Background: TGF-β drives renal fibrosis and mesenchymal gene expression in epithelial cells.
Results: Targets of TGF-β include Wnt11, which enhances mesenchymal gene expression through non-canonical signaling.
Conclusion: TGF-β activates signaling pathways that promote the mesenchymal phenotype in an autocrine manner.
Significance: Understanding the effects of TGF-β and downstream signaling is essential for developing new anti-fibrotic therapies for chronic renal disease.
Keywords: Epithelial Cell, Fibrosis, Kidney, Transforming Growth Factor β (TGFβ), Wnt Signaling
Abstract
Transforming growth factor β1 (TGF-β) promotes renal interstitial fibrosis in vivo and the expression of mesenchymal genes in vitro; however, most of its direct targets in epithelial cells are still elusive. In a screen for genes directly activated by TGF-β, we found that components of the Wnt signaling pathway, especially Wnt11, were targets of activation by TGF-β and Smad3 in primary renal epithelial cells. In gain and loss of function experiments, Wnt11 mediated the actions of TGF-β through enhanced activation of mesenchymal marker genes, such as Zeb1, Snail1, Pai1, and αSMA, without affecting Smad3 phosphorylation. Inhibition of Wnt11 by receptor knockdown or treatment with Wnt inhibitors limited the effects of TGF-β on gene expression. We found no evidence that Wnt11 activated the canonical Wnt signaling pathway in renal epithelial cells; rather, the function of Wnt11 was mediated by the c-Jun N-terminal kinase (JNK) pathway. Consistent with the in vitro results, all the TGF-β, Wnt11, and JNK targets were activated in a unilateral ureteral obstruction (UUO) model of renal fibrosis in vivo. Our findings demonstrated cooperativity among the TGF-β, Wnt11, and JNK signaling pathways and suggest new targets for anti-fibrotic therapy in renal tissue.
Introduction
Renal interstitial fibrosis is a common pathology in most chronic and progressive kidney diseases (1). By replacing the kidney parenchyma with increased numbers of fibroblasts, myofibroblasts, and extracellular matrix, normal renal tubular architecture and function are compromised, and end stage renal disease is imminent. The function of the profibrotic cytokine TGF-β in the initiation and progression of fibrosis has been intensively studied in the kidney and other tissues (2–4). In almost all animal models examined, increased renal fibrosis correlates with increased expression of TGF-β ligands. Renal fibrosis is observed upon overexpression of TGF-β or application of recombinant TGF-β in mice, whereas inhibition of the TGF-β pathway can alleviate the severity of progressive renal fibrosis (5–7).
In mammals the binding of TGF-β ligand to its receptor, TGF-β receptor type II, leads to the recruitment and phosphorylation of TGF-β receptor type I (8). The activated TGFβRI is a serine/threonine kinase that transduces the signal through phosphorylating receptor-activated Smad2 and Smad3. Phosphorylated Smad2/3 form a heteromeric complex with a common partner, Smad4, and translocate to the nucleus. Normally, the Smad complex requires other transcription factors to activate or repress target gene expression (9, 10). Both Smad2 and Smad3 are activated in TGF-β signaling pathway, but their targets and functions are distinct (11, 12). Genetic experiments point to a critical role for Smad3 in promoting TGF-β mediated renal fibrosis. Despite the evidence pointing to TGF-β as a profibrotic agent, its direct target genes and detailed mechanisms that promote fibrosis are still not well characterized.
Renal tubular epithelial cells are target cells for TGF-β in kidney fibrosis (3). In the unilateral ureteral obstruction (UUO)2 mouse model, expression of both TGF-β and its type I receptor increased rapidly and specifically in renal tubular epithelia. In vitro, epithelial cells treated with TGF-β lost expression of epithelial markers and assumed a more mesenchymal phenotype. This epithelial-to-mesenchymal transition (EMT) was thought to occur in animal models of interstitial fibrosis to help explain the increased number of fibroblasts and the loss of epithelial tubular integrity (13). However, more recent cell lineage tracing experiments disputed the EMT model of fibrosis in the kidney and other tissues (14, 15). Although the existence of EMT in renal fibrosis remains controversial, the direct impact of TGF-β on the renal epithelial cells appears critical for initiation and progression of fibrosis.
The Wnt signaling pathways have also been linked to TGF-β and to EMT during normal development and diseases. There are 19 Wnt ligands in the mouse and human genomes. These different Wnt ligands can signal through the canonical, β-catenin-dependent pathway or the non-canonical, β-catenin-independent pathway. In the canonical pathway, activated Wnt signaling prevents the degradation of β-catenin by the GSK3β·Axin·Adenomatous Polyposis Coli (APC) complex. The accumulating nuclear β-catenin then interacts with T Cell Factor/Lymphoid Enhancer binding Factor (TCF/LEF) proteins to regulate gene expression (16). One branch of the non-canonical Wnt pathway involves calcium influx and further activation of Ca2+/calmodulin-dependent kinase II (CaMKII) and protein kinase C (17, 18). Another branch of the non-canonical pathway transduces its signal by activating the c-Jun N-terminal kinase (JNK) pathway either through small GTPase or other mechanisms (18).
Although the Wnt signaling pathways were shown to function in EMT in vitro and in fibrosis in vivo (19, 20), the relationships with the profibrotic cytokine TGF-β are not well defined. A limited number of studies addressing the cross-talk of TGF-β and Wnt signaling pathways converged on the β-catenin, as TGF-β could stabilize β-catenin by inhibiting its GSK3β-dependent degradation through p38 MAPK and Akt (1, 21, 22). Also, β-catenin could physically interact with Smad proteins to regulate target gene expression (23–25). Yet, little is known about the function of non-canonical Wnt signaling pathway in fibrosis and its relation to TGF-β.
In this report we defined the targets of TGF-β in renal epithelial cells in vitro by global gene expression analysis. We showed that components of the Wnt signaling pathways were activated by TGF-β. Among these, the non-canonical signaling protein Wnt11 was directly regulated by TGF-β through Smad3 in both primary and immortalized renal epithelial cells. Wnt11 enhanced the effects of TGF-β and was necessary for maximal activation of mesenchymal genes such as Zeb1, Snail1, Pai1, and the myofibroblast marker αSMA. Wnt11 did not enhance P-Smad3 nor activate the canonical Wnt signaling pathway; rather, it appeared to increase mesenchymal gene expression through the non-canonical JNK pathway. These results pointed to a critical role for non-canonical Wnt signaling in TGF-β-mediated fibrosis and suggested that autocrine and paracrine mechanisms could mediate TGF-β-dependent effects in epithelial cells and adjacent cells.
MATERIALS AND METHODS
Animals
C57BL/6 mice were kept according to National Institutes of Health guidelines. Animal use was approved by the University Committee on Use and Care of Animals at the University of Michigan.
For the induction of renal fibrosis, the UUO model was utilized. Mice were anesthetized with intraperitoneal injection of ketamine and xylozine. Through a midline abdominal incision, the right ureter was exposed and tied off at the mid-ureteral level with fine suture materials (4–0 silk) to induce a complete obstruction. Mice were allowed to recover from anesthesia and were kept with ad libitum supply of food and water until the indicated time of sacrifice (7, 14, and 28 days). Both obstructed and contralateral kidneys were harvested for RNA and protein analysis.
Primary and Immortalized Renal Epithelial Cells
Primary renal epithelial cells were isolated from the cortex of 5–6-week-old female mice. Briefly, the medulla was manually removed, and cortex was digested by liberase DH (Roche Applied Science) in Dulbecco's modified Eagle's medium (DMEM, Lonza). The tissue fragments were sieved through a 212-μm pore size mesh. After 3 washes with cold DMEM, cells were expanded in UltraMDCK serum-free medium (Lonza) supplied with 0.5× insulin-transferrin-ethanolamine-selenium (Lonza), 60 μg/liter epidermal growth factor (R&D Systems), 10−9 m triiodothyronine, and 1× antibiotic antimycotic (Invitrogen). Cells were split and frozen in fetal bovine serum (FBS, Invitrogen) with 10% dimethyl sulfoxide. Recombinant human TGF-β1 and Wnt11 were from R&D systems.
To inhibit translation, cycloheximide (5 μg/ml, Sigma) was added half an hour before TGF-β treatment (10 ng/ml) for the indicated times. To inhibit Smad3 phosphorylation, specific inhibitor of Smad3 (SIS3, Sigma) was added into the medium at the concentration of 5 μm 1 h before 10 ng/ml TGF-β treatment for 24 h. To inhibit JNK signaling, 20 μm SP600125 (Sigma) or 10 μm JNK inhibitor III (EMD) was added into the medium 1 h before 10 ng/ml TGF-β treatment for 24 h. To inhibit Wnt signaling, Sfrp1 (R&D Systems) was added at 0.5 μg/ml together with 10 ng/ml TGF-β for 24 h.
Immortalized Transgenic Kidney Proximal Tubule Cells (TKPTS) were a kind gift from Dr. Bello-Reuss. Cells were cultured in Dulbecco's modified Eagle's medium:nutrient mixture F-12 (DMEM/F-12, Invitrogen) with 2% FBS, 1× insulin-transferring-ethanolamine-selenium, and penicillin-streptomycin (Invitrogen). UltraMDCK serum-free medium was used when serum starvation was necessary.
To overexpress Smad3 or Wnt11, TKPTS cells were cultured on 6-well plates in UltraMDCK serum-free medium and transfected with 3 μg of DNA of Smad3 or Wnt11 expressing vector or sonicated herring sperm (SHS) DNA control using FuGENE 6 (Roche Applied Science) per the manufacturer's instructions. TGF-β at the indicated concentrations was added into the medium 24 h after transfection, and cells were cultured for an additional 24 h.
Microarray Expression Analysis
Primary renal epithelial cells (PRECs) were grown on 100-mm dishes until confluency reached 80%. Cycloheximide (5 μg/ml) was added half an hour before TGF-β treatment (10 ng/ml) for 4 h. RNA was extracted using the TRIzol RNA isolation system (Invitrogen). All samples were done in triplicate. Gene expression microarray analysis was done by the University of Michigan Comprehensive Cancer Center Affymetrix and Microarray Core Facility. Briefly, the FL-Ovation cDNA Biotin Module V2 kit (NuGEN Technologies, San Carlos, CA) was used to produce biotin-labeled cRNA, which was then fragmented and hybridized to a Mouse 430 2.0 Affymetrix GeneChip 3 expression arrays (Affymetrix, Santa Clara, CA). Array hybridization, washes, staining, and scanning procedures were carried out according to standard Affymetrix protocols. Expression data were normalized by the robust multiarray average (RMA) method and fitted to weighted linear models in R using the affy and limma packages of Bioconductor, respectively (26, 27). Only probe sets with a variance over all samples superior to 0.1, a p value inferior or equal to 0.05 after adjustment for multiplicity using the false discovery rate (28), and a minimum 2-fold difference in expression were selected for the analysis. The complete data set is available upon request.
Western Blot Analysis
Cells were directly lysed in 2× SDS buffer (4% sodium dodecyl sulfate, 20% glycerol, 0.2 m dithiothreitol, 125 mm Tris, pH 6.8) and boiled at 94 °C. Samples were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antibodies as indicated. Rabbit anti-phosphorylated Smad3, rabbit anti-Wnt11, and rabbit anti-phosphorylated CaMKII are from Abcam. Rabbit anti-Smad2/3, rabbit anti-phosphorylated Smad2, rabbit anti-phosphorylated JNK, rabbit anti-phosphorylated c-Jun, and rabbit anti-c-Jun are from Cell Signaling. Mouse anti-αSMA, mouse anti-FLAG, and mouse anti-β-tubulin are from Sigma. Mouse anti-active-β-catenin is from Millipore. Mouse anti-β-catenin is from BD transduction Laboratories. Mouse anti-N-cadherin is from Upstate Biotechnology. HRP-linked secondary antibodies and ECL reagent are from GE Healthcare.
RNA Reverse Transcription and Real-time PCR
2–3 μg of total RNA was reverse-transcribed into cDNA with SuperScript First Strand kit (Invitrogen). The cDNA products were diluted 5 times and amplified with the iTaq SYBR Green master mix (Bio-Rad) in a Prism 7500 (Applied Biosystems). Primers pairs for PCR are as follows: Wnt11 5′-GGGCCAAGTTTTCCGATGCT, 5′-TTCGTGGCTGACAGGTAGCG; ZEB1 5′-TCAAGTACAAACACCACCTG, 5′-TGGCGAGGAACACTGAGA; PAI1 5′-ACATGTTTAGTGCAACCCTG, 5′-GGTCTATAACCATCTCCGTG; Snai1 5′-GGAAGCCCAACTATAGCGA, 5′-AGCGAGGTCAGCTCTACG; Fzd7 5′-GAAGCTGGAGAAGCTGATGG, 5′-ATCTCTCGCCCCAAACTCT; Axin2 5′-TGAGCTGGTTGTCACCTACT, 5′-CACTGTCTCGTCGTCCCA; Wisp1 5′-GCCAGAGCAGGAAAGTCG, 5′-TACTTGGGTCGGTAGGTGC; GAPDH 5′-ACCACAGTCCATGCCATCAC, 5′-TCCACCACCCTGTTGCTGTA.
Northern Blotting
An aliquot of 10 μg of total RNA was electrophoresed in 1% agarose gel containing formaldehyde, blotted on a Hybond-N membrane (overnight), and probed with cDNA fragments that were labeled with [32P]dCTP via the random prime reaction. Blots were prehybridized with 5 ml of Rapid-Hyb (Amersham Biosciences), and 5 × 106 cpm of the radiolabeled probe was added. After 4 h at 65 °C, blots were washed in 2× SSC (0.3 m NaCl, 30 mm sodium citrate), 1% SDS twice, and 0.2× SSC, 0.1% SDS once at 65 °C each.
shRNA-mediated Gene Knockdown
TKPTS cells were seeded on the 6-well plate 1 day before transfection. Cells were transfected with 2 μg of DNA of Wnt11 shRNA lentivirus vector 54666, 53302, and a scrambled shRNA lentivirus vector (Open Biosystems) using FuGENE 6. For Smad3 or Fizzled7 knockdown, TKPTS cells were infected with lentivirus expressing Smad3 shRNA 54904 or Fzd7 64762 (Open Biosystems) in the presence of 8 μg/ml Polybrene and kept overnight. Puromycin (Sigma) was added into the medium at 10 μg/ml and kept in culture medium for constitutive selection. Survival cells were cultured, expanded, and frozen for further experiments.
Wnt11 53302 shRNA lentivirus was used to knock down in PRECs. Cells were seeded on 100-mm dishes for 24 h. Lentivirus was added with 8 μg/ml Polybrene and kept overnight. Puromycin was added for selection for 10 days before experiments.
Luciferase Assays
TKPTS cells were seeded on 12-well plates. 3TP-lux reporter vector was transfected (1 μg/well) together with SHS or Smad3-expressing vector (2 μg/well) into the cells in triplicate 24 h later. TOPFLASH or FOPFLASH reporter vector was transfected (1 μg/well) together with SHS or Wnt11-expressing vector (2 μg/well). Cells were lysed 48 h after transfection with the dual luciferase assay kit (Promega), and results were read. In assays requiring TGF-β or LiCl treatment, TGF-β (10 ng/ml) or LiCl (20 mm) was administrated 24 h after the transfection and kept for another 24 h.
Cellular Fractionation
PRECs were treated with Wnt11 (500 ng/ml) or LiCl (20 mm) for 24 h. To make cytoplasmic extracts, cells were washed with PBS once, scraped off, and lysed in cell lysis buffer (5 mm PIPES at pH 8.0, 85 mm KCl, 0.5% Nonidet P-40, and 1× protease inhibitors) on ice for 10 min. Then the cells were spun at 3000 rpm for 5 min, and supernatant was collected as cytoplasmic fraction. To extract nuclear fractions, cells were scraped off, resuspended in 5 ml of nuclear buffer 1 (10 mm Tris at pH 8.0, 10 mm NaCl, 3 mm MgCl2, 0.5 mm DTT, 0.1% Triton X-100, 0.1 m sucrose, and 1× protease inhibitors) and Dounce-homogenized 20 times with a loose fitting (type A) homogenizer. Then 5 ml of nuclear buffer 2 (10 mm Tris at pH 8.0, 10 mm NaCl, 3 mm MgCl2, 0.5 mm DTT, 0.1% Triton X-100, 0.25 m sucrose, and 1× protease inhibitors) was added and mixed. Then 2.5 ml of nuclear buffer 3 (10 mm Tris at pH 8.0, 5 mm MgCl2, 0.5 mm DTT, 0.33 m sucrose, and 1× protease inhibitor) was layered underneath, and the nuclei were collected by centrifugation at 2000 rpm for 5 min at 4 °C through the sucrose step-gradient. The nuclei pellet was boiled in 2× SDS/PAGE sample buffer, and extracts were separated on gels.
RESULTS
Primary Renal Epithelial Cells Respond to TGF-β in Dose- and Time-dependent Manner
The effects of TGF-β on various cells in culture have been studied in detail (3, 4, 20). To confirm that PRECs from the adult kidneys respond to TGF-β, we isolated cells from adult mouse kidneys and cultured them in a defined serum-free medium. Normally, PRECs formed islands with clear boundaries (Fig. 1A). They also expressed high levels of epithelial markers, E-cadherin, and low levels of mesenchymal markers, N-cadherin and αSMA (Fig. 1, B and C). Other cell types, such as podocytes and blood cells, cannot grow in this conditional medium and were lost after the first passage.
FIGURE 1.
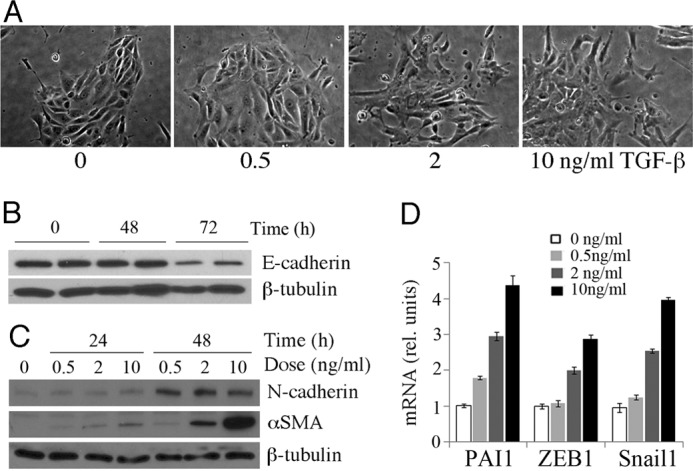
Effects of TGF-β on primary renal epithelial cells. A, shown are phase contrast micrographs of PRECs treated with increasing concentrations of TGF-β for 24 h. B, shown is a Western blot of cells treated for 0, 48, and 72 h with 10 ng/ml TGF-β and probed for E-cadherin. C, shown is time and dose dependence of αSMA and N-cadherin expression with increasing amounts of TGF-β. D, shown is qRT-PCR of mesenchymal markers after 24 h of TGF-β treatment at the indicated doses. All samples were done in triplicate with error bars representing 1 S.D.
When PRECs were exposed to TGF-β, cells detached from each other and migrated out of the islands (Fig. 1A). Mesenchymal markers were activated in those cells, although the kinetics of activation varied among different markers. The genes encoding αSMA, Zeb1, Pai1, and Snail1 were more sensitive to TGF-β with a response in 24 h, whereas the up-regulation of N-cadherin became obvious by 48 h (Fig. 1, C and D). A significant decrease of E-cadherin was not observed until 72 h (Fig. 1B). The effect of TGF-β at a low dose (0.5 ng/ml) was limited. When the dose of TGF-β1 was increased to 2 or 10 ng/ml, its ability to change cell morphology and gene expression profile was significantly increased (Fig. 1). These data indicated that the primary isolated cells showed a consistent and dose-dependent response to TGF-β by activating genes associated with a more mesenchymal phenotype.
Once we had established the effects of TGF-β on PRECs in vitro, we sought to determine more precisely what genes are directly under the control of TGF-β. Thus, we derived PRECs from adult (5–6 weeks) mouse kidneys and cultured them in serum-free media for 2 passages before splitting into replicates for our screening strategy. Cells were then cultured with cycloheximide to inhibit further protein synthesis so only mRNAs of direct TGF-β targets would be activated. Subsequently, TGF-β was added, and RNA was prepared after 4 h of incubation. Total RNA from PRECs treated with TGF-β and cycloheximide was compared with RNA from cells cultured with cycloheximide alone using Affymetrix Mouse 430 2.0 cDNA microarrays. Three independent samples for each group were hybridized, and statistically significant changes were noted (Table 1).
TABLE 1.
Select genes regulated by TGF-β in renal epithelial cells
* log2 scale.
Overall, 454 Affymetrix probe sets representing at least 318 annotated unique genes showed significantly altered RNA expression levels of at least 2-fold or greater. Of these, 248 genes exhibited increased expression levels, whereas 70 genes showed a decrease in RNA levels 4 h post-TGF-β treatment. The gene list was analyzed by Genomatix pathway analysis for common elements of known molecular pathways (Table 2). Not surprisingly, the TGF-β pathway was at the top of the list. However, other signaling pathway components were also regulated by TGF-β, especially the WNT/β-catenin, parathyroid hormone (PTH), Notch, fibroblast growth factor, and mitogen-activated protein kinase (MAPK) pathways. In terms of molecular functions, 127 genes were assigned to functional groups involved in DNA binding or transcription regulation. More than 21 kinases showed changed expression levels, whereas 19 receptor binding proteins were altered. Given the effects of TGF-β on cell migration and dissociation, it was also noted that 12 GTPase regulatory proteins showed changed expression levels. Conspicuously absent on our list of primary target genes were cell adhesion molecules and extracellular matrix proteins that are known to be expressed in response to TGF-β treatment. This suggests that suppression of E-cadherin and the activation of matrix metalloproteases and collagens, for example, may require new protein synthesis and thus may be mediated by the transcription factors activated by TGF-β rather than directly by receptor-activated Smads.
TABLE 2.
Pathway analysis of TGF-β target genes in renal epithelial cells
| Pathway | No. of genes |
|||
|---|---|---|---|---|
| p Value | Observed | Expected | Total | |
| TGF superfamily | ||||
| TGF-β | 6.33E−08 | 61 | 32.14 | 1360 |
| Mothers against DPP homolog | 1.26E−04 | 33 | 17.06 | 722 |
| Other signaling | ||||
| Wingless type | 2.02E−06 | 45 | 22.57 | 955 |
| Notch | 7.09E−05 | 29 | 13.68 | 579 |
| Dual specificity phosphatase | 9.58E−05 | 15 | 4.87 | 206 |
| Fibroblast growth factor | 2.14E−04 | 35 | 19.07 | 807 |
| Leukemia inhibitory factor receptor | 2.32E−04 | 12 | 3.59 | 152 |
| Protooncogene/protein kinase PIM | 2.51E−04 | 9 | 2.13 | 90 |
| Mitogen-activated protein kinase | 4.72E−04 | 93 | 72.27 | 3058 |
| STAT | 8.09E−04 | 39 | 23.68 | 1002 |
| Insulin-like growth factor 2 receptor | 1.69E−03 | 27 | 14.98 | 634 |
| Janus kinase | 2.89E−03 | 24 | 13.24 | 560 |
| Platelet-derived growth factor receptor | 3.50E−03 | 22 | 11.91 | 504 |
| EGF receptor family member ERBB4 | 4.98E−03 | 42 | 28.69 | 1214 |
| Transcription | ||||
| Parathyroid hormone receptor | 1.75E−06 | 17 | 4.44 | 188 |
| β-Catenin | 9.28E−05 | 35 | 18.29 | 774 |
| VEGF receptor | 3.20E−04 | 27 | 13.45 | 569 |
| Lymphoid enhancer binding factor 1 (TCF/LEF) | 3.87E−04 | 10 | 2.74 | 116 |
| CAMP-responsive element binding protein 1 | 1.13E−03 | 27 | 14.58 | 617 |
| Peroxisome proliferator-activated receptor γ | 1.73E−03 | 18 | 8.41 | 356 |
| CCAAT/enhancer-binding protein, α | 2.40E−03 | 8 | 2.36 | 100 |
| Hairy and enhancer of split 1 | 4.58E−03 | 8 | 2.62 | 111 |
| Extracelllular matrix-related | ||||
| Matrix metalloproteinase | 7.30E−04 | 22 | 10.54 | 446 |
| Integrin | 5.34E−03 | 20 | 10.82 | 458 |
| Immune mediators | ||||
| IL-6 signal transducer | 1.23E−04 | 10 | 2.39 | 101 |
| Interleukin 1 | 2.58E−04 | 29 | 14.72 | 623 |
| Interleukin 4 | 4.51E−04 | 18 | 7.49 | 317 |
| TNF receptor-associated factor | 9.55E−04 | 16 | 6.66 | 282 |
| Interleukin 6 (interferon, β2) | 1.02E−03 | 26 | 13.73 | 581 |
| Interleukin 7 | 1.22E−03 | 11 | 3.73 | 158 |
| TNF superfamily, member 2 | 2.85E−03 | 38 | 24.46 | 1035 |
| Interleukin 10 | 9.67E−03 | 13 | 6.26 | 265 |
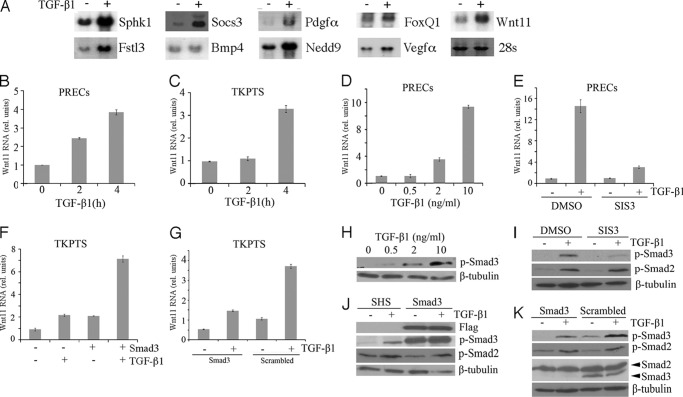
Activation of Wnt11 by TGF-β1 Was Smad3-dependent in Renal Epithelial Cells
A subset of the genes directly activated by TGF-β identified in the array screens were confirmed by Northern blotting and/or qRT-PCR to ensure that changes in mRNA levels were accurately reflected in the Affymetrix datasets (Fig. 2, A and B). Of all the Wnt pathway components identified as TGF-β targets, we focused on Wnt11 because previous work suggested the cross-talk between Wnt11 and TGF-β (19, 29). Up-regulation of Wnt11 in the presence of cycloheximide was confirmed in PRECs and also in an immortalized renal epithelial cell line TKPTS that expresses SV40 large T antigen. The TKPTS cells were useful for a variety of assays that required a long time selection, as these cells maintained their epithelial character for more than 25 passages (30). Although Wnt11 up-regulation could be seen as early as 2 h after TGF-β addition in PRECs, up-regulation in TKPTS cells was a bit slower but still robust (Fig. 2, B and C).
FIGURE 2.
Activation of Wnt11 and TGF-β targets. A, shown are Northern blots of total RNA from control cells (−) or 4 h TGF-β treated cells (+) cultured in the presence of cycloheximide and probed for the indicated RNAs. B, shown are Wnt11 RNA levels in PRECs cells cultured with TGF-β for the indicated time in hours in the presence of cycloheximide. C, shown are Wnt11 RNA levels in TKPTS cells cultured with TGF-β for the indicated time in hours in the presence of cycloheximide. D, shown are Wnt11 RNA levels after 24 h with varying does of TGF-β as indicated. E, shown is Wnt11 RNA activation in response to TGF-β in the presence or absence of the Smad3 inhibitor SIS3. F, shown are Wnt11 RNA levels measured after Smad3 transfection and/or TGF-β treatment. G, shown is Wnt11 RNA activation in response to TGF-β in the presence or absence of Smad3 shRNAs or scrambled controls. H, shown is a Western blot of P-Smad3 in response to increasing doses of TGF-β. I, shown are Western blots of P-Smad3 and P-Smad2 after inhibition by SIS3 and treatment with TGF-β. J, shown is a Western blot for P-Smad3 and P-Smad2 after FLAG-Smad3 transfection or control transfection (SHS) and TGF-β treatment. K, shown is a Western blot of P-Samd3, P-Smad2, and total Smad2/3 after culture with Smad3 shRNAs and TGF-β treatment. It is noted that Smad3 proteins were shown nearly totally gone in Smad2/3 panel, whereas they were still detectable in the P-Smad3 panel. This resulted from different exposure times and different affinities for these two antibodies to their respective antigens.
Smad2 and Smad3 are the most critical mediators in TGF-β signaling pathway. Although both Smad2 and Smad3 are phosphorylated after TGF-β stimulation, their functions are not necessarily similar. In recent studies utilizing TGF-β-mediated animal models of fibrosis, Smad2 activation was protective, whereas Smad3 functions as an enhancer of the disease phenotype (12). Thus, we asked whether Wnt11 up-regulation was mediated by Smad3. In PRECs, the induction of Wnt11 mRNA was TGF-β dose-dependent, in a range from 2 to 10 ng/ml (Fig. 2D). The fold change of Wnt11 mRNA level correlated with the amount of phosphorylated Smad3 (Fig. 2H). A specific Smad3 inhibitor, SIS3, which blocked its phosphorylation upon TGF-β treatment (31), abolished the activation of Wnt11 by TGF-β (see Fig. 2, E and I). In TKPTS, Wnt11 mRNA level was up-regulated by transient transfection of FLAG-tagged Smad3 vector. This activation was due to constitutive phosphorylation of transfected Smad3 without TGF-β and was further enhanced with TGF-β treatment (Fig. 2, F and J). Next, we used an shRNA lentivirus to specifically knock down Smad3 in TKPTS. After puromycin selection, Smad3 protein level was significantly knocked down, whereas Smad2 protein level remained the same (Fig. 2K). Both the basal and activated Wnt11 expression levels were reduced in Smad3 knockdown group (Fig. 2G), consistent with the decreased basal and TGF-β activated P-Smad3 in this group. We also checked the P-Smad2 level under different conditions mentioned above. Neither the Smad3 inhibitor in PRECs nor the modulation of Smad3 proteins in TKPTS affected the response of Smad2 to TGF-β (Fig. 2, I–K). Considering the Wnt11 expression change in those conditions, our results suggested that TGF-β up-regulated Wnt11 in renal epithelial cells mainly through P-Smad3 proteins.
Wnt11 Enhances Activation of TGF-β-dependent Mesenchymal Markers
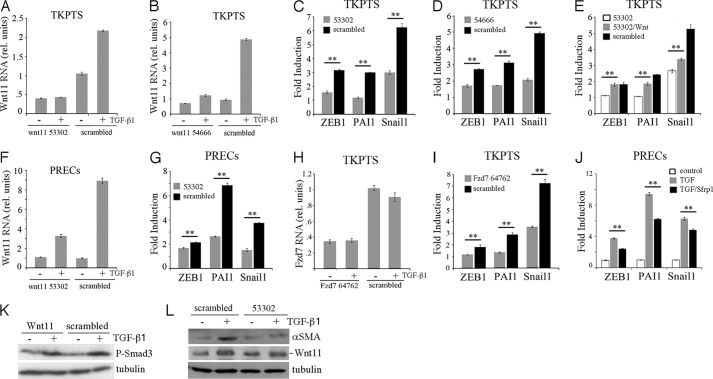
In epithelial cell culture, TGF-β activates a program of mesenchymal marker gene expression associated with the transition of epithelial cells to a more mesenchymal phenotypes. We thus tested whether Wnt11 induction helped mediate this TGF-β-dependent EMT in vitro (Fig. 3). In addition to the myofibroblast marker αSMA, we examined cells for activation of the mesenchymal markers Zeb1, Pai1, and Snail1. Recombinant Wnt11 by itself did not activate any mesenchymal marker gene. At a high dose of TGF-β (10 ng/ml) all of the mesenchymal markers were activated by 24 h. However, at a lower dose of TGF-β (2 ng/ml), recombinant Wnt11 significantly enhanced the expression of αSMA (Fig. 3A) and other mesenchymal marker genes (Fig. 3B). Using a Wnt11 expression plasmid, a similar effect was observed as both Pai1 and Snail1 expression increased significantly upon TGF-β treatment and Wnt11 expression (Fig. 3C).
FIGURE 3.
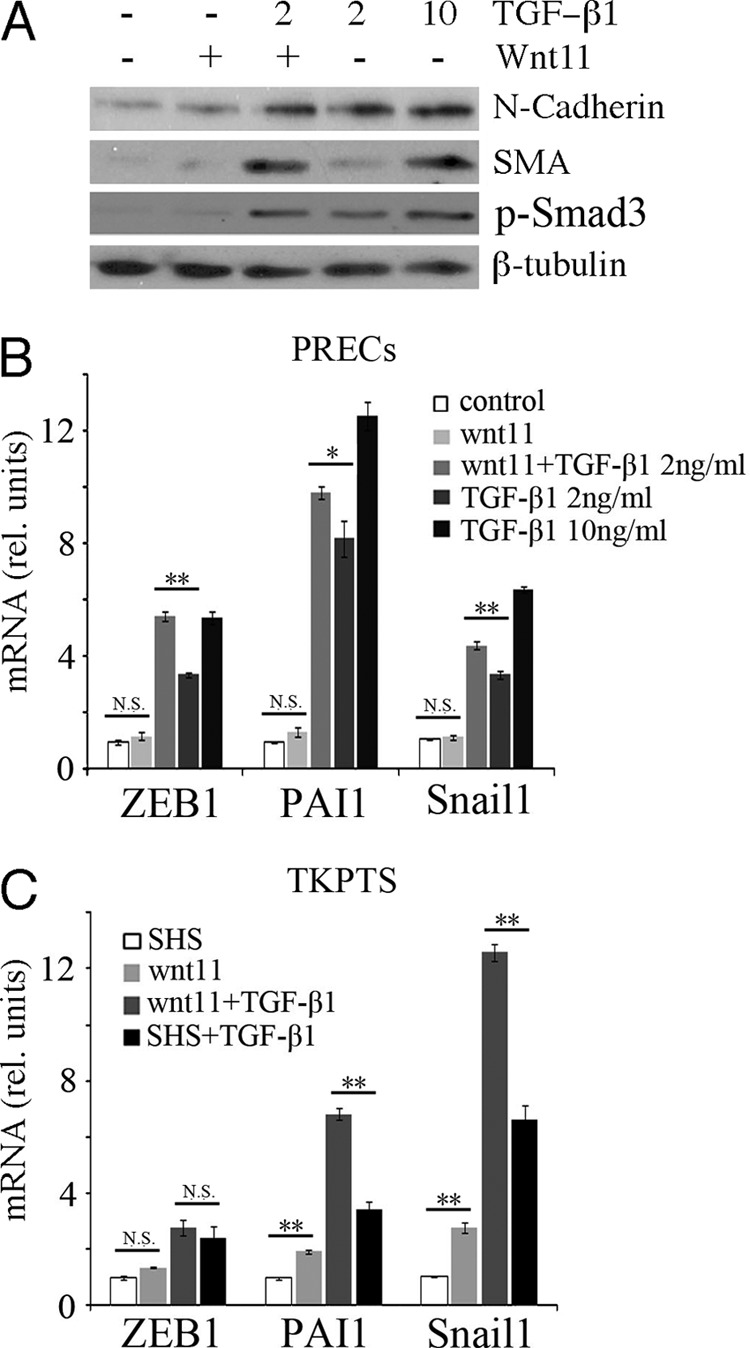
Wnt11 increases TGF-β-dependent activation of mesenchymal genes. A, Western blots for N-cadherin, αSMA, P-Samd3, and β-tubulin from cells treated with recombinant Wnt11 and different doses of TGF-β are indicated. Note that Wnt11 increases αSMA levels at low doses of TGF-β. B, quantitative RT-PCR for genes are indicated under similar conditions as in A. C, quantitative RT-PCR for mesenchymal genes after transfection with Wnt11 expression plasmids and/or TGF-β addition. SHS, sonicated herring sperm DNA transfection control (*, p < 0.05; **, p < 0.01; N.S., not significant; Student's t test for independent variables).
Because endogenous Wnt11 is activated by TGF-β, a better test for the Wnt11 contribution to EMT in vitro is to knock down endogenous Wnt11 and test for TGF-β activation of target genes. Thus, we used shRNA lentivirus to produce two stable cell lines, 53302 and 54666, derived from the immortalized renal cell line TKPTS. In cell line 53302, basal Wnt11 mRNA expression was reduced by ∼60% compared with a scrambled control line, and Wnt11 was not activated by TGF-β (Fig. 4A). The up-regulation of Pai1 was abolished in these Wnt11 knockdown cells, whereas up-regulation of Zeb1 and Snail1 were also reduced (Fig. 4C). In the 54666 cell line, basal Wnt11 expression was reduced by ∼40% (Fig. 4B). However, these cells up-regulated Wnt11 about 2-fold in response to TGF-β1, although this was still significantly less than the scrambled control cells. Nevertheless, the up-regulation of EMT marker genes was also attenuated in the 54666 Wnt11 knockdown cells (Fig. 4D). Moreover, administration of Wnt11 recombinant protein totally rescued the TGF-β dependent up-regulation of Zeb1 and partially rescued the PAI1 and Snail1 in the 53302 cells (Fig. 4E). The Wnt11 knockdown did not by itself affect the ability of TGF-β to phosphorylate Smad3 (Fig. 4K). We also used the Wnt11-specific 53302 shRNA lentivirus for knockdown in PRECs. Although the basal Wnt11 expression was not affected, its up-regulation upon TGF-β treatment was largely reduced, as were the response of EMT marker genes (Fig. 4, F and G) and the expression of αSMA (Fig. 4L).
FIGURE 4.
Wnt11 is necessary for the TGF-β-dependent activation of mesenchymal genes. A, shown are Wnt11 RNA levels after culture with shRNA #53302 or a scrambled control in TKPTS cells with or without TGF-β. B, shown is a similar experiment as in A but using the Wnt11 shRNA #54666. C, shown is TGF-β induction of RNAs for the indicated genes in the presence or absence of shRNA #53302. Relative amount of RNA is compared before or after TGF-β addition and expressed as fold induction. D, shown is a similar experiment as in C but using the Wnt11 shRNA #54666. E, the induction -fold change of indicated genes by TGF-β is measured in cells cultured with shRNA 53302, with shRNA 53302 and recombinant Wnt11, or with scrambled shRNA. Note that recombinant Wnt11 increases the induction of mesenchymal genes in the presence of 53302. F, Wnt11 shRNA 53302 in PRECs reduces TGF-β-dependent Wnt11 RNA induction. G, in PRECs, Wnt11 shRNA 53302 reduced the TGF-β-mediated fold induction of mesenchymal marker genes. H, in TKPTS, inhibition of Fzd7 RNA by shRNA #64762 is independent of TGF-β. I, Fzd7 shRNA #64762 inhibits the TGF-β-mediated induction of mesenchymal marker genes. J, the Wnt secreted inhibitor Sfrp1 reduces the TGF-β-mediated induction of mesenchymal marker genes in PRECs. K, shown is a Western blot of P-Smad3 from cells cultured with Wnt11 53302 shRNAs and/or TGF-β as indicated in TKPTS. L, Western blots for αSMA cells show that inhibition of Wnt11 by shRNAs reduces the accumulation of αSMA in response to TGF-β in PRECs. Levels of Wnt11 protein induction by TGF-β are reduced by 53302 treatment, although basal levels without TGF-β are similar. *, p < 0.05; **, p < 0.01, Student's t test for independent variables.
We also determined if Frizzled7 (Fzd7), a well characterized Wnt11 receptor (32, 33), was important for mediating the TGF-β effects in EMT. Although Fzd7 was expressed in TKPTS, its expression was not affected by TGF-β treatment. Fzd7 shRNA lentivirus knocked down ∼60% of its mRNA expression (Fig. 4H). Similar to Wnt11 knockdown, the activation of mesenchymal marker genes, especially Zeb1 and Pai1, in response to TGF-β was reduced in the Fzd7 knockdown cell lines (Fig. 4I). Lastly, we inhibited Wnt signaling by the addition of the secreted frizzled-related protein Sfrp1, which sequesters Wnt away from its receptors (Fig. 4J). Similar to Fzd7 knockdowns, Sfrp1 addition significantly inhibited the expression of mesenchymal genes after TGF-β addition. Taken together, these data indicated that Wnt11 was an important TGF-β target for promoting the activation of mesenchymal marker genes in renal epithelial cell lines.
Mechanisms of Wnt11-mediated Increase in TGF-β Responses
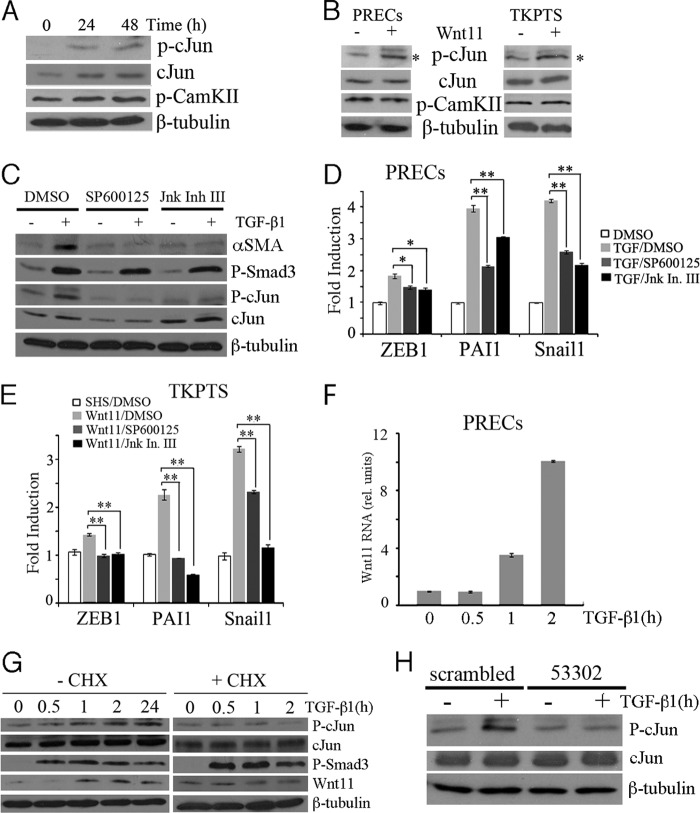
Our data suggested that Wnt11 was an important autocrine mediator of the TGF-β response. To rule out that Wnt11 functions by modulating the levels of P-Smad3, we performed additional assays. We had shown that recombinant Wnt11 did not increase the levels of P-Smad3 with or without TGF-β (Fig. 3A). This was confirmed using a Wnt11 expression plasmid (Fig. 5A) in TKPTS. If anything, Wnt11 overexpression reduced P-Smad3 slightly. We also tested the effects of Wnt11 on the 3TP-Luc reporter plasmid that responds to TGF-β (Fig. 5, B and C). Overexpression of Wnt11 did not activate 3TP-luc by itself (Fig. 5B), and Wnt11 knockdown did not affect the ability of TGF-β to activate 3TP-Luc (Fig. 5C). Thus, the effects of Wnt11 on TGF-β activation of mesenchymal markers were not mediated through alterations of P-Smad3 levels.
FIGURE 5.

Wnt11 does not mediate Smad2/3 phosphorylation or β-catenin-dependent gene activation. A, Western blots from cells overexpressing Wnt11 and treated with TGF-β show no effects of Wnt11 on P-Smad3 levels. B, the P-Smad2/3 reporter 3TP-Luc was assayed after co-transfection with Wnt11 or treatment with TGF-β. Note that Wnt11 does not increase 3TP-dependent luciferase. C, Wnt11 53302 shRNA knockdown does not affect the ability of TGF-β to activate 3TP-luc. D, a Western blot using antibodies against activated β-catenin shows no effects of Wnt11 on active β-catenin accumulation in press. E, shown is a Western blot using antibody against total β-catenin in the cytoplasmic and nuclear fractions of PRECs under Wnt11 or LiCl treatment for 24 h. Fractionation controls are β-tubulin for cytoplasma and ptip for nuclear fractions. F, Wnt11 does not activate Wisp1 or Axin, two known β-catenin target genes, as assayed by qRT-PCR in renal epithelial cells. N.S., not significant. G, cells were transfected with the β-catenin reporter TOPFLASH then treated with TGF-β or LiCl or co-transfected with Smad3/Wnt11. Only the known GSK3 kinase inhibitor LiCl significantly activated the TOPFLASH reporter. FOPFLASH was used as an internal control plasmid, and statistical significance used Student's t test for independent variables.
Alternatively, Wnt11 could function through the canonical Wnt/β-catenin pathway either as a target or as an activator thereof (34, 35). Previous studies indicated that in mouse L cells and human embryonic stem cells, the secreted Xenopus Wnt11/5a complex has more canonical Wnt signaling activity than secreted Wnt11 or Wnt5a alone (36). Thus, we checked whether Wnt11 activated canonical signaling pathway by Western blotting, gene expression, and reporter assays. In PRECs, administration of recombinant Wnt11 did not increase the levels of active β-catenin (Fig. 5D) nor the β-catenin levels in the nuclear fraction (Fig. 5E). However, the basal nuclear β-catenin level was relative high in PRECs, which was consistent with a previous report (37). Two target genes for canonical Wnt signaling pathway, Axin2 and Wisp1, were also unresponsive to Wnt11 (Fig. 5F). In TKPTS, Wnt11 did not activate the TOPFLASH reporter for canonical Wnt signaling pathway (Fig. 5G). Conversely, Wnt11 overexpression slightly reduced the basal expression level of TOPFLASH, consistent with previous studies that show non-canonical Wnt signaling pathway could inhibit the canonical pathway (38). These data showed that Wnt11 was not activating canonical Wnt signaling in renal epithelial cell cultures. Furthermore, there was little evidence that TGF-β activated canonical Wnt signaling when using TOPFLASH reporters (Fig. 5G) or by assaying levels of Axin expression after 4 h in the microarray-based screens.
We then asked whether the non-canonical Wnt signaling pathway was activated in TGF-β-mediated responses. The JNK signaling pathway is one branch of non-canonical Wnt signaling. We found that phosphorylation of c-Jun and total c-Jun was elevated after TGF-β administration (Fig. 6A). The calcium-dependent signaling pathway is a second branch in which calcium influx will eventually cause the activation and autophosphorylation of CaMKII (17, 39). To our surprise, the basal phosphorylation level of CaMKII in PRECs was high even without TGF-β treatment. After TGF-β administration, the amount of phosphorylated CaMKII increased only slightly (Fig. 6A). However, recombinant Wnt11 administration in PRECs or overexpression in TKPTS by itself could activate the JNK signaling pathway as evidenced by phospho-c-Jun levels (Fig. 6B). To test the role of JNK signaling more directly, we used two specific JNK inhibitors, SP600125 and JNK inhibitor III, to block JNK signaling in PRECs before TGF-β administration. The JNK inhibitor III was a cell-permeable 37-mer peptide by fusing the human c-Jun σ-domain (amino acids 33–57) sequence with that of HIV-TAT protein transduction domain (amino acids 47–57). It has been shown to specifically disrupt c-Jun·JNK complex formation and the subsequent phosphorylation and activation of c-Jun by JNK and could be used as a complement inhibitor for SP600125 (40, 41). Both the inhibitors blocked the up-regulation of mesenchymal genes induced by TGF-β without affecting P-Smad3 levels (Fig. 6, C and D). However, despite their common blockade effects on c-Jun phosphorylation, SP600125 decreased the total c-Jun, whereas JNK inhibitor III slightly increased it. This may reflect the different characteristics of the two inhibitors. Similarly, in TKPTS, both the inhibitors reduced the up-regulation of mesenchymal genes induced by Wnt11 overexpression (Fig. 6E). A more precise examination of Wnt11 expression upon the TGF-β treatment showed that Wnt11 was induced as early as 1 h after TGF-β administration, which was just the time point that the amount of phosphorylated c-Jun began to increase (Fig. 6, F and G). The addition of cycloheximide abolished c-Jun phosphorylation (Fig. 6G), suggesting that the activation of JNK signaling pathway upon TGF-β treatment required new protein synthesis. Also, Wnt11 knockdown suppressed TGF-β-induced c-Jun phosphorylation (Fig. 6H), which is consistent with a role for Wnt11 in activating the JNK pathway. Taken together, our results demonstrated that up-regulation of Wnt11 by TGF-β drove expression of mesenchymal genes through the non-canonical, JNK signaling pathway.
FIGURE 6.
Activation of JNK signaling by TGF-β/Wnt11 activates mesenchymal marker genes. A, a Western blot for (Ser(P)-63)-c-Jun (p-cJun), total c-Jun, and P-CaMKII after treatment of PRECs for 24 or 48 h with TGF-β shows increased P- c-Jun and total c-Jun but not P-CaMKII. B, the addition of recombinant Wnt11 in PRECs or Wnt11 overexpression in TKPTS increases levels of phospho-c-Jun but not phospho-CaMKII. C, Western blots of cell lysates show that inhibition of the c-Jun kinase (JNK) by SP600125 or JNK inhibitor III reduces expression of αSMA in response to TGF-β. Note there is no affect on phospho-Smad3 levels. D, qRT-PCR of mesenchymal marker genes from samples treated with TGF-β with or without the JNK inhibitors show reduced expression of all mesenchymal markers tested upon JNK inhibition in PRECs. E, activation of mesenchymal marker gene expression in response to Wnt11 is reduced upon JNK inhibition in TKPTS, as determined by qRT-PCR. F, shown are Wnt11 RNA levels in PRECs cells cultured with TGF-β for the indicated time in hours. G, shown are Western blots for phospho-c-Jun, total c-Jun, P-Smad3, and Wnt11 with TGF-β treatment for the indicated times in the absence or in the presence of cycloheximide (CHX). Cycloheximide abolished the elevated phospho-c-Jun and Wnt11 induction upon TGF-β treatment. H, Western blots for phospho-c-Jun show reduced levels upon TGF-β treatment when cells are cultured with shRNA 53302 against Wnt11. *, p < 0.05; **, p < 0.01; Student's t test for independent variables.
Finally, we asked whether Wnt11 and the JNK pathway were activated in the UUO model of renal fibrosis. Ligation of a single ureter results in rapidly progressing interstitial fibrosis with increased TGF-β and P-Smad3 levels. This is readily apparent 7 days after UUO, as described previously (42, 43). We isolated both total RNA and protein from the obstructed kidney of 3 different mice 7 days after obstruction and checked for mesenchymal gene expression and protein phosphorylation (Fig. 7). Consistent with our in vitro cell studies, UUO kidneys showed significant induction of Wnt11, Snail1, and Zeb1 RNA. Additionally, we detected increased P-Smad3 and increased P-JNK levels by Western blotting. Active β-catenin was slightly increased in the UUO kidneys but was still a minor fraction of the total amount of β-catenin detected. These data suggested that Wnt11 and P-JNK may contribute to the pathology associated with ureteral obstruction, which included increased fibroblast proliferation, extracellular matrix deposition, and epithelial cell malfunction.
FIGURE 7.
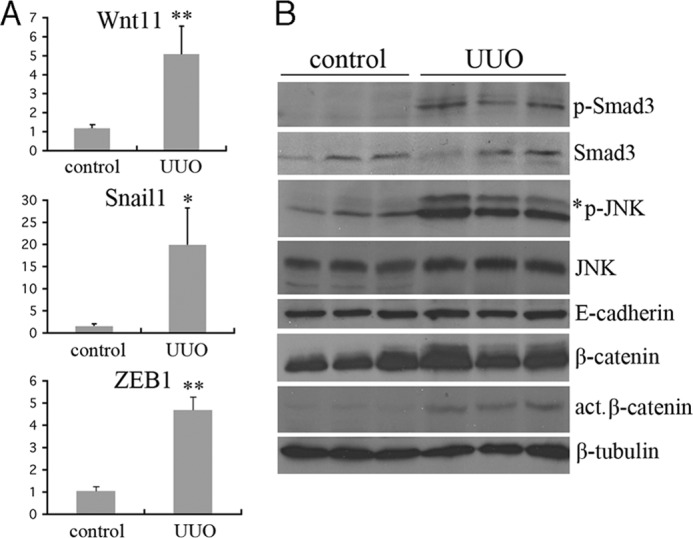
Activation of Wnt11 and JNK in the UUO model of renal fibrosis. A, Gene expression levels were assayed by qRT-PCR for the indicated genes in control (n = 3) and 7-day UUO kidney (n = 3) RNA isolates. RNA levels are expressed as relative units with the control kidney values set at 1 (*, p < 0.05; **, p < 0.01; Student's t test for independent variables). B, shown are Western blots of kidney protein lysates from three independent control or 7-day UUO kidneys. Identical blots were probed with the indicated antibodies. Note the increased levels of P-Smad3 and P-JNK in the UUO kidney lysates.
DISCUSSION
Despite the critical role of TGF-β in renal fibrosis, many of its target genes and biological effects in renal epithelial cells remain poorly characterized. We systematically analyzed the effects of TGF-β on transcription of direct target genes using renal epithelial cells and translation inhibition. From the microarray result, we found that more than 300 genes changed their RNA expression levels of at least 2-fold. In the presence of cycloheximide, not all mesenchymal markers, such as Pai1 and Zeb1, were up-regulated by TGF-β, suggesting that these genes are not direct targets of Smad protein-dependent transcription. This was consistent with previous studies indicating that some mesenchymal markers, such as Pai1, were not directly regulated by Smad proteins but the targets of other transcriptional factors induced by TGF-β signaling (44).
Among the genes activated by TGF-β were many associated with the Wnt signaling pathway. Recent evidence implied that Wnt signaling, especially the canonical Wnt/β-catenin pathway, was involved in EMT- and TGF-β-mediated fibrosis (19, 25, 45, 46). In our studies Wnt11 showed the greatest response to TGF-β both in microarray and real-time PCR assays and was thus studied further. Prior studies also suggested that Wnt11 was directly regulated by β-catenin (34, 35). However, TGF-β did not activate the expression of Axin2, a typical β-catenin target, in our microarray studies. In fact, we saw little evidence that β-catenin activation or reporter gene expression is enhanced by TGF-β or Wnt11 directly (Fig. 5). In the Smad3 knockdown TKPTS cells, the Smad2 phosphorylation upon TGF-β treatment was not affected, but Wnt11 up-regulation was largely reduced, suggesting that Smad2 alone is not sufficient to activate Wnt11 (Fig. 2, G and K). In Smad3-overexpressed TKPTS, the transfected Smad3 was phosphorylated and activated Wnt11 expression without affecting Smad2 phosphorylation status (Fig. 3, F and J). However, the kinases that phosphorylated Smad3 were unknown. Taken together, our data clearly showed that increased Wnt11 expression was regulated by a Smad3-dependent mechanism, consistent with the idea that Wnt11 is a direct TGF-β target.
Wnt11 has multiple functions in regulating cell properties, such as proliferation, migration, and differentiation. However, its precise function in different cell types was context-dependent and sometimes contradictory. For example, Wnt11 enhanced tight and gap junction formation in a quail mesodermal cell line QCE6 to promote its differentiation to cardiomyocytes (47). In contrast, Wnt11 conditional medium induced E-cadherin internalization in a rat intestinal epithelial cell line IEC6, thus increasing its proliferation and migration (48). These data suggested that Wnt11 effects were highly dependent on the cellular context. Although it was shown that Wnt11 was involved in EMT during dorsal fin development in Xenopus (49), its detailed function and mechanisms of action were not well characterized. Here, we reported that Wnt11 participated in TGF-β-mediated induction of mesenchymal marker genes in both primary and immortalized renal epithelial cells. Although both Wnt11 administration in PRECs and transfection in TKPTS could enhance TGF-β effects, its treatment alone in two cell types was different. In PRECs, Wnt11 alone did not activate mesenchymal markers (Fig. 3, A and B); however, in TKPTS, Wnt11 up-regulated some of the mesenchymal genes (Fig. 3C). This could result from subtle differences of cell characteristics between primary and immortalized cells and Wnt11 delivery methods. Recombinant protein administration is more like a paracrine model, whereas expressing vector transfection may be more autocrine. Compared with the Wnt11 recombinant protein, the autocrine Wnt11 in TKPTS may interact with other secreted ligands, thus acquiring additional properties. For example, the secreted Xenopus Wnt11 physically interacts with Wnt5a, and the complexes have more canonical Wnt signaling activity than secreted Wnt11 or Wnt5a acting alone (36). Alternatively, in the TKPTS cells, transfected Wnt11 might enhance low levels of endogenous TGF-β function, similar to what we observed at low TGF-β doses in PREC cells.
Whether Wnt11 activates the canonical Wnt signaling pathway is also controversial. For example, Cha et al. (36) showed that the Wnt11/5a complex could enhance canonical Wnt11 signaling through accumulating cytosolic β-catenin in Xenopus oocytes, mouse L cells, and human embryonic stem cells. Other reports showed that Wnt11 had no effects or even down-regulated β-catenin signaling (50, 51). To date, most studies are consistent with Wnt11 activating the non-canonical Wnt signaling pathways through the JNK or CaMKII kinases (34, 52, 53). In our renal epithelial cells, Wnt11 alone did not activate canonical Wnt signaling nor was the CaMKII protein activated by TGF-β or Wnt11 directly (Fig. 6, A and B). In fact, CaMKII reportedly can inactivate Smad/TGF-β signaling through blocking the accumulation of nuclear Smad proteins (54). Clearly this was not the case given the robust TGF-β responses observed. However, we did measure activation of the JNK signaling pathway by TGF-β and Wnt11. The lack of c-Jun phosphorylation by TGF-β in the presence of cycloheximide indicated that the activation of JNK signaling was a secondary effect for TGF-β, which required new protein synthesis (Fig. 6G). Activation of JNK was critical for mediating the Wnt11/TGF-β response because inhibition of JNK signaling could decrease up-regulation of the mesenchymal genes induced by TGF-β and Wnt11 (Fig. 6, C–E). Although JNK signaling was activated by Wnt11 alone in PRECs, this was not sufficient to induce expression of mesenchymal genes. The JNK inhibitor only attenuated the effects of TGF-β but did not entirely block the activation of mesenchymal genes. In the UUO model, TGF-β, Wnt11, and JNK signaling pathways were activated in the injured kidneys. Although we detected the activation of canonical/β-catenin signaling, this may result from up-regulation of other Wnt ligands, such as Wnt1 and Wnt3a (19), or other cell types, such as fibroblasts, endothelial cells, infiltrating immune cells, and podocytes (55).
Taken together, our results showed that TGF-β activated multiple signaling pathways in renal epithelial cells through enhanced expression of secreted signaling proteins and transcription factors. Of these, Wnt11 activation by TGF-β enhanced the overall effects attributed to TGF-β on epithelial cells, such as expression of genes associated with more mesenchymal phenotypes. A recent study on EMT in breast tumor suggested the importance of TGF-β, canonical, and non-canonical Wnt signaling pathways in maintaining the mesenchymal state, indicating an interactive communication model between different signaling pathways in a biological process (20). Our findings suggested a transregulation model whereby TGF-β directly activated signaling factors and effectors necessary for the phenotypic changes observed. Furthermore, by activating families of secreted signaling molecules, TGF-β acting on epithelial cells could impact the environment and the adjacent cells in the renal interstitium, such as fibroblasts, myofibroblasts, and the pericytes associated with the vasculature. Indeed, the effects of Wnt11 on other cell types could be an equally important determinant in the pathology mediated by TGF-β in the kidney and must be investigated in subsequent studies.
Acknowledgments
We thank Elsa Bello-Reuss (Texas Tech University Health Science Center) for the TKPTS immortalized cell line, Min Wang for help with primary cell isolation techniques, and Craig Johnson (University of Michigan Cancer Center Microarray Core Facility) for bioinformatics support.
This work was supported, in whole or in part, by National Institutes of Health Grants DK062914 and DK073722 (to G. R. D.).
- UUO
- unilateral ureteral obstruction
- EMT
- epithelial-to-mesenchymal transition
- CaMKII
- Ca2+/calmodulin-dependent kinase II
- SHS
- sonicated herring sperm
- PREC
- primary renal epithelial cell
- RMA
- robust multiarray average
- Fzd7
- Frizzled7
- qPCR
- quantitative PCR.
REFERENCES
- 1. Liu Y. (2010) New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am. Soc. Nephrol. 21, 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Böttinger E. P., Bitzer M. (2002) TGF-β signaling in renal disease. J. Am. Soc. Nephrol. 13, 2600–2610 [DOI] [PubMed] [Google Scholar]
- 3. Yang J., Liu Y. (2001) Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 159, 1465–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeisberg E. M., Tarnavski O., Zeisberg M., Dorfman A. L., McMullen J. R., Gustafsson E., Chandraker A., Yuan X., Pu W. T., Roberts A. B., Neilson E. G., Sayegh M. H., Izumo S., Kalluri R. (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 [DOI] [PubMed] [Google Scholar]
- 5. Kopp J. B., Factor V. M., Mozes M., Nagy P., Sanderson N., Böttinger E. P., Klotman P. E., Thorgeirsson S. S. (1996) Transgenic mice with increased plasma levels of TGF-β 1 develop progressive renal disease. Lab. Invest. 74, 991–1003 [PubMed] [Google Scholar]
- 6. Ledbetter S., Kurtzberg L., Doyle S., Pratt B. M. (2000) Renal fibrosis in mice treated with human recombinant transforming growth factor-β2. Kidney Int. 58, 2367–2376 [DOI] [PubMed] [Google Scholar]
- 7. Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. (1990) Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature 346, 371–374 [DOI] [PubMed] [Google Scholar]
- 8. Feng X. H., Derynck R. (2005) Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 [DOI] [PubMed] [Google Scholar]
- 9. Sano Y., Harada J., Tashiro S., Gotoh-Mandeville R., Maekawa T., Ishii S. (1999) ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J. Biol. Chem. 274, 8949–8957 [DOI] [PubMed] [Google Scholar]
- 10. Labbé E., Letamendia A., Attisano L. (2000) Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-β and wnt pathways. Proc. Natl. Acad. Sci. U.S.A. 97, 8358–8363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phanish M. K., Wahab N. A., Colville-Nash P., Hendry B. M., Dockrell M. E. (2006) The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFβ1 responses in human proximal-tubule epithelial cells. Biochem. J. 393, 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meng X. M., Huang X. R., Chung A. C., Qin W., Shao X., Igarashi P., Ju W., Bottinger E. P., Lan H. Y. (2010) Smad2 protects against TGF-β/Smad3-mediated renal fibrosis. J. Am. Soc. Nephrol. 21, 1477–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwano M., Plieth D., Danoff T. M., Xue C., Okada H., Neilson E. G. (2002) Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Humphreys B. D., Lin S. L., Kobayashi A., Hudson T. E., Nowlin B. T., Bonventre J. V., Valerius M. T., McMahon A. P., Duffield J. S. (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taura K., Miura K., Iwaisako K., Osterreicher C. H., Kodama Y., Penz-Osterreicher M., Brenner D. A. (2010) Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology 51, 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling. Components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kühl M., Sheldahl L. C., Malbon C. C., Moon R. T. (2000) Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275, 12701–12711 [DOI] [PubMed] [Google Scholar]
- 18. Veeman M. T., Axelrod J. D., Moon R. T. (2003) A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 5, 367–377 [DOI] [PubMed] [Google Scholar]
- 19. He W., Dai C., Li Y., Zeng G., Monga S. P., Liu Y. (2009) Wnt/β-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheel C., Eaton E. N., Li S. H., Chaffer C. L., Reinhardt F., Kah K. J., Bell G., Guo W., Rubin J., Richardson A. L., Weinberg R. A. (2011) Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145, 926–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwang I., Seo E. Y., Ha H. (2009) Wnt/β-catenin signaling. A novel target for therapeutic intervention of fibrotic kidney disease. Arch. Pharm. Res. 32, 1653–1662 [DOI] [PubMed] [Google Scholar]
- 22. Masszi A., Fan L., Rosivall L., McCulloch C. A., Rotstein O. D., Mucsi I., Kapus A. (2004) Integrity of cell-cell contacts is a critical regulator of TGF-β 1-induced epithelial-to-myofibroblast transition. Role for β-catenin. Am. J. Pathol. 165, 1955–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim K. K., Wei Y., Szekeres C., Kugler M. C., Wolters P. J., Hill M. L., Frank J. A., Brumwell A. N., Wheeler S. E., Kreidberg J. A., Chapman H. A. (2009) Epithelial cell α3β1 integrin links β-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J. Clin. Invest. 119, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang M., Wang M., Tan X., Li T. F., Zhang Y. E., Chen D. (2010) Smad3 prevents β-catenin degradation and facilitates β-catenin nuclear translocation in chondrocytes. J. Biol. Chem. 285, 8703–8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou B., Liu Y., Kahn M., Ann D. K., Han A., Wang H., Nguyen C., Flodby P., Zhong Q., Krishnaveni M. S., Liebler J. M., Minoo P., Crandall E. D., Borok Z. (2012) Interactions between β-catenin and transforming growth factor-β signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J. Biol. Chem. 287, 7026–7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irizarry R. A., Wu Z., Jaffee H. A. (2006) Comparison of Affymetrix GeneChip expression measures. Bioinformatics 22, 789–794 [DOI] [PubMed] [Google Scholar]
- 27. Smyth G. K. (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article3 [DOI] [PubMed] [Google Scholar]
- 28. Benjamini Y., Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy Stat. Soc. B. Met. 57, 289–300 [Google Scholar]
- 29. Schiro M. M., Stauber S. E., Peterson T. L., Krueger C., Darnell S. J., Satyshur K. A., Drinkwater N. R., Newton M. A., Hoffmann F. M. (2011) Mutations in protein-binding hotspots on the hub protein Smad3 differentially affect its protein interactions and Smad3-regulated gene expression. PLoS One 6, e25021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ernest S., Bello-Reuss E. (1995) Expression and function of P-glycoprotein in a mouse kidney cell line. Am. J. Physiol. 269, C323–C333 [DOI] [PubMed] [Google Scholar]
- 31. Jinnin M., Ihn H., Tamaki K. (2006) Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β1-induced extracellular matrix expression. Mol. Pharmacol. 69, 597–607 [DOI] [PubMed] [Google Scholar]
- 32. Djiane A., Riou J., Umbhauer M., Boucaut J., Shi D. (2000) Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 127, 3091–3100 [DOI] [PubMed] [Google Scholar]
- 33. Yamanaka H., Nishida E. (2007) Wnt11 stimulation induces polarized accumulation of Disheveled at apical adherens junctions through Frizzled7. Genes Cells 12, 961–967 [DOI] [PubMed] [Google Scholar]
- 34. Zhou W., Lin L., Majumdar A., Li X., Zhang X., Liu W., Etheridge L., Shi Y., Martin J., Van de Ven W., Kaartinen V., Wynshaw-Boris A., McMahon A. P., Rosenfeld M. G., Evans S. M. (2007) Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFβ2. Nat. Genet. 39, 1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dwyer M. A., Joseph J. D., Wade H. E., Eaton M. L., Kunder R. S., Kazmin D., Chang C. Y., McDonnell D. P. (2010) WNT11 expression is induced by estrogen-related receptor α- and β-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res. 70, 9298–9308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cha S. W., Tadjuidje E., White J., Wells J., Mayhew C., Wylie C., Heasman J. (2009) Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr. Biol. 19, 1573–1580 [DOI] [PubMed] [Google Scholar]
- 37. Jian H., Shen X., Liu I., Semenov M., He X., Wang X. F. (2006) Smad3-dependent nuclear translocation of β-catenin is required for TGF-β1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 20, 666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abdul-Ghani M., Dufort D., Stiles R., De Repentigny Y., Kothary R., Megeney L. A. (2011) Wnt11 promotes cardiomyocyte development by caspase-mediated suppression of canonical Wnt signals. Mol. Cell. Biol. 31, 163–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barria A., Muller D., Derkach V., Griffith L. C., Soderling T. R. (1997) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long term potentiation. Science 276, 2042–2045 [DOI] [PubMed] [Google Scholar]
- 40. Holzberg D., Knight C. G., Dittrich-Breiholz O., Schneider H., Dörrie A., Hoffmann E., Resch K., Kracht M. (2003) Disruption of the c-JUN-JNK complex by a cell-permeable peptide containing the c-JUN δ domain induces apoptosis and affects a distinct set of interleukin-1-induced inflammatory genes. J. Biol. Chem. 278, 40213–40223 [DOI] [PubMed] [Google Scholar]
- 41. Wang Y., Crisostomo P. R., Wang M., Markel T. A., Novotny N. M., Meldrum D. R. (2008) TGF-α increases human mesenchymal stem cell-secreted VEGF by MEK- and PI3-K- but not JNK- or ERK-dependent mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1115–R1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin J., Patel S. R., Wang M., Dressler G. R. (2006) The cysteine-rich domain protein KCP is a suppressor of transforming growth factor β/activin signaling in renal epithelia. Mol. Cell. Biol. 26, 4577–4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin J., Patel S. R., Cheng X., Cho E. A., Levitan I., Ullenbruch M., Phan S. H., Park J. M., Dressler G. R. (2005) Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat. Med. 11, 387–393 [DOI] [PubMed] [Google Scholar]
- 44. He W., Tan R., Dai C., Li Y., Wang D., Hao S., Kahn M., Liu Y. (2010) Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J. Biol. Chem. 285, 24665–24675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Surendran K., Schiavi S., Hruska K. A. (2005) Wnt-dependent β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 16, 2373–2384 [DOI] [PubMed] [Google Scholar]
- 46. Akhmetshina A., Palumbo K., Dees C., Bergmann C., Venalis P., Zerr P., Horn A., Kireva T., Beyer C., Zwerina J., Schneider H., Sadowski A., Riener M. O., MacDougald O. A., Distler O., Schett G., Distler J. H. (2012) Activation of canonical Wnt signaling is required for TGF-β-mediated fibrosis. Nat. Commun. 3, 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eisenberg C. A., Gourdie R. G., Eisenberg L. M. (1997) Wnt-11 is expressed in early avian mesoderm and required for the differentiation of the quail mesoderm cell line QCE-6. Development 124, 525–536 [DOI] [PubMed] [Google Scholar]
- 48. Ouko L., Ziegler T. R., Gu L. H., Eisenberg L. M., Yang V. W. (2004) Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J. Biol. Chem. 279, 26707–26715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garriock R. J., Krieg P. A. (2007) Wnt11-R signaling regulates a calcium-sensitive EMT event essential for dorsal fin development of Xenopus. Dev. Biol. 304, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maye P., Zheng J., Li L., Wu D. (2004) Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J. Biol. Chem. 279, 24659–24665 [DOI] [PubMed] [Google Scholar]
- 51. Anton R., Kestler H. A., Kühl M. (2007) β-Catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 581, 5247–5254 [DOI] [PubMed] [Google Scholar]
- 52. Westfall T. A., Brimeyer R., Twedt J., Gladon J., Olberding A., Furutani-Seiki M., Slusarski D. C. (2003) Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J. Cell Biol. 162, 889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Flaherty M. P., Abdel-Latif A., Li Q., Hunt G., Ranjan S., Ou Q., Tang X. L., Johnson R. K., Bolli R., Dawn B. (2008) Noncanonical Wnt11 signaling is sufficient to induce cardiomyogenic differentiation in unfractionated bone marrow mononuclear cells. Circulation 117, 2241–2252 [DOI] [PubMed] [Google Scholar]
- 54. Wicks S. J., Lui S., Abdel-Wahab N., Mason R. M., Chantry A. (2000) Inactivation of smad-transforming growth factor β signaling by Ca2+-calmodulin-dependent protein kinase II. Mol. Cell. Biol. 20, 8103–8111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang D., Dai C., Li Y., Liu Y. (2011) Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int. 80, 1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]