Abstract
Two discoveries have put D-serine in the spotlight of neuroscience. First, D-serine was detected in brain tissue at high levels. Second, it was found to act on the N-methyl-D-aspartate receptor (NMDAR). This receptor is central to use-dependent synaptic plasticity, the cellular process which is widely believed to underlie learning. The ensuing quest for the mechanisms of D-serine synthesis, release and clearance, as well as for its physiological significance has provided a wealth of experimental evidence implicating D-serine in synaptic plasticity. However some key questions remain unanswered. Which cells release D-serine and upon what stimuli? Is D-serine supply dynamically regulated? What is the fate of released D-serine? Answering these questions appears to be an essential step in our understanding of how NMDARs trigger synaptic plasticity and learning. This review will highlight some recent advances and avenues of enquiry in dynamic D-serine signaling in the mammalian brain with emphasis on neurophysiology.
Keywords: Astrocytes, Synaptic plasticity, D-Serine, Serine racemase
1. Introduction
Enantiomers of the ubiquitous L-amino acids were widely believed to play little role in the neurophysiology of higher mammals until high concentrations of D-serine were detected unexpectedly in the brain (Hashimoto et al., 1992). This prompted urgent reevaluation of the earlier observations of excitatory actions of exogenous D-serine (Curtis et al., 1961) and studies suggesting D-serine binding to NMDARs (Danysz et al., 1990). Taken together, these early data seemed to indicate that, by binding to the NMDAR co-agonist site, endogenous D-serine could support NMDAR-dependent synaptic plasticity, a common type of use-dependent changes in synaptic connections thought to represent a cellular mechanism of learning. D-serine could therefore play the role previously attributed to the NMDAR co-agonist glycine (Johnson and Ascher, 1987) which gave the original name to the NMDAR co-agonist site.
In addition to the co-agonist action, the opening of the NMDAR ion channel in situ classically requires the binding of its “main” ligand, the excitatory neurotransmitter glutamate (released mainly by presynaptic terminals) as well as depolarization of the host cell to remove the Mg2+ block. In contrast to many other receptors in the brain the NMDAR therefore integrates three potentially independent signals each of which could thus gate receptor function. For instance, changes of co-agonist concentrations in space or time could dynamically determine whether NMDAR activation upon coinciding presynaptic glutamate release and cell depolarization will occur at a synapse and whether that synapse will undergo plastic changes.
2. Structure
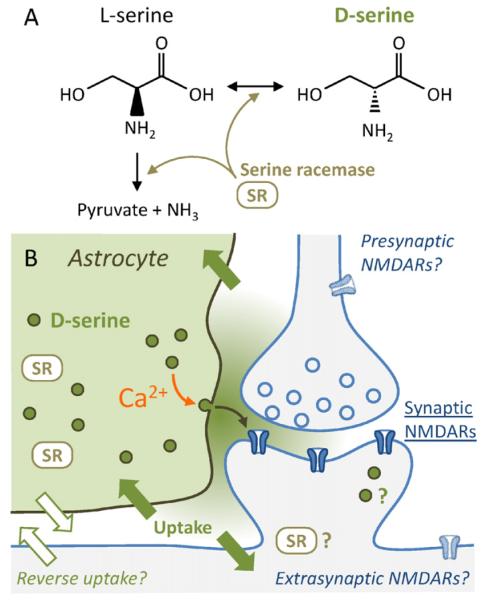
D-Serine ((R)-2-amino-3-hydroxypropanoic acid, C3H7NO3, CAS Registry Number 312-84-5) is the enantiomer of the ubiquitous neutral amino acid L-serine (Fig. 1A). The water soluble amino acid has an isoelectric point of 5.68 suggesting that it might in principle interact with electric fields detected in the microenvironment of excitatory synapses (Sylantyev et al., 2008).
Fig. 1.
cellular pathways of D-serine in brain tissue at a single glutamatergic synapse. (A) L-Serine is metabolized by the serine racemase (SR) and either broken down into pyruvate and ammonia or converted into D-serine. (B) Serine racemase containing glial cells, specifically astrocytes, contain high concentrations of D-serine (green) and their processes closely enwrap synapses. Ca2+ elevations in astrocytes are potent triggers of vesicular release of D-serine into the extracellular space. Released D-serine can act on synaptic and potentially also on extrasynaptic and presynaptic NMDARs. Removal of D-serine (solid green arrows) from the extracellular space is mediated by neutral amino acid transporters (ASCT, Asc-1 and similar), which may contribute to D-serine release as well by reversal uptake. Neurons express serine racemase too and could therefore represent an alternative cellular source of D-serine. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3. Expression, activation and turnover
D-Serine is particularly abundant in the cerebral cortex, hippocampus, anterior olfactory nucleus, olfactory tubercle and amygdala (Schell et al., 1995). Its distribution in the rodent brain mirrors the expression of NMDARs, as one might expect for an NMDAR co-agonist, during development and in the adult animal (Schell et al., 1995; Schell, 2004). On a microscopic scale, D-serine has been predominantly localized to astrocytes (Schell et al., 1995), a non-neuronal glial cell type, and, within astrocytes, to the secretory vesicles (Mothet et al., 2005) suggesting that astrocytes are a primary source of endogenous D-serine. In this case, astrocytes would need to either accumulate D-serine or synthesize it through racemization of L-serine (Fig. 1). However, amino acid racemases were thought to be present in bacteria and insects only, until a specific serine racemase (SR) was identified in the brain (Wolosker et al., 1999). Indeed, the levels of D-serine, but not other amino acids, decrease in the cortex and hippocampus when SR is genetically deleted (Horio et al., 2011). Furthermore, SR expression not only followed the distribution of D-serine across brain regions but also featured prominently in astrocytes (Wolosker et al., 1999). The exact cellular expression pattern has remained controversial, however, as more recent studies have indicated that SR could also be expressed in both neurons and astrocytes (Kartvelishvily et al., 2006) or even predominantly in neurons as demonstrated by immunohistochemistry using antibodies against SR tested in SR-knockout mice (Miya et al., 2008). Although one could probably attribute the conflicting results to different antibodies used to localize SR or to identify neurons and astrocytes, a tempting alternative explanation could be the active (e.g. circadian) regulation of SR expression and distribution. Furthermore, both neurons and astrocytes may have to be considered as the site of synthesis of D-serine because differential regulation of SR activity – for instance, by metabotropic glutamate receptors in glia (Mustafa et al., 2009) – might shift the relative contributions of neurons and glia irrespective of SR expression. Interestingly, SR also mediates the conversion of L-serine into pyruvate, and its activity depends on the presence of metabolites, ATP and Mg2+ thus relating SR not only to NMDAR function but also to energy metabolism (De Miranda et al., 2002; Wolosker, 2011). It is therefore conceivable that changes of astrocytes metabolism, substrate availability or of the aforementioned glutamate signaling could translate into rapid changes of D-serine production.
In order to bind to the NMDAR co-agonist site D-serine has to be present in the extracellular space. Early studies demonstrated that activation of glutamate receptors in cultured glial cells or increasing Ca2+ concentrations in glia using Ca2+-ionophores could deplete intracellular while increasing extracellular D-serine levels (Schell et al., 1995; Cook et al., 2002; Mothet et al., 2005). Conversely, disrupting Ca2+ signaling inside astrocytes reduces D-serine release from astrocytes in culture and in organized hippocampal tissue (Mothet et al., 2005; Henneberger et al., 2010), which is consistent with Ca2+-mediated vesicular exocytosis of D-serine (see Henneberger and Rusakov, 2010 for a recent survey). D-serine release can also be suppressed by inhibiting the vesicular proton pump that generates the proton gradient essential for loading secretory vesicles (Mothet et al., 2005) or by tetanus toxin (Mothet et al., 2005; Henneberger et al., 2010). Conversely, the expression of a dominant negative SNARE protein in astrocytes impairs NMDAR function, which can be restored by exogenous D-serine (Fellin et al., 2009). Together these studies provide compelling evidence that astrocytes can release D-serine (Fig. 1B), most likely in a vesicular, Ca2+-dependent manner. Because the underlying mechanisms may not always be detectable as the classical Ca2+ store-mediated global astrocyte Ca2+ waves (Agulhon et al., 2010), it would seem important to focus on the microscopic Ca2+ signals in thin astrocyte protrusions (Di Castro et al., 2011) which could potentially involve a variety of local Ca2+ sources.
Extracellular D-serine is taken up by neutral amino acid transporters in a manner which is either sodium-dependent (ASCT-like, Ribeiro et al., 2002) or sodium-independent (e.g. Asc-1, Rutter et al., 2007). An important implication of the former is that activity-dependent increases of intracellular sodium may reduce transporter efficiency and thereby increase extracellular D-serine levels independently of vesicular D-serine release. Finally, degradation of D-serine is accomplished by either SR itself or by the D-amino oxidase (Schell, 2004; Wolosker, 2011) the latter providing an important tool for the investigation of D-serine signaling in the brain (Mothet et al., 2000; Panatier et al., 2006).
4. Biological function
As a candidate endogenous co-agonist of NMDARs, D-serine has attracted increasing attention in its possible role as a gate for NMDAR-dependent synaptic plasticity. An important challenge has been to unequivocally demonstrate that a potential co-agonist like D-serine is an endogenous NMDAR co-agonist in situ. A first major breakthrough was the successful use of the D-amino oxidase (DAAO) to degrade endogenously produced D-serine (Mothet et al., 2000). When D-serine levels were reduced with DAAO, NMDA-currents and spontaneous NMDAR-dependent synaptic transmission in cultured hippocampal neurons were diminished whereas subsequent application of D-serine restored NMDAR function (Mothet et al., 2000). Together with the pre-dominant localization of D-serine in astrocytes, this observation predicted that NMDAR-dependent plasticity should depend on astrocytes. Indeed, a particularly well-studied form of NMDAR-dependent plasticity, long-term potentiation (LTP), turned out to depend on the presence of astrocytes in culture (Yang et al., 2003) implying that astrocyte coverage of neurons could determine if neuronal NMDAR-dependent LTP can occur.
This hypothesis was subsequently tested in the hypothalamic nucleus where astrocyte processes physiologically retract from neurons with the onset of lactation. Strikingly, with lactation the NMDAR-dependent synaptic transmission dropped and LTP virtually disappeared (Panatier et al., 2006). This phenotype was fully reversible when D-serine levels were exogenously restored. Conversely, lowering D-serine levels with DAAO in virgin rats mimicked the effects of lactation on NMDAR function and LTP (Panatier et al., 2006). These results indicated that the spatial relationship between astrocyte processes and the target NMDARs could be critical for NMDAR function. However, it is not known whether astrocytes release D-serine at specialized release zones and, if so, where such zones are located. Astrocytes could in principle deliver D-serine to distinct populations of NMDARs (e.g. synaptic, extrasynaptic) characterized by different subunit compositions and with variable affinity to co-agonists (Fig. 1B). Since these NMDAR populations may potentially trigger different forms of plasticity (Liu et al., 2004) selective astrocyte supply of D-serine may support varying types of plasticity. This scenario would require astrocytes to release D-serine locally as opposed to setting a global D-serine ‘tone’. At first glance, the latter could be expected from astrocytes that are inter-connected via gap junctions allowing intracellular signals, such as release triggering Ca2+ transients, to propagate from cell to cell. However, hippocampal astrocytes in brain slices retain the ability to control LTP within or near their individual territories involving Ca2+-dependent, possibly vesicular D-serine release (Henneberger et al., 2010). This implies that astrocytes are at least capable of regulating local D-serine supply and might indeed be able to deliver D-serine to specific NMDAR populations. These astrocytes also require SR activity to support NMDAR function and are likely to store D-serine in vesicles (Henneberger et al., 2010) in line with earlier studies in culture (Mothet et al., 2005).
In summary, experimental evidence indicates that the D-serine produced by SR, stored and released from astrocytes acts on neuronal NMDARs and is thus capable of modulating NMDAR-dependent synaptic plasticity (Mothet et al., 2000, 2005; Yang et al., 2003; Panatier et al., 2006; Henneberger et al., 2010). Nonetheless, a recurring observation is that experimental approaches designed to suppress endogenous D-serine supply do not necessarily inhibit NMDAR function completely. One possible explanation in the case of DAAO (Mothet et al., 2000; Panatier et al., 2006) is that enzymatic degradation is incomplete, or that the enzyme does not fully penetrate the preparation or is outcompeted by D-serine release. Yet, even when astrocytes are metabolically poisoned, are not present in culture or have their release mechanism suppressed the NMDAR-dependent synaptic transmission is far from abolished (Yang et al., 2003; Fellin et al., 2009; Henneberger et al., 2010). This observation indicates that either other cells types, most notably neurons, provide NMDAR co-agonist(s) too or the classic co-agonist glycine (or another yet unknown co-agonist) supports the residual NMDAR function. Since the levels of D-serine vary across brain regions (Schell, 2004) it appears likely that the relative contribution of different co-agonists to NMDAR activation is heterogeneous throughout the brain.
An important pre-requisite for the NMDAR sensitivity to its co-agonist supply is the non-saturation of the NMDAR co-agonist binding site in situ in baseline conditions (Panatier et al., 2006; Li et al., 2009; Henneberger et al., 2010): this should enable changes of extracellular (local) co-agonist concentrations to have an immediate impact on NMDAR function. In that case, the balance between co-agonist supply by different mechanisms and from different cellular sources and the co-agonist clearance mechanisms will ultimately determine the availability of NMDARs for activation.
5. Potential implications for neurological conditions
It is conceivable that D-serine signaling could be involved in brain disorders in which NMDARs contributes to the pathophysiology, such as stroke. Here, an indirect reduction of NMDAR-mediated excitotoxicity through modified D-serine supply may in principle suggest new therapeutic avenues. It is encouraging in that respect that SR deletion was recently reported to reduce tissue damage in a mouse stroke model (Mustafa et al., 2010). However, increasing D-serine levels might on the other hand help to overcome cognitive impairments related to the dysfunction of NMDAR signaling. Promising early studies showed that D-cycloserine, a partial agonist at the NMDAR co-agonist site, can improve spatial learning in rats (Monahan et al., 1989). Therapeutic approaches targeting D-serine signaling will therefore have to strike the balance between preventing NMDAR-dependent damage and avoiding cognitive impairment.
References
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–4. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca(2+) detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–84. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- Cook SP, Galve-Roperh I, Martínez del Pozo A, Rodríguez-Crespo I. Direct calcium binding results in activation of brain serine racemase. J Biol Chem. 2002;277:27782–92. doi: 10.1074/jbc.M111814200. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Phillis JW, Watkins JC. Actions of aminoacids on the isolated hemisected spinal cord of the toad. Br J Pharmacol Chemother. 1961;16:262–83. doi: 10.1111/j.1476-5381.1961.tb01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Fadda E, Wroblewski JT, Costa E. [3H]D-serine labels strychnine-insensitive glycine recognition sites of rat central nervous system. Life Sci. 1990;46:155–64. doi: 10.1016/0024-3205(90)90100-6. [DOI] [PubMed] [Google Scholar]
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci USA. 2009;106:15037–42. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. The presence of free D-serine in rat brain. FEBS Lett. 1992;296:33–6. doi: 10.1016/0014-5793(92)80397-y. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–6. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Rusakov DA. Synaptic plasticity and Ca2+ signalling in astrocytes. Neuron Glia Biol. 2010;6:141–6. doi: 10.1017/S1740925X10000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio M, Kohno M, Fujita Y, Ishima T, Inoue R, Mori H, Hashimoto K. Levels of D-serine in the brain and peripheral organs of serine racemase (Srr) knock-out mice. Neurochem Int. 2011;59:853–9. doi: 10.1016/j.neuint.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281:14151–62. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- Li Y, Krupa B, Kang J-S, Bolshakov VY, Liu G. Glycine site of NMDA receptor serves as a spatiotemporal detector of synaptic activity patterns. J Neurophysiol. 2009;102:578–89. doi: 10.1152/jn.91342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA Receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–4. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- De Miranda J, Panizzutti R, Foltyn VN, Wolosker H. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonistd-serine. Proc Natl Acad Sci USA. 2002;99:14542–7. doi: 10.1073/pnas.222421299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 2008;510:641–54. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- Monahan JB, Handelmann GE, Hood WF, Cordi AA. D-cycloserine, a positive modulator of the N-methyl-D-aspartate receptor, enhances performance of learning tasks in rats. Pharmacol Biochem Behav. 1989;34:649–53. doi: 10.1016/0091-3057(89)90571-6. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97:4926–31. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet J-P, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci USA. 2005;102:5606–11. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Ahmad AS, Zeynalov E, Gazi SK, Sikka G, Ehmsen JT, Barrow RK, Coyle JT, Snyder SH, Dore S. Serine racemase deletion protects against cerebral ischemia and excitotoxicity. J Neurosci. 2010;30:1413–6. doi: 10.1523/JNEUROSCI.4297-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, van Rossum DB, Patterson RL, Maag D, Ehmsen JT, Gazi SK, Chakraborty A, Barrow RK, Amzel LM, Snyder SH. Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc Natl Acad Sci USA. 2009;106:2921–6. doi: 10.1073/pnas.0813105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet J-P, Touquet B, Pollegioni L, Poulain DA, Oliet SHR. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–84. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Reis M, Panizzutti R, de Miranda J, Wolosker H. Glial transport of the neuromodulator D-serine. Brain Res. 2002;929:202–9. doi: 10.1016/s0006-8993(01)03390-x. [DOI] [PubMed] [Google Scholar]
- Rutter AR, Fradley RL, Garrett EM, Chapman KL, Lawrence JM, Rosahl TW, Patel S. Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating D-serine reuptake in the mouse CNS. Eur J Neurosci. 2007;25:1757–66. doi: 10.1111/j.1460-9568.2007.05446.x. [DOI] [PubMed] [Google Scholar]
- Schell MJ. The N-methyl-D-aspartate receptor glycine site and D-serine metabolism: an evolutionary perspective. Philos Trans R Soc Lond, B: Biol Sci. 2004;359:943–64. doi: 10.1098/rstb.2003.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–52. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylantyev S, Savtchenko LP, Niu YP, Ivanov AI, Jensen TP, Kullmann DM, Xiao MY, Rusakov DA. Electric fields due to synaptic currents sharpen excitatory transmission. Science. 2008;319:1845–9. doi: 10.1126/science.1154330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H. Serine racemase and the serine shuttle between neurons and astrocytes. Biochim Biophys Acta. 2011;1814:1558–66. doi: 10.1016/j.bbapap.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96:13409–14. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci USA. 2003;100:15194–9. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]