many tissues and across many species. α-Dystroglycan (α-DG), a protein linked to the extracellular matrix, whose glycosylation status is associated with human muscular dystrophies, displays two predominant types of O-glycosylation, O-linked mannose (O-Man) and O-linked N-acetylgalactosamine (O-GalNAc), in its highly conserved mucin-like domain. The O-Man is installed by an enzyme complex present in the endoplasmic reticulum. O-GalNAc modifications are initiated subsequently in the Golgi apparatus by the UDP-GalNAc polypeptide N-acetylgalactosaminyltransferase (ppGalNAc-T) enzymes. How the presence and position of O-Man influences the action of the ppGalNAc-Ts on α-DG and the distribution of the two forms of glycosylation in this domain is not known. Here, we investigated the interplay between O-Man and the addition of O-GalNAc by examining the activity of the ppGalNAc-Ts on peptides and O-Man-containing glycopeptides mimicking those found in native α-DG. These synthetic glycopeptides emulate intermediate structures, not otherwise readily available from natural sources. Through enzymatic and mass spectrometric methods, we demonstrate that the presence and specific location of O-Man can impact either the regional exclusion or the site of O-GalNAc addition on α-DG, elucidating the factors contributing to the glycosylation patterns observed in vivo. These results provide evidence that one form of glycosylation can influence another form of glycosylation in α-DG and suggest that in the absence of proper O-mannosylation, as is associated with certain forms of muscular dystrophy, aberrant O-GalNAc modifications may occur and could play a role in disease presentation.
Introduction
α-Dystroglycan (α-DG)5 is an evolutionarily conserved glycoprotein component of the dystrophin complex, which is responsible for anchoring the cellular cytoskeleton to the extracellular matrix (ECM) (1–3). α-DG is known to be required for proper skeletal muscle cell integrity and attachment to the ECM. Loss of α-DG function is responsible for certain forms of muscular dystrophy in humans, a number of which are due to mutations in the glycosyltransferases that modify α-DG (2, 4). Furthermore, changes in α-DG glycosylation are also associated with various neurological and eye defects, and abnormal kidney morphology (3, 5), indicating its role across multiple organs and tissues. In addition to being required for proper cell/ECM attachment in diverse organ systems, α-DG is a point of engagement in infection by several viruses, including lymphocytic choriomeningitis virus and Lassa virus (6, 7).
The diseases that result from the loss of α-DG function are referred to as dystroglycanopathies (8, 9) and fall under the category of congenital disorders of glycosylation. One glycosylation event of particular interest is the addition of α-O-Man on serines and threonines within the mucin-like domain of α-DG. This has been the focus of considerable attention not only because defects in the O-Man glycans have been associated with disease (4), but also because it was the first, and remains the only well characterized example of this type of protein modification in higher animals (10–12), although other instances have been indicated (13, 14). This glycosylation is initiated by a pair of enzymes, protein O-mannosyltransferase 1 or 2, which act in concert in the endoplasmic reticulum to catalyze the addition of O-Man (2, 15, 16). O-Man sugars are then further extended, commonly to form the tetrasaccharide NeuAc-α2–3Gal-β1–4GlcNAc-β1–2Man-α-O-S/T (3). Although mutations in the enzymes responsible for the assembly of this O-Man glycan are associated with various forms of muscular dystrophy (2, 4), the specific mechanism by which this glycan impacts function of α-DG is not yet known. Interestingly, recent studies have uncovered yet another glycan based on an O-Man-6-phosphate structure on α-DG that has been shown to interact with laminin (17, 18), a function associated with the ability of α-DG to mediate attachment to the ECM. Altogether these data highlight both the importance and complexity of glycan modifications on α-DG.
The presence of another sugar modification, O-GalNAc, on serines and threonines of α-DG has been identified as well (10–12, 19). In contrast to the addition of O-Man, which occurs in the endoplasmic reticulum, O-GalNAc sites are initiated subsequently in the Golgi apparatus by a family of enzymes known as the ppGalNAc-Ts (20–22). The ppGalNAc-T family of enzymes are type II transmembrane proteins that contain a catalytic domain and a lectin-like carbohydrate-recognition domain found within the lumen of the Golgi apparatus (20–22). This family is subdivided into members that will add GalNAc to unmodified peptides (peptide transferases) and those that will only add GalNAc to substrates that already contain a GalNAc sugar (glycopeptide transferases). How the extant GalNAc on glycopeptide substrates is recognized by the ppGalNAc-T enzymes is not completely understood. However, previous studies suggest that both the catalytic and lectin domains can influence the sites of subsequent GalNAc addition (23). In the case of ppGalNAc-T2, the lectin domain appears to be responsible for recognizing extant GalNAcs, whereas the catalytic domain takes on this function in ppGalNAc-T10 (23).
Aberrations in the O-GalNAc glycans of α-DG have not yet been associated with muscular dystrophy, although evidence from cell culture studies have implicated these glycans as potentially participating in the elaboration of the structure on α-DG that contributes to its laminin binding capacity (24, 25). At present the molecular basis for only a little more than half of the dystroglycanopathies have been defined (9), suggesting that defects in other activities, perhaps involving synthesis of O-GalNAc glycans, may be contributing factors. The O-Man and O-GalNAc glycans are predominantly restricted to certain respective regions of the α-DG mucin-like domain, implying regulation of the action of the ppGalNAc-Ts. Furthermore, O-GalNAc addition is required for viability in Drosophila (26–28) and is known to affect ECM protein secretion (29) and protease sensitivity (30). Additionally, the presence of O-GalNAc glycosylation can influence peptide and protein structural properties, stabilizing more extended structures in regions where these glycans are present (31, 32), in contrast to the effects of O-Man modifications that have more modest conformational effects (33, 34). The molecular morphology of α-DG as revealed by electron microscopy (35, 36) indicates N- and C-terminal globular domains separated by a central extended region. The latter is similar to that found in conventional mucins. Glycosylation mapping (10–12) indicates that the N-terminal portion of the mucin domain is predominantly modified by O-Man, which would allow a globular character, whereas the region that follows has a greater prevalence of O-GalNAc, likely contributing to the extended region. If O-GalNAc modifications were aberrantly present at the extreme N terminus, the organization and interactions might be perturbed. It is expected that disruption of the tertiary structure of the N-terminal domain would be detrimental to function. The highly conserved sequence and the organization of the O-glycosylation suggest a significant structure/function relationship in these features for α-DG.
Given the biological importance of O-Man and O-GalNAc glycans and the regional distribution of both in α-DG (10–12), we set out to investigate the interplay of these two modifications and address the extent to which O-Man modifications impact the action of ppGalNAc-Ts. Because the installation of O-GalNAc normally occurs in the Golgi and is subsequent to O-Man addition in the endoplasmic reticulum, the O-Man-modified form represents a relevant intermediate stage for examining how the presence of O-Man at specific residues in α-DG influences the addition of O-GalNAc by the ppGalNAc-Ts. Although the role of the underlying amino acid sequence, as well as pre-existing sites of α-O-GalNAc on the activity of various ppGalNAc-T isoforms has been investigated (37–42), the impact of O-Man on O-GalNAc addition is unknown. Because isolation of homogeneous intermediate glycoproteins from natural sources is not practical, synthetic O-Man glycopeptides based on glycoforms of native α-DG (10) were synthesized and used as substrates in vitro. Two sequence segments of α-DG, each of which has clusters of four adjacent potential sites of glycosylation, were investigated with this strategy. The first is from a region of α-DG where only O-Man modifications are detected in vivo and the second is from a region populated with both O-GalNAc and O-Man sites (10, 11). From our in vitro results, which recapitulate patterns observed in the native rabbit skeletal muscle α-DG (10), we demonstrate that O-Man modifications at certain sites can substantially suppress, as well as alter sites of GalNAc addition. Thus one form of glycosylation can affect the presence and position of other forms of glycosylation. These studies shed light on the interplay between distinct forms of glycosylation on the same protein and suggest that changes in one form of glycosylation may impact the abundance and position of other forms of glycosylation in vivo, possibly contributing to variations in disease severity or presentation.
EXPERIMENTAL PROCEDURES
Expression of Secreted, Recombinant ppGalNAc-Ts
ppGalNAc-T isoforms were expressed as recombinant enzymes as described previously (43). Briefly, COS7 cells were grown to ∼90% confluence and transfected with each recombinant ppGalNAc-T as described. Recombinant enzymes were harvested directly from the media of transfected cells and used in enzyme assays.
Glycosyltransferase Assays
Assays for ppGalNAc-T activity were performed as described previously (28), but in the absence of Triton X-100. Briefly, media from COS7 cells expressing recombinant enzymes were harvested and equal volumes were used in the in vitro reactions with: 7.3 μm [14C]UDP-GalNAc (54.7 mCi/mmol; 0.02 mCi/ml), 44 μm cold UDP-GalNAc, 40 mm cacodylate (pH 6.5), 40 mm 2-mercaptoethanol, 10 mm MnCl2, and 500 μm of the acceptor substrates. All reactions were performed in duplicate at 37 °C for 1 h. Reaction products were purified by anion exchange chromatography and [14C]GalNAc incorporation was measured. Reactions using media from cells expressing empty vector alone were used as negative controls and yielded background values that were subtracted from each experimental value. Additional negative control reactions were run for each enzyme in the absence of acceptor peptide. These background values were also subtracted from each experimental value. The adjusted experimental values were then averaged, and standard deviations were calculated. Enzyme activity is expressed as dpm/h. ppGalNAc-T isoforms that displayed activity against appropriate positive control peptide substrates were used in this study, including ppGalNAc-T1 (44), -T2 (45), -T3 (46), -T4 (43), -T5 (47), -T7 (48), -T10 (49), -T11 (26), and -T16 (22). Positive control peptide substrates were EA2 (PTTDSTTPAPTTK) (50) and MUC5AC-3/13 (GTT*PSPVPTTSTT*SAP) (where * denotes a GalNAc modified residue) (51).
Quantitative Real-time PCR
Mouse skeletal muscle and kidney total RNA were purchased from Clontech Laboratories. cDNA synthesis was performed using the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR primers for the genes (Galnts) encoding the ppGalNAc-T enzymes were designed using Beacon Designer software (Premier Biosoft) and are shown in supplemental Table S1. Quantitative real-time PCR was performed on a CFX96 Real-time system (Bio-Rad) using SYBR Green PCR Master Mix (Bio-Rad). Gene expression was normalized to the 29 S rRNA. Assays were run in triplicate.
Glycosyltransferase Assays for Mass Spectrometric Analysis
Media from COS7 cells expressing recombinant enzymes were harvested and equal volumes of media were used in the in vitro reactions with: 440 μm cold UDP-GalNAc, 40 mm cacodylate (pH 6.5), 40 mm 2-mercaptoethanol, 10 mm MnCl2, and 500 μm of the acceptor substrates. All reactions were performed for 24 h in the presence of protease inhibitors (Sigma P8340 and P8849).
Analysis of Sites of Modification via Mass Spectrometry
With the exception of some products from the enzyme reactions on the peptide Ac-PPTTTTKKP-NH2 for which collision-induced dissociation mass spectrometry (MS) methods were used, electron transfer dissociation (ETD) mass spectrometry methods were employed. For ETD, the resulting peptides were resuspended in 1% formic acid, 50% acetonitrile and directly infused at 0.5 μl/min into a linear ion trap equipped with ETD (LTQ XL-ETD from ThermoFisher). The substrate peptide as well as m/z ions corresponding to the addition of 1 to 2 GalNAc residues were manually trapped and fragmented via activated ETD (using a 100-ms reaction time with fluoranthene). The resulting fragmentation spectra were analyzed using Bioworks (ThermoFisher) and assigned sites of modification were confirmed via manual inspection. A parent ion monitoring mode via LC-MS/MS (LTQ-Orbitrap XL; ThermoFisher) was used to study the products of Ac-PPTTTTKKP-NH2. The resulting fragmentation spectra were analyzed using Proteome Discover (ThermoFisher) and validated by manual inspection.
Solid Phase Peptide Synthesis of Ac-RIRT(α-d-Man)TTSGVPR-NH2, Ac-RIRTT(α-d-Man)TSGVPR-NH2, Ac-RIRTTT(α-d-Man)SGVPR-NH2, Ac-RIRTTTS(α-d-Man)GVPR-NH2, and Ac-RIRTTTSGVPR-NH2
The peptide chain assembly was carried out with solid phase techniques, starting with Fmoc-PAL-PEG-PS resin (150 mg, 0.18 mmol/g). Side chain protection was provided by Pbf for Arg and t-Bu for Ser and Thr. Fmoc removal was achieved with piperidine:N,N′-dimethylformamide (1:4), and N-acetyl capping was carried out with Ac2O:N,N′-dimethylformamide (1:4). Couplings of Fmoc-amino acid derivatives were mediated by 2-(1H-6-chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate/1-hydroxybenzotriazole/N,N′-diisopropylethylamine in N,N′-dimethylformamide. Constructs Ac-RIRT(α-d-Man)TTSGVPR-NH2, Ac-RIRTTT(α-d-Man)SGVPR-NH2, and Ac-RIRTTTS(α-d-Man)GVPR-NH2 were synthesized manually without stirring in a CEM Discover Microwave reactor. Fmoc removal and N-acetyl capping were carried out for 4 min at 75 °C and 30 W. Couplings of Fmoc-amino acids (3 eq), including Fmoc-Ser(Ac4-α-d-Man)-OPfp and Fmoc-Thr(Ac4-α-d-Man)-OPfp (33), were achieved for 9.5 min at 75 °C and 25 W. Ac-RIRTT(α-d-Man)TSGVPR-NH2 was synthesized manually at room temperature on a bench-top facility at 25 °C. Double couplings of Fmoc-Thr(Ac4-α-d-Man)-OPfp (3 and 1.2 eq) were carried out for 8 and 4 h, respectively. Double couplings were also applied to Fmoc-Thr(t-Bu)-OH and Fmoc-Arg(Pbf)-OH (both 3 eq). Ac-RIRTTTSGVPR-NH2 was synthesized on a ABI 433A peptide synthesizer using standard protocol with 10 eq of Fmoc-amino acids. Peptide cleavage from the corresponding resin was realized in a glass vessel (25 ml) by treatment with Reagent B, TFA:phenol:H2O:triisopropylsilane (88:5:5:2, 3 ml), under stirring for 2 h at 25 °C. The peptides were precipitated by adding cold anhydrous ether (100 ml) to the filtrates, and purified by semi-preparative RP-HPLC. For O-acetylated glycopeptides, de-acetylation reactions were carried out with NaOMe in methanol (pH was adjusted to ∼9, as detected by wet litmus paper), and monitored by analytical RP-HPLC. The de-acetylation reactions were complete within 5 h and quenched by adding powdered CO2 (dry ice) to pH ∼ 6. The corresponding glycopeptides were obtained after a second purification. The final peptide yields were 15–45% based on the initial loading of the resin. All the peptides were confirmed by MALDI-TOF (observed for glycopeptides, [M + H]+, 1446.8; for plain peptide, [M + H]+, 1284.7). The synthesis of constructs in the Ac-PPTTTTKKP-NH2 series has been reported (33).
RESULTS
O-Mannose Affects Sites of GalNAc Addition
To define the effect of the presence of O-Man on the activity of the ppGalNAc-T isoforms, we began by chemical synthesis of peptides and glycopeptides based on α-DG, because isolation of homogeneous glycoprotein intermediates from natural sources is not practical. We focused on two regions. The first set was from the peptide sequence Ac-PPTTTTKKP-NH2 incorporating residues 419–427 of α-DG derived from the larger 403–425 tryptic fragment, ATVTIPGYVEPTAVATPPTTTTK. MS mapping of this fragment from native rabbit skeletal muscle α-DG showed a total of 3 sites that were occupied by O-Man substitutions (10), but the specific sites within the segment were not established. The second set of constructs was based on Ac-RIRTTTSGVPR-NH2 covering positions 479–489 of α-DG, where both O-Man and O-GalNAc modifications were noted in the naturally derived tryptic glycopeptides (10).
Assay of Enzyme Activity on Defined Peptide and Glycopeptide Substrates
Initially we tested the ability of various members (ppGalNAc-T1, -T2, -T3, -T4, -T5, -T7, -T10, -T11, and -T16) to glycosylate each set of peptides and glycopeptides derived from α-DG, along with appropriate positive controls (Figs. 1, 2, and supplemental Figs. S2 and S3). Quantitative real-time PCR analysis of the expression of the genes encoding members of the ppGalNAc-T enzyme family (Galnts) in mouse skeletal muscle and kidney demonstrated that multiple family members are present in each tissue, with ppGalNAc-T1, -T2, and -T11 being most abundant in muscle, and ppGalNAc-T11, -T1, and -T3 being most abundant in kidney (supplemental Fig. S1). Of the enzymes tested, only ppGalNAc-T1, -T3, and -T5 exhibited activity against any of the α-DG peptides or glycopeptides (Figs. 1 and 2). The other ppGalNAc-T isoforms tested, including glycopeptide transferases ppGalNAc-T7 and -T10, did not show GalNAc incorporation into any of the α-DG substrates, although they did incorporate GalNAc into previously defined positive controls (supplemental Figs. S2 and S3).
FIGURE 1.
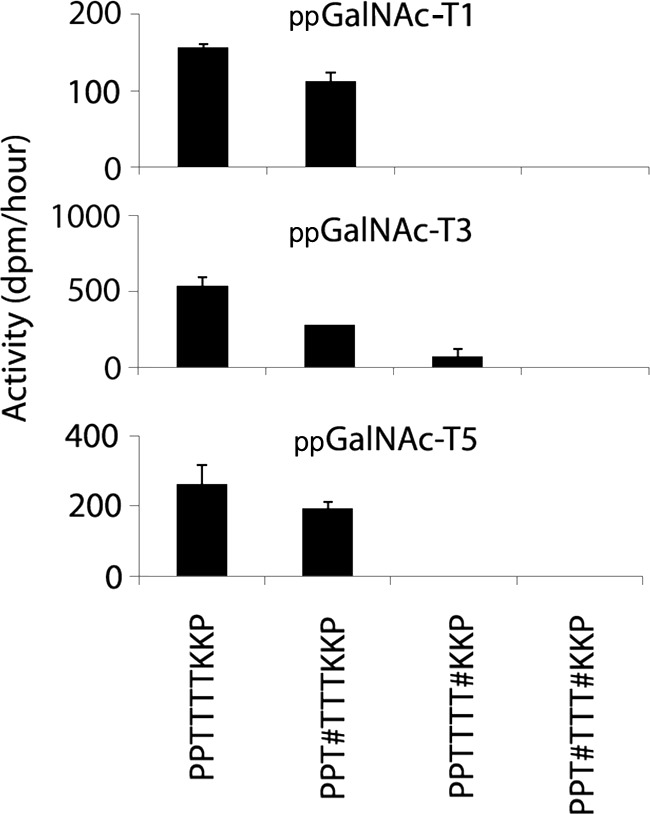
Initial rates of ppGalNAc -T1, -T3, and -T5 against peptides and glycopeptides derived from the PPTTTTKKP region of α-DG. Initial rates (expressed as dpm/h) are shown on the vertical axes. Acceptor substrates are shown along the X axes. # denotes position of mannose on the preceding amino acid. Error bars = standard deviation.
FIGURE 2.
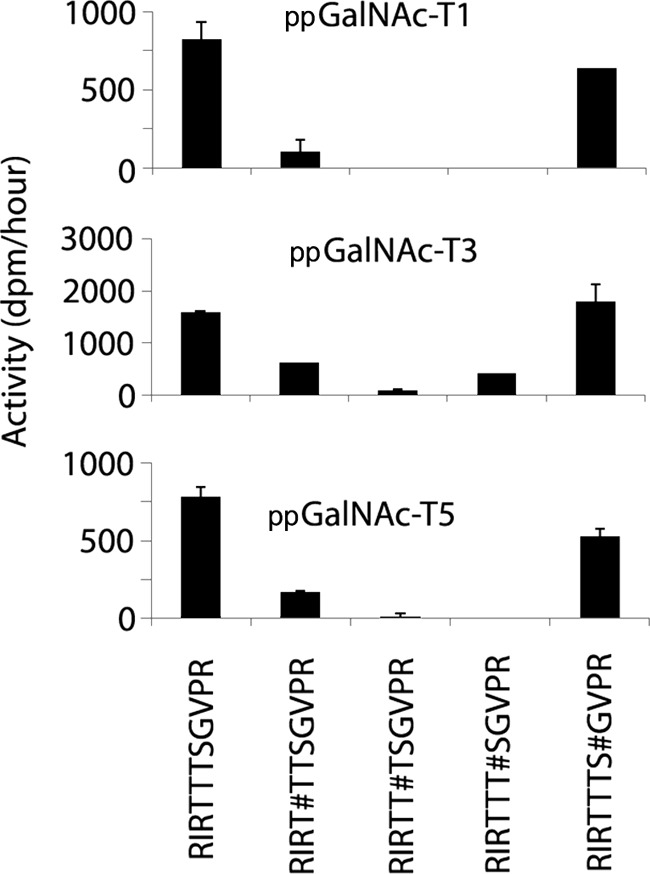
Initial rates of ppGalNAc -T1, -T3, and -T5 against peptides and glycopeptides derived from the RIRTTTSGVPR region of α-DG. Initial rates (expressed as dpm/h) are shown on the vertical axes. Acceptor substrates are shown along the X axes. # denotes the position of mannose on the preceding amino acid.
Initial rates of GalNAc addition by ppGalNAc-T1, -T3, or -T5 to unmodified α-DG peptides as well as O-mannosylated versions are shown in Figs. 1 and 2. ppGalNAc-T1 readily glycosylated the unmodified version of PPTTTTKKP as well as PPT#TTTKKP (where # denotes the presence of O-Man on the first threonine) but did not act appreciably on PPTTTT#KKP or PPT#TTT#KKP (Fig. 1). Similar results were seen with ppGalNAc-T3 and -T5 (Fig. 1). These data indicate that ppGalNAc-T1, -T3, or -T5 can glycosylate this region of α-DG in the absence of O-Man or when O-Man is present on the first threonine. However, the presence of O-Man on the fourth threonine of this peptide abrogated the ability of ppGalNAc-T1, -T3, or -T5 to transfer GalNAc to any of the remaining positions.
We next examined the peptide RIRTTTSGVPR and its monomannosylated derivatives. The choice of substrates was based on analysis of glycoforms from this fragment of native α-DG that had shown a maximum of one O-Man present in them (10). ppGalNAc-T1 added GalNAc to the unmodified peptide as well as RIRTTTS#GVPR (where O-Man is on the serine) to equivalent levels (Fig. 2). Additionally, ppGalNAc-T1 also added a small amount of GalNAc to RIRT#TTSGVPR. However, ppGalNAc-T1 did not add GalNAc to RIRTT#TSGVPR or RIRTTT#SGVPR, indicating that the presence of O-Man on the second or third threonine of this sequence interfered with GalNAc addition by ppGalNAc-T1. Similar results were obtained for ppGalNAc-T5 (Fig. 2), indicating that its activity is also sensitive to the presence of O-Man on the second or third threonine. ppGalNAc-T3, however, added GalNAc to RIRTTTS#GVPR, RIRT#TTSGVPR, and RIRTTT#SGVPR but not RIRTT#TSGVPR, indicating that ppGalNAc-T3, like ppGalNAc-T1 and -T5, is significantly inhibited by O-Man on the second threonine (Fig. 2). These results are fully consistent with what is found in rabbit skeletal muscle, where at most one O-Man was present even in glycoforms of this segment with GalNAc, and O-Man is the unique modification when it is on the second threonine (10). The other glycoforms from this region, where O-Man was not on the second threonine, had a total of two modifications, one each of O-Man and O-GalNAc. These observations suggest that our in vitro experiments can recapitulate the patterns of α-DG glycosylation found in vivo.
Identification of Sites of Enzymatic GalNAc Modification
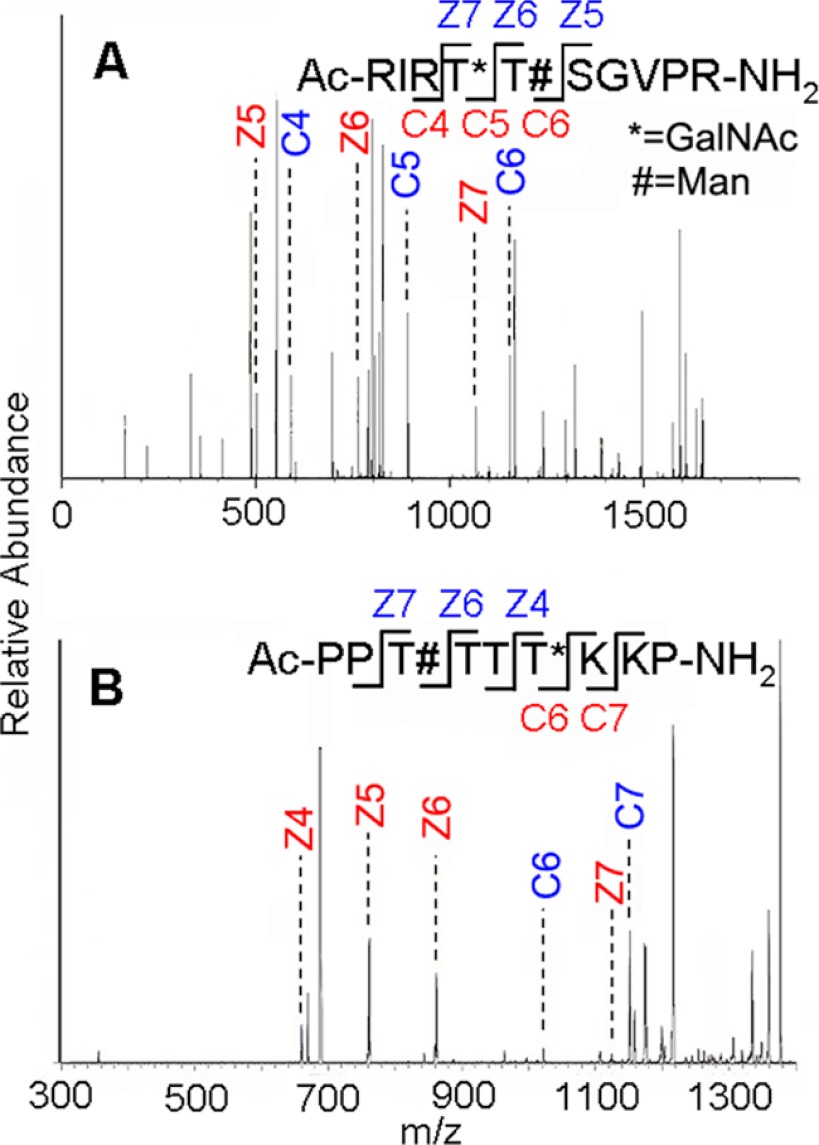
In addition to identifying which substrates were acceptors, we also determined how O-Man modifications influences the specific site of O-GalNAc addition by each ppGalNAc-T enzyme using ETD MS/MS (52, 53) or collision-induced dissociation MS/MS analysis on the products of the enzymatic reactions. Fig. 3 shows the MS/MS-based analysis of the structures of O-Man glycopeptide 479–489 (Fig. 3A) after reaction with ppGalNAc-T3, and O-Man glycopeptide 419–427 (Fig. 3B) after the first GalNAc addition by ppGalNAc-T1. Fig. 4 summarizes the sites of O-GalNAc addition found by ppGalNAc-T1, -T3, and -T5 on all nonmannosylated and O-mannosylated α-DG peptides PPTTTTKKP (A series) and RIRTTTSGVPR (B series). Sites of pre-installed O-Man are indicated by filled green circles and sites of O-GalNAc addition by the ppGalNAc-T enzymes are denoted by filled yellow squares.
FIGURE 3.
ETD-MS/MS fragmentation of O-Man glycopeptides products after action by ppGalNAc-T3 on glycopeptide 479–489 (A) and the initial product of action by ppGalNAc-T1 on glycopeptide 418–427 (B) of α-DG. The # symbol indicates sites where O-Man modifications were incorporated by chemical synthesis, and * indicates sites modified by GalNAc as a result of subsequent enzymatic addition. Key fragments peaks for site mapping are noted. The large number of lines in A arise from additional fragments of the singly charged form and also others from +2 and +3 multiply charged species.
FIGURE 4.
Summary of the sites of O-GalNAc addition by ppGalNAc-T1, -T3, and -T5 on peptide and O-mannosylated glycopeptides from regions of α-DG detected by mass spectrometry. Sites of preinstalled O-Man are indicated by filled green circles and sites of O-GalNAc addition by the ppGalNAc-T enzymes are denoted by filled yellow squares. A, results for the PPTTTTKKP-derived peptides and glycopeptides, and B, results for the RIRTTTSGVPR-derived peptides and glycopeptide.
In the absence of O-Man, ppGalNAc-T1, -T3, and -T5 added GalNAc at the fourth threonine on the PPTTTTKKP sequence (Fig. 4). As mentioned previously, when O-Man was present on the fourth threonine, GalNAc addition by ppGalNAc-T1, -T3, or -T5 was inhibited. When O-Man was present on the first threonine (PPT#TTTKKP), the preferred site of GalNAc incorporation by ppGalNAc-T1 or -T5 continued to be the fourth threonine, but the preferred site of GalNAc incorporation by ppGalNAc-T3 was changed to the third threonine (Fig. 4). These were the only singly modified forms detected in the MS/MS experiments. Diglycosylated forms were detected as well with ppGalNAc-T1 adding to the third threonine and ppGalNAc-T3 adding to the fourth threonine. The absence of evidence for singly glycosylated sites at these latter sites indicate that these were secondary modifications. Thus, whereas O-Man at specific sites is compatible with subsequent GalNAc addition by ppGalNAc-T1, -T3, and -T5, at others it is not. Also, O-Man on the first threonine can alter the subsequent position of GalNAc addition by ppGalNAc-T3.
For the unmodified RIRTTTSGVPR peptide, the third threonine was the primary site of addition by ppGalNAc-T1, -T3, or -T5 (Fig. 4). This is a residue identified in native α-DG as bearing an O-GalNAc (10). When mannose was present at the first threonine (RIRT#TTSGVPR) or the serine (RIRTTTS#GVPR), the third threonine continued to be the primary site of addition by ppGalNAc-T1, -T3, or -T5 (Fig. 4). As noted above, when O-Man was present on the third threonine (RIRTT#TSGVPR), addition of GalNAc by ppGalNAc-T1 and -T5 was inhibited. Interestingly, ppGalNAc-T3 was still able to add GalNAc to this glycopeptide, but now on the second threonine (Fig. 4). This was the only instance we found where this threonine residue was modified with GalNAc. These results provide additional examples where the presence of O-Man can inhibit the subsequent addition of GalNAc by certain members of the ppGalNAc-T family as well as alter the site of GalNAc addition.
DISCUSSION
The emerging appreciation of the importance of O-Man glycans and their coexistence with O-GalNAc modification in the biologically important glycoprotein α-DG (10–13), raises the question of how the initial O-mannosylation process affects the disposition of subsequent O-GalNAc glycans. The importance of α-DG glycosylation is illustrated by the human muscular dystrophies, many of which are due to mutations in glycosyltransferases that normally modify α-DG to mediate its ability to attach to the ECM (2). Although installation of O-Man and the enzymes responsible for its elaboration are primarily implicated in α-DG function, there are a number of examples of dystroglycanopathies where mutations are not found in either α-DG or enzymes presently known to be responsible for the synthesis of O-Man glycans, potentially implicating other forms of glycosylation in this disease (reviewed in Ref. 9). Additionally, the heterogeneity of clinical presentation, even in cases where the mutation has been identified, suggests there are other factors that contribute to α-DG function and disease severity. Thus, understanding how the diverse forms of glycosylation present on α-DG can influence one another is the first step in understanding the phenotypic diversity present in muscular dystrophies. In exploring the relationship of these two forms of O-glycosylation in α-DG we present evidence that one form of O-glycosylation can influence the subsequent addition as well as location of another form of glycosylation.
Our findings with the 419PPTTTTKKP427 segment of α-DG, a region normally devoid of O-GalNAc modifications in vivo, indicate that in the absence of a particular pattern of O-Man modification, O-GalNAc incorporation by certain ppGalNAc-Ts can occur. Extending this line of reasoning one would postulate that disruption of O-mannosylation in vivo, as in muscular dystrophy (2), may be accompanied by ectopic addition of O-GalNAc to portions of α-DG where it is normally absent. This may have a significant impact, as our structural studies have shown that O-GalNAc modifications can alter the peptide backbone conformation (33, 34). How these changes in glycosylation may affect the functional properties of α-DG in vivo remains to be determined.
In contrast, the second sequence we examined, 479RIRTTTSGVPR489, has a similar contiguous cluster of four potential sites for O-glycosylation, but shows a more complex pattern of glycoforms with both O-Man and O-GalNAc structures intermingled within this cluster (10). Here we have demonstrated that ppGalNAc-T1, -T3, and -T5 isoforms are able to reproduce the salient features of native glycoforms associated with this segment, thus supporting the validity of our approach using glycopeptides and recombinant enzymes. The comparative results of the two sequences examined indicate a role both for the underlying peptide and the sites of pre-existing glycan modifications in delineating the eventual sites of O-GalNAc addition. Although preferences of ppGalNAc-T activity as a function of other amino acids adjacent to the single threonine glycosylation acceptor site have been reported (37, 40), no data on the action or specificity of these enzymes on O-mannosylated glycopeptides has been examined previously.
From the results described here, it is evident that O-Man modifications on neighboring loci can significantly bias the subsequent activity and site selectivity of ppGalNAc-T enzymes. The influence of pre-existing O-GalNAc sites on ensuing GalNAc addition by ppGalNAc-Ts has been reported for several short glycopeptides with clusters of acceptor sites. The findings in the cases of isoforms -T1, and -T3, generally indicate a preference for addition to a neighbor on the N-terminal side of an existing site of GalNAc modification (41, 42). In contrast, for the 419–427 fragment, we found that O-mannosylation suppresses addition to Thr residues on the N-terminal side, but allows addition to C-terminal sites. In the case of the 479–489 glycopeptides, ppGalNAc-T activity could add to either side of the O-Man (Fig. 4). In each instance, the effects are qualitatively similar for all three active isoforms, suggesting similarities in the environment adjacent to the catalytic site. Interestingly, two members of the ppGalNAc-T family that require pre-existing O-GalNAc modifications on glycopeptide substrates, ppGalNAc-T7 and -T10 (40, 48, 49), showed no activity on any of the O-mannosylated substrates used in the present study. It is anticipated that O-Man and O-GalNAc glycopeptides would interact differently with the enzymes, given the differences in the structure of the sugar residue and the conformational properties of the glycopeptides (33, 34).
Because the short length of the substrates used would not accommodate binding by both the catalytic and lectin domains of the ppGalNAc-Ts, the preferences we have detected would predominantly arise from the specificity imparted by the catalytic domains of the enzymes. In the in vivo environment there are likely additional factors and components that impact this process, including potential interactions involving the lectin domains of the ppGalNAc-Ts, which may preferentially direct activity to sites based on pre-existing glycosylation on α-DG (23). This may explain why we did not observe GalNAc added to Ser485 in our experiments, although it was observed in one of the glycoforms of the 479–489 tryptic fragment from rabbit muscle α-DG (10). Nevertheless, the close correspondence between native glycosylation patterns found for rabbit muscle and those produced on our glycopeptides suggests that the preferences of the catalytic domains of the ppGalNAc-Ts have a significant influence. The ppGalNAc-T1 isoform is the most abundant in muscle (supplemental Fig. 1), and is the only isoform present in appreciable amounts in that tissue that we found active with α-DG-derived peptides and glycopeptides, suggesting its importance in processing α-DG in tissue. There are no data presently available that address the additional issue of whether extension of the O-Man, which in most cases would involve action of the POMGnT1 enzyme adding a GlcNAc through a β1–2 linkage (2), occurs before or after ppGalNAc-T modifications, and how it might impact the latter. In conventional mucin structures, extension of a pre-existing GalNAc can have a detrimental effect on ppGalNAc-T activity at nearby sites (41). The influence of more extended O-Man glycans of α-DG on GalNAc addition will be a fruitful area for future investigation.
The spatial and temporal separation of O-mannosylation and O-GalNAcylation processes effectively minimizes competition for the same sites of addition on α-DG. However, it has recently been suggested that under situations where Src kinase is activated, retrograde trafficking can relocate certain ppGalNAc-Ts to the endoplasmic reticulum (55), potentially allowing competition for sites of O-Man and O-GalNAc addition. Thus in various diseases associated with overexpression of Src (56) and/or altered glycosylation of α-DG (54, 57), one should consider how disruption of sequential glycosylation may be participating. Not only should the primary perturbation of O-mannosylation pattern be considered, but the effective gain of function for the ppGalNAc-Ts and/or changes in the site of GalNAc addition should be investigated as well. The specific glycosylation changes occurring in vivo where O-mannosylation is altered can be explored with cell culture systems using cell lines where glycosyltransferase enzyme expression has been manipulated (25).
In summary, we have investigated the interplay of two distinct forms of glycosylation found on α-DG. We demonstrate that the presence and position of O-Man influences the presence and location of subsequent O-GalNAc addition by the ppGalNAc-Ts, and can account for significant and in some regions dominant features in the pattern of O-GalNAc glycosylation observed in α-DG. We anticipate that such effects will be seen in other O-Man containing glycoproteins as they are characterized and examined in more detail. Our results suggest that loss or changes in one form of glycosylation may influence other forms of glycosylation in vivo, potentially contributing to the diversity of clinical presentations seen in human muscular dystrophies.
This work was supported, in whole or in part, by National Institutes of Health Grants R21AR056055 and R01GM066148 and Resource for Integrated Glycotechnology Grant P41GM103390 (to D. L.), Integrated Technology Resource for Biomedical Glycomics Grant P41GM103490 (to L. W.), and by the Intramural Research Program of NIDCR at the National Institutes of Health (to K. T. H.).

This article contains supplemental Figs. S1–S3 and Table S1.
- α-DG
- α-dystroglycan
- ppGalNAc-T
- UDP-GalNAc polypeptide N-acetylgalactosaminyltransferase
- ETD-MS
- electron transfer dissociation-mass spectrometry
- t-Bu
- tert-butyl
- Ac2O
- acetic anhydride
- Fmoc
- 9-fluorenylmethyloxycarbonyl
- PAL
- 5-[[(4-amino)-methyl]-3,5-dimethoxyphenoxy]valeric acid
- PEG-PS
- polyethylene glycol-polystyrene
- Pbf
- 2,2,4,6,7-pentamethyldihydrobenzo-furan-5-sulfonyl
- Pfp
- pentafluorophenyl.
REFERENCES
- 1. Moore C. J., Winder S. J. (2010) Dystroglycan versatility in cell adhesion. A tale of multiple motifs. Cell Commun. Signal. 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barresi R., Campbell K. P. (2006) Dystroglycan. From biosynthesis to pathogenesis of human disease. J. Cell Sci. 119, 199–207 [DOI] [PubMed] [Google Scholar]
- 3. Nakamura N., Lyalin D., Panin V. M. (2010) Protein O-mannosylation in animal development and physiology. From human disorders to Drosophila phenotypes. Semin. Cell Dev. Biol. 21, 622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore C. J., Hewitt J. E. (2009) Dystroglycan glycosylation and muscular dystrophy. Glycoconj. J. 26, 349–357 [DOI] [PubMed] [Google Scholar]
- 5. Kojima K., Nosaka H., Kishimoto Y., Nishiyama Y., Fukuda S., Shimada M., Kodaka K., Saito F., Matsumura K., Shimizu T., Toda T., Takeda S., Kawachi H., Uchida S. (2011) Defective glycosylation of α-dystroglycan contributes to podocyte flattening. Kidney Int. 79, 311–316 [DOI] [PubMed] [Google Scholar]
- 6. Cao W., Henry M. D., Borrow P., Yamada H., Elder J. H., Ravkov E. V., Nichol S. T., Compans R. W., Campbell K. P., Oldstone M. B. (1998) Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282, 2079–2081 [DOI] [PubMed] [Google Scholar]
- 7. Oldstone M. B., Campbell K. P. (2011) Decoding arenavirus pathogenesis. Essential roles for α-dystroglycan-virus interactions and the immune response. Virology 411, 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michele D. E., Campbell K. P. (2003) Dystrophin-glycoprotein complex. Post-translational processing and dystroglycan function. J. Biol. Chem. 278, 15457–15460 [DOI] [PubMed] [Google Scholar]
- 9. Godfrey C., Foley A. R., Clement E., Muntoni F. (2011) Dystroglycanopathies. Coming into focus. Curr. Opin. Genet. Dev. 21, 278–285 [DOI] [PubMed] [Google Scholar]
- 10. Stalnaker S. H., Hashmi S., Lim J. M., Aoki K., Porterfield M., Gutierrez-Sanchez G., Wheeler J., Ervasti J. M., Bergmann C., Tiemeyer M., Wells L. (2010) Site mapping and characterization of O-glycan structures on α-dystroglycan isolated from rabbit skeletal muscle. J. Biol. Chem. 285, 24882–24891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nilsson J., Nilsson J., Larson G., Grahn A. (2010) Characterization of site-specific O-glycan structures within the mucin-like domain of α-dystroglycan from human skeletal muscle. Glycobiology 20, 1160–1169 [DOI] [PubMed] [Google Scholar]
- 12. Harrison R., Hitchen P. G., Panico M., Morris H. R., Mekhaiel D., Pleass R. J., Dell A., Hewitt J. E., Haslam S. M. (2012) Glycoproteomic characterization of recombinant mouse α-dystroglycan. Glycobiology 22, 662–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chai W., Yuen C. T., Kogelberg H., Carruthers R. A., Margolis R. U., Feizi T., Lawson A. M. (1999) High prevalence of 2-mono- and 2,6-di-substituted manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur. J. Biochem. 263, 879–888 [DOI] [PubMed] [Google Scholar]
- 14. Stalnaker S. H., Aoki K., Lim J. M., Porterfield M., Liu M., Satz J. S., Buskirk S., Xiong Y., Zhang P., Campbell K. P., Hu H., Live D., Tiemeyer M., Wells L. (2011) Glycomic analyses of mouse models of congenital muscular dystrophy. J. Biol. Chem. 286, 21180–21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manya H., Chiba A., Yoshida A., Wang X., Chiba Y., Jigami Y., Margolis R. U., Endo T. (2004) Demonstration of mammalian protein O-mannosyltransferase activity. Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. U.S.A. 101, 500–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breloy I., Schwientek T., Gries B., Razawi H., Macht M., Albers C., Hanisch F. G. (2008) Initiation of mammalian O-mannosylation in vivo is independent of a consensus sequence and controlled by peptide regions within and upstream of the α-dystroglycan mucin domain. J. Biol. Chem. 283, 18832–18840 [DOI] [PubMed] [Google Scholar]
- 17. Yoshida-Moriguchi T., Yu L., Stalnaker S. H., Davis S., Kunz S., Madson M., Oldstone M. B., Schachter H., Wells L., Campbell K. P. (2010) O-Mannosyl phosphorylation of α-dystroglycan is required for laminin binding. Science 327, 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hara Y., Kanagawa M., Kunz S., Yoshida-Moriguchi T., Satz J. S., Kobayashi Y. M., Zhu Z., Burden S. J., Oldstone M. B., Campbell K. P. (2011) Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc. Natl. Acad. Sci. U.S.A. 108, 17426–17431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sasaki T., Yamada H., Matsumura K., Shimizu T., Kobata A., Endo T. (1998) Detection of O-mannosyl glycans in rabbit skeletal muscle α-dystroglycan. Biochem. Biophys. Acta 1425, 599–606 [DOI] [PubMed] [Google Scholar]
- 20. Tian E., Ten Hagen K. G. (2009) Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj. J. 26, 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tabak L. A. (2010) The role of mucin-type O-glycans in eukaryotic development. Semin. Cell Dev. Biol. 21, 616–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raman J., Guan Y., Perrine C. L., Gerken T. A., Tabak L. A. (2012) UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferases. Completion of the family tree. Glycobiology 22, 768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raman J., Fritz T. A., Gerken T. A., Jamison O., Live D., Liu M., Tabak L. A. (2008) The catalytic and lectin domains of UDP-GalNAc:polypeptide α-N-acetylgalactosaminyltransferase function in concert to direct glycosylation site selection. J. Biol. Chem. 283, 22942–22951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patnaik S. K., Stanley P. (2005) Mouse large can modify complex N- and mucin O-glycans on α-dystroglycan to induce laminin binding. J. Biol. Chem. 280, 20851–20859 [DOI] [PubMed] [Google Scholar]
- 25. Aguilan J. T., Sundaram S., Nieves E., Stanley P. (2009) Mutational and functional analysis of Large in a novel CHO glycosylation mutant. Glycobiology 19, 971–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwientek T., Bennett E. P., Flores C., Thacker J., Hollmann M., Reis C. A., Behrens J., Mandel U., Keck B., Schäfer M. A., Haselmann K., Zubarev R., Roepstorff P., Burchell J. M., Taylor-Papadimitriou J., Hollingsworth M. A., Clausen H. (2002) Functional conservation of subfamilies of putative UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases in Drosophila, Caenorhabditis elegans, and mammals. One subfamily composed of l(2)35Aa is essential in Drosophila. J. Biol. Chem. 277, 22623–22638 [DOI] [PubMed] [Google Scholar]
- 27. Tian E., Ten Hagen K. G. (2007) O-Linked glycan expression during Drosophila development. Glycobiology 17, 820–827 [DOI] [PubMed] [Google Scholar]
- 28. Ten Hagen K. G., Tran D. T. (2002) A UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is essential for viability in Drosophila melanogaster. J. Biol. Chem. 277, 22616–22622 [DOI] [PubMed] [Google Scholar]
- 29. Zhang L., Tran D. T., Ten Hagen K. G. (2010) An O-glycosyltransferase promotes cell adhesion during development by influencing secretion of an extracellular matrix integrin ligand. J. Biol. Chem. 285, 19491–19501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schjoldager K. T., Vester-Christensen M. B., Bennett E. P., Levery S. B., Schwientek T., Yin W., Blixt O., Clausen H. (2010) O-Glycosylation modulates proprotein convertase activation of angiopoietin-like protein 3. Possible role of polypeptide GalNAc-transferase-2 in regulation of concentrations of plasma lipids. J. Biol. Chem. 285, 36293–36303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coltart D. M., Royyuru A. K., Williams L. J., Glunz P. W., Sames D., Kuduk S. D., Schwarz J. B., Chen X. T., Danishefsky S. J., Live D. H. (2002) Principles of mucin architecture. Structural studies on synthetic glycopeptides bearing clustered mono-, di-, tri-, and hexasaccharide glycodomains. J. Am. Chem. Soc. 124, 9833–9844 [DOI] [PubMed] [Google Scholar]
- 32. Borgert A., Heimburg-Molinaro J., Song X., Lasanajak Y., Ju T., Liu M., Thompson P., Ragupathi G., Barany G., Smith D. F., Cummings R. D., Live D. H. (2012) Deciphering structural elements of mucin glycoprotein recognition. ACS Chem. Biol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu M., Borgert A., Barany G., Live D. (2008) Conformational consequences of protein glycosylation. Preparation of O-mannosyl serine and threonine building blocks, and their incorporation into glycopeptide sequences derived from α-dystroglycan. Biopolymers 90, 358–368 [DOI] [PubMed] [Google Scholar]
- 34. Mo K. F., Fang T., Stalnaker S. H., Kirby P. S., Liu M., Wells L., Pierce M., Live D. H., Boons G. J. (2011) Synthetic, structural, and biosynthetic studies of an unusual phospho-glycopeptide derived from α-dystroglycan. J. Am. Chem. Soc. 133, 14418–14430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brancaccio A., Schulthess T., Gesemann M., Engel J. (1995) Electron microscopic evidence for a mucin-like region in chick muscle α-dystroglycan. FEBS Lett. 368, 139–142 [DOI] [PubMed] [Google Scholar]
- 36. Kunz S., Calder L., Oldstone M. B. (2004) Electron microscopy of an α-dystroglycan fragment containing receptor sites for lymphocytic choriomeningitis virus and laminin, and use of the receptoid body as a reagent to neutralize virus. Virology 325, 207–215 [DOI] [PubMed] [Google Scholar]
- 37. Gerken T. A., Jamison O., Perrine C. L., Collette J. C., Moinova H., Ravi L., Markowitz S. D., Shen W., Patel H., Tabak L. A. (2011) Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J. Biol. Chem. 286, 14493–14507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gerken T. A., Tep C., Rarick J. (2004) Role of peptide sequence and neighboring residue glycosylation on the substrate specificity of the uridine 5′-diphosphate-α-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyl transferases T1 and T2. Kinetic modeling of the porcine and canine submaxillary gland mucin tandem repeats. Biochemistry 43, 9888–9900 [DOI] [PubMed] [Google Scholar]
- 39. Gerken T. A., Zhang J., Levine J., Elhammer A. (2002) Mucin core O-glycosylation is modulated by neighboring residue glycosylation status. Kinetic modeling of the site-specific glycosylation of the apo-porcine submaxillary mucin tandem repeat by UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases T1 and T2. J. Biol. Chem. 277, 49850–49862 [DOI] [PubMed] [Google Scholar]
- 40. Perrine C. L., Ganguli A., Wu P., Bertozzi C. R., Fritz T. A., Raman J., Tabak L. A., Gerken T. A. (2009) Glycopeptide-preferring polypeptide GalNAc transferase 10 (ppGalNAc T10), involved in mucin-type O-glycosylation, has a unique GalNAc-O-Ser/Thr-binding site in its catalytic domain not found in ppGalNAc T1 or T2. J. Biol. Chem. 284, 20387–20397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takeuchi H., Kato K., Hassan H., Clausen H., Irimura T. (2002) O-GalNAc incorporation into a cluster acceptor site of three consecutive threonines. Distinct specificity of GalNAc-transferase isoforms. Eur. J. Biochem. 269, 6173–6183 [DOI] [PubMed] [Google Scholar]
- 42. Kato K., Takeuchi H., Miyahara N., Kanoh A., Hassan H., Clausen H., Irimura T. (2001) Distinct orders of GalNAc incorporation into a peptide with consecutive threonines. Biochem. Biophys. Res. Commun. 287, 110–115 [DOI] [PubMed] [Google Scholar]
- 43. Hagen F. K., Ten Hagen K. G., Beres T. M., Balys M. M., VanWuyckhuyse B. C., Tabak L. A. (1997) cDNA cloning and expression of a novel UDP-N-acetyl-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase. J. Biol. Chem. 272, 13843–13848 [DOI] [PubMed] [Google Scholar]
- 44. Hagen F. K., Van Wuyckhuyse B., Tabak L. A. (1993) Purification, cloning, and expression of a bovine UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase. J. Biol. Chem. 268, 18960–18965 [PubMed] [Google Scholar]
- 45. White T., Bennett E. P., Takio K., Sørensen T., Bonding N., Clausen H. (1995) Purification and cDNA cloning of a human UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase. J. Biol. Chem. 270, 24156–24165 [DOI] [PubMed] [Google Scholar]
- 46. Zara J., Hagen F. K., Ten Hagen K. G., Van Wuyckhuyse B. C., Tabak L. A. (1996) Cloning and expression of mouse UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase-T3. Biochem. Biophys. Res. Commun. 228, 38–44 [DOI] [PubMed] [Google Scholar]
- 47. Ten Hagen K. G., Hagen F. K., Balys M. M., Beres T. M., Van Wuyckhuyse B., Tabak L. A. (1998) Cloning and expression of a novel, tissue specifically expressed member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family. J. Biol. Chem. 273, 27749–27754 [DOI] [PubMed] [Google Scholar]
- 48. Ten Hagen K. G., Tetaert D., Hagen F. K., Richet C., Beres T. M., Gagnon J., Balys M. M., VanWuyckhuyse B., Bedi G. S., Degand P., Tabak L. A. (1999) Characterization of a UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase that displays glycopeptide N-acetylgalactosaminyltransferase activity. J. Biol. Chem. 274, 27867–27874 [DOI] [PubMed] [Google Scholar]
- 49. Ten Hagen K. G., Bedi G. S., Tetaert D., Kingsley P. D., Hagen F. K., Balys M. M., Beres T. M., Degand P., Tabak L. A. (2001) Cloning and characterization of a ninth member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family, ppGaNTase-T9. J. Biol. Chem. 276, 17395–17404 [DOI] [PubMed] [Google Scholar]
- 50. Albone E. F., Hagen F. K., VanWuyckhuyse B. C., Tabak L. A. (1994) Molecular cloning of a rat submandibular gland apomucin. J. Biol. Chem. 269, 16845–16852 [PubMed] [Google Scholar]
- 51. Ten Hagen K. G., Tran D. T., Gerken T. A., Stein D. S., Zhang Z. (2003) Functional characterization and expression analysis of members of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family from Drosophila melanogaster. J. Biol. Chem. 278, 35039–35048 [DOI] [PubMed] [Google Scholar]
- 52. Zhao P., Viner R., Teo C. F., Boons G. J., Horn D., Wells L. (2011) Combining high-energy C-trap dissociation and electron transfer dissociation for protein O-GlcNAc modification site assignment. J. Proteome Res. 10, 4088–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Z., Udeshi N. D., O'Malley M., Shabanowitz J., Hunt D. F., Hart G. W. (2010) Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol. Cell Proteomics 9, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shimojo H., Kobayashi M., Kamigaito T., Shimojo Y., Fukuda M., Nakayama J. (2011) Reduced glycosylation of α-dystroglycans on carcinoma cells contributes to formation of highly infiltrative histological patterns in prostate cancer. Prostate 71, 1151–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gill D. J., Chia J., Senewiratne J., Bard F. (2010) Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J. Cell Biol. 189, 843–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ottenhoff-Kalff A. E., Rijksen G., van Beurden E. A., Hennipman A., Michels A. A., Staal G. E. (1992) Characterization of protein tyrosine kinases from human breast cancer. Involvement of the c-src oncogene product. Cancer Res. 52, 4773–4778 [PubMed] [Google Scholar]
- 57. Singh J., Itahana Y., Knight-Krajewski S., Kanagawa M., Campbell K. P., Bissell M. J., Muschler J. (2004) Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 64, 6152–6159 [DOI] [PubMed] [Google Scholar]