Abstract
Measurement of the survival and dispersal rates of mosquito vectors is an important step in designing and implementing control strategies. Vector survival plays a key role in determining the intensity of pathogen transmission, and vector movement determines the spatial scale on which control efforts must operate to be effective. We provide the first estimates of field survival and dispersal rates for Culex pipiens in North America, an important enzootic and bridge vector for West Nile virus (WNV). We conducted mark-release-recapture studies in a residential area near Washington DC in two consecutive years and fit nonlinear regression models to the recapture data that incorporate weather information into survival and recapture probabilities. We found that daily survival rates were not significantly different between the two years but were negatively affected by rainfall. The daily survival rate was 0.904 ± 0.037 (SE), which implies an average longevity of 10.4 days. As with other vector-borne pathogens, the measured survival rate suggests that at our site the majority of WNV-infected Cx. pipiens mosquitoes may perish before becoming infectious (being able to transmit WNV to hosts). We found relatively little evidence of dispersal following the initial night after release. Our results suggest that transmission of WNV and other pathogens transmitted by Cx. pipiens may be highly local and they highlight the importance of factors that influence survival of mosquito vectors.
KEWORDS: Culex pipiens, dispersal, mosquito, survival, West Nile virus
Introduction
Longevity of adult female mosquitoes is a key variable in the transmission of pathogens. After ingesting an infectious blood meal, a mosquito must survive the extrinsic incubation period of the pathogen before it can transmit it to a susceptible host (Davis 1932, Milby and Reisen 1989). In some cases, variation in mosquito survival may be the dominant factor regulating transmission intensity, and increasing mortality of infected females through adulticiding is one strategy used to control some vector-borne diseases. It follows that determining the survival rate of vectors can allow a better understanding of pathogen transmission, can aid in control efforts and, if the factors influencing survival can be identified, may help predict the occurrence of outbreaks.
At the same time, dispersal of adult mosquitoes from their larval habitats and mosquito movement between feeding and oviposition sites play key roles in the spatial scale of transmission of vector-borne pathogens. Thus, understanding mosquito movement is critical in determining effective control strategies because if mosquitoes move over large distances (e.g. several kilometers) then larviciding and adulticiding efforts must cover a large area to effectively decrease mosquito abundance and/or the intensity of a local epidemic. In contrast, if mosquitoes have low dispersal rates, and move little between feeding and oviposition sites, then if hosts are also sedentary transmission may be highly heterogeneous and very focal with transmission hotspots near key larval habitats or competent hosts.
The introduction of West Nile virus (WNV) to North America in 1999 brought renewed importance to understanding the vector ecology of North American mosquitoes (Lanciotti et al. 1999). WNV has since become the most important mosquito-borne disease in North America, with 35,231 reported cases, 12,852 cases of encephalitis, 1308 deaths, and an estimated 360,000 illnesses from 1999–2010 in the USA and Canada (Centers for Disease Control and Prevention 2011, Health Canada 2011). WNV is a member of the Japanese encephalitis antigenic complex of the genus Flavivirus, family Flaviviridae and is transmitted worldwide primarily by Culex mosquitoes (Kramer et al. 2008, Kilpatrick 2011). In North America, the dominant enzootic and bridge vectors of WNV across many regions are Cx. tarsalis Coquillett, Cx. quinquefasciatus Say, and Cx. pipiens L. (Turell et al. 2002, Kilpatrick et al. 2005, Hamer et al. 2008) and Cx. pipiens also appears to be the predominant vector in urban epidemics of WNV in Romania, and southern Russia (Hubalek and Halouzka 1999). Cx. tarsalis and Cx. quinquefasciatus have been relatively well studied over the past 60 years due to their important roles in the transmission of St. Louis Encephalitis virus and Western Equine Encephalitis virus (Reeves 1965, Reisen et al. 1991, Reisen et al. 1992b, Reisen et al. 1995, Reisen et al. 2003). However, despite its global importance in transmission of WNV and other arboviruses (e.g. St. Louis Encephalitis virus), the field survival rate and dispersal of Cx. pipiens have not been measured in North America.
Culex pipiens mosquitoes are abundant in urban environments, using storm drains and other sources of organically rich, stagnant water for oviposition and larval development (Spielman and D’Antonio 2001, Andreadis et al. 2004). They often feed primarily on birds, which makes them an ideal vector for avian pathogens like WNV and SLEV (Kilpatrick et al. 2006a, Molaei et al. 2006, Hamer et al. 2009). However, they also feed on humans and other mammals, especially in the late summer, which results in their serving as an important bridge vector (Kilpatrick et al. 2005, 2006b; Hamer et al. 2008). Thus, measuring the dispersal and survival of this species could offer important insights into the transmission of WNV.
Measuring survival and dispersal rates of mosquitoes in the field is a logistically challenging task, and can be especially difficult in urban and residential areas where releasing thousands of laboratory-reared mosquitoes might have detrimental impacts on public health. However, previous work has shown that measurements of survival in a laboratory setting are frequently very different from those obtained in the field, making field studies necessary. For example, the life expectancy of Cx. quinquefasciatus in the lab at 25–28°C was estimated to be 74 days (Suleman and Reisen 1979), whereas a field study estimated longevity to be 6.25 days in a residential area of California when temperatures averaged 23.9°C (Reisen et al. 1991).
The purpose of our study was thus to determine the survival and dispersal rates of Cx. pipiens mosquitoes in a residential area where WNV has been actively transmitted yearly since at least 2003. We hypothesized that the feeding and larval habitat of Cx. pipiens would result in low dispersal rates because both avian hosts and larval habitat are locally available. As a result, mosquitoes need not fly long distances for either resource, especially in comparison with mosquito species whose vertebrate hosts and larval habitats may be separated by several kilometers (Walton et al. 1999).
Materials and Methods
We performed mark-release-recapture (MRR) studies at a residential site in Takoma Park, MD during the summer in two years, 2008 and 2009. The 2008 study was performed from 19 August until 2 September and the 2009 study was performed at the same site from 25 July until 8 August. The study site in Takoma Park, MD, about 1.5km northwest of Washington, DC (Latitude: 38.98°N, Longitude: 77.01°W) is in a residential neighborhood (Fig. 1). This site consists primarily of houses with yards and overhanging trees, and also includes a grassy park. The land use in a 500 m radius around the site is 26% impermeable surface and 28% forest cover, with the rest composed primarily of grass/lawns.
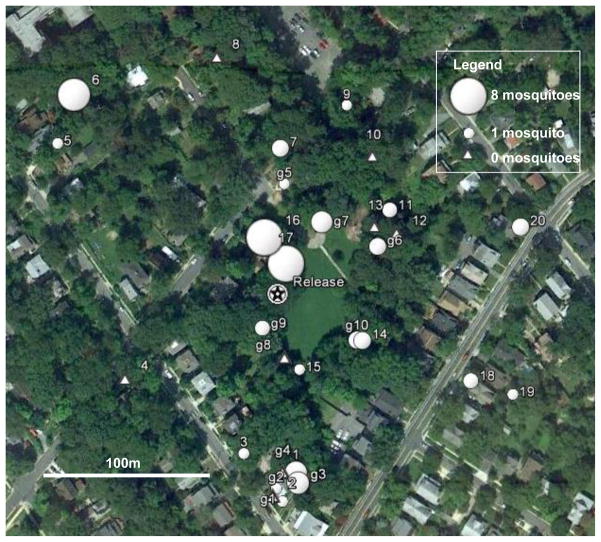
Fig. 1.
Map of the study area in Takoma Park, MD with release site and trap locations, and number of mosquitoes caught in each trap in 2009. Numbers preceded by the letter “g” show CDC gravid trap locations (note: only g1–g4 were run during the 2008 study), and numbers not preceded by “g” refer to CDC light traps baited with dry ice (CO2). The star shows the release site.
Egg Rafts
Egg rafts were collected for the two studies on 6 August 2008 and on 12 and 13 July 2009 by placing 10–15 navy blue, 5.7-liter plastic containers containing 2.5–5 cm organically enriched water (~19 liters water mixed with 0.2–0.4 liters of rabbit chow in a plastic container and placed in the sun for >1 wk before use to stimulate bacterial growth). Tubs were covered with one-inch mesh chicken-wire screening to attract egg-laying Cx. pipiens and deter other species (Walther and Weber 1996). Egg rafts were collected daily and placed individually into 15 × 23 × 5 cm containers, inside a 0.23 m3 cage that was placed in a shaded location at the site. The cages had screen on all sides to allow airflow, and plastic covers over the top of the cage to protect mosquitoes from direct rainfall.
Rearing Mosquitoes
After 2–3 days, two larvae were removed from each container for species identification (Darsie and Ward 1981, Andreadis et al. 2005). Containers containing Cx. pipiens were retained and allowed to develop to adulthood, whereas containers containing larvae of other species were removed from the cage. Larvae were fed 0.5 g of fish food (Koi’s premium choice fish food; Kaytee Inc., Chilton, WI) daily and adults were offered a 10% sucrose solution on cotton balls.
Spermathecal dissections
After emergence, males and females were held in cages for three days in order to allow mating. On the third day, ten females were removed from each cage and dissected to determine mating status by the presence or absence of sperm in spermathecae. Spermathecal dissections showed the presence of sperm in 10/10 (100%) of the reared mosquitoes from each year, suggesting that a large fraction of the marked and released mosquitoes had mated.
Marking Mosquitoes
We marked female mosquitoes on the day of release approximately an hour before dusk. Mosquitoes were removed from the cage using aspirators, counted in a clear plastic tube, and transported to 0.027m3 cuboidal cages. Mosquitoes were dusted with Bioquip (Rancho Dominguez, CA) fluorescent powders dispensed from 4 oz handheld bulbs. In 2008, mosquitoes were released on a single night and dusted with red. In 2009, mosquitoes were released on two consecutive nights and were dusted with either yellow or blue to differentiate the two nights.
Releasing Mosquitoes
The marked mosquitoes were released at dusk at the approximate center of the study site and mosquito traps (Fig. 1). In 2008 we released 223 marked female mosquitoes on a single day (19 August, 2008), whereas in 2009, 500 marked female mosquitoes were released on each of two consecutive days (25 and 26 July, 2009).
Recapturing Mosquitoes
Twenty CDC light traps (baited with ~1 kg of dry ice (solidified CO2) in a 1.5-liter plastic thermos) and four (2008) or ten (2009) gravid traps (baited with the organically enriched water used in the ovitraps described above) were distributed throughout the site. Two CDC light traps were hung from parachute cord at 1.5m above the ground and the remainder were placed 2–10m above the ground to attract mosquitoes feeding in the foliage and canopies of trees. Mosquitoes were removed from all traps every afternoon for 15 consecutive days. All trapped mosquitoes were examined for fluorescent dusts with UV light under a dissecting microscope. We obtained daily rainfall, temperature and wind speed and direction estimates for the duration of the study period from a nearby weather station ~3 km away (http://www.wunderground.com/history/airport/KCGS/).
Statistical Analysis
We used non-linear least squares regression to estimate survival rates (or more correctly, “retention rates” since mosquitoes that disperse outside the trapping area cannot be distinguished from death) and recapture probability by adapting a previous approach (Buonaccorsi et al. 2003). In the simplest model, we fit the number of marked female mosquitoes captured on each night j, Cj, to the following equation:
| (1) |
Here N is the number of mosquitoes of that color released, θ is the probability that a live marked mosquito is recaptured, π is the daily survival probability, j is the trapping occasion, and tj is the number of days between release and trapping occasion j (in our case, tj = j, because each trapping occasion was 1 d and mosquitoes were trapped every day). The summation of trapped mosquitoes from previous days (ΣCi, i = 1 to j−1), removes mosquitoes from the marked population that were caught and removed in previous time steps (this was approximated by Buonaccorsi et al. as (1−θ)j−1 but eq. 1 uses the exact number removed rather than an estimate).
We analyzed the influence of year and rain on recapture probability and survival using the equation:
| (2) |
Here c5 measured the effect of rain on night j, Rj, on recapture probability, and c6 measured the effect of rain on survival (note that the effects of previous rain, if any, must be compounded as indicated by the summation), y was a dummy variable (0 for 2008, and 1 for 2009), the coefficient c3 measured the difference in survival between the years, and c4 measured the difference in recapture probability between the two years. It is important to note that this model assumes the impact of rain on survival is nonlinear and saturating. Comparisons between years were then made by determining whether the year coefficients (c3 and c4) were significantly different from 0. The non-linear regression models were fit to the data using function nls in R (R Development Core Team 2011). The variance of the parameters in eq. 2 is estimated from a linearized version of the model (Bates and Watts 1988). Finally, we calculated an estimate of the average longevity by inverting the daily mortality rate (average longevity = 1/(1−survival rate)).
Mosquito Dispersal
The average daily dispersal distance was estimated by calculating the effort- weighted average distance that marked individuals were trapped away from the release site. We estimated trapping effort by calculating the number of traps in a disc with the distance between outer and inner circles of 20 m and dividing by the area in this disc. For example, if there were three traps that were between 20 m and 40 m from the release site, the trapping effort in this disc would be 3/(π(402−202)). We then calculated the distance dispersed on day j, Dj, using the equation:
| (4) |
Here is the number of mosquitoes trapped on day j in trap k that is within disc i (i = 1 to n, with i = 1 being a 20 m radius circle, i = 2, a disc with outer diameter 40 m and inner diameter 20 m, etc., and i = n being the disc containing the traps furthest from the release site; k = 1 to m, where m is the number of traps within disk i), dk is the distance between trap k and the release site, and Ei is the trapping effort in that disc, as described above. We estimated the dispersal rate by regressing the effort-weighted average distance that marked mosquitoes were caught each day against the number of days since the release, starting with day 1 post-release (we did not include a distance of 0 on day 0). Thus, dispersal from the release location on the night of release does not influence this calculation. We also performed a set of simulations of mosquitoes dispersing across our trapping grid in order to assess whether our relatively narrow-distance trapping grid could underestimate the rate of dispersal (Supplemental Online Material).
The direction traveled by the mosquitoes was determined by averaging the direction (in degrees) of the release site for each site to a trap that captured a marked mosquito. A chi-squared test was used to determine if the mosquitoes dispersed in random directions or showed evidence of directional movement. We separated the site into six sectors, the largest number that could be used and still satisfy the assumptions of a chi-squared test. We used the following equation to calculate the χ2 statistic:
| (5) |
Here O is the observed number of marked mosquitoes trapped in the sector, and the term in parentheses is the expected number in sector i, which is the product of C, the total number of mosquitoes recaptured and xi, the number of traps in a sector divided by n, the total number of traps at the site, and q the number of sectors. Numbers of recaptured mosquitoes were large enough to permit analysis for the study in 2009, but were too small for 2008.
Results
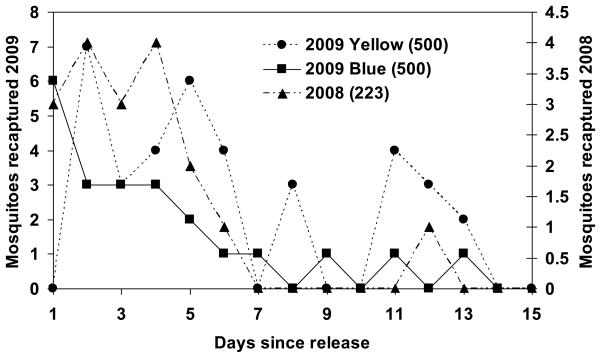
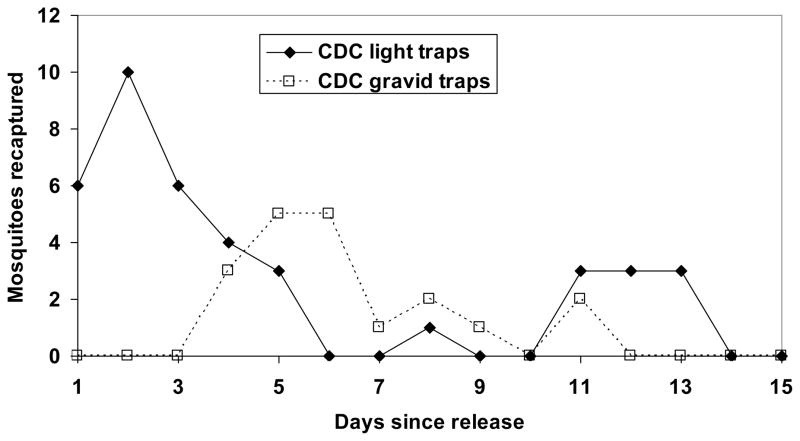
We captured a total of 1818 mosquitoes in 2008 and 5493 mosquitoes in 2009. Thus, the overall impact of our study on local mosquito populations was to reduce them at least 5-fold relative to the 223 and 1000 mosquitoes that were locally reared from egg rafts laid at the site. Marked female mosquitoes were captured throughout the study area with 38% of the traps recapturing at least one marked mosquito in 2008 and 73% in 2009 (Fig. 1). The fraction of female mosquitoes recaptured was slightly higher in 2008 (8.1%, 18 of 223) than in 2009 (5.8%, or 58 of 1000) but there was no evidence that survival or recapture probability differed between the two years (survival: c3 = −0.0055± 0.006 (SE); t = −0.88; df = 1; P = 0.38; recapture probability: c4 = 0.060±0.078; t= 0.776; df = 1; P = 0.44). The number of recaptured marked female mosquitoes was highest in the first few days the release and declined thereafter, with mosquitoes being trapped through day 11 in 2008 and day 13 in 2009 (Figs. 2 and 3). The temporal pattern of recaptures in CDC light and CDC gravid traps in 2009 varied through time with no recaptures in gravid traps until day 4 post-release and a second rise in recaptures in light traps on days 11–13 (Fig. 3). In both years more mosquitoes were recaptured by CO2-baited CDC light traps than gravid traps possibly because of the larger number of CDC light traps than CDC gravid traps (2008: 20 light and 4 gravid traps; all 18 recaptures in light traps; χ2 = 3.6; df = 1; P = 0.058; 2009: 20 light vs. 10 gravid traps; 39 of 58 recaptures were in light traps: χ2 = 0.01; df = 1; P > 0.95) and because mosquitoes had to survive feeding and egg development before becoming gravid.) and because mosquitoes had to survive feeding and egg development before becoming gravid.
Fig. 2.
Number of Culex pipiens recaptured each day in 2008 and 2009. In 2009, 500 mosquitoes were marked and released on each of two consecutive days and were dusted with different colors (yellow and blue). In 2008, a single batch of 223 mosquitoes were released. Numbers recaptured in 2009 and 2008 are given on the left and right Y-axes, respectively, and the two Y-axes are scaled so the numbers recaptured are comparable.
Fig. 3.
Number of Culex pipiens recaptured each day post-release in CDC light traps baited with dry ice (CO2) and CDC gravid traps in 2009.
Rainfall occurred on several days during the study in both years (Fig. S3), and there was evidence that rain negatively affected mosquito survival (Table 1) but only weak and non-significant evidence that rain reduced the recapture probability (c5 = −5.42±3.25; t = −1.67; df = 1; P = 0.10). The estimated coefficient for rain, −0.042, (Table 1) implies that a 1cm rainstorm in 24 h would decrease daily survival by 34% from 0.904 to 0.594.
Table 1.
Daily survival and recapture probability (theta) for Cx. pipiens mosquitoes calculated using nonlinear least squares on data from both years combined.
| Parameter | Estimate | SE | t | P |
|---|---|---|---|---|
| Recapture probability, θ | 0.013 | 0.002 | 6.48 | 8.8×10−08 |
| Survival, π | 0.905 | 0.037 | 24.56 | 1.00×10−16 |
| Rain on survival, c2a | −0.042 | 0.015 | −2.90 | 0.0068 |
Note: There was no evidence for differences in survival or recapture probability between the two years, 2008 and 2009; see text for further details.
Parameter c2 indicates the effect of rainfall, R, in mm, on mosquito survival given by the expression survival = survival*ec2*R. See Methods for further explanation.
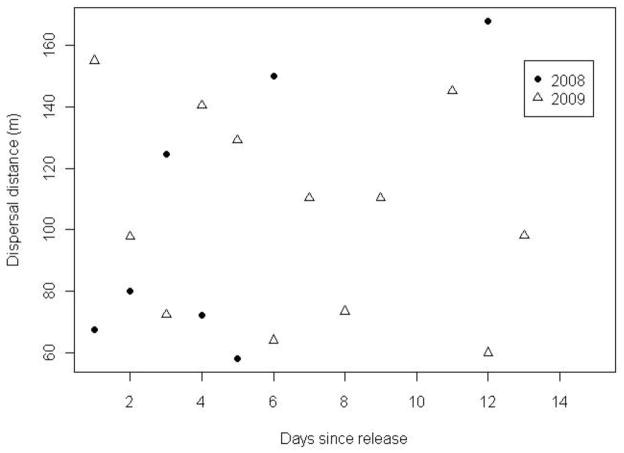
Dispersal rates (mean distance of marked mosquitoes recaptured each day, weighted by trapping effort) were estimated for both years (Fig. 4). For the 2008 study, there was marginal support for Cx. pipiens mosquitoes dispersing at a very low rate (Fig. 4; 8.8 ± 3.3 m/d). However, in 2009 there was no evidence for dispersal in that the distance of trapped mosquitoes from the release site did not increase with days since release (Fig. 4; −2.2 ± 2.5 m/d). These low estimates for dispersal rate do not appear to be an artifact of the small spatial extent of our trapping grid (Supplemental Online Material). There was some evidence that the direction of dispersal of mosquitoes in 2009 was not random, with more mosquitoes being caught, after adjusting for trapping effort, in traps in a NNW direction, and fewer in the NNE direction from the release site (χ2 = 12.8; df = 5; P = 0.025). This is opposite the predominant wind direction during the study, which was southwest (Fig. S4). The number of recaptured mosquitoes was too low to determine if the direction of dispersal was different from random in 2008.
Fig. 4.
Average distance of recaptured mosquitoes on each day post-release, corrected for trapping effort. In 2008 mosquitoes dispersed ~9 m per day (Distance from release site (m) = 61.3 + 8.8*Day; n = 7; P = 0.06). In 2009 there was little statistical evidence for positive dispersal (Distance from release site (m) = 119.4 − 2.1*Day; n = 12; P = 0.10).
Discussion
Variation in the survival of Cx. pipiens mosquitoes could play an important role in the transmission of several arboviruses including WNV and SLEV. Our MRR study suggests that the daily survival rate for Cx. pipiens females in a suburban area in the mid-Atlantic was 90.4% with a 95% confidence interval (C.I.) of 83.1–97.9%. This is somewhat higher than survival estimates for other WNV vectors. For example, the 95% C.I. for Cx. pipiens from our study barely includes the estimate of 83.8% obtained for Cx. quinquefasciatus in Rossmoor, Orange County, California (Milby and Reisen 1989), and the mean for Cx. pipiens is also substantially higher than estimates from MRR studies of Cx. tarsalis (47–75%) in the Coachella Valley of California (Reisen and Lothrop 1995). However, estimates of daily survival of Cx. tarsalis at the same sites based on parity dissections, which are less biased by dispersal but require other assumptions, were much higher (80–92% in 4 of 5 months). These latter estimates are similar to our estimate for Cx. pipiens and to those for Cx. erythrothorax across three months in southern California (84–89%) (Walton et al. 1999).
Trapping of mosquitoes with significant numbers of host seeking and gravid traps in 2009 allows a preliminary estimate of the lengths of the stages of a single gonotrophic cycle in the field including the period of host seeking, digestion, and egg laying (Fig. 3). All mosquitoes caught in the first four days were caught in host-seeking traps, suggesting that the released mosquitoes, which had already mated, may have taken up to 4 d to find and feed on a host. In the subsequent four days, recaptured mosquitoes were primarily captured in gravid traps suggesting they had successfully fed and were ready to lay eggs. Finally, a second “wave” of mosquitoes was caught in host-seeking traps between days 11 and 13. These data provide a crude estimate of 8–10 d for the length of the gonotrophic cycle over a two week period when the average temperature was 25.2 °C. This is longer than the five days estimated for Cx. tarsalis in May when temperatures were similar (Reisen and Lothrop 1995), but we acknowledge that our estimate is somewhat crude and based on limited sample sizes.
Mosquitoes appeared to disperse throughout the study areas in the initial night after the release but there was only weak evidence of very limited dispersal afterward. Although some mosquitoes likely moved beyond our furthest traps (200 m from the release site) on the first night and continued to disperse, the remaining mosquitoes showed little evidence of movement after the first night. Our simulations suggest that despite the limited spatial extent of our trapping grid, our data are inconsistent with significant mosquito dispersal (Supplemental Online Material). If our low estimates of dispersal after the release night are valid, they contrast markedly with mark-recapture studies of Cx. tarsalis in which mosquitoes appeared to be actively dispersing for at least 3 days and moved over 1 km during this time (Reisen and Lothrop 1995). In another study Cx. tarsalis moved ~110 m/d and Cx. quinquefasciatus dispersed ~180 m/d (Reisen et al. 1992a). The difference in dispersal rates may be due to differences in larval habitat and host preferences of the three species. In a residential area with an ample supply of larval habitats, and 15.3 birds/ha (Kilpatrick et al. 2006a) little movement would be required to find both hosts and larval habitat. Limited dispersal by Cx. pipiens would result in highly focal WNV transmission where this species the dominant vector.
Our estimate for the survival rate of Cx. pipiens suggests an interesting implication for WNV transmission that is likely common to many vector-borne pathogens: approximately 2.3% of Cx. pipiens that feed on competent hosts (e.g. those with a viremia of 108 PFU/ml such as an American robin; (Kilpatrick et al. 2007)) on day 3 after adult emergence would survive and be able to transmit WNV 7 days later. This scenario is based on the fact that during the time of our study, the average temperature was 25.2°C, and the average longevity of Cx. pipiens mosquitoes was 10.5 days (assuming a constant daily survival rate, 0.905). Under this model of exponentially distributed longevity, only ~49.7% of mosquitoes would survive 7 days, and other research suggests that at 25.2°C, approximately 4.7% would likely transmit WNV on their second feeding on day 10 if they fed on an infected host in their first feeding on day 3 (Kilpatrick et al. 2008). Thus, only a small fraction of mosquitoes would be capable of transmitting WNV, and decreases in adult mosquito survival, such as a severe rainstorm (which may also reduce larval survival; (Koenraadt and Harrington 2008)), could decrease transmission in the short term. It remains to be determined whether survival of Cx. pipiens varies among sites, regionally or seasonally, and how factors like mosquito predators, adulticiding, temperature, and humidity might influence both mosquito survival and pathogen transmission.
Supplementary Material
Acknowledgments
We thank Mary Ashley Laine, Alex Martin, Alex Arp, Katie Jensen, and Juraj Cech for assistance in the field, George O’Meara for helpful advice and discussion, and the many residents of Takoma Park who allowed us to trap mosquitoes in their yards. We thank K. Feldman and R. Gibbs for support, guidance, and/or permission to perform this study. This work was funded by NSF grant EF-0914866 as part of the joint NSF-NIH Ecology of Infectious Disease program, and NIH grant 1R01AI090159-01.
References Cited
- Andreadis TG, Thomas MC, Shepard JJ. Identification guide to the mosquitoes of Connecticut. The Connecticut Agricultural Experiment Station; New Haven, CT: 2005. [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: A five-year analysis of mosquito data 1999–2003. Vector-Borne and Zoonotic Diseases. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- Bates DM, Watts DG. Nonlinear Regression Analysis and Its Applications. Wiley; New York: 1988. [Google Scholar]
- Buonaccorsi JP, Harrington LC, Edman JD. Estimation and comparison of mosquito survival rates with release-recapture-removal data. J Med Entomol. 2003;40:6–17. doi: 10.1603/0022-2585-40.1.6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed on January 16, 2009];West Nile virus. 2011 Available from: http://www.cdc.gov/ncidod/dvbid/westnile/index.htm.
- Darsie RFJ, Ward RA. Identification and geographic distribution of mosquitoes of North America, north of Mexico. Mosq Syst Suppl. 1981;1:1–313. [Google Scholar]
- Davis NC. The effect of various temperatures in modifying the extrinsic incubation period of the yellow fever virus. Am J Hyg. 1932;16:163–75. [Google Scholar]
- Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Walker ED. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol. 2008;45:125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culex pipiens mosquitoes and West Nile Virus amplification. Am J Trop Med Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- Health Canada. [Accessed on January 16, 2009];West Nile virus. 2011 Available from: http://www.phac-aspc.gc.ca/wnv-vwn/mon_e.html.
- Hubalek Z, Halouzka J. West Nile fever - a reemerging mosquito-borne viral disease in Europe. Emerging Infectious Diseases. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM. West Nile virus: Globalization, land use, and the emergence of an infectious disease. Science. 2011;334:323–7. doi: 10.1126/science.1201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, LaDeau SL, Marra PP. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk. 2007;124:1121–1136. [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathogens. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proceedings of the Royal Society B: Biological Sciences. 2006a;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biology. 2006b;4:606–610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell S, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerging Infectious Diseases. 2005;11:425–9. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenraadt CJM, Harrington LC. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae) J Med Entomol. 2008;45:28–35. doi: 10.1603/0022-2585(2008)45[28:feoroc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annual Review of Entomology. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Milby MM, Reisen WK. Estimation of vectorial capacity: vector survivorship. Bulletin of the Society for Vector Ecology. 1989;14:47–54. [Google Scholar]
- Molaei G, Andreadis T, Armstrong P, Anderson J, Vossbrinck C. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerging Infectious Diseases. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. [Accessed on April 25];R: A Language and Environment for Statistical Computing. 2011 Available from: http://www.R-project.org.
- Reeves WC. Ecology of mosquitoes in relation to arboviruses. Annual Review of Entomology. 1965;10:25–46. [Google Scholar]
- Reisen WK, Lothrop HD. Population ecology and dispersal of Culex tarsalis (Diptera, Culicidae) in the Coachella Valley of California. J Med Entomol. 1995;32:490–502. doi: 10.1093/jmedent/32.4.490. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Milby MM, Meyer RP. Population dynamics of adult Culex mosquitoes (Diptera: Culiciade) along the Kern River, Kern County, California, in 1990. J Med Entomol. 1992a;29:531–543. doi: 10.1093/jmedent/29.3.531. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Hardy JL. Bionomics of Culex tarsalis (Diptera, Culicidae) in relation to arbovirus transmission in southeastern California. J Med Entomol. 1995;32:316–327. doi: 10.1093/jmedent/32.3.316. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Lothrop B. Factors influencing the outcome of mark-release-recapture studies with Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2003;40:820–829. doi: 10.1603/0022-2585-40.6.820. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Milby MM, Presser SB, Hardy JL. Ecology of mosquitos and St. Louis Encephalitis Virus in the Los Angeles Basin of California. 1992b:1987–1990. doi: 10.1093/jmedent/29.4.582. [DOI] [PubMed] [Google Scholar]; J Med Entomol. 29:582–598. doi: 10.1093/jmedent/29.4.582. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Milby MM, Meyer RP, Pfuntner AR, Spoehel J, Hazelrigg JE, Webb JP. Mark-release-recapture studies with Culex mosquitoes (Diptera, Culicidae) in southern California. J Med Entomol. 1991;28:357–371. doi: 10.1093/jmedent/28.3.357. [DOI] [PubMed] [Google Scholar]
- Spielman A, D’Antonio M. Mosquito: a natural history of our most persistent and deadly foe. Hyperion; New York: 2001. [Google Scholar]
- Suleman M, Reisen WK. Culex quinqufasciatus Say: life table characteristics of adults reared from wild-caught pupae from North West frontier province, Pakistan. Mosq News. 1979;39:756–762. [Google Scholar]
- Turell MJ, Sardelis MR, O’Guinn ML, Dohm DJ. Potential vectors of West Nile virus in North America. In: Mackenzie J, Barrett A, Deubel V, editors. Japanese Encephalitis and West Nile Viruses. Vol. 267. Springer-Verlag; Berlin: 2002. pp. 241–252. Current Topics in Microbiology and Immunology. [Google Scholar]
- Walther DA, Weber RG. Simple structural modifications to ovitraps affect oviposition response by wild Culex pipiens and Culex restuans. Proc NJ Mosq Control Assoc. 1996;83:106–111. [Google Scholar]
- Walton WE, Workman PD, Tempelis CH. Dispersal, survivorship, and host selection of Culex erythrothorax (Diptera: Culicidae) associated with a constructed wetland in southern California. J Med Entomol. 1999;36:30–40. doi: 10.1093/jmedent/36.1.30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.