Abstract
Purpose
This study aimed to perform a prospective evaluation of 18F-NaF and 18F-FDG PET/CT in the detection of occult metastatic disease in men with prostate cancer and biochemical relapse.
Methods
Thirty-seven men with prostate-specific antigen (PSA) relapse (median, 3.2 ng/mL; range, 0.5–40.2 ng/mL) after definitive therapy for localized prostate cancer [26 radical prostatectomy (RP), 11 external beam radiation therapy] and negative conventional imaging underwent 18F-FDG and 18F-NaF PET/CT on 2 separate days within the same week. Studies were interpreted by 2 experienced radiologists in consensus for abnormal uptake suspicious for metastatic disease. The reference standard was a combination of imaging and clinical follow-up. Rank of PSA values for positive and negative PET/CT was compared using analysis of variance adjusting for primary therapy. Association between PSA and scan positivity in patients with RP was evaluated using Wilcoxon rank sum test.
Results
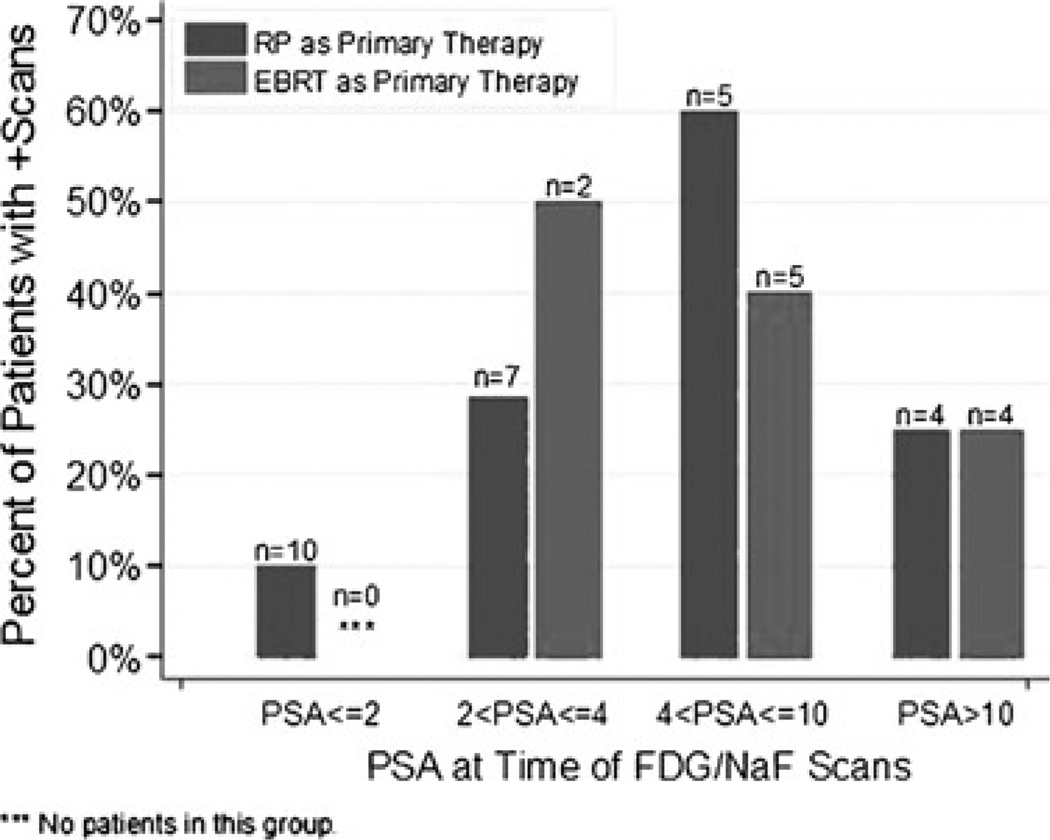
Result of the 18F-FDG PET/CT scan was positive for nodal disease in 2 patients. True-positive detection rate for occult osseous metastases by 18F-NaF PET/CT was 16.2%. Median PSA levels for positive versus negative PET/CT scans were 4.4 and 2.9 ng/mL, respectively, with the difference marginally significant in prostatectomized men (P = 0.072). Percentages of patients with either 18F-NaF– or 18F-FDG–positive PET/CT in RP and external beam radiation therapy were 10% (n = 10) and undefined (n = 0) for a PSA of 2 ng/mL or less, 29% (n = 7) and 50% (n = 2) for PSA greater than 2 ng/mL but 4 ng/mL or less,60% (n = 5) and 40%(n = 5) for PSA greater than 4 ng/mL but 10 ng/mL or less, and 25% (n = 4) and 25% (n = 4) for PSA greater than 10 ng/mL, respectively.
Conclusions
In biochemical relapse of prostate cancer, 18F-NaF PET/CT is useful in the detection of occult osseous metastases, whereas the yield of 18F-FDG PET/CT is relatively limited. 18F-NaF PET/CT positivity tends to associate with increasing PSA level in prostatectomized men and may occur in lower PSA ranges than conventionally recognized.
Keywords: 18F-NaF, 18F-FDG, prostate, cancer, PSA
Prostate cancer is the most common cancer and the second leading cause of cancer death affecting men in the United States.1 Despite successful treatments for localized prostate cancer, up to 40% of men will eventually (most within 10 y from primary treatment) experience a detectable rise in the serum prostate-specific antigen (PSA) level (biochemical failure), suggesting that prostate cancer can metastasize early in the course of the disease.2 However, biochemical failure may occur years before metastases become clinically evident, and the clinical course can be markedly different. The stratification of men with biochemical recurrence is an important unmet clinical need.
Conventional imaging evaluation with CT and 99mTc-based bone scintigraphy (BS) may be negative or indeterminate in a substantial number of men with biochemical failure. 111In-capromab pendetide (ProstaScint; EUSA Pharma, Inc, Langhorne, Pa), which is a radiolabeled antibody targeted at the prostate-specific membrane antigen, has low sensitivity for detecting osseous metastases and poor specificity, making it of limited clinical value.3 More sensitive and specific imaging procedures may provide valuable information because treatment options are primarily based on detection and location of disease, for instance, observation in the absence of frank metastases, and the use of bone-targeted therapy for bone metastases.
There is an increasing interest in the potential role of PET in prostate cancer. Several promising radiotracers are currently being investigated in the imaging evaluation of prostate cancer including 18F- or 11C-choline, 18F- or 11C-acetate, 16β-18F-fluoro-5α -dihydrotestosterone, targeted to the androgen receptor, anti–1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, a synthetic l-leucine analog, and prostate-specific membrane antigen–based PET radiotracers.4 However, the contribution of these radiotracers to decision-making in prostate cancer remains undefined, and this will require continued investigation.
18F-FDG is the most common PET radiotracer used for oncologic applications. The ability of 18F-FDG PET to detect cancer is based on elevated glucose metabolism in malignant tissue in comparison to that in normal tissue.5 Current literature suggests that 18F-FDG PET/CT may be useful in the imaging evaluation of men with metastatic prostate cancer.6 Similarly, 18F-NaF PET/CT offers significantly higher sensitivity and specificity for identifying bone metastases in comparison to 99mTc-based BS.7,8 Recent decision by the Center for Medicare and Medicaid Services to reimburse sites participating in the National Oncologic PET Registry for 18F-NaF PET/CT scans has facilitated use of this sensitive imaging method in patients with cancer.9
There is lack of data regarding the potential utility of 18F-NaF and 18F-FDG PET/CT in men with biochemical recurrence of prostate cancer, who have negative conventional imaging studies. In view of the current critical need for early and accurate localization of occult metastatic disease in biochemical failure of prostate cancer, we set out to perform a prospective evaluation of 18F-NaF and 18F-FDG PET/CT imaging in this population.
SUBJECTS AND METHODS
Patients
Patients were enrolled prospectively between September 22, 2010, and June 23, 2011, after approval of the research protocol by our institutional review board and the radiation safety committee. All patients signed the informed consent in adherence to the Health Insurance Portability and Accountability Act regulations.
The study cohort included 37 men (median age, 71.1 y; range, 53.5–86.9 y) with inclusion criteria of originally localized biopsy-proven prostate cancer (stage T1c, T2, or T3) and presentation with PSA relapse (median, 3.2 ng/mL; range, 0.5–40.2 ng/mL) after definitive local therapy [radical prostatectomy (RP) in 26 men and external radiation beam therapy (EBRT) in 11 men]. Four prostatectomized men were receiving androgen deprivation therapy with castrate levels of serum testosterone. The median and range for the time interval between primary therapy and biochemical failure was 5.7 years and from 0.07 to 22.3 years, respectively. All PSA levels were obtained within 8 weeks of enrollment. The primary tumor Gleason sum score was less than 7 in 7 patients, 7 or higher in 27 patients, and unattainable in 3 patients.
Exclusion criteria included history of cancer other than prostate cancer, active infection, poorly controlled diabetes mellitus, active inflammatory conditions, recent or complicated nonhealing fracture, and hip or knee arthroplasty.
Definition of PSA Relapse
Treatment-specific American Urological Association and American Society for Therapeutic Radiology and Oncology consensus definitions of PSA relapse were used for those who had undergone either RP or EBRT, respectively. The American Urological Association’s definition considers biochemical failure when an initial serum PSA level is 0.2 ng/mL or higher with a second confirmatory rise in PSA.10 The American Society for Therapeutic Radiology and Oncology’s definition considers biochemical failure when there is a rise by 2 ng/mL or more above the nadir PSA level (“nadir + 2’’).11,12
Conventional Imaging
The inclusion criteria for our prospective study required negative or indeterminate conventional planar 99mTc-based BS and contrast-enhanced or unenhanced chest, abdomen, and pelvis CT that had been performed within 6 weeks of the PET/CT scans. Because our PET Imaging Center is a large tertiary referral site, a uniform approach was used to examine the imaging studies to confirm the negative official reports. Bone scintigraphy reports were unattainable in 7 patients, but the CT scans were negative for metastatic bone disease and the patients did not report bone pain.
PET/CT Imaging
Patients underwent both 18F-NaF [10.5 (1.1) mCi, 388.5 (40.7) MBq] and 18F-FDG [14.2 (0.7) mCi, 525.4 (25.9) MBq] PET/CT scans on 2 separate days in random order within 1 week. Each hybrid PET/CT (Biograph Duo LSO; Siemens, Erlangen, Germany) was performed 1 hour after IV administration of the radiotracer. Low-dose helical CT transmission scan [pitch 0.8, 50 mA s, 120 kV (peak)] was performed first for each of the 2 scans. Only oral contrast material but no IV contrast was used. PET was then performed with 3 minutes per bed position at a sufficient number of bed positions to cover the anatomic regions from the top of the head to the feet. Raw CT data were reconstructed into 5-mm-thick section of transverse images, and reformatted sagittal and coronal CT images were generated. CT-based attenuation-corrected PET images were reconstructed and viewed on a high-resolution colored monitor. PET and CT images could be viewed on a continuous fusion scale from PET only to CT only images using image fusion software (E-soft; Siemens).
All patients fasted for 4 to 6 hours before 18F-FDG PET/CT imaging only, and water intake was encouraged before both scans. Blood glucose level was determined in all patients before 18F-FDG administration and, in all cases, was less than 200 mg/dL.
Image Interpretation
Fused PET/CT images for each scan were interpreted by 2 board-certified fellowship-trained nuclear radiologists with more than 11 years of experience in interpreting hybrid PET/CT studies. No reader for a particular scan was aware of the findings on the other paired scan. All interpreted pairs of PET/CT studies were again reexamined for agreement by consensus.
Maximum-intensity-projection images were examined to help facilitate lesion detection. For 18F-FDG PET studies, SUVmax values of suspicious lesions (defined as foci of nonphysiological uptake above regional background activity) were obtained using 3D region of interest using vendor-provided software (Siemens). For 18F-NaF studies, suspicious skeletal lesions were identified based on published guidelines.13
REFERENCE STANDARD
The findings on PET/CT were validated by a 2-tier combination of follow-up (median, 24 weeks; range, 1–49 weeks) imaging and clinical management data. Follow-up imaging included 99mTc-based BS; contrast-enhanced CT scans of the chest, abdomen, and pelvis; regional MR; and 18F-NaF PET/CT.
True-positive finding was defined as either when follow-up imaging showed newly apparent or enlarging lesion at the same site as noted on PET/CT or in cases without relevant follow-up imaging, when the clinician felt compelled to continue or initiate new therapy (androgen deprivation therapy or chemotherapy) based on a total assessment of the clinical case including the PET results. True-negative finding was declared when result of the follow-up imaging was also negative or, in cases without follow-up imaging, when there was no change in clinical management (change to clinical trial with herbal or sipuleucel-T therapy was allowed). False-positive finding was defined when result of the follow-up imaging was negative for the lesions that were considered abnormal on PET/CT or, in cases without follow-up imaging, when the patient was continued to be observed or followed onto clinical trial therapy. False-negative finding was defined when PET/CT failed to capture lesions present on follow-up imaging or, in cases without follow-up imaging, when the patient was, nevertheless, started or continued on hormonal or chemotherapeutic regimen owing to continued rise in serum PSA level.
Statistical Data Analysis
The PET/CT imaging results were reported on a per-patient basis. For comparison of PSA levels between patients with positive scans and those with negative scans, the rank of the PSA values was compared between the 2 groups of patients using analysis of variance adjusting for patients’ primary therapy. We further evaluated the association between PSA levels and either 18F-FDG or 18F-NaF (or both) PET/CT scan positivity using the Wilcoxon rank sum test in the larger group of patients whose primary therapy was RP.
RESULTS
PET/CT Imaging
Table 1 is a summary of baseline patient characteristics, PET/CT T1 imaging results, and follow-up data for all patients. Both 18F-FDG PET/CT and 18F-NaF PET/CT were negative in 26 patients (70.3% of total). In 1 prostatectomized patient (2.7% of total; patient 1 in Table 1), only 18F-FDG PET/CT was positive for retroperitoneal lymph node metastases, and there were no suspicious hypermetabolic osseous lesions. In 8 patients (21.6% of total), only 18F-NaF PET/CT was positive, demonstrating randomly distributed skeletal lesions. Careful magnified examination of CT at bone window level occasionally demonstrated only subtle sclerosis and/or rarefaction at sites of PET-detected lesions (Fig. 2A, patient 17). Both 18F-NaF PET/CT and 18F-FDG PET/CT were positive in 2 patients (5.4% of total). In one of these patients (patient 28 in Table 1), only 1 of 5 osseous lesions identified on 18F-NaF PET/CT was also positive on 18F-FDG PET/CT (Fig. 1). In the other patient (patient 17 in Table 1), 18F-FDG PET/CT was positive for lymph nodes, whereas 18F-NaF PET/CT showed a positive osseous lesion in the left acetabulum that was not 18F-FDG avid (Fig. 2). There was at least 1 positive PET/CT scan in 11 (29.7%) of all 37 patients, in 7 (26.9%) of 26 patients in the RP group, and in 4 (36.4%) of 11 patients in the EBRT group. When positive PET/CT findings were characterized based on the defined validation criteria and per-patient basis, there were 7 true-positive, 4 false-positive, 7 false-negative, and 19 true-negative studies, yielding a positive predictive value of 64% and a negative predictive value of 73%.
TABLE 1.
Summary of Baseline Patient Characteristics, PET/CT Imaging Results, and Post-PET/CT Management Plan for all Patients
| No. | Age, y | GS | Primary Therapy |
Hormone Therapy at PET/CT |
Time From Primary Therapy to Biochemical Recurrence, y |
PSA at PET/CT, ng/mL |
PET/CT FDG/NaF |
Follow-up Period, wk |
Therapy Changes After PET/CT |
Follow-up Imaging |
Final PET/CT Result |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67.7 | 7 | RP | No | 3.76 | 7.22 | +/− | 31 | Started on ADT | N/A | TP |
| 2 | 67.1 | 7 | RP | Yes | 6.23 | 22 | −/− | 49 | Continue on ADT | CT C/A/P (−) | TN |
| 3 | 64.7 | 7 | EBRT | No | 2.09 | 4.1 | −/+ | 23 | Started on ADT | CT C/A/P (+) | TP |
| 4 | 59.8 | 7 | RP | No | 1.78 | 6.32 | −/− | 11 | Continue clinical trial with herbal therapy | MRI A/P (−) | TN |
| 5 | 70.8 | 9 | RP | No | 0.98 | 4.9 | −/− | 38 | Started on ADT | BS (−) CT C/A/P (−) |
TN |
| 6 | 63.2 | 9 | RP | No | 5.85 | 2.84 | −/− | 36 | Observation | BS (−) CT C/A/P (−) |
TN |
| 7 | 73.4 | 7 | EBRT | No | 10.99 | 2.68 | −/+ | 27 | Continue clinical trial with herbal therapy | N/A | FP |
| 8 | 67.3 | 8 | RP | No | 0.31 | 0.5 | −/− | 29 | Observation | CT C/A/P (−) | TN |
| 9 | 61.8 | 7 | RP | No | 11.90 | 1.9 | −/− | 24 | Stared on clinical trial with herbal therapy | N/A | TN |
| 10 | 83.4 | 7 | EBRT | No | 5.66 | 18.2 | −/− | 34 | Started on ADT | N/A | FN |
| 11 | 71.1 | 7 | RP | No | 0.62 | 2.1 | −/− | 5 | Observation | N/A | TN |
| 12 | 78 | 8 | RP | No | 16.31 | 1.53 | −/− | 37 | Started on clinical trial with herbal therapy | N/A | TN |
| 13 | 75.2 | N/A | RP | No | 8.00 | 4.61 | −/+ | 5 | Observation | N/A | FP |
| 14 | 82.6 | 6 | EBRT | No | 11.39 | 7.78 | −/− | 29 | Started on clinical trial with herbal therapy | CT C/A/P (−) | TN |
| 15 | 65.1 | 7 | RP | No | 2.92 | 2.1 | −/− | 23 | Started on clinical trial with herbal therapy | N/A | TN |
| 16 | 65.8 | 9 | EBRT | No | 4.93 | 3.4 | −/− | 31 | Started on clinical trial with herbal therapy | BS (−) CT C/A/P (−) |
TN |
| 17 | 83.9 | 9 | RP | Yes | 0.07 | 3.1 | +/+ | 36 | Continue ADT | N/A | TP |
| 18 | 71.2 | 6 | RP | No | 9.97 | 11.7 | −/− | 22 | Observation | MRI Hip/LS (−) | TN |
| 19 | 78.1 | 6 | RP | No | 9.45 | 2.67 | −/− | 37 | Observation | N/A | TN |
| 20 | 79.8 | 6 | EBRT | No | 17.76 | 10.1 | −/− | 36 | Started on ADT | N/A | FN |
| 21 | 72.8 | 7 | RP | No | 17.31 | 2.88 | −/− | 36 | Observation | MRI LS (−) | TN |
| 22 | 82.6 | 5 | RP | Yes | 22.33 | 27.4 | −/− | 36 | Continue ADT | N/A | FN |
| 23 | 53.5 | 9 | RP | No | 2.40 | 1.9 | −/+ | 11 | Continue clinical trial with herbal therapy | MRI Ribs (−) | FP |
| 24 | 86.9 | 5 | EBRT | No | 12.36 | 25.7 | −/+ | 44 | Started on ADT | NaF PET/CT (+) | TP |
| 25 | 81.5 | 7 | EBRT | No | 9.81 | 4.4 | −/+ | 24 | Observation | MRI shoulder (+) | TP |
| 26 | 68.6 | N/A | EBRT | No | 8.92 | 40.24 | −/− | 24 | Started on clinical trial with sipuleucel-T | N/A | TN |
| 27 | 65.4 | 7 | RP | No | 4.03 | 0.8 | −/− | 12 | Started on ADT | N/A | FN |
| 28 | 65.1 | 8 | RP | Yes | 7.54 | 29.3 | +/+ | 25 | Started on chemotherapy | BS (−) CT C/A/P (+) |
TP |
| 29 | 73.9 | 7 | RP | No | 3.03 | 0.97 | −/− | 1 | Observation | N/A | TN |
| 30 | 73.4 | 7 | RP | No | 3.31 | 5.83 | −/+ | 14 | Observation | N/A | FP |
| 31 | 78.8 | 9 | RP | No | 0.10 | 2.48 | −/+ | 16 | Started on ADT | N/A | TP |
| 32 | 66.2 | 9 | RP | No | 7.46 | 1.18 | −/− | 2 | Started on ADT | N/A | FN |
| 33 | 86.7 | 6 | EBRT | No | 15 | 9.49 | −/− | 15 | Observation | N/A | TN |
| 34 | 58.5 | 9 | RP | No | 2.80 | 1.8 | −/− | 19 | Started on ADT | N/A | FN |
| 35 | 54.3 | N/A | RP | No | 1.07 | 1.47 | −/− | 15 | Started on ADT | N/A | FN |
| 36 | 84.5 | 7 | EBRT | No | 2.17 | 6.6 | −/− | 7 | Started on clinical trial with herbal therapy | N/A | TN |
| 37 | 55.2 | 7 | RP | No | 1.34 | 1 | −/− | 9 | Started on clinical trial with sipuleucel-T | N/A | TN |
ADT indicates androgen deprivation therapy; BS = 99mTc-based planar BS; C/A/P, chest, abdomen, pelvis; FN, false-negative; FP, false-positive; GS, Gleason score; LN, lymph node; LS, lumbar spine; N/A, not attainable; TN, true-negative; TP, true-positive.
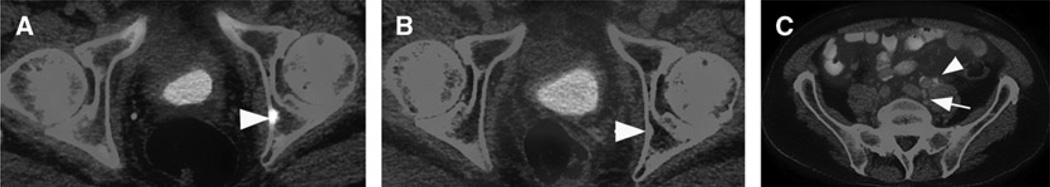
FIGURE 2.
Patient 17 in Table 1: Images of an 84-year-old man with biochemically recurrent prostate cancer post RP (PSA, 3.1 ng/mL; Gleason score 4+5). 18F-NaF PET/CT (A) showed a lesion in posterior left acetabulum (arrowheads) that was negative on 18F-FDG PET/CT (B). However, 18F-FDG PET/CT (C) also demonstrated 2 isolated hypermetabolic (SUVmax, 3) subcentimeter left common iliac lymph nodes (arrow) and left ureteral urine activity (arrowhead). The patient was continued on androgen deprivation therapy.
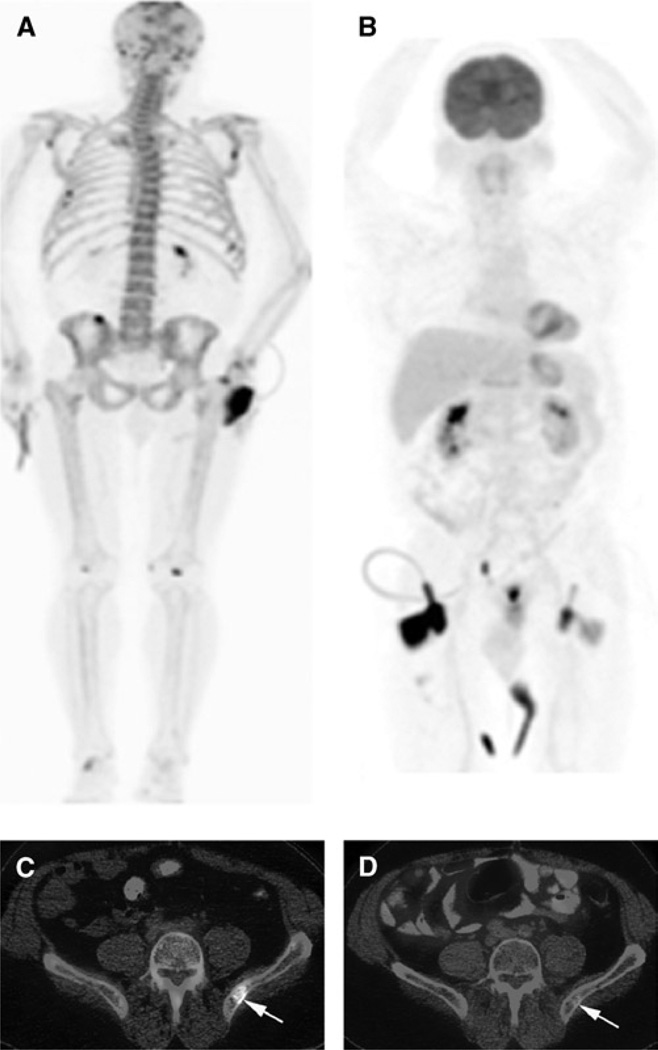
FIGURE 1.
Patient 28 in Table 1: Images of a 65-year-old man with biochemically recurrent prostate cancer post RP (PSA, 29.3 ng/mL; Gleason score 3+5). A, 18F-NaF PET/CT maximum intensity projection. B, 18F-FDG PET/CT maximum intensity projection. C, Fused 18F-NaF PET/CT. D, Fused 18F-FDG PET/CT. 18F-NaF PET/CT demonstrated randomly distributed osseous metastases. However, only the lesion in left posterior ilium was concordantly active on 18F-FDG PET/CT (arrows). The patient was started on docetaxel with a subsequent fall in serum PSA level to 4.25 ng/mL at 3 months after PET/CT scans.
PET/CT and PSA Relationship
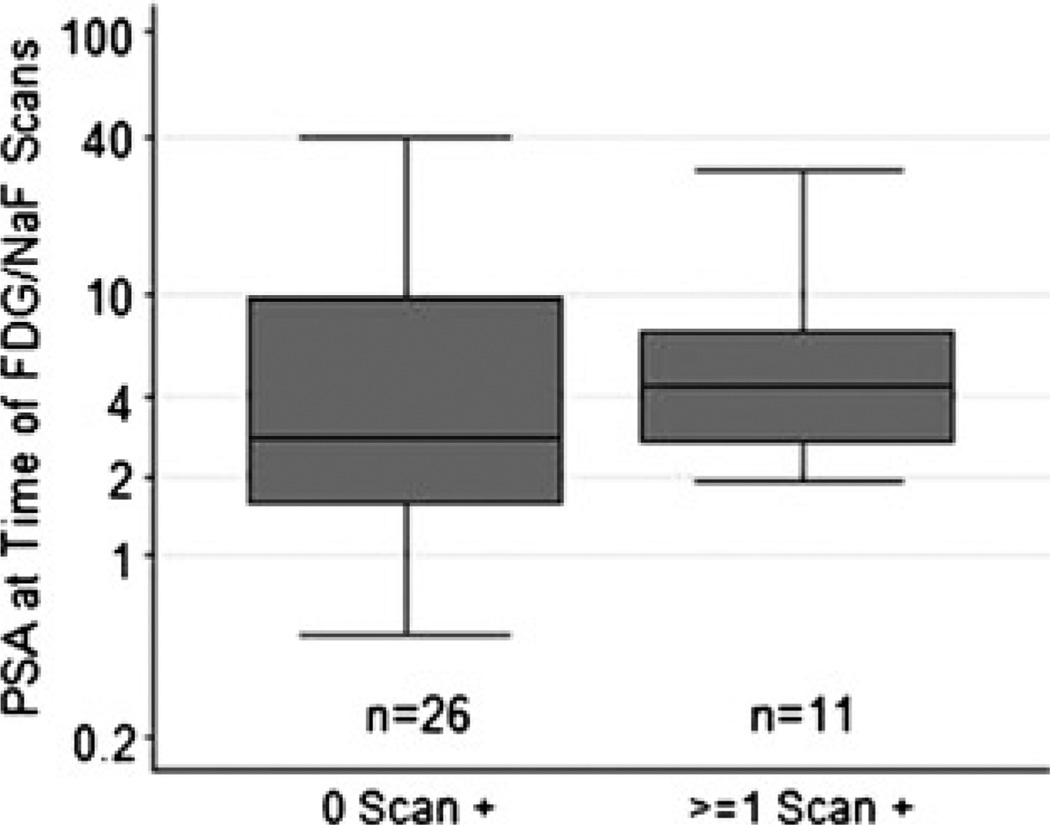
For the 8 patients with only 18F-NaF PET/CT positive scan, the median and range of PSA level were 4.1 ng/mL and from 1.9 to 5.8 ng/mL, respectively. For the 1 patient with only 18F-FDG PET/CT positive scan, the PSA level was 7.2 ng/mL. Interestingly, in the 2 prostatectomized men with both PET/CT scans positive, one had a relatively low PSA level of 3.1 ng/mL, whereas the PSA level was relatively high at 29.3 ng/mL in the other patient. Figure 3 summarizes the relationship between PSA and PET/CT results. The median PSA level for the 26 patients with negative scan results was 2.9 ng/mL and ranged from 0.5 to 40.2 ng/mL. The median PSA level for the 11 patients with at least 1 positive PET/CT scan was 4.4 ng/mL and ranged from 1.9 to 29.3 ng/mL. The difference in median PSA levels between the groups was not significant (P = 0.31).
FIGURE 3.
Box-and-whisker plot of the PSA values (log scale) at the time of PET/CT scans for patients with negative PET/CT scans comparing to those with 1 or more positive PET/CT scans. The difference in median PSA levels between the groups was not significant (P = 0.42). The line inside the box, box margins, and whisker margins represent the median value, interquartile ranges, and ranges, respectively.
Stratification Based on Primary Therapy
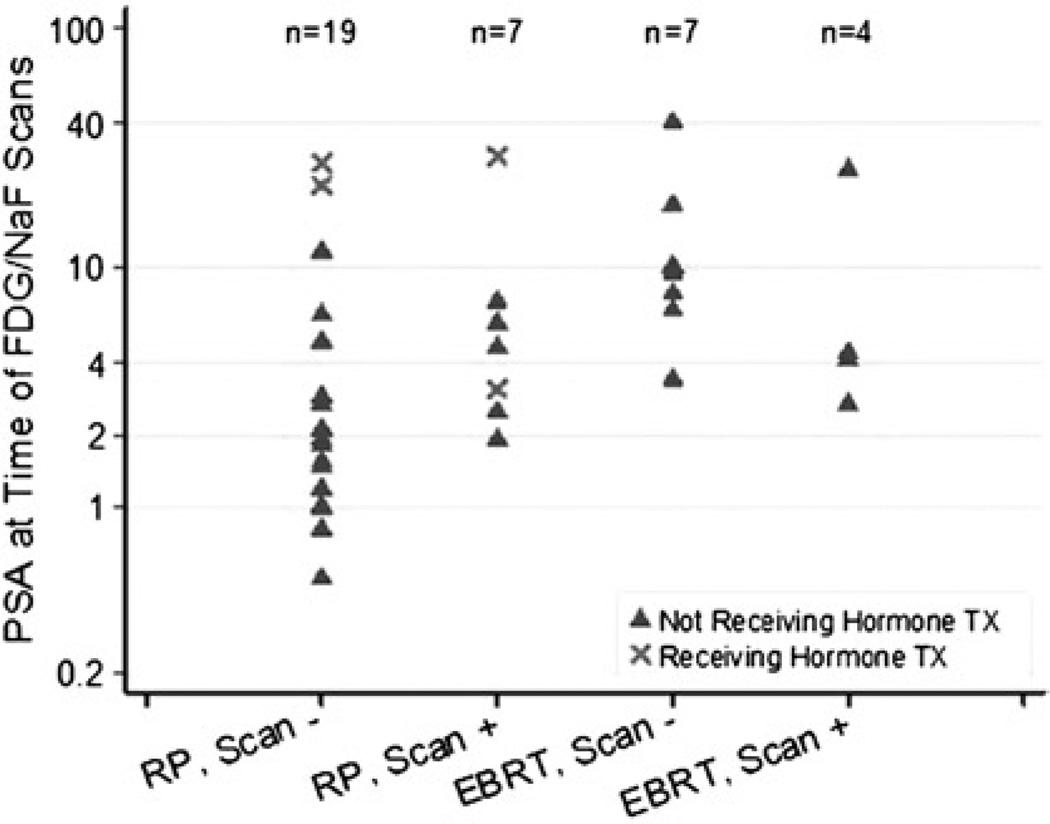
When analyzing patients whose primary therapy was RP and those whose primary therapy was EBRT separately, only patients with prior RP showed marginally higher median PSA levels in the positive PET/CT group in comparison to those with negative scans (4.6 vs 2.1 ng/mL, P = 0.072; Fig. 4). This result was unaffected by including or excluding the 4 patients on hormonal therapy (2 with positive PET/CT and 2 with negative PET/CT). Percentages of patients with at least 1 positive PET/CT in the RP group were 10% (n = 10) for PSA of 2 ng/mL or less, 29% (n = 7) for PSA greater than 2 ng/mL but 4 ng/mL or less, 60% (n = 5) for PSA greater than 4 ng/mL but 10 ng/mL or less, and 25% (n = 4) for PSA greater than 10 ng/mL. For the EBRT group, a PSA level of 2 ng/mL or less is undefined, but the percentages of patients with at least 1 positive PET/CT were 50% (n = 2) for PSA greater than 2 ng/mL but 4 ng/mL or less, 40% (n = 5) for PSA greater than 4 ng/mL but 10 ng/mL or less, and 25% (n = 4) for PSA greater than 10 ng/mL (Fig. 5).
FIGURE 4.
Values (log scale) of PSA at the time of PET/CT scans stratified by the patients’ primary therapy (RP or EBRT) and scan positivity. Patients with previous RP showed a marginally higher median PSA level in the positive PET/CT group in comparison to those with negative PET/CT scans (4.6 vs 2.1 ng/mL, P = 0.072).
FIGURE 5.
Percent of patients with 1 or more positive PET/CT scans grouped by PSA range (ng/mL).
Post PET/CT Management Plan
Although beyond the scope of our initial study design, we collected data on the management plans of patients immediately after completion of the imaging trial with PET/CT. In those 11 patients with at least 1 positive PET/CT, 4 were started and 1 was continued on androgen-deprivation therapy, 1 was started on chemotherapy (previously on androgen deprivation therapy), and 2 continued on clinical trial with herbal therapy and 3 on observation. In the 26 patients with negative PET/CT studies, 7 were started and 2 continued on androgen-deprivation therapy, 7 were started and/or maintained on clinical trial with herbal therapy, 8 were on observation, and 2 were on clinical trial with sipuleucel-T vaccine therapy. Overall, in those patients with available follow-up data, significant therapeutic management change was noted in 5 (45.4%) of 11 patients within the positive PET/CT group and 9 (34.6%) of 26 patients within the negative PET/CT group (Fisher exact test, P = 0.71). In the negative group, hormonal therapy was prompted by rapidly rising PSA levels despite negative conventional and PET/CT imaging studies.
DISCUSSION
There continues to be a diagnostic dilemma for early detection of recurrent and metastatic disease in a substantial number of men who present with biochemical failure after definitive therapy for localized primary prostate cancer. Accurate detection and localization of disease is pivotal because it affects the type of treatment. Salvage therapy may be considered in those men with only local recurrence, whereas systemic treatment will be needed for metastatic disease. Identifying bone metastases may be particularly important, given that treatment with bone-targeted pharmaceuticals have been associated with reduction in clinically meaningful skeletal-related events.14 Locally recurrent cancer is eventually detected in approximately 25% to 35%, metastatic disease only in approximately 20% to 25%, and both local recurrence and metastatic disease in 45% to 55% of men with biochemical failure.1,15
In the specific clinical setting of biochemically recurrent prostate cancer, radiolabeled (11C or 18F) choline has received most attention, particularly in Japan and Europe, with a reported sensitivity ranging between 38% and 98% for detection of locally recurrent and metastatic disease.16 Although there seems to be a direct association between PSA level and detection rate of choline PET, the wide range of reported sensitivity is probably reflective of the heterogeneity of patient population in terms of type of primary therapy, PSA ranges, and type and quality of the validation criteria, which, in some cases, have included positive standard imaging in violation of the definition of biochemical recurrence. Nevertheless, based on cumulative experience and when available, choline PET is expected to play an important role in the imaging evaluation of men with biochemical failure after definitive treatment of primary prostate cancer.
To our knowledge, this is the first systematic investigation to prospectively evaluate PET/CT with the commonly available 18F-NaF and 18F-FDG in men with prostate cancer who present with biochemical failure. We found that, in 18.9% (7 true-positive of 37 total) of our patients, at least one of the PET/CT scans was positive for occult metastatic disease. Although the incidence of positive scan for occult metastatic disease in our investigation is generally in line with the expected prevalence as stated previously, in some of our patients, local recurrence may have been present. Our study was not designed to exclude those with possible local recurrence nor was it designed to include a search for local recurrence. However, none of the patients received prostatic bed salvage therapy during the follow-up period.
Limited data are available regarding the potential utility of 18F-FDG PET/CT in men with biochemical recurrence of prostate cancer. In a retrospective study of 91 patients with PSA relapse after prostatectomy and validation of tumor presence by biopsy or clinical and imaging follow-up, a disease detection rate of 31% was reported.17 However, confidence in the accuracy of this figure is guarded in view of the heterogeneity and limitation of the validation criteria (eg, use of other concurrent positive imaging studies as criterion standard because it violated the definition of PSA relapse only condition). Our prospective study was, however, specifically designed to only include those men with biochemical failure who had negative conventional imaging with the goal of deciphering if nonstandard imaging may yield useful diagnostic information that will otherwise be unavailable by conventional imaging. We feel that our substantially lower detection rate of 8.1% (3/37 patients) for 18F-FDG PET/CT is more realistic in this clinical setting.
In another study of 24 patients with rising serum PSA levels after treatment of localized prostate tumors, 18F-FDG PET was performed before pelvic lymph nodes were dissected.18 In none of the patients did BS or pelvic CT yield positive findings. However, histology of pelvic lymph nodes confirmed the presence of metastases in 67% of patients with increased 18F-FDG uptake at sites of histopathologically proven metastases. In our investigation, hypermetabolic subcentimeter retroperitoneal lymph nodes were noted in 2 patients with PSA levels of 3.1 ng/mL (patient 17 in Table 1) and 7.2 ng/mL (patient 1 in Table 1); these patients were deemed to have true-positive findings. In another patient with a higher PSA level (29.3 ng/mL; patient 28 in Table 1), 18F-NaF PET/CT was positive for 5 bone lesions, of which only 1 was 18F-FDG avid.
Our study showed that, in 16.2% (6 true-positive of 37 total) of men with biochemical failure only, 18F-NaF PET/CT may reveal sites of occult osseous metastases. Moreover, in 8 of 10 patients with positive 18F-NaF PET/CT, the PSA level was relatively low (range, 1.9–5.83 ng/mL) at levels where conventional BS is often negative.19 Interestingly, recently it has also been shown that, soon after initiation of treatment, bone metastases may be identified in 11% of men with prostate cancer who were otherwise thought not to have skeletal involvement based on normal pretreatment conventional planar BS.20 This observation has been attributed to the early posttreatment “amplification of signal through flare” after successful therapy allowing the early posttreatment planar BS to reveal these otherwise occult lesions that may have in fact been visible on the more sensitive 18F-NaF PET scan.
Combination imaging with simultaneous 18F-FDG and 18F-NaF injection has been reported.21 However, currently, there is insufficient evidence to support its use in routine clinical practice, and there is some suggestion that it may lead to uncertainty in separating the contribution of each radiotracer for accurate interpretation.13,22,23 To avoid this potential limitation, we performed 18F-FDG and 18F-NaF PET/CT on separate days.
With the realization that decision to change clinical management is multifactorial, we noted that therapy was prompted in 45.4% of patients with positive PET/CT results. However, in 3 patients (13, 25, and 30 in Table 1), no immediate change in management was adopted in view of the perceived need for follow-up verification. In addition, change in treatment was noted in 9 of 26 patients with negative PET/CT (and conventional) imaging results that were influenced by rapidly rising PSA level. Within the limitations of our observational study, these data suggest that PET/CT might be useful in clinical decision making.
A potential limitation of our study was the lack of histologic verification by biopsy for all the imaging findings. However, we were constrained by practical, economical, and ethical issues. Because negative conventional imaging was the inclusion criterion, we used a combination of follow-up imaging and clinical management data for verification of PET/CT findings, which has commonly been used in the clinical imaging literature. We applied these criteria uniformly based on multidisciplinary consensus on all the available clinical information. Another potential limitation is with regard to the definition of conventional imaging that included planar BS without requirement for potential optimization with single-photon CT and/or correlation with anatomic imaging. However, given that our PET Imaging Center is a tertiary referral site, we had little control over the local community imaging practices, and as a result, our experience represents a more “real-world” situation.
In conclusion, our prospective study suggests that, although 18F-NaF PET/CT may be diagnostically informative in the detection and localization of occult osseous metastases in men with prostate cancer and biochemical failure, the role of 18F-FDG PET/CT may be limited. We also showed that there might be an association between PSA at the time of relapse to the probability of positive PET/CT scan in prostatectomized men and that positive findings occur in lower PSA ranges than conventionally recognized.
ACKNOWLEDGMENTS
The authors thank Julia Quillen, Banafsheh Peyvandi, Diana Shycoff, Syed Rahmanuddin, and Jessica Gomez for their administrative help with patient accrual.
This work was supported by the National Institutes of Health, National Cancer Institute Grant number R01-CA111613. The data collection and study management for this study were developed using CAFÉ (Common Application Framework Extensible) developed at University of Southern California’s Norris Comprehensive Cancer Center with support in part by award number P30CA014089 from the National Cancer Institute.
Footnotes
Conflicts of interest and sources of funding: none declared.
REFERENCES
- 1.National Cancer Institute. SEER: The Surveillance, Epidemiology, and End Results Program—based within the Surveillance Research Program at the National Cancer Institute. Baltimore, MD: National Cancer Institute; Available at: http://seer.cancer.gov. [Google Scholar]
- 2.Dong JT, Rinker-Schaeffer CW, Ichikawa T, et al. Prostate cancer—biology of metastasis and its clinical implications. World J Urol. 1996;14:182–189. doi: 10.1007/BF00186898. [DOI] [PubMed] [Google Scholar]
- 3.Haseman MK, Rosenthal SA, Polascik TJ. Capromab pendetide imaging of prostate cancer. Cancer Biother Radiopharm. 2000;15:131–140. doi: 10.1089/cbr.2000.15.131. [DOI] [PubMed] [Google Scholar]
- 4.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med. 2008;49:2031–2041. doi: 10.2967/jnumed.108.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillies RJ, Robey I, Catenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49:24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 6.Jadvar H. Molecular imaging of prostate cancer with [F-18]-fluorodeoxyglucose PET. Nat Rev Urol. 2009;6:317–323. doi: 10.1038/nrurol.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iagaru A, Mittra E, Dick DW, et al. Prospective evaluation of (99m)Tc MDP scintigraphy, (18)F NaF PET/CT, and (18)F FDG PET/CT for detection of skeletal metastases. Mol Imaging Biol. 2012;14:252–259. doi: 10.1007/s11307-011-0486-2. [DOI] [PubMed] [Google Scholar]
- 8.Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services. CMS Manual. Pub 100-03 Medicare National Coverage Determinations. Baltimore, MD: Centers for Medicare & Medicaid Services; Available at: http://www.cms.gov/transmittals/downloads/R119NCD.pdf. [Google Scholar]
- 10.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 11.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segall G, Delbeke D, Stabin MG, et al. SNM guideline for sodium 18F-fluoride PET/CT bone scan 1.0. J Nucl Med. 2010;51:1813–1820. doi: 10.2967/jnumed.110.082263. [DOI] [PubMed] [Google Scholar]
- 14.Bracarda S, Logothetis C, Sternberg CN, et al. Current and emerging treatment modalities for metastatic castration-resistant prostate cancer. BJU Int. 2011;107(suppl 2):13–20. doi: 10.1111/j.1464-410X.2010.10036.x. [DOI] [PubMed] [Google Scholar]
- 15.Carroll P. Rising PSA after a radical treatment. Eur Urol. 2001;40:9–16. doi: 10.1159/000049879. [DOI] [PubMed] [Google Scholar]
- 16.Picchio M, Briganti A, Fanti S, et al. The role of choline positron emission tomography/computed tomography in the management of patienst with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol. 2011;59:51–60. doi: 10.1016/j.eururo.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Schöder H, Herrmann K, Gönen M, et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for detection of disease in patients with prostatespecific antigen relapse after radical prostatectomy. Clin Cancer Res. 2005;11:4761–4769. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Wu HC, Tsai JJ, et al. Detecting metastatic pelvic lymph nodes by (18)F-2-deoxyglucose positron emission tomography in patients with prostate-specific antigen relapse after treatment for localized prostate cancer. Urol Int. 2003;70:311–315. doi: 10.1159/000070141. [DOI] [PubMed] [Google Scholar]
- 19.Gomez P, Manoharan M, Kim SS, et al. Radionuclide bone scintigraphy in patients with biochemical recurrence after radical prostatectomy: when is it indicated? BJU Int. 2004;94:299–302. doi: 10.1111/j.1464-410X.2004.04927.x. [DOI] [PubMed] [Google Scholar]
- 20.Cook GJR, Venkitaraman R, Sohaib AS, et al. The diagnostic utility of the flare phenomenon on bone scintigraphy in staging prostate cancer. Eur J Nucl Med Mol Imaging. 2011;38:7–13. doi: 10.1007/s00259-010-1576-0. [DOI] [PubMed] [Google Scholar]
- 21.Iagaru A, Mittra E, Yaghoubi S, et al. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy: results of the pilot-phase study. J Nucl Med. 2009;50:501–505. doi: 10.2967/jnumed.108.058339. [DOI] [PubMed] [Google Scholar]
- 22.Wade AA, Scott JA, Kuter I, et al. Flare response in 18F-fluoride ion PET bone scanning. AJR Am J Roentgenol. 2006;186:1783–1786. doi: 10.2214/AJR.05.0225. [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Rao R, Bailey DL. Combined 18F-FDG and fluoride approach in PET/CT imaging: is there a clinical future? [letter] J Nucl Med. 2010;51:165–167. doi: 10.2967/jnumed.109.066910. [DOI] [PubMed] [Google Scholar]