Abstract
Purpose
Previous epidemiological works have reported that obesity is a risk factor for kidney stone disease. However, the effect of increasing degrees of obesity on stone formation has yet to be defined. To address this question we examined how an increasing body mass index affects the risk of kidney stone disease.
Materials and Methods
We evaluated claims from a 5-year period (2002 to 2006) in a national private insurance database to identify subjects diagnosed with or treated for kidney stones. From a data set of 95,598 patients, subjects were identified by ICD-9 or CPT codes specific to kidney stone disease. Descriptive analyses were performed and odds ratios were calculated.
Results
Gender distribution of the 3,257 stone formers was 42.9% male and 57.1% female. Obesity (body mass index greater than 30 kg/m2) was associated with a significantly greater likelihood of being diagnosed with a kidney stone. However, when obese patients were stratified by body mass index there were no significant differences in the likelihood of a kidney stone diagnosis, suggesting a stabilization of risk once body mass index increased above 30 kg/m2. The association of body mass index and a stone removal procedure was significant only for men and women with a body mass index between 30 and 45 kg/m2 relative to a body mass index less than 25 kg/m2 (p < 0.001).
Conclusions
An obese body mass index is associated with an increased risk of kidney stone disease. However, the magnitude of this risk appears to be stable in the morbidly obese population. Once body mass index is greater than 30 kg/m2, further increases do not appear to significantly increase the risk of stone disease.
Keywords: kidney calculi, obesity, epidemiology
The obesity epidemic threatens to redefine diagnostic and treatment algorithms throughout medicine. Recent evidence suggests that more than 30% of American adults may already be called obese while the prevalence of obesity has been reported to be increasing at an alarming rate.1 Obesity is an important public health concern as it creates a deferred societal burden of type II diabetes, heart disease, hypertension, pregnancy complications, sleep apnea and other health problems. To this list of morbidities one may add nephrolithiasis, as previous epidemiological studies have described an association between obesity and kidney stone disease.2–4 Interestingly just as recent epidemiological investigations have noted the prevalence of obesity to be increasing, so too has the prevalence of kidney stone disease been increasing, a coincidence suggesting the possibility that these disorders share a common pathophysiology.3
Little is known about the relationship between obesity and nephrolithiasis. In particular we have no insight into the effect that increasing degrees of obesity might have on the prevalence of nephrolithiasis. It may be that the effect of obesity on stone disease is one of an exposure-response relationship, a process by which the prevalence of stone disease increases as the magnitude of obesity increases. Alternatively the converse may be true, that the prevalence of stone disease may not increase in concert with increasingly obese BMI values once a certain threshold is attained. A better understanding of these unique relationships may ultimately lead to improved therapies for stone formers. Therefore, we performed a study to define the prevalence of clinically diagnosed and surgically treated kidney stone disease in obese patients, and to stratify these data by BMI.
MATERIALS AND METHODS
The data and in-kind database development support and guidance were provided by the BCBS Association, BCBS of Tennessee, BCBS of Hawaii, BCBS of Michigan, BCBS of North Carolina, Highmark, Inc. of Pennsylvania, Independence Blue Cross of Pennsylvania, Wellmark BCBS of Iowa and Wellmark BCBS of South Dakota. All individuals with 1 of these 7 plans as their primary insurer were eligible for inclusion in the data set. The claims data used in this study were de-identified in accordance with the Health Insurance Portability and Accountability Act of 1996 definition of a limited data set, and were used in accordance with federal standards for protecting confidentiality of the personal health information of the enrollee. The institutional review board of The Johns Hopkins University found this analysis to be exempt from the requirement for review.
The data set included approximately 3.4 million insured lives during a 5-year period (2002 to 2006), with information on enrollee age, gender, enrollment dates and claims for reimbursement for billable health care services. Included in these data were patient diagnoses as identified by ICD-9 codes and Diagnosis Related Groups, and medical procedures classified by CPT codes and ICD-9 procedure codes. A subset of patients (95,598) also completed a HRA which included BMI, and these assessments provided the data used for this study. A substantial proportion of patients (30.6%) completed HRAs on more than 1 occasion during the 5-year period. For these subjects with multiple BMI recordings the BMI closest in time to the stone diagnosis was used. Age was calculated as of the date of the HRA used to obtain the BMI reading. We excluded from analysis the female subjects with a code indicating pregnancy the year before, the year of or the year after an obese BMI value.
For statistical analysis BMI strata were set at less than 20, 20.00 to 24.99, 25.00 to 29.99, 30.00 to 34.99, 35.00 to 39.99, 40.00 to 44.99, 45.00 to 49.99, and 50 kg/m2 or more. The main dependent variables in this study were a diagnosis of upper urinary tract calculus or a stone removal procedure (SWL, ureteroscopy or percutaneous nephrolithotomy). The codes used to define these conditions and procedures are listed in the Appendix. Simple descriptive statistics were obtained, and logistic regression was performed to model the odds of a diagnosis or procedure by age, gender and BMI. SAS® version 9.13 was used for all analyses.
RESULTS
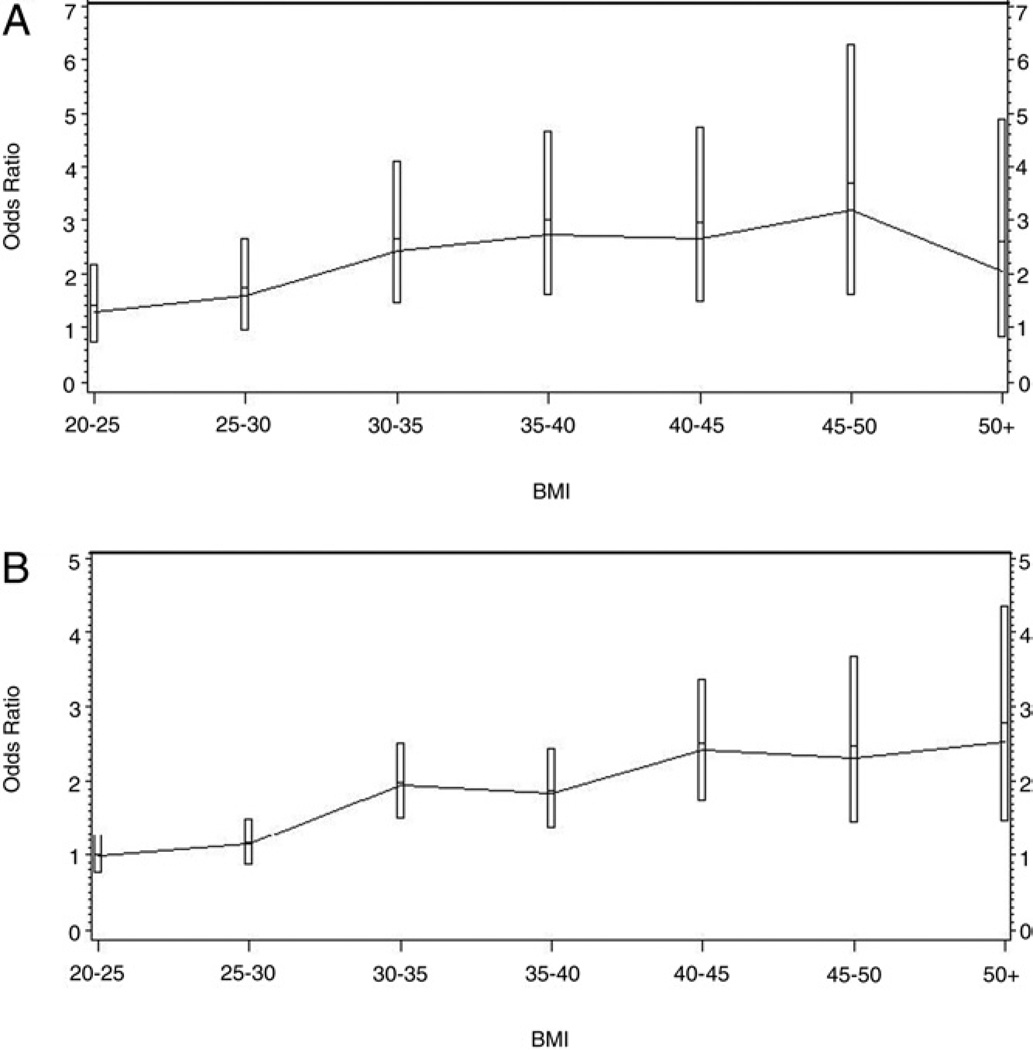
Of the 95,598 subjects identified for evaluation 54,572 were female (57.1%) and 41,026 were male (42.9%). Mean patient age was 48.9 years (SD 20.2) for males and 48.2 (SD 18.1) for females. Median observation period was 3.6 years (SE 0.005) for males and 3.7 (SE 0.004) for females. Overall the diagnosis of a kidney stone occurred in 3.4% of the evaluated group. The likelihood of being diagnosed with a kidney stone was twice as high in men as it was in women, and increased with age from 1.44 for those between 35 and 44 years old to 2.44 for those between 55 and 64 years old. Of the cohort with a BMI less than 30 kg/m2, 2.6% were diagnosed with a kidney stone during the evaluation period. In contrast, 4.9% of subjects with a BMI greater than 30 kg/m2 were diagnosed with a kidney stone during the evaluation period (table 1). At all stratifications of obesity (except for men with a BMI greater than 50 kg/m2) obese patients were significantly more likely to be diagnosed with a kidney stone than were the nonobese (BMI less than 30 kg/m2) (see figure).
Table 1.
Diagnosis of a kidney stone
| BMI (kg/m2) | No. Pts | Mean Age | No. Diagnosed With Kidney Stone (%) | Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| Males: | |||||
| Less than 20 | 674 | 43.5 | 15 (2.2) | — | — |
| 20.0–24.9 | 9,135 | 47.9 | 293 (3.2) | 1.28 (0.76–2.17) | 0.3550 |
| 25.0–29.9 | 16,389 | 48.4 | 661 (4.0) | 1.58 (0.94–2.66) | 0.0829 |
| 30.0–34.9 | 10,538 | 50.8 | 672 (6.4) | 2.44 (1.45–4.10) | 0.0008 |
| 35.0–39.9 | 2,938 | 49.2 | 204 (6.9) | 2.74 (1.61–4.67) | 0.0002 |
| 40.0–44.9 | 923 | 48.2 | 61 (6.6) | 2.66 (1.49–4.73) | 0.0009 |
| 45.0–49.9 | 269 | 47.6 | 21 (7.8) | 3.18 (1.61–6.29) | 0.0009 |
| 50.0 or Greater | 160 | 46.4 | 8 (5.0) | 2.03 (0.84–4.09) | 0.1135 |
| Females: | |||||
| Less than 20 | 4,338 | 43.8 | 74 (1.7) | — | — |
| 20.0–24.9 | 19,165 | 46.5 | 335 (1.7) | 0.99 (0.77–1.28) | 0.9443 |
| 25.0–29.9 | 12,846 | 48.7 | 265 (2.1) | 1.15 (0.99–1.49) | 0.3009 |
| 30.0–34.9 | 11,100 | 51.5 | 389 (3.5) | 1.95 (1.51–2.51) | <0.0001 |
| 35.0–39.9 | 4,379 | 49.6 | 143 (3.3) | 1.84 (1.38–2.44) | <0.0001 |
| 40.0–44.9 | 1,751 | 47.9 | 74 (4.2) | 2.42 (1.75–3.37) | <0.0001 |
| 44.0–49.9 | 609 | 49.0 | 25 (4.1) | 2.31 (1.45–3.67) | 0.0004 |
| 50.0 or Greater | 384 | 47.8 | 17 (4.4) | 2.54 (1.48–4.35) | 0.0007 |
Figure 1.
Odds ratios with 95% CI of diagnosis of kidney stone for males (A) and females (B).
There were no significant differences among BMI stratifications less than 30 kg/m2 and there were no significant differences among BMI stratifications that were greater than 30 kg/m2 (p = 0.9246). These findings remained true even when the cohorts were further analyzed by gender and age as confirmed by logistic regression. Although the odds ratios do increase up to a BMI of 50 kg/m2, the confidence intervals overlap for all BMI intervals greater than 30 kg/m2, implying the nonsignificant nature of the trend. Similarly the odds ratios for subjects with a BMI less than 30 kg/m2 also overlap.
When the use of stone removal procedures was examined certain cohorts of obese subjects had an increased likelihood of undergoing a surgical intervention whereas other cohorts did not (table 2). Specifically men with a BMI between 30 and 45 kg/m2 were significantly more likely to undergo a stone removal procedure compared to nonobese men. However, men with a BMI greater than 45 kg/m2 were no more likely to undergo a stone removal procedure than were nonobese men. Similarly women with a BMI between 30 and 45 kg/m2 were significantly more likely to undergo a stone removal procedure than were nonobese women. However, women with a BMI greater than 45 kg/m2 were no more likely to undergo a stone removal procedure than were nonobese women.
Table 2.
Kidney stone removal procedures performed
| BMI (kg/m2) | No. Pts | No. Treated for Kidney Stone (%) |
Odds Ratio (95% CI) |
p Value |
|---|---|---|---|---|
| Males: | ||||
| Less than 25 | 9,809 | 53 (0.5) | — | — |
| 25.0–29.9 | 16,389 | 149 (0.9) | 1.62 (1.18–2.22) | 0.0027 |
| 30.0–34.9 | 10,538 | 132 (1.3) | 2.14 (1.55–2.95) | <0.0001 |
| 35.0–39.9 | 2,938 | 43 (1.5) | 2.55 (1.70–3.83) | <0.0001 |
| 40.0–44.9 | 923 | 11 (1.2) | 2.12 (1.10–4.07) | 0.0247 |
| 45.0–49.9 | 269 | 3 (1.1) | 1.95 (0.61–6.30) | 0.2621 |
| 50.0 or Greater | 160 | 1 (0.6) | 1.11 (0.15–8.09) | 0.9175 |
| Females: | ||||
| Less than 25 | 23,503 | 54 (0.2) | — | — |
| 25.0–29.9 | 12,846 | 44 (0.3) | 1.44 (0.97–2.15) | 0.0742 |
| 30.0–34.9 | 11,100 | 83 (0.7) | 3.12 (2.21–4.42) | <0.0001 |
| 35.0–39.9 | 4,379 | 35 (0.8) | 3.38 (2.21–5.19) | <0.0001 |
| 40.0–44.9 | 1,751 | 17 (1.0) | 4.14 (2.40–7.16) | <0.0001 |
| 45.0–49.9 | 609 | 4 (0.7) | 2.71 (0.98–7.51) | 0.0553 |
| 50.0 or Greater | 384 | 3 (0.8) | 3.31 (1.03–10.62) | 0.0447 |
Ureteroscopic interventions and SWL were used to a similar extent at 11% and 10%, respectively (table 3). Nephrolithotomy was much less commonly performed (0.9%). The use of all surgical procedures peaked in subjects with a BMI between 35 and 39.9 kg/m2.
Table 3.
Detailed breakdown of kidney stone procedures performed
| BMI (kg/m2) | No. Pts |
No. SWL (%) |
No. Ureteroscopy (%) |
No. Nephrolithotomy (%) |
|---|---|---|---|---|
| Less than 25 | 718 | 64 (8.9) | 57 (7.9) | 0 (0.0) |
| 25–29.9 | 933 | 96 (10.3) | 114 (12.2) | 6 (0.6) |
| 30–34.9 | 1,064 | 112 (10.5) | 125 (11.7) | 10 (0.9) |
| 35–39.9 | 348 | 43 (12.4) | 42 (12.1) | 10 (2.9) |
| 40–44.9 | 136 | 14 (10.3) | 15 (11.0) | 2 (1.5) |
| 44–49.9 | 46 | 3 (6.5) | 4 (8.7) | 0 (0.0) |
| 50 or Greater | 25 | 2 (8.0) | 2 (8.0) | 0 (0.0) |
In those 3,270 patients diagnosed with a kidney stone only.
DISCUSSION
Curhan et al performed one of the earliest studies characterizing the effect of body size on kidney stone risk in a cross-sectional analysis of the NHS I and II, and the HPFS.4 For men and women they found that the prevalence of stone disease was directly associated with BMI, although the magnitude of the association was consistently greater for women than for men. Taylor et al subsequently advanced this work when they prospectively evaluated the effect of obesity and weight gain on kidney stone risk.2 Using the NHS I, NHS II and HPFS data sets they also found that BMI was positively associated with the risk of kidney stone formation. The authors further characterized the effect of an increasing magnitude of obesity. The relative risk for women with a BMI greater than 35 kg/m2 was significantly greater than for women with a BMI greater than 30 kg/m2. The study had inadequate data to permit any analysis of stone risk in men with a BMI greater than 35 kg/m2. Nonetheless the data from the female cohort suggest an increased risk of kidney stone disease with an increasing BMI.
What the present work uniquely adds to our understanding of the relationship between obesity and kidney stone disease is the subcategorization of a large cohort with extreme, or morbid, obesity. Our analysis included 33,051 subjects with a BMI greater than 30 kg/m2. Even among those with class 2 (BMI 35 to 39.9 kg/m2) or class 3 (BMI greater than 40 kg/m2) obesity we were able to evaluate the prevalence of stone disease for 11,413 subjects. Therefore, we can provide a robust description of the prevalence of stone disease among all categories of obesity. Indeed to our knowledge there has been no similar report stratifying the effect of increasing degrees of obesity on stone risk, particularly in those with extreme obesity.
Although our analyses confirm that obesity is associated with an increased risk of kidney stone disease, we also found that once a BMI of 30 kg/m2 is achieved, the risk of stone disease stabilizes. It is not clear why the likelihood of being diagnosed with a kidney stone does not continuously increase in concert with an increasing BMI. The urinary milieu may be contributory in that an increasing BMI is associated with an increase in the excretion of promoters and inhibitors of calcium oxalate stone formation.5,6
Ekeruo et al,7 and Taylor and Curhan8 studied the effect of body size on urine chemistry, and found a significant association between increasing BMI and lithogenic risk factors such as calcium, oxalate, sodium, phosphate and uric acid excretion as well as urinary pH levels. However, they also noted that potentially protective factors such as urine volume and urinary citrate concentrations also increased in concert with an increasing BMI. While Ekeruo et al did not calculate urinary supersaturation values which permit the most accurate estimation of stone risk,7 Taylor and Curhan found that the urinary supersaturation of calcium oxalate had no significant relationship with BMI in any population.8 It may be that promoters and inhibitors of calcium oxalate stone formation increase in concert with an increasing BMI so that the overall risk of calcium oxalate stone formation does not increase significantly in the obese population.
Interestingly Taylor and Curhan did note a significant association between BMI and urinary supersaturation of uric acid, a finding which would increase the risk of uric acid stone formation.8 These data are consistent with previous studies demonstrating an increased rate of uric acid stone formation in obese patients. 9–11 Insulin resistance, which develops due to obesity, may be a part of the common pathophysiology driving the underlying formation of uric acid stones. Insulin resistance alters urinary parameters such as pH and uric acid.12 Additionally obese subjects are at increased risk for gouty diathesis, which may further promote uric acid stone formation.6,7 Unfortunately the database used in our study does not contain information on stone composition, thus limiting the conclusions that can be made about the role of stone composition.
The divergence between stone prevalence and stone removal procedures was unexpected. Intuitively one would expect that as more kidney stones are diagnosed, more stone removal procedures would be performed. However, in this study despite our finding that kidney stones were more commonly diagnosed in men with a BMI between 40 and 50 kg/m2, stone removal procedures were not significantly increased in this cohort. Rather stone removal procedures were only increased in those patients with a BMI between 30 and 45 kg/m2. Similarly although all obese women were at increased likelihood for a kidney stone, stone removal procedures were significantly more common only in women with a BMI less than 45 kg/m2. Although we cannot provide a definitive explanation for this phenomenon, a conjecture worth considering is that uric acid stone disease is reported to be more common in the obese. Uric acid calculi are one of the few types of stones that can be chemically dissolved with medical therapy. Therefore, it may be that fewer obese subjects required surgical intervention because this cohort had a greater proportion of stones treated successfully with medical dissolution, rendering surgery unnecessary.
Alternative hypotheses may also be readily developed. As obesity is associated with significant medical comorbidities, the extremely obese may have an increased use of health care resources and encounters. Such use may lead to a detection bias, resulting in an increase in incidental, asymptomatic kidney stones that do not require treatment. It may also be that urologists will offer a longer course of conservative management with a trial of passage to the very obese because they may be hesitant to perform a surgical procedure. There may also be an as yet uncharacterized increased rate of stone passage in obese patients. Lastly it is possible that we were simply unable to detect a significant difference in the higher BMI categories because the event rates for these groups were so small (between 1 and 11). Ostensibly further investigation to better characterize this finding is welcome and necessary.
The limitations of our work merit discussion. Given the nature of an administrative claims database we did not have access to certain clinical data including stone composition, laboratory studies or diagnostic circumstances. Another limitation intrinsic to administrative claims databases is potentially erroneous and incomplete coding (ie failing to list codes for all diagnoses relevant to a given admission, or missing claims).
CONCLUSIONS
Obesity is associated with an increased risk of kidney stone formation, a concerning finding considering that obesity and nephrolithiasis are increasing at a great rate. However, the risk of stone disease in the obese population does remain stable with increasing degrees of obesity as stratified by BMI based on our present analysis. Dietary modification and weight loss should be encouraged in the obese population for a multitude of reasons but also to reduce stone risk.
ACKNOWLEDGMENTS
The Hariri Family Foundation, Mr. and Mrs. Chad and Nissa Richison, the Blue Cross and Blue Shield Plans, and the many staff members at these sites actively contributed to this study by providing data and expert advice. These organizations included BCBS of Tennessee, Highmark BCBS of Pennsylvania, BCBS of Michigan, BCBS of North Carolina, Independence Blue Cross (of Pennsylvania), Wellmark BCBS of Iowa and South Dakota, and the Hawaii Medical Service Association. Eric Bass and Jonathan Weiner provided study support.
Supported by The Hariri Family Foundation, and Mr. and Mrs. Chad and Nissa Richison.
Supported by Grant T32DK07552 from the National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases. The contents are solely the responsibility of the author, and do not necessarily represent the official views of the National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases.
The data set used in this study was originally created for a different research project on patterns of obesity care within selected BCBS plans. The previous research project (but not the current study) was funded by unrestricted research grants from Ethicon Endo-Surgery, Inc. (a Johnson & Johnson company); Pfizer, Inc and GlaxoSmith-Kline.
Abbreviations and Acronyms
- BCBS
Blue Cross/Blue Shield
- BMI
body mass index
- HPFS
Health Professional Follow-Up Study
- HRA
Health Risk Assessment
- NHS
Nurses’ Health Study
- SWL
shock wave lithotripsy
APPENDIX
Codes Used to Define Urology Procedures and Conditions
| Indicator | Type of Code | Description |
|---|---|---|
| Urological Procedures | ||
| SWL | CPT code | 50590 Lithotripsy, extracorporeal shock wave |
| S0400 Global fee for extracorporeal shock wave lithotripsy treatment of kidney stone(s) | ||
| ICD-9 procedure code | 98.5 Extracorporeal shock wave lithotripsy | |
| 98.51 Extracorporeal shock wave lithotripsy of the kidney, ureter and/or bladder | ||
| Ureteroscopy | CPT code | 52352 Cystourethroscopy, with ureteroscopy and/or pyeloscopy; with removal or manipulation of calculus |
| 52353 Cystourethroscopy, with ureteroscopy and/or pyeloscopy; with lithotripsy | ||
| Nephrolithotomy (percutaneous and open) | CPT code | 50060 Nephrolithotomy; removal of calculus |
| 50065 Nephrolithotomy; secondary surgical operation for calculus | ||
| 50070 Nephrolithotomy; complicated by congenital kidney abnormality | ||
| 50075 Nephrolithotomy; removal of large staghorn calculus filling renal pelvis and calices | ||
| 50080 Percutaneous nephrostolithotomy or pyelostolithotomy, up to 2 cm | ||
| 50081 Percutaneous nephrostolithotomy or pyelostolithotomy, over 2 cm | ||
| ICD-9 procedure code | 55.03 Nephrostomy | |
| Renal Disease Indicators | ||
| Urinary Calculi | Diagnosis Related Group | 323 Urinary stones with complication or comorbidity and/or extracorporeal shock wave lithotripsy |
| 324 Urinary stones without complication or comorbidity | ||
| ICD-9 diagnosis code | 274.11 Uric acid nephrolithiasis | |
| 592 Calculus of kidney and ureter | ||
| 592.0 Calculus of kidney – nephrolithiasis not otherwise specified | ||
| 592.1 Calculus of ureter | ||
| 592.9 Urinary calculus, unspecified |
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 3.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States, 1976–1994. Kidney Int. 2003;63:1817. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 4.Curhan GC, Willett WC, Rimm EB, et al. Body size and risk of kidney stones. J Am Soc Nephrol. 1998;9:1645. doi: 10.1681/ASN.V991645. [DOI] [PubMed] [Google Scholar]
- 5.Powell CR, Stoller ML, Schwartz BF, et al. Impact of body weight on urinary electrolytes in urinary stone formers. Urology. 2000;55:825. doi: 10.1016/s0090-4295(99)00617-2. [DOI] [PubMed] [Google Scholar]
- 6.Duffey BG, Pedro RN, Kriedberg C, et al. Lithogenic risk factors in the morbidly obese population. J Urol. 2008;179:1401. doi: 10.1016/j.juro.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 7.Ekeruo WO, Tan YH, Young MD, et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J Urol. 2004;172:159. doi: 10.1097/01.ju.0000128574.50588.97. [DOI] [PubMed] [Google Scholar]
- 8.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Maalouf NM, Sakhaee K, Parks JH, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 10.Daudon M, Lacour B, Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res. 2006;34:193. doi: 10.1007/s00240-006-0042-8. [DOI] [PubMed] [Google Scholar]
- 11.Asplin JR. Obesity and urolithiasis. Adv Chronic Kidney Dis. 2009;16:11. doi: 10.1053/j.ackd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Sakhaee K, Maalouf NM. Metabolic syndrome and uric acid nephrolithiasis. Semin Nephrol. 2008;28:174. doi: 10.1016/j.semnephrol.2008.01.010. [DOI] [PubMed] [Google Scholar]