Abstract
Immunologic memory reflects the ability of a host to more effectively respond to a re-encounter with a particular pathogen than the first encounter, and when a vaccine mimics the first encounter, comprises the basis of vaccine efficacy. For T cells, memory is often equated with the anamnestic response, the ability of secondary lymphoid tissue (SLT)-based(central) memory T cells to respond to pathogen exposure with a more rapid and higher magnitude production and infection-site delivery of pathogen-specific effector cells than observed in naïve hosts. However, increasing evidence supports a fundamentally different kind of T cell memory in which differentiated, long-lived effector memory T cells (TEM), pre-positioned in sites of potential pathogen invasion or rapidly mobilized to such sites from blood and marginated pools, intercept and potentially control/eliminate pathogen within hours of infection. Here, we review the evidence for this “hidden” T cell memory, and its implication for vaccine development.
Introduction
Most pathogens are initially encountered at body surfaces. While innate immune mechanisms may exert immediate control over microbes at the point of entry, elimination and/or control of microbes with pathogenic potential often requires the enlistment of adaptive, pathogen-specific T cell responses. Compared to immediate innate immune responses, T cell responses are slow to develop upon a first-time infection because in the naïve host, pathogen-specific T cells are extraordinarily low in frequency, manifest restricted anatomic localization (SLT only), and lack differentiated effector function [1]. Before contributing to pathogen control, the rare, quiescent naïve T cells specific for a pathogen not previously encountered must be activated within SLTs that drain sites of infection and then undergo a relatively prolonged period of proliferation and differentiation to both expand the size of the population and provide it with relevant effector functions. Moreover, since T cell effector functions – such as production of antiviral cytokines, killing of host cells harboring cytoplasmic infections, or Th1-mediated control of phagosomal infections – act locally, the expanded, differentiated, pathogen-specific T cells arising in SLT must also migrate to all sites of infection [2]. This process can take anywhere from a few days to a few weeks in primary infection, an inherent delay that provides a temporal window of opportunity for continued pathogen replication, and for some pathogens, time for full implementation of immune evasion strategies that permit establishment of persistent infection (see below). However, the majority of first-time infections are ultimately cleared by these primary immune responses, and the host T cell system retains a long-term “memory” of the initial pathogen encounter manifested by elevated frequencies of pathogen-specific T cell clones [3–5]. These memory T cell populations also exhibit phenotypic differences from their naïve counterparts (for example, increased expression of adhesion molecules [6]) that allow them to more efficiently respond to Ag a second time. Early models for T cell-dependent immunity highlighted the contribution of quiescent populations of memory T cells in SLTs that in the event of secondary Ag exposure, would re-initiate a second round of expansion and differentiation -- the so-called anamnestic response. Owing to the increase in frequency and enhanced intrinsic responsiveness of pathogen-specific (SLT-based) memory populations over their naïve counterparts, anamnestic T cell responses were more rapid and higher in magnitude than primary responses, and thus more efficient in controlling infection [7]. However, such memory responses were viewed largely as just a faster and larger recapitulation of the primary response, still reliant upon expansion and re-acquisition of effector functions each time the pathogen is encountered.
While this system of “reserve” memory with rapid anamnestic mobilization is an efficient mechanism of increasing the efficiency of secondary T cell responses, it has now become clear that this process is only part of the story. Further characterization of T cell populations in extra-lymphoid tissues have revealed novel memory subsets that are poised for immediate effector function, rather than expansion and differentiation [8]. These memory T cell populations are anatomically based in tissues that can be collectively designated front-line, extra-lymphoid sites of pathogen exposure (immune “effector” sites, as opposed to immune inductive sites) [9]. Indeed, some of these effector site-based memory T cell populations appear to reside permanently within such sites, without evidence of tissue to lymph to blood re-circulation, a characteristic that has hidden their existence from conventional analysis [10]. These effector site-based, differentiated subsets comprise a long-term memory compartment that makes distinct and crucial contributions to T cell-dependent pathogen control upon re-infection. This review will summarize the known properties of various memory T cell subsets and their development, highlight the intimate relationship between T cell location and function, and hypothesize how each subset contributes to the integrated process of T cell-dependent pathogen control upon re-infection. The essential parameter of T cell location to rapid infection containment will be emphasized. Ramifications for vaccine-elicited memory T cells will also be discussed.
T cell expansion and differentiation – the cellular substrate of memory
Naïve T cells constantly re-circulate between SLTs using the blood and lymph as conduits, but are largely, if not completely, excluded from extra-lymphoid effector sites [11–13]. Thus, naïve T cells directly scan a relatively small fraction of host tissues, and depend on the Ag acquisition and collection strategies of SLTs to detect pathogen invasion. This restricted naïve T cell re-circulation pattern is mediated by expression of CD62L and CCR7 (required for migration from blood into lymph nodes across the high endothelial venules -- HEVs), the expression of S1p receptors (allowing T cells to leave lymph nodes by following a chemotactic gradient towards efferent lymph vessels), and the lack of the homing/chemokine receptor combinations required to extravasate into extra-lymphoid sites [12–15]. Naïve T cells become activated only after recognition of cognate Ag presented by APCs within lymphoid organs, which drain tissue sites of infection. Naïve T cells contain very limited cytoplasm, and must undergo an extended period of activation, associated with an ~6-fold increase in cell volume within 1–2 days of Ag stimulation, before entering the metabolically demanding program of proliferation [16]. Once this program is initiated, activated T cells divide as rapidly as every 6 hours, resulting in as much as an ~10,000-fold amplification in the number of Ag-specific T cells [3, 4, 17]. This proliferation program is coupled with differentiation into effector cells, characterized by expression of important mediators of pathogen control (including IFNγ, TNFα, IL-5, IL-13 and IL-17 that directly inhibit pathogen replication and/or recruit and activate non-T cell effector populations), IL-2 which supports continued T cell proliferation and differentiation, as well as cytotoxic granules that mediate infected host cell lysis. As non-lymphoid tissues represent common sites of infection, a cardinal feature of the response is the re-distribution of many of the effector cells developing in the SLTs to these sites [12, 18, 19]. This altered T cell trafficking pattern is accomplished via changes in the expression of T cell homing molecules. Most activated T cells down-regulate CD62L and CCR7, which prevents reentry into resting lymph nodes across HEVs [13, 20]. In turn, they up-regulate various non-lymphoid homing lectins, integrins, and chemokine receptors. These may include receptors that support T cell entry into general sites of inflammation, such as CXCR3 and CCR5, as well as molecules that target T cells to specific organs, such as α4β7 integrin/CCR9 and CLA/CCR4, which mediate selective entry into the small intestinal mucosa and skin, respectively [12, 13, 15]. This change in anatomic distribution, from SLT sites of priming to sites of infection, is critical for the exertion of local T cell effector mechanisms.
In situations in which the initial antigenic/microbial challenge is eliminated, 90–95% of the Ag-specific T cell population undergoes apoptosis (although this proportion is highly dependent upon the nature of the pathogen or vaccine modality)[4]. Those that remain, memory T cells, are diversified into functionally heterogeneous subsets that play distinct, but cooperative roles in protecting the host from re-infection [8]. Notably, the functional specializations of each subset are coordinately regulated with homing properties and anatomic distribution [9]. Central memory T cells (TCM) are fundamentally defined by their trafficking properties. This subset retains or has re-expressed CD62L and CCR7, lacks CCR5 expression [21], and selectively and constitutively re-circulates through SLTs. TCM typically express co-stimulatory receptors for enhanced re-activation by professional antigen presenting cells. TCM also retain a great deal of proliferation potential, and exhibit a high capacity to secrete IL-2 upon re-stimulation [9, 22]. However, TCM are largely excluded from non-lymphoid effector sites, and thus are anatomically sequestered away from the epithelial surfaces and underlying stroma that constitute the most common sites of pathogen exposure [18, 21]. Moreover, TCM require a period of differentiation to acquire certain effector functions, such as the capacity to kill infected host cells within minutes via the targeted secretion of pre-formed, perforin- and granzyme-containing granules [23, 24]. In summary, TCM comprise a population that shares many features with naïve T cells; they patrol SLTs rather than front-line sites of infection and they are subdued in immediate effector function relative to more differentiated effectors. In the event that pathogens are not immediately intercepted at the point of entry by other immune system components, TCM remain poised to proliferate and differentiate upon cognate Ag recognition on APCs that present tissue Ags within the draining SLTs, thus producing a wave of differentiated effector daughter cells. Relative to naïve T cells, they exhibit a dramatic increase in clonal abundance and express effector functions more rapidly. Thus, TCM comprise a reserve force of antigen-specific T cell clones that is responsible for recapitulating a primary response in the host, albeit much more quickly, but is not capable of responding immediately to secondary challenges in non-lymphoid tissues. The advantage of TCM-based memory is that a relatively small population of pathogen-specific TCM can “pack a large punch” in terms of the ultimate size of its anamnestic effector response, and therefore a myriad of different TCM specificities can be maintained in an overall SLT T cell compartment of finite size.
In contrast to TCM, effector memory T cells (TEM) lack CD62L and/or CCR7 expression, molecules directing lymph node entry via HEVs, and express chemokine receptors such as CCR5 that are associated with homing to inflammatory sites [9, 21]. Accordingly, TEM are preferentially distributed within non-lymphoid tissues and/or remain blood-associated, either circulating or contained within marginated pools in splenic red pulp and hepatic sinusoids [2, 25]. Further definition of TEM is challenging because they comprise a remarkably heterogeneous pool of memory T cells, but, in general, TEM are poised for more immediate effector function. For example, both CD8+ and CD4+ TEM may maintain pre-formed cytotoxic granules for rapid cytolysis of infected host cells [23, 24]. TEM are also poised to coordinately secrete high levels of effector cytokines such as IFNγ and TNFα and chemokines such as MIP-1β [26]. However, upon re-stimulation TEM undergo relatively little proliferation and secrete little IL-2 [9, 22]. Thus, compared to TCM, TEM are unlikely to contribute as significantly to anamnestic expansions of effector cells [27]. However, owing to their constitutively maintained heightened capacity for effector functions, and more importantly, their anatomic localization, TEM are specialized to more rapidly respond to re-infection, particularly within non-lymphoid sites of pathogen exposure.
Considerable effort has been made to delineate the mechanisms responsible for the development and maintenance of TCM vs. TEM. The theme that has emerged is that the degree of tissue damage and inflammation, and the density and persistence of antigen play a major role in setting the ratio of TEM to TCM maintained at various stages of the immune response against any particular pathogen. Under priming conditions in which foreign antigen is rapidly eliminated, maintenance of TCM is favored over TEM, and over time, the response becomes increasingly TCM-biased [28]. For example, vaccination with replication-deficient vectors, infection with agents that are very modestly pathogenic, or experimental situations in which mice contain particularly abundant antigen-specific T cells in the naïve population, all favor the production of TCM [29–31]. More pathogenic infections, typically corresponding to more durable antigen presentation, increase the number of resulting TEM. Preferential differentiation of TEM is enhanced when pathogens are not immediately contained or Ag exposure is recurrent, resulting in iterative waves of T cell activation and proliferation, a process exploited by heterologous prime-boost vaccination [32, 33]. TEM differentiation and long-term maintenance of large TEM populations is particularly pronounced during certain persistent infections, such as CMV, typified by continuous, low level or repeated recrudescent pathogen replication that is controlled, but never eliminated [26, 34]. Teleologically, one may surmise that only under conditions in which infections are particularly pathogenic, persistently or repetitively experienced, and/or exert a high fitness cost without rapid control, is it evolutionarily advantageous to maintain large numbers of TEM.
Evidence for effector site-based memory
TEM are present in blood, splenic red pulp and comprise the dominant, if not exclusive, T cell population within non-lymphoid tissues [2, 18, 19, 35]. In healthy adult nonhuman primates (NHPs), fully differentiated (CD28−) TEM are highly represented in blood and spleen, but comprise a very modest component of CD8+ T cells in lymph node and thoracic duct lymph (<20% of blood levels), suggesting that the majority of fully differentiated TEM recirculate through lymphatics slowly or not at all ([36] and L. Picker, unpublished observations). In addition, these observations suggest that blood or compartments of organs that are contiguous with blood and contain large populations of memory T cells (e.g., splenic red pulp and liver sinusoids) comprise a distinct compartment that may be rapidly recruitable to sites of pathogen invasion. Recent evidence adds to this paradigm by demonstrating that certain tissue compartments are populated by resident effector memory T cells that do not exit non-lymphoid tissues in the resting host [37–46]. We will refer to this recently identified “resident memory” T cell subset [10] as “resident effector memory T cells” (rTEM) to emphasize their location in non-lymphoid tissues and conceptual relationship with other populations of TEM. Such rTEM populations typically adopt tissue-specific differentiation states, and are not identified by peripheral blood analysis. Tissue TEM within the small intestinal epithelium, which comprise a population of CD8αβ+ memory intraepithelial lymphocytes (IELs), have been particularly well characterized in mice. Months following clearance of infection in mice, pathogen-specific small intestinal memory IELs constitutively retain cytolytic function and express high levels of granzyme B, yet express relatively low levels of the co-stimulatory ligand CD27 and cytokine receptors for IL-15 and IL-7 [47, 48]. In addition, they constitutively maintain CD103/β7 integrin expression as well as CD69 [47–49]. This CD103/CD69+ phenotype was not expressed among pathogen-specific memory CD8 T cells in blood, and this discordance was later explained by the observation that small intestinal IEL are resident and do not re-circulate through blood [37, 38]. In mice, stable populations of non-recirculating CD8 rTEM have been identified within intestinal epithelium, the CNS, the skin epidermis, and potentially the salivary gland and a subset of cells within the respiratory mucosa [37–46]. While functional properties vary, rTEM isolated from any of these compartments typically express CD69 and often CD103/β7 integrin. Interestingly, pathogen-specific memory CD8 T cells isolated from pancreas, stomach, kidney, heart, and female reproductive tract also contain CD69+/CD103- and CD69+/CD103+ subsets [50]. Evidence supports the hypothesis that CD69 prevents S1P-mediated egress from tissues [51, 52] and that CD103/β7 integrin enforces maintenance in epithelia via E-cadherin binding [50, 53, 54], suggesting that CD103/CD69 expression may be a defining feature of non-recirculating rTEM within many non-lymphoid tissues.
Questions regarding rTEM ontogeny remain. These populations may be very long-lived; for example, mouse and NHP studies reported minimal decay over ~1–2y [18, 55]. CD69 expression is often interpreted as a surrogate for recent TCR engagement and recent studies have proposed that CD103 expression by rTEM is induced or maintained by local cognate-Ag [39, 43]. Removal of pathogen-specific rTEM from small intestinal IEL can permit re-differentiation into TCM and loss of immediate cytolytic function, suggesting that the population exhibits developmental plasticity, and maintenance of an effector-like phenotype is dependent upon anatomic location [47]. In situations in which antigen is cleared, it remains possible that the effector-like properties of rTEM are maintained by local cross-reactivity with environmental antigens at body surfaces. Alternatively, certain tissue compartments may regulate T cell effector differentiation state independently of TCR ligation [50]. Discriminating between these possibilities has obvious ramifications for generating long-lived rTEM through vaccination.
While new evidence points to the broad distribution of rTEM, other TEM populations appear to constitutively re-circulate through non-lymphoid tissues. The dichotomy between these subsets was elegantly illustrated in a recent report on murine skin memory T cell populations [41]. In vivo dynamic imaging revealed that during the resting state, memory CD8 T cells within the epidermis were resident and exhibited little motility. In contrast, memory T cells within the underlying dermis (mostly containing memory CD4 T cells) lacked CD103/β7 integrin expression and were motile and recirculated between blood and tissue. Indeed, migratory memory CD8 and CD4 T cells (mTEM) can be isolated from the afferent lymphatics of primary lymph nodes, suggesting constitutive T cell recirculation occurs through certain tissue compartments [11, 56, 57]. Thus, two pathways exist for routine surveillance of non-lymphoid compartments; manifested by the maintenance of both resident and recirculating migratory memory T cells. Further work is required to catalogue the complete distribution and numerical contribution of rTEM and mTEM to the memory T cell pool. Importantly, analysis of blood will not reveal rTEM populations, and thus these populations will remain hidden from view without the direct examination of non-lymphoid tissues.
Function of effector site-based memory
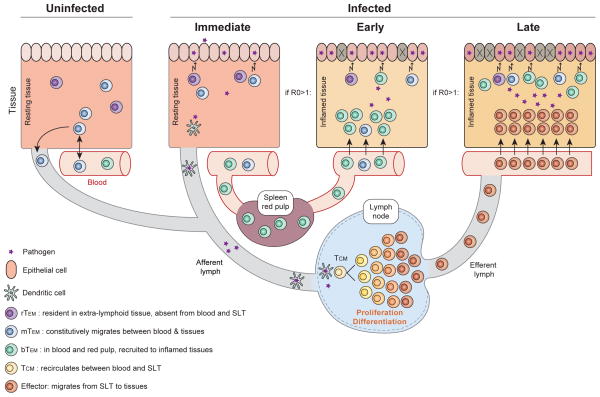
T cell-dependent protective immunity likely results from the concerted actions of multiple memory subsets, and identifying the distinct roles of TCM, blood-borne TEM, tissue recirculating mTEM, and sessile rTEM is experimentally arduous. However, recent evidence supports the hypothesis that discrete memory T cell subsets operate at different phases of re-infection. We propose that rTEM and tissue recirculating mTEM already present at the site of infection are the only subsets poised to provide an immediate (within minutes to hours) contribution to protective immunity in situ. Local inflammation resulting from infection would promote a second wave of early (after several hours) adaptive effector functions via the local recruitment of pre-formed blood-borne TEM (bTEM). In the event that immediate and early responses contain, but do not eliminate, the infection, TCM would then undergo a period of proliferation and effector differentiation within SLTs, and provide a late (days to weeks) wave of abundant effectors that would contribute to control of pathogens that escape TEM-mediated elimination (Figure 1).
Figure 1. Contribution of each memory T cell subset varies at each phase of response and is mandated by anatomic location and functional specialization.
In the uninfected host, resident (rTEM) and migrating (mTEM) effector memory T cells are the only subsets present within non-lymphoid effector sites, which represent the most common sites of initial pathogen exposure. Immediately upon infection, the density of local memory T cells is critical and proliferation potential is irrelevant. rTEM and mTEM present at the point of entry are the only subsets that participate in protection at this time. Early (<3–4 days) after infection, recruitment to sites of inflammation is critical and proliferation potential is still irrelevant. The presence of recruitable TEM cell populations from blood (bTEM) may substantially increase the local effector to infected target ratio. If a secondary infection is not contained rapidly at the portal of entry by TEM subsets (e.g., a reproductive ratio - R0 - of infection of > 1), TCM undergo re-activation in draining lymph nodes (or other SLTs), which results in extensive proliferation, differentiation and production of an abundant new wave of effectors, which migrate to the sites of infection. This phase of the response occurs late (≥4 days), and is particularly important when TEM densities in tissues are too low to contain infection.
Mounting evidence highlights the particular role of effector site-based memory to rapid protection from re-infection. For instance, after contraction of the T cell response, systemic memory T cells remain stable, while those within the lung airways gradually wane [25]. This loss of local memory correlates with a gradual loss of T cell-dependent protective immunity against a heterosubtypic respiratory influenza virus challenge, providing evidence for a site-based role in protection [58]. This interpretation was strengthened by later studies demonstrating that transfer of memory CD4 T cells directly into the lung airways of naïve mice conferred partial, but rapid, protection against a local viral challenge [59]. A recent study demonstrated the potential to generate tissue-retentive influenza virus-specific memory CD4+ T cells within the lung tissue. Interestingly, this population preferentially homed to lung upon transfer to naïve mice and conferred more rapid and efficacious control in response to local challenge compared to memory T cells derived from spleen [46]. Studies by Swain and colleagues demonstrated that lung CD4 T cell memory may contribute to early influenza control by enhancing the magnitude of innate responses, highlighting the integration of frontline adaptive T cell memory with the innate immune system [60]. A protective role for effector site-based memory has been demonstrated in several models of skin infection in mice. rTEM established within epidermis are essential for rapid protective immunity against epidermal re-challenge with either HSV-1 or vaccinia virus [40, 44, 45]. Importantly, TCM make little contribution to immediate protection in these models, although they do make late contributions to virus control in the event the infection is not controlled rapidly at the site of infection.
Mouse studies have made highly reductionist comparisons of intravenously transferred CD8+ TCM vs. TEM to protect against various pathogen challenges. It is important to note that transferred TEM will not reconstitute rTEM populations, but may comprise mTEM and/or bTEM. Nevertheless, transferred CD8+ TEM elicited a greater degree of protection than TCM against a variety of infectious challenges when measured within a few days of challenge [61–63]. In cases in which the pathogen was rapidly eliminated, TEM were sufficient and faster than TCM on a per cell basis. However, in cases where the pathogen overwhelmed the number of transferred memory T cells, TCM exhibited far greater control than TEM when measured 7 days or more after infection, almost certainly due to the enhanced ability of TCM to proliferate and differentiate into a second wave of effectors that migrate to sites of infection [28, 61]. Thus, the relative contributions of TCM and TEM to protective immunity will depend upon the dose, route, replication rate, and pathogenesis of the infectious agent in question, as well as the quantity, function, and anatomic distribution of memory T cells throughout the host [8].
The theme that emerges from these studies is that the contribution of each memory T cell subset is mandated by their location and functional specialization, and varies at each phase of an anamnestic response (Figure 1). For ‘immediate’ responses (which may commence as soon as foreign peptide has been presented on host MHC molecules), location is critical, as is rapid effector function. In contrast, proliferation potential is completely irrelevant. Only rTEM and mTEM that are actually present at the peripheral site of infection can contribute to ‘immediate’ protection, and their local quantity is a critical determinant of immediate pathogen control. If pathogen is not immediately eliminated at the point of entry, inflammation (which may be augmented by re-activation of tissue TEM) will result in the specific extravasation of greater numbers of mTEM as well as bTEM at the site(s) of infection. In principle, this ‘early’ mTEM/bTEM recruitment would greatly increase the local density of TEM, an augmentation of effector potential that would almost certainly be critical for rapid control of all but the most low dose (or benign) infections. At the same time of this early TEM response, tissue Ags will localize to draining lymph nodes, and if presented on APCs in sufficient quantity, will elicit a second round of activation, proliferation, and differentiation among TCM. This process, which takes several to many days, will result in a numerically robust dissemination of new effectors (even from a rare population of TCM) into the site of infection. This ‘late’ phase of the anamnestic effector response may explain why TCM make little impact on protective immunity until several days following challenge. However, TCM can ultimately make very potent contributions to protective immunity, and suffice under conditions in which very rapid control is unnecessary.
Implications of effector site-based memory for vaccination
The goal of any prophylactic vaccine is to safely mimic an initial pathogen encounter so as to elicit protective immune memory: Ab and/or T cell responses that prevent infection with the bona fide pathogen, or abrogate such infection prior to overt disease. For pathogens that are highly susceptible to Ab- or T cell-mediated mechanisms, typically those agents that naturally cause acute infections, any vaccine that is capable of maintaining protective Ab responses or eliciting robust anamnestic Ab and T cell responses upon pathogen exposure will usually meet this goal. Indeed, none of the vaccine approaches in clinical use today specifically target high frequency TEM-biased T cell responses, and given the dependence of the generation and maintenance of such responses on higher/longer Ag exposure and/or increased tissue damage/inflammation, such targeting would almost certainly increase vaccine complexity, and potentially pose safety and tolerability issues. However, conventional vaccine approaches have not yielded highly protective vaccines for a number of critical human pathogens, in particular pathogens with sophisticated immune evasion capabilities that allow chronic infection. Notably, 3 such pathogens – HIV, Mycobacterium tuberculosis and the plasmodium species responsible for malaria – are among the top 5 most deadly global infectious diseases. HIV, in particular, has resisted vaccine development for more than 25 years. HIV and its simian counterpart SIV combine an intrinsic resistance to Ab-mediated neutralization with a replication strategy that provides for rapid establishment of a large, systemic viral population, capable of overwhelming or dynamically adapting/evading almost all immune selection pressures [64]. While many conventional vaccines, most notably approaches using replication-deficient vectors, have proven capable of eliciting TCM-biased virus-specific memory that generates robust anamnestic T cell responses upon viral exposure, these responses, though considerably more rapid and of higher magnitude than primary responses in naïve subjects, have largely failed to mediate long-term control of HIV/SIV infection [64]. Significantly, when highly augmented effector T cell responses occur only after peak systemic viral replication, they are still subject to the dynamic immune evasion mechanisms operating in naïve individuals.
Given that sexually/mucosally transmitted HIV infections in humans and experimental SIV infections in NHPs after limiting dose mucosal challenge are typically initiated by one or very few transmitted/founder viral variants, and that establishment of a systemic, progressive infection capable of dynamic evasion takes many days of local viral amplification and spread [64], it was hypothesized that establishment of a high frequency virus-specific TEM response might be an effective way to control these infections. This hypothesis was recently tested with the development of SIV protein-expressing vectors based on the persistent β-herpesvirus Rhesus CMV. Rhesus CMV/SIV vectors elicit and indefinitely maintain high frequency SIV-specific TEM responses at potential sites of early viral replication after mucosal challenge, and strikingly, over 50% of monkeys vaccinated with these vectors manifested early, stringent control of mucosally administered SIV [27]. This protection, which correlated with the peak SIV-specific CD8+ TEM response magnitude during the vaccine phase, was characterized by an initial peak of viremia of variable, but usually low, magnitude, followed by near immediate control to below quantifiable levels. Although many of the protected monkeys showed periodic, low level viral “blips” of measurable viremia over the first 6 months of follow up, these blips waned, and after one year, replication-competent SIV was not recoverable from the tissues of protected animals. Notably, this early onset TEM-mediated protection occurred without an anamnestic response.
Such “windows of TEM opportunity” – periods in early infection with chronic pathogens in which immune evasion mechanisms are not fully developed and the infection may be relatively susceptible to TEM-mediated control – may well exist for other vaccine-resistant pathogens as well. Malaria parasites would be most vulnerable to T cell-mediated immunity during the intrahepatic stage of its lifecycle, but the length of this stage (~5 days) is sufficiently short to largely avoid T cell effectors arising from a vaccine-elicited anamnestic response. In keeping with this, recent studies have suggested that protection mediated by malaria vaccines is associated with TEM-biased responses [65]. Similarly, a vaccine that elicits and maintains high frequency mycobacterium tuberculosis-specific TEM populations in lung might well allow for earlier and more efficient control of this pathogen. Finally, maintenance of robust vaccine-elicited TEM responses might also be effective in therapeutic vaccination against reactivation of latent viruses such as herpes simplex, allowing earlier intercept of reactivation foci, perhaps before clinical manifestations. Taken together, these considerations suggest that vaccines exploiting TEM have the potential to address some of the most pressing problems in clinical infectious disease today, a potential that merits the prioritization of development of safe, potent, TEM-generating vaccine modalities.
Concluding remarks
A central theme of the immune system is the concentric layering of protective mechanisms of increasing complexity and specificity: adaptive immunity is layered on innate immunity, αβ T cells and B2 B cells are layered over γδ T cells and B1 B cells, and memory populations are layered over naïve populations. As this review indicates, it is increasingly clear that a similar concentric layering exists within the memory T cell compartment itself. Diverse, differentiated and specialized TEM that form a first line of T cell-mediated defense to pathogen re-encounters are layered over TCM populations that serve as a strategic reserve. T cell memory cannot be equated to the anamnestic effector response, but must be considered a complex composite of functionally and anatomically diverse populations, many of which are hidden from routine analysis of blood, that make distinct contributions to host defense. Understanding the interplay of these populations and their specific functional contribution to immunity will be crucial for definition of the mechanisms underlying protective T cell immunity and for the rational development of next generation immunotherapeutics, particularly vaccines, that exploit these mechanisms.
Acknowledgments
The authors wish to thank Vaiva Vezys (U of Minnesota) for critical reading of the manuscript.
References
- 1.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaech SM, Wherr EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 4.Harty JT, V, Badovinac P. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 6.Picker LJ, Terstappen LW, Rott LS, Streeter PR, Stein H, Butcher EC. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990;145:3247–3255. [PubMed] [Google Scholar]
- 7.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 10.Bevan MJ. Memory T cells as an occupying force. Eur J Immunol. 2011;41:1192–1195. doi: 10.1002/eji.201041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 13.Von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 14.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 15.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 19.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 20.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 21.Grossman Z, Picker LJ. Pathogenic mechanisms in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2008;3:380–386. doi: 10.1097/COH.0b013e3282fbaae6. [DOI] [PubMed] [Google Scholar]
- 22.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 23.Suni MA, Ghanekar SA, Houck DW, Maecker HT, Wormsley SB, Picker LJ, Moss RB, Maino VC. CD4(+)CD8(dim) T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur J Immunol. 2001;31(8):2512–2520. doi: 10.1002/1521-4141(200108)31:8<2512::aid-immu2512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Harari A, Enders FB, Cellerai C, Bart PA, Pantaleo G. Distinct profiles of cytotoxic granules in memory CD8 T cells correlate with function, differentiation stage, and antigen exposure. J Virol. 2009;83:2862–2871. doi: 10.1128/JVI.02528-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 26.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 29.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- 31.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 33.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easeterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 36.Pitcher CJ, Hagen SL, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 39.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 41.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4(+) and CD8(+) T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci USA. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: Gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 48.Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163(8):4125–4132. [PubMed] [Google Scholar]
- 49.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 50.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D. Antigen independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 52.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J Virol. 2011;85:11007–11015. doi: 10.1128/JVI.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 58.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 59.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16:558–564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34:781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- 63.Huster KM, Koffler M, Stemberger C, Schiemann M, Wagner H, Busch DH. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur J Immunol. 2006;36:1453–1464. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]
- 64.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reyes-Sandoval A, Wyllie DH, Bauza K, Milicic A, Forbes EK, Rollier CS, Hill AV. CD8+ T effector memory cells protect against liver-stage malaria. J Immunol. 2011;187:1347–1357. doi: 10.4049/jimmunol.1100302. [DOI] [PMC free article] [PubMed] [Google Scholar]