Background: Fluid shear increases intracellular calcium and NO/cGMP signaling in osteoblasts.
Results: Focal adhesion kinase and protein kinase G are independently activated downstream of calcium; both kinases cooperatively activate Src, leading to Akt/GSK3/β-catenin signaling in shear-stressed osteoblasts.
Conclusion: Osteoblast mechanotransduction requires cross-talk between FAK and PKG to regulate Akt.
Significance: cGMP-elevating agents may prove useful for the treatment of osteoporosis.
Keywords: beta-Catenin, Calcium, Focal Adhesion Kinase, Mechanotransduction, Osteoblasts, Protein Kinase G (PKG), Fluid Shear Stress, cGMP-dependent Protein Kinase
Abstract
Mechanical loading of bone induces interstitial fluid flow, leading to fluid shear stress (FSS) of osteoblasts. FSS rapidly increases the intracellular calcium concentration ([Ca2+]) and nitric oxide (NO) synthesis in osteoblasts and activates the protein kinase Akt. Activated Akt stimulates osteoblast proliferation and survival, but the mechanism(s) leading to Akt activation is not well defined. Using pharmacological and genetic approaches in primary human and mouse osteoblasts and mouse MC3T3 osteoblast-like cells, we found that Akt activation by FSS occurred through two parallel pathways; one required calcium stimulation of NO synthase and NO/cGMP/protein kinase G II-dependent activation of Src, and the other required calcium activation of FAK and Src, independent of NO. Both pathways cooperated to increase PI3K-dependent Akt phosphorylation and were necessary for FSS to induce nuclear translocation of β-catenin, c-fos, and cox-2 gene expression and osteoblast proliferation. These data explain how mechanical stimulation of osteoblasts leads to increased signaling through a growth regulatory pathway essential for maintaining skeletal integrity.
Introduction
Mechanical loading of the skeleton is crucial for maintaining bone mass and strength. Decreased mechanical loading due to prolonged immobilization, muscle paresis, or weightlessness in space causes bone loss, whereas increased loading promotes bone formation (1). Mechanical loading of bone increases interstitial fluid flow through the lacunar-canalicular system, and the resulting fluid shear stress (FSS)4 stimulates osteocytes embedded in lacunae/canaliculi and osteoblasts lining bone surfaces, although both cell types are also subjected to other types of mechanical forces (1–4). Osteoblasts and osteocytes translate the mechanical signal into biochemical responses, resulting in enhanced proliferation and survival, respectively (1, 5). The biochemical responses include increases in the intracellular calcium concentration ([Ca2+]), nitric oxide (NO) production, ATP release, and prostaglandin synthesis; in addition, Akt and extracellular signal-regulated kinases (Erk1/2) are activated and increase the expression of c-fos, cox-2 (cyclooxygenase-2), and other genes involved in bone formation (2, 4–6).
Two of the earliest events in osteoblast mechanotransduction are a rapid influx of extracellular Ca2+ through mechanosensitive calcium channels and mobilization of intracellular Ca2+ through inositol 1,4,5-triphosphate-mediated release from intracellular Ca2+ stores (5, 7, 8). Pharmacological agents that inhibit Ca2+ influx or release from intracellular stores prevent FSS-induced downstream events, but the mechanism(s) whereby calcium regulates these events is not well defined (6, 8–10).
FSS increases NO production rapidly through calcium activation of NO synthase(s), although enhanced NO synthesis may be sustained through calcium-independent mechanisms (6, 11–13). NO synthase inhibitors block FSS-induced bone formation and c-fos mRNA expression in vivo, and experiments in NO synthase-deficient mice suggest a crucial role of NO in osteoblast mechanotransduction (14–17). We showed that increased NO in FSS-stimulated osteoblasts activates soluble guanylate cyclase, leading to increased cGMP production and activation of cytosolic (type I) and membrane-bound (type II) protein kinase G (PKG) (6). We subsequently showed that FSS triggers recruitment of PKG II, Src, and Shp-1/2 phosphatases to a focal adhesion-associated complex containing β3 integrins and that PKG II activates Src bound to the cytoplasmic tail of β3 integrins (18). We found that PKG II activation of Src is required for FSS-induced Erk activation, fos family gene expression, and osteoblast proliferation and that PKG II-deficient mice have defective Src and Erk activation in osteoblasts and decreased c-fos expression in bone (18).
For FSS to induce osteoblast proliferation, Akt must be activated in addition to Erk, but it is unclear how this occurs (19). Previous work suggests that focal adhesion kinase (FAK) is upstream of Akt, because a dominant negative FAK blocks FSS-induced Akt and Erk activation (19, 20). In many cell types, FAK and Src function within a network of integrin-stimulated signaling pathways leading to activation of the Ras/Raf/MEK/Erk and phosphatidylinositol 3-kinase (PI3K)/Akt signaling cascades (21–23). However, whether FAK and Src interact to regulate Akt in shear-stressed osteoblasts is unknown, and whether FAK is activated by FSS is controversial (19, 20, 24). Akt activation by growth factors requires PI3K-mediated synthesis of phosphatidylinositol 3,4,5-triphosphate, which recruits Akt and its activating kinases to the plasma membrane (25). PI3K-independent Akt activation can occur, and there are conflicting reports on whether PI3K is required for FSS-induced Akt activation (19, 26).
The Wnt/β-catenin pathway is important for all aspects of skeletal physiology, including bone mass accrual and maintenance, and response to mechanical stimulation (27–31). Downstream of Wnt co-receptors, glycogen synthase kinase-3 (GSK-3) is inhibited, allowing β-catenin stabilization, translocation to the nucleus, and regulation of gene transcription (5, 27, 29). PKG II and Akt can directly phosphorylate and inactivate GSK-3, potentially leading to Wnt-independent β-catenin stabilization; therefore, we asked whether PKG II and/or Akt play a role in FSS-induced β-catenin nuclear translocation in osteoblasts (30, 32, 33).
We now report that FSS activates a Ca2+/NO/cGMP/PKG II and a Ca2+/FAK pathway. These two pathways converge on Src, which then activates PI3K/Akt/GSK-3/β-catenin signaling; the latter is necessary for the anabolic effects of mechanical stimulation.
EXPERIMENTAL PROCEDURES
Materials
Antibodies against total Src, Src Tyr(P)418, Src-non-phosphorylated Tyr529, total FAK, FAK Tyr(P)397, FAK Tyr(P)576, total Akt, Akt Ser(P)473, total GSK-3β, and GSK-3β Ser(P) were from Cell Signaling. Antibodies against total Erk1 and phospho-specific antibodies for Erk1/2 (Tyr(P)204) were from Santa Cruz Biotechnology, Inc. The total β-catenin-specific antibody and a secondary anti-mouse antibody conjugated to AlexaFluor 555 were from Invitrogen. Bromodeoxyuridine (BrdU), DNase, and a BrdU-specific antibody were from Sigma. The calcium ionophore A23187, the intracellular calcium chelator BAPTA-AM, the Src family kinase inhibitor PP2 and its inactive cogener PP3, and the PI3K inhibitors LY294002 and wortmannin were from Calbiochem/EMD. The cGMP agonist 8-pCPT-cGMP and cGMP antagonist (Rp)-8-pCPT-PET-cGMPS were from Biolog. The NO synthase inhibitor l-NAME, and the sGC inhibitor ODQ were from Cayman. The sGC activator cinaciguat was from AdipoGen.
Cell Culture
Human primary osteoblasts (hPOBs) were explant cultures established from trabecular bone fragments obtained at the time of knee replacement surgery according to an institutionally approved protocol (6, 34). Outgrowing cells were used at passages 1–3 and were routinely cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS). They were characterized by alkaline phosphatase activity assay and histochemical staining, and osteocalcin mRNA expression in response to 1,25-dihydroxyvitamin D3 (10 nm) as shown in supplemental Fig. 1, A–C (34). Murine primary osteoblasts were isolated from the calvariae of 5–7-day-old Prkg2−/− mice and their wild type littermates as described (18). Murine MC3T3-E1 (clone 4) osteoblastic cells with high differentiation potential were from the American Type Culture Collection. They were used at <12 passages and cultured in ascorbate-free medium as described (6).
FSS Experiments
Cells were plated on etched glass slides 48 h prior to the experiments to allow secretion of extracellular matrix proteins (35); cells were transferred to medium containing 0.1% FBS for the last 24 h, and preincubated with pharmacological inhibitors or vehicle for 1 h. They were then subjected to laminar FSS at 12 dynes/cm2 for 5 min unless noted otherwise, in minimum essential medium with 1 mg/ml fatty acid free bovine serum albumin (Sigma) at 37 °C in a parallel plate flow chamber (Cytodyne Inc., San Diego, CA), as described (6, 36). Shear stress was calculated as described by Stevens and Frangos (36). “Static control” cultures were grown under identical conditions and mounted onto the flow chamber but were not subjected to shear stress.
siRNA Transfections
Sequences targeted by siRNA in the common C terminus of PKG Iα/β or in PKG II were described previously (6). Target sequences in FAK and Src were 5′-TGCAATGGAACGAGTATTAAA-3′ and 5′-CACCACGAGGGTTGCCATCAA-3′, respectively. The oligoribonucleotides, including a control siRNA targeting green fluorescent protein (GFP), were produced by Qiagen. MC3T3 cells were plated at 1.3 × 105 cells/well of a 6-well dish or at 5 × 105 cells/glass slide. Cells were transfected 18 h later at ∼40% confluence with 100 pmol of siRNA and 3 μl of Lipofectamine 2000TM (Invitrogen) in 1 ml of 10% FBS-containing medium. After 24 h, cells were transferred to medium containing 0.1% FBS.
Western Blot Analyses
Western blots were generated using horseradish peroxidase-conjugated secondary antibodies and detected with an enhanced chemiluminescence system as described previously (6). Films in the linear range of exposure were scanned using ImageJ software.
Quantitation of NOx
NO production was measured based on nitrite and nitrate accumulation in the medium using a two-step colorimetric assay kit (6).
BrdU Labeling and Immunofluorescence Staining
hPOBs were plated on glass coverslips, serum-starved for 24 h, treated with pharmacological inhibitors for 1 h, and exposed to orbital FSS (120 rpm) for 1 h; this method of generating FSS induces responses very similar to those of laminar or oscillating FSS produced in a parallel plate flow chamber (18, 37). Cells were fixed and permeabilized and incubated with a β-catenin-specific antibody, followed by a secondary antibody conjugated to AlexaFluor 555; nuclei were counterstained using Hoechst 33342. For BrdU incorporation, cells were exposed to orbital FSS for 10 min and treated with 200 μm BrdU for 18 h; then cells were fixed, permeabilized, and incubated with DNase I prior to staining with anti-BrdU antibody and Hoechst 33342 (18). Cells were viewed with a Leica fluorescent microscope, and images were analyzed as described (18).
Quantitative RT-PCR
Quantitative RT-PCR was performed using an MX3005P real-time PCR detection system (Stratagene); primers for c-fos were described previously (6). Primers for cox-2 were 5′-TCCTCACATCCCTGAGAACC-3′ and 5′-AAGTGGTAACCGCTCAGGTG-3′; primers for osteocalcin were 5′-TGACGAGTTGGCTGACCACATC-3′ and 5′-GCAAGGGGAAGAGGAAAGAAGG-3′. Relative changes in mRNA expression were analyzed using the 2−ΔΔCt method, with glyceraldehyde 3-phosphate dehydrogenase serving as an internal reference.
Statistical Analyses
Pairwise comparison of data groups was done by two-tailed Student's t test, and comparison of multiple groups was done by analysis of variance, with a Bonferroni post-test comparison with the control group; a p < 0.05 was considered statistically significant. Results shown in bar graphs represent the mean ± S.E. of at least three independent experiments, and all other results are for representative experiments reproduced at least three times, unless stated otherwise.
RESULTS
FSS Activation of Akt Requires NO/cGMP/PKG Signaling
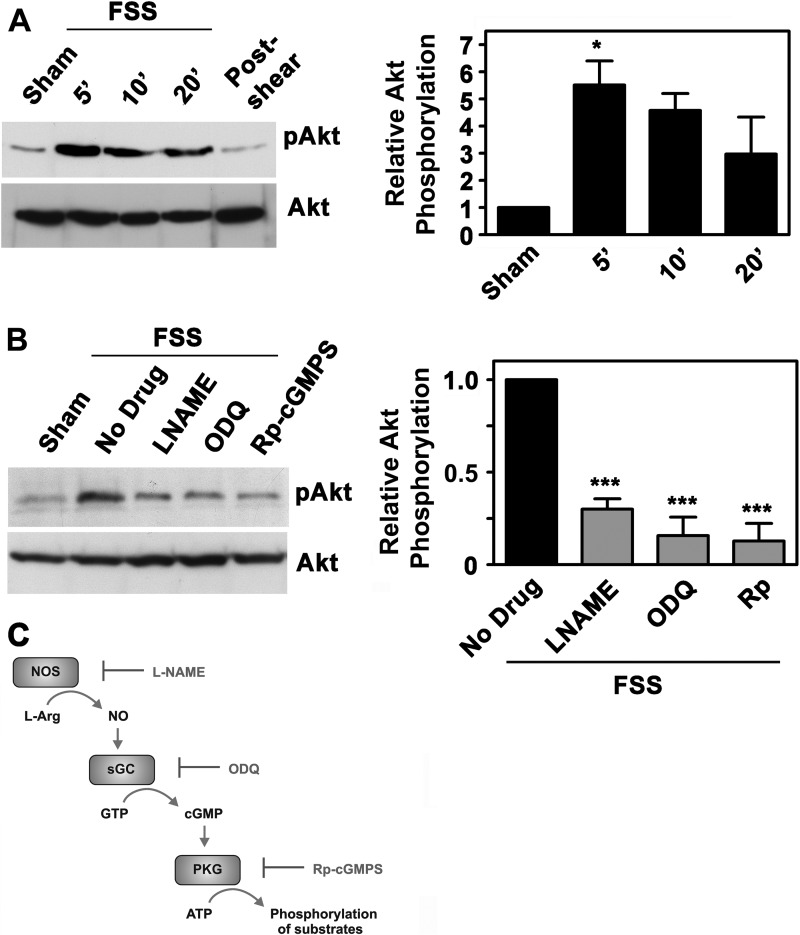
We previously showed that FSS increased NO production, leading to NO/cGMP/PKG II-dependent Erk activation (6, 18). We now asked whether NO/cGMP/PKG signaling plays a role in FSS-induced Akt activation. In hPOBs and murine MC3T3 osteoblast-like cells, laminar FSS increased Akt phosphorylation on an activating site (Ser473) 5–6-fold compared with sham-treated cells; Akt activation occurred within 5 min and declined thereafter (Fig. 1A for hPOBs and supplemental Fig. 1, D and E for MC3T3 cells). When flow was stopped, the amount of phospho-Akt (pAkt) decreased back to basal levels within 10–15 min (in the last lane of Fig. 1A, hPOBs were exposed to FSS for 20 min and harvested at 30 min; in supplemental Fig. 1E, flow was stopped after 5 min, and cells were harvested at 20 min). Although there was some variability in the amount of pAkt at 20 min, the kinetics of Akt phosphorylation were similar in shear-stressed hPOBs and MC3T3 cells.
FIGURE 1.
FSS-induced Akt activation requires NO/cGMP/PKG signaling. A, serum-deprived hPOBs were mounted in a parallel plate flow chamber and kept under static conditions for 30 min (sham-treated; lane 1) or were subjected to laminar FSS at 12 dynes/cm2 for 5 min (lane 2), 10 min (lane 3), or 20 min (lanes 4 and 5); cells in lane 5 were kept in the flow chamber for an additional 10 min after cessation of flow. Western blots were analyzed with antibodies specific for Akt phosphorylated on Ser473 (pAkt; upper panel) or total Akt (lower panel) as described under “Experimental Procedures.” The bar graph on the right represents relative intensities of the pAkt bands normalized to total Akt, as measured by densitometry (mean ± S.E. (error bars) of three independent experiments; *, p < 0.05 for the comparison between sham- and shear-treated cells). B, hPOBs were preincubated for 1 h in the presence of 4 mm l-NAME, 10 μm ODQ, or 100 μm (Rp)-8-pCPT-PET-cGMPS, as indicated, and then exposed to FSS for 5 min or kept static (sham). Relative Akt phosphorylation was measured by Western blotting and densitometry as described in A, but the amount of pAkt in shear-stressed cells preincubated with medium only was assigned a value of 1 (***, p < 0.001 for the comparison between drug-treated cells and cells treated with medium only). C, the NO/cGMP/PKG signaling pathway with pharmacological inhibitors: l-NAME for NO synthase (NOS), ODQ for soluble guanylate cyclase (sGC), and (Rp)-8-pCPT-PET-cGMPS (Rp-cGMPS) for PKG.
Pretreating cells with l-NAME (to inhibit NO synthases; Fig. 1C), ODQ (to inhibit soluble guanylate cyclase), or (Rp)-8-pCPT-PET-cGMPS (to inhibit PKGs) reduced shear-induced Akt phosphorylation by ∼75% (Fig. 1B for hPOBs and supplemental Fig. 1F for MC3T3 cells). We previously optimized inhibitor concentrations and preincubation times to reduce FSS-induced NO production by l-NAME to basal levels and to ensure >95% inhibition of PKG-dependent phosphorylation of vasodilator-stimulated phosphoprotein in shear-stressed osteoblasts by all three inhibitors (6). We conclude that Akt activation by FSS requires signaling through NO/cGMP/PKG.
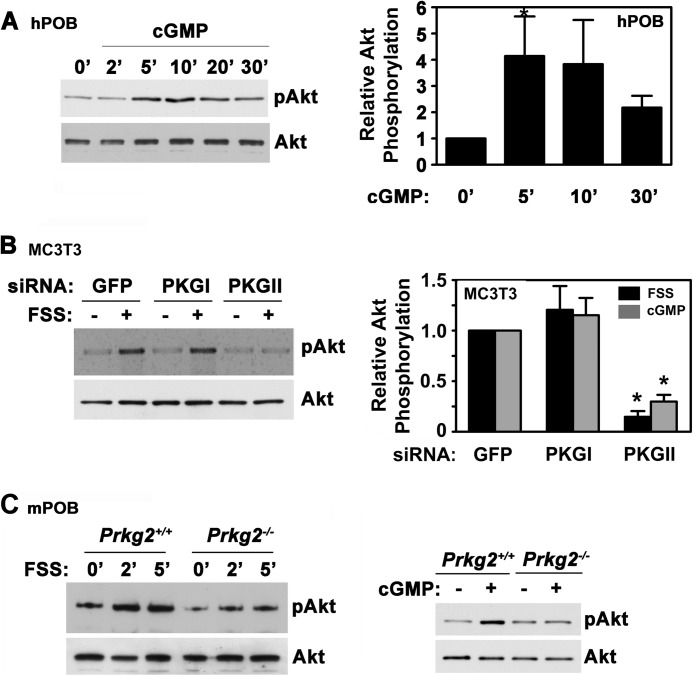
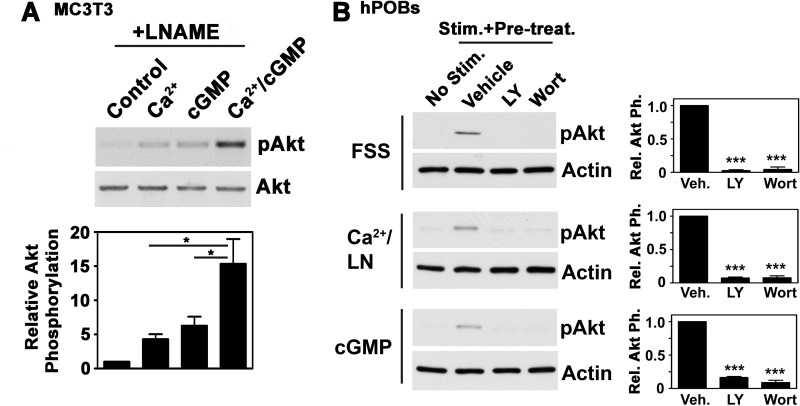
cGMP and FSS Activate Akt through PKG II
To determine whether direct PKG activation can reproduce the effects of FSS on Akt phosphorylation, we treated osteoblasts with the membrane-permeable cGMP agonist 8-pCPT-cGMP and found a 4-fold increase in Akt phosphorylation (Fig. 2A for hPOBs; supplemental Fig. 2A shows similar results in MC3T3 cells). The NO-releasing agent sodium nitroprusside and the direct sGC activator cinaciguat similarly increased Akt phosphorylation in hPOBs (supplemental Fig. 2B).
FIGURE 2.
cGMP and FSS activate Akt through PKG II. A, serum-deprived hPOBs cells were treated with 50 μm 8-pCPT-cGMP for the indicated times, and Akt phosphorylation was assessed as described in the legend to Fig. 1A. The bar graph on the right summarizes three experiments. *, p < 0.05 for the comparison between cells cultured in the absence and presence of cGMP. B, MC3T3 cells were transfected with siRNAs targeting GFP (control), PKG I, or PKG II. After 48 h, cells were either subjected to FSS for 10 min (+, black bars in the graph on the right) or were kept under static conditions (−), as described under “Experimental Procedures.” Some cells were treated with 50 μm 8-pCPT-cGMP (cGMP, gray bars) for 10 min under static conditions. Akt phosphorylation was measured as in Fig. 1A, with the relative amount of pAkt found in FSS- or cGMP-treated cells transfected with GFP siRNA assigned a value of 1 (*, p < 0.05 for the comparison between cells transfected with PKG II siRNA versus GFP siRNA). C, primary calvarial osteoblasts isolated from wild type (Prkg2+/+) or PKG II-deficient (Prkg2−/−) mice were stimulated with either FSS for the indicated times (left panels) or with 100 μm 8-CPT-cGMP for 5 min (right panels). Akt phosphorylation was analyzed as in Fig. 1A (representative of two (for FSS) or three (for cGMP) experiments). Error bars, S.E.
FSS- and cGMP-induced Akt activation was largely blocked in MC3T3 cells transfected with a PKG II-specific siRNA, whereas knockdown of PKG I had no effect (Fig. 2B and supplemental Fig. 2C). Efficient and specific siRNA depletion of PKG I and II was shown in parallel experiments (supplemental Fig. 2D). Calvarial osteoblasts isolated from mice with a homozygous deletion of the PKG II gene showed impaired Akt phosphorylation in response to FSS and cGMP compared with osteoblasts isolated from wild type littermates (Fig. 2C). These results indicate that Akt activation in shear-stressed osteoblasts is mediated by cGMP activation of PKG II.
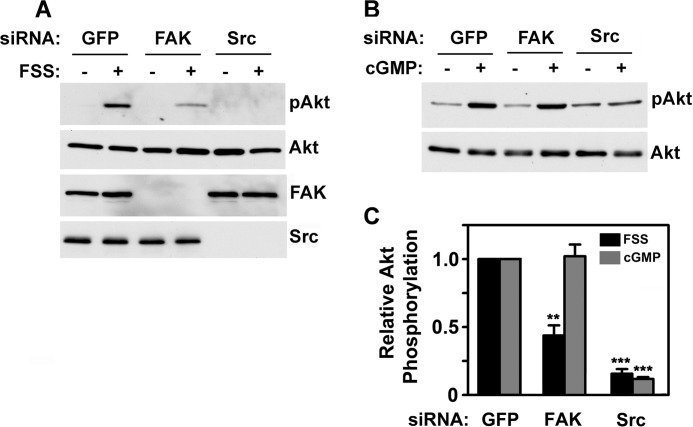
FSS Activation of Akt Requires both Src and FAK, whereas cGMP-induced Akt Activation Requires Src but Is Independent of FAK
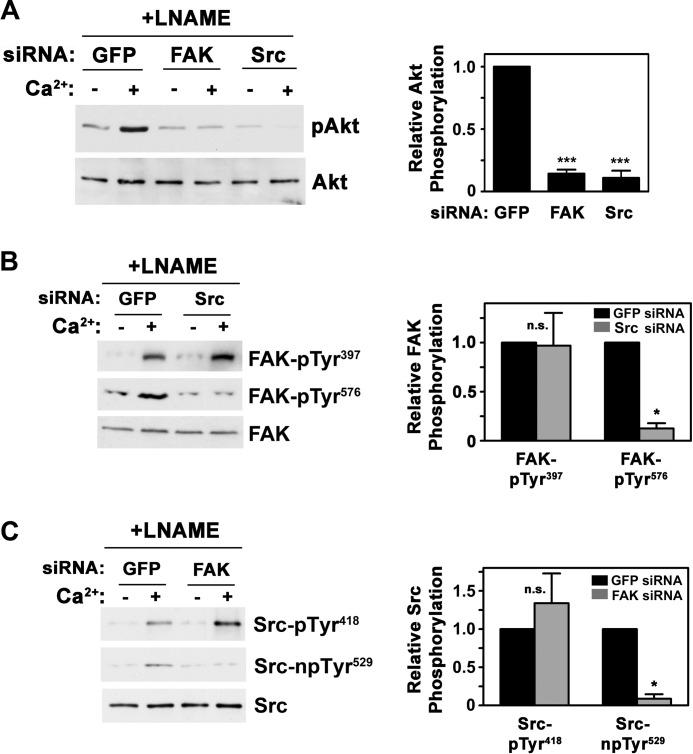
In human osteosarcoma cells, FSS activation of Akt and Erk is blocked by expression of a dominant negative FAK, but the latter may disrupt scaffolding functions of FAK (19, 24, 38); osteoblasts from FAK-deficient mice show reduced FSS-induced Erk phosphorylation, but Akt was not examined (20, 37). We sought to determine the roles of FAK and Src in FSS- and cGMP-induced Akt phosphorylation using an siRNA approach, which allowed specific depletion of each protein (Fig. 3A, bottom two panels).
FIGURE 3.
FSS-induced Akt activation requires both Src and FAK, whereas cGMP-induced Akt activation is FAK-independent. MC3T3 cells were transfected with siRNAs targeting GFP (control), FAK, or Src, as described under “Experimental Procedures.” After 48 h, cells were exposed for 5 min to either FSS (A), or 50 μm 8-pCPT-cGMP (B), and Akt phosphorylation was determined as in Fig. 1A. Western blots showing expression of FAK and Src are shown in the bottom two panels of A. C, the graph summarizes three independent experiments for each treatment, with the relative amount of pAkt found in FSS- or cGMP-treated cells transfected with GFP siRNA assigned a value of 1 (**, p < 0.01 for the comparison between FAK and GFP siRNA-transfected cells; ***, p < 0.001 for the comparison between Src and GFP siRNA-transfected cells). Error bars, S.E.
Depleting MC3T3 cells of FAK reduced FSS-induced Akt phosphorylation by about 50% (Fig. 3, A and C). We found a similar decrease in FSS-induced Erk phosphorylation (supplemental Fig. 3, A and D), confirming previous reports in FAK-deficient primary rodent osteoblasts exposed to FSS (20, 37). In stark contrast, cGMP-induced Akt and Erk phosphorylation were unaffected by FAK knockdown (Fig. 3, B and C; cGMP-induced Erk activation is shown in supplemental Fig. 3, B and D). Depleting cells of Src completely blocked Akt and Erk phosphorylation induced by both FSS and cGMP (Fig. 3, A–C, for Akt, and supplemental Fig. 3, A, B, and D, for Erk). Thus, FSS activation of Akt and Erk proceeds through two pathways, one FAK-dependent and one FAK-independent, with both pathways converging on Src. cGMP/PKG-mediated Akt and Erk activation occurs through the FAK-independent pathway.
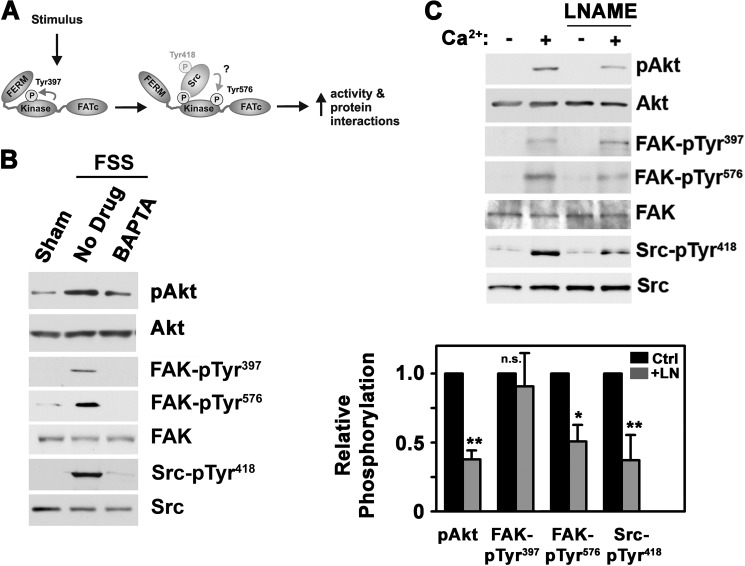
FSS Activates FAK, Src, and Akt in a Calcium-dependent Manner
In response to FSS, FAK is recruited to integrin-containing focal adhesion complexes, but it is controversial whether this increases its activity (19, 20, 24). Upon activation, FAK autophosphorylates on Tyr397, which promotes association with Src and other proteins (22). Full activation of FAK requires phosphorylation on Tyr576 by Src or other kinases, depending on the cell type (Fig. 4A) (22). We found that FSS increased FAK autophosphorylation on Tyr397 and trans-phosphorylation on Tyr576 in addition to stimulating Src autophosphorylation on Tyr418 (Fig. 4B).
FIGURE 4.
FSS activates FAK, Src, and Akt in a calcium-dependent manner, and a calcium ionophore mimics the effects of FSS. A, model depicting FAK activation by various stimuli, resulting in sequential autophosphorylation on Tyr397, recruitment of Src to this site, and phosphorylation of FAK Tyr576 by Src or other kinases. B, hPOBs were exposed to 5 min of FSS or kept static; some cells were pretreated for 1 h with vehicle or 10 μm BAPTA-AM. Western blots were analyzed with antibodies specific for pAkt, Akt, FAK autophosphorylated on Tyr397, FAK phosphorylated on Tyr576, total FAK, Src autophosphorylated on Tyr418, and total Src. C, hPOBs were preincubated for 1 h in the presence or absence of 4 mm l-NAME prior to receiving 0.3 μm A23187 (Ca2+) for 5 min. Akt, FAK, and Src phosphorylation were assessed as in B. The bar graph below summarizes three experiments; black bars represent calcium plus vehicle treatment (Ctrl), and gray bars represent cells treated with calcium plus l-NAME (LN). *, p < 0.05; **, p < 0.01 for the comparison between cells treated with calcium in the absence or presence of l-NAME. pTyr, phosphotyrosine; Error bars, S.E.
Because FSS rapidly increases intracellular [Ca2+] in primary osteoblasts and MC3T3 cells (7, 8, 39) and increased [Ca2+] is required for FSS-induced Erk activation (6, 10), we asked if FSS-induced Akt, FAK, and Src activation also require calcium. When hPOBs were treated with the intracellular calcium chelator BAPTA-AM, shear-induced Akt, FAK, and Src phosphorylation were largely prevented (Fig. 4B; similar results were obtained in MC3T3 cells (data not shown)). BAPTA-AM had no effect on cGMP-induced Akt phosphorylation, excluding a nonspecific toxic effect (supplemental Fig. 4A). Thus, FSS activation of FAK, Src, and Akt requires intracellular calcium.
Calcium Mimics Effects of FSS on Akt, FAK, and Src
To examine calcium effects independently of mechanical stimulation, we used the calcium ionophore A23187 to increase intracellular [Ca2+]. Consistent with previous results (12), we found that A23187 stimulated NO synthesis, but treating cells with l-NAME prior to A23187 maintained NO concentrations at basal levels, allowing us to examine calcium effects in the absence and presence of NO synthase activation (supplemental Fig. 4B).
Treating hPOBs with A23187 without l-NAME increased Akt phosphorylation, FAK and Src autophosphorylation, and FAK phosphorylation on Tyr576, consistent with calcium-dependent effects of FSS (Fig. 4C). FAK autophosphorylation was the same in the absence or presence of l-NAME, but calcium-induced Akt phosphorylation, Src autophosphorylation, and FAK phosphorylation on Tyr576 were reduced by l-NAME (Fig. 4C, compare lanes 2 and 4); these results were consistent with Ca2+/NO/cGMP/PKG-dependent Akt and Src activation (Figs. 1 and 2) (18). Similar results were obtained in MC3T3 cells (supplemental Fig. 4, C and D). These data indicate that calcium activates Src and Akt through both NO-dependent and -independent mechanisms and that calcium-induced FAK autophosphorylation is NO-independent.
Calcium-induced Akt Activation Requires FAK and Src
The differential sensitivity of FSS- and cGMP-induced Akt phosphorylation to FAK depletion suggested a FAK-dependent but cGMP-independent mechanism of Akt activation during FSS; therefore, we asked whether calcium activates Akt through FAK. Treating osteoblasts with A23187 in the presence of l-NAME increased Akt phosphorylation in control siRNA-transfected cells, but this effect was completely blocked in cells transfected with FAK- or Src-specific siRNAs (Fig. 5A). Supplemental Fig. 3, C and D, shows similar results for calcium-induced Erk phosphorylation. Thus, FAK is required for Akt and Erk activation by calcium via an NO/cGMP-independent pathway.
FIGURE 5.
Calcium-induced Akt activation requires FAK and Src; full FAK activation by calcium requires Src, and vice versa. A, MC3T3 cells were transfected with siRNAs targeting GFP, FAK, or Src; 48 h later, they were treated for 1 h with 4 mm l-NAME prior to receiving 0.3 μm A23187 (Ca2+) for 5 min as indicated. pAkt was quantified as described in the legend to Fig. 3C (***, p < 0.001 for the comparison with GFP siRNA-transfected cells). B and C, MC3T3 cells were transfected with siRNAs targeting GFP and Src (B) or FAK (C) and were treated with l-NAME and A23187 as described in A. Phosphorylation of FAK (B) and Src (C) was assessed as described in the legend to Fig. 4B (*, p < 0.05 for the comparison with GFP siRNA-transfected cells). n.s., non-significant. pTyr, phosphotyrosine. npTyr, non-phosphorylated tyrosine. Error bars, S.E.
Full FAK Activation by Calcium Requires Src and Vice Versa
Because calcium-induced Akt activation in the presence of l-NAME was completely blocked by depletion of either FAK or Src, we asked whether FAK and Src activation by calcium were interdependent. Again, osteoblasts were treated with A23187 plus l-NAME to increase the intracellular calcium concentration without stimulating NO synthesis. Calcium-induced FAK phosphorylation on Tyr576 was prevented when Src was depleted by siRNA (Fig. 5B) or inhibited by the Src family kinase inhibitor PP2 (supplemental Fig. 5A), indicating that FAK Tyr576 is targeted by Src in osteoblasts. However, FAK autophosphorylation on Tyr397 was independent of Src (Fig. 5B and supplemental Fig. 5A). Thus, full FAK activation by calcium requires Src-mediated phosphorylation of FAK Tyr576, although calcium induces FAK autophosphorylation in the absence of Src.
Full Src activation requires dephosphorylation of Src at Tyr529 (40). Depleting cells of FAK prevented Src dephosphorylation on Tyr529 in cells treated with A23187 and l-NAME, although calcium-induced Src autophosphorylation was not decreased (Fig. 5C).
Thus, full Src activation by calcium requires FAK. In contrast, treating cells with 8-CPT-cGMP resulted in both Src autophosphorylation of Tyr418 and dephosphorylation on Tyr529 in FAK-depleted cells, indicating full Src activation, consistent with FAK-independent Akt activation by cGMP (supplemental Fig. 5B). We previously showed that cGMP/PKG II directly activates the Shp-1/2 phosphatase complex, which dephosphorylates Src on the inhibitory Tyr529 (19).
Calcium and cGMP Cooperate to Activate Akt
To determine if calcium and cGMP cooperate in activating Akt, we treated cells with the calcium ionophore A23187 and a membrane-permeable cGMP analog in the presence of l-NAME. We found an at least additive effect on Akt phosphorylation (Fig. 6A). Because FSS-induced Akt phosphorylation required calcium (Fig. 4B) and was largely blocked by inhibiting NO/cGMP synthesis (Fig. 1B), these results suggest that calcium cooperates with NO/cGMP to induce Akt phosphorylation during FSS.
FIGURE 6.
Calcium and cGMP cooperate to activate Akt, and Akt activation by FSS, calcium, or cGMP requires PI3K. A, MC3T3 cells were pretreated for 1 h with 4 mm l-NAME and then received either vehicle, calcium ionophore (0.3 μm A23187), cGMP (100 μm 8-pCPT-cGMP), or both agents for 10 min. Akt phosphorylation was measured by densitometry, and the amount of pAkt in vehicle-treated cells was assigned a value of 1 (*, p < 0.05). B, hPOBs were pretreated for 1 h with vehicle, 10 μm LY294002 (LY), or 30 nm wortmannin (Wort); cells shown in the middle two panels additionally received 4 mm l-NAME (LN). Cells shown in lanes 2–4 were then exposed for 5 min to the indicated stimulus (FSS in the top two panels, 0.3 μm A23187 in the middle two panels, and 100 μm 8-pCPT-cGMP in the bottom two panels). Western blots developed with β-actin antibody served as a loading control. pAkt was quantified as described in the legend to Fig. 3C (***, p < 0.001 for the comparison with vehicle-pretreated cells). Error bars, S.E.
Akt Activation by FSS, Calcium, or cGMP Requires PI3K
Previous work in osteocarcoma cells suggests that FSS-induced Akt activation occurs through PI3K, which associates with FAK (19), but other workers found PI3K-independent Akt phosphorylation in rat primary osteoblasts exposed to FSS (26). We found that two structurally unrelated PI3K inhibitors (LY294002 and wortmannin) completely blocked shear-induced Akt phosphorylation in hPOBs (Fig. 6B). Similarly, calcium- and cGMP-induced Akt phosphorylation were dependent on PI3K activity (Fig. 6B). Thus, FSS-induced Akt activation by both calcium and cGMP requires PI3K.
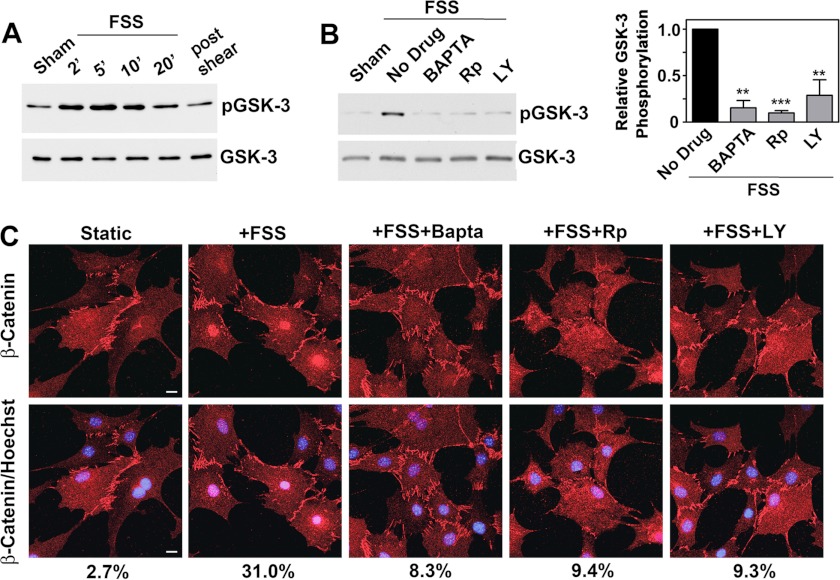
FSS-induced GSK-3 Inhibition and Nuclear Translocation of β-Catenin Are Dependent on Calcium, cGMP/PKG, and PI3K/Akt
Wnt/β-catenin signaling is essential for the anabolic response to mechanical loading in vivo and in vitro, and β-catenin regulates osteoblast differentiation and survival (27, 30, 41). FSS induces nuclear translocation of β-catenin and enhances β-catenin transcriptional activity, but the role of Akt in FSS-induced β-catenin regulation has not been clearly defined (33). Akt can stabilize β-catenin by phosphorylating and inactivating GSK-3; Akt can also directly phosphorylate β-catenin, leading to nuclear translocation in different cell types (41, 42).
FSS rapidly increased GSK-3β phosphorylation on the inhibitory site (Ser9) targeted by Akt (Fig. 7A and supplemental Fig. 6A). Chelating intracellular calcium with BAPTA, inhibiting PKG with (Rp)-8-pCPT-PET-cGMPS, or blocking PI3K with LY294002 largely prevented shear-induced GSK-3 phosphorylation and β-catenin nuclear translocation (Fig. 7, B and C). Thus, FSS-induced β-catenin nuclear translocation requires signaling through calcium, NO/cGMP/PKG, and PI3K/Akt.
FIGURE 7.
FSS-induced GSK-3 inhibition and nuclear translocation of β-catenin require calcium, cGMP/PKG, and PI3K/Akt. A, hPOBs were kept static or were exposed to FSS for the indicated times, as described in the legend to Fig. 1A. Western blots were analyzed with antibodies specific for GSK-3β phosphorylated on Ser9 (inhibitory phosphorylation site; top) or total GSK-3β (bottom). B, hPOBs were pretreated for 1 h with vehicle, 10 μm BAPTA-AM, 100 μm (Rp)-8-pCPT-PET-cGMPS (Rp), or 10 μm LY294002 (LY), as indicated, and were then exposed to 5 min of FSS. GSK-3β phosphorylation was assessed as in A. The graph on the right summarizes three experiments. **, p < 0.01; ***, p < 0.001 for the comparison between the absence and presence of each inhibitor. C, MC3T3 cells were pretreated as in B and exposed to FSS for 1 h prior to detecting total β-catenin by immunofluorescence staining. In the bottom panels, DNA was counterstained with Hoechst 33342. Scale bar, 5 μm. Numbers below indicate the percentage of cells showing nuclear staining for β-catenin; the experiment was repeated two times with similar results. Similar results were also obtained with hPOBs. Error bars, S.E.
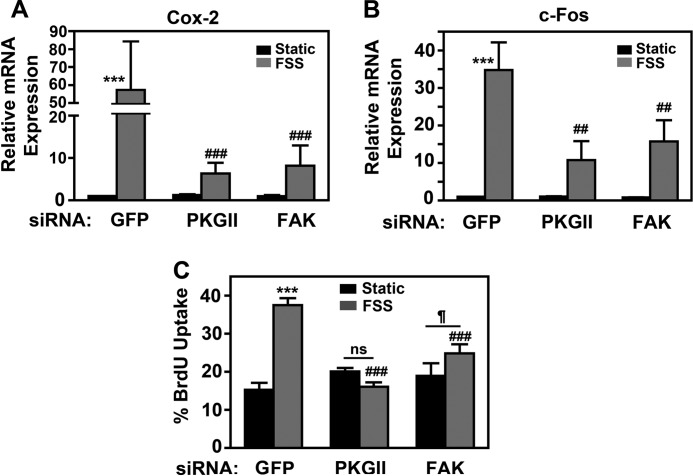
PKG II and FAK Are Both Required for FSS-induced Gene Expression and Osteoblast Proliferation
To determine the relative importance of PKG II and FAK signaling in shear-stressed osteoblasts, we measured the effects of PKG II or FAK depletion on FSS-induced gene expression and osteoblast proliferation. We examined cyclooxygenase-2 (cox-2) and c-fos, genes important for the anabolic response to FSS downstream of Erk, Akt, and β-catenin (1–5). Using PKG II and FAK siRNAs with comparable knockdown efficiencies (Fig. 3A and supplemental Fig. 2D), we found that PKG II or FAK depletion each reduced FSS-induced cox-2 and c-fos mRNA expression by ∼85 and 70%, respectively (Fig. 8, A and B). PKG II or FAK depletion also inhibited FSS-induced osteoblast proliferation as measured by BrdU incorporation into DNA; FAK-deficient cells showed a small, residual proliferative response to FSS, whereas PKG II-deficient cells were completely unresponsive (Fig. 8C and supplemental Fig. 6B). We conclude that both PKG II and FAK have major, equally important effects on regulating gene expression in shear-stressed osteoblasts but that PKG II plays a greater role in regulating proliferation; both pathways are required for maximal anabolic effects of FSS.
FIGURE 8.
PKG II and FAK are both required for FSS-induced gene expression and osteoblast proliferation. A and B, MC3T3 cells were transfected with siRNAs targeting GFP, PKG II, or FAK, and 48 h later they were either kept static (black bars) or exposed to FSS for 1 h (gray bars). Expression of cox-2 (A) or c-fos (B) mRNA was quantified by real-time RT-PCR and normalized to glycerol 3-phosphate dehydrogenase mRNA as described under “Experimental Procedures”; relative cox-2 or c-fos mRNA levels measured in static, GFP siRNA-transfected cells were assigned a value of 1. C, MC3T3 cells were transfected as in A and B and exposed to FSS for 10 min prior to receiving BrdU for 18 h. BrdU incorporation into DNA was detected by immunofluorescence, with >300 cells analyzed/condition. A–C, ***, p < 0.001 for the comparison between static and shear-stressed cells. ###, p < 0.001; ##, p < 0.01 for the comparison with shear-stressed cells transfected with GFP siRNA. ¶, p < 0.05. ns, non-significant.
DISCUSSION
Akt and Erk activation are essential for the anabolic response of bone to mechanical loading; both pathways are required for osteoblast proliferation and differentiation (2, 5, 18, 19). Both pathways relay signals from mechanosensors present at the plasma membrane (e.g. integrins and mechanosensitive calcium channels) to the nucleus, where they induce changes in gene expression.
A rapid increase in intracellular [Ca2+] induced by FSS and other mechanical stimuli is one of the earliest events in osteoblast mechanotransduction and appears to be required for most downstream signaling events, including stimulation of NO synthesis and changes in gene expression (5, 6, 8–10, 12). We found that FSS, by increasing intracellular [Ca2+], induced NO/cGMP/PKG II-dependent Akt and Erk activation via Src. In addition, we found that calcium activated Akt and Erk through a second, NO/cGMP-independent pathway involving Src and FAK (Fig. 9). The NO/cGMP/PKG II-dependent pathway can be explained by PKG II directly regulating the phosphatases Shp-1/2, which dephosphorylate the inhibitory Tyr529 of Src (18); this allows full Src activation by cGMP/PKG II in the absence of FAK (supplemental Fig. 5B). In contrast, Src activation via the NO-independent pathway was incomplete in the absence of FAK, lacking Src dephosphorylation on Tyr529. FSS and calcium increased FAK autophosphorylation, indicating partial FAK activation (22). FAK autophosphorylation recruits Src to FAK, and we found FSS- and calcium-induced trans-phosphorylation of FAK Tyr576 by Src. This will further enhance FAK activity and induce FAK association with other proteins involved in activation of the PI3K/Akt and Ras/Raf/MEK/Erk pathways (22). siRNA-mediated depletion of FAK reduced FSS-induced Akt and Erk phosphorylation by about half, similar to the partial loss of shear-induced Erk activation in osteoblasts from FAK-deficient mice reported previously (20, 37). In contrast, siRNA-mediated depletion of Src completely blocked shear-induced Akt and Erk phosphorylation, indicating that Src serves as a nodal point for both PKG II and FAK, leading to downstream activation of Akt and Erk. Our findings connect the mechanically induced increase in intracellular [Ca2+] with activation of FAK and Src; they are consistent with reports of calcium-dependent Src activation in shear-stressed endothelial cells (43) and reports of local increases in [Ca2+] triggering FAK autophosphorylation and association with focal adhesion complexes in neuronal cells (44). We propose a model in which the FSS-induced increase in intracellular [Ca2+] directly activates FAK and Src and indirectly activates Src via NO/cGMP/PKG II regulation of Shp-1/2 (Fig. 9). Both pathways cooperate to activate Akt and Erk, resulting in osteoblast proliferation and changes in gene expression. In a positive feedback loop, increased Akt activity may increase phosphorylation and activity of endothelial NO synthase, leading to a prolonged increase in NO production, as shown previously (6, 12, 45).
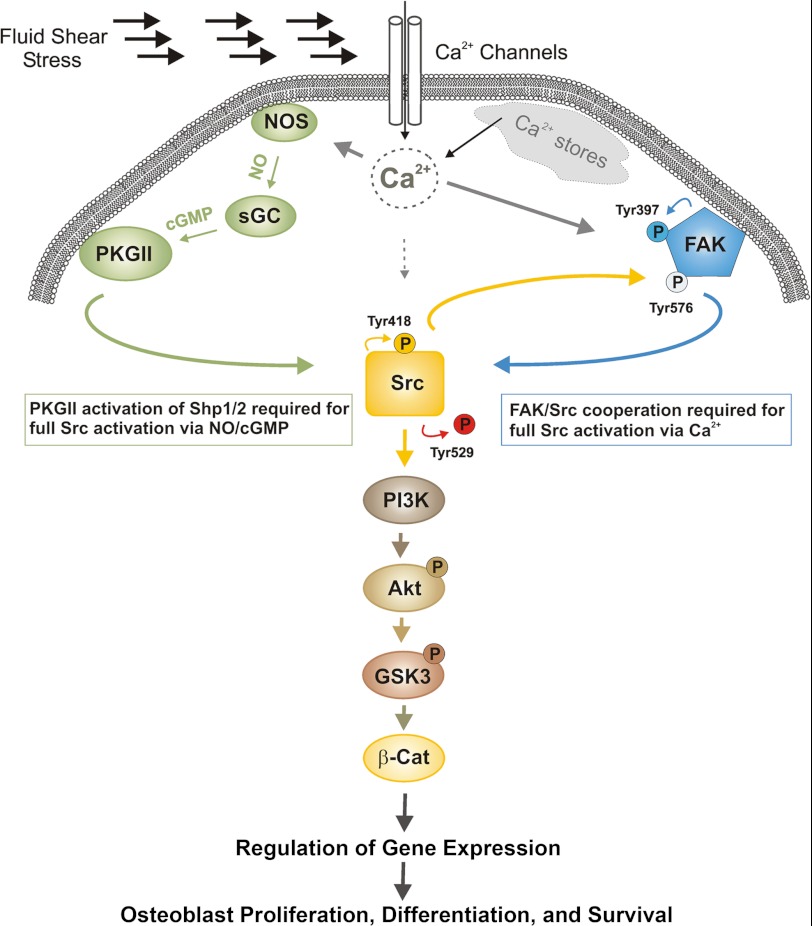
FIGURE 9.
Model of PI3K/Akt/GSK-3/β-catenin pathway activation in fluid shear-stressed osteoblasts. FSS stimulates calcium influx through membrane channels and release from intracellular stores. Calcium stimulates NO synthase (NOS) and activates Akt via NO/cGMP/PKG II-dependent activation of Src; the latter occurs through dephosphorylation of the inhibitory Tyr529 on Src by the Shp-1/2 phosphatase complex (18), and Src then activates PI3K independently of FAK. Calcium also activates Akt through a second, NO-independent pathway that proceeds through FAK and Src; autophosphorylated FAK recruits Src, and Src phosphorylates FAK on Tyr576; Src and FAK cooperate to activate PI3K and Akt. Akt phosphorylates and inhibits GSK-3, thereby releasing the inhibitory effect of GSK-3 on β-catenin (β-Cat); this leads to β-catenin nuclear translocation and transcriptional regulation of genes involved in osteoblast proliferation, differentiation, and survival (27).
Integrin-based cell adhesion complexes are important mechanosensors on the cell surface (46, 47). FSS activates osteoblast β1 and β3 integrins, resulting in integrin clustering and recruitment of various signaling molecules, including FAK and Src, to integrin-containing focal adhesion complexes (4, 5, 18). FSS induces association of FAK, the adaptor protein Shc, and the regulatory subunit of PI3K with β3 integrins in osteoblasts and other mechanosensitive cells (19, 48–50). Concomitantly, FSS induces activation of Src associated with β3 integrins in osteoblasts through PKG II regulation of Shp-1/2 (18). Src phosphorylates Shc, thereby activating PI3K (48, 49, 51). In the basal state, PI3K activity is suppressed by binding of the regulatory to the catalytic subunit; this inhibition is relieved when the PI3K regulatory subunit binds to Tyr(P) residues on FAK, Src, and adaptor proteins, such as Shc, and the enzyme is recruited to the plasma membrane (25). There, PI3K mediates Akt activation by producing phosphatidylinositol 3,4,5-triphosphate, which in turn leads to recruitment of Akt together with its activating kinases PDK1 and mTORC-2 to the plasma membrane (25). We found that Src activation by NO/cGMP was FAK-independent and that full FAK activation by calcium required Src; therefore, it appears that Src and FAK function together in a non-redundant fashion in osteoblast mechanotransduction.
Stabilization and nuclear translocation of β-catenin occurs in primary and immortalized rodent osteoblasts and osteocyte-like cells exposed to FSS or four-point bending (30, 33, 52). Osteoblasts and osteocytes differ in their sensitivity to FSS or stretching and demonstrate different kinetics of β-catenin nuclear translocation, but both cell types respond to mechanical stimulation with an increase in intracellular calcium and NO production (53–57). Nuclear β-catenin together with its binding partner Lef1 regulates genes in osteoblasts and osteocytes necessary for maintenance of normal bone mass in vivo, including cox-2, and control osteoblast as well as osteoclast differentiation and survival (27–30, 41, 58). We found that nuclear translocation of β-catenin in shear-stressed osteoblasts required calcium- and NO/cGMP/PKG-dependent PI3K/Akt signaling, placing this pathway parallel to calcium- and NO/cGMP/PKG-dependent Erk activation, which regulates fos family gene expression and osteoblast proliferation (6, 18). Kamel et al. (53) found that cox-2 induction and prostaglandin E2 (PGE2) synthesis in fluid shear-stressed osteoblasts and osteocytes correlate with β-catenin nuclear translocation, and other workers showed that FSS-induced cox-2 expression and PGE2 synthesis are dependent on calcium and NO signals (8, 59). Thus, increased PGE2 synthesis may contribute to activation of PI3K/Akt/GSK-3/β-catenin signaling in shear-stressed osteoblasts and osteocytes, especially at later time points. The skeletal phenotypes of mice with genetic alterations in the PI3K/Akt/GSK-3 pathway confirm the importance of this pathway for regulating bone formation and stimulating osteoblast differentiation (58, 60, 61).
In conclusion, our work provides a mechanism for Akt activation by FSS, involving increased intracellular [Ca2+] activating two parallel pathways that converge on Src. One pathway requires NO/cGMP/PKG II, and the other requires FAK. Whether the same pool or different pools of Src are activated by the two pathways remains to be seen. Either way, we have defined two mechanisms of FSS activation of the PI3K/Akt/GSK-3/β-catenin signaling module, which is essential for the maintenance of skeletal integrity. Because preclinical and clinical studies support osteogenic functions of NO (62, 63), our results provide a rationale for using PKG-activating drugs as “mechano-mimetics” in treating osteoporosis.
Acknowledgments
We thank Drs. S. Ball, W. Bugbee, G. Firestein, and D. Boyle for providing operative bone specimens and A. Pfeifer and B. Haas for providing primary osteoblasts from PKG II-deficient mice.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-AR051300 (to R. B. P., H. R., and S. Z.).

This article contains supplemental Figs. 1–6.
- FSS
- fluid shear stress
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethylester)
- FAK
- focal adhesion kinase
- GSK
- glycogen synthase kinase
- hPOB
- human primary osteoblast
- l-NAME
- N-nitro-l-arginine methyl ester
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- 8-pCPT-cGMP
- 8-(4-chlorophenylthio)- guanosine-3′,5′-cyclic monophosphate
- PKG
- cGMP-dependent protein kinase
- (Rp)-8-pCPT-PET-cGMPS
- 8-(4-chlorophenylthio)-β-phenyl-1,N2-ethenoguanosine-3′,5′-cyclic monophosphorothioate (Rp isomer)
- pAkt
- phospho-Akt
- PGE2
- prostaglandin E2.
REFERENCES
- 1. Ozcivici E., Luu Y. K., Adler B., Qin Y. X., Rubin J., Judex S., Rubin C. T. (2010) Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol. 6, 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hughes-Fulford M. (2004) Signal transduction and mechanical stress. Sci. STKE 2004, RE12. [DOI] [PubMed] [Google Scholar]
- 3. Riddle R. C., Donahue H. J. (2009) From streaming potentials to shear stress. 25 years of bone cell mechanotransduction. J. Orthop. Res. 27, 143–149 [DOI] [PubMed] [Google Scholar]
- 4. Turner C. H., Warden S. J., Bellido T., Plotkin L. I., Kumar N., Jasiuk I., Danzig J., Robling A. G. (2009) Mechanobiology of the skeleton. Sci. Signal. 2, pt3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papachristou D. J., Papachroni K. K., Basdra E. K., Papavassiliou A. G. (2009) Signaling networks and transcription factors regulating mechanotransduction in bone. BioEssays 31, 794–804 [DOI] [PubMed] [Google Scholar]
- 6. Rangaswami H., Marathe N., Zhuang S., Chen Y., Yeh J. C., Frangos J. A., Boss G. R., Pilz R. B. (2009) Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J. Biol. Chem. 284, 14796–14808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hung C. T., Allen F. D., Pollack S. R., Brighton C. T. (1996) Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J. Biomech. 29, 1411–1417 [DOI] [PubMed] [Google Scholar]
- 8. Chen N. X., Ryder K. D., Pavalko F. M., Turner C. H., Burr D. B., Qiu J., Duncan R. L. (2000) Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am. J. Physiol. Cell Physiol. 278, C989–C997 [DOI] [PubMed] [Google Scholar]
- 9. You J., Reilly G. C., Zhen X., Yellowley C. E., Chen Q., Donahue H. J., Jacobs C. R. (2001) Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J. Biol. Chem. 276, 13365–13371 [DOI] [PubMed] [Google Scholar]
- 10. Liu D., Genetos D. C., Shao Y., Geist D. J., Li J., Ke H. Z., Turner C. H., Duncan R. L. (2008) Activation of extracellular signal-regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3-E1 osteoblasts. Bone 42, 644–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klein-Nulend J., Helfrich M. H., Sterck J. G., MacPherson H., Joldersma M., Ralston S. H., Semeins C. M., Burger E. H. (1998) Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem. Biophys. Res. Commun. 250, 108–114 [DOI] [PubMed] [Google Scholar]
- 12. McAllister T. N., Frangos J. A. (1999) Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J. Bone Miner. Res. 14, 930–936 [DOI] [PubMed] [Google Scholar]
- 13. Rawlinson S. C., Pitsillides A. A., Lanyon L. E. (1996) Involvement of different ion channels in osteoblasts' and osteocytes' early responses to mechanical strain. Bone 19, 609–614 [DOI] [PubMed] [Google Scholar]
- 14. Fox S. W., Chambers T. J., Chow J. W. (1996) Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am. J. Physiol. 270, E955–E960 [DOI] [PubMed] [Google Scholar]
- 15. Chow J. W., Fox S. W., Lean J. M., Chambers T. J. (1998) Role of nitric oxide and prostaglandins in mechanically induced bone formation. J. Bone Miner. Res. 13, 1039–1044 [DOI] [PubMed] [Google Scholar]
- 16. Watanuki M., Sakai A., Sakata T., Tsurukami H., Miwa M., Uchida Y., Watanabe K., Ikeda K., Nakamura T. (2002) Role of inducible nitric-oxide synthase in skeletal adaptation to acute increases in mechanical loading. J. Bone Miner. Res. 17, 1015–1025 [DOI] [PubMed] [Google Scholar]
- 17. Bergula A. P., Haidekker M. A., Huang W., Stevens H. Y., Frangos J. A. (2004) Venous ligation-mediated bone adaptation is NOS3-dependent. Bone 34, 562–569 [DOI] [PubMed] [Google Scholar]
- 18. Rangaswami H., Schwappacher R., Marathe N., Zhuang S., Casteel D. E., Haas B., Chen Y., Pfeifer A., Kato H., Shattil S., Boss G. R., Pilz R. B. (2010) Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci. Signal. 3, ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee D. Y., Li Y. S., Chang S. F., Zhou J., Ho H. M., Chiu J. J., Chien S. (2010) Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by αvβ3 and β1 integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6K pathway. J. Biol. Chem. 285, 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young S. R., Gerard-O'Riley R., Kim J. B., Pavalko F. M. (2009) Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J. Bone Miner. Res. 24, 411–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Howe A. K., Aplin A. E., Juliano R. L. (2002) Anchorage-dependent ERK signaling. Mechanisms and consequences. Curr. Opin. Genet. Dev. 12, 30–35 [DOI] [PubMed] [Google Scholar]
- 22. Mitra S. K., Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 23. Li S., Kim M., Hu Y. L., Jalali S., Schlaepfer D. D., Hunter T., Chien S., Shyy J. Y. (1997) Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J. Biol. Chem. 272, 30455–30462 [DOI] [PubMed] [Google Scholar]
- 24. Wang B., Du T., Wang Y., Yang C., Zhang S., Cao X. (2011) Focal adhesion kinase signaling pathway is involved in mechanotransduction in MG-63 cells. Biochem. Biophys. Res. Commun. 410, 671–676 [DOI] [PubMed] [Google Scholar]
- 25. Manning B. D., Cantley L. C. (2007) AKT/PKB signaling. Navigating downstream. Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pavalko F. M., Gerard R. L., Ponik S. M., Gallagher P. J., Jin Y., Norvell S. M. (2003) Fluid shear stress inhibits TNF-α-induced apoptosis in osteoblasts. A role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J. Cell Physiol. 194, 194–205 [DOI] [PubMed] [Google Scholar]
- 27. Westendorf J. J., Kahler R. A., Schroeder T. M. (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341, 19–39 [DOI] [PubMed] [Google Scholar]
- 28. Glass D. A., 2nd, Bialek P., Ahn J. D., Starbuck M., Patel M. S., Clevers H., Taketo M. M., Long F., McMahon A. P., Lang R. A., Karsenty G. (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 [DOI] [PubMed] [Google Scholar]
- 29. Galli C., Passeri G., Macaluso G. M. (2010) Osteocytes and WNT. The mechanical control of bone formation. J. Dent. Res. 89, 331–343 [DOI] [PubMed] [Google Scholar]
- 30. Lau K. H., Kapur S., Kesavan C., Baylink D. J. (2006) Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J. Biol. Chem. 281, 9576–9588 [DOI] [PubMed] [Google Scholar]
- 31. Robinson J. A., Chatterjee-Kishore M., Yaworsky P. J., Cullen D. M., Zhao W., Li C., Kharode Y., Sauter L., Babij P., Brown E. L., Hill A. A., Akhter M. P., Johnson M. L., Recker R. R., Komm B. S., Bex F. J. (2006) Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 281, 31720–31728 [DOI] [PubMed] [Google Scholar]
- 32. Zhao X., Zhuang S., Chen Y., Boss G. R., Pilz R. B. (2005) Cyclic GMP-dependent protein kinase regulates CCAAT enhancer-binding protein β functions through inhibition of glycogen synthase kinase-3. J. Biol. Chem. 280, 32683–32692 [DOI] [PubMed] [Google Scholar]
- 33. Norvell S. M., Alvarez M., Bidwell J. P., Pavalko F. M. (2004) Fluid shear stress induces β-catenin signaling in osteoblasts. Calcif. Tissue Int. 75, 396–404 [DOI] [PubMed] [Google Scholar]
- 34. Dillon J. P., Waring-Green V. J., Taylor A. M., Wilson P. J., Birch M., Gartland A., Gallagher J. A. (2012) Primary human osteoblast cultures. Methods Mol. Biol. 816, 3–18 [DOI] [PubMed] [Google Scholar]
- 35. Ponik S. M., Pavalko F. M. (2004) Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. J. Appl. Physiol. 97, 135–142 [DOI] [PubMed] [Google Scholar]
- 36. Stevens H. Y., Frangos J. A. (2003) Bone cell responses to fluid flow. Methods Mol. Med. 80, 381–398 [DOI] [PubMed] [Google Scholar]
- 37. Young S. R., Hum J. M., Rodenberg E., Turner C. H., Pavalko F. M. (2011) Non-overlapping functions for Pyk2 and FAK in osteoblasts during fluid shear stress-induced mechanotransduction. PLoS One 6, e16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carragher N. O., Westhoff M. A., Fincham V. J., Schaller M. D., Frame M. C. (2003) A novel role for FAK as a protease-targeting adaptor protein. Regulation by p42 ERK and Src. Curr. Biol. 13, 1442–1450 [DOI] [PubMed] [Google Scholar]
- 39. Jacobs C. R., Yellowley C. E., Davis B. R., Zhou Z., Cimbala J. M., Donahue H. J. (1998) Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 31, 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roskoski R., Jr. (2005) Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 331, 1–14 [DOI] [PubMed] [Google Scholar]
- 41. Almeida M., Han L., Bellido T., Manolagas S. C., Kousteni S. (2005) Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 280, 41342–41351 [DOI] [PubMed] [Google Scholar]
- 42. Fang D., Hawke D., Zheng Y., Xia Y., Meisenhelder J., Nika H., Mills G. B., Kobayashi R., Hunter T., Lu Z. (2007) Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J. Biol. Chem. 282, 11221–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Okuda M., Takahashi M., Suero J., Murry C. E., Traub O., Kawakatsu H., Berk B. C. (1999) Shear stress stimulation of p130cas tyrosine phosphorylation requires calcium-dependent c-Src activation. J. Biol. Chem. 274, 26803–26809 [DOI] [PubMed] [Google Scholar]
- 44. Giannone G., Rondé P., Gaire M., Beaudouin J., Haiech J., Ellenberg J., Takeda K. (2004) Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J. Biol. Chem. 279, 28715–28723 [DOI] [PubMed] [Google Scholar]
- 45. Fleming I. (2010) Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 459, 793–806 [DOI] [PubMed] [Google Scholar]
- 46. Schwartz M. A. (2009) Cell biology. The force is with us. Science 323, 588–589 [DOI] [PubMed] [Google Scholar]
- 47. Geiger B., Spatz J. P., Bershadsky A. D. (2009) Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 [DOI] [PubMed] [Google Scholar]
- 48. Chen K. D., Li Y. S., Kim M., Li S., Yuan S., Chien S., Shyy J. Y. (1999) Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J. Biol. Chem. 274, 18393–18400 [DOI] [PubMed] [Google Scholar]
- 49. Tzima E., Irani-Tehrani M., Kiosses W. B., Dejana E., Schultz D. A., Engelhardt B., Cao G., DeLisser H., Schwartz M. A. (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 [DOI] [PubMed] [Google Scholar]
- 50. Shyy J. Y., Chien S. (2002) Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 91, 769–775 [DOI] [PubMed] [Google Scholar]
- 51. Lieskovska J., Ling Y., Badley-Clarke J., Clemmons D. R. (2006) The role of Src kinase in insulin-like growth factor-dependent mitogenic signaling in vascular smooth muscle cells. J. Biol. Chem. 281, 25041–25053 [DOI] [PubMed] [Google Scholar]
- 52. Armstrong V. J., Muzylak M., Sunters A., Zaman G., Saxon L. K., Price J. S., Lanyon L. E. (2007) Wnt/β-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor α. J. Biol. Chem. 282, 20715–20727 [DOI] [PubMed] [Google Scholar]
- 53. Kamel M. A., Picconi J. L., Lara-Castillo N., Johnson M. L. (2010) Activation of β-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2. Implications for the study of mechanosensation in bone. Bone 47, 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mikuni-Takagaki Y., Suzuki Y., Kawase T., Saito S. (1996) Distinct responses of different populations of bone cells to mechanical stress. Endocrinology 137, 2028–2035 [DOI] [PubMed] [Google Scholar]
- 55. Ponik S. M., Triplett J. W., Pavalko F. M. (2007) Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J. Cell Biochem. 100, 794–807 [DOI] [PubMed] [Google Scholar]
- 56. Kamioka H., Sugawara Y., Murshid S. A., Ishihara Y., Honjo T., Takano-Yamamoto T. (2006) Fluid shear stress induces less calcium response in a single primary osteocyte than in a single osteoblast. Implication of different focal adhesion formation. J. Bone Miner. Res. 21, 1012–1021 [DOI] [PubMed] [Google Scholar]
- 57. Bakker A. D., Silva V. C., Krishnan R., Bacabac R. G., Blaauboer M. E., Lin Y. C., Marcantonio R. A., Cirelli J. A., Klein-Nulend J. (2009) Tumor necrosis factor α and interleukin-1β modulate calcium and nitric oxide signaling in mechanically stimulated osteocytes. Arthritis Rheum. 60, 3336–3345 [DOI] [PubMed] [Google Scholar]
- 58. Noh T., Gabet Y., Cogan J., Shi Y., Tank A., Sasaki T., Criswell B., Dixon A., Lee C., Tam J., Kohler T., Segev E., Kockeritz L., Woodgett J., Müller R., Chai Y., Smith E., Bab I., Frenkel B. (2009) Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLoS One 4, e5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kanematsu M., Ikeda K., Yamada Y. (1997) Interaction between nitric-oxide synthase and cyclooxygenase pathways in osteoblastic MC3T3-E1 cells. J. Bone Miner. Res. 12, 1789–1796 [DOI] [PubMed] [Google Scholar]
- 60. Liu X., Bruxvoort K. J., Zylstra C. R., Liu J., Cichowski R., Faugere M. C., Bouxsein M. L., Wan C., Williams B. O., Clemens T. L. (2007) Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proc. Natl. Acad. Sci. U.S.A. 104, 2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ulici V., Hoenselaar K. D., Agoston H., McErlain D. D., Umoh J., Chakrabarti S., Holdsworth D. W., Beier F. (2009) The role of Akt1 in terminal stages of endochondral bone formation. Angiogenesis and ossification. Bone 45, 1133–1145 [DOI] [PubMed] [Google Scholar]
- 62. Wimalawansa S. J. (2007) Rationale for using nitric oxide donor therapy for prevention of bone loss and treatment of osteoporosis in humans. Ann. N.Y. Acad. Sci. 1117, 283–297 [DOI] [PubMed] [Google Scholar]
- 63. Jamal S. A., Hamilton C. J., Eastell R., Cummings S. R. (2011) Effect of nitroglycerin ointment on bone density and strength in postmenopausal women. A randomized trial. JAMA 305, 800–807 [DOI] [PubMed] [Google Scholar]