Background: HTRA1 has been associated with intervertebral disc (IVD) degeneration although its role is unknown.
Results: HTRA1 up-regulated matrix metalloproteinase (MMP) production by IVD cells via the generation of fibronectin fragments.
Conclusion: HTRA1 plays a detrimental role in the pathogenesis of IVD degeneration.
Significance: HTRA1 may represent a novel therapeutic target for the treatment of spinal disc degeneration.
Keywords: Extracellular Matrix, Fibronectin, Genetic Polymorphism, Matrix Metalloproteinase (MMP), Serine Protease, HTRA1, Intervertebral Disc (IVD) Degeneration
Abstract
Human HTRA1 is a highly conserved secreted serine protease that degrades numerous extracellular matrix proteins. We have previously identified HTRA1 as being up-regulated in osteoarthritic patients and as having the potential to regulate matrix metalloproteinase (MMP) expression in synovial fibroblasts through the generation of fibronectin fragments. In the present report, we have extended these studies and investigated the role of HTRA1 in the pathogenesis of intervertebral disc (IVD) degeneration. HTRA1 mRNA expression was significantly elevated in degenerated disc tissue and was associated with increased protein levels. However, these increases did not correlate with the appearance of rs11200638 single nucleotide polymorphism in the promoter region of the HTRA1 gene, as has previously been suggested. Recombinant HTRA1 induced MMP production in IVD cell cultures through a mechanism critically dependent on MEK but independent of IL-1β signaling. The use of a catalytically inactive mutant confirmed these effects to be primarily due to HTRA1 serine protease activity. HTRA1-induced fibronectin proteolysis resulted in the generation of various sized fragments, which when added to IVD cells in culture, caused a significant increase in MMP expression. Furthermore, one of these fragments was identified as being the amino-terminal fibrin- and heparin-binding domain and was also found to be increased within HTRA1-treated IVD cell cultures as well as in disc tissue from patients with IVD degeneration. Our results therefore support a scenario in which HTRA1 promotes IVD degeneration through the proteolytic cleavage of fibronectin and subsequent activation of resident disc cells.
Introduction
Degeneration of the intervertebral disc (IVD)6 is now regarded as one of the major causes of lower back pain, which is a highly prevalent, debilitating, and costly disorder (1, 2). The pathogenesis of degeneration is a highly complex and poorly understood process with many different genetic, biological, and mechanical influences playing key roles in the breakdown of extracellular matrix (ECM) components (3). The predominant means by which ECM is degraded is thought to be due to the proteolytic actions of matrix metalloproteinases (MMPs) and aggrecanases (ADAMTS). A number of MMPs, including MMP-1, -3, -7, -9, and -13, as well as ADAMTS-4, have been shown to increase in the IVD during disc degeneration and are responsible for the breakdown of several matrix components, the most notable being aggrecan and collagen (4–7). Both MMPs and their inhibitors (TIMP-1, -2, and -3) have been localized to the resident chondrocyte-like cells of the nucleus pulposus and inner fibrosus compartments of the IVD (4), thus implicating these cells in disease pathogenesis. The secretion of MMPs by human IVD cells is mediated in part through the stimulatory effects of various pro-inflammatory cytokines, the most prominent of which is IL-1β (8). In addition, fibronectin peptide fragments of the ECM have also been shown to induce MMP production by IVD cells (9) and are potent instigators of experimental disc degeneration (10). Moreover, fibronectin fragments have been shown to accumulate in the IVD during degeneration (11), although the proteases responsible for their formation remain elusive.
Human HTRA1 (high temperature requirement serine protease A1) belongs to a well defined family of serine proteases originally identified in bacteria (12). Although primarily regarded as a key regulator of tumor development and subsequent malignancies (13–15), a growing body of evidence now exists to suggest that HTRA1 may also play a central role in determining the outcome of various musculoskeletal disease pathologies, including Duchenne muscular dystrophy (16), osteoarthritis (17–19), and rheumatoid arthritis (19, 20). It has recently been shown in a Japanese population study that a single nucleotide polymorphism (SNP) located within the HTRA1 promoter is associated with spinal disc degeneration, where increases in spinal disc narrowing were observed in patients without the G allele (AA) as compared with those bearing at least one G allele (GG + GA) (21). It would therefore appear that HTRA1 may also play a role in disc pathology, although its influence on disease and its mechanism of action have not yet been elucidated.
Observations from our own studies examining the role of HTRA1 in osteoarthritis imply that HTRA1 may actually have a detrimental effect on the pathogenesis of musculoskeletal disease. Elevated levels of HTRA1 protein were measured in the synovial fluid from osteoarthritic patients, and primary synovial fibroblasts isolated from diseased patients were identified as being a major source of HTRA1 (19). The addition of proteolytically active HTRA1 to fibroblast cultures resulted in marked up-regulation of various MMPs, including MMP-1 and MMP-3, both of which have been implicated in cartilage and joint destruction in arthritic patients. Further experiments confirmed that the stimulatory effects of HTRA1 on MMP expression were protease-dependent and were related to the formation of fibronectin fragments due to extracellular degradation. Such observations are therefore strongly suggestive of a central role for HTRA1 in joint degeneration through its proteolytic actions on ECM components and thus may also be indicative of its role in other disease pathologies, such as disc degeneration.
In the current study, we used IVD tissue and cell samples from surgical patients in order to further investigate the potential involvement of HTRA1 in IVD degeneration. Our findings implicate HTRA1 as a key factor in the underlying pathology associated with IVD degeneration. HTRA1 may therefore represent a novel target for the development of more effective therapeutic strategies to treat this debilitating condition.
EXPERIMENTAL PROCEDURES
Materials
Human fibronectin and rabbit IgG were purchased from R & D Systems (Abingdon, UK). IL-1 receptor antagonist (IL-1RA) was obtained from Abcam (Cambridge, UK), and MEK1/2 inhibitors PD98059 and U0126 were from Sigma-Aldrich (Buchs, Switzerland). Monoclonal antibodies against the fibronectin carboxyl-terminal heparin-binding domain (Mab1935) and the amino-terminal fibrin- and heparin-binding domain (Mab1936) were from Chemicon International. A polyclonal anti-HTRA1 antibody was generated as described previously (19). All anti-IgG horseradish peroxidase (HRP)-conjugated, fluorescence-conjugated secondary antibodies and normal serum were from Jackson ImmunoResearch (Suffolk, UK). DAPI was purchased from Sigma-Aldrich. Collagenase NB4 was purchased from Serva/Promega (Duebendorf, Switzerland), and dispase II was from Roche Applied Science (Rotkreuz, Switzerland).
Tissue Harvesting
IVD tissue and/or blood was obtained from a total of 39 patients undergoing spinal surgery for symptomatic degenerative disc disease, disc herniation, or spinal trauma following informed consent in accordance with the local ethical guidelines (carried out at the SRH Clinic Karlsbad-Langensteinbach, Karlsbad, Germany). The degree of IVD degeneration in patients was assessed prior to surgical intervention by magnetic resonance imaging (MRI) using a four-level grading system based on Pfirrmann's classification of disc degeneration (22). Degeneration grades were assigned as follows: grade 1, non-degenerated (normal disc height); grade 2, mild degeneration (slight decrease in disc height); grade 3, moderate degeneration (moderate decrease in disc height); grade 4, severe degeneration (collapsed disc space).
Isolation and Culture of IVD Cells
Human IVD cells were isolated from the discs of a total of 14 patients undergoing spinal surgery for disc herniation (carried out at Balgrist University Hospital, Zürich, Switzerland) as described previously (23). Briefly, IVD tissue was enzymatically digested (0.2% collagenase NB4, 0.3% dispase II) for 4–8 h, and cells were thereafter cultured in growth medium consisting of DMEM/F-12 supplemented with 10% FCS, penicillin (50 units/ml), streptomycin (50 μg/ml), and amphotericin B (25 μg/ml) and incubated at 37 °C with 5% CO2 and used at passages 2–3.
Recombinant Human HTRA1
Purified recombinant His-tagged HTRA1 in which the amino-terminal mac25 homology domain was absent (termed HTRA1Δmac) was produced in Escherichia coli and purified using Ni2+-NTA chromatography as described previously (19, 24). The enzymatically inactive mutated form of HTRA1Δmac, termed HTRA1ΔmacSA, was generated through conversion of residue serine 328 to alanine by mutagenesis.
Stimulation of IVD Cells with Recombinant HTRA1
IVD cells were cultured in 6-well plates at 3.5 × 105 cells/well and serum starved for 2 h prior to stimulation. Cells were incubated in medium alone or in medium supplemented with either HTRA1Δmac (5 μg/ml) or HTRA1ΔmacSA (5 μg/ml) for up to 24 h. Concentrations used were based on previous observations using human synovial fibroblasts (19). After this time, RNA and culture supernatants were harvested for further analysis. In the case of inhibition studies, IVD cells were preincubated with either PD98059 (10 μm), U0126 (10 μm), or IL-1RA (250 ng/ml) for 2 h prior to stimulation.
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from either intact IVD tissue or cells and purified using TRIzol reagent (Invitrogen AG, Basel, Switzerland) according to the manufacturer's instructions. RNA (0.5 μg) was reverse transcribed to cDNA using Superscript II (Invitrogen AG) and random hexanucleotide primers (Promega AG, Dübendorf, Switzerland). Quantification of mRNA expression was performed with TaqMan Gene Expression Assays (Applied Biosystems, Rotkreuz, Switzerland) (supplemental Table 1) using the StepOnePlus real-time PCR system (Applied Biosystems), and values were normalized to GAPDH mRNA levels and presented as -fold change according to the 2−ΔΔCT method. In cases where individual patients (n = 36) were compared for expression levels of HTRA1 and FN mRNA in IVD tissue, data were normalized to TBP and presented as 2−ΔCT. Each 10-μl reaction consisted of 1× TaqMan fast universal PCR master mix (Applied Biosystems), 1× TaqMan gene expression assay, and 10 ng of cDNA (based upon initial RNA concentrations). All reactions were performed in triplicate in fast optical 96-well reaction plates (Applied Biosystems) at 95 °C for 20 s and 40 cycles of 95 °C for 1 s and 60 °C for 20 s.
SNP Analysis
A total of 35 patients were genotyped using a TaqMan SNP genotyping assay specific for the SNP, rs11200638, according to the manufacturer's instructions (Applied Biosystems). Patients were grouped according to their individual genotypes, and association studies were performed in order to determine the influence of the rs11200638 (A) risk allele on susceptibility to IVD degeneration. The influence of rs11200638 on HTRA1 expression in IVD tissue was also assessed in patients from whom both RNA and DNA samples were obtained (n = 32).
Western Blot Analysis of Patient IVD Tissue
Patient IVD samples (n = 12) were selected based on degeneration grade. Protein was extracted using CelLytic M (Sigma-Aldrich) containing a protease inhibitor mixture (Sigma-Aldrich), and protein amounts were determined initially by a Bio-Rad protein assay (Bio-Rad, Reinach, Switzerland). Protein samples were boiled for 5 min in loading buffer (50 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 100 mm DTT, 0.002% bromphenol blue), and equal amounts of protein were loaded onto 12% SDS-polyacrylamide gels. Further corrections to the loading volumes were made following densitometric analysis of Coomassie Blue-stained gels, thus allowing for accurate comparisons to be made between individual patient samples. Protein was then electroblotted onto PVDF membranes using the Trans-Blot Turbo blotting system (Bio-Rad) and incubated in 5% skim milk, 50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 0.1% Tween 20 (TBST) for 1 h at room temperature. Membranes were then incubated for 1 h at room temperature with either rabbit anti-human HTRA1 (1:2000) or mouse anti-fibronectin amino-terminal fibrin- and heparin-binding domain (Mab1936) (1 μg/ml). After washing in TBST three times for 5 min each, membranes were incubated with a HRP-conjugated anti-mouse or anti-rabbit IgG (1:10,000) for 1 h at room temperature. Following a further washing step, peroxidase activity was detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Lausanne, Switzerland).
Immunofluorescence Microscopy
Unfixed frozen IVD tissue sections were air-dried for 20 min, blocked with normal goat serum (1:10), and incubated with polyclonal anti-HTRA1 (1:50) or control rabbit IgG (2 μg/ml) in phosphate-buffered saline (PBS), pH 7.3, 1% BSA for 16 h at 4 °C. Tissue samples containing HTRA1 were then identified using goat anti-rabbit-Cy3 (1:400). Sections were mounted in Mowiol/DABCO (1,4-diazabicyclo[2.2.2]octane) (Sigma-Aldrich) containing DAPI (0.5 g/ml), and images were captured using the Leica DMI6000B automated inverted research microscope system (Leica Microsystems).
Quantification of Secreted MMP-3
MMP-3 protein levels in culture supernatants were determined using an MMP-3-specific ELISA kit according to the manufacturer's instructions (R & D Systems).
Proteolytic Enzyme Assays
Degradation of fibronectin by HTRA1Δmac was determined using methods described previously (19). Briefly, HTRA1Δmac and recombinant human fibronectin, in an equimolar ratio, were incubated together in Tris-buffered saline (TBS), pH 8.5, for 16 h at 37 °C. In some reactions, HTRA1Δmac was replaced by proteolytically inactive HTRA1ΔmacSA. Fibronectin, HTRA1Δmac, and HTRA1ΔmacSA were also incubated separately under the same conditions and served as controls. In order to assess whether HTRA1Δmac could also generate fibronectin fragments in IVD cell cultures, supernatants (20 ml) were harvested from cells treated with HTRA1Δmac (5 μg/ml) or HTRA1ΔmacSA (5 μg/ml) for 24 h and concentrated using Amicon Ultra-15, 10,000 molecular weight cut-off filter units (Millipore). Fibronectin fragments were analyzed on 4–15% Mini-PROTEAN TGX Precast gels (Bio-Rad) by Coomassie Blue staining and immunoblotting using either mouse anti-fibronectin carboxyl-terminal heparin-binding domain (Mab1935) (1 μg/ml) or mouse-anti-fibronectin amino-terminal fibrin- and heparin-binding domain (Mab1936) (1 μg/ml) as described above. The EnzCheck elastase kit (Molecular Probes, Basel, Switzerland) was used to confirm the proteolytic activity of recombinant HTRA1 proteins according to the manufacturer's protocol.
Effect of Fibronectin Fragments on MMP Expression
Equimolar concentrations of fibronectin (20 μg) and HTRA1Δmac (5 μg) were incubated under the conditions described above. Control reactions were also performed and included TBS, pH 8.5, alone or in combination with either fibronectin or HTRA1Δmac. Samples were then diluted into equilibration buffer containing TBS, pH 7.6, with 20 mm imidazole and incubated with 50 μl of pre-equilibrated HisPur Ni2+-NTA resin (Qiagen, Hombrechtikon, Switzerland) in spin columns (Thermo Scientific) for 1 h at 4 °C. Columns were then centrifuged for 5 min at 1600 rpm, and the affinity column flow-through fraction was collected, dialyzed in TBS for 4 h at 4 °C using Slide-A-Lyzer MINI dialysis devices (3500 molecular weight cut-off) (Thermo Scientific), and then incubated with IVD cells for up to 24 h, after which time MMP expression was evaluated by qRT-PCR.
Statistical Analysis
All statistical analyses were carried out using SPSS19.0 (SPSS Inc., Chicago, IL). Parametric analysis of normally distributed data were performed using the two-tailed unpaired Student's t test or one-way analysis of variance (ANOVA) followed by Tukey's post hoc tests for multiple-group comparisons. Pearson's correlation coefficient was used to evaluate the relationship between the expression levels of selected genes in patient tissue samples. The χ2 test was used to compare allele frequencies in patients with or without IVD degeneration (1 degree of freedom). In all cases, a p value of <0.05 was considered statistically significant.
RESULTS
We have previously demonstrated that HTRA1 plays a central role in the regulation of MMP expression in synovial fibroblasts from arthritic patients and that its stimulatory effects may be linked to the generation of fibronectin fragments (19). In the present report, we further investigated this property of HTRA1 in IVD cell cultures and aimed to establish its potential role in IVD degeneration.
Identification of HTRA1 in Patient Tissue
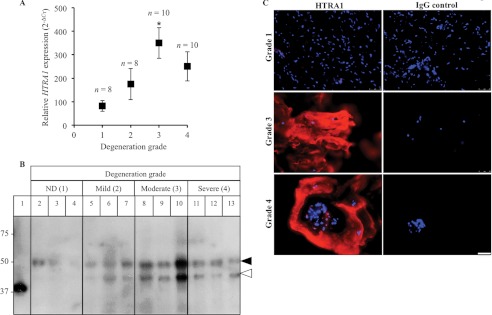
HTRA1 mRNA levels within IVD tissue samples from patients with varying degrees of disc degeneration were normalized to TBP and presented as 2−ΔCT. Expression levels significantly correlated (r = 0.375; p = 0.024) with patient degeneration grade and were found to be markedly increased in patients with severity scores of 3 (4-fold; p = 0.015) and 4 (3-fold; p = 0.2) as compared with control patients (Fig. 1A). Western blot analysis of IVD protein samples using a polyclonal antibody against HTRA1 identified two main species of HTRA1 protein migrating at ∼50 kDa (closed arrowhead) and ∼42 kDa (open arrowhead), which most likely represented the full-length and processed forms of HTRA1, respectively (Fig. 1B and supplemental Fig. 1) (25). The 50-kDa HTRA1 was found at varying levels in the majority samples tested, whereas the 42-kDa form of HTRA1 was identified in degenerated IVD protein samples only and was noticeably increased in the more severely affected discs. HTRA1 protein was also identified within both the cells and ECM of frozen IVD tissue sections, as determined by immunofluorescence staining, and levels were found to be increased in the more severely affected patients as compared with the control trauma patients (Fig. 1C).
FIGURE 1.
Detection of HTRA1 in human IVD tissue. A, HTRA1 mRNA levels in intact IVD tissue samples from patients (n = 36) with varying degrees of IVD degeneration were determined by qRT-PCR and presented as 2−ΔCT. *, p < 0.05, as determined by one-way ANOVA Error bars, S.E. B, protein extracts from patient IVD tissues (n = 12) were loaded onto a 12% SDS-polyacrylamide gel, and immunoblotting was performed using a polyclonal antibody specific for HTRA1. Lane 1, HTRA1Δmac (4 ng); lanes 2–4, non-degenerated (ND) discs; lanes 5–7, mildly degenerated discs; lanes 8–10, moderately degenerated discs; lanes 11–13, severely degenerated discs. Closed arrowhead, 50-kDa HTRA1; open arrowhead, 42-kDa HTRA1. C, representative images of HTRA1 protein within frozen IVD tissue sections as identified by immunofluorescence staining. HTRA1 was detected using a Cy3-labeled secondary antibody (red), and nuclei were labeled with DAPI (blue). The specificity of staining was confirmed through the use of a nonspecific rabbit IgG control (IgG). Grade 1 represents a normal, non-degenerated IVD, whereas grades 3 and 4 signify moderate and severe degeneration, respectively. Scale bar, 50 μm.
Analysis of Patient rs11200638 SNP Genotype
In light of the recent evidence linking the rs11200638 SNP (G>A) in the HTRA1 gene promoter and spinal disc degeneration in Japanese women (21), we investigated whether the rs11200638 risk allele frequency between patient groups used in the current study was associated with susceptibility to IVD degeneration. DNA from a total of 35 patients was subjected to SNP genotyping using the TaqMan SNP genotyping assay specific for rs11200638 SNP. Contrary to previous expectations, we were unable to demonstrate any significant differences in SNP allele frequencies between control patients without disc degeneration (25%) and patients with degeneration grades of 2 (14.3%; p = 0.4), 3 (15%; p = 0.37), or 4 (15%; p = 0.37) (Table 1). We also performed a comparative analysis of HTRA1 expression levels in IVD tissue from 32 patients of known genotype. However, no significant associations could be made between HTRA1 expression levels and the rs11200638 genotype (Table 1).
TABLE 1.
rs11200638 SNP Genotyping of European patients with and without IVD degeneration
| Genotype | Degeneration grade |
n | Relative HTRA1 expressiona | p valueb | |||
|---|---|---|---|---|---|---|---|
| 1 (n = 8) | 2 (n = 7) | 3 (n = 10) | 4 (n = 10) | ||||
| GG | 5 | 5 | 7 | 7 | 23 | 204.1 ± 35.6 | |
| GA | 2 | 2 | 3 | 3 | 8 | 307 ± 87.3 | 0.20 |
| AA | 1 | 0 | 0 | 0 | 1 | 14.9 | |
| GA + AA | 3 | 2 | 3 | 3 | 9 | 274.5 ± 83.5 | 0.37 |
| G allele | 12 | 12 | 17 | 17 | |||
| A allelec | 4 (25%) | 2 (14.3%) | 3 (15%) | 3 (15%) | |||
| p = 0.4d | p = 0.37d | p = 0.37d | |||||
a Comparisons were made between HTRA1 expression levels ± S.E. in IVD tissue and genotype frequency in patients where both RNA and DNA samples were available (n = 32).
b Student's t test was used to compare HTRA1 expression levels between patients carrying the risk allele (GA/AA) and those homozygous for the wild type allele (GG).
c Percentages refer to risk allele (A) frequency.
d The χ2 test was used to evaluate the significance of differences in risk allele (A) frequency between patients with IVD degeneration (grades 2–4) and control patients without IVD degeneration (grade 1) (n = 35).
Effect of Recombinant HTRA1 on MMP Production by IVD Cells
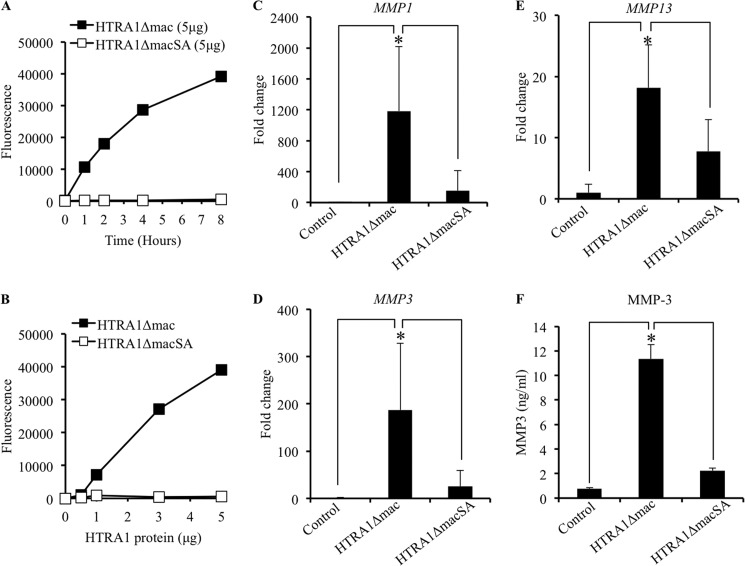
In order to investigate how HTRA1 may contribute to IVD degeneration, we generated both proteolytically active and inactive recombinant forms of HTRA1 lacking the amino-terminal mac25 homology domain as described previously (19). The protease activity of the active protein, termed HTRA1Δmac, was confirmed by its ability to cleave purified bovine elastin and was dependent on both incubation time (Fig. 2A) and protein amount (Fig. 2B). No such activity was observed with the proteolytically inactive form of HTRA1 (HTRA1ΔmacSA) in which serine 328, part of the serine protease catalytic triad domain, had been substituted for alanine. The effects of these recombinant proteins on MMP production by cultured IVD cells were then investigated. Cells were incubated for up to 24 h with either HTRA1Δmac (5 μg/ml) or HTRA1ΔmacSA (5 μg/ml), and the -fold change in mRNA expression levels of MMP1, MMP3, and MMP13 was determined by qRT-PCR. HTRA1Δmac induced a significant increase in the expression levels of MMPs tested as compared with untreated cells and cells treated with the proteolytically inactive HTRA1ΔmacSA (Fig. 2, C–E). In addition, HTRA1Δmac also enhanced the expression of ADAMTS4 (aggrecanase-1), although no increase was observed in MMP2 (gelatinase A) expression, and ACAN (aggrecan) expression was actually reduced (supplemental Fig. 2). MMP expression was also up-regulated in HTRA1ΔmacSA-treated cells, although expression levels were over 3–7-fold less than those observed in cells incubated with HTRA1Δmac and were not deemed statistically significant as compared with untreated control cells (MMP1, p = 0.19; MMP3, p = 0.41; MMP13, p = 0.41). Clearly, therefore, HTRA1-induced MMP expression in IVD cells is primarily a protease-dependent phenomenon, although it would appear that other routes of activation may also exist. In addition to its stimulatory effects on MMP mRNA expression, HTRA1Δmac also enhanced MMP protein production, as evidenced by results obtained from the MMP-3 ELISA (Fig. 2F). Low levels of secreted MMP-3 were detected in the supernatants of untreated cells (0.76 ng/ml ± 0.04) but became significantly elevated following stimulation with HTRA1Δmac (11.33 ng/ml ± 0.68; p < 0.001). As with our previous findings, these stimulatory effects of HTRA1 were significantly diminished following inactivation of its protease activity, although levels remained elevated as compared with untreated cells (2.23 ± 0.13 ng/ml; p = 0.089).
FIGURE 2.
Regulation of MMP expression in IVD cells by recombinant HTRA1. A and B, recombinant HTRA1 proteolytic activity was determined using soluble bovine BODIPY FL-labeled DQ-elastin (100 μg/ml) as a substrate. Digestion of the DQ-elastin yielded fluorescent fragments detectable at 530 nm by a fluorescence microplate reader. The amount of DQ-elastin digestion was measured at selected time points (0, 1, 2, 4, and 8 h) using a defined amount of HTRA1 (5 μg) (A) or was determined after incubation for 8 h with varying amounts of HTRA1 (0, 0.5, 1, 3, and 5 μg) (B). C–E, the effects of recombinant HTRA1 (5 μg/ml) on MMP expression levels in IVD cells after a 24-h incubation period were determined by qRT-PCR, and the -fold change as compared with untreated controls was determined using the 2−ΔΔCT method (n = 4–6 patients). F, a specific MMP-3 ELISA was used to investigate the effects of recombinant HTRA1 on MMP protein secretion in supernatants obtained from IVD cells. Shown are results of triplicate determinations ± S.D. (error bars). *, p < 0.01, as determined by one-way ANOVA.
Influence of MEK and IL-1β Inhibition on HTRA1-induced MMP Production
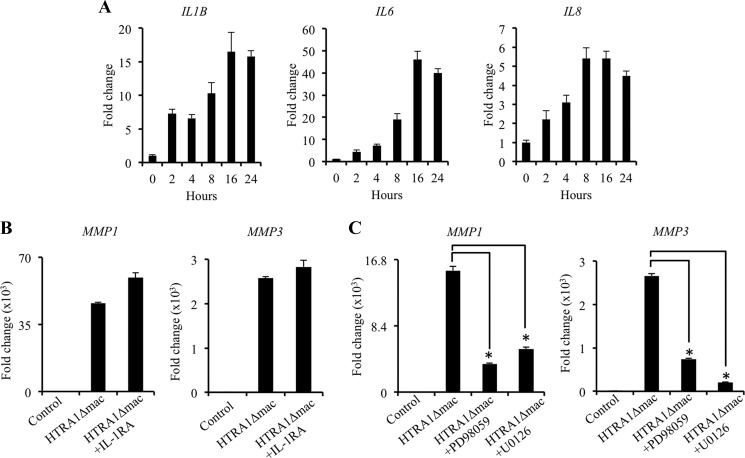
Of the known instigators of MMP expression by IVD cells, IL-1β is considered to be the most potent and, as such, is centrally involved in both IVD cell activation and IVD degeneration (8). Furthermore, IL-1β has previously been shown to play a central role in mediating MMP production by fibronectin fragments in cultures of bovine articular cartilage (26). We therefore investigated if the expression of IL-1β, along with several other cytokines, including IL-6 and IL-8, was up-regulated in IVD cells by HTRA1Δmac and whether this had any influence on the induction of MMP expression by HTRA1Δmac. Indeed, expression levels of the cytokines tested were up-regulated in IVD cells stimulated with HTRA1Δmac (5 μg/ml) in a time-dependent manner (Fig. 3A). However, additional studies focusing on the inhibition of IL-1β signaling using the IL-1RA (250 ng/ml) failed to demonstrate any significant alterations in the ability of HTRA1 to induce MMP expression in IVD cells (Fig. 3B and supplemental Fig. 3). This would suggest that HTRA1 does not mediate its stimulatory effects through IL-1β production, although we cannot exclude the possibility that other cytokines may be involved.
FIGURE 3.
Down-regulation of HTRA1-induced MMP expression in IVD cells by MEK inhibition. A, IL1B, IL6, and IL8 mRNA expression levels were measured in IVD cell cultures by qRT-PCR in response to HTRA1Δmac (5 μg/ml) stimulation over the course of 24 h, and the -fold change as compared with untreated cells was determined using the 2−ΔΔCT method. B, the influence of IL-1β inhibition on HTRA1-induced MMP1 and MMP3 expression by IVD cells was evaluated by qRT-PCR after a 24-h incubation with HTRA1Δmac (5 μg/ml) in combination with the IL-1RA (250 ng/ml), and the -fold change as compared with untreated controls was determined using the 2−ΔΔCT method. C, the effects of MEK inhibitors PD98059 (10 μm) and U0126 (10 μm) on HTRA1-induced MMP1 and MMP3 expression by IVD cells were evaluated by qRT-PCR after a 24-h incubation, and the -fold change as compared with untreated controls was determined using the 2−ΔΔCT method. In each case, data are representative of at least two separate experiments performed using IVD cells isolated from a total of n = 5 patients. Shown are results of triplicate determinations ± S.D. (error bars). *, p < 0.01, as determined by one-way ANOVA.
We have previously demonstrated that MMP expression by human synovial fibroblasts is up-regulated in response to HTRA1 stimulation and that this was at least partly dependent on the generation of fibronectin fragments (19). Furthermore, fibronectin fragments were recently confirmed as being potent inducers of MMP expression in IVD cells, mediating their effects through activation of the MEK pathway (27). We therefore investigated whether inhibition of MEK could influence the actions of HTRA1 on IVD MMP expression. Indeed, inclusion of either of the specific MEK inhibitors PD98059 (10 μm) or U0126 (10 μm) 2 h prior to treatment with HTRA1Δmac (5 μg/ml) significantly abrogated its stimulatory effects on the expression of MMP1 and MMP3 (Fig. 3C), thereby supporting the theory that fibronectin fragments may represent one possible route through which HTRA1 mediates its stimulatory effects on IVD cells.
Effect of Fibronectin Fragments on MMP Expression by IVD Cells
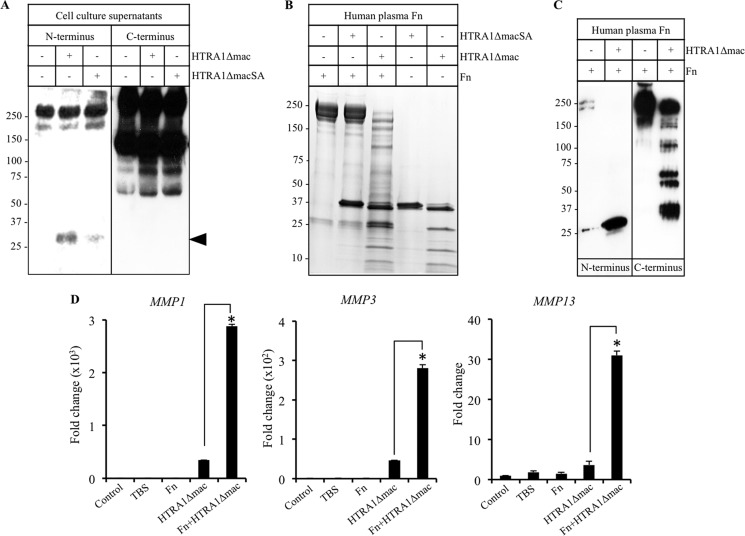
Based on the above results, we further investigated the potential involvement of fibronectin fragments in mediating the actions of HTRA1 on IVD cells. We observed a noticeable increase in the level of a 27–29-kDa fibronectin fragment containing the amino-terminal fibrin- and heparin-binding domain in the supernatants from IVD cell cultures previously treated with HTRA1Δmac (5 μg/ml) as compared with cells incubated in medium alone or with the inactive HTRA1ΔmacSA (5 μg/ml) (Fig. 4A and supplemental Fig. 4A). Interestingly, increases in fibronectin fragments containing the carboxyl-terminal heparin-binding domain were evident in both HTRA1Δmac- and HTRA1ΔmacSA-treated cell cultures as compared with untreated controls. Further analyses confirmed that HTRA1Δmac, but not HTRA1ΔmacSA, could digest equimolar concentrations of human plasma-derived fibronectin after a 16-h incubation period, resulting in various sized fragments being generated, as determined by SDS-PAGE (Fig. 4B). The appearance of HTRA1Δmac as several bands is due to its autoproteolytic activity, as described previously (19), an effect not observed with HTRA1ΔmacSA. Western blot analysis of the products generated from HTRA1Δmac-induced fibronectin proteolysis revealed the presence of numerous fragments containing the carboxyl-terminal heparin-binding domain as well as the 27–29-kDa fragment containing the amino-terminal fibrin- and heparin-binding domain previously identified in the supernatants from HTRA1Δmac-treated IVD cells (Fig. 4C). We next investigated whether the fibronectin fragments generated by HTRA1 were also capable of activating IVD cells. Following an overnight incubation of human plasma-derived fibronectin with HTRA1Δmac (equimolar concentration), digested and undigested fibronectin was purified by affinity chromatography using Ni2+-NTA to remove the majority of His-tagged HTRA1Δmac (supplemental Fig. 4B) and then incubated for up to 24 h with IVD cells. The expression levels of MMP1, MMP3, and MMP13 (Fig. 4D) were all significantly increased in IVD cells incubated with the purified mixture of fibronectin fragments. By comparison, MMP expression in control cultures stimulated with the affinity column flow-through fraction from HTRA1Δmac samples in the absence of fibronectin was 6–9-fold less (p < 0.01), thus confirming that fibronectin fragments were the predominant cause of IVD cell activation.
FIGURE 4.
Stimulation of IVD cells with HTRA1-generated fibronectin fragments. A, concentrated protein supernatants (15 μg) from IVD cells treated for 24 h without or with HTRA1Δmac (5 μg/ml) or HTRA1ΔmacSA (5 μg/ml) were subjected to immunoblotting using antibody Mab1935 specific for the fibronectin carboxyl-terminal heparin-binding domain (C terminus) or Mab1936 specific for the fibronectin amino-terminal fibrin- and heparin-binding domain (N terminus). Fibronectin fragments containing the amino-terminal fibrin- and heparin-binding domain are identified by the closed arrowhead. B, purified human plasma-derived fibronectin (Fn) was incubated with HTRA1Δmac or HTRA1ΔmacSA at equimolar concentrations in TBS, pH 8.5, for 16 h at 37 °C, and samples were loaded onto a 4–15% gradient gel and stained with Coomassie Blue. Fibronectin and recombinant HTRA1 alone were also loaded and served as controls. C, an equimolar concentration of human plasma-derived fibronectin and HTRA1Δmac were incubated for 16 h, and fibronectin fragments were visualized by Western blot analysis using the antibodies described in A. D, equimolar concentrations of fibronectin (20 μg) and HTRA1Δmac (5 μg) were incubated for 16 h, and fibronectin fragments were purified by affinity chromatography. IVD cells were incubated with purified HTRA1-digested fibronectin (Fn+HTRA1Δmac) for 24 h, and expression levels of MMP1, MMP3, and MMP13 mRNA were determined by qRT-PCR and the -fold change as compared with untreated controls was determined using the 2−ΔΔCT method. Additional cultures were incubated with either affinity-purified Tris-buffered saline, pH 7.6 (TBS), fibronectin (Fn), or HTRA1 (HTRA1Δmac) or left untreated (Control). Data are representative of two separate experiments performed using IVD cells from two patients. Shown are results of triplicate determinations ± S.D. *, p < 0.01, as determined by one-way ANOVA.
Association of Fibronectin Fragments with Disc Degeneration
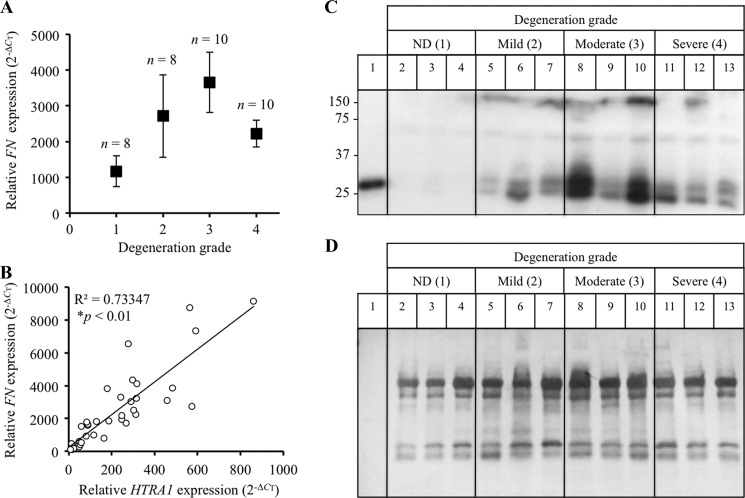
Both fibronectin and fibronectin fragments are known to increase with increasing disc degeneration and are thus considered to be an integral part of the underlying pathology (11). In the present study, fibronectin mRNA levels within IVD tissue samples from patients with varying degrees of disc degeneration were normalized to TBP and presented as 2−ΔCT. Fibronectin expression levels within patient disc tissue samples were indeed elevated in response to increases in degeneration grade (Fig. 5A) and correlated significantly with HTRA1 mRNA expression levels (r = 0.856; p < 0.01) (Fig. 5B). Furthermore, Western blot analysis of IVD protein samples revealed an increase in fibronectin fragments containing the amino-terminal fibrin- and heparin-binding domain (Fig. 5C) in degenerated discs. Moreover, the majority of amino-terminal fragments identified were found to be of a similar size (27–29 kDa) to the fragment identified within samples of HTRA1-digested human fibronectin (lane 1). Membranes were counterstained with Coomassie Blue to confirm equal protein loading (Fig. 5D).
FIGURE 5.
Detection of fibronectin fragments in degenerated IVD tissue. A, fibronectin (FN) mRNA levels in intact IVD tissue samples from patients (n = 36) with varying degrees of IVD degeneration were determined by qRT-PCR and presented as 2−ΔCT ± S.E. (error bars). B, correlation study between FN and HTRA1 mRNA levels (2−ΔCT) in patient IVD tissue samples (n = 36). R2, square of correlation coefficient; p < 0.01 as determined from Pearson's correlation coefficient. C, protein extracts from patient IVD tissues (n = 12) were loaded onto a 12% SDS-polyacrylamide gel, and immunoblotting was performed using a monoclonal antibody (Mab1936) specific for the amino-terminal fibrin- and heparin-binding domain. D, the PVDF membrane used in C was stained with Coomassie Blue in order to confirm equal protein loading. Lane 1, HTRA1-digested human plasma-derived fibronectin; lanes 2–4, non-degenerated (ND) discs; lanes 5–7, mildly degenerated discs; lanes 8–10, moderately degenerated discs; lanes 11–13, severely degenerated discs.
DISCUSSION
In the current study, we have identified HTRA1 mRNA and protein in the IVDs of human subjects and demonstrated an association between expression levels and severity of IVD degeneration. HTRA1 has previously been linked with disc degeneration following the association of a single nucleotide polymorphism, rs11200638, in the HTRA1 gene and loss of disc height in postmenopausal Japanese women (21). In the present study, we were unable to show any significant association between the frequency of the risk allele (A) of SNP rs11200638 and IVD degeneration in a small European surgical patient population (n = 35). Furthermore, we found no evidence to suggest that the A allele of SNP rs11200638 has any significant influence on HTRA1 expression levels in IVD tissue. This is supported by findings from studies investigating the role of HTRA1 in age-related macular degeneration, where, although closely associated with disease risk, rs11200638 alone was unable to significantly alter HTRA1 expression (28, 29). It was subsequently reported that the additional disruption of the adjacent gene, LOC387715, was necessary in order for rs11200638 to have a positive influence on HTRA1 expression (28).
We have previously shown that HTRA1 has the potential to stimulate MMP production by human synovial fibroblasts, thus implicating it in the pathogenesis of arthritis (19). As in the joints of arthritic patients, MMPs play a central role in orchestrating ECM breakdown in discs of patients with IVD degeneration (4–7). Therefore, we next investigated whether HTRA1 could also influence MMP production in human IVD cells. Consistent with our earlier findings with human synovial fibroblasts, recombinant HTRA1 was able to induce the expression of MMP1, MMP3, and MMP13 mRNA, as well as MMP-3 protein, in short term IVD cell cultures. In addition, we also observed a significant increase in ADAMTS4 expression in IVD cells stimulated with HTRA1. As with MMP-1, -3, and -13, ADAMTS-4 is also up-regulated in degenerated discs (4) and may therefore represent an additional route through which HTRA1 could influence ECM breakdown. These stimulatory effects were found to be primarily reliant on HTRA1 protease activity as evidenced by the fact that the proteolytically inactive form of HTRA1 (HTRA1ΔmacSA) was unable to significantly enhance MMP production. Nevertheless, increases in MMP production were apparent in cultures treated with HTRA1ΔmacSA and may be indicative of an additional, and as yet unidentified, protease-independent mechanism through which HTRA1 mediates its stimulatory effects. It is possible that further studies utilizing other mutant forms of HTRA1 may further assist in trying to decipher the exact cause of these stimulatory effects.
Degradation of the ECM within degenerated IVDs can give rise to fibronectin fragments of various sizes, which themselves are capable of activating resident disc cells to produce matrix-degrading proteases in a MEK-dependent manner (9, 27). Furthermore, the stimulatory properties of certain fibronectin fragment species on cellular MMP production are known to be mediated through the actions of IL-1β (26). In the current report, we demonstrated that the stimulatory effects of HTRA1 on MMP production by IVD cells were indeed MEK-dependent. Moreover, despite the fact HTRA1 induced the expression of IL1B, along with several other cytokines, inhibition of IL-1β signaling through the use of the IL-1RA failed to have any significant impact on HTRA1-induced MMP expression in IVD cells. On closer examination, it was revealed that IVD cell cultures and purified human plasma-derived fibronectin incubated with HTRA1Δmac both consisted of increased levels of a fibronectin fragment containing the 27–29-kDa amino-terminal fibrin- and heparin-binding domain. Furthermore, MMP expression was enhanced in IVD cells following stimulation with the proteolytic products of HTRA1-digested human plasma-derived fibronectin. Considering this, together with the additional observation that HTRA1-induced MMP expression was also dependent on MEK activity, we conclude that the stimulatory effects of HTRA1 on MMP production by IVD cells are mediated, at least in part, through the generation of fibronectin fragments. Interestingly, increases in fibronectin fragments containing the carboxyl-terminal heparin-binding domain, were also evident in the culture supernatants of cells treated with the HTRA1ΔmacSA. The fact that HTRA1ΔmacSA was proteolytically inactive and only able to generate fibronectin fragments in the presence of cells would suggest that these effects were most likely mediated through the stimulated secretion of other active proteases. This may therefore offer some explanation as to why MMP production by IVD cells was enhanced with HTRA1ΔmacSA, although the mechanism of action still remains to be determined. Furthermore, the observation that levels of the 27–29-kDa fibronectin fragment containing the amino-terminal fibrin- and heparin-binding domain were elevated predominantly in cell cultures treated with HTRA1Δmac would further imply that these fragment species are of particular relevance with regard to IVD cell activation.
Increased production of fibronectin by resident IVD cells has been observed within degenerated disc tissue samples and is considered to be central to the ongoing reparative and remodeling processes associated with IVD degeneration (30, 31). In the present study, we observed a marked increase in fibronectin expression in IVD tissue from patients with moderate to severe disc degeneration, which correlated significantly with changes in HTRA1 expression levels. At present, it is not known whether fibronectin and HTRA1 share common regulatory pathways, although it would appear that both of their expression patterns are dependent on factors intrinsic to the degenerative process. Given the fact that HTRA1-generated fibronectin fragments serve as potent inducers of MMP production, overexpression of fibronectin and HTRA1 within IVDs is likely to have a significant influence on the development of disc degeneration. This is supported by our finding that small molecular mass fragments (27–29 kDa) containing the amino-terminal fibrin- and heparin-binding domain of fibronectin could also be identified within degenerated disc samples. The involvement of such fragments in disc degeneration has already been alluded to in a recent report by Anderson et al. (32), where a single fibronectin fragment with an estimated size of 25 kDa was detected in degenerated discs by immunoblotting with the same monoclonal antibody as used in the current study. It is possible, therefore, that HTRA1 protease activity may in fact be one of the main causative agents responsible for generating such fibronectin fragments within degenerated discs.
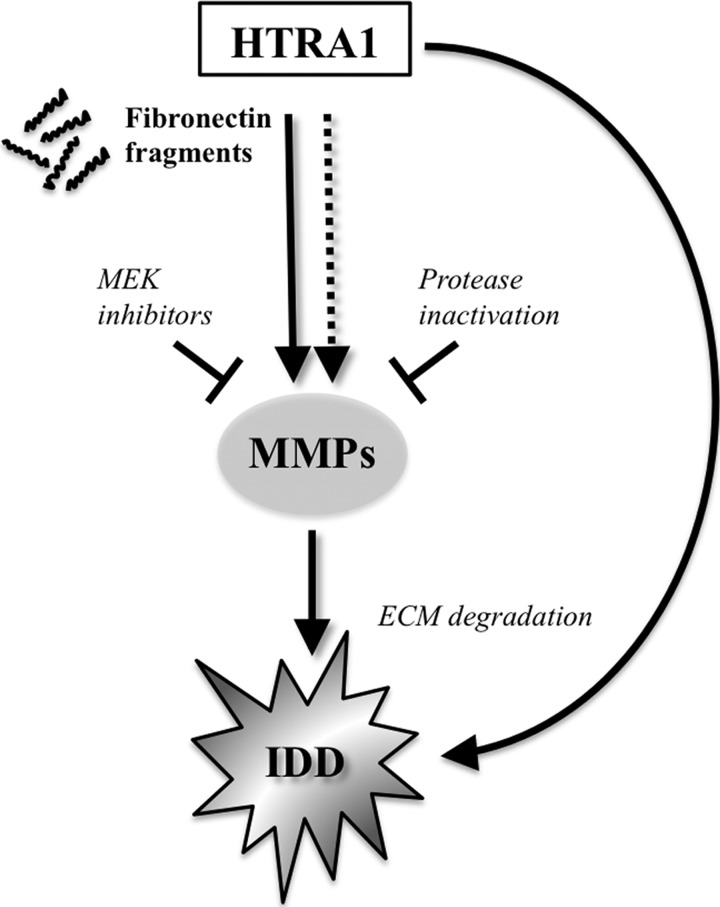
Taken together, these findings led us to propose a working model for the biological role of HTRA1 in IVD degeneration (Fig. 6). In addition to its already well characterized ability to directly degrade ECM proteins known to be present within IVDs (19, 20, 33–35), HTRA1 may also further modulate ECM breakdown indirectly through fibronectin fragment production and subsequent up-regulation of MMPs by resident disc cells. Clearly, further investigations are required in order to identify which particular fragments are responsible for the activation of IVD cells in vitro and to clarify the involvement of fibronectin fragments in IVD degeneration. Furthermore, additional studies are needed to fully evaluate the potential stimulatory effects of other soluble mediators (e.g. cytokines) released by IVD cells following incubation with HTRA1. Moreover, examination of the possible interplay between inflammatory mediators and fibronectin fragments in the regulation of Toll-like receptor signaling pathways in IVD cells may also lend further insight into how HTRA1 contributes to the catabolic response in IVD degeneration (36). These results therefore encourage the design of specific HTRA1 inhibitors for the treatment of patients with disc degeneration, an endeavor that will no doubt be facilitated by information gleaned from the recently solved crystal structure of HTRA1 (37).
FIGURE 6.
A theoretical model for the role of HTRA1 in IVD degeneration. Based on our findings, we propose that HTRA1 accumulates in IVD tissue undergoing degeneration and stimulates MMP production by resident cells in a predominantly protease-dependent manner, via activation of the MEK pathway. Furthermore, we suggest that the stimulatory effects of HTRA1 on IVD cells are mediated indirectly through its ability to generate fibronectin fragments, although other routes of cellular activation cannot be ruled out. IDD, intervertebral disc degeneration.
This work was supported by a grant from the Center for Applied Biotechnology and Molecular Medicine Start-up Grant/Mäxi Foundation.

This article contains supplemental Table 1 and Figs. 1–4.
- IVD
- intervertebral disc
- ECM
- extracellular matrix
- SNP
- single nucleotide polymorphism
- MMP
- matrix metalloproteinase
- IL-1RA
- IL-1 receptor antagonist
- ANOVA
- analysis of variance
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Peterson C. K., Bolton J. E., Wood A. R. (2000) A cross-sectional study correlating lumbar spine degeneration with disability and pain. Spine 25, 218–223 [DOI] [PubMed] [Google Scholar]
- 2. Luoma K., Riihimäki H., Luukkonen R., Raininko R., Viikari-Juntura E., Lamminen A. (2000) Low back pain in relation to lumbar disc degeneration. Spine 25, 487–492 [DOI] [PubMed] [Google Scholar]
- 3. Freemont A. J., Watkins A., Le Maitre C., Jeziorska M., Hoyland J. A. (2002) Current understanding of cellular and molecular events in intervertebral disc degeneration. Implications for therapy. J. Pathol. 196, 374–379 [DOI] [PubMed] [Google Scholar]
- 4. Le Maitre C. L., Freemont A. J., Hoyland J. A. (2004) Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J. Pathol. 204, 47–54 [DOI] [PubMed] [Google Scholar]
- 5. Le Maitre C. L., Freemont A. J., Hoyland J. A. (2006) Human disc degeneration is associated with increased MMP 7 expression. Biotech. Histochem. 81, 125–131 [DOI] [PubMed] [Google Scholar]
- 6. Roberts S., Caterson B., Menage J., Evans E. H., Jaffray D. C., Eisenstein S. M. (2000) Matrix metalloproteinases and aggrecanase. Their role in disorders of the human intervertebral disc. Spine 25, 3005–3013 [DOI] [PubMed] [Google Scholar]
- 7. Bachmeier B. E., Nerlich A., Mittermaier N., Weiler C., Lumenta C., Wuertz K., Boos N. (2009) Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur. Spine J. 18, 1573–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Millward-Sadler S. J., Costello P. W., Freemont A. J., Hoyland J. A. (2009) Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells. Implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther. 11, R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson D. G., Li X., Balian G. (2005) A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine 30, 1242–1246 [DOI] [PubMed] [Google Scholar]
- 10. Greg Anderson D., Li X., Tannoury T., Beck G., Balian G. (2003) A fibronectin fragment stimulates intervertebral disc degeneration in vivo. Spine 28, 2338–2345 [DOI] [PubMed] [Google Scholar]
- 11. Oegema T. R., Jr., Johnson S. L., Aguiar D. J., Ogilvie J. W. (2000) Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine 25, 2742–2747 [DOI] [PubMed] [Google Scholar]
- 12. Clausen T., Kaiser M., Huber R., Ehrmann M. (2011) HTRA proteases. Regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 12, 152–162 [DOI] [PubMed] [Google Scholar]
- 13. Shridhar V., Sen A., Chien J., Staub J., Avula R., Kovats S., Lee J., Lillie J., Smith D. I. (2002) Identification of underexpressed genes in early and late stage primary ovarian tumors by suppression subtraction hybridization. Cancer Res. 62, 262–270 [PubMed] [Google Scholar]
- 14. Chien J., Staub J., Hu S. I., Erickson-Johnson M. R., Couch F. J., Smith D. I., Crowl R. M., Kaufmann S. H., Shridhar V. (2004) A candidate tumor suppressor HtrA1 is down-regulated in ovarian cancer. Oncogene 23, 1636–1644 [DOI] [PubMed] [Google Scholar]
- 15. Baldi A., De Luca A., Morini M., Battista T., Felsani A., Baldi F., Catricalà C., Amantea A., Noonan D. M., Albini A., Natali P. G., Lombardi D., Paggi M. G. (2002) The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene 21, 6684–6688 [DOI] [PubMed] [Google Scholar]
- 16. Bakay M., Zhao P., Chen J., Hoffman E. P. (2002) A Web-accessible complete transcriptome of normal human and DMD muscle. Neuromuscul. Disord. 12, S125–S141 [DOI] [PubMed] [Google Scholar]
- 17. Hu S. I., Carozza M., Klein M., Nantermet P., Luk D., Crowl R. M. (1998) Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J. Biol. Chem. 273, 34406–34412 [DOI] [PubMed] [Google Scholar]
- 18. Wu J., Liu W., Bemis A., Wang E., Qiu Y., Morris E. A., Flannery C. R., Yang Z. (2007) Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis Rheum. 56, 3675–3684 [DOI] [PubMed] [Google Scholar]
- 19. Grau S., Richards P. J., Kerr B., Hughes C., Caterson B., Williams A. S., Junker U., Jones S. A., Clausen T., Ehrmann M. (2006) The role of human HtrA1 in arthritic disease. J. Biol. Chem. 281, 6124–6129 [DOI] [PubMed] [Google Scholar]
- 20. Tsuchiya A., Yano M., Tocharus J., Kojima H., Fukumoto M., Kawaichi M., Oka C. (2005) Expression of mouse HtrA1 serine protease in normal bone and cartilage and its up-regulation in joint cartilage damaged by experimental arthritis. Bone 37, 323–336 [DOI] [PubMed] [Google Scholar]
- 21. Urano T., Narusawa K., Kobayashi S., Shiraki M., Horie-Inoue K., Sasaki N., Hosoi T., Ouchi Y., Nakamura T., Inoue S. (2010) Association of HTRA1 promoter polymorphism with spinal disc degeneration in Japanese women. J. Bone Miner. Metab. 28, 220–226 [DOI] [PubMed] [Google Scholar]
- 22. Pfirrmann C. W., Metzdorf A., Zanetti M., Hodler J., Boos N. (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26, 1873–1878 [DOI] [PubMed] [Google Scholar]
- 23. Wuertz K., Urban J. P., Klasen J., Ignatius A., Wilke H. J., Claes L., Neidlinger-Wilke C. (2007) Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J. Orthop. Res. 25, 1513–1522 [DOI] [PubMed] [Google Scholar]
- 24. Grau S., Baldi A., Bussani R., Tian X., Stefanescu R., Przybylski M., Richards P., Jones S. A., Shridhar V., Clausen T., Ehrmann M. (2005) Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. U.S.A. 102, 6021–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nie G., Li Y., Salamonsen L. A. (2005) Serine protease HtrA1 is developmentally regulated in trophoblast and uterine decidual cells during placental formation in the mouse. Dev. Dyn. 233, 1102–1109 [DOI] [PubMed] [Google Scholar]
- 26. Yasuda T., Poole A. R. (2002) A fibronectin fragment induces type II collagen degradation by collagenase through an interleukin-1-mediated pathway. Arthritis Rheum. 46, 138–148 [DOI] [PubMed] [Google Scholar]
- 27. Xia M., Zhu Y. (2011) Fibronectin fragment activation of ERK increasing integrin α and β subunit expression to degenerate nucleus pulposus cells. J. Orthop. Res. 29, 556–561 [DOI] [PubMed] [Google Scholar]
- 28. Yang Z., Tong Z., Chen Y., Zeng J., Lu F., Sun X., Zhao C., Wang K., Davey L., Chen H., London N., Muramatsu D., Salasar F., Carmona R., Kasuga D., Wang X., Bedell M., Dixie M., Zhao P., Yang R., Gibbs D., Liu X., Li Y., Li C., Li Y., Campochiaro B., Constantine R., Zack D. J., Campochiaro P., Fu Y., Li D. Y., Katsanis N., Zhang K. (2010) Genetic and functional dissection of HTRA1 and LOC387715 in age-related macular degeneration. PLoS Genet. 6, e1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanda A., Chen W., Othman M., Branham K. E., Brooks M., Khanna R., He S., Lyons R., Abecasis G. R., Swaroop A. (2007) A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 104, 16227–16232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nerlich A. G., Bachmeier B. E., Boos N. (2005) Expression of fibronectin and TGF-β1 mRNA and protein suggest altered regulation of extracellular matrix in degenerated disc tissue. Eur. Spine J. 14, 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gruber H. E., Hoelscher G. L., Ingram J. A., Bethea S., Zinchenko N., Hanley E. N., Jr. (2011) Variations in aggrecan localization and gene expression patterns characterize increasing stages of human intervertebral disk degeneration. Exp. Mol. Pathol. 91, 534–539 [DOI] [PubMed] [Google Scholar]
- 32. Anderson D. G., Markova D., Adams S. L., Pacifici M., An H. S., Zhang Y. (2010) Fibronectin splicing variants in human intervertebral disc and association with disc degeneration. Spine 35, 1581–1588 [DOI] [PubMed] [Google Scholar]
- 33. Tocharus J., Tsuchiya A., Kajikawa M., Ueta Y., Oka C., Kawaichi M. (2004) Developmentally regulated expression of mouse HtrA3 and its role as an inhibitor of TGF-β signaling. Dev. Growth Differ. 46, 257–274 [DOI] [PubMed] [Google Scholar]
- 34. Hadfield K. D., Rock C. F., Inkson C. A., Dallas S. L., Sudre L., Wallis G. A., Boot-Handford R. P., Canfield A. E. (2008) HtrA1 inhibits mineral deposition by osteoblasts. Requirement for the protease and PDZ domains. J. Biol. Chem. 283, 5928–5938 [DOI] [PubMed] [Google Scholar]
- 35. Chamberland A., Wang E., Jones A. R., Collins-Racie L. A., LaVallie E. R., Huang Y., Liu L., Morris E. A., Flannery C. R., Yang Z. (2009) Identification of a novel HtrA1-susceptible cleavage site in human aggrecan. Evidence for the involvement of HtrA1 in aggrecan proteolysis in vivo. J. Biol. Chem. 284, 27352–27359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Su S. L., Tsai C. D., Lee C. H., Salter D. M., Lee H. S. (2005) Expression and regulation of Toll-like receptor 2 by IL-1β and fibronectin fragments in human articular chondrocytes. Osteoarthr. Cartil. 13, 879–886 [DOI] [PubMed] [Google Scholar]
- 37. Truebestein L., Tennstaedt A., Mönig T., Krojer T., Canellas F., Kaiser M., Clausen T., Ehrmann M. (2011) Substrate-induced remodeling of the active site regulates human HTRA1 activity. Nat. Struct. Mol. Biol. 18, 386–388 [DOI] [PubMed] [Google Scholar]