Background: γ-Secretase-mediated intramembrane proteolysis generates Aβ42/43, pathogenic peptides implicated in causing Alzheimer disease (AD).
Results: Reconstitution of γ-secretase in model membranes reveals that Aβ42/43 generation can be lowered in thick membranes.
Conclusion: Membrane thickness is a crucial factor influencing the activity of γ-secretase to generate Aβ42/43.
Significance: Targeting the lipid environment of γ-secretase by increasing membrane thickness may provide a therapeutic strategy for AD.
Keywords: Alzheimer Disease, Amyloid, Membrane Bilayer, Presenilin, Secretases
Abstract
Pathogenic generation of amyloid β-peptide (Aβ) by sequential cleavage of β-amyloid precursor protein (APP) by β- and γ-secretases is widely believed to causally underlie Alzheimer disease (AD). β-Secretase initially cleaves APP thereby generating a membrane-bound APP C-terminal fragment, from which γ-secretase subsequently liberates 37–43-amino acid long Aβ species. Although the latter cleavages are intramembranous and although lipid alterations have been implicated in AD, little is known of how the γ-secretase-mediated release of the various Aβ species, in particular that of the pathogenic longer variants Aβ42 and Aβ43, is affected by the lipid environment. Using a cell-free system, we have directly and systematically investigated the activity of γ-secretase reconstituted in defined model membranes of different thicknesses. We found that bilayer thickness is a critical parameter affecting both total activity as well as cleavage specificity of γ-secretase. Whereas the generation of the pathogenic Aβ42/43 species was markedly attenuated in thick membranes, that of the major and rather benign Aβ40 species was enhanced. Moreover, the increased production of Aβ42/43 by familial AD mutants of presenilin 1, the catalytic subunit of γ-secretase, could be substantially lowered in thick membranes. Our data demonstrate an effective modulation of γ-secretase activity by membrane thickness, which may provide an approach to lower the generation of the pathogenic Aβ42/43 species.
Introduction
Alzheimer disease (AD)3 is the most common neurodegenerative disease affecting the elderly. Neurofibrillary tangles as well as amyloid plaques are invariant, major pathological hallmarks of the disease. The principal component of the amyloid plaques is the ∼4-kDa amyloid β-peptide (Aβ), which represents a heterogeneous mixture of hydrophobic peptides consisting of 37–43-amino acid Aβ species, Aβ40 being the major species (1). The longer variants Aβ42 and Aβ43 are highly aggregation-prone. Aβ42, and as demonstrated recently Aβ43 as well (2), is highly pathogenic and believed to cause AD by triggering a pathogenic series of events, the so-called amyloid cascade that ultimately leads to neurodegeneration and dementia (1, 3). Aβ is generated by proteolytic processing of the β-amyloid precursor protein (APP), a type I membrane protein, by the combined action of β-secretase and γ-secretase (4). β-Secretase removes the large portion of the APP ectodomain and leaves a 99-amino acid C-terminal fragment in the membrane, which is subsequently cleaved by γ-secretase in the transmembrane domain (TMD). γ-Secretase cleavage occurs in a stepwise manner thereby liberating the APP intracellular domain (AICD) and the various Aβ species from the membrane (5, 6). The initial AICD-releasing cleavage at the ϵ-site occurs close to the cytoplasmic border of the membrane and is followed by further ζ- and γ-site cuts downstream the TMD ultimately releasing the different Aβ species, which become secreted (5, 6).
γ-Secretase belongs to the few unusual proteases that cleave their substrates within the core of the membrane (7). The protease is a complex composed of four integral membrane proteins, which are essential and necessary for activity (8–10). Presenilin (PS) 1 or its homolog PS2, respectively, act as catalytic subunits and mediate the intramembrane cleavages of the APP C-terminal fragment substrate (11). The potential substrate receptor nicastrin, APH-1 and PEN-2, are subunits required for stabilization and activation of PS (4, 12). Although drug targeting γ-secretase to lower Aβ generation may prove difficult as γ-secretase has many other substrates besides APP including Notch1, a major regulator of cell differentiation in development and adulthood (13), APP-selective inhibitors and modulators of γ-secretase have been developed that are in clinical trials for AD treatment (14).
Apart from the common sporadic forms, rare familial forms of AD (FAD) exist, which are characterized by an early disease onset and in the vast majority of the cases caused by mutations in PS1 and to a lesser extent by mutations in PS2. PS mutations cause an increase in the relative amounts of Aβ42 and/or Aβ43 species that are generated by γ-secretase cleavage (15, 16). Few FAD mutations have also been found in APP, most of them are located in the APP TMD at the γ-secretase cleavage sites and increase the ratio of Aβ42 to total Aβ (15).
Because APP processing is a membrane-associated process, alterations in the lipid membrane environment would naturally have an impact on the generation of Aβ and may thus potentially constitute a risk factor for AD. Alterations of a large variety of lipids have been documented in AD brain (17–19). However, whether and how known AD-associated lipid alterations affect APP processing and to what extent this may affect Aβ generation have remained unclear. Apart from previous studies demonstrating that amyloidogenic APP processing occurs predominantly in lipid rafts, i.e. cholesterol and sphingomyelin-rich membrane microdomains (20, 21), and is modulated by cholesterol (21–24), our knowledge on the lipid dependence of Aβ generation has remained limited (19, 25).
Reconstitution of purified membrane proteins has been an established and powerful tool to study directly the mechanism of action and structure-function relationships of a variety of integral membrane proteins including receptors, channels, carriers, as well as membrane-bound enzymes and enzyme complexes under well defined environmental conditions such as the membrane lipids in which the protein is embedded (26, 27). Recent advances in the purification of the enzymes involved in APP processing and their reconstitution in liposomes now provide a platform not only to identify lipids that modulate Aβ generation but also to study directly the mechanism by which lipids might affect Aβ generation. These studies already provided a first insight into the lipid classes, which affect β- and γ-secretase activities directly and identified, among other lipids, cholesterol as an activity-promoting lipid of both secretases (28, 29).
As has been reported for a number of membrane proteins and membrane protein complexes (30, 31), alteration of basic membrane properties such as hydrophobic thickness, chain order, and the packing of the lipids of the membrane core may likely modulate the activities of the secretases, in particular that of γ-secretase being a large intramembrane-cleaving protease complex containing 19 TMDs. To understand better the role of the lipid microenvironment of γ-secretase for the generation of Aβ, in particular that of the pathogenic Aβ42/43 species, we set out to investigate systematically whether and how acyl chain length as well as unsaturation of phosphatidylcholine (PC) lipids would influence γ-secretase activity and cleavage specificity in reconstituted proteoliposomes (PLs). Overall, we found that the activity of γ-secretase is directly affected by bilayer thickness, and we show that its modulation may be exploited to lower the generation of the pathogenic Aβ42/43 species.
EXPERIMENTAL PROCEDURES
Antibodies
Monoclonal and polyclonal antibodies, respectively, to Aβ1–16 (2D8) and to the N terminus of PEN-2 (1638) have been described (32, 33). Monoclonal and polyclonal antibodies to Aβ17–24 (4G8), to the ectodomain (clone 35), and to the C terminus of nicastrin (N1660) were obtained from Covance, BD Biosciences, and Sigma, respectively. Monoclonal anti-His5 antibody was obtained from Qiagen. For additional reagents used, such as γ-secretase inhibitors (34), see supplemental Materials and Methods.
Cell Lines
Human embryonic kidney 293 (HEK293) cells, HEK293 cells stably expressing Swedish mutant APP (HEK293/sw) or coexpressing wt PS1, PS1 Δexon9, or PS1 L166P have been described before (35).
Lipids
Synthetic lipids (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), diCn:0 PC, diCn:1(Δ9-cis) PC, Cn:0/Cm:0 PC, Cn:0/Cm:1 PC, diC18:2 PC, C18:0/C18:2 PC, C18:0/C20:4 PC, and C18:0/C22:6 PC) were purchased from Avanti Polar Lipids.
Preparation and Biophysical Characterization of Lipid Vesicles
12 mg of lipid was hydrated with 1.2 ml of distilled water for 1 h at room temperature. Hydrated lipids were sonicated with a Branson sonifier in pulsed mode (duty cycle 20%, output level 3) for 1 h at temperatures above the chain-melting temperature, Tm, of the lipids under a stream of argon gas. The clear suspension was centrifuged for 1 min with a table centrifuge to remove Ti particles and analyzed with dynamic light scattering (Malvern Instruments High Performance Particle Sizer) to confirm that small unilamellar vesicles (SUVs) with a diameter of about 30 nm had been formed. SUVs were diluted in assay buffer (35 mm sodium citrate, 3.5% glycerol, pH 6.4) to a final lipid concentration of 3.45 mg/ml and stored in aliquots of 200 μl at −20 °C until further use. For certain batches of diC14:0 PC, 15 mg of lipid was hydrated in 1.2 ml of distilled water to achieve robust activity of reconstituted γ-secretase.
Purification and Reconstitution of γ-Secretase into Lipid Vesicles
Native human γ-secretase was isolated from HEK293 cells by a multistep purification protocol as described before (36). The final enzyme preparations (Q-Sepharose eluates) contained 30–150 μg/ml protein in 5% glycerol, 1 mm EDTA, 1% CHAPSO, 25 mm caproic acid, 18.75 mm BisTris, 500 mm NaCl, pH 7.0. To reconstitute the enzyme into SUVs, 200 μl of the lipid vesicles were thawed, sonicated for 1 min as described above, and diluted 5 times with buffer (35 mm sodium citrate, 3.5% glycerol, 30 mm DTT, pH 6.4). Next, 1 volume of the purified enzyme was diluted with 4 volumes of the above prepared lipid vesicle suspension and incubated at 4 °C as described before (36) to allow the formation of PLs. The final PL mixture contained 6–30 μg/ml protein, 0.55 mg/ml lipids, and 0.2% CHAPSO (lipid/protein ratio (w/w): 20–100; detergent/lipid ratio (w/w): 3.6, for all lipids used). In each experiment identical aliquots of the same enzyme preparation were taken. One aliquot was always used for the POPC control, and the other aliquots were used for the lipids under investigation.
γ-Secretase in Vitro Assay
Purified recombinant C100-His6 APP substrate was prepared as described (9). In a standard assay, C100-His6 was added to 20 μl of PLs to a final substrate concentration of 0.5 μm. Following overnight incubation at 37 °C, in vitro generated Aβ and AICD substrate cleavage products were analyzed by immunoblotting and quantified as described (36) by measuring the respective chemiluminescence signal intensities using the FluorchemTM 8900 detection system (Alpha Innotech).
Tris-Bicine Urea SDS-PAGE
To analyze individual Aβ species by immunoblotting, Tris-Bicine urea SDS-PAGE was used (37), which was adjusted for the separation of short Aβ species from substrate (36) by using a 11% separation gel.
For additional procedures, see supplemental Materials and Methods.
Statistical Analysis
For each lipid, independent enzyme reconstitutions using independent γ-secretase preparations and independent PC SUVs were analyzed. Quantitative data were collected from at least three independent assays. For some lipids, the corresponding data sets were repeatedly presented when used in more than one experimental context. Statistical significance of quantitative data were calculated by two-tailed unpaired Student's t test with Bonferroni correction for multiple testing.
RESULTS
To investigate the role of the lipid environment of γ-secretase on APP substrate processing we made advantage of our previously established cell-free reconstitution system comprised of purified enzyme, purified substrate, and lipid vesicles (36), allowing us to test the activity of the endogenous protease isolated from HEK293 cells in various defined model membranes. Because we had previously shown that our purified CHAPSO-solubilized γ-secretase displays robust activity and physiological cleavage specificity when reconstituted in PC vesicles (36), we based our studies on this lipid focusing on the influence of hydrophobic membrane thickness using model membranes of synthetic PC lipids with various acyl chains. POPC was used as control lipid in our studies because it represents the major phospholipid of biological membranes. Serving as general reference, a more detailed analysis of γ-secretase reconstituted in this lipid was undertaken, demonstrating robust enzyme activity, and as shown by electron microscopy and dynamic light scattering analysis, formation of PLs with a diameter of 30 nm (supplemental Fig. 1). We further found that consistent with earlier results (29), the reconstituted enzyme required the presence of residual detergent for activity, probably to allow the release of cleavage products from the PLs and/or to facilitate substrate availability (supplemental Fig. 2).
γ-Secretase Activity and Cleavage Specificity Depend on Membrane Thickness
The hydrophobic thickness of lipid membranes is determined by many parameters including temperature, the state of the lipids (liquid-crystalline or gel phase), the distance between the head groups, the length and the amount of double bonds of the acyl chains, the angle of the acyl chains with respect of the plane of the bilayer, and the intercalation of the acyl chains (38). We started to investigate γ-secretase activity in PLs composed of PC with two monounsaturated acyl chains of increasing length allowing us to maintain the liquid-crystalline state of the bilayers at assay temperature. As in supplemental Fig. 1A, γ-secretase activity was generally analyzed after overnight incubation at 37 °C by immunoblot analysis of the formation of the cleavage products Aβ and AICD from purified recombinant APP C100-His6 substrate added to the PLs. To assess changes in the whole profile of the individual Aβ species generated, Tris-Bicine urea SDS-PAGE was used, which allowed us to separate shorter (Aβ37–40) and longer (Aβ42/43) Aβ species from each other as well as from C100-His6 (36).
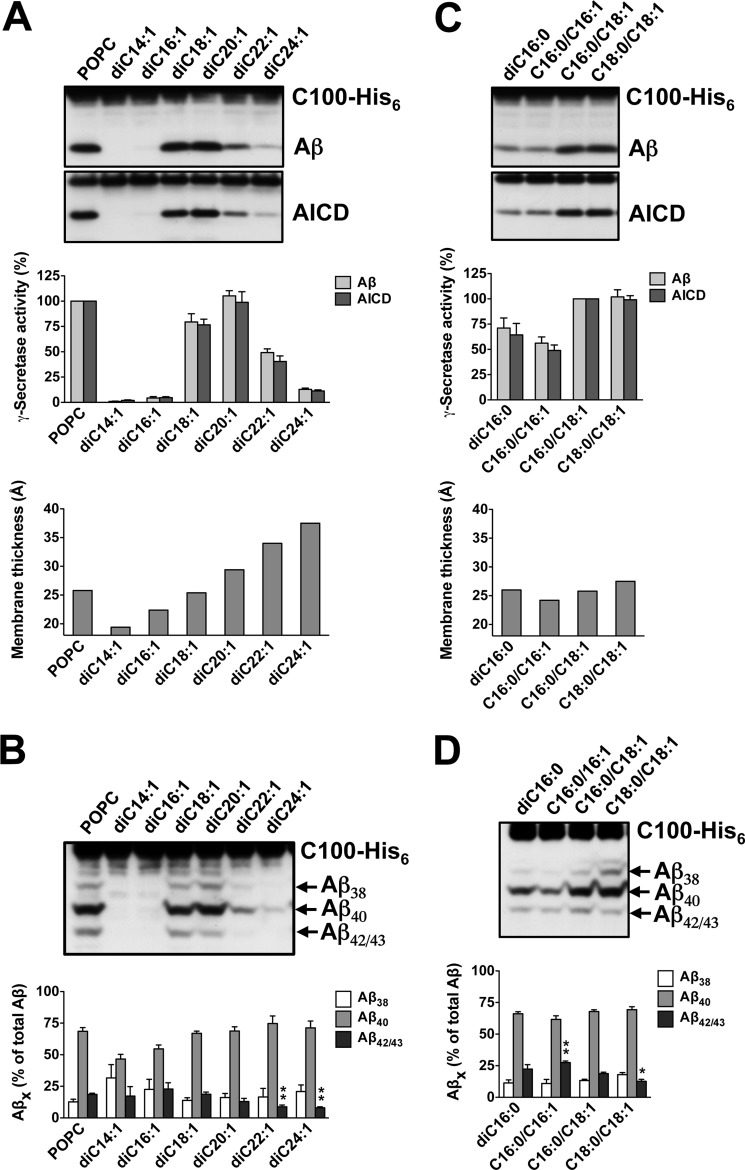
As shown in Fig. 1A, only poor γ-secretase activity was observed with the short-chain lipids diC14:1 and di16:1, remaining below 5% of the POPC control. γ-Secretase activity increased sharply with diC18:1 PC, reached an optimum comparable with control with diC20:1 PC, and gradually decreased down to ∼10% with the long chain lipids diC22:1 and diC24:1 PC.4 Thus, γ-secretase activity depends on the length of the lipid acyl chains, with an optimum at 20 carbon atoms for monounsaturated acyl chains corresponding to a hydrophobic thickness of ∼29 Å. We next investigated the cleavage specificity of γ-secretase with respect to the increase in the hydrophobic thicknesses of these bilayers. Assessment of the individual Aβ species generated revealed that the relative production of Aβ42/43 was lowered with increasing acyl chain length (Fig. 1B). Remarkably, at bilayers thicker than di20:1 PC, i.e. thicker than a POPC bilayer, the Aβ42/43/Aβtotal ratio dropped significantly below that found for the POPC control.
FIGURE 1.
Dependence of γ-secretase activity and cleavage specificity on membrane thickness. A, γ-secretase activity was assessed following reconstitution of the purified enzyme into preformed SUVs composed of POPC or the indicated PCs containing monounsaturated acyl chains of increasing length. Upon addition of C100-His6 substrate and overnight incubation at 37 °C, samples were analyzed for Aβ and AICD cleavage products by immunoblotting using antibodies 2D8 (Aβ) and anti-His5 (AICD) (top). Quantitation of total Aβ and AICD cleavage products is shown in the middle. γ-Secretase activity was expressed relative to the control POPC PLs, which was set to 100%. Data are represented as mean ± S.E. (error bars) (POPC, n = 7; all other PCs n = 3–7). The hydrophobic membrane thickness of the bilayers comprising the corresponding lipids is shown in the bottom panel. B, aliquots of samples analyzed in A were subjected to Tris-Bicine urea SDS-PAGE to allow the separation of individual Aβ species and analyzed by immunoblotting using antibody 2D8 (upper). Note that Aβ42 and Aβ43 are not separated under our gel electrophoresis conditions and migrate as one band (Aβ42/43). Quantitation of Aβ38, Aβ40, Aβ42/43 species is shown in the lower panel. Data are represented as mean ± S.E. (POPC, n = 7; all other PCs, n = 3–7; **, p < 0.01 relative to POPC). C and D, conditions were as in A and B for diC16:0, C16:0/C16:1, C16:0/C18:1 (i.e. POPC), and C18:0/C18:1 PC. Quantitative data are represented as mean ± S.E. (POPC, n = 13, all other PCs, n = 3–5; *, p < 0.05; **, p < 0.01 relative to POPC).
To substantiate further the modulatory influence of membrane thickness on the cleavage specificity of γ-secretase, we next analyzed saturated and monounsaturated PC in the C16–C18 acyl chain length range. As expected from our analysis above, substantial γ-secretase activity was observed for the diC16 lipids compared with the mixed-chain C16/C18 POPC control lipid, which was comparable with the diC18 lipid (Fig. 1C). In further support of the findings above, we found that compared with the diC16:0 membrane, the relative production of Aβ42/43 by γ-secretase was increased in the thinner C16:0/C16:1 PC bilayer (Fig. 1D). Thickening the membrane by prolonging one of the acyl chains using C16:0/C18:1 PC (i.e. POPC) reverted this effect. Consistent with the data above, further thickening the bilayer using C18:0/C18:1 PC resulted in the lowest Aβ42/43/Aβtotal ratio measured for these bilayers, lower than that produced by γ-secretase in POPC (Fig. 1D).
Taken together, we conclude that γ-secretase has an activity optimum for APP substrate cleavage in membranes with a hydrophobic core of 26–29 Å. The relative production of Aβ42/43 species decreases with increasing thickness of the bilayer, suggesting that lipid chain length molulates not only total activity of γ-secretase, but also its cleavage specificity.
γ-Secretase Activity Is Affected by Lipid Saturation State
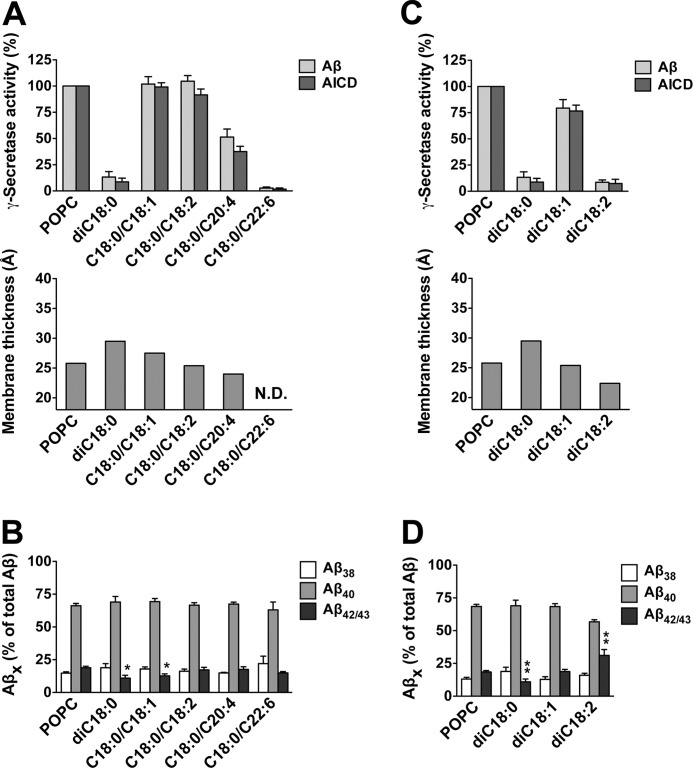
We next analyzed the impact of the saturation state of the lipid acyl chain on γ-secretase activity by comparing diC18:0, C18:0/C18:1, C18:0/C18:2, and two C18:0/polyunsaturated fatty acid PCs, i.e. lipids with an increasing degree of unsaturation in one of the acyl chains (Fig. 2A). γ-Secretase displayed poor activity in bilayers composed of diC18:0 PC, which might be due its gel state at the assay temperature (Tm of diC18:0 PC = 55 °C) (38). However, adding one or two double bounds in one of the acyl chains recovered γ-secretase activity toward the POPC control level. Arachidonic acid (C20:4 ω-6) reduced the activity to 50% of control, whereas docosahexaenoic acid (C22:6 ω-3) dropped the activity to <10%. As shown in Fig. 2B, while the Aβ42/43 levels were not much different from POPC for most of these bilayers, the lowest productions of Aβ42/43 were found for diC18:0 and C18:0/C18:1 PC, i.e. for the thickest bilayers of this series. Interestingly, addition of one double bond to both acyl chains (diC18:1 PC) also rescued the strongly reduced activity observed with diC18:0 PC, whereas addition of two double bonds in each acyl chain (diC18:2 PC) dropped the activity again to <10% (Fig. 2C). Consistent with the decrease in membrane thickness, Aβ42/43 production was elevated with the latter lipid (Fig. 2D). Thus, overall, γ-secretase activity showed a trend of an increased relative production of Aβ42/43 as the membrane thickness was reduced in consequence of an increasing degree of acyl chain unsaturation.
FIGURE 2.
Dependence of γ-secretase activity and cleavage specificity on lipid saturation state. A and B, γ-secretase activity was assessed following reconstitution of the purified enzyme into preformed POPC, diC18:0, C18:0/C18:1, C18:0/C18:2, C18:0/C20:4, and C18:0/C22:6 PC SUVs as in Fig. 1, A and B. Quantitation of total Aβ and AICD and representation of hydrophobic membrane thicknesses are shown in A (N.D., not determined), and quantitation of Aβ38, Aβ40, Aβ42/43 species in is shown in B. Quantitative data are represented as mean ± S.E. (error bars) (POPC, n = 10, all other PCs, n = 3–6; *, p < 0.05 relative to POPC). C and D, conditions were as in A and B for POPC, diC18:0, diC18:1, and diC18:2 PC. Quantitative data are represented as mean ± S.E. (POPC, n = 11, all other PCs, n = 3–7; **, p < 0.01 relative to POPC).
DiC14:0 PC Robustly Elevates Aβ42/43 Generation, Which Can Be Reverted by Acyl Chain Length Elongation
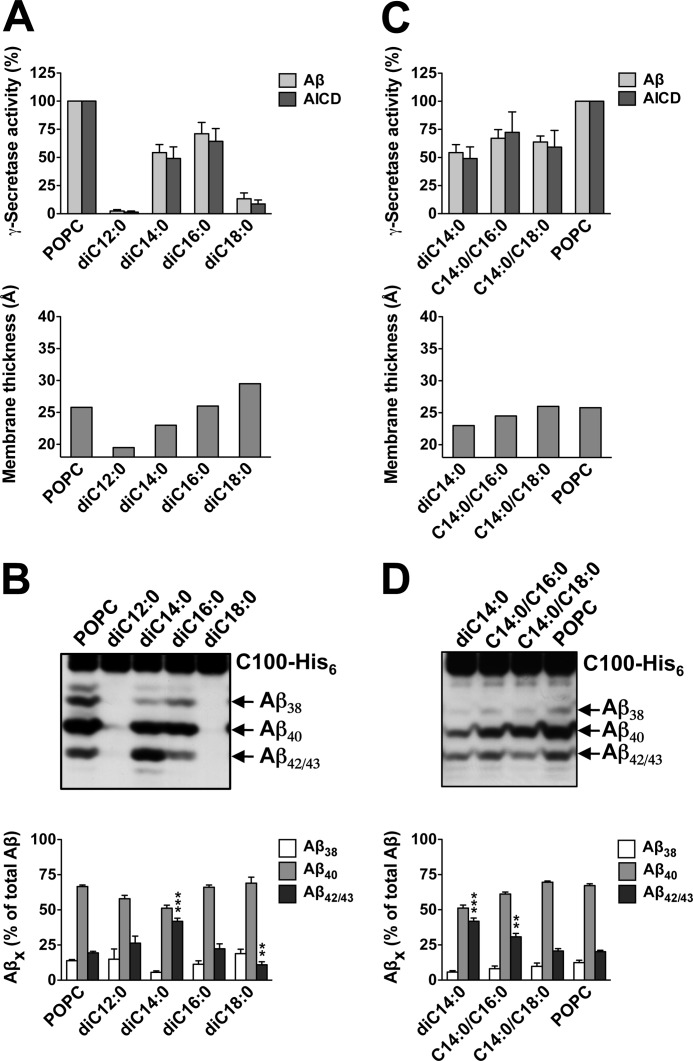
We also studied the influence of bilayer thickening on γ-secretase activity in SUVs composed of PC containing two fully saturated acyl chains of increasing length. γ-Secretase activity was strongly reduced for diC12:0 PC, increased robustly for diC14:0 and diC16:0 PC, nearly reaching the activity of the POPC control, and as shown above, dropped strongly down again for diC18:0 PC (Fig. 3A). Whereas the profile of Aβ species produced by γ-secretase for diC16:0 PC was similar to that of the POPC control, a striking change was observed for diC14:0 PC (Fig. 3B). In these thin bilayers, generation of the pathogenic Aβ42/43 species was markedly increased, whereas Aβ38 and Aβ40 levels were reduced (Fig. 3B). As shown in supplemental Fig. 3, Aβ43 was the predominant pathogenic Aβ species generated by γ-secretase in this bilayer. In addition, the relative production of Aβ42/43 was reduced as the membrane thickness increased from diC16:0 toward diC18:0 PC bilayers.
FIGURE 3.
Increased Aβ42/43 generation in diC14:0 membranes. A, γ-secretase activity was assessed following reconstitution of the purified enzyme into preformed SUVs composed of POPC or the indicated PCs containing two saturated acyl chains of increasing length as in Fig. 1A. Quantitation of total Aβ and AICD as well as hydrophobic membrane thicknesses are shown in the upper and lower panels, respectively. Quantitative data are represented as mean ± S.E. (error bars) (POPC, n = 12, all other PCs, n = 3–7). B, aliquots of samples of A were analyzed as in Fig. 1B. Quantitative data are represented as mean ± S.E. (POPC, n = 12, all other PCs, n = 3–7; **, p < 0.01; ***, p < 0.001 relative to POPC). C and D, conditions were as in A and B for POPC, diC14:0, C14:0/C16:0, and C14:0/C18:0 PC. Quantitative data are represented as mean ± S.E. (POPC, n = 9, all other PCs, n = 3–7; **, p < 0.01; ***, p < 0.001 relative to POPC).
We next wondered whether the strikingly high relative production of Aβ42/43 species found for γ-secretase reconstituted in diC14:0 PC could be reverted by elongating one of the acyl chains of this lipid thereby increasing the membrane thickness. As shown in Fig. 3C, γ-secretase displayed comparable activities in diC14:0, C14:0/C16:0, and C14:0/C18:0 PC bilayers. Indeed, the relative production of Aβ42/43 was attenuated by C14:0/C16:0 PC and reached POPC control levels for C14:0/C18:0 PC (Fig. 3D). Thus, the increase in membrane thickness induced by the acyl chain elongation gradually normalized the “pathogenic” cleavage of γ-secretase in diC14:0 PC.
Increased Aβ42/43 Generation by PS1 FAD Mutant γ-Secretases Is Attenuated in Thick Membranes
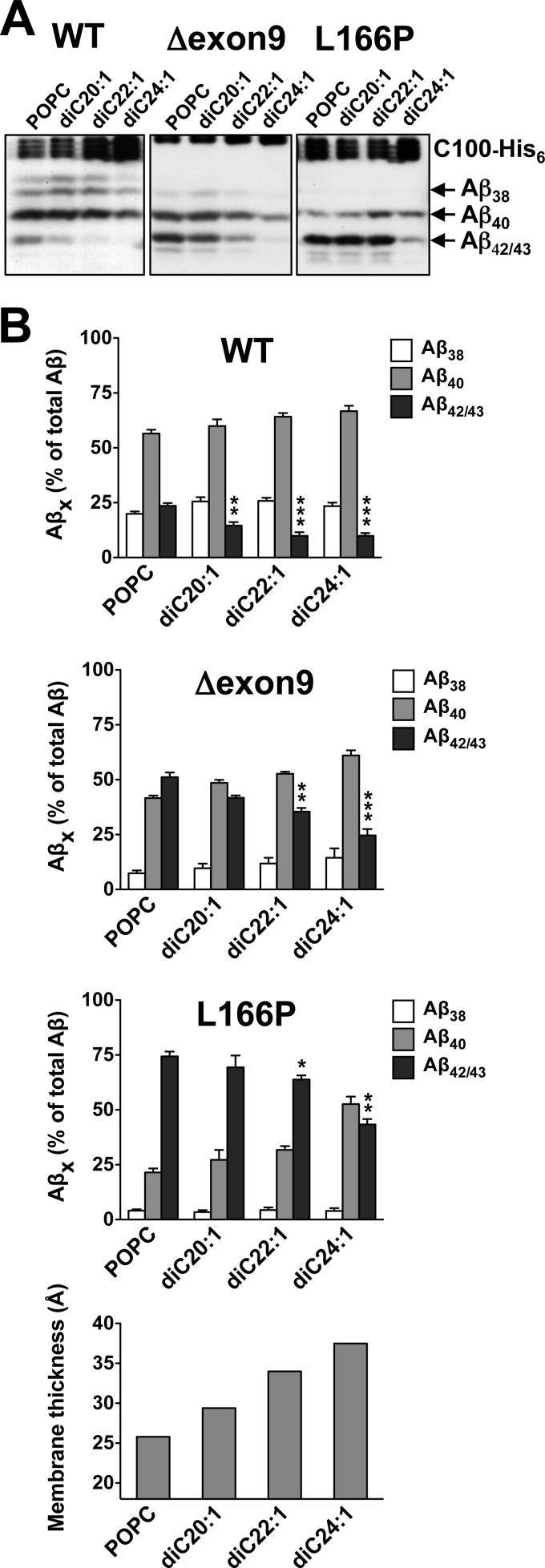
Having established that membrane thickness influences γ-secretase cleavage specificity modulating the generation of Aβ42/43, we next investigated whether increasing membrane thickness could attenuate the pathogenic generation of Aβ42/43 by FAD mutants within PS1, the catalytic subunit of γ-secretase. To this end, γ-secretase was purified from HEK293 cells stably expressing WT or FAD mutant PS1 and reconstituted in diC20:1-diC24:1 PC bilayers. FAD mutants of moderate (PS1 Δexon9) and strong (PS1 L166P) pathogenic activity (35) were chosen for analysis and changes in cleavage specificity were compared with WT PS1. Both FAD mutants showed the expected increases of Aβ42/43 compared with the WT enzyme in POPC control conditions (Fig. 4). Comparable with their behavior in cultured cells, PS1 Δexon9 produced roughly similar amounts of Aβ42/43 as Aβ40, whereas the PS1 L166P mutant generated substantially more Aβ42/43 than Aβ40. In agreement with recent results (39), these FAD mutants generated higher amounts of Aβ43 than Aβ42 in the cell free assay system (supplemental Fig. 4). Increasing the lipid chain length caused a reduction of total activity for the PS1 Δexon9 mutant, which was accompanied by a change of the Aβ42/43/Aβtotal ratios. In diC22:1 PC and diC24:1 PC lipids, Aβ42/43 levels were lower than that of Aβ40. Even for the strong PS1 L166P mutant, changes in the Aβ42/43/Aβtotal ratios became apparent, which were most prominent in the diC24:1 PC bilayer. In this bilayer, a clear reduction of Aβ42/43 was observed. Thus, the pathogenic activity of PS1 FAD mutants is attenuated in thick membranes and the increased production of Aβ42/43 can be substantially attenuated under such conditions.
FIGURE 4.
Attenuation of the pathogenic activity of PS1 FAD mutants in thicker membranes. A, γ-secretase cleavage specificity was assessed following reconstitution of the purified PS1 WT as well as Δexon9 and L166P FAD mutant enzymes into preformed POPC, diC20:1, diC22:1, and diC24:1 PC SUVs as in Fig. 1B. B, quantification of Aβ38, Aβ40, Aβ42/43 species in samples of A and hydrophobic membrane thicknesses (lower panel) are shown. Quantitative data are represented as mean ± S.E. (error bars) (POPC, n = 5–8, all other PCs, n = 3–4; *, p < 0.05; **, p < 0.01; ***, p < 0.001 relative to the corresponding POPC).
DISCUSSION
Despite being both an intramembrane-cleaving protease and a key AD drug target, only few studies have addressed the role of lipids for γ-secretase with respect to its activity so far (29, 40–42). Studies by Fraering et al. (40) and others (41, 42) showed that the purified detergent-solubilized protease is inactive unless it is reconstituted with membrane lipids. Investigating the influence of mixtures of PC with sphingolipids, or glycerophospholipids containing different head groups, on the activity of γ-secretase, Osenkowski et al. (29) could further show that while the latter had little effect, sphingolipids could stimulate γ-secretase activity, which was boosted by soluble cholesterol.
Building on these initial studies, we systematically studied the role of membrane thickness and lipid chain order by reconstituting the purified enzyme into defined model membranes made of synthetic PC lipids with acyl chains of various length and saturation thereby allowing us to identify the optima for total activity and cleavage specificity of γ-secretase. γ-Secretase displayed robust (i.e. >75% compared with POPC) activity in membranes with a hydrophobic thickness of about 26–29 Å, almost regardless of whether PLs composed of C18:0/C18:1, C18:0/C18:2, diC18:1, or diC20:1 were used. The only exception was diC18:0 PC, a lipid in which γ-secretase displayed very low activity. It should be noted, however, that diC18:0 PC is the only lipid that we used which is in the rigid gel state at 37 °C. We thus conclude that a requirement for robust γ-secretase activity is that the lipid is in the liquid-crystalline state. Interestingly, lipids with polyunsaturated fatty acids, which are common in the brain, were not beneficial. Also adding double bonds in both acyl chains was not beneficial for its activity. Thus, besides hydrophobic thickness being an important parameter for robust γ-secretase activity, too many double bonds in the acyl chains of the hydrophobic core of the bilayer reduces γ-secretase activity. γ-Secretase displayed better activity when lipids were used with one saturated and one unsaturated acyl chain. POPC (C16:0/C18:1 PC) has one relatively stiff unsaturated and one more mobile saturated acyl chain (43) and apparently builds a bilayer with optimal hydrophobic thickness and packing of the lipids allowing a conformation of γ-secretase with optimal activity.
An important observation of this study was that the Aβ42/43/Aβtotal ratio profile observed for the various bilayers did not correlate with that of the corresponding total γ-secretase activity. Although there was an optimum membrane thickness for total Aβ production for POPC PLs, the tendency with regard to the Aβ42/43/Aβtotal ratio was that it decreased with increasing thickness of the bilayer. This was even observed for bilayers in which total activity of γ-secretase was very low. Interestingly, a decrease of Aβ42/43 species was typically accompanied by an increase of the shorter Aβ40 and/or Aβ38 species, consistent with the sequential-cleavage model (5).
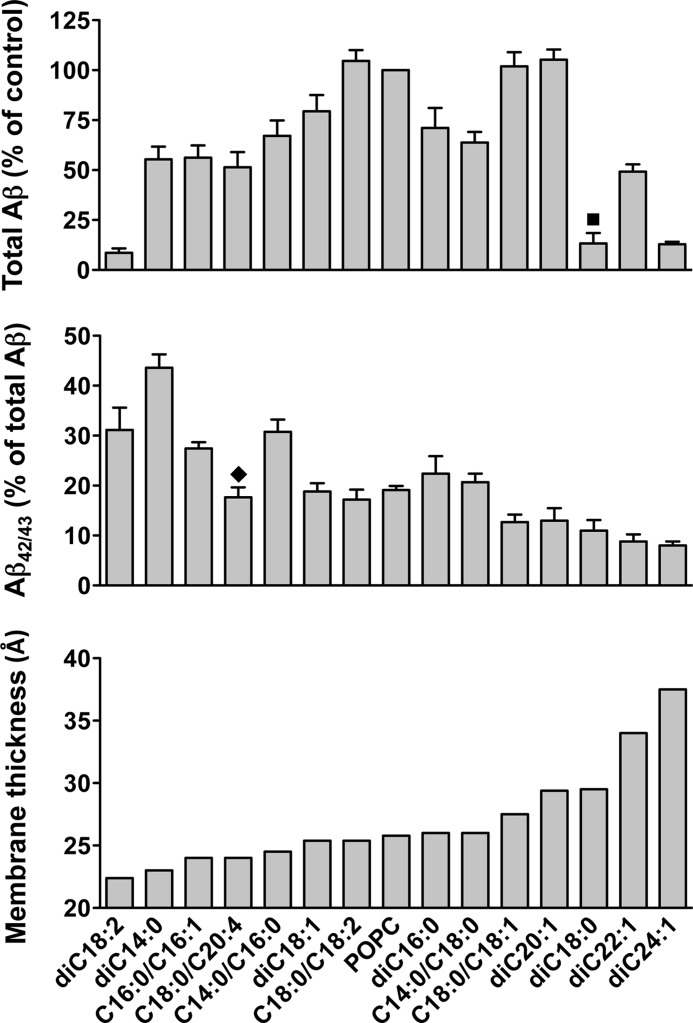
To distinguish the effects of membrane thickness from changes in lipid membrane fluidity on the cleavage specificity of γ-secretase, we used classes of phospholipids with similar membrane fluidity and lipid packing. Thus, we used PC with identical saturated acyl chains, saturated acyl chains with mismatching chain lengths, identical acyl chains having one cis double bond, and one saturated and one monounsaturated acyl chain. Within each class we found the same trend with regard to a reduced relative production of Aβ42/43 for thicker membranes, justifying our conclusion that this effect is due to changes in thickness and not due to changes in fluidity. This is further illustrated in Fig. 5, which gives a summary of γ-secretase activity and cleavage specificity with respect to membrane thickness of in all used lipids in which the activity was >5% compared with the POPC control.
FIGURE 5.
Summary of the dependence of γ-secretase activity and cleavage specificity on membrane thickness. Data of Figs. 1–3 were combined to represent all lipids in which the activity was higher than 5% compared with the POPC control. Total Aβ generation by γ-secretase in each lipid relative to the control POPC PLs is shown in the top panel. Activity is low for very thin and very thick membranes. Optimal activity is achieved for membranes with a thickness between 26 and 29 Å. Note that the activity varies for lipids with similar thickness probably due to differences in fluidity and lipid packing. Particularly lipids with one saturated and one unsaturated acyl chain have optimal activity. Relative Aβ42/43 generation is shown in the middle panel. Aβ42/43/Aβtotal ratios decrease with increasing membrane thickness, irrespective to variations of total γ-secretase activity. The hydrophobic membrane thickness of the bilayers comprising the corresponding lipids is shown in the bottom panel. ■, diC18:0 PC is the only lipid investigated in our study, which is in the rigid gel state at 37 º C. ⧫, estimation of the hydrophobic thickness of a bilayer composed of C18:0/C20:4 PC might be inaccurate due to the large mismatch of the C18:0 and C20:4 acyl chains (see supplemental Table 1).
The highest Aβ42/43/Aβtotal ratio was found in diC14:0 PC bilayers. The Aβ42/43/Aβtotal ratio for diC14:0 PC PLs was about two times higher compared with the POPC control, similar to ratios found for weak FAD mutants. The high Aβ42/43/Aβtotal ratios found for diC14:0 PC PLs could be reverted to the values observed for the POPC control by increasing one of the acyl chains of the lipid. In these experiments, the total γ-secretase activity hardly changed whereas the Aβ42/43/Aβtotal ratios dramatically decreased. However, Aβ42/43/Aβtotal ratios were not as much elevated as in diC14:0 PC in other thin bilayers of comparable thickness, such as diC18:2, or C16:0/C16:1 PC. This suggests that for diC14:0 PC, in addition to its property of forming a thin membrane bilayer, its two short saturated chains may lack the mobility to adjust to hydrophobic domains of the γ-secretase leading to a pathogenic change of the cleavage specificity of γ-secretase.
Because γ-secretase generated lower absolute and relative amounts of Aβ42/43 when reconstituted in thicker membranes, we finally asked whether the same also applied for PS FAD mutant enzymes. As expected, when PS1 FAD mutant γ-secretase complexes were reconstituted in POPC vesicles, the Aβ42/43/Aβtotal ratios were elevated compared with the WT PS1 γ-secretase control. Strikingly, Aβ42/43/Aβtotal ratios were strongly attenuated when FAD mutants were reconstituted in thick membranes. Absolute Aβ42/43 production was dramatically reduced for these PLs showing that the pathogenic activity of FAD mutant γ-secretase is strongly influenced by the thickness of its surrounding lipid bilayer and attenuated in thicker membranes. This was shown for both the PS1 Δexon9 and PS1 L166P FAD mutants, which display a moderate-strong elevation of Aβ42/43 generation, respectively.
How does the lipid environment affect the activity of γ-secretase? Lipid properties affecting activities of integral membrane proteins include the head group, the chain order, the lipid packing, and the thickness of the hydrophobic part of the membrane. Particularly the extent of hydrophobic matching between the hydrophobic thickness of the bilayer and the length of the membrane-spanning part of the protein determines the amino acid composition, packing, and tilting of the TMDs of the protein thereby also influencing enzyme activity (30, 31). Interestingly, with respect to γ-secretase, changes of the tilt and elongation of the helical TMDs in bilayers of different thickness have recently been revealed by molecular dynamics simulations of the PS1 C-terminal fragment (44).
Altered hydrophobic matching between the bilayer and the TMDs of γ-secretase and/or of the substrate TMD and its surrounding membrane lipids might both influence the positioning of the substrate cleavage sites relative to the active site. As a result, an alteration of membrane thickness, which increases from early to late compartments of the secretory pathway (45–47), will directly affect hydrophobic matching and thereby not only influence total γ-secretase activity but also the ratios of Aβ40 versus the pathogenic Aβ42 and Aβ43 species generated. As shown in our study, the exposure of the γ42/43 cleavage sites toward the catalytic site is apparently increased in thin membranes leading to an enhanced generation of Aβ42/43, whereas in thicker membranes the γ40 cleavage site is more exposed to the active site. Our data may thus also provide a direct explanation for the previously observed preferential generation of Aβ42 in the early compartments of the secretory pathway (48, 49).
Taken together, using defined model membranes, we have shown that γ-secretase activity and cleavage specificity toward its substrate APP are modulated by membrane thickness including inhibition of total Aβ generation and/or that of the pathogenic Aβ42/43 species. Our findings thus suggest that alteration of membrane thickness to lower the generation of the pathogenic Aβ42/43 species by γ-secretase could represent a target for potential therapeutic intervention of AD.
Acknowledgments
We thank Christian Haass for critical reading of the manuscript, helpful discussion, and support; and Donald Small for assistance with membrane thickness calculations.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB596), the Bundesministerium für Bildung und Forschung (Kompetenznetz Degenerative Demenzen), the Alzheimer Research Award of the Hans and Ilse Breuer Foundation (to H. S.), and the Center of Integrated Protein Science Munich.

This article contains supplemental Materials and Methods, additional references, Figs. 1–4, and Table 1.
To allow easy assessment of Aβ generation in relation to the thicknesses of the respective model membranes, the values of the hydrophobic thickness of the membranes were graphically represented for all data sets. These values were taken from the literature using well documented experimental data or were otherwise calculated (see supplemental Table 1).
- AD
- Alzheimer disease
- Aβ
- amyloid β-peptide
- AICD
- APP intracellular domain
- APP
- β-amyloid precursor protein
- Bicine
- N,N-bis(2-hydroxyethyl)glycine
- BisTris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- CHAPSO
- 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonic acid
- FAD
- familial AD
- PC
- phosphatidylcholine
- PL
- proteoliposome
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- PS
- presenilin
- SUV
- small unilamellar vesicle
- TMD
- transmembrane domain.
REFERENCES
- 1. Haass C., Selkoe D. J. (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat. Rev. Mol. Cell. Biol. 8, 101–112 [DOI] [PubMed] [Google Scholar]
- 2. Saito T., Suemoto T., Brouwers N., Sleegers K., Funamoto S., Mihira N., Matsuba Y., Yamada K., Nilsson P., Takano J., Nishimura M., Iwata N., Van Broeckhoven C., Ihara Y., Saido T. C. (2011) Potent amyloidogenicity and pathogenicity of Aβ43. Nat. Neurosci. 14, 1023–1032 [DOI] [PubMed] [Google Scholar]
- 3. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 4. Lichtenthaler S. F., Haass C., Steiner H. (2011) Regulated intramembrane proteolysis: lessons from amyloid precursor protein processing. J. Neurochem. 117, 779–796 [DOI] [PubMed] [Google Scholar]
- 5. Qi-Takahara Y., Morishima-Kawashima M., Tanimura Y., Dolios G., Hirotani N., Horikoshi Y., Kametani F., Maeda M., Saido T. C., Wang R., Ihara Y. (2005) Longer forms of amyloid β protein: implications for the mechanism of intramembrane cleavage by γ-secretase. J. Neurosci. 25, 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takami M., Nagashima Y., Sano Y., Ishihara S., Morishima-Kawashima M., Funamoto S., Ihara Y. (2009) γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl-terminal fragment. J. Neurosci. 29, 13042–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolfe M. S. (2009) Intramembrane-cleaving proteases. J. Biol. Chem. 284, 13969–13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimberly W. T., LaVoie M. J., Ostaszewski B. L., Ye W., Wolfe M. S., Selkoe D. J. (2003) γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc. Natl. Acad. Sci. U.S.A. 100, 6382–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edbauer D., Winkler E., Regula J. T., Pesold B., Steiner H., Haass C. (2003) Reconstitution of γ-secretase activity. Nat. Cell Biol. 5, 486–488 [DOI] [PubMed] [Google Scholar]
- 10. Takasugi N., Tomita T., Hayashi I., Tsuruoka M., Niimura M., Takahashi Y., Thinakaran G., Iwatsubo T. (2003) The role of presenilin cofactors in the γ-secretase complex. Nature 422, 438–441 [DOI] [PubMed] [Google Scholar]
- 11. Steiner H. (2008) The catalytic core of γ-secretase: presenilin revisited. Curr. Alzheimer Res. 5, 147–157 [DOI] [PubMed] [Google Scholar]
- 12. Dries D. R., Yu G. (2008) Assembly, maturation, and trafficking of the γ-secretase complex in Alzheimer's disease. Curr. Alzheimer Res. 5, 132–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wakabayashi T., De Strooper B. (2008) Presenilins: members of the γ-secretase quartets, but part-time soloists too. Physiology 23, 194–204 [DOI] [PubMed] [Google Scholar]
- 14. Imbimbo B. P., Giardina G. A. (2011) γ-Secretase inhibitors and modulators for the treatment of Alzheimer's disease: disappointments and hopes. Curr. Top. Med. Chem. 11, 1555–1570 [DOI] [PubMed] [Google Scholar]
- 15. Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad E., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. (1996) Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 2, 864–870 [DOI] [PubMed] [Google Scholar]
- 16. Citron M., Westaway D., Xia W., Carlson G., Diehl T., Levesque G., Johnson-Wood K., Lee M., Seubert P., Davis A., Kholodenko D., Motter R., Sherrington R., Perry B., Yao H., Strome R., Lieberburg I., Rommens J., Kim S., Schenk D., Fraser P., St George Hyslop P., Selkoe D. J. (1997) Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nat. Med. 3, 67–72 [DOI] [PubMed] [Google Scholar]
- 17. Han X. (2005) Lipid alterations in the earliest clinically recognizable stage of Alzheimer's disease: implication of the role of lipids in the pathogenesis of Alzheimer's disease. Curr. Alzheimer Res. 2, 65–77 [DOI] [PubMed] [Google Scholar]
- 18. Hartmann T., Kuchenbecker J., Grimm M. O. (2007) Alzheimer's disease: the lipid connection. J. Neurochem. 103, 159–170 [DOI] [PubMed] [Google Scholar]
- 19. Di Paolo G., Kim T. W. (2011) Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat. Rev. Neurosci. 12, 284–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S. J., Liyanage U., Bickel P. E., Xia W., Lansbury P. T., Jr., Kosik K. S. (1998) A detergent-insoluble membrane compartment contains Aβ in vivo. Nat. Med. 4, 730–734 [DOI] [PubMed] [Google Scholar]
- 21. Ehehalt R., Keller P., Haass C., Thiele C., Simons K. (2003) Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 160, 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simons M., Keller P., De Strooper B., Beyreuther K., Dotti C. G., Simons K. (1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 95, 6460–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wahrle S., Das P., Nyborg A. C., McLendon C., Shoji M., Kawarabayashi T., Younkin L. H., Younkin S. G., Golde T. E. (2002) Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 9, 11–23 [DOI] [PubMed] [Google Scholar]
- 24. Grimm M. O., Grimm H. S., Tomic I., Beyreuther K., Hartmann T., Bergmann C. (2008) Independent inhibition of Alzheimer disease β- and γ-secretase cleavage by lowered cholesterol levels. J. Biol. Chem. 283, 11302–11311 [DOI] [PubMed] [Google Scholar]
- 25. Zinser E. G., Hartmann T., Grimm M. O. (2007) Amyloid β-protein and lipid metabolism. Biochim. Biophys. Acta 1768, 1991–2001 [DOI] [PubMed] [Google Scholar]
- 26. Rigaud J. L., Pitard B., Levy D. (1995) Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta 1231, 223–246 [DOI] [PubMed] [Google Scholar]
- 27. Seddon A. M., Curnow P., Booth P. J. (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta 1666, 105–117 [DOI] [PubMed] [Google Scholar]
- 28. Kalvodova L., Kahya N., Schwille P., Ehehalt R., Verkade P., Drechsel D., Simons K. (2005) Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J. Biol. Chem. 280, 36815–36823 [DOI] [PubMed] [Google Scholar]
- 29. Osenkowski P., Ye W., Wang R., Wolfe M. S., Selkoe D. J. (2008) Direct and potent regulation of γ-secretase by its lipid microenvironment. J. Biol. Chem. 283, 22529–22540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen O. S., Koeppe R. E., 2nd (2007) Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36, 107–130 [DOI] [PubMed] [Google Scholar]
- 31. Lee A. G. (2004) How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta 1666, 62–87 [DOI] [PubMed] [Google Scholar]
- 32. Shirotani K., Tomioka M., Kremmer E., Haass C., Steiner H. (2007) Pathological activity of familial Alzheimer's disease-associated mutant presenilin can be executed by six different γ-secretase complexes. Neurobiol. Dis. 27, 102–107 [DOI] [PubMed] [Google Scholar]
- 33. Steiner H., Winkler E., Edbauer D., Prokop S., Basset G., Yamasaki A., Kostka M., Haass C. (2002) PEN-2 is an integral component of the γ-secretase complex required for coordinated expression of presenilin and nicastrin. J. Biol. Chem. 277, 39062–39065 [DOI] [PubMed] [Google Scholar]
- 34. Beher D., Fricker M., Nadin A., Clarke E. E., Wrigley J. D., Li Y. M., Culvenor J. G., Masters C. L., Harrison T., Shearman M. S. (2003) In vitro characterization of the presenilin-dependent γ-secretase complex using a novel affinity ligand. Biochemistry 42, 8133–8142 [DOI] [PubMed] [Google Scholar]
- 35. Moehlmann T., Winkler E., Xia X., Edbauer D., Murrell J., Capell A., Kaether C., Zheng H., Ghetti B., Haass C., Steiner H. (2002) Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Aβ42 production. Proc. Natl. Acad. Sci. U.S.A. 99, 8025–8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Winkler E., Hobson S., Fukumori A., Dümpelfeld B., Luebbers T., Baumann K., Haass C., Hopf C., Steiner H. (2009) Purification, pharmacological modulation, and biochemical characterization of interactors of endogenous human γ-secretase. Biochemistry 48, 1183–1197 [DOI] [PubMed] [Google Scholar]
- 37. Wiltfang J., Smirnov A., Schnierstein B., Kelemen G., Matthies U., Klafki H. W., Staufenbiel M., Hüther G., Rüther E., Kornhuber J. (1997) Improved electrophoretic separation and immunoblotting of β-amyloid (Aβ) peptides 1–40, 1–42, and 1–43. Electrophoresis 18, 527–532 [DOI] [PubMed] [Google Scholar]
- 38. Small D. M. (1986) The Physical Chemistry of Lipids: from Alkanes to Phospholipids (Hanahan D. J., ed), Plenum Press, New York [Google Scholar]
- 39. Quintero-Monzon O., Martin M. M., Fernandez M. A., Cappello C. A., Krzysiak A. J., Osenkowski P., Wolfe M. S. (2011) Dissociation between the processivity and total activity of γ-secretase: implications for the mechanism of Alzheimer's disease-causing presenilin mutations. Biochemistry 50, 9023–9035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fraering P. C., Ye W., Strub J. M., Dolios G., LaVoie M. J., Ostaszewski B. L., van Dorsselaer A., Wang R., Selkoe D. J., Wolfe M. S. (2004) Purification and characterization of the human γ-secretase complex. Biochemistry 43, 9774–9789 [DOI] [PubMed] [Google Scholar]
- 41. Zhou H., Zhou S., Walian P. J., Jap B. K. (2010) Dependency of γ-secretase complex activity on the structural integrity of the bilayer. Biochem. Biophys. Res. Commun. 402, 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wrigley J. D., Schurov I., Nunn E. J., Martin A. C., Clarke E. E., Ellis S., Bonnert T. P., Shearman M. S., Beher D. (2005) Functional overexpression of γ-secretase reveals protease-independent trafficking functions and a critical role of lipids for protease activity. J. Biol. Chem. 280, 12523–12535 [DOI] [PubMed] [Google Scholar]
- 43. Huber T., Rajamoorthi K., Kurze V. F., Beyer K., Brown M. F. (2002) Structure of docosahexaenoic acid-containing phospholipid bilayers as studied by 2H NMR and molecular dynamics simulations. J. Am. Chem. Soc. 124, 298–309 [DOI] [PubMed] [Google Scholar]
- 44. Sobhanifar S., Schneider B., Löhr F., Gottstein D., Ikeya T., Mlynarczyk K., Pulawski W., Ghoshdastider U., Kolinski M., Filipek S., Güntert P., Bernhard F., Dötsch V. (2010) Structural investigation of the C-terminal catalytic fragment of presenilin 1. Proc. Natl. Acad. Sci. U.S.A. 107, 9644–9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bretscher M. S., Munro S. (1993) Cholesterol and the Golgi apparatus. Science 261, 1280–1281 [DOI] [PubMed] [Google Scholar]
- 46. van Meer G., Voelker D. R., Feigenson G. W. (2008) Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell. Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitra K., Ubarretxena-Belandia I., Taguchi T., Warren G., Engelman D. M. (2004) Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc. Natl. Acad. Sci. U.S.A. 101, 4083–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hartmann T., Bieger S. C., Brühl B., Tienari P. J., Ida N., Allsop D., Roberts G. W., Masters C. L., Dotti C. G., Unsicker K., Beyreuther K. (1997) Distinct sites of intracellular production for Alzheimer's disease Aβ40/42 amyloid peptides. Nat. Med. 3, 1016–1020 [DOI] [PubMed] [Google Scholar]
- 49. Wild-Bode C., Yamazaki T., Capell A., Leimer U., Steiner H., Ihara Y., Haass C. (1997) Intracellular generation and accumulation of amyloid β-peptide terminating at amino acid 42. J. Biol. Chem. 272, 16085–16088 [DOI] [PubMed] [Google Scholar]