Background: The mechanism for differential control of gonadotropin gene induction by GnRH is not established.
Results: GnRH activates Gαs and Gαq/11, which modulate LH and FSH synthesis, respectively, by a mechanism including secreted factors.
Conclusion: Different G proteins and autocrine signaling regulate the pattern of FSH and LH expression by GnRH.
Significance: A novel G protein and autocrine signaling mechanism has been identified.
Keywords: Cell Signaling, G Protein-coupled Receptors (GPCRs), G Proteins, Gene Regulation, siRNA, Autocrine, Gonadotrope, Gonadotropin, Paracrine, Signaling Circuit
Abstract
Gonadotropin-releasing hormone (GnRH) acts at gonadotropes to direct the synthesis of the gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). The frequency of GnRH pulses determines the pattern of gonadotropin synthesis. Several hypotheses for how the gonadotrope decodes GnRH frequency to regulate gonadotropin subunit genes differentially have been proposed. However, key regulators and underlying mechanisms remain uncertain. We investigated the role of individual G proteins by perturbations using siRNA or bacterial toxins. In LβT2 gonadotrope cells, FSHβ gene induction depended predominantly on Gαq/11, whereas LHβ expression depended on Gαs. Specifically reducing Gαs signaling also disinhibited FSHβ expression, suggesting the presence of a Gαs-dependent signal that suppressed FSH biosynthesis. The presence of secreted factors influencing FSHβ expression levels was tested by studying the effects of conditioned media from Gαs knockdown and cholera toxin-treated cells on FSHβ expression. These studies and related Transwell culture experiments implicate Gαs-dependent secreted factors in regulating both FSHβ and LHβ gene expression. siRNA studies identify inhibinα as a Gαs-dependent GnRH-induced autocrine regulatory factor that contributes to feedback suppression of FSHβ expression. These results uncover differential regulation of the gonadotropin genes by Gαq/11 and by Gαs and implicate autocrine and gonadotrope-gonadotrope paracrine regulatory loops in the differential induction of gonadotropin genes.
Introduction
Mammalian reproductive processes are controlled by a complex interplay between functionally and spatially discrete tissues. Pituitary gonadotropes play a central role in the hypothalamic-pituitary-gonadal system and in controlling reproduction. Gonadotropin-releasing hormone (GnRH)2 is released in discrete pulses by the hypothalamus and acts at the gonadotrope to regulate the synthesis and release of the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The gonadotropins regulate various gonadal functions including gametogenesis, sex hormone production, and the phases of the female reproductive cycle.
GnRH is released in discrete pulses. The pattern of gonadotropin synthesis and release in the pituitary gonadotrope is influenced by the frequency of hypothalamic GnRH release. In the female reproductive cycle, for example, lower frequency hypothalamic GnRH promotes preferential FSH synthesis and release that mediate ovarian follicular recruitment and growth in the early follicular phase. Higher frequency hypothalamic GnRH stimulates preferential LH synthesis and release, triggering ovulation in the late follicular phase (1, 2). This frequency encoding is exploited therapeutically to assist reproduction and to cause chemical castration using long acting GnRH analogues for the treatment of gonadal steroid-dependent cancers. Abnormalities in gonadotropin synthesis and release are involved in several reproductive disorders, such as Kallmann syndrome and polycystic ovarian syndrome (1, 3–6). The mechanisms and associated regulatory structures underlying the decoding of GnRH signaling patterns by the gonadotrope are incompletely understood.
An important goal of gonadotropin research over the previous decade has been to define the topology of gonadotropin signaling, i.e. the positive and negative signaling and gene regulatory connections and cis-acting gene control processes linking receptor activation to gonadotropin gene regulation (7). There have been several efforts to develop mathematical models of gonadotrope frequency-decoding mechanisms (8–11). Modeling is valuable for understanding information processing in a system of this complexity, and refining the understanding of the regulatory loops and network topology is important for guiding this modeling effort.
The GnRH receptor (GnRHR) is a member of the rhodopsin-like G protein-coupled receptor superfamily that activates heterotrimeric GTP-binding proteins (G proteins) to transmit extracellular information to the intracellular signaling network (12). GnRHR activates G proteins by facilitating GDP to GTP exchange of Gα, which in turn activates downstream effectors (13, 14). There are four Gα subfamilies in the mammalian genome: Gαs, Gαq/11, Gα12/13, and Gαi/o (15). Each Gα subfamily has been associated with principal downstream effectors. For example, Gαs activates adenylyl cyclase catalyzing cAMP production. Gαq/11 activates phopholipase Cβ, producing diacylglycerol and inositol triphophate. Gα12/13 activates RhoGEFs (16). Gαi/o inhibits adenylyl cyclase and regulates potassium channels. The GnRHR is believed to regulate several Gα proteins as downstream effectors. However, it has not been fully determined which G proteins are involved in the GnRHR signaling of the gonadotrope. How each G protein contributes to signaling and gonadotropin transcription has not been studied.
Although early studies implicated predominantly Gαq/11 in gonadotrope GnRHR signaling, recent work from Webster's laboratory based on GTP loading assays and cell-permeable inhibitory peptides suggests that both Gαq and Gαs contribute to regulation of ERK and LHβ expression (17–19). Notably, Gαs and Gαq show differential desensitization in response to pulsatile GnRH stimulation (18). No evidence for involvement of Gαi/o in the GnRHR signaling of gonadotropes has been reported (17). On the other hand, in reproductive tumor cells, Gαi/o played an important role in GnRHR signaling by regulating MAPK activation and in inhibiting cell proliferation in response to GnRH stimulation (20–23). In the GnRH-producing neurons of the hypothalamus, Gαi and Gαq were involved in the pulsatile GnRH release in response to autocrine/paracrine GnRH stimulation (24). G protein activation by GnRHR may vary, as the GnRHR-activated G protein shifted from Gαs to Gαi under increasing GnRH concentration in hypothalamic neurons (24). The involvement of Gα12/13 in GnRHR signaling has not been explored. Therefore, GnRHR is capable of activating multiple Gα subfamilies depending on cell type or cell context.
An important technique for studying signaling topology relies on the use of interfering RNA (RNAi). This approach has not been widely utilized in LβT2 cells because of difficulty in inducing a high level of suppression. We have found that nucleofection protocols can provide efficient suppression of specific transcripts and proteins in LβT2 cells (25, 26). In this study we used specific RNAi-mediated suppression of individual G proteins in conjunction with bacterial toxin experiments to probe the topology of GnRHR signaling. These experiments identified differential induction of LHβ and FSHβ by Gαs and Gαq G proteins, respectively. The unexpected discovery of a Gαs-dependent suppression of FSH expression led to the identification of autocrine/paracrine signaling mechanisms involved in differential gonadotropin expression.
EXPERIMENTAL PROCEDURES
Materials
G protein antibodies (Gαs, Gαq, Gα11, and Gα12) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-PKCα was from BD Biosciences. Phosphor-JNK, total JNK, phosphor-PKCμ, total PKCμ, and phosphor-PKA substrates antibodies were purchased from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase-coupled secondary antibodies were from Santa Cruz Biotechnology. Cholera toxin (CTX) and pertussis toxin (PTX) were purchased from Calbiochem. GnRH was purchased from Bachem. Proteinase K was purchased from Roche Applied Science. Molecular weight cutoff filters (Amicon centrifugal filter) and Transwells were purchased from Millipore.
Cell Culture
LβT2 cells obtained from Professor Pamella Mellon (University of California, San Diego) were maintained at 37 ºC in 5% CO2 in humidified air in DMEM (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Gemini, Calabasas, CA).
siRNA Interference
1 million LβT2 cells were transfected with 0.5 μg of siRNA using Amaxa shuttle with SG buffer and DS-137 nucleofection program, following the manufacturer's instructions (Lonza Walkersville Inc., Walkersville, MD). Gαs, Gαq, Gα11, Gα12, and Gα13 siRNAs were ordered from a commercial supplier (On-Target plus siRNA SMARTpool; Dharmacon, Thermo Fisher Scientific). After siRNA transfection, cells were seeded on a cell culture plate supplemented with 10% FBS + DMEM and incubated at 37 ºC in 5% CO2 incubator. 48 h after siRNA transfection, medium was exchanged from 10% FBS + DMEM to serum-free DMEM for an overnight serum starvation. 72 h after siRNA transfection, cells were treated with 1 nm GnRH or 5 μg/ml CTX. Later, cells were harvested for Western blotting or real-time PCR analysis.
Western Blot Analysis
siRNA-transfected LβT2 cells (2 million) were grown in 6-well plates. After Nonidet P-40 lysis (20 mm Tris-HCl, 1% Nonidet P-40, NaCl), cell lysates were centrifuged at 14,000 rpm for 10 min to remove cell debris. Cell lysates were mixed with an equal volume of 2× Laemmli sample buffer and boiled for 5 min at 95 ºC. 50 μg of protein/well was loaded onto 10–20% Tris-HCl ready gradient gel (Bio-Rad Laboratories) and underwent electrophoresis for 1.5 h at 100 V. Proteins were transferred to H-Bond membrane (Hybond ECL; Amersham Biosciences), and the membrane was blocked for 40 min with 5% nonfat dry milk (Bio-Rad) in Tris-buffered saline. The membrane was incubated with primary antibody (1:1000) at 4 ºC for overnight. Incubation with the secondary antibody (1:5000) coupled to horseradish peroxidase (Santa Cruz Biotechnology) was performed at room temperature for 45 min, which was followed by three 5-min washes with Tris-buffered saline in 1% Tween 20. Target protein bands were visualized by enhanced chemiluminescence (Amersham Biosciences) according to the manufacturer's instructions.
Quantitative Real-time PCR
For quantitative real-time PCR experiments, cells were seeded in 24-well plates at 1 million cells/well. For cell harvest, 300 μl of lysis buffer (4 m guanidinium thiocyanate, 25 mm sodium citrate, pH 7.0, 0.5% N-lauroyl-sarcosine, and 0.1 m 2-mercaptoethanol) were added to each well of a 24-well plate. Total RNA was isolated using Absolutely RNA 96 micropreparation kit (Stratagene) following the manufacturer's instructions. RNA concentration was measured using NanoDrop (Thermo Scientific), and 1 μg of total RNA was used for reverse transcription using affinity script reverse transcriptase (Agilent, Santa Clara, CA). After reverse transcription, cDNA samples were diluted 1:20 in distilled H2O. 5 μl of diluted cDNA and 5 μl of master mix (DNA polymerase, primers, dNTP, PCR buffer, and MgCl2) were mixed and used for PCR. Quantitative real-time PCR assays were performed in an ABI Prism 7900HT with SYBR Green according to the manufacturer's protocol. The results were exported as cycle threshold (CT) values, and CT values of target genes were normalized to that of rps11 in subsequent analysis. Data were expressed as arbitrary units by using the formula, E = 2500 × 1.93(rps11 CT value − gene of interest CT value), where E is the expression level in arbitrary units.
Luciferase Assay
0.5 μg of FSHβ or LHβ firefly luciferase reporter, 0.005 μg of thymidine kinase Renilla luciferase reporter (internal control), and 0.5 μg of siRNAs (control or Gαs) were co-transfected into 1 million LβT2 cells using Amaxa shuttle with SG buffer and DS-137 nucleofection program (Lonza Walkersville). After the transfection, 0.5 million LβT2 cells were seeded to each well of 24-well cell culture plates containing 1 ml of DMEM supplemented with 10% FBS. 48 h after transfection, the medium was exchanged to 1 ml of serum-free DMEM for overnight serum starvation. 72 h after transfection, cells were stimulated with 1 nm GnRH for 6 h to induce FSHβ and LHβ firefly luciferase reporter expression. A Sirius single tube luminometer (Berthold Detection Systems, Huntsville, AL) with dual luciferase reporter assay system reagents (Promega) was used for measuring FSHβ and LHβ promoter activity.
Conditioned Media Experiment
1 million LβT2 cells were transfected with either 1 μg of control or Gαs siRNA using Amaxa shuttle. Then cells were seeded on each well of 24-well plates supplemented with 1 ml of 10% FBS+DMEM. 48 h after the transfection, medium was exchanged to 1 ml of serum-free DMEM for overnight serum starvation. 72 h after the transfection, conditioned medium was harvested and added to naïve cells for 6 h.
Purification of Primary Gonadotropes
H2Kk mice were generously provided by Professor William Miller (University of North Carolina). Gonadotrope purification procedures were described previously (27). Briefly, pituitaries from 10–20 mice were dispersed by digestion with collagenase and pancreatin. Undigested tissue and cell aggregates were removed using a 27-μm pore size nylon mesh. Cells were then incubated with 20 μl of biotin anti-mouse H2Kk antibody (BD Pharmingen) in 180 μl of degassed PBS (BSA/EDTA) at 4 ºC for 10 min. Then excess H2Kk antibodies were washed off, and 20 μl of anti-biotin paramagnetic microbeads (Miltenyi Biotec) were added to cells and rotated for 15 min at 4 ºC. The cells were added to magnetic separation column (Miltenyi Biotec) and washed three times with 0.5 ml of PBS (BSA/EDTA). Cells attracted to the column were enriched gonadotropes, and other pituitary cells were removed by PBS washing. The enriched gonadotropes were subsequently eluted by removing the magnetic field. The eluted gonadotropes were reapplied to a fresh magnetic separation column, rewashed with 0.5 ml of PBS (BSA/EDTA) three times, and eluted again from the column. This second column purification ensured gonadotropes reaching >90% purity. Then cells were seeded on 96-well plates at 20,000 cells/well in M-199 Complete (Invitrogen) and incubated at 37 ºC in humidified air in 5% CO2.
Inhibinα ELISA
An inhibinα ELISA kit was purchased from a commercial supplier (MyBioSource, San Diego, CA). 1 million LβT2 cells or 80,000 purified primary gonadotropes were stimulated with 1 nm GnRH or 5 μg/ml CTX for 10 h to induce inhibin synthesis and secretion into the medium. Conditioned medium was then harvested and processed for ELISA following the manufacturer's instructions.
Perfusion Experiment
The laminar flow perfusion system was custom designed and built by the laboratory, and its performance has been extensively tested. Internal temperature is maintained at 37 ºC by heating blocks. The system holds 16 coverslips in four cassettes. 1 million gonadotrope cells cultured for 2 days on the coverslide were placed in each chamber. Each chamber of the cassette is independently perfused with two electronically controlled microfluidic valves. One valve provided medium (DMEM), and another valve provided 5 nm GnRH in DMEM. Gonadotrope cells were exposed to vehicle (no GnRH control), slow pulse frequency GnRH (5-min GnRH pulse exposure every 2 h), or high pulse frequency GnRH (5-min GnRH pulse exposure every 30 min) for a 10-h period. After 10 h, cells were harvested for RNA extraction.
Statistical Analysis
Statistical calculations were performed using the Prism statistical software package version 5 (GraphPad, San Diego, CA). Data were analyzed for normality followed by calculation of ANOVA.
RESULTS
G Protein Expression in Gonadotropes
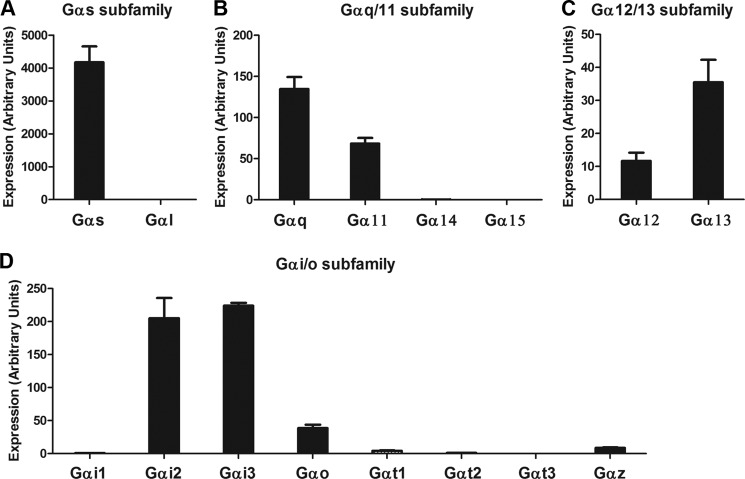
In various cell types, the GnRHR has been shown to regulate Gαs, Gαq/11, and Gαi/o family G proteins (17–24). To guide the study of G protein coding of gonadotropin expression, we determined which G protein mRNAs were expressed in gonadotrope cells. Two independent primer sets were designed for each Gα mRNA. The data from independent primer sets were concordant. In the Gαs subfamily, Gαs mRNA was abundantly expressed, but the expression of Gαl mRNA was not detected (Fig. 1A). In the Gαq/11 subfamily, Gαq and Gα11 mRNAs were moderately expressed, but the expression of Gα14 and Gα15 mRNAs was not detected (Fig. 1B). In the Gα12/13 subfamily, both Gα12 and Gα13 mRNAs were detected (Fig. 1C). In the Gαi/o subfamily, the expression of Gαi2, Gαi3, Gαo, Gαt1, and Gαz mRNAs were detected, but not Gαi1, Gαt2, and Gαt3 mRNAs (Fig. 1D). Overall, among 18 specific Gα genes in the mammalian genome, 10 Gα mRNAs were expressed in LβT2 cells: Gαs, Gαq, Gα11, Gα12, Gα13, Gαi2, Gαi3, Gαo1, Gαt1, and Gαz.
FIGURE 1.
Expression profile of Gα proteins in LβT2 gonadotropes. mRNA expression levels of each Gα protein were determined by real-time PCR analysis. The mRNA expression of Gαs subfamily (A), Gαq/11 subfamily (B), Gα12/13 subfamily (C), and Gαi/o subfamily (D) is indicated. Two independent primer sets used for each gene gave similar results. One representative dataset is presented. Error bars, S.E.
Gαi/o Subfamily Is Not Involved in Expression of Either Early Genes or Gonadotropin Genes in Response to GnRH Stimulation
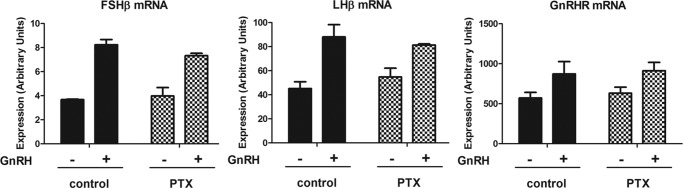
To test the role of Gαi/o subfamily G proteins in GnRHR signaling, gonadotropes were treated with PTX, an inhibitor of Gαi/o subfamily activation, and the effects on early gene and gonadotropin subunit induction in response to GnRH stimulation were assayed. PTX had no significant effect on early gene induction by GnRH (supplemental Fig. 1). Similarly, the induction of gonadotropin subunits in response to GnRH stimulation was unaltered (Fig. 2). These data indicate that Gαi/o subfamily proteins are not significantly involved in GnRHR signaling leading to downstream gene expression in the pituitary gonadotrope. These results are consistent with a previous report (17).
FIGURE 2.
Effect of Gαi/o subfamily inhibition on GnRH-induced gonadotropin transcription. LβT2 gonadotrope cells were pretreated with either vehicle (control) or 100 ng/ml PTX for 2 h. Cells were then exposed to 1 nm GnRH for 2 h and harvested 4 h later. FSHβ, LHβ, and GnRHR mRNA expression levels were determined by real-time PCR analysis. For statistical analysis, two-way ANOVA with Bonferroni post test was used, and the effect of PTX was not significant. Error bars, S.E.
Role of Specific G Proteins in Early Gene Induction
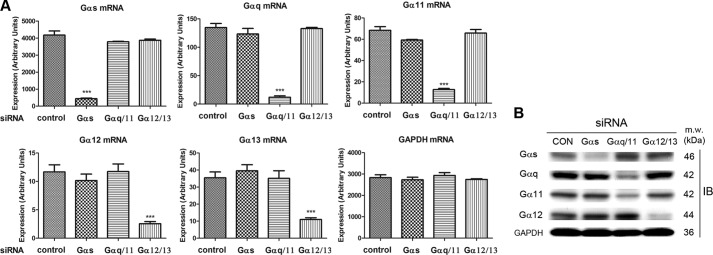
The involvement of Gαs, Gαq/11, and Gα12/13 subfamilies in GnRH-dependent gene expression was investigated using specific siRNAs against individual Gα proteins. We first examined the specificity and the efficiency of siRNA-mediated Gα mRNA knockdown in LβT2 gonadotropes by real-time PCR and Western blot analysis. Chemically modified siRNA pools were used to reduce off-target effects, and nucleofection was employed to increase efficiency. Each Gα siRNA efficiently and specifically down-regulated its target Gα mRNA, and protein. mRNA expression was reduced by 80–90% (Fig. 3A), and protein expression was reduced by 60–80% (Fig. 3B and supplemental Fig. 2). Importantly, no nonspecific Gα mRNA or protein effects were observed, suggesting that the siRNA-mediated knockdown was both efficient and specific.
FIGURE 3.
Specificity and efficiency of Gα siRNA-mediated knockdown in LβT2 gonadotrope cells. LβT2 gonadotrope cells were transfected with control or Gα siRNAs via nucleofection. Cells were harvested, 3 days after siRNA transfection, and the expression levels of individual Gα were monitored by real-time PCR (mRNA) and Western blot (protein) analysis. A, real-time PCR quantified the expression level of individual Gα mRNAs under specific Gα siRNA transfections. The mRNA expression of Gα siRNA-transfected samples was compared with that of control siRNA-treated samples. For statistical analysis, one-way ANOVA with Bonferroni post test was used. ***, p < 0.001. Error bars, S.E. B, Western blot analysis determined the expression level of individual Gα proteins with specific Gα siRNA transfections. GAPDH was used as a loading control. One representative dataset from three independent experiments is presented.
GnRHR activation induces various early genes (26, 28). We examined the effects of G protein knockdown on the pattern of early gene induction by GnRH. Either control or Gα siRNAs were transfected into LβT2 gonadotropes, and 3 days after siRNA transfection, cells were exposed to GnRH. Gαs knockdown significantly attenuated the induction by GnRH of several early genes, including egr3, fra1, fosB, cjun, n10, and rgs2. Gαq/11 knockdown significantly attenuated the induction by GnRH of all early genes assayed. Gα12/13 knockdown slightly increased the induction of cfos, and pip92 by GnRH suggesting that activation of Gα12/13 might contribute to suppression of those genes (supplemental Fig. 3).
Gαs and Gαq/11 Differentially Regulate FSHβ and LHβ Transcription in Response to GnRH
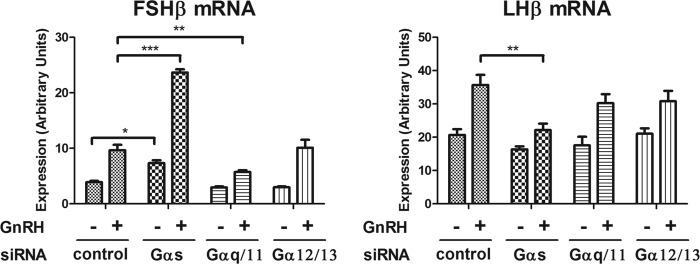
We studied the effects of G protein knockdown on gonadotropin subunit gene induction. Gαs knockdown reduced LHβ mRNA induction by GnRH by 52%. Gαq/11 and Gα12/13 knockdown had no significant effect on LHβ induction (Fig. 4). Gαq/11 knockdown reduced FSHβ mRNA induction by GnRH by 60%, but Gα12/13 knockdown had no effect on FSHβ mRNA induction by GnRH (Fig. 4). Gαs knockdown strongly increased both basal and GnRH-stimulated FSHβ mRNA expression. G protein knockdown studies using promoter activity reporter constructs for FSHβ and LHβ were not entirely consistent with the effects seen on mRNA expression (supplemental Fig. 4A; see “Discussion”). The Gαs knockdown results raised the possibility that Gαs activation leads to the suppression of FSHβ mRNA expression.
FIGURE 4.
Effect of Gαs, Gαq/11, and Gα12/13 subfamily knockdown on GnRH-induced gonadotropin subunit transcription. LβT2 cells were transfected with control or Gα siRNA by nucleofection. Three days after siRNA transfection, cells were stimulated with either vehicle (no GnRH) or 1 nm GnRH for 2 h, followed by a 4-h incubation without GnRH, to induce gonadotropin subunit expression. The effect of Gαs, Gαq/11, and Gα12/13 knockdown on GnRH-induced gonadotropin subunit expression was determined by real-time PCR analysis. For statistical analysis, two-way ANOVA with Bonferroni post test was used. ***, p < 0.001; **, p < 0.01; *, p < 0.05. Error bars, S.E.
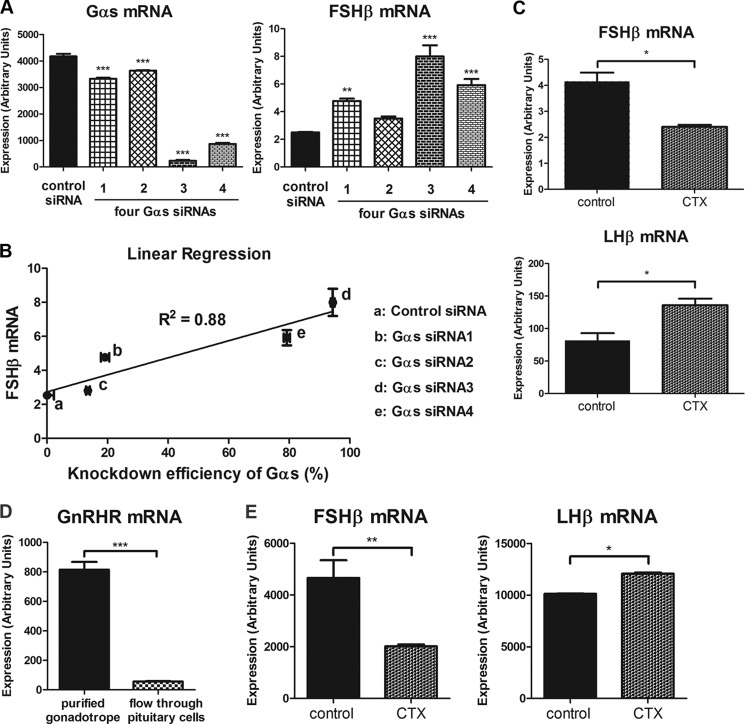
To test further the surprising observation that Gαs knockdown increased FSHβ mRNA levels, the assays were repeated using four sequence-independent FSHβ primer sets, which generated essentially identical results (supplemental Fig. 5). To exclude the possibility that this effect resulted from off-target knockdown of some other gene, each of the sequence-independent siRNAs comprising the pool used for the previous experiments were tested separately. If the FSHβ mRNA induction resulted from off-target effects, then a similar response would be unlikely using different independent siRNAs. However, each of the four Gαs siRNAs was found to up-regulate FSHβ mRNA expression (Fig. 5A). Further supporting the involvement of Gαs in this effect, the efficiency of Gαs knockdown and the increase in basal FSHβ were correlated (r2 = 0.88, p < 0.01; Fig. 5B). These data indicate that the increased FSHβ mRNA expression was due to Gαs knockdown.
FIGURE 5.
Verification of Gαs-dependent FSHβ and LHβ mRNA regulation. LβT2 cells were transfected with control or Gαs siRNAs via nucleofection. Cells were harvested for real-time PCR analysis, 3 days after siRNA transfection. A, four independent Gαs siRNAs were used to test the specificity of Gαs siRNA-mediated knockdown and its effect on FSHβ mRNA expression. B, linear regression analysis was conducted to examine the correlation between Gαs knockdown efficiency and increased FSHβ mRNA expression. C, LβT2 cells were exposed to 5 μg/ml CTX for 7 h, and the effect of specific Gαs stimulation on gonadotropin subunit mRNA expression was monitored by real-time PCR. D and E, primary gonadotrope cells were purified from the pituitaries of H2Kk transgenic mice. D, the quality of primary gonadotrope purification was determined by measuring GnRHR mRNA expression of purified gonadotropes and nonpurified flow-through pituitary cells. E, purified primary gonadotropes were stimulated with either vehicle (no CTX) or 5 μg/ml CTX for 7 h. FSHβ and LHβ mRNA expression levels were determined by real-time PCR analysis. For statistical analysis, one-way ANOVA (A) and two-tailed t test with Bonferroni post test (C–E) were used. ***, p < 0.001; **, p < 0.01; *, p < 0.05. Error bars, S.E.
We also studied the effects of Gαs stimulation using CTX, which specifically activates Gαs subfamily proteins via ADP-ribosylation (29). Gαs stimulation by CTX strongly suppressed FSHβ mRNA expression and enhanced LHβ mRNA expression (Fig. 5C). These data indicate that Gαs activity suppresses FSHβ expression and induces LHβ expression.
We then tested whether the observations in LβT2 gonadotrope cells could be extended to the primary gonadotrope cells. We purified primary gonadotropes from the pituitaries of H2Kk transgenic mice via affinity purification as described previously (27). Among various cell types in the pituitary, only gonadotropes express GnRHR, thus GnRHR was used to determine the quality of the gonadotrope cell purification. The purification procedures resulted in >90% enrichment in primary gonadotropes (Fig. 5D). The purified primary gonadotropes were then tested for Gαs activity dependence on the regulation of FSHβ and LHβ mRNA expression. Consistently, FSHβ mRNA expression was significantly down-regulated by Gαs activation using CTX, whereas LHβ mRNA expression was up-regulated (Fig. 5E). These data indicate that Gαs activity suppressed FSHβ mRNA expression and enhanced LHβ mRNA expression in both LβT2 gonadotropes and purified primary gonadotropes.
Gαs-dependent FSHβ mRNA Suppression Is Mediated by Secreted Factors
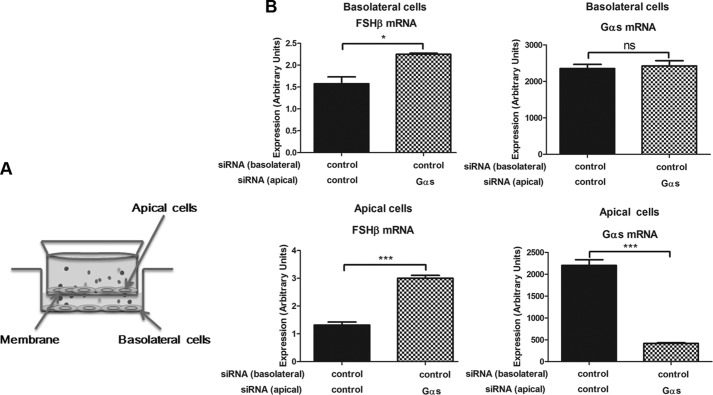
The studies described above show that activation of Gαs protein by GnRH or CTX suppressed FSHβ gene expression and induced LHβ gene expression. Several secreted proteins are known to regulate gonadotropin expression (30–34). We speculated that the Gαs-mediated suppression of FSH might result from secreted autocrine and paracrine factors. To test this idea, we first conducted a Transwell experiment (Fig. 6A). Gonadotrope cells transfected with control or Gαs siRNAs were seeded on each side of apical or basolateral chambers. We observed that regardless of cell seeding position (either apical or basolateral) control siRNA-transfected gonadotropes had increased FSHβ mRNA expression, when Gαs siRNA-transfected cells were seeded on the chamber across the membrane (Fig. 6B and supplemental Fig. 6). Gαs mRNA expression in control-transfected cells was not changed.
FIGURE 6.
Transwell experiment testing the involvement of Gαs-dependent secreted factors regulating FSHβ transcription. LβT2 gonadotrope cells were transfected with control or Gαs siRNAs via nucleofection. siRNA-transfected gonadotrope cells were then seeded on apical or basolateral chambers of the Transwell. Medium was replaced with fresh medium, 2 days after siRNA transfection. At day 3, cells were harvested, and the expression levels of FSHβ and Gαs mRNAs were determined by real-time PCR analysis. A, schematic shows Transwell experiment. B, expression levels of FSHβ and Gαs mRNAs from apical and basolateral cells in the Transwell were determined by real-time PCR analysis. For statistical analysis, two-tailed t test with Bonferroni post test was used. ns, nonsignificant. ***, p < 0.001; *, p < 0.05. Error bars, S.E.
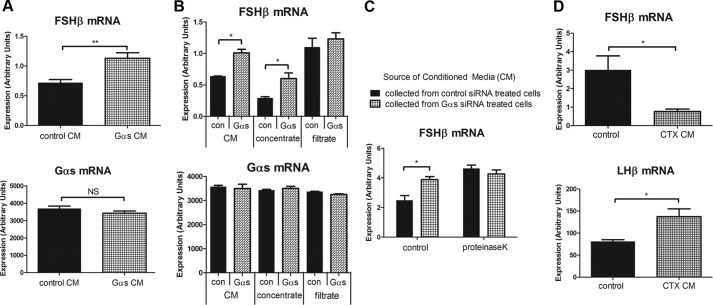
We next tested whether the conditioned medium from Gαs knockdown cells regulated FSHβ mRNA levels in naïve cells. Fresh medium was incubated with control or Gαs siRNA-transfected cells overnight, and this conditioned medium was transferred to naïve gonadotrope cells. We found that the conditioned medium from Gαs knockdown cells significantly increased FSHβ mRNA expression in untransfected cells. Also consistent with the Transwell results, Gαs mRNA expression was not altered by conditioned medium (Fig. 7A).
FIGURE 7.
Gαs-dependent gonadotropin mRNA regulation is mediated by released autocrine/paracrine peptide(s). A–C, LβT2 gonadotrope cells were transfected with control or Gαs siRNAs via nucleofection. Two days after siRNA transfection, medium was replaced with fresh medium. At day 3, overnight conditioned medium (CM) was collected from siRNA-transfected cells, and it was added to non-siRNA-treated recipient cells. A, conditioned medium from control and Gαs knockdown cells was added to the non-siRNA-treated recipient cells for 6 h, and the effect on FSHβ and Gαs transcription was monitored by real-time PCR analysis. B, conditioned medium from control and Gαs knockdown cells passed through 3-kDa molecular mass cutoff filter. Concentrate fraction (enriched with more than 3-kDa proteins) and filtrate fraction (depleted from more than 3-kDa proteins) were added to non-siRNA-treated recipient cells for 6 h. C, conditioned medium from control and Gαs knockdown cells was treated with proteinase K and then heat-denatured. Both control and proteinase K-treated conditioned media were added to the recipient cells, and the effect on FSHβ transcription was monitored by real-time PCR. D, conditioned medium from control and CTX-pretreated cells was collected and was added to non-CTX-treated recipient cells for 6 h. FSHβ and LHβ mRNA expression was monitored by real-time PCR. For statistical analysis, two-tailed t test was used. **, p < 0.01; *, p < 0.05. Error bars, S.E.
We next characterized the size of the secreted factors using a 3-kDa molecular mass cutoff filter to process conditioned medium from control and Gαs siRNA-treated cells and exposing naïve cells to each heavy and light fraction. The filtrate fractions did not contain FSHβ regulatory activity, suggesting that the factor or factors were likely to be proteins larger than 3 kDa (Fig. 7B). We also found that proteinase K, a broad spectrum endopeptidase, eliminated regulatory activity in conditioned medium (Fig. 7C). Proteinase K-treated conditioned medium did not impair the capacity of cells to respond to GnRH (data not shown). Conditioned medium from CTX-treated cells suppressed FSHβ mRNA and stimulated LHβ mRNA (Fig. 7D). These results support the presence of secreted, Gαs-regulated proteins that suppress FSHβ mRNA in an autocrine and paracrine fashion.
Role of Inhibin as a Gαs-dependent Autocrine/Paracrine Factor Suppressing FSHβ mRNA Expression
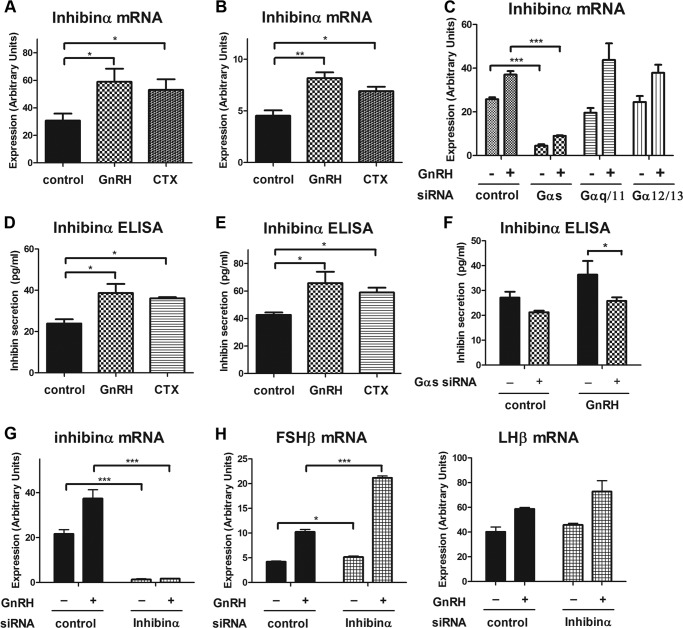
We used a candidate approach to search for regulated, secreted factors involved in FSHβ regulation. We first studied whether the TGFβ superfamily member follistatin played a role in Gαs-dependent FSHβ suppression. Titrated follistatin experiments looking for competitive inhibition of FSHβ expression and PCR assays for follistatin regulation excluded its role. Follistatin expression level was low, and it was not Gs-dependent (data not shown). We next studied inhibin which is a heterodimeric peptide hormone produced in the ovary that inhibits FSH synthesis in the pituitary. Inhibinα subunit interacts with the activin type I receptor, antagonizes the activin signaling in the pituitary gonadotrope (35), and has been reported to be regulated by GnRH in the gonadotrope (36, 37). Therefore, we tested whether inhibinα expression could be regulated by GnRH stimulation in a Gαs-dependent manner. We observed that inhibinα mRNA expression was significantly up-regulated by GnRH stimulation and also by Gαs activation using CTX both in the gonadotrope cell line and in primary gonadotrope cells (Fig. 8, A and B). Inhibinα mRNA expression was markedly down-regulated in Gαs knockdown gonadotrope cells under basal and GnRH-stimulated conditions, but it was not affected by the knockdown of other Gα proteins (Fig. 8C). In addition, we observed that inhibinα protein secretion was stimulated by GnRH and by Gαs activation both in LβT2 cells and in purified primary gonadotropes (Fig. 8, D and E). Both basal and GnRH-stimulated inhibinα protein secretion were significantly down-regulated by Gαs knockdown (Fig. 8F). Together, these data indicate that inhibinα mRNA expression and protein secretion were induced by GnRH stimulation in a Gαs-dependent manner.
FIGURE 8.
Inhibin is a Gαs-dependent autocrine/paracrine factor involved in the feedback suppression of FSHβ expression in response to GnRH stimulation. A and B, LβT2 gonadotrope cells (A) or purified primary gonadotrope cells (B) were stimulated with vehicle, 1 nm GnRH, or 5 μg/ml CTX for 8 h. The level of inhibinα mRNA expression was determined by real-time PCR. C, LβT2 gonadotrope cells were transfected with either control siRNA or Gα siRNAs via nucleofection. Three days after siRNA transfection, cells were stimulated with either vehicle or 1 nm GnRH for 6 h. Real-time PCR was used to determine inhibinα mRNA expression. D and E, LβT2 gonadotropes (D) or purified primary gonadotrope cells (E) were stimulated with vehicle, 1 nm GnRH, or 5 μg/ml CTX for 10 h. Conditioned medium was harvested, and secreted inhibinα protein was measured by ELISA. F–H, LβT2 gonadotrope cells were transfected with either control siRNA or Gαs siRNA (F) or inhibinα siRNA (G and H) via nucleofection. Three days after siRNA transfection, cells were stimulated with either vehicle or 1 nm GnRH for 6 h and tested for inhibinα ELISA (F) or the mRNA expression of inhibinα, LHβ, and FSHβ (G and H) by real-time PCR. For statistical analysis, one-way ANOVA (A, B, D, and E) and two-way ANOVA with Bonferroni post test (C and F–H) were used. ***, p < 0.001; **, p < 0.01; *, p < 0.05. Error bars, S.E.
To determine whether inhibin acted as an endogenous autocrine/paracrine factor suppressing FSHβ mRNA expression in the gonadotrope, we down-regulated inhibinα expression by inhibinα siRNA and monitored the effect on gonadotropin gene expression. Inhibinα mRNA expression was efficiently down-regulated (>90%) by inhibinα siRNA (Fig. 8G). With inhibinα knockdown, both basal and GnRH-induced FSHβ mRNA expression were significantly up-regulated (Fig. 8H), supporting the role of inhibin as a GnRH-stimulated, Gαs-dependent endogenous autocrine/paracrine regulatory factor.
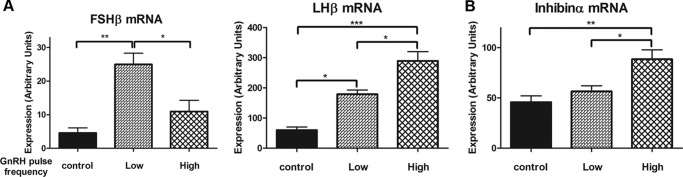
Inhibin Expression Is Regulated by GnRH in Pulse Frequency-sensitive Manner
We used a custom perfusion system to monitor the mRNA expression patterns of FSHβ, LHβ, and inhibinα in response to pulsatile GnRH stimulation. Consistent with previous studies, the expression of LHβ and FSHβ mRNAs was differentially regulated under low and high pulse frequencies of GnRH stimulation. FSHβ mRNA expression strongly favored low frequency GnRH pulses, whereas LHβ mRNA expression favored high frequency GnRH pulses (Fig. 9A). Notably, inhibinα mRNA expression is specifically up-regulated under high pulse frequency GnRH stimulation, but not under slow pulse frequency GnRH stimulation (Fig. 9B). Overall, our data suggest that inhibin contributes to the autocrine/paracrine-mediated negative feedback control of FSHβ expression in response to GnRH stimulation and to the suppression of FSHβ expression under high pulse frequency GnRH stimulation.
FIGURE 9.
Inhibin expression is regulated in a GnRH pulse frequency-specific manner. LβT2 gonadotrope cells were stimulated with vehicle or high or low frequency GnRH pulses using a custom designed cell perfusion system. For high pulse frequency GnRH stimulation, gonadotrope cells were exposed to 5 min of 5 nm GnRH pulses every 30 min for a 10-h period; and for low pulse frequency GnRH stimulation, cells were exposed to 5 min of 5 nm GnRH pulses every 2 h for a 10-h period. The levels of FSHβ and LHβ (A) and inhibinα (B) mRNA expression were monitored by real-time PCR analysis. For statistical analysis, one-way ANOVA with Bonferroni post test was used. ***, p < 0.001; **, p < 0.01; *, p < 0.05. Error bars, S.E.
DISCUSSION
Specific perturbation of Gαs and Gαq/11 differentially affected the GnRH-stimulated early gene and gonadotropin gene expression patterns. Gαi/o and Gα12/13 played relatively minor roles. Our results are consistent with previous reports suggesting that GnRH signaling in gonadotrope cells involves mainly Gαq/11 and Gαs (17, 18, 38, 39), whereas Gαi/o proteins are less important (17). Notably, we found distinct roles for Gαs and Gαq/11 in controlling gonadotropin subunit expression. Gαq/11 predominantly mediated FSHβ transcription, whereas Gαs mediated LHβ transcription and suppressed FSHβ transcription.
The finding that Gαs contributed to suppression of FSHβ mRNA was unexpected, as the FSHβ promoter has been reported to have activating CREB sites that could be stimulated by Gαs signaling (40). Experiments using separate individual Gαs siRNAs and stimulatory experiments in the gonadotrope cell line and in primary mouse gonadotrope cells using CTX confirmed that the major effect of Gαs signaling on FSHβ expression was inhibitory. Notably, a previous study indicated that Gαs activation using CTX in dispersed rat pituitary cells significantly down-regulated FSH secretion and up-regulated LH secretion (41).
The regulation of gonadotropin gene promoter activity, as determined using reporter assays, often diverged from the regulation of the endogenous mRNA levels. For example, Gαs knockdown enhanced GnRH-stimulated FSHβ mRNA expression, whereas it reduced FSHβ reporter activity (see supplemental Fig. 4A). LHβ mRNA induction was eliminated by Gαs knockdown, whereas the induction of LHβ reporter activity persisted (supplemental Fig. 4A). Furthermore, the concentration response curve of LHβ and FSHβ mRNA induction by GnRH was U-shaped whereas the response of the each promoter construct was sigmoidal (supplemental Fig. 4B). These results suggest that the mechanisms underlying control of mRNA levels are more complex than can be accounted for by regulation of the proximal promoter. Expression and stability of mRNAs may be controlled by diverse mechanisms such as upstream promoter region, distant enhancer elements, chromatin structure (42), mRNA processing (43), and mRNA stability and degradation (44, 45). mRNA processing has been implicated in regulating gonadotropin mRNA levels (46).
Several studies have suggested paracrine factors that influence gonadotropin expression (30–34),45–50). We studied whether the Gαs-mediated suppression of FSH might result from secreted autocrine factors. Transwell and conditioned media experiments indicated that secreted proteinase K-sensitive factors larger than 3 kDa suppressed FSHβ expression. Several known autocrine/paracrine factors may be involved. Using a candidate approach, we have identified inhibin as a GnRH-induced, Gαs-dependent autocrine/paracrine factor that mediates FSHβ suppression in gonadotropes.
We identified expression of all Gα G protein genes in gonadotrope cells except Gαl, Gα14, Gα15/16, Gαi1, Gαt2, and Gαt3. Many nonexpressed G proteins are tissue-specific genes, thus the absence of their expression in gonadotropes is not surprising. For example, Gαl is involved in odorant transduction and is exclusively expressed in the olfactory receptor neurons (47), and Gαt2 and Gαt3 are involved in light detection and found predominantly in photoreceptor cells of the eye (48). Gα15/16 expression is restricted to tissues rich in hematopoietic cell types such as spleen, thymus, and bone marrow (49, 50).
Experimentally, high frequency stimulation favors LH gene induction, whereas low frequency favors FSH gene induction. The differential induction of inhibin by Gαs and FSHβ by Gαq provides a simple, yet novel mechanism to explain the preferential induction of FSHβ by low frequency GnRH stimulation of the gonadotrope. High frequency stimulation favors inhibin expression which consequently suppresses FSHβ gene induction. Low frequency stimulation reduces the inhibin level and allows higher levels of FSHβ expression.
Our results suggest that the signaling network between the cell surface GnRHR and the gonadotropin genes includes previously unrecognized extracellular regulatory loops. We propose that differential targeting of LH and FSH gene regulation by distinct G protein pathways and autocrine factors such as inhibin underlies the frequency-dependent regulation of the gonadotropin genes by GnRH. Further experimental studies and mathematical simulations will be required to test this hypothesis.
Acknowledgments
We thank Dr. E. Stern and Dr. H. Pincas for advice and critically reading this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R01DK046943

This article contains supplemental Figs. 1–6.
- GnRH
- gonadotropin-releasing hormone
- CT
- cycle threshold
- CTX
- cholera toxin
- GnRHR
- GnRH receptor
- LH
- luteinizing hormone
- PTX
- pertussis toxin.
REFERENCES
- 1. Marshall J. C., Eagleson C. A., McCartney C. R. (2001) Hypothalamic dysfunction. Mol. Cell. Endocrinol. 183, 29–32 [DOI] [PubMed] [Google Scholar]
- 2. Marshall J. C., Dalkin A. C., Haisenleder D. J., Paul S. J., Ortolano G. A., Kelch R. P. (1991) Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles. Recent Prog. Horm. Res. 47, 155–187; discussion 188–159 [DOI] [PubMed] [Google Scholar]
- 3. Hall J. E., Taylor A. E., Hayes F. J., Crowley W. F., Jr. (1998) Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J. Endocrinol. Invest. 21, 602–611 [DOI] [PubMed] [Google Scholar]
- 4. Achermann J. C., Weiss J., Lee E. J., Jameson J. L. (2001) Inherited disorders of the gonadotropin hormones. Mol. Cell. Endocrinol. 179, 89–96 [DOI] [PubMed] [Google Scholar]
- 5. Brothers S. P., Janovick J. A., Conn P. M. (2003) Unexpected effects of epitope and chimeric tags on gonadotropin-releasing hormone receptors: implications for understanding the molecular etiology of hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 88, 6107–6112 [DOI] [PubMed] [Google Scholar]
- 6. Ehrmann D. A. (2005) Polycystic ovary syndrome. N. Engl. J. Med. 352, 1223–1236 [DOI] [PubMed] [Google Scholar]
- 7. Fink M. Y., Pincas H., Choi S. G., Nudelman G., Sealfon S. C. (2010) Research resource. Gonadotropin-releasing hormone receptor-mediated signaling network in LβT2 cells: a pathway-based web-accessible knowledgebase. Mol. Endocrinol. 24, 1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armstrong S. P., Caunt C. J., Fowkes R. C., Tsaneva-Atanasova K., McArdle C. A. (2009) Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the Ca2+/NFAT signaling pathway decode GnRH pulse frequency? J. Biol. Chem. 284, 35746–35757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson R. J., Lim J. W., Moritz R. L., Mathivanan S. (2009) Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteomics 6, 267–283 [DOI] [PubMed] [Google Scholar]
- 10. Krakauer D. C., Page K. M., Sealfon S. (2002) Module dynamics of the GnRH signal transduction network. J. Theor. Biol. 218, 457–470 [PubMed] [Google Scholar]
- 11. Washington T. M., Blum J. J., Reed M. C., Conn P. M. (2004) A mathematical model for LH release in response to continuous and pulsatile exposure of gonadotrophs to GnRH. Theor. Biol. Med. Model 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sealfon S. C., Weinstein H., Millar R. P. (1997) Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr. Rev. 18, 180–205 [DOI] [PubMed] [Google Scholar]
- 13. Lambert N. A. (2008) Dissociation of heterotrimeric G proteins in cells. Sci. Signal. 1, re5. [DOI] [PubMed] [Google Scholar]
- 14. Oldham W. M., Hamm H. E. (2008) Heterotrimeric G protein activation by G protein-coupled receptors. Nat. Rev. Mol. Cell. Biol. 9, 60–71 [DOI] [PubMed] [Google Scholar]
- 15. Simon M. I., Strathmann M. P., Gautam N. (1991) Diversity of G proteins in signal transduction. Science 252, 802–808 [DOI] [PubMed] [Google Scholar]
- 16. Siehler S. (2009) Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J. Pharmacol. 158, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu F., Usui I., Evans L. G., Austin D. A., Mellon P. L., Olefsky J. M., Webster N. J. (2002) Involvement of both Gq/11 and Gs proteins in gonadotropin-releasing hormone receptor-mediated signaling in LβT2 cells. J. Biol. Chem. 277, 32099–32108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsutsumi R., Mistry D., Webster N. J. (2010) Signaling responses to pulsatile gonadotropin-releasing hormone in LβT2 gonadotrope cells. J. Biol. Chem. 285, 20262–20272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stanislaus D., Janovick J. A., Brothers S., Conn P. M. (1997) Regulation of Gq/11α by the gonadotropin-releasing hormone receptor. Mol. Endocrinol. 11, 738–746 [DOI] [PubMed] [Google Scholar]
- 20. Kimura A., Ohmichi M., Kurachi H., Ikegami H., Hayakawa J., Tasaka K., Kanda Y., Nishio Y., Jikihara H., Matsuura N., Murata Y. (1999) Role of mitogen-activated protein kinase/extracellular signal-regulated kinase cascade in gonadotropin-releasing hormone-induced growth inhibition of a human ovarian cancer cell line. Cancer Res. 59, 5133–5142 [PubMed] [Google Scholar]
- 21. Gründker C., Schlotawa L., Viereck V., Emons G. (2001) Protein kinase C-independent stimulation of activator protein-1 and c-Jun N-terminal kinase activity in human endometrial cancer cells by the LHRH agonist triptorelin. Eur. J. Endocrinol. 145, 651–658 [DOI] [PubMed] [Google Scholar]
- 22. Gründker C., Völker P., Emons G. (2001) Antiproliferative signaling of luteinizing hormone-releasing hormone in human endometrial and ovarian cancer cells through G protein α(I)-mediated activation of phosphotyrosine phosphatase. Endocrinology 142, 2369–2380 [DOI] [PubMed] [Google Scholar]
- 23. Cheung L. W., Wong A. S. (2008) Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 275, 5479–5495 [DOI] [PubMed] [Google Scholar]
- 24. Krsmanovic L. Z., Mores N., Navarro C. E., Arora K. K., Catt K. J. (2003) An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc. Natl. Acad. Sci. U.S.A. 100, 2969–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bowles R., Patil S., Pincas H., Sealfon S. C. (2011) Optimized protocol for efficient transfection of dendritic cells without cell maturation. J. Vis. Exp. 53, e2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimoni Y., Fink M. Y., Choi S. G., Sealfon S. C. (2010) Plato's cave algorithm: inferring functional signaling networks from early gene expression shadows. PLoS Comput. Biol. 6, e1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu J. C., Su P., Safwat N. W., Sebastian J., Miller W. L. (2004) Rapid, efficient isolation of murine gonadotropes and their use in revealing control of follicle-stimulating hormone by paracrine pituitary factors. Endocrinology 145, 5832–5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wurmbach E., Yuen T., Ebersole B. J., Sealfon S. C. (2001) Gonadotropin-releasing hormone receptor-coupled gene network organization. J. Biol. Chem. 276, 47195–47201 [DOI] [PubMed] [Google Scholar]
- 29. Tang S. K., Zhi X. Y., Wang Y., Wu J. Y., Lee J. C., Kim C. J., Lou K., Xu L. H., Li W. J. (2010) Haloactinobacterium album gen. nov., sp. nov., a halophilic actinobacterium, and proposal of Ruaniaceae fam. nov. Int. J. Syst. Evol. Microbiol. 60, 2113–2119 [DOI] [PubMed] [Google Scholar]
- 30. Ho C. C., Bernard D. J. (2010) Bone morphogenetic protein 2 acts via inhibitor of DNA-binding proteins to synergistically regulate follicle-stimulating hormone β transcription with activin A. Endocrinology 151, 3445–3453 [DOI] [PubMed] [Google Scholar]
- 31. Lee K. B., Khivansara V., Santos M. M., Lamba P., Yuen T., Sealfon S. C., Bernard D. J. (2007) Bone morphogenetic protein 2 and activin A synergistically stimulate follicle-stimulating protein β subunit transcription. J. Mol. Endocrinol. 38, 315–330 [DOI] [PubMed] [Google Scholar]
- 32. Huang H. J., Wu J. C., Su P., Zhirnov O., Miller W. L. (2001) A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology 142, 2275–2283 [DOI] [PubMed] [Google Scholar]
- 33. Pernasetti F., Vasilyev V. V., Rosenberg S. B., Bailey J. S., Huang H. J., Miller W. L., Mellon P. L. (2001) Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology 142, 2284–2295 [DOI] [PubMed] [Google Scholar]
- 34. Bernard D. J. (2004) Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone β subunit in mouse gonadotrope cells. Mol. Endocrinol. 18, 606–623 [DOI] [PubMed] [Google Scholar]
- 35. Zhu J., Lin S. J., Zou C., Makanji Y., Jardetzky T. S., Woodruff T. K. (2012) Inhibin α-subunit N terminus interacts with activin type IB receptor to disrupt activin signaling. J. Biol. Chem. 287, 8060–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Popovics P., Rekasi Z., Stewart A. J., Kovacs M. (2011) Regulation of pituitary inhibin/activin subunits and follistatin gene expression by GnRH in female rats. J. Endocrinol. 210, 71–79 [DOI] [PubMed] [Google Scholar]
- 37. Bilezikjian L. M., Blount A. L., Leal A. M., Donaldson C. J., Fischer W. H., Vale W. W. (2004) Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol. Cell. Endocrinol. 225, 29–36 [DOI] [PubMed] [Google Scholar]
- 38. Hsieh K. P., Martin T. F. (1992) Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol. Endocrinol. 6, 1673–1681 [DOI] [PubMed] [Google Scholar]
- 39. Shah B. H., Milligan G. (1994) The gonadotrophin-releasing hormone receptor of αT3–1 pituitary cells regulates cellular levels of both of the phosphoinositidase C-linked G proteins, Gqα and G11α, equally. Mol. Pharmacol. 46, 1–7 [PubMed] [Google Scholar]
- 40. Ciccone N. A., Lacza C. T., Hou M. Y., Gregory S. J., Kam K. Y., Xu S., Kaiser U. B. (2008) A composite element that binds basic helix-loop-helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone β gene. Mol. Endocrinol. 22, 1908–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanyicska B., Burris T. P., Freeman M. E. (1991) The effects of endothelins on the secretion of prolactin, luteinizing hormone, and follicle-stimulating hormone are mediated by different guanine nucleotide-binding proteins. Endocrinology 129, 2607–2613 [DOI] [PubMed] [Google Scholar]
- 42. Berger S. L. (2007) The complex language of chromatin regulation during transcription. Nature 447, 407–412 [DOI] [PubMed] [Google Scholar]
- 43. Licatalosi D. D., Darnell R. B. (2010) RNA processing and its regulation: global insights into biological networks. Nat. Rev. Genet. 11, 75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Houseley J., Tollervey D. (2009) The many pathways of RNA degradation. Cell 136, 763–776 [DOI] [PubMed] [Google Scholar]
- 45. Ross J. (1995) mRNA stability in mammalian cells. Microbiol. Rev. 59, 423–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burger L. L., Dalkin A. C., Aylor K. W., Haisenleder D. J., Marshall J. C. (2002) GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes: assessment by primary transcript assay provides evidence for roles of GnRH and follistatin. Endocrinology 143, 3243–3249 [DOI] [PubMed] [Google Scholar]
- 47. Ronnett G. V., Moon C. (2002) G proteins and olfactory signal transduction. Annu. Rev. Physiol. 64, 189–222 [DOI] [PubMed] [Google Scholar]
- 48. Smyth V. A., Di Lorenzo D., Kennedy B. N. (2008) A novel, evolutionarily conserved enhancer of cone photoreceptor-specific expression. J. Biol. Chem. 283, 10881–10891 [DOI] [PubMed] [Google Scholar]
- 49. Amatruda T. T., 3rd, Steele D. A., Slepak V. Z., Simon M. I. (1991) Gα16, a G protein α subunit specifically expressed in hematopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 88, 5587–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mapara M. Y., Bommert K., Bargou R. C., Leng C., Beck C., Ludwig W. D., Gierschik P., Dörken B. (1995) G protein subunit Gα16 expression is restricted to progenitor B cells during human B-cell differentiation. Blood 85, 1836–1842 [PubMed] [Google Scholar]