Abstract
Renal failure remains a principal cause of morbidity for patients with multiple myeloma. Once reversible factors such as hypercalcemia have been corrected, the most common cause of severe renal failure in these patients is a tubulointerstitial pathology that results from the very high circulating concentrations of monoclonal immunoglobulin free light chains. These endogenous proteins can result in isolated proximal tubule cell cytotoxicity, tubulointerstitial nephritis and cast nephropathy (myeloma kidney). Less frequently, high levels of free light chains can lead to immunoglobulin light chain amyloidosis and light chain deposition disease, although these conditions are usually associated with insidious progression of renal failure rather than acute kidney injury. Unless there is rapid intervention, progressive and irreversible damage occurs, particularly interstitial fibrosis and tubular atrophy. Despite advances in our understanding of the pathogenesis of these processes there has been a gap in translating these achievements into improved patient outcomes. The International Kidney and Monoclonal Gammopathy Research Group was formed to address this need. In this Review, we discuss the mechanisms of disease and diagnostic approaches to patients with acute kidney injury complicating multiple myeloma.
Introduction
Despite 40 years of advances in the management of both multiple myeloma and acute kidney injury (AKI), the outcomes for patients with severe AKI complicating multiple myeloma remain poor. A report from the UK in the early 1970s found that patients with multiple myeloma and severe AKI had a median survival of 2 months compared with 3 years for patients presenting with moderate renal impairment.1 More recent data from the UK have shown that the survival of patients with severe AKI compli cating multiple myeloma has improved, but only to 10 months.2 There is therefore still a great unmet clinical need to improve outcomes for this population. A potential starting point is to develop treatment strategies that enable an early recovery of renal function in this setting, as this approach alone can greatly improve patient survival.3–5 Essential to achieving such renal recovery is a rapid reduction in serum concentrations of immunoglobulin (Ig) free light chains (FLCs),4,6 the nephrotoxic proteins that are responsible for the majority of severe AKI in patients with multiple myeloma. In addition, there is great potential to further reduce the incidence and severity of AKI in patients with multiple myeloma by improving our understanding of the underlying disease processes, thereby identifying potential targets for early intervention. Subsequent strategies and care pathways designed to protect patients’ kidneys from monoclonal proteins could then be developed.
As with many rare diseases, one of the key barriers to changing clinical practice has been the lack of suitable evidence on which to base any recommendations. Research in this field has largely been confined to small populations and is often uncontrolled. The International Kidney and Monoclonal Gammopathy Research Group was founded in late 2010 to enable a more coordinated, cross-disciplinary and multicenter approach to future research in the field of AKI complicating multiple myeloma. In this Review, we provide an update on the pathogenesis and diagnostic approaches to patients with AKI and monoclonal FLC.
Mechanisms of injury
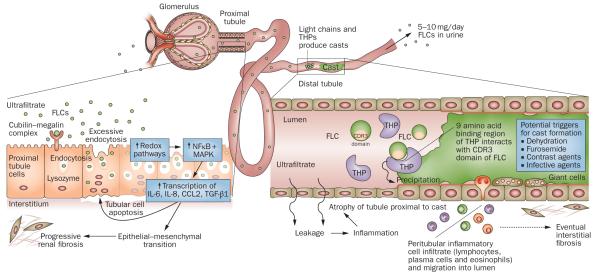
The past 25 years have witnessed considerable advances in the characterization and pathogenesis of tubulointerstitial renal injury caused by Ig FLCs, and in elucidation of the structure–function relationships involved in FLC-mediated renal tubular damage (Figure 1).7–33 FLCs were originally described by Henry Bence Jones in the urine of a patient with mollities ossium as a protein that precipitated on warming, but then redissolved when heated to 75 °C.34 These proteins constitute a family of low-molecular-weight proteins that, unlike most endogenously produced proteins, have a strong propensity to cause tubular damage. Patterns of tubulointerstitial renal injury occurring in the setting of plasma cell dyscrasias include isolated proximal tubule epithelial cell cytotoxicity, tubulointerstitial nephritis and cast nephropathy (also known as myeloma kidney).21
Figure 1.
Mechanisms of FLC-induced acute kidney injury. The very high concentrations of FLCs present in the ultrafiltrate of patients with multiple myeloma can result in direct injury to PTCs. The excessive endocytosis of FLCs by the cubilin–megalin complex expressed on PTCs can trigger apoptotic, proinflammatory and fibrotic pathways. Activation of redox pathways occurs, with increased expression of NFκB and MAPK, which in turn leads to the transcription of both inflammatory and profibrotic cytokines, such as IL-6, IL-8, CCL2 and TGF-β1. In the distal tubules, FLCs can bind to a specific binding domain on THPs and co-precipitate to form casts. These casts result in tubular atrophy proximal to the cast and lead to progressive interstitial inflammation and fibrosis. Abbreviations: CCL2, C-C motif chemokine 2; CDR, complementarity determining region; FLC, free light chain; IL, interleukin; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor κB; PTC, proximal tubule cell; TGF-β1, transforming growth factor β1; THP, Tamm-Horsfall protein.
About 500 mg of polyclonal FLCs are produced daily by the normal lymphoid system and catabolized by the proximal tubule. This system is highly efficient and only about 1–10 mg of polyclonal FLCs normally appear in the urine each day.35 However, in the setting of a plasma cell dyscrasia, FLC production increases considerably, producing circulating levels of monoclonal FLCs that can be hundreds of fold higher than normal.36 When this increase occurs, the capacity of the multiligand endocytic receptor complex of the proximal tubule is quickly exceeded, and high concentrations of FLCs appear in the tubular fluid and finally in the urine. Substantial accumulation of FLCs within the proximal tubular epithelium can occur; the FLCs that appear in the urine are traditionally termed Bence Jones proteins.35
Monoclonal FLCs are known to induce isolated proximal tubular injury, cast nephropathy or a combination of both. FLC interaction with proximal tubule cells (PTCs) can activate inflammatory cascades that lead to tubulointerstitial fibrosis, a major feature of myeloma kidney. Similarly, FLC interaction with Tamm-Horsfall proteins (THPs; also known as uromodulin) and cast formation in the distal tubule can block glomerular flow and produce tubular atrophy and also contribute to interstitial fibrosis. Insight into the pathophysiological mechanisms involved in the toxic effects of FLCs in the kidney is likely to improve our ability to treat kidney disease associated with multiple myeloma.
Proximal tubule cell injury
FLCs can exert direct toxic effects on PTCs, the most abundant cell type in the kidney, and many of the renal consequences of myeloma involvement of the kidney are related to proximal tubular injury.10,15–19,28–30,37–46 Studies have shown that FLCs purified from the urine of myeloma patients without glomerular disease inhibited substrate transport in isolated brush border membrane vesicles, cultured PTCs in vitro10,11,41,42 and in perfused proximal tubules in rats in vivo.19,38,39 These studies shed light on the pathophysiology of the clinical proximal tubulopathy occasionally seen in patients with multiple myeloma also referred to as light chain Fanconi syndrome.10,11,37,41–43,45,46 Although FLCs can be directly toxic to PTCs by blocking transport of glucose, amino acids or phosphate,10,11,41–43 and by activating redox signaling upon contact with PTCs,28–30 most of the toxicity seems to be mediated after endocytosis of FLCs through the tandem endocytic receptors cubilin and megalin.12–14,29,42 Excessive FLC endocytosis can induce a spectrum of inflammatory effects that include activation of redox pathways and expression of nuclear factor κB (NFκB) and mitogen-activated protein kinases (MAPKs), leading to transcription of inflammatory and profibrotic cyto kines, such as IL-6, C-C motif chemokine 2 (CCL2; also known as monocyte chemo attractant protein), IL-8 and transforming growth factor (TGF)-β1.15–18,28,30,42 Excessive FLC endocytosis can also trigger apoptotic pathways and alter the phenotype of PTCs towards a fibroblastic one through epithelial– mesenchymal transition in vitro15,16,43 and in vivo.44 Studies have shown that blocking FLC endo cytosis, either by inhibition of endocytosis9,17,18 or by silencing the endocytic receptors cubilin and megalin, abrogates cytotoxicity.14,29 These observations support the principal that endocytosis is a prerequisite for these inflammatory processes and are the basis of three potential therapeutic strategies to prevent tubular injury: first, to eliminate or reduce the FLC burden in myeloma patients with renal involvement; second, to block the inflammatory pathways that are activated as a result of FLC toxicity; and third, to potentially block FLC endocytosis.
Cast nephropathy
A major mechanism of FLC-mediated tubule damage is intratubular obstruction from precipitation of FLCs in the lumen of the distal nephron, which leads to interstitial inflammation and fibrosis.21 The clinical relevance of cast formation was initially revealed by intravenous infusion of nephrotoxic human FLCs in rats, which increased proximal tubule pressure and simultaneously decreased single-nephron glomerular filtration rate. Intraluminal protein casts were identified in these kidneys.47 Persistence of intraluminal casts in vivo reduces single-nephron glomerular blood flow to the obstructed nephron and results in atrophy of the nephron proximal to the obstruction.48,49 When infused directly into the rat nephron in vivo, monoclonal FLCs from patients with cast nephropathy produced dose-dependent intraluminal obstruction by precipitating in the distal nephron; casts were not observed before the tip of the loop of Henle.22 Obstruction was accelerated by the presence of furosemide. Pretreatment of rats with colchicine decreased urinary levels of THP and prevented intraluminal cast formation and obstruction.22 Additional studies demonstrated an integral relationship between monoclonal FLCs and THPs in cast formation and the associated kidney injury. In humans, casts are generally observed in the distal portion of the nephron, although they have also been found in proximal tubular segments and even in glomeruli in renal biopsy specimens.50 However, these casts also contained THP, suggesting intraluminal reflux of co-precipitated THP and FLC into the proximal nephron.50
Cast formation in vivo is a complex process that is dictated by multiple variables, including the ionic composition of the tubule fluid, tubule fluid flow rates, the concentration of THP and FLC, the strength of binding interaction between THP and FLC, and the presence of furosemide.19,20,22,23,25,31,32,51 These observations have direct clinical relevance as many of these factors (except the intrinsic binding interaction between THP and FLC) can be modified with current treatment modalities.
Identifying nephrotoxic light chains
Not all monoclonal FLCs are nephrotoxic. Although the risk of AKI in patients with multiple myeloma is increased when FLC proteinuria reaches 2 g per day, some patients do not develop kidney disease despite high FLC urine concentrations.52 As no tool to predict toxicity of a given FLC is currently available, preventive measures and removal of precipitating factors are mandatory.
The mechanisms involved in the renal pathogenic effects of individual monoclonal FLCs remain incompletely understood. Nephrotoxicity appears to be an intrinsic property of some FLCs, as indicated by the recurrence of similar renal lesions after kidney transplantation, and by animal studies that have specifically reproduced human FLC-related nephropathies using injections of purified human FLCs,22 intraperitoneal injections of transfected plasmacytomas secreting a pathogenic human FLC,53,54 or gene-targeted insertion.55 Growing evidence shows that the pattern of renal injury is governed by both structural peculiarities of mono clonal FLCs, particularly of the variable (V) domain, and is influenced by environmental factors, such as pH, urea concentration or local tissue proteo lysis. In addition, intrinsic host factors are likely to have an important role in determining both the type and severity of any renal response to a given FLC.
Pathogenic FLCs purified from patients’ urine are character ized by their propensity to form high-order aggregates or polymers in vitro, which differ according to the sequence variability of the V domain.56 The peculiarities of the V domain are observed in many types of renal disease induced by light chains. Myeloma-associated Fanconi syndrome, for example, is characterized by proximal tubule dysfunction secondary to FLC reabsorption and crystallization within the lysosomal compartment of PTCs. FLCs associated with Fanconi syndrome are nearly always of the Vκ1 subgroup and are derived from only two germ line genes, IGKV1-39 and IGKV1-33. These proteins display unique sequence peculiarities in their complementarity determining regions (CDRs); replacement of polar residues by nonpolar or hydro phobic residues in the CDR can induce resistance of the Vκ domain to proteolysis and result in light chain crystalliza tion.57–60 In one study, an experimental mouse model of Fanconi syndrome was developed by administering intraperitoneal injections of murine hybridoma SP2/O cells transfected with DNA encoding a human Fanconi syndrome Vκ1 FLC.54 Using site-directed mutagenesis in these mice, sequential replacement of hydrophobic residues at position 30 or 94 of the V region by their germ line polar counterparts abolished crystal forma tion in the PTCs.54
In immunoglobulin light chain (AL) amyloidosis and light chain deposition disease, the pathogenic role of V regions is suggested by overrepresentation of the Vλ661 and Vκ462 subgroups, respectively, N-glycosylation of the V region,61 and substitutions of key amino acids induced by somatic mutations that might account for the propensity of certain FLCs to aggregate and influence tropism of deposition.53,63–67 In AL amyloidosis, a role for V sequences is also suggested by the high potential of V domain dimerization in vitro68 and in vivo.69 FLCs associated with light chain deposition disease are characterized by cationic isoelectric points, whereas the isoelectric point profile of FLCs involved in AL amyloidosis is heterogeneous. This observation suggests that fibrillar amyloid deposits form by electrostatic interaction between oppositely charged polypeptides, whereas granular deposits in light chain deposition disease result from the binding of cationic polypeptides to anionic basement membranes.70 Two studies have also highlighted a previously underestimated role for the FLC constant domains in amyloid fibril formation.71,72 Finally, differences in FLC structure probably influence cellular effects, as demonstrated by human mesangial cells that undergo either a myofibroblastic or a macrophage-like phenotypic transformation in vitro after incubation with FLCs involved in light chain deposition disease or AL amyloidosis, respectively.73
The role of the molecular characteristics of FLCs in myeloma kidney is less clear. In high-mass myeloma, the capacity of the proximal tubule to reabsorb and degrade FLCs is rapidly overwhelmed by the dramatic increase in the burden of filtered FLCs. Large amounts of FLCs reach the distal tubule lumen where they interact with THP. Huang and Sanders identified a binding domain for FLCs on THP, which consisted of nine amino acids and was termed LCBD.25 Importantly, all FLCs tested bound to this FLC-binding domain.25 In turn, the CDR3 domain in the variable region of both κ and λ FLCs interacted with THP.26 The binding affinities of FLCs for THP are related to the amino acid composition of the CDR3 domain.26
The other key mechanism of injury in myeloma kidney is the massive reabsorption of FLCs in PTCs, which induces the generation of hydrogen peroxide,29 activation of NFκB and phosphorylation of MAPKs, resulting in the release of IL-6, IL-8, CCL2 and TGF-β1.7,30 Oxidative stress, morphological changes,15 and tubulointerstitial inflammation that rapidly progresses to fibrosis,74 partici pate in the development of renal failure. Pote et al. have shown that purified monoclonal FLCs cultured with human PTCs induced cytoskeletal injury and DNA damage consistent with apoptosis, followed by secondary necrosis.16 Toxicity was variable among the different FLCs, but the effect was dose-dependent, suggesting that both FLC structure and concentration determine cellular toxicity in cast nephropathy.16
Despite our growing understanding of the pathogenic mechanisms by which Ig FLCs induce renal injury, there are currently no clinically relevant tools for identifying the potential nephrotoxicity of a specific monoclonal FLC.
Diagnostic approaches
Identifying monoclonal FLCs by immunoassays
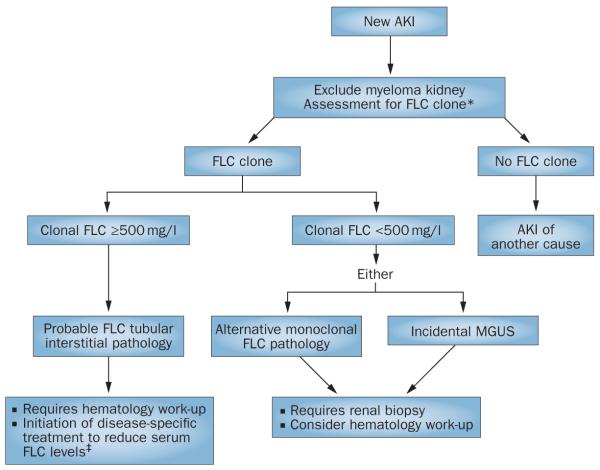
During the assessment of an individual with AKI it is often appropriate and necessary to screen for the presence of a potentially nephrotoxic monoclonal FLC. In such cases, it is essential that the nephrologist and laboratories work together to minimize diagnostic delays to enable the rapid initiation of disease-specific treatment (Figure 2). This assessment is usually undertaken by evaluating the patient’s urine by protein electro phoresis (with or without immunofixation). An alternative to this technique is the quantitative measurement of FLC in the serum by nephelometric immunoassays.75,76 These immunoassays provide a quantitative measurement of both κ and λ FLCs; an overproduction of one of these monoclonal FLCs will lead to a ratio of the two FLCs that deviates outside the normal range (0.26–1.65).77 Use of these assays as a screening tool can help overcome logistical delays and analytical inaccuracies associated with other laboratory methods used for the identification of monoclonal FLCs (such as serum and urine protein electrophoresis and immunofixation).78 In an assessment of 1,877 patients with plasma cell dyscrasias, Katzmann et al. found that serum protein electrophoresis and a quantitative serum FLC assay identified 100% of patients with multiple myeloma and macroglobulinemia, 99.5% of patients with smoldering multiple myeloma, 96.5% of patients with AL amyloidosis and 78% of patients with light chain deposition disease.79 International guidelines now recommend that screening of serum alone (with serum protein electrophoresis and a quantitative serum FLC assay) for plasma cell dyscrasias is a viable alternative to urinary assessment.80,81 Despite the accumulation of serum polyclonal FLCs in renal impairment, the assay remains useful in patients with renal failure, but absolute values are raised and the normal range for the FLC ratio is changed from that in the general population81 to 0.37–3.17.82 By utilizing this renal range in a dialysis-dependent AKI population the number of false positives was reduced.76 In addition to these diagnostic advantages, the FLC immunoassays offer the ability to monitor clonal disease response,80 which has particular relevance in patients with myeloma kidney in whom an early reduction in serum FLC concentrations is associated with renal recovery.4,6
Figure 2.
Screening algorithm for monoclonal disease in AKI. Patients with AKI can be initially screened for a monoclonal protein by serum FLC assays, which enable the rapid identification of a monoclonal FLC as the possible cause of a tubular interstitial process. *To exclude the presence of an intact monoclonal immunoglobin, serum FLC assays should be combined with serum protein electrophoresis (many units would also include immunofixation electrophoresis as standard of care). For the assessment of immunoglobulin light chain amyloidosis and light chain deposition disease, urinary assessment is required. Where serum FLC assays are not used, urgent urinary assessment for monoclonal FLCs is necessary. ‡Emergency treatment designed to achieve a rapid reduction in serum FLC levels is based on high-dose dexamethasone. The full benefit of adding FLC removal strategies to this therapy remains to be determined. Abbreviations: AKI, acute kidney injury; FLC, free light chain; MGUS, monoclonal gammopathy of undetermined significance.
Renal biopsy and monoclonal FLC
Clinical indications
Renal disorders relating to plasma cell dyscrasias are the primary diagnosis in approximately 3% of native renal biopsy samples. A renal biopsy in a patient with a monoclonal gammopathy and renal dysfunction helps to define the type of renal injury present, determines the degree of activity of the pathological process, and defines chroni city status, which can influence how aggressive therapy should be and predict possible outcome and eventual prognosis.21,24,37,83–88
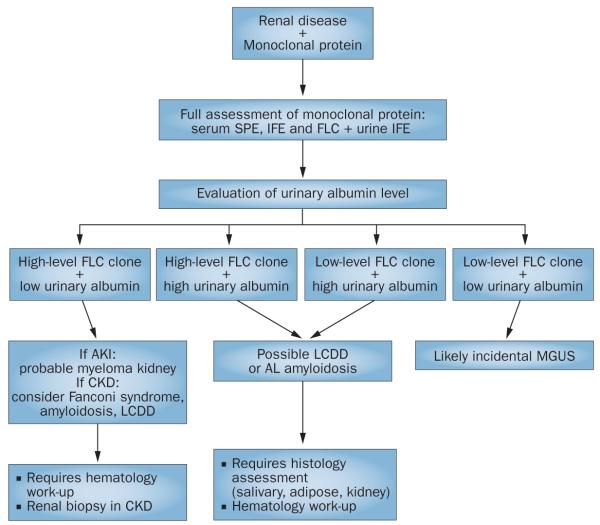
Renal biopsies are indicated in various clinical settings in patients with a circulating monoclonal FLC (Figure 3). Some of the indications are perhaps much more apparent than others. For example, a patient with a diagnosed lymphoproliferative disorder and a circulating monoclonal FLC who develops abrupt renal dysfunction would benefit from a renal biopsy to identify the type of injury present, which can be used to design an appropriate therapeutic intervention. Similarly, a patient with a diagnosis of monoclonal gammopathy of undetermined significance who develops AKI should be a prime candidate for a biopsy to determine whether the circulating monoclonal FLC is responsible for the renal injury. A patient with a known lymphoproliferative disorder and renal dysfunction may also be considered for renal transplantation. A renal biopsy in this setting would enable identification of the renal lesion present, and assessment of the probability of recurrence and eventual prognosis after transplantation.89,90
Figure 3.
Diagnostic approach to a patient with renal disease and a monoclonal protein. Combining the evaluation of urinary albumin concentration with the level of the FLC clone can guide the management of a patient with renal injury and a monoclonal protein. Tubulointerstitial pathologies are more likely when urinary albumin levels are low and FLC levels are high. By contrast, patients with AL amyloidosis and LCDD frequently have high urinary albumin levels. These pathologies are often associated with a lower level of FLC clone, but can occur with any FLC level. Where diagnostic uncertainty remains, assessment of histology is essential. Abbreviations: AKI, acute kidney injury; AL, amyloid light chain; CKD, chronic kidney disease; FLC, free light chain; IFE, immunofixation electrophoresis; LCDD, light chain deposition disease; MGUS, monoclonal gammopathy of undetermined significance; SPE, serum protein electrophoresis.
Technical considerations
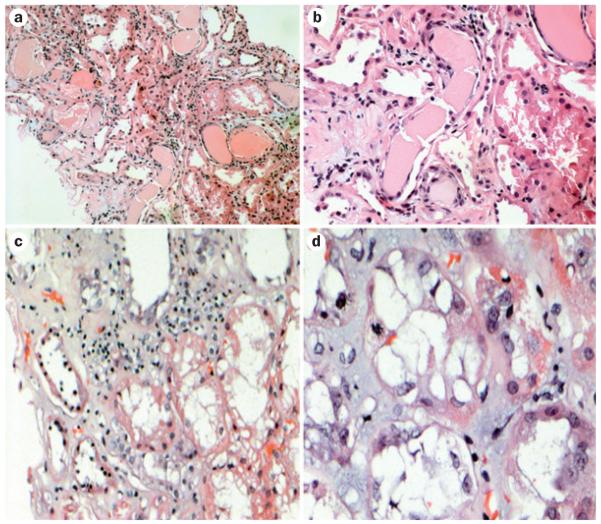
The most common renal lesions seen in patients with circulating monoclonal FLC and AKI are tubular interstitial lesions (Figure 4). FLC cast nephropathy is the most frequent of these lesions and is fairly well recognized by pathologists. However, the other two principal lesions are not always correctly identified. These lesions include proximal tubulopathy (also referred to as acute tubular necrosis or acute tubulopathy)37,83,84,86–88 and an inflamma tory tubular interstitial process without casts that has morphological features identical to those seen in classical acute tubulointerstitial nephritis (and is sometimes referred to as such).91 Although the main pathological processes seen in the latter two entities may be recognized by pathologists in renal biopsy samples, the association of those lesions with an underlying plasma cell dyscrasia is frequently missed. Even in the diag nosis of FLC cast nephropathy there is currently a need to define definitive criteria as to how many distal nephron casts are needed to make a diagnosis, with the understanding that sampling can have an important role in this situation.83 Carefully controlled studies are therefore required to define parameters in renal biopsy samples that correlate with recovery or irreversibility of renal damage although some parameters, such as the degree of interstitial damage, are intuitive. Another question that remains is whether the obstructive process in FLC cast nephropathy is the main pathological process responsible for renal failure or whether the accompanying inflammatory reaction is the real culprit. Thus, the entity of inflammatory tubulointerstitial nephritis, which is seen in association with plasma cell dyscrasias merges conceptually speaking with FLC cast nephropathy. Other renal lesions related to monoclonal light and heavy chains, such as AL amyloidosis, heavy chain (AH) amyloidosis or light/heavy chain deposition disease, tend to present less acutely, but can also exhibit (or mimic) AKI and need to be considered in the differential diagnosis of AKI in patients with monoclonal gammopathies.
Figure 4.
Renal injuries induced by free light chains in patients with acute kidney injury. All samples were stained with hematoxylin and eosin. The classic appearance of cast nephropathy can be seen in a | and b | (magnification ×150 and ×350, respectively). Casts with fracture planes in distal nephrons and surrounding reactive tubular cells can be seen (center). Note adjacent proximal tubular damage and mild inflammation surrounding the casts. c | Marked tubular damage is observed, with inflammatory infiltrate that contains a mixture of mononuclear inflammatory cells and scattered eosinophils (magnification ×250). d | Tubular interstitial injury without casts (magnification ×750). A mitotic figure can be seen in a tubular cell, indicative of tubular regeneration.
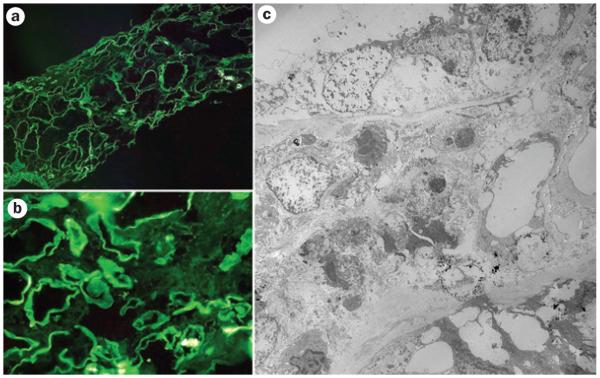
The work-up of these renal biopsy samples must include staining for κ and λ FLCs and careful examination of the Ig stains used in the routine evaluation of renal biopsy samples. This approach will help to determine isotype restriction for light and/or heavy chains and enable the morphological manifestations to be directly related to the underlying plasma cell dyscrasia.24,83,84 Heavy chains have not been documented to be involved in tubular interstitial pathology although, in rare cases, a coexisting FLC can result in a cast nephropathy.92 The evaluations must carefully assess not only immunofluorescence, but also ultrastructural findings93 and correlate these with the light microscopic features (Figure 5). Many of the clues or diagnostic findings leading to a correct diagnosis are found in the careful evaluation of these ancillary diagnostic techniques. The pathologists evaluating these renal biopsy samples must be aware of the subtle and early renal manifestations of these conditions so as to not misinterpret them. In a few selected cases, the use of other diagnostic techniques, such as ultrastructural immunolabeling94 or mass spectroscopy is needed, for instance in some cases of amyloidosis, with the purpose of determining the precursor protein and typing the amyloid.95 Mass spectroscopy could also be used to detect a monotypical light or heavy chain deposited in the renal parenchyma. The utilization of these sophisticated diagnostic techniques may require sending the biopsy sample to a center where these techniques are available. In the case of ultrastructural labeling there are specific fixation protocols that are needed to achieve satisfactory results.94,96
Figure 5.
Immunofluorescence and electron microscopy evaluation of tubulointerstitial damage associated with circulating monoclonal free light chains. a | and b | show examples of immunofluorescence for κ light chains. Linear staining along the tubular basement membranes represents κ light chains (negative for λ light chains). c | Electron microscopy findings of prominent tubular damage and interstitial inflammatory infiltrate. Focal punctate electron-dense material around tubular basement membranes represents deposits of free light chains.
Immunofluorescence evaluation is key in defining the presence of monoclonal light or heavy chain deposi tion in the renal biopsy sample to make a definitive diagnosis. However, available commercial antibodies do not detect some of the abnormal monoclonal Igs that may be deposited in the various renal compartments and that are directly responsible for renal dysfunction. This lack of recognition is due to the fact that the monoclonal proteins can be quite abnormal physicochemically or truncated to such an extent that the epitopes recognized by routine polyclonal antibodies are no longer present.
In selected cases, a re-biopsy should be seriously considered. The results from a biopsy sample can establish what therapy has accomplished and whether additional therapy is recommended. In this situation, a renal biopsy can provide evidence of improvement (or lack thereof) by comparing the findings in the initial biopsy sample with repeat biopsies. This approach has been documented in the literature primarily in patients with plasma cell dyscrasias and glomerular lesions.97–99 A renal biopsy may also provide evidence that further treatment may be of no, or rather limited, value as the renal parenchymal damage is deemed to be extensive and/or irreversible.74
Avoiding AKI in multiple myeloma
A number of factors, including dehydration, hyper-calcemia, nephrotoxic drugs and infection, are frequently associated with reversible tubular injury in patients with multiple myeloma.100–102 However, as discussed above, these factors can also trigger tubulointerstitial lesions associated with cast nephropathy in patients who are at risk. Patients’ risk can be stratified by both serum FLC level and urinary FLC excretion.103,104 Only 2% of patients without urinary FLC excretion have renal impairment whereas this percentage increases to 50% when urinary FLC concentrations are high.104 In the case of AL amyloid osis, FLC concentrations do not need to be high, but the location and mechanism of damage is typically very different from that of the AKI seen in patients with multiple myeloma. As renal impairment worsens, absolute serum concentrations of FLCs rise as a result of reduced renal clearance. Higher concentrations of FLC are therefore delivered to the remaining functional tubules, which increases the burden on proximal tubules and the risk of precipitation in the distal tubules. If correction of dehydration and hypercalcemia, removal of nephrotoxic agents and treatment of infection is timely, this vicious cycle can be broken.
Clinicians need access to the best diagnostic tools and must establish clear clinical pathways to enable rapid and accurate diagnosis and an early initiation of disease-specific treatment.105 For presentations of new AKI in patients not known to have multiple myeloma, adaptations of the screening algorithm in Figure 2 will almost certainly reduce diagnostic delays. For those patients who are known to have multiple myeloma, but have no significant renal impairment, close monitoring is key to avoiding AKI. Particular attention should be paid to patients with FLC only myeloma in whom serial monitoring of FLC levels is required. Further work is required to determine how this monitoring should be undertaken, with serum immunoassays or 24 h urine collections.
Clinicians should also be aware of FLC escape, which can occur in patients with an intact Ig multiple myeloma (5% of patients with IgG multiple myeloma and 15% of patients with IgA multiple myeloma). This condition refers to the rise in levels of FLCs independent of intact Ig, which can be easily missed if urine or serum are not examined for FLCs.106 In addition, serum FLCs are abnormal in 95% of patients with intact Ig multiple myeloma at presentation. Those patients with FLC concentrations of >1,000 mg/l (10–15% of IgG and IgA cases) are at increased risk of developing renal failure.104
Strategies to improve early diagnosis are urgently needed to identify patients at a time when FLC concentrations are low in order to avoid AKI. In 2007, 38% of patients with multiple myeloma in the UK were diagnosed as a result of an emergency admission or referral, compared with an average of 23% across all cancer types.107 This delayed diagnosis of myeloma is probably the result of the relative rarity of the disease and the nonspecific nature of its early symptoms. Switching off production of FLCs by eradication of the plasma cell clone is the most effective way of stopping progressive renal damage. High-dose dexamethasone alone is effective as a single agent in this setting and can lead to a 100-fold fall in serum FLC levels within 2 weeks, in sensitive disease. Oral high-dose dexamethasone can be started without delay while decisions about definitive chemotherapy are being made.
The past decade has seen a range of novel chemo-therapies developed for myeloma that can be used without dose reduction in renal impairment. These agents include bortezomib and thalidomide combinations, and should be commenced as soon as possible in this setting. Use of these new agents in combination with close monitoring should lead to improved patient outcomes. For this purpose, serum FLC measurements in the first 2 weeks might identify patients who are not responding to their antimyeloma therapy and in whom early change of treatment might be indicated in order to rescue the kidney.103
Conclusions
AKI remains an important cause of morbidity and mortality in patients with multiple myeloma. Severe cases are frequently a direct consequence of the high clonal production rates of Ig FLCs. The resulting high serum concentrations of these proteins often lead to tubular interstitial injuries as endocytic receptors in the proximal tubules are overwhelmed. Direct proximal tubule epithelial cell cytotoxicity, tubulointerstitial nephritis and cast nephropathy can occur in isolation or together. The past 20 years has seen great advances in our detailed understanding of these disease processes. Coordinated translational research programs are now required to translate these advances into improved outcomes for patients with multiple myeloma.
Key points.
▪ The tubulointerstitial injury, cast nephropathy, is the most common cause of severe acute kidney injury in patients with multiple myeloma
▪ Histology findings of acute tubular necrosis and acute tubulointerstitial nephritis should raise a ‘red flag’ for potential injury from high levels of free light chains in patients with multiple myeloma
▪ Standard assessment of renal histology by light microscopy, immunofluorescence and electron microscopy might require the addition of specialist techniques to detect subtle injuries in patients with a monoclonal protein
▪ Serum immunoassays can provide a rapid alternative to urine electrophoresis for the identification of monoclonal free light chains
▪ Early diagnosis and intervention remain key to preventing irreversible renal injuries in patients with multiple myeloma
Acknowledgments
The authors of this manuscript would like to thank the other members of the International Kidney and Monoclonal Gammopathy Research Group for their intellectual support in the review process for this manuscript: J. Bladé, P. Cockwell, M. Cook, M. Drayson, J.-P. Fermand, S. Kastritis, R. Kyle, N. Leung and C. Winearls. P. W. Sanders’ research was supported by National Institutes of Health grant R01 DK46199 and P30 DK079337 (George M. O’Brien Kidney and Urological Research Centers Program) and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
Footnotes
Review criteria PubMed and MEDLINE were searched using the terms “multiple myeloma”, “monoclonal protein”, “free light chain”, “acute kidney injury”, “renal/kidney impairment” and “cast nephropathy”. No language restrictions were placed on the search and all publications from 1970 to 2010 were considered.
Author contributions C. A. Hutchison, V. Batuman, J. Behrens, F. Bridoux, C. Sirac, A. Dispenzieri, G. A. Herrera and P. W. Sanders researched data to include in the manuscript. All authors contributed equally to discussion of content for the article, writing the manuscript and reviewing and editing of the manuscript before submission.
Competing interests C. A. Hutchison declares an association with the following company: Binding Site. See the article online for full details of the relationship. The other authors declare no competing interests.
Contributor Information
Colin A. Hutchison, Renal Institute of Birmingham, University Hospital Birmingham and University of Birmingham, Birmingham, UK
Vecihi Batuman, Section of Nephrology and Hypertension, Department of Medicine, Tulane University School of Medicine, New Orleans, LA, USA.
Judith Behrens, Department of Hematology, St Helier Hospital, Carshalton, Surrey, UK.
Frank Bridoux, Department of Nephrology, Poitiers University Hospital, Poitiers, France.
Christophe Sirac, CNRS UMR 6101, Limoges University Hospital, Limoges, France.
Angela Dispenzieri, Department of Medicine, Division of Hematology, Mayo Clinic, Rochester, MN, USA.
Guillermo A. Herrera, Nephrocor, Bostwick Laboratories, Orlando, FL, USA
Helen Lachmann, National Amyloidosis Centre and Centre for Nephrology, University College London Medical School, London, UK.
Paul W. Sanders, Division of Nephrology, Department of Medicine, University of Alabama, Birmingham, AL, USA
References
- 1.Peto R. Factors of prognostic significance in myelomatosis. J. Clin. Pathol. 1972;25:555. doi: 10.1136/jcp.25.6.555-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes RJ, Read S, Collins GP, Darby SC, Winearls CG. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20-year experience from a single centre. Nephrol. Dial. Transplant. 2010;25:419–426. doi: 10.1093/ndt/gfp488. [DOI] [PubMed] [Google Scholar]
- 3.Bladé J, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch. Intern. Med. 1998;158:1889–1893. doi: 10.1001/archinte.158.17.1889. [DOI] [PubMed] [Google Scholar]
- 4.Leung N, et al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int. 2008;73:1282–1288. doi: 10.1038/ki.2008.108. [DOI] [PubMed] [Google Scholar]
- 5.Hutchison CA, et al. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin. J. Am. Soc. Nephrol. 2009;4:745–754. doi: 10.2215/CJN.04590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchison CA, et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J. Am. Soc. Nephrol. 2011;22:1129–1136. doi: 10.1681/ASN.2010080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arimura A, Li M, Batuman V. Potential protective action of pituitary adenylate cyclase-activating polypeptide (PACAP38) on in vitro and in vivo models of myeloma kidney injury. Blood. 2006;107:661–668. doi: 10.1182/blood-2005-03-1186. [DOI] [PubMed] [Google Scholar]
- 8.Batuman V, Dreisbach AW, Cyran J. Light-chain binding sites on renal brush-border membranes. Am. J. Physiol. 1990;258:F1259–F1265. doi: 10.1152/ajprenal.1990.258.5.F1259. [DOI] [PubMed] [Google Scholar]
- 9.Batuman V, Guan S. Receptor-mediated endocytosis of immunoglobulin light chains by renal proximal tubule cells. Am. J. Physiol. 1997;272:F521–F530. doi: 10.1152/ajprenal.1997.272.4.F521. [DOI] [PubMed] [Google Scholar]
- 10.Batuman V, Guan S, O’Donovan R, Puschett JB. Effect of myeloma light chains on phosphate and glucose transport in renal proximal tubule cells. Ren. Physiol. Biochem. 1994;17:294–300. doi: 10.1159/000173861. [DOI] [PubMed] [Google Scholar]
- 11.Batuman V, Sastrasinh M, Sastrasinh S. Light chain effects on alanine and glucose uptake by renal brush border membranes. Kidney Int. 1986;30:662–665. doi: 10.1038/ki.1986.237. [DOI] [PubMed] [Google Scholar]
- 12.Batuman V, et al. Myeloma light chains are ligands for cubilin (gp280) Am. J. Physiol. 1998;275:F246–F254. doi: 10.1152/ajprenal.1998.275.2.F246. [DOI] [PubMed] [Google Scholar]
- 13.Klassen RB, Allen PL, Batuman V, Crenshaw K, Hammond TG. Light chains are a ligand for megalin. J. Appl. Physiol. 2005;98:257–263. doi: 10.1152/japplphysiol.01090.2003. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Balamuthusamy S, Simon EE, Batuman V. Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am. J. Physiol. Renal Physiol. 2008;295:F82–F90. doi: 10.1152/ajprenal.00091.2008. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Hering-Smith KS, Simon EE, Batuman V. Myeloma light chains induce epithelial-mesenchymal transition in human renal proximal tubule epithelial cells. Nephrol. Dial. Transplant. 2008;23:860–870. doi: 10.1093/ndt/gfm670. [DOI] [PubMed] [Google Scholar]
- 16.Pote A, Zwizinski C, Simon EE, Meleg-Smith S, Batuman V. Cytotoxicity of myeloma light chains in cultured human kidney proximal tubule cells. Am. J. Kidney Dis. 2000;36:735–744. doi: 10.1053/ajkd.2000.17620. [DOI] [PubMed] [Google Scholar]
- 17.Sengul S, Zwizinski C, Batuman V. Role of MAPK pathways in light chain-induced cytokine production in human proximal tubule cells. Am. J. Physiol. Renal Physiol. 2003;284:F1245–F1254. doi: 10.1152/ajprenal.00350.2002. [DOI] [PubMed] [Google Scholar]
- 18.Sengul S, et al. Endocytosis of light chains induces cytokines through activation of NF-κB in human proximal tubule cells. Kidney Int. 2002;62:1977–1988. doi: 10.1046/j.1523-1755.2002.00660.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanders PW, Herrera GA, Chen A, Booker BB, Galla JH. Differential nephrotoxicity of low molecular weight proteins including Bence Jones proteins in the perfused rat nephron in vivo. J. Clin. Invest. 1988;82:2086–2096. doi: 10.1172/JCI113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders PW, Booker BB, Bishop JB, Cheung HC. Mechanisms of intranephronal proteinaceous cast formation by low molecular weight proteins. J. Clin. Invest. 1990;85:570–576. doi: 10.1172/JCI114474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders PW, Herrera GA, Kirk KA, Old CW, Galla JH. Spectrum of glomerular and tubulointerstitial renal lesions associated with monotypical immunoglobulin light chain deposition. Lab. Invest. 1991;64:527–537. [PubMed] [Google Scholar]
- 22.Sanders PW, Booker BB. Pathobiology of cast nephropathy from human Bence Jones proteins. J. Clin. Invest. 1992;89:630–639. doi: 10.1172/JCI115629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang ZQ, Kirk KA, Connelly KG, Sanders PW. Bence Jones proteins bind to a common peptide segment of Tamm-Horsfall glycoprotein to promote heterotypic aggregation. J. Clin. Invest. 1993;92:2975–2983. doi: 10.1172/JCI116920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders PW, Herrera GA. Monoclonal immunoglobulin light chain-related renal diseases. Semin. Nephrol. 1993;13:324–341. [PubMed] [Google Scholar]
- 25.Huang ZQ, Sanders PW. Localization of a single binding site for immunoglobulin light chains on human Tamm-Horsfall glycoprotein. J. Clin. Invest. 1997;99:732–736. doi: 10.1172/JCI119218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying WZ, Sanders PW. Mapping the binding domain of immunoglobulin light chains for Tamm-Horsfall protein. Am. J. Pathol. 2001;158:1859–1866. doi: 10.1016/S0002-9440(10)64142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera GA, Sanders PW. Paraproteinemic renal diseases that involve the tubulointerstitium. Contrib. Nephrol. 2007;153:105–115. doi: 10.1159/000096763. [DOI] [PubMed] [Google Scholar]
- 28.Wang PX, Sanders PW. Immunoglobulin light chains generate hydrogen peroxide. J. Am. Soc. Nephrol. 2007;18:1239–1245. doi: 10.1681/ASN.2006111299. [DOI] [PubMed] [Google Scholar]
- 29.Basnayake K, Ying WZ, Wang PX, Sanders PW. Immunoglobulin light chains activate tubular epithelial cells through redox signaling. J. Am. Soc. Nephrol. 2010;21:1165–1173. doi: 10.1681/ASN.2009101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying WZ, Wang PX, Aaron KJ, Basnayake K, Sanders PW. Immunoglobulin light chains activate NF-κB in renal epithelial cells through a Src-dependent mechanism. Blood. 2011;117:1301–1307. doi: 10.1182/blood-2010-08-302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland MD, Galla JH, Sanders PW, Luke RG. Effect of urinary pH and diatrizoate on Bence Jones protein nephrotoxicity in the rat. Kidney Int. 1985;27:46–50. doi: 10.1038/ki.1985.8. [DOI] [PubMed] [Google Scholar]
- 32.Huang ZQ, Sanders PW. Biochemical interaction between Tamm-Horsfall glycoprotein and Ig light chains in the pathogenesis of cast nephropathy. Lab. Invest. 1995;73:810–817. [PubMed] [Google Scholar]
- 33.Solomon A, Weiss DT, Kattine AA. Nephrotoxic potential of Bence Jones proteins. N. Engl. J. Med. 1991;324:1845–1851. doi: 10.1056/NEJM199106273242603. [DOI] [PubMed] [Google Scholar]
- 34.Jones H. Bence. Papers on clinical pathology. Lancet. 1847;50:88–92. [Google Scholar]
- 35.Berggård I, Peterson PA. Polymeric forms of free normal κ and λ chains of human immunoglobulin. J. Biol. Chem. 1969;244:4299–4307. [PubMed] [Google Scholar]
- 36.Mead GP, et al. Serum free light chains for monitoring multiple myeloma. Br. J. Haematol. 2004;126:348–354. doi: 10.1111/j.1365-2141.2004.05045.x. [DOI] [PubMed] [Google Scholar]
- 37.Markowitz GS. Dysproteinemia and the kidney. Adv. Anat. Pathol. 2004;11:49–63. doi: 10.1097/00125480-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Sanders PW, Herrera GA, Galla JH. Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int. 1987;32:851–861. doi: 10.1038/ki.1987.286. [DOI] [PubMed] [Google Scholar]
- 39.Galla JH, Herrera GA, Sanders PW. Differential toxicity of human Bence-Jones proteins in the rat proximal convoluted tubule in vivo. Contrib. Nephrol. 1988;68:198–202. doi: 10.1159/000416513. [DOI] [PubMed] [Google Scholar]
- 40.Sanders PW, Herrera GA, Lott RL, Galla JH. Morphologic alterations of the proximal tubules in light chain-related renal disease. Kidney Int. 1988;33:881–889. doi: 10.1038/ki.1988.80. [DOI] [PubMed] [Google Scholar]
- 41.Guan S, el-Dahr S, Dipp S, Batuman V. Inhibition of Na-K-ATPase activity and gene expression by a myeloma light chain in proximal tubule cells. J. Investig. Med. 1999;47:496–501. [PubMed] [Google Scholar]
- 42.Batuman V. Proximal tubular injury in myeloma. Contrib. Nephrol. 2007;153:87–104. doi: 10.1159/000096762. [DOI] [PubMed] [Google Scholar]
- 43.Sengul S, Li M, Batuman V. Myeloma kidney: toward its prevention—with new insights from in vitro and in vivo models of renal injury. J. Nephrol. 2009;22:17–28. [PubMed] [Google Scholar]
- 44.Khan AM, et al. Myeloma light chain-induced renal injury in mice. Nephron Exp. Nephrol. 2010;116:e32–e41. doi: 10.1159/000317129. [DOI] [PubMed] [Google Scholar]
- 45.Maldonado JE, et al. Fanconi syndrome in adults. A manifestation of a latent form of myeloma. Am. J. Med. 1975;58:354–364. doi: 10.1016/0002-9343(75)90601-4. [DOI] [PubMed] [Google Scholar]
- 46.Herlitz LC, Roglieri J, Resta R, Bhagat G, Markowitz GS. Light chain proximal tubulopathy. Kidney Int. 2009;76:792–797. doi: 10.1038/ki.2008.666. [DOI] [PubMed] [Google Scholar]
- 47.Weiss JH, et al. Pathophysiology of acute Bence-Jones protein nephrotoxicity in the rat. Kidney Int. 1981;20:198–210. doi: 10.1038/ki.1981.122. [DOI] [PubMed] [Google Scholar]
- 48.Tanner GA, Knopp LC. Glomerular blood flow after single nephron obstruction in the rat kidney. Am. J. Physiol. 1986;250:F77–F85. doi: 10.1152/ajprenal.1986.250.1.F77. [DOI] [PubMed] [Google Scholar]
- 49.Tanner GA, Evan AP. Glomerular and proximal tubular morphology after single nephron obstruction. Kidney Int. 1989;36:1050–1060. doi: 10.1038/ki.1989.300. [DOI] [PubMed] [Google Scholar]
- 50.Start DA, Silva FG, Davis LD, D’Agati V, Pirani CL. Myeloma cast nephropathy: immunohistochemical and lectin studies. Mod. Pathol. 1988;1:336–347. [PubMed] [Google Scholar]
- 51.Ying WZ, Sanders PW. Dietary salt regulates expression of Tamm-Horsfall glycoprotein in rats. Kidney Int. 1998;54:1150–1156. doi: 10.1046/j.1523-1755.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- 52.Woodruff R, Sweet B. Multiple myeloma with massive Bence Jones proteinuria and preservation of renal function. Aust. NZ J. Med. 1977;7:60–62. doi: 10.1111/j.1445-5994.1977.tb03359.x. [DOI] [PubMed] [Google Scholar]
- 53.Khamlichi AA, et al. Role of light chain variable region in myeloma with light chain deposition disease: evidence from an experimental model. Blood. 1995;86:3655–3659. [PubMed] [Google Scholar]
- 54.Decourt C, et al. Mutational analysis in murine models for myeloma-associated Fanconi’s syndrome or cast myeloma nephropathy. Blood. 1999;94:3559–3566. [PubMed] [Google Scholar]
- 55.Sirac C, et al. Role of the monoclonal κ chain V domain and reversibility of renal damage in a transgenic model of acquired Fanconi syndrome. Blood. 2006;108:536–543. doi: 10.1182/blood-2005-11-4419. [DOI] [PubMed] [Google Scholar]
- 56.Myatt EA, et al. Pathogenic potential of human monoclonal immunoglobulin light chains: relationship of in vitro aggregation to in vivo organ deposition. Proc. Natl Acad. Sci. USA. 1994;91:3034–3038. doi: 10.1073/pnas.91.8.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aucouturier P, et al. Monoclonal Ig L chain and L chain V domain fragment crystallization in myeloma-associated Fanconi’s syndrome. J. Immunol. 1993;150:3561–3568. [PubMed] [Google Scholar]
- 58.Rocca A, et al. Sequences of V kappa L subgroup light chains in Fanconi’s syndrome. Light chain V region gene usage restriction and peculiarities in myeloma-associated Fanconi’s syndrome. J. Immunol. 1995;155:3245–3252. [PubMed] [Google Scholar]
- 59.Messiaen T, et al. Adult Fanconi syndrome secondary to light chain gammopathy. Clinicopathologic heterogeneity and unusual features in 11 patients. Medicine (Baltimore) 2000;79:135–154. doi: 10.1097/00005792-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Déret S, et al. Kappa light chain-associated Fanconi’s syndrome: molecular analysis of monoclonal immunoglobulin light chains from patients with and without intracellular crystals. Protein Eng. 1999;12:363–369. doi: 10.1093/protein/12.4.363. [DOI] [PubMed] [Google Scholar]
- 61.Comenzo RL, Zhang Y, Martinez C, Osman K, Herrera GA. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig VL germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–720. doi: 10.1182/blood.v98.3.714. [DOI] [PubMed] [Google Scholar]
- 62.Denoroy L, Déret S, Aucouturier P. Overrepresentation of the V kappa IV subgroup in light chain deposition disease. Immunol. Lett. 1994;42:63–66. doi: 10.1016/0165-2478(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 63.Cogné M, Preud’homme JL, Bauwens M, Touchard G, Aucouturier P. Structure of a monoclonal kappa chain of the V kappa IV subgroup in the kidney and plasma cells in light chain deposition disease. J. Clin. Invest. 1991;87:2186–2190. doi: 10.1172/JCI115252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevens FJ. Four structural risk factors identify most fibril-forming kappa light chains. Amyloid. 2000;7:200–211. doi: 10.3109/13506120009146835. [DOI] [PubMed] [Google Scholar]
- 65.Enqvist S, Sletten K, Stevens FJ, Hellman U, Westermark P. Germ line origin and somatic mutations determine the target tissues in systemic AL-amyloidosis. PLoS ONE. 2007;2:e981. doi: 10.1371/journal.pone.0000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Déret S, et al. Molecular modeling of immunoglobulin light chains implicates hydrophobic residues in non-amyloid light chain deposition disease. Protein Eng. 1997;10:1191–1197. doi: 10.1093/protein/10.10.1191. [DOI] [PubMed] [Google Scholar]
- 67.del Pozo Yauner L, et al. Influence of the germline sequence on the thermodynamic stability and fibrillogenicity of human lambda 6 light chains. Proteins. 2008;72:684–692. doi: 10.1002/prot.21934. [DOI] [PubMed] [Google Scholar]
- 68.Stevens PW, et al. Recombinant immunoglobulin variable domains generated from synthetic genes provide a system for in vitro characterization of light-chain amyloid proteins. Protein Sci. 1995;4:421–432. doi: 10.1002/pro.5560040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan B, et al. Free light chains in plasma of patients with light chain amyloidosis and nonamyloid light chain deposition disease. High proportion and heterogeneity of disulfide-linked monoclonal free light chains as pathogenic features of amyloid disease. Br. J. Haematol. 2009;144:705–715. doi: 10.1111/j.1365-2141.2008.07522.x. [DOI] [PubMed] [Google Scholar]
- 70.Kaplan B, Livneh A, Gallo G. Charge differences between in vivo deposits in immunoglobulin light chain amyloidosis and nonamyloid light chain deposition disease. Br. J. Haematol. 2007;136:723–728. doi: 10.1111/j.1365-2141.2006.06488.x. [DOI] [PubMed] [Google Scholar]
- 71.Klimtchuk ES, et al. The critical role of the constant region in thermal stability and aggregation of amyloidogenic immunoglobulin light chain. Biochemistry. 2010;49:9848–9857. doi: 10.1021/bi101351c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto K, et al. The amyloid fibrils of the constant domain of immunoglobulin light chain. FEBS Lett. 2010;584:3348–3353. doi: 10.1016/j.febslet.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 73.Keeling J, Teng J, Herrera GA. AL-amyloidosis and light-chain deposition disease light chains induce divergent phenotypic transformations of human mesangial cells. Lab. Invest. 2004;84:1322–1338. doi: 10.1038/labinvest.3700161. [DOI] [PubMed] [Google Scholar]
- 74.Basnayake K, et al. Differential progression of renal scarring and determinants of late renal recovery in sustained dialysis dependent acute kidney injury secondary to myeloma kidney. J. Clin. Pathol. 2010;63:884–887. doi: 10.1136/jcp.2010.079236. [DOI] [PubMed] [Google Scholar]
- 75.Bradwell AR, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin. Chem. 2001;47:673–680. [PubMed] [Google Scholar]
- 76.Hutchison CA, et al. Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol. 2008;9:11. doi: 10.1186/1471-2369-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katzmann JA, et al. Serum reference intervals and diagnostic ranges for free κ and free λ immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin. Chem. 2002;48:1437–1444. [PubMed] [Google Scholar]
- 78.Hutchison CA, Basnayake K, Cockwell P. Serum free light chain assessment in monoclonal gammopathy and kidney disease. Nat. Rev. Nephrol. 2009;5:621–628. doi: 10.1038/nrneph.2009.151. [DOI] [PubMed] [Google Scholar]
- 79.Katzmann JA, et al. Screening panels for detection of monoclonal gammopathies. Clin. Chem. 2009;55:1517–1522. doi: 10.1373/clinchem.2009.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durie BG, et al. on behalf of the International Myeloma Working Group. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 81.Dispenzieri A, et al. on behalf of the International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 82.Hutchison CA, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008;3:1684–1690. doi: 10.2215/CJN.02290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herrera GA. Renal lesions associated with plasma cell dyscrasias: practical approach to diagnosis, new concepts, and challenges. Arch. Pathol. Lab. Med. 2009;133:249–267. doi: 10.5858/133.2.249. [DOI] [PubMed] [Google Scholar]
- 84.Herrera GA. Renal manifestations of plasma cell dyscrasias: an appraisal from the patients’ bedside to the research laboratory. Ann. Diagn. Pathol. 2000;4:174–200. doi: 10.1016/s1092-9134(00)90042-x. [DOI] [PubMed] [Google Scholar]
- 85.Korbet SM, Schwartz MM. Multiple myeloma. J. Am. Soc. Nephrol. 2006;17:2533–2545. doi: 10.1681/ASN.2006020139. [DOI] [PubMed] [Google Scholar]
- 86.Herrera GA, Picken MM. In: Heptinstall’s Pathology of the Kidney. Jennette JC, Olson JL, Schwartz MM, editors. Lippincott-Raven; Philadelphia: 2006. pp. 853–910. [Google Scholar]
- 87.Herrera GA. In: Silva’s Diagnostic Renal Pathology. Zhou XJ, Laszik Z, Nadasdy T, D’Agati VD, Silva F, editors. Cambridge University Press; Cambridge: 2009. pp. 345–406. [Google Scholar]
- 88.Kapur U, Barton K, Fresco R, Leehey DJ, Picken MM. Expanding the pathologic spectrum of immunoglobulin light chain proximal tubulopathy. Arch. Pathol. Lab. Med. 2007;131:1368–1372. doi: 10.5858/2007-131-1368-ETPSOI. [DOI] [PubMed] [Google Scholar]
- 89.Leung N, et al. Long-term outcome of renal transplantation in light-chain deposition disease. Am. J. Kidney Dis. 2004;43:147–153. doi: 10.1053/j.ajkd.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 90.Short AK, O’Donoghue DJ, Riad HN, Short CD, Roberts IS. Recurrence of light chain nephropathy in a renal allograft. A case report and review of the literature. Am. J. Nephrol. 2001;21:237–240. doi: 10.1159/000046254. [DOI] [PubMed] [Google Scholar]
- 91.Gu X, Herrera GA. Light-chain-mediated acute tubular interstitial nephritis: a poorly recognized pattern of renal disease in patients with plasma cell dyscrasia. Arch. Pathol. Lab. Med. 2006;130:165–169. doi: 10.5858/2006-130-165-LATINA. [DOI] [PubMed] [Google Scholar]
- 92.Isaac J, Herrera GA. Cast nephropathy in a case of Waldenström’s macroglobulinemia. Nephron. 2002;91:512–515. doi: 10.1159/000064299. [DOI] [PubMed] [Google Scholar]
- 93.Herrera GA. The contributions of electron microscopy to the understanding and diagnosis of plasma cell dyscrasia-related renal lesions. Med. Electron Microsc. 2001;34:1–18. doi: 10.1007/s007950100000. [DOI] [PubMed] [Google Scholar]
- 94.Herrera GA, Turbat-Herrera EA. Ultrastructural immunolabeling in the diagnosis of monoclonal light-and heavy-chain-related renal diseases. Ultrastruct. Pathol. 2010;34:161–173. doi: 10.3109/01913121003672873. [DOI] [PubMed] [Google Scholar]
- 95.Picken MM, Herrera GA. The burden of “sticky” amyloid: typing challenges. Arch. Pathol. Lab. Med. 2007;131:850–851. doi: 10.5858/2007-131-850-TBOSAT. [DOI] [PubMed] [Google Scholar]
- 96.Sethi S, et al. Mass spectrometry-based proteomic diagnosis of renal immunoglobulin heavy chain amyloidosis. Clin. J. Am. Soc. Nephrol. 2010;5:2180–2187. doi: 10.2215/CJN.02890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Montseny JJ, et al. Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol. Dial. Transplant. 1998;13:1438–1445. doi: 10.1093/ndt/13.6.1438. [DOI] [PubMed] [Google Scholar]
- 98.Komatsuda A, et al. Disappearance of nodular mesangial lesions in a patient with light chain nephropathy after long-term chemotherapy. Am. J. Kidney Dis. 2000;35:E9. doi: 10.1016/s0272-6386(00)70221-6. [DOI] [PubMed] [Google Scholar]
- 99.Hotta O, Taguma Y. Resolution of nodular glomerular lesions in a patient with light-chain nephropathy. Nephron. 2002;91:504–505. doi: 10.1159/000064296. [DOI] [PubMed] [Google Scholar]
- 100.Clark AD, Shetty A, Soutar R. Renal failure and multiple myeloma: pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Rev. 1999;13:79–90. doi: 10.1016/s0268-960x(99)90014-0. [DOI] [PubMed] [Google Scholar]
- 101.Haubitz M, Peest D. Myeloma—new approaches to combined nephrological-haematological management. Nephrol. Dial. Transplant. 2006;21:582–590. doi: 10.1093/ndt/gfi318. [DOI] [PubMed] [Google Scholar]
- 102.Penfield JG. Multiple myeloma in end-stage renal disease. Semin. Dial. 2006;19:329–334. doi: 10.1111/j.1525-139X.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- 103.Mead GP, Drayson MT. Sensitivity of serum free light chain measurement of residual disease in multiple myeloma patients. Blood. 2009;114:1717. doi: 10.1182/blood-2009-06-225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Drayson M, et al. Effects of paraprotein heavy and light chain types and free light chain load on survival in myeloma: an analysis of patients receiving conventional-dose chemotherapy in Medical Research Council UK multiple myeloma trials. Blood. 2006;108:2013–2019. doi: 10.1182/blood-2006-03-008953. [DOI] [PubMed] [Google Scholar]
- 105.Cockwell P, Hutchison CA. Management options for cast nephropathy in multiple myeloma. Curr. Opin. Nephrol. Hypertens. 2010;19:550–555. doi: 10.1097/MNH.0b013e32833ef72c. [DOI] [PubMed] [Google Scholar]
- 106.Pratt G. The evolving use of serum free light chain assays in haematology. Br. J. Haematol. 2008;141:413–422. doi: 10.1111/j.1365-2141.2008.07079.x. [DOI] [PubMed] [Google Scholar]
- 107.Elliss-Brookes L, et al. Routes to Diagnosis: NCIN Data Briefing. National Cancer Intelligence Network. 2010 [online], http:// www.ncin.org.uk/publications/data_briefings/routes_to_diagnosis.aspx. [Google Scholar]