Abstract
Dental composites do not hinder bacteria colonization and plaque formation. Caries at the restoration margins is a frequent reason for replacement of existing restorations, which accounts for 50 to 70% of all restorations. The objectives of this study were to examine the filler level effect on nanocomposite containing nanoparticles of amorphous calcium phosphate (NACP) and investigate the load-bearing and acid-neutralizing properties and bacteria inhibition. NACP with 116-nm particle size were synthesized via a spray-drying technique and incorporated into a resin. Flexural strength of nanocomposite with 10 to 30% NACP fillers matched the strength of a commercial hybrid composite (p > 0.1). Nanocomposite with 40% NACP matched the strength of a microfill composite, which was 2-fold that of a resin-modified glass ionomer. Nanocomposite with 40% NACP neutralized a lactic acid solution of pH 4 by rapidly increasing the pH to 5.69 in 10 min. In contrast, the commercial controls had pH staying at near 4. Using Streptoccocus mutans, an agar disk-diffusion test showed no inhibition zone for commercial controls. In contrast, the inhibition zone was (2.5 ± 0.7) mm for nanocomposite with 40% NACP. Crystal violet staining showed that S. mutans coverage on nanocomposite was 1/4 that on commercial composite. In conclusion, novel calcium–phosphate nanocomposite matched the mechanical properties of commercial composite and rapidly neutralized lactic acid of pH 4. The nanocomposite appeared to moderately reduce the S. mutans growth, and further study is needed to obtain strong antimicrobial properties. The new nanocomposite may have potential to reduce secondary caries and restoration fracture, two main challenges facing tooth cavity restorations.
Keywords: dental nanocomposite, calcium phosphate, acid neutralization, S. mutans, stress bearing, tooth caries inhibition
INTRODUCTION
Tooth caries is a dietary carbohydrate-modified bacterial infectious disease and is one of the most common bacterial infections in humans.1–3 Resin composites have been increasingly used for tooth cavity restorations because of their esthetics and direct-filling capability.4–8 However, reports showed that “The two main challenges are secondary caries and bulk fracture.”7,8 Caries at the restoration margins is a frequent reason for replacing the existing restorations,9 accounting for 50 to 70% of all restorations.10,11 Replacement dentistry costs $5 billion annually in the U.S.12 Resin composites in general do not prevent secondary caries, because they do not hinder bacteria colonization and plaque formation. In fact, several studies indicated that composites allowed more accumulation of dental plaque on their surfaces than other restorative materials.13–15 In addition, frequent occurrence of gingivitis was reported when composites were placed at the subgingival area.16 Therefore, there is a need to develop dental composites that can inhibit the adherence and growth of bacteria which are responsible for tooth decay.
The basic mechanism of dental caries is demineralization through the attack by acid generated by bacteria.17–19 Therefore, acidogenic bacteria growth and biofilm formation with exposure to fermentable carbohydrates are responsible for dental caries.1–3,20–22 Plaque formation has been described to have three steps: pellicle formation, bacteria colonization, and biofilm maturation.23 In the initial stage, a proteinaceous film called pellicle forms on the tooth surface with adsorbed components from saliva, mucosa, and bacteria.24 Bacteria then adhere and colonize on this surface to grow into a biofilm, which is a heterogeneous structure consisting of clusters of various types of bacteria embedded in an extracellular matrix.25 Cariogenic bacteria such as Streptococcus mutans (S. mutans) and lactobacilli in the plaque metabolize carbohydrates to acids. Acid production causes demineralization to the tooth structure beneath the biofilm. Microscopic examinations showed that rough surfaces, cracks, and pits enhanced bacteria adherence and plaque formation,24,26 which likely contributed to secondary caries at the often microcracked, chipped, and ditched margins at the composite–tooth interfaces.19 Therefore, efforts were made to develop composites with antibacterial properties to inhibit caries formation. Composites fabricated from an antibacterial monomer were shown to inhibit the growth and plaque accumulation by S. mutans.14,27,28 Fluoride releasing restoratives were shown to have antibacterial properties to some extent,29,30 although conflicting results were reported.31,32
Calcium phosphate (CaP) composites were developed with remineralizing capabilities.33–36 The filler particle sizes ranged from about 1 to 55 μm.33–35 These novel composites released supersaturating levels of calcium (Ca) and phosphate (PO4) ions and remineralized tooth lesions in vitro.34,35 One drawback of the CaP composites was that they were mechanically weak, with flexural strength about half that of unfilled resin.33,34 Such a low strength was “inadequate to make these composites acceptable as bulk restoratives.”33 Recently, novel CaP nanoparticles of sizes of about 100 nm were synthesized via a spray-drying technique and filled into dental composites.36,37 These nanocomposites achieved Ca and PO4 release similar to those of previous CaP composites, while their mechanical properties were much higher.36,37 However, the effects of these nanocomposites on oral bacteria growth have not been investigated.
Accordingly, the objectives of this study were to examine the filler level effect on nanocomposite containing amorphous calcium phosphate nanoparticles, and investigate its load-bearing, acid-neutralizing, and antimicrobial properties for the first time. It would be beneficial for the nanocomposite to be mechanically stronger than a resin-modified glass ionomer, to match the mechanical properties of a commercial microfill composite, and yet be able to neutralize cariogenic acids and decrease the growth of S. mutans on the composite surface.
MATERIALS AND METHODS
Spray-drying ACP nanoparticles
The spray-drying apparatus was described previously.36,38 In this study, nanoparticles of ACP (Ca3[PO4]2), referred to as NACP, were synthesized via spray drying. A solution was prepared by adding 1.5125 g of glacial acetic acid (J.T. Baker, Phillipsburg, NJ) into 500 mL of distilled water. Then, 0.8 g of calcium carbonate (CaCO3, Fisher, Fair Lawn, NJ) and 5.094 g of DCPA (Baker) were dissolved into the acetic acid solution. This solution was added with distilled water to a total of 1 L. The final Ca and PO4 ionic concentrations were 8 mmol/L and 5.333 mmol/L, respectively. This yields a Ca/P molar ratio of 1.5, the same as that for ACP. This solution was sprayed into a heated glass column to be dried. An electrostatic precipitator (AirQuality, Minneapolis, MN) was connected to the lower end of the column to collect the dried particles. A separate study showed that the collected NACP particles had a size of 116 nm.39
Resin composite fabrication
Four filler mass fractions of NACP were used: 10%, 20%, 30%, and 40%. As a cofiller, barium boroaluminosilicate glass particles of a median diameter of 1.4 μm (Caulk/Dentsply, Milford, DE) were silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine. A resin of Bis-GMA (bisphenol glycidyl dimethacrylate) and TEGDMA (triethylene glycol dimethacrylate) at 1:1 mass ratio was rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoate.
Four nanocomposites were fabricated with the following fillers (1): 10% NACP + 65% glass; (2): 20% NACP + 50% glass; (3): 30% NACP + 35% glass; and (4) 40% NACP + 20% glass. The total filler level was gradually reduced when the NACP amount was increased to maintain a similar working viscosity for the paste by the same operator. This was because nanoparticles with a higher surface area required more resin to form a cohesive paste.
A commercial composite (TPH, Caulk/Dentsply, Milford, DE) was used as a control and referred to as “hybrid composite.” It consisted of barium glass and fumed silica with a mean size of about 0.8 μm, at 78% filler level by mass in a urethane-modified Bis-GMA-TEGDMA resin. A resin-modified glass ionomer (referred to as “RMGI”) (Vitremer, 3M, St. Paul, MN) served as a second control. It consisted of fluoroaluminosilicate glass and a light-sensitive, aqueous polyalkenoic acid. A powder/liquid ratio of 2.5/1 was used according to the manufacturer. As a third control, a microfill composite with nanofillers (40–200 nm) (Heliomolar, Ivoclar, Ontario, Canada) was used (referred to as “microfill composite”). The Heliomolar fillers consisted of silica and ytterbium-trifluoride (total filler mass fraction = 66.7%).
Flexural testing
For flexural testing, each paste was placed into a mold of 2 × 2 × 25 mm. All materials were photocured (Triad 2000, Dentsply, York, PA) for 1 min on each side, including specimens for acid neutralization and bacteria experiments. The specimens were incubated in a humidor at a relative humidity of 95% at 37° C for 24 h, and then immersed in distilled water at 37° C for 1 day. Specimens were fractured in three-point flexure with a 20-mm span at a crosshead speed of 1 mm/min on a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC). Flexural strength was calculated by as follows: S = 3PmaxL/(2bh2), where Pmax is the fracture load, L is span, b is specimen width, and h is thickness. Elastic modulus was calculated by as follows: E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope of the load-displacement curve.
Acid neutralization
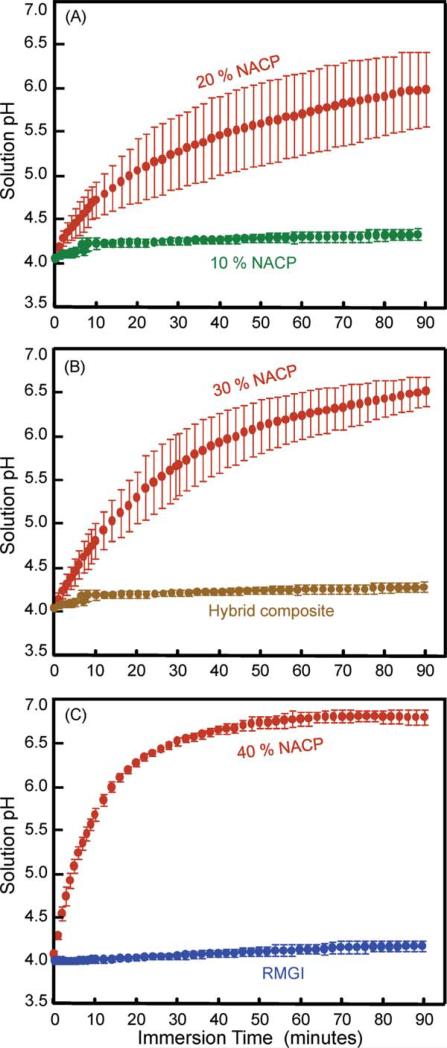
To characterize the acid neutralization ability of the composites, sodium chloride (NaCl) solution (133 mmol/L) was buffered to pH 4 with 5 mmol/L lactic acid. Three specimens of 2 × 2 × 12 mm were immersed in each vial, following previous studies.36 One milliliter of the pH 4 solution was used to submerge the three bars, yielding a composite volume/acid volume ratio of 0.14/1. As soon as the specimens were submerged in the acid solution, the pH of the solution was monitored with a combination pH electrode (Orion, Cambridge, MA). The pH was recorded versus time which was continued for 90 min. In addition, the acid neutralization capacity of the composite was calculated using a software (Chemist, Micromath Research, St. Louis, MO). The calculation used a simulation in which potassium hydroxide (KOH), a typical base, was added to the 1 mL solution of pH 4 to raise the pH. Increasing the pH from 4 to, say, a pH of 5, would require a certain amount of KOH to be added to the solution. This amount of KOH was calculated using the Chemist software. For any experimentally measured pH point in Figure 2, for example, the solution pH for the 40% NACP nanocomposite at 90 min, the Chemist software could calculate the amount of KOH needed to reach that pH. The effect of this KOH would be equivalent to the base released from the nanocomposite in neutralizing the acid and raising the pH. The amount of KOH that would need to be added, in order to increase the pH from 4 to the measured pH, was used to represent the acid neutralization capacity of the composite.
FIGURE 2.
Increasing the pH of a cariogenic acid solution. (A) Nanocomposite containing 10% and 20% NACP, (B) nanocomposite with 30% NACP, and hybrid composite, (C) nanocomposite with 40% NACP, and RMGI. The data for the microfill composite were similar to those of the hybrid composite and RMGI and were not included in the plot for clarity. Each datum is mean ± sd; n = 4. NACP nanocomposite increased the pH and neutralized the acid that otherwise could dissolve the tooth structure. In comparison, the commercial hybrid composite and RMGI did not raise the pH of the acid solution. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Inhibition of S. mutans growth
S. mutans was used because it is a major species of bacteria that produces organic acids responsible for dental caries.1,17S. mutans was obtained from American Type Culture Collection (ATCC 700610, Manassas, VA). The use of S. mutans was approved by the University of Maryland (IBC-00000794). S. mutans was cultured at 37° C with the infusion of 5% supplemented CO2 in a Brain Heart Infusion broth (BHI, Difco, Detroit, MI) from stock culture and used for the experiments.
Three materials were tested in the agar disk-diffusion test: Nanocomposite with 40% NACP and 20% glass; hybrid composite (TPH); and RMGI (Vitremer). Disks were fabricated in molds of 9 mm diameter and 2 mm thickness and photocured. The disks were sterilized in an ethylene oxide sterilizer (Andersen, Haw River, NC) for 1 day and degassed for 3 days. The sterilized disk was placed onto a BHI agar plate inoculated with 350 μL of 1 × 108 CFU/mL of S. mutans suspension,14 and the plate was incubated at 37° C for 48 h.
To examine bacterial growth on the bottom surface of the disk, the disk was removed from the agar plate. The bottom surface of the disk was examined via the method of crystal violet staining (Sigma, St. Louis, MO), in which the S. mutans adhering on the specimen stained a purple color.14,40 Photographs were taken of stained specimens with a camera (Nikon Digital Sight, Nikon, Melville, NY) attached to a microscope (Nikon Eclipse TE 2000-S). To quantify the area of the disk that was covered by bacteria, a NIS-Elements BR software (Nikon) was used to estimate the percentage of area that was covered by the bacteria (= the area stained purple/the total surface area of the disk).
Disk specimens were placed in a BHI agar plate inoculated with 350 μL of 1 × 108 CFU/mL of S. mutans suspension. After incubation for 48 h, the disks were removed and prepared for examination with scanning electron microscopy (SEM). The specimen was rinsed with phosphate buffered saline (PBS), and then immersed in 1% glutaraldehyde in PBS for 4 h at 4° C. The specimens were rinsed with PBS and subjected to graded ethanol dehydrations. They were then rinsed twice with 100% hexamethyldisilazane. The specimens were then sputter coated with gold and examined via SEM (JEOL 5300, Peabody, MA).
One-way and two-way ANOVA were performed to detect the significant effects of the variables. Tukey's multiple comparison test was used at a p value of 0.05 to compare the data.
RESULTS
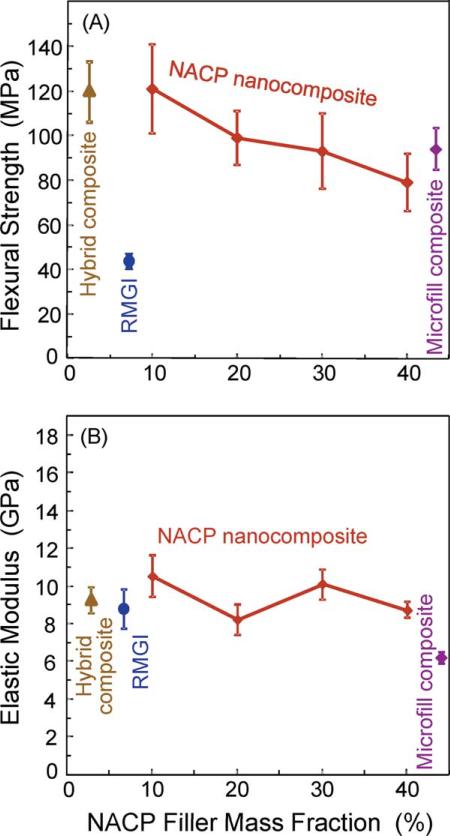
Figure 1 plots (A) flexural strength and (B) elastic modulus of the NACP nanocomposites with different NACP mass fractions, along with the microfill composite, hybrid composite, and RMGI. The nanocomposite strength at 10 to 30% NACP fillers matched the strength of microfill composite and hybrid composite (p > 0.1). Increasing the NACP filler level, while decreasing the glass filler level, significantly decreased the strength (p < 0.05). The nanocomposite at 40% NACP had a strength of (79 ± 5) MPa, significantly (p < 0.05) lower than (121 ± 10) MPa at 10% NACP, and (120 ± 13) MPa of the hybrid composite. However, the nanocomposite strength at 40% NACP was not significantly different from the (93 ± 10) MPa of the microfill composite (p > 0.1). All these composites had strengths that were 2- to 3-fold that of the RMGI. The elastic moduli were generally similar between the different materials (p > 0.1), except the microfill composite which had a lower modulus (p < 0.05).
FIGURE 1.
Mechanical properties of composites: (A) flexural strength, and (B) elastic modulus of NACP nanocomposites, microfill composite, hybrid composite, and RMGI. Each value is the mean of five measurements, with the error bar showing one standard deviation (mean ± sd; n = 5). The nanocomposite at 40% NACP had a strength that matched the commercial microfill composite (p > 0.1). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The acid neutralization results are plotted in Figure 2 for: (A) Nanocomposite with 10% and 20% NACP, (B) nanocomposite with 30% NACP, and hybrid composite, (C) nanocomposite with 40% NACP, and RMGI. The pH curve for the hybrid composite, RMGI, and microfill composite (not shown) were similar to each other, with little increase in pH. In contrast, the nanocomposite with 20 to 40% NACP greatly increased the pH. Therefore, the NACP nanocomposite had a strong ability to neutralize the acid and increase the pH. At 10 min after the specimens were immersed in the pH 4 solution, the pH became 5.69 ± 0.07 for nanocomposite with 40% NACP, higher than all other materials (p < 0.05). RMGI reached a pH of 4.06 ± 0.03. The hybrid composite reached a pH of 4.05 ± 0.02. At 90 min, the solution containing the nanocomposite with 40% NACP reached a pH of 6.80 ± 0.08, much higher than 4.21 ± 0.05 for RMGI, and 4.14 ± 0.03 for hybrid composite (p < 0.05).
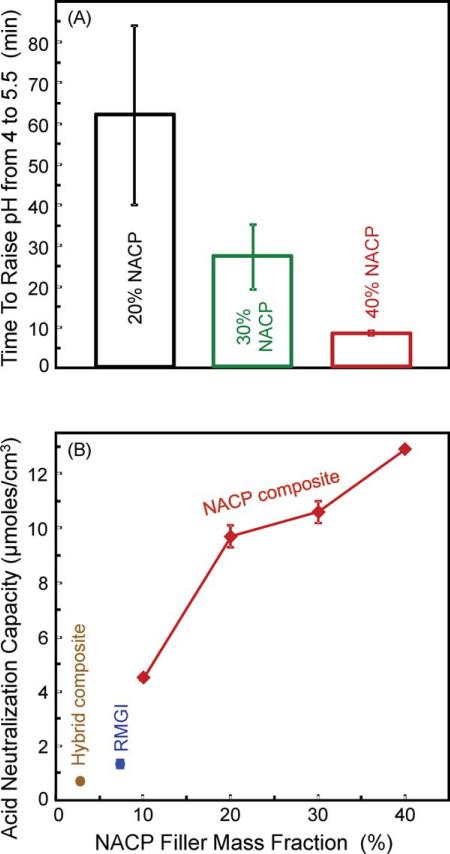
Because it is desirable for the composite to quickly raise the pH from a cariogenic pH to the critical pH of 5.5, Figure 3(A) plots the time it took for the composite to increase the solution pH from 4 to 5.5. It took the nanocomposite with 40% NACP (8.2 ± 0.5) min to reach pH 5.5, much faster than (27 ± 8) min for the 30% NACP composite, and (62 ± 22) min for the 20% NACP composite (p < 0.05). In comparison, the solutions containing RMGI, microfill, and hybrid composites never reached a pH of 5.5.
FIGURE 3.
Acid neutralization capacity. (A) The time it took for the composite to increase the solution pH from a cariogenic pH of 4 to a critical pH of 5.5, above which it is relatively safe for the tooth structure. (B) Acid neutralization capacity. It was evaluated by calculating the amount of a base (potassium hydroxide) that would need to be added to the pH 4 solution, in order to increase the pH to the experimentally measured value. The μmoles of base was normalized by the composite volume to yield the acid neutralization capacity, with units of μmoles/(cm)3 (mean ± sd; n = 4). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The acid neutralization capacity was evaluated by calculating the amount of a base (potassium hydroxide) that would need to be added to the pH 4 solution, in order to increase the pH to the measured value. In Figure 3(B), the measured pH at 10 min was used to calculate the μmoles of potassium hydroxide that would be needed to reach the same pH. The composite volume in the 1 mL solution was 0.144 (cm)3. The calculated μmoles of base was normalized by the composite volume to yield the acid neutralization capacity. The acid neutralization capacity was (12.9 ± 0.1) μmoles/cm3 for the nanocomposite with 40% NACP, higher than those of all other materials (p < 0.05). This value was 10-fold the (1.3 ± 0.1) μmoles/cm3 for RMGI.
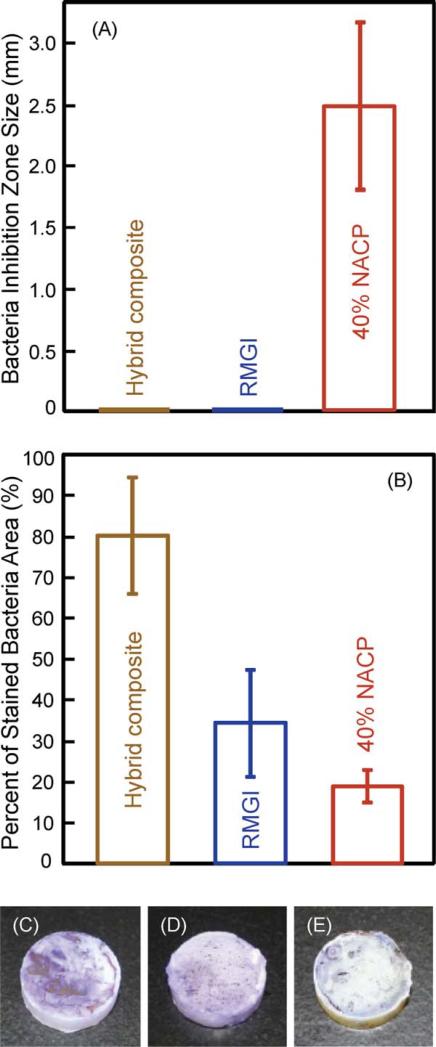
Figure 4 shows the results on bacteria-material interactions: (A) Bacteria inhibition zone size, and (B) percentage of stained bacterial area on the disk. The photos in (C–E) showed that the hybrid composite had the most purple staining, RMGI had an intermediate amount of staining, and the nanocomposite with 40% NACP had the least staining.
FIGURE 4.
Bacteria growth on composites. (A) Bacteria inhibition zone size from the agar disk-diffusion test. (B) Percentage of stained bacteria area on the bottom surface of the disk. (C) More bacteria staining on hybrid composite, (D) intermediate staining on RMGI, and (E) minimal staining on the nanocomposite with 40% NACP. In (A), the hybrid composite and RMGI showed no zone of bacteria inhibition, while the inhibition zone was (2.5 ± 0.7) mm for the nanocomposite with 40% NACP (mean ± sd; n = 3). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 5 shows SEM micrographs of S. mutans adherence on: (A) Hybrid composite, (B) nanocomposite with 40% NACP, and (C) and (D) hybrid composite at higher magnification. The hybrid composite surface had numerous bacteria. The nanocomposite had minimal bacterial growth. The bacteria body was of the order of 1 μm in length and 0.5 μm in diameter. The bacterial cells appeared to have extensions (arrows in C) that formed junctions between neighboring bacteria and adhered to the composite surface. These features are shown more clearly in (D) as indicated by the long arrows. In addition, the S. mutans cell body had the shape of a short rod with wall bands on its surface (short arrows).
FIGURE 5.
SEM micrographs of S. mutans on: (A) Hybrid composite, (B) nanocomposite with 40% NACP, (C) hybrid composite at an intermediate magnification, and (D) hybrid composite at a high magnification. The hybrid composite was nearly entirely covered by bacteria. The RMGI (not shown) had less bacteria coverage. The nanocomposite (B) had the least bacteria. The bacterial cells had developed extensions (indicated by arrows in C). These extensions are shown more clearly in (D) as indicated by the long arrows. S. mutans had the shape of a short rod with wall bands on its surface (indicated by the short arrows). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
In this study, novel nanocomposites were developed to inhibit caries. The nanocomposite could neutralize the acids that otherwise would be dissolving the tooth enamel. Acidogenic bacteria ferment carbohydrates and produce organic acids including lactic, formic, acetic, and propionic acids.17 As a result, the oral plaque pH after a sucrose rinse can decrease to 4.5 or even 4.41 There appears to be a critical pH, below which demineralization dominates, leading to a net enamel mineral dissolution.42 This critical pH varies with ionic concentrations of the oral fluid, but is ~5.5 for most individuals.42 The Stephan Curve shows that the plaque pH, following a glucose mouthrinse, stays in the cariogenic area for about 30 min, and then increases back to a safe pH of 5.5 or higher, after the bacteria have completed their metabolization of the glucose and the saliva has buffered the acid.41 Hence, the damage is done within the first 30 min after a glucose rinse. Therefore, it would be highly desirable for the composite restoration to quickly neutralize the local acids and raise the cariogenic pH from 4 to a safe pH of 5.5 or above, thereby to help inhibit caries.
This study demonstrated that when a commercial hybrid composite, a microfill composite, and a resin-modified glass ionomer were immersed in a lactic acid solution of pH 4, they all failed to raise the pH to the critical pH of 5.5. In fact, the pH stayed at near 4. In contrast, the novel NACP nanocomposite was able to rapidly increase the pH. The ability of acid neutralization greatly increased with higher NACP filler levels. This was manifested by the much higher solution pH that was reached at 10 min as well as at 90 min, and by a much shorter time it took for the solution to reach the critical pH of 5.5. Literature searches indicated that this is the first time a dental nanocomposite was shown to be able to rapidly increase the pH of a cariogenic solution and to neutralize the acid. This pilot study showed that NACP nanocomposite has a unique and highly desirable acid neutralization feature which the commercial restoratives do not have. Hence, the NACP nanocomposite is promising as a new type of restoration to protect tooth structure and inhibit caries.
Besides neutralizing the bacterial acids, another strategy to inhibit caries is to develop antibacterial composites, which directly eradicates the cause of bacterial acids and hence caries. Commercial resin composites typically show no inhibitory effect against plaque formation. Efforts were made to develop silver-containing resin composites which were shown to kill oral bacterial such as S. mutans.43,44 However, a common problem with silver-containing materials is their color stability. Other studies developed novel composites containing an antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide (MDPB).14,27 MDPB copolymerized with other monomers in the composite and acted as a contact inhibitor against bacteria attachment. This decreased the attachment of S. mutans, reduced the amount of plaque accumulation on the composite,14 and inhibited the progression of artificial secondary root caries.28 However, MDPB was immobilized and not released, and hence it could not penetrate the cell walls of the bacteria. In addition, the adsorption of proteins on the composite surfaces reduced the efficacy of contact inhibition of bacteria.27 Hence, recent study has explored novel composite with protein-repellent surface to improve the antibacterial effect.45 While the silver-containing composites and MDPB composites were meritorious with antibacterial properties, they did not release calcium and phosphate ions for tooth lesion remineralization. Furthermore, they are not expected to have acid neutralization capacity to raise cariogenic pH and protect the tooth structure.
The novel NACP nanocomposites of this study possessed acid neutralization capacity and appeared to moderately reduce bacteria growth. A separate study showed that the nanocomposite released calcium and phosphate ions.39 The calcium and phosphate ion releases are not expected to have antibacterial properties. This study demonstrated that the NACP nanocomposite rapidly increased the pH of a lactic acid. It is possible that the alkalinity may reduce the bacteria growth. It is also possible that other components of the nanocomposite such as residual unpolymerized monomers were also released,15 which could affect the longevity of the adequate mechanical properties of the composite. In addition, while the traditional ACP material had a white color, the ACP nanopowder had a whitish-grayish color. This could indicate that the spray drying system had some impurity in the nanopowder which could also affect the bacteria growth. While this study focused on the nanocomposite fabrication and its mechanical and acid neutralization properties, further study is needed to investigate in depth the mechanisms of bacteria inhibition.
Besides reducing bacterial growth, another potential benefit of a composite that can raise the local pH is the modification of the microenvironment of the dental plaque. The presence of organic acids in the plaque can result in an increase in the proportion of acidogenic bacteria which have a high acid tolerance (aciduric), at the expense of the other benign bacteria that are less acid tolerant.1,23 The normal plaque contains less than 1% of acidogenic bacteria in the oral flora.17 The repeated acidification in the plaque with an increasingly more acidic milieu results in the predominance of acidogenic and aciduric bacteria such as S. mutans that can preferentially survive well.2 Therefore, a composite that can raise the local pH may help promote the survival of the benign bacteria and maintain a normal oral flora, which can exert a protective effect on the tooth by preventing the dominance of cariogenic bacteria. Further study is needed to investigate the effect of the NACP nanocomposite on maintaining a nearly neutral plaque pH, and thereby maintaining a healthy proportion of various organisms in the biofilm.
As shown in Figure 5, the hybrid composite was nearly entirely covered by S. mutans cells, consistent with previous reports that dental composites readily had bacteria accumulation.13–15 The reduced bacteria growth in Figure 5(B) on the 40% NACP composite agrees with the bacteria inhibition in Figure 4. The S. mutans showed a rod shape with wall bands on the surface [Figure 5(D)]. A coccus (plural cocci) is any bacterium that has a circular shape. A diplococci consists of two cocci connected by a wall band. S. mutans is formed by the linkage of diplococci and, hence, has a rod shape with wall bands on its surface.46 The S. mutans shape and wall band features in Figure 5 are consistent with previous observations.46 The number of wall bands suggested that these S. mutans cells each consisted to two to three diplococci. Although the nanocomposite had less S. mutans coverage than the hybrid composite, SEM observations did not reveal noticeable morphological differences between the bacteria on the two composites. The S. mutans cells on the nanocomposite did not show damage such as broken membranes or separated wall bands, indicating a relatively weak effect of the nanocomposite. Incorporation of other antibacterial agents into the nanocomposite is needed to develop strong antibacterial composite.
The two main challenges facing dental composite restorations are secondary caries and bulk fracture. Therefore, besides antibacterial properties, there is also a need for the composite to have load-bearing properties. The photocured nanocomposites with 10–30% NACP matched the strength of a hybrid composite for posterior and anterior restorations. The nanocomposite at 40% NACP had a strength that was not significantly different from a microfill composite (Heliomolar). Since Heliomolar is indicated for Class I and II posterior restorations and Class III and IV anterior restorations, the new nanocomposite may also be suitable for these applications, with two important benefits of acid neutralization and antibacterial properties. All the NACP nanocomposites had strengths much higher than that of the resin-modified glass ionomer. However, a preliminary S. mutans plaque accumulation experiment, similar to that described in a previous study,14 showed no significant reduction in bacteria accumulation on the NACP composite compared with the control composite. In addition, planktonic bacteria behave differently than bacteria in mature biofilms, and studies on oral biofilm–nanocomposite interactions are yet to be performed. Therefore, it should be noted that the main focus of this study was the development of material compositions and the investigation of acid neutralization capacity for the first time. Further studies are needed to characterize the bacteria and biofilm interactions of the nanocomposite and to develop antibacterial nanocomposite.
SUMMARY
Novel nanocomposites containing calcium phosphate nanoparticles were investigated for acid neutralization and antibacterial properties for the first time. Several commercial restoratives failed to increase the pH of a cariogenic pH 4 solution. The NACP nanocomposite rapidly raised the pH and neutralized the acid that otherwise would demineralize the tooth enamel. The acid neutralization capacity increased with increasing NACP filler level. S. mutans readily attached to and covered the surface of a commercial hybrid composite. In contrast, the NACP nanocomposite reduced the S. mutans growth. The S. mutans coverage on the nanocomposite with 40% NACP was 1/2 that of a resin-modified glass ionomer, and only 1/4 that of a hybrid composite. The nanocomposites with 10–30% NACP matched the strength of the commercial hybrid composite. The nanocomposite at 40% NACP matched the strength of a microfill composite used in Class I to IV restorations. All the NACP nanocomposites had strengths much higher than the resin-modified glass ionomer cement. The new nanocomposites may have the potential to reduce secondary caries and restoration fracture. Future study is needed to further characterize the interactions of the nanocomposites with bacteria and biofilms and to improve their antibacterial properties.
ACKNOWLEDGMENTS
The authors thank Drs. Joseph M. Antonucci, Sabine Dickens, and Drago Skrtic for discussions and Mr. Anthony A. Giuseppetti for experimental assistance.
Contract grant sponsor: NIH/NIDCR; contract grant numbers: R01 DE17974, R01 DE11789
Contract grant sponsors: University of Maryland Dental School, NIST; American Dental Association Foundation
REFERENCES
- 1.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–681. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 3.Featherstone JDB. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 4.Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 5.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater. 2002;18:436–444. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 6.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: Clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 10.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 11.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Prim Dent Care. 2002;9:31–36. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 12.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commision Projects 2–95. Int Dent J. 2001;51:117–158. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 13.Svanberg M, Mjör IA, Ørstavik D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J Dent Res. 1990;69:861–864. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 14.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RRB. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Imazato S, Russell RRB, Noiri Y, Ebisu S. Influence of resin monomers on growth of oral streptococci. J Dent Res. 2004;83:302–306. doi: 10.1177/154405910408300406. [DOI] [PubMed] [Google Scholar]
- 16.van Dijken JWV, Sjostrom S. The effect of glass ionomer cement and composite resin filling on marginal gingival. J Clin Periodontol. 1991;18:200–203. doi: 10.1111/j.1600-051x.1991.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 17.Featherstone JDB The continuum of dental caries—Evidence for a dynamic disease process. J Dent Res. 2004;83:C39–C42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 18.Deng DM, ten Cate JM. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Res. 2004;38:54–61. doi: 10.1159/000073921. [DOI] [PubMed] [Google Scholar]
- 19.Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41:467–473. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 20.Zero DT. In situ caries models. Adv Dent Res. 1995;9:214–230. doi: 10.1177/08959374950090030501. [DOI] [PubMed] [Google Scholar]
- 21.Deng DM, van Loveren C, ten Cate JM. Caries-preventive agents induce remineralization of dentin in a biofilm model. Caries Res. 2005;39:216–223. doi: 10.1159/000084801. [DOI] [PubMed] [Google Scholar]
- 22.Cenci MS, Pereira-Cenci T, Cury JA, ten Cate JM. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009;43:97–102. doi: 10.1159/000209341. [DOI] [PubMed] [Google Scholar]
- 23.Burne RA. Oral streptococci. . . products of their environment. J Dent Res. 1998;77:445–452. doi: 10.1177/00220345980770030301. [DOI] [PubMed] [Google Scholar]
- 24.Carlén A, Nikdel K, Wennerberg A, Holmberg K, Olsson J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials. 2001;22:481–487. doi: 10.1016/s0142-9612(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 25.Stoodley P, Wefel J, Gieseke A, deBeer D, von Ohle C. Biofilm plaque and hydrodynamic effects on mass transfer, fluoride delivery and caries. J Am Dent Assoc. 2008;139:1182–1190. doi: 10.14219/jada.archive.2008.0333. [DOI] [PubMed] [Google Scholar]
- 26.Quirynen M. The clinical meaning of the surface roughness and the surface free energy of intro-oral hard substrata on the microbiology of the supra- and subgingival plaque: Results from in vitro and in vivo experiments. J Dent. 1994;22(Suppl 1):S13–S16. doi: 10.1016/0300-5712(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 27.Imazato S. Review: Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 28.Thome T, Mayer MPA, Imazato S, Geraldo-Martins VR, Marques MM. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. J Dent. 2009;37:705–711. doi: 10.1016/j.jdent.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Yap AUJ, Khor E, Foo SH. Fluoride release and antibacterial properties of new-generation tooth colored restoratives. Oper Dent. 1999;24:297–305. [PubMed] [Google Scholar]
- 30.Hengtrakool C, Pearson GJ, Wilson M. Interaction between GIC and S. sanguis biofilms: Antibacterial properties and changes of surface hardness. J Dent. 2006;34:588–597. doi: 10.1016/j.jdent.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Eick S, Glockmann E, Brandl B, Pfister W. Adherence of Streptococcus mutans to various restorative materials in a continuous flow system. J Oral Rehabil. 2004;31:278–285. doi: 10.1046/j.0305-182X.2003.01233.x. [DOI] [PubMed] [Google Scholar]
- 32.Fucio SBP, Carvalho FG, Sobrinho LC, Sinhoreti MAC, Puppin-Rontani RM. The influence of 30-day-old Streptoccucus mutans biofilm on the surface of esthetic restorative materials—An in vitro study. J Dent. 2008;36:833–839. doi: 10.1016/j.jdent.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53B:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 34.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 35.Langhorst SE, O'Donnell JNR, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative microradiographic study. Dent Mater. 2009;25:884–891. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu HHK, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC. Nano dicalcium phosphate anhydrous-whisker composites with high strength and Ca and PO4 release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu HHK, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, Antonucci JM. Strong nanocomposites with Ca. PO4 and F release for caries inhibition. J Dent Res. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chow LC, Sun L, Hockey B. Properties of nanostructured hydroxyapatite prepared by a spray drying technique. J Res NIST. 2004;109:543–551. doi: 10.6028/jres.109.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu HHK, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011 doi: 10.1016/j.dental.2011.03.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hostacka A, Ciznar I, Stefkovicova M. Temperature and pH affect the production of bacterial biofilm. Folia Microbiol. 2010;55:75–78. doi: 10.1007/s12223-010-0012-y. [DOI] [PubMed] [Google Scholar]
- 41.Thylstrup A, Fejerskov O. Textbook of Cariology. Munksgaard; Copenhagen, Denmark: 1986. pp. 145–146. [Google Scholar]
- 42.Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69:722–724. [PubMed] [Google Scholar]
- 43.Yamamoto K, Ohashi S, Aono M, Kokubo T, Yamada I, Yamauchi J. Antibacterial activity of silver ions implanted in SiO2 filler on oral streptococci. Dent Mater. 1996;12:227–229. doi: 10.1016/s0109-5641(96)80027-3. [DOI] [PubMed] [Google Scholar]
- 44.Kawahara K, Tsuruda K, Morishita M, Uchida M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent Mater. 2000;16:452–455. doi: 10.1016/s0109-5641(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 45.Muller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–4929. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 46.Lee BS, Lin YW, Chia JS, Hsieh TT, Chen MH, Lin CP, Lan WH. Bactericidal effects of diode laser on streptococcus mutans after irradiation through different thickness of dentin. Lasers Surg Med. 2006;38:62–69. doi: 10.1002/lsm.20279. [DOI] [PubMed] [Google Scholar]