Abstract
Tissue-engineered vascular grafts (TEVGs) hold great promise for the improvement of outcomes in pediatric patients with congenital cardiac anomalies. Currently used synthetic grafts have several limitations, including thrombogenicity, increased risk of infection, and lack of growth potential. The first pilot clinical trial of TEVGs demonstrated the feasibility of this new technology and revealed an excellent safety profile. However, long-term follow-up from this trial revealed the primary graft-related complication to be stenosis, affecting 16 percent of grafts within 7 years post-implantation. In order to determine the mechanism behind TEVG stenosis and ultimately to create improved second generation TEVGs, our group has returned to the bench to study vascular neotissue formation in a variety of large and small animal models. The purpose of this report is to review the recent advances in the understanding of neotissue formation and vascular tissue engineering.
Keywords: tissue-engineered vascular grafts, congenital heart disease, translational research, tissue engineering, vascular remodeling, bone marrow-derived mononuclear cells, extracellular matrix
Introduction
Native vessels remain the gold standard in revascularization procedures; however, such autologous grafts are not always obtainable secondary to limited availability or concurrent disease. While synthetic grafts such as expanded polytetrafluoroethylene (Gortex®, W.L. Gore & Associates, Newark, DE) and polyethylene terephthalate (Dacron®, Dupont, Wilmington, DE) have been used in the past, they have an increased risk of thrombosis and intimal hyperplasia [1-3]. Moreover, the lack of growth potential associated with synthetic grafts is particularly limiting in the pediatric population, where multiple operations and graft oversizing become necessary to ensure adequate blood flow. Tissue-engineered vascular grafts (TEVGs) represent a potentially limitless supply of autologous grafts with the added benefit of growth potential. In its most basic form, vascular tissue engineering involves seeding autologous cells onto a biodegradable tubular scaffold which serves as a site of cell attachment and neotissue formation. Once the graft is implanted, neotissue begins to form while the biodegradable scaffold slowly dissolves. The end result is a neovascular conduit composed entirely of the autologous tissue [4]. Multiple large animal studies have demonstrated the feasibility of tissue engineering in the construction of vascular grafts [5-8].
Tissue engineering has been described as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ” [4]. As the field moves from the laboratory to the clinic, all aspects of TEVG construction (scaffold materials, cells for seeding grafts, and seeding techniques) will need to be re-evaluated to ensure adequate safety and efficacy. Just as importantly, clinical experience should dictate laboratory investigation of the process of neotissue formation in a concerted effort to move beyond first generation TEVG and onto second generation TEVG with improved hemodynamic functionality and in vivo performance.
Clinical Significance
Even with the implementation of innovative medical and surgical treatment modalities, congenital heart disease (CHD) remains the most common form of death in the newborn period [9]. Estimates of the current birthrate indicate that approximately 1.35 million infants worldwide are born with CHD each year, representing a major global health burden [10]. The development of a man-made vascular conduit for use in pediatric patients with cardiac anomalies holds particular promise for the advancement of the field.
Single ventricle anomalies are comprised of a large and diverse group of structural irregularities that result in a life-threatening form of CHD characterized by chronic hypoxia and cyanosis. This classification includes diseases such as hypoplastic left heart syndrome, double inlet left ventricle, and pulmonary atresia. The unifying feature of each condition is the mixing of the oxygenated systemic circulation with the deoxygenated pulmonary circulation, leading to volume overload and heart failure with a mortality of up to 70 percent during the first year of life if left untreated [11]. Surgical reconstruction is the preferred approach in the management of single ventricle physiology. One of the more commonly performed procedures is a series of staged operations termed the modified Fontan operation with extracardiac total cavopulmonary connection (EC TCPC), which allows the single ventricle to pump oxygenated blood through the systemic circulation. The EC TCPC procedure is designed to directly connect the inferior vena cava (IVC) to the pulmonary artery and is the most commonly performed congenital heart operation in which a large caliber vascular graft is implanted in a high-flow, low-pressure circulation. While the operation has resulted in substantial improvements in long-term survival, it is ultimately considered a palliative procedure with significant morbidity and mortality [12,13], and it is widely accepted that the ideal conduit remains to be developed [14-16].
The most significant disadvantage of synthetic conduits is their lack of growth potential. Consequently, surgical repair may be delayed until the patient grows to the point where an adult-sized conduit can be used. Performing the surgery prior to this point risks the need for reoperation when the child outgrows the original conduit, a precarious proposition associated with increased risk of complications and early post-operative mortality rates of up to 5 percent [17]. Implanting an oversized graft at an earlier age avoids the complications of delayed treatment and mitigates the possibility of the patient outgrowing the conduit, but this approach carries an increased risk of complications [18]. Notably, a small subset of patients born with CHD is amenable to primary repair without the use of synthetic grafts. These patients are not susceptible to the inherent risks of synthetic conduits, and their outcomes are markedly improved [19]. Similarly, the use of autografts — vascular conduits created from an individual’s own tissue — results in long-term patency rates that exceed 80 percent [20]. This superior level of effectiveness compared to synthetic grafts is mitigated by limited availability, prompting the search for a man-made graft that retains growth potential [17,19,20-22].
Pilot Clinical Trial
Promising results in animal models led to the initiation of a clinical pilot study in Japan in 2001. The study was designed to evaluate the safety and efficacy of TEVGs for use in congenital heart surgery, specifically the EC TCPC operation in patients born with single ventricle cardiac anomalies [23-25]. TEVGs were created by seeding a biodegradable scaffold with autologous bone marrow mononuclear cells (BM-MNCs) followed by implantation in a cohort of 25 patients with congenital cardiac anomalies. The initial follow-up period was set at 7 years and included imaging studies such as contrast angiography and computed tomography (CT) scanning in order to monitor the progression of the implanted conduits. Each patient received between 3 and 6 months of antiplatelet and anticoagulation therapy immediately following the procedure [6,25]. Early results from the study were encouraging. The implanted conduits were universally functional with no evidence of failure due to thrombosis, stenosis, or obstruction of the TEVGs. Additionally, angiography and CT imaging revealed no evidence of aneurysm formation or calcification. All grafts were patent, and serial scans confirmed that the diameter of the neovessel increased with time. None of the patients required graft replacement, and there was no mortality relating to the implanted conduits. Antiplatelet and anticoagulant medications were successfully discontinued by all patients within 6 months of the procedure, a marked contrast to the lifetime requirement for anticoagulation when synthetic grafts are used. Additionally, 40 percent of patients did not require any daily medication regimen. Late analysis of the cohort again illustrated the absence of graft-related mortality. However, 16 percent of patients were revealed to have TEVG stenosis at long-term follow-up. This finding was frequently asymptomatic, and all patients were successfully treated with angioplasty or angioplasty and stenting [25].
Data from the initial clinical trial suggest that TEVGs are indeed safe and effective for use in congenital heart surgery, but the mechanisms of neovessel formation and the processes leading to graft failure are incompletely understood. Both phenomena must be investigated thoroughly if an improved tissue-engineered vascular graft is to become a reality. Additionally, the results of the trial indicate that stenosis is the primary means of graft-related failure. It is therefore critical to gain a better understanding of the mechanisms underlying TEVG stenosis in order to identify the potential targets and strategies that could prevent this complication. Ultimately, this would allow for the rational design of a second generation TEVG with improved safety and efficacy.
TEVG Construction
Scaffold Materials
Ideal scaffold material should possess three characteristics, namely: (1) biodegradability, (2) non-immunogenicity, and (3) functionality as a temporary vascular conduit until neotissue formation takes place. Current scaffold material can be broken up into the subgroups of synthetic and biological. Traditionally, synthetic materials have been used, and scaffolds have been constructed using woven polymers of polyglycolic acid (PGA), polylactic acid (PLA), and polycaprolactone (PCL). By combining and varying the concentrations of these polymers, scaffolds can be generated to meet the compliance specifications of the vascular environment in which they are to be introduced [26-28]. However, the degree to which the compliance and biomechanical properties of woven polymer scaffolds can be fine-tuned is significantly less than that found in newer construction techniques such as electrospinning, in which an electrical field is used to mold individual polymer fibers into a tubular structure upon a rotating metal rod [29]. Other groups have focused on the use of biologic scaffolds, such as decellularized human and porcine vessels and human umbilical vein. These materials have shown a high degree of matching of compliance between graft and natural vessels with little risk of immunogenic response [30,31]. However, these methods have not yet been evaluated in clinical trials, and their applicability in TEVG constructions requires further study.
Cells for Seeding
Several different cell types have been considered for use in TEVGs, including endothelial cells, smooth muscle cells, embryonic stem cells, induced pluripotent stem cells, and BM-MNCs. The ideal seeded cell should be easily harvested, require a short incubation time, and rapidly give rise to neotissue formation. While the use of endothelial cells and smooth muscles cells is enticing due to their matching of natural vessels, they require long in vitro incubation times and thus increased risk of contamination [32,33]. Similarly, embryonic stem cells and induced pluripotent stem cells have been considered due to their potential to differentiate into the endothelial and smooth muscle cells of a mature vessel. However, these cells are not always available, carry significant risk of teratoma formation, and require more investigation before clinical use can be considered [34,35]. Conversely, BM-MNCs are readily available via bone marrow aspiration and require a relatively short, 2-hour scaffold incubation time prior to implantation. Our group has pioneered a novel process of separating BM-MNCs from the aspirate through the use of a specially designed filter to separate cells of a particular size [36]. While the process of harvesting bone marrow mononuclear cells is not without some risk to sterility and cell viability, its relative speed and safety makes it the current frontrunner in the construction of TEVGs.
Seeding Techniques
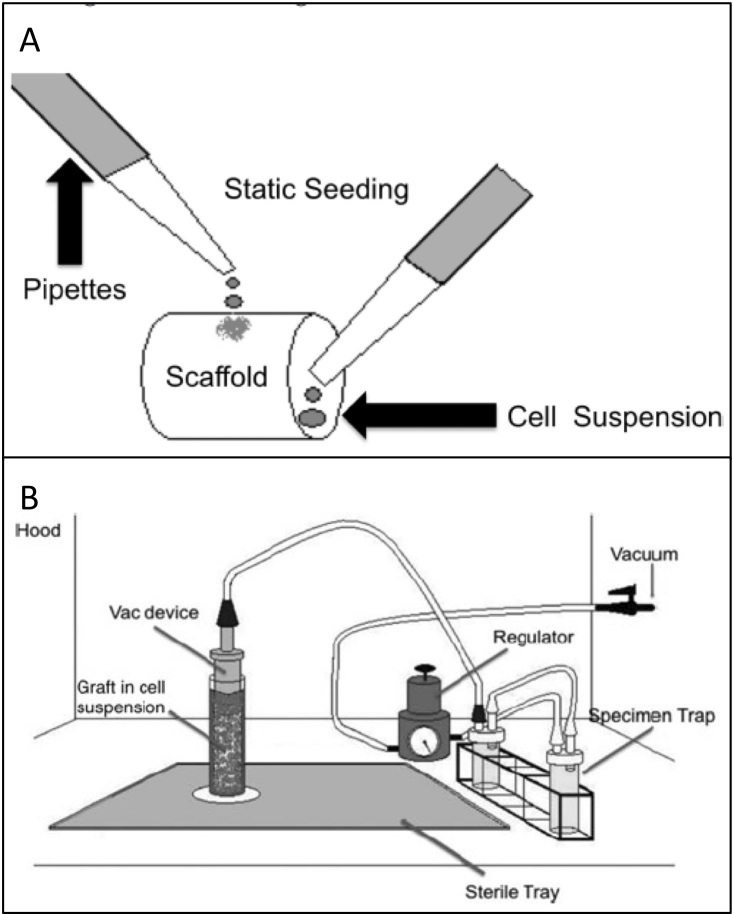
Traditionally, scaffolds have been statically seeded through a process in which cells in media are pipetted onto the outer lumen and into the inner lumen of the scaffold. The cells are allowed several hours to attach to the scaffold in vitro prior to implantation. The drawbacks of this process have been long recognized and include low seeding efficiency and the potential for intra- and inter-operator variability. While many techniques have been proposed, including dynamic, magnetic, and electrostatic [37], none of these techniques have been evaluated in clinical trials or fulfill the characteristics of an ideal seeding system, which should be rapid, automated, and standardized, while maximizing cell viability and minimizing risk of contamination. In an effort to reach this ideal, our group utilizes a disposable vacuum-seeding device in which negative pressure is used to rapidly lodge cells in the pores of a scaffold fastened to a perforated tubular device connected to suction [38]. This vacuum-seeding device has been approved for use in a pilot clinical study and achieves similar cell seeding viability and efficiency as that seen in static seeding, but with the advantage of automaticity and self-containment (Figure 1).
Figure 1.
Cell seeding of TEVG. a) Static seeding. A cell suspension is pipetted onto the outer surface and inner lumen of the scaffold. b) Vacuum seeding. A cell suspension is forced through a scaffold by internal pressure, thus lodging the cells within the pores of the scaffold. Remaining aspirate is collected in specimen traps. (Adapted with permission from Villalona (2010) [37] and Udelsman (2011) [38]).
Back to Bench
Large Animal Studies
In an effort to better understand the mechanisms behind TEVG stenosis, further bench work studies are necessary in an effort to create improved second generation TEVGs. Using an ovine model, TEVGs have been implanted as interpositional IVC grafts in juvenile lambs and monitored with bimonthly magnetic resonance angiography over a 6-month time course. At the end of the time course, all grafts were patent and demonstrated an increase in volume proportional to the native right pulmonary artery. Explanted grafts at 6 months resembled native IVC in that they contained a similar wall thickness with an inner monolayer of endothelial cells surrounded by several layers of smooth muscle cells in concentric layers. These explanted grafts contained an extracellular layer with collagen, elastin, and glycosaminoglycan (GAG) contents comparable to native IVC and a similar biomechanical profile. Equally important, by 6 months, the TEVG expressed Eph-B4, a marker of venous differentiation, suggesting that implantation of TEVG in venous circulation leads to formation of venous vascular neotissue [39].
Small Animal Studies
While this ovine model marked the first time a TEVG demonstrated molecular evidence of normal vascular development, a mouse model was sought after to better characterize the process of neotissue formation. In this effort, sub-1mm tubular scaffolds composed of similar materials used in the pilot clinical trial were constructed. These TEVGs had the advantage of being implantable in immunodeficient SCID-beige mice, enabling the use of TEVGs seeded with human cells without the need for immunosuppression [40]. TEVGs seeded with human BM-MNCs and implanted as IVC interpositional grafts remained fully patent and functional as venous conduits over a 6- month time course. By 6 months, TEVGs contained a mature vascular architecture consisting of a confluent endothelial cell lined intima and one to two layers of smooth muscle cell media, while the original scaffold material had degraded and was replaced by a supportive adventitial layer of collagen fibrils [41].
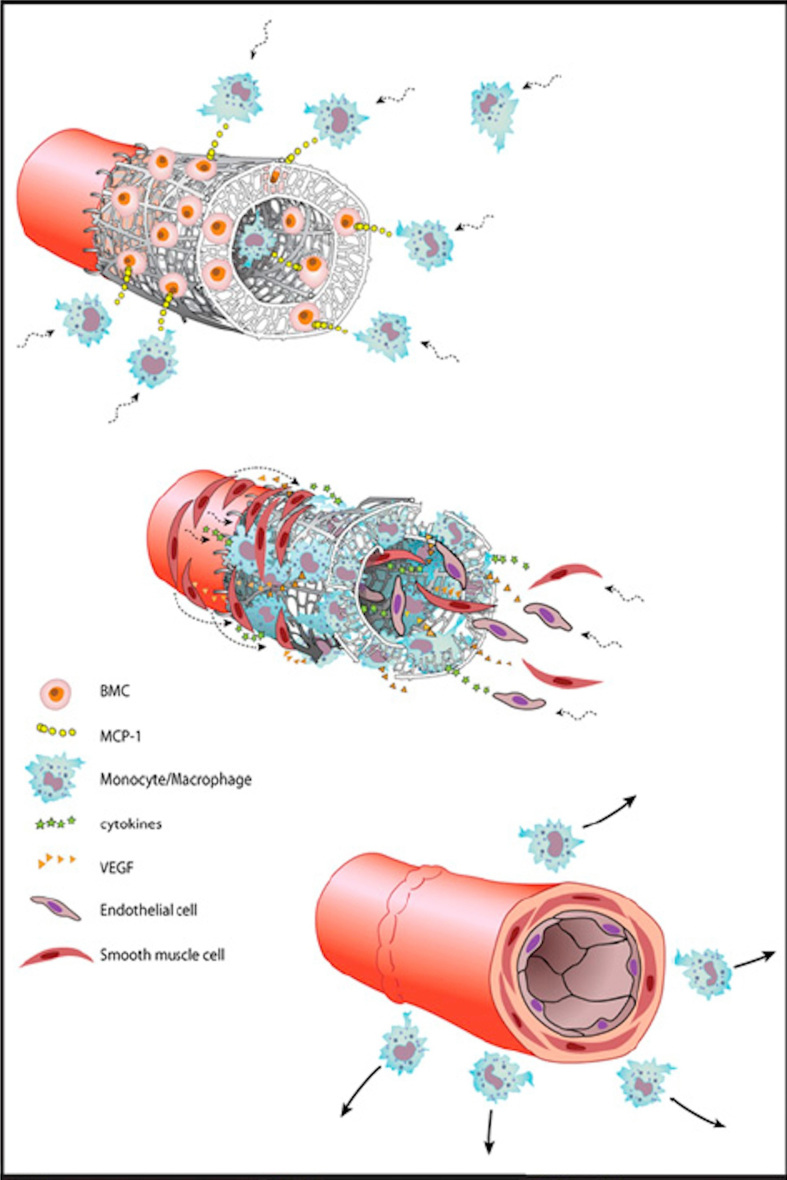
Extensive histological and immunohistochemical characterization was performed to investigate the mechanism by which neotissue formation within the TEVGs occurred. Although it has been hypothesized that stem cells within the seeded BM-MNC population differentiate into the cells of the neotissue [42], by 1 week after implantation, human BM-MNCs were no longer detectable within the TEVGs. Rather, it is hypothesized that these seeded cells augment a host inflammatory process via a paracrine mechanism through the secretion of chemokines that recruit host cells to the scaffold, ultimately leading to neovascularization. When compared to unseeded grafts, seeded grafts demonstrated a higher concentration of macrophages during early graft development. Notably, interleukin 1-beta (IL-1B) and monocyte chemoattractant protein-1 (MCP-1) were both found in abundant quantity. Later experiments utilized alginate microspheres containing MCP-1. Implanted TEVGs with alginate microspheres incorporated into their walls developed and functioned similar to human BM-MNC-seeded scaffolds with an internal lumen containing organized vascular neotissue [41] (Figure 2).
Figure 2.
TEVG development in mouse model. Early pulse of cytokine (MCP-1) release from seeded BM-MNC enhances monocyte recruitment to the scaffold. Monocytes infiltrate the scaffold and direct neotissue formation, leading to the recruitment of smooth muscle cells from neighboring native vessel wall. This process results in concentric layers of smooth muscle cells embedded in an extracellular matric with a monolayer of endothelial cells lining the luminal surface. (Adapted with permission from Roh (2010) [41]).
Regenerative Model of Neotissue Formation
The early abundance of host-derived inflammatory cells led to further investigations on the proposed role for monocytes/macrophages in the process of neovessel formation and the development of graft stenosis. These studies were performed by isolating monocytes from the bone marrow of syngeneic C57BL/6 mice followed by seeding onto the synthetic conduits and implantation [43]. Histological and immunohistochemical characterization revealed that this model successfully recapitulated the results of the human trial, with stenosis remaining the primary graft-related complication. Early stenosis was observed in 80 percent of unseeded TEVGs, but only 20 percent of seeded TEVGs, with all stenoses occurring within 2 weeks of implantation. Additionally, luminal diameter was significantly higher in the seeded grafts. Quantification of F4/80-positive macrophages revealed significantly higher levels of macrophage infiltration in the unseeded grafts compared to seeded grafts. To determine whether these relationships were causative or correlative, additional TEVGs were implanted into CD11b-DTR transgenic mice treated with diphtheria toxin to inhibit macrophage infiltration [44-46]. This conditional knockout decreased the total number of macrophages in the graft, increased rates of patency, and increased luminal diameters. Macrophage inhibition resulted in a decrease in neovessel formation, with histological analysis revealing the absence of both the endothelial and smooth muscle layers, as well as reduced collagen deposition. These experiments illustrate a direct correlation between the degree of macrophage infiltration and the formation of stenosis. The data also highlight the well-recognized role of macrophages in the processes of vascular repair and remodeling.
This model of seeded cells augmenting the host inflammatory response rather than playing a direct role in the formation of neotissue contrasts with the classic tissue engineering theory of seeded cells “as building block of neotissue” [28]. Rather, these studies suggest a regenerative medicine paradigm, in which seeded cells merely promote the host’s natural healing process. In line with this paradigm are recent studies showing that male-derived vessel segments implanted in female wild type mice previously given green fluorescent protein bone marrow transplants give rise to neovessel tissue originating from migration of the adjacent vessel segment rather than from the seeded cells or bone marrow [47].
This finding was further substantiated by studies investigating the use of serial magnetic resonance imaging (MRI) for noninvasive monitoring of implanted vascular grafts in a mouse model. The technique was based upon the uptake of ultra-small superparamagnetic iron oxide (USPIO) particles by monocyte derivatives which were then seeded onto a biodegradable scaffold. MRI of the grafts confirmed the presence of the seeded cells immediately after implantation, but subsequent imaging revealed a rapid return to control levels within 2 hours. The return to baseline correlated with the loss of USPIO-labeled cells from the scaffolds as verified with Prussian blue staining for the presence of iron oxide-labeled cells. These data support the theory of rapid loss of seeded cells from the TEVGs post-implantation and argues against their role as direct cellular precursors in the process of neovessel formation [48].
Extracellular Matrix
In addition to the cellular composition of the neovessel, the characteristics of the extracellular matrix (ECM) play a critical role in the determination of the vessel’s biomechanical properties and overall structural integrity. Deviations from the normal physiological processes of ECM deposition and remodeling can lead to alterations in the biomechanical properties of the graft, resulting in complications such as aneurismal dilatation and stenosis. The primary components of the ECM include collagen, elastin, and GAG [39]. Zinc-dependent enzymes referred to as matrix metalloproteinases (MMP) also have been shown to be intimately involved in the process of ECM degradation and remodeling [49]. Using a C57BL/6 murine model, the quantitative and qualitative characteristics of these ECM components were assessed in implanted TEVGs over a 4-week period. Results indicated a trend of early deposition of Type I and Type III collagen in the neovessel, peaking at 2 weeks. Gene expression levels of both Type I and Type III collagen were significantly higher in the TEVG versus native IVC at that time point, but there was no statistical difference by 4 weeks. Type IV collagen was expressed at a lower level in the graft when compared to native IVC at early time points but approximated that of vena cava by 4 weeks. Similarly, analysis of the total collagen content of the neovessel revealed no difference between the implanted graft and normal vena cava by the 4-week time point [50].
Additional experiments revealed that gene expression levels of elastin increased within the TEVGs over the course of the experiment, and overall production of GAG was also significantly greater when compared to earlier time points. Gene expression studies on the metalloproteinases MMP-9 and MMP-2 revealed high early expression of MMP-9 followed by a gradual decline, while MMP-2 expression increased steadily over time [50]. These patterns are consistent with previously described models of vascular injury [51,52]. The presumed roles of MMP-9 and MMP-2 include induction of an early inflammatory foreign body reaction coupled with ECM degradation and remodeling, thereby allowing the vascular graft to adapt to the specific biomechanical environment in which it is implanted. These investigations provide some of the first qualitative and quantitative data clarifying the sequence of ECM deposition and remodeling in TEVGs. These processes play a critical role in neovessel formation and may be implicated in many of the complications arising from deficiencies in the structural integrity of the implanted conduit. Further work is needed in order to more fully characterize these mechanisms so that an improved vascular graft can be designed.
Future Directions
As TEVGs reach clinical fruition, further investigation will be necessary to ensure the long-term success and efficacy of this technology. The pilot clinical trial conducted in Japan is promising in finding growth potential, but also challenging in that it points to areas in need of improvement, namely prevention of long-term graft stenosis. The future of TEVG development will lie in both clinical and laboratory investigation. An FDA-approved clinical trial is currently under way at the Yale School of Medicine to establish a benchmark for evaluating the safety of tissue-engineered vascular grafts for use in the surgical management of patients born with single ventricle anomalies. This clinical trial and additional investigations on the mechanisms of neotissue formation and stenosis will be instrumental in improving outcomes in patients with CHD [53,54]. At the same time, the recent development of a mouse model and subsequent laboratory work has pointed toward the mediators of neotissue formation and mechanisms of controlling graft stenosis. Second generation TEVGs will benefit from the unfolding understanding of how these cytokines dictate neotissue formation. Ultimately, cytokine-eluting TEVGs may replace current technology and eliminate the need for seeding altogether. Equally important, current investigations into the development and structuring of extracellular matrix will improve upon scaffold design and open the door for novel methods of preventing stenosis.
Abbreviations
- TEVGs
tissue-engineered vascular grafts
- CHD
congenital heart disease
- EC TCPC
extracardiac total cavopulmonary connection
- IVC
inferior vena cava
- BM-MNCs
bone marrow mononuclear cells
- CT
computed tomograpic
- PGA
polyglycolic acid
- PLA
polylactic acid
- PCL
polycaprolactone
- GAG
glycosaminoglycan
- MRI
magnetic resonance imaging
- USPIO
superparamagnetic iron oxide
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- IL-1B
interleukin-1 beta
- MCP-1
monocyte chemoattractant protein-1
References
- Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA. et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3(1):104–114. doi: 10.1067/mva.1986.avs0030104. [DOI] [PubMed] [Google Scholar]
- Sapsford RN, Oakley GD, Talbot S. Early and late patency of expanded polytetrafluoroethylene vascular grafts in aorta-coronary bypass. J Thorac Cardiovasc Surg. 1981;81(6):860–864. [PubMed] [Google Scholar]
- Klinkert P, Post PN, Breslau PJ, Van bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27(4):357–362. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Shinoka T, Shum-tim D, Ma PX, Tanel RE, Isogai N, Langer R. et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115(3):536–545. doi: 10.1016/S0022-5223(98)70315-0. [DOI] [PubMed] [Google Scholar]
- Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin’oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24(13):2303–2308. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- Matsumura G, Ishihara Y, Miyagawa-tomita S, Ikada Y, Matsuda S, Kurosawa H. et al. Evaluation of tissue-engineered vascular autografts. Tissue Eng. 2006;12(11):3075–3083. doi: 10.1089/ten.2006.12.3075. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Shin’oka T, Tohyama S, Hibino N, Konuma T, Matsumura G. et al. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7(4):429–439. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- American Heart Associatin. 2008 Annual Report
- Van der linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ. et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Samánek M. Children with congenital heart disease: probability of natural survival. Pediatr Cardiol. 1992;13(3):152–158. doi: 10.1007/BF00793947. [DOI] [PubMed] [Google Scholar]
- Giannico S, Hammad F, Amodeo A, Michielon G, Drago F, Turchetta A. et al. Clinical outcome of 193 extracardiac Fontan patients: the first 15 years. J Am Coll Cardiol. 2006;47(10):2065–2073. doi: 10.1016/j.jacc.2005.12.065. [DOI] [PubMed] [Google Scholar]
- Petrossian E, Reddy VM, Collins KK, Culbertson CB, MacDonald MJ, Lamberti JJ. et al. The extracardiac conduit Fontan operation using minimal approach extracorporeal circulation: early and midterm outcomes. J Thorac Cardiovasc Surg. 2006;132(5):1054–1063. doi: 10.1016/j.jtcvs.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Conte MS. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998;12(1):43–45. doi: 10.1096/fasebj.12.1.43. [DOI] [PubMed] [Google Scholar]
- Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41(2):349–354. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Dearani JA, Danielson GK, Puga FJ, Schaff HV, Warnes CW, Driscoll DJ. et al. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg. 2003;75(2):399–410. doi: 10.1016/s0003-4975(02)04547-2. [DOI] [PubMed] [Google Scholar]
- Alexi-meskishvili V, Ovroutski S, Ewert P, Dähnert I, Berger F, Lange PE. et al. Optimal conduit size for extracardiac Fontan operation. Eur J Cardiothorac Surg. 2000;18(6):690–695. doi: 10.1016/s1010-7940(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Van son JA, Reddy M, Hanley FL. Extracardiac modification of the Fontan operation without use of prosthetic material. J Thorac Cardiovasc Surg. 1995;110(6):1766–1768. doi: 10.1016/s0022-5223(95)70043-9. [DOI] [PubMed] [Google Scholar]
- Bermudez CA, Dearani JA, Puga FJ, Schaff HV, Warnes CA, O’Leary PW. et al. Late results of the peel operation for replacement of failing extracardiac conduits. Ann Thorac Surg. 2004;77(3):881–887. doi: 10.1016/j.athoracsur.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Jonas RA, Freed MD, Mayer JE, Castaneda AR. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72(3 Pt 2):1177–1183. [PubMed] [Google Scholar]
- Homann M, Haehnel JC, Mendler N, Paek SU, Holper K, Meisner H. et al. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardiothorac Surg. 2000;17(6):624–630. doi: 10.1016/s1010-7940(00)00414-0. [DOI] [PubMed] [Google Scholar]
- Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344(7):532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- Naito Y, Imai Y, Shin’oka T, Kashiwagi J, Aoki M, Watanabe M. et al. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J Thorac Cardiovasc Surg. 2003;125(2):419–420. doi: 10.1067/mtc.2003.134. [DOI] [PubMed] [Google Scholar]
- Hibino N, Mcgillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C. et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139(2):431-6, 436.e1-2. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- Gui L, Zhao L, Spencer RW, Burghouwt A, Taylor MS, Shalaby SW. et al. Development of novel biodegradable polymer scaffolds for vascular tissue engineering. Tissue Eng Part A. 2011;17(9-10):1191–200. doi: 10.1089/ten.tea.2010.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Shinoka T, Duncan D, Hibino N, Solomon D, Cleary M. et al. Vascular tissue engineering: towards the next generation vascular grafts. Adv Drug Deliv Rev. 2011;63(4-5):312–323. doi: 10.1016/j.addr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Duncan DR, Breuer CK. Challenges in translating vascular tissue engineering to the pediatric clinic. Vasc Cell. 2011;3(1):23. doi: 10.1186/2045-824X-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Yong T, Teo WE, Ma Z, Ramakrishna S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: potential vascular graft for blood vessel tissue engineering. Tissue Eng. 2005;11(9-10):1574–1588. doi: 10.1089/ten.2005.11.1574. [DOI] [PubMed] [Google Scholar]
- Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci USA. 2011;108(22):9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenicka M, Lehle K, Jacobs VR, Schmid FX, Birmbaum DE. Properties of the human umbilical vein as a living scaffold for a tissue-engineered vessel graft. Tissue Eng. 2007;13(1):219–229. doi: 10.1089/ten.2006.0121. [DOI] [PubMed] [Google Scholar]
- Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y. et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3(68):68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- Weber B, Emmert MY, Schoenauer R, Brokopp C, Baumgartner L, Hoerstrup SP. et al. Tissue engineering on matrix: future of autologous tissue replacement. Semin Immunopathol. 2011;33(3):307–315. doi: 10.1007/s00281-011-0258-8. [DOI] [PubMed] [Google Scholar]
- Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19(6):321–331. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Hibino N, Duncan DR, Nalbandian A, Yi T, Qyang Y, Shinoka T. et al. Evaluation of the use of an induced puripotent stem cell sheet for the construction of tissue-engineered vascular grafts. J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2011.06.046. Forthcoming 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino N, Nalbandian A, Devine L, Martinez RS, McGillicuddy E, Yi T. et al. Comparison of human bone marrow mononuclear cell isolation methods for creating tissue-engineered vascular grafts: novel filter system versus traditional density centrifugation method. Tissue Eng Part C Methods. 2011;17(10):993–998. doi: 10.1089/ten.tec.2011.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalona GA, Udelsman B, Duncan DR, McGillicuddy E, Sawh-Martinez RF, Hibino N. et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng Part B Rev. 2010;16(3):341–350. doi: 10.1089/ten.teb.2009.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udelsman B, Hibino N, Villalona GA, McGillicuddy E, Nieponice A, Sakamoto Y. et al. Development of an operator-independent method for seeding tissue-engineered vascular grafts. Tissue Eng Part C Methods. 2011;17(7):731–736. doi: 10.1089/ten.tec.2010.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan MP, Dardik A, Hibino N, Roh JD, Nelson GN, Papademitris X. et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg. 2008;248(3):370–377. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JD, Nelson GN, Brennan MP, Mirensky TL, Yi T, Hazlett TF. et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials. 2008;29(10):1454–1463. doi: 10.1016/j.biomaterials.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JD, Sawh-martinez R, Brennan MP, Jay SM, Devine L, Rao DA. et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci USA. 2010;107(10):4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura G, Miyagawa-tomita S, Shin’oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108(14):1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- Hibino N, Yi T, Duncan DR, Dean E, Naito Y, Dardik A. et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 2011;25(12):4253–4263. doi: 10.1096/fj.11-186585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM. et al. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80(1):59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101(1):40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danenberg HD, Fishbein I, Gao J. et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- Hibino N, Villalona G, Pietris N, Duncan DR, Schoffner A, Roh JD. et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J. 2011;25(8):2731–2739. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington JK, Chahboune H, Criscione JM, Li AY, Hibino N, Yi T. et al. Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI. FASEB J. 2011;25(12):4150–4161. doi: 10.1096/fj.11-185140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Williams-fritze M, Duncan DR, Church SN, Hibino N, Madri JA. et al. Characterization of the natural history of extracellular matrix production in tissue-engineered vascular grafts during neovessel formation. Cells Tissues Organs (Print) 2012;195(1-2):60–72. doi: 10.1159/000331405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75(3):539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation. 2000;102(23):2861–2866. doi: 10.1161/01.cir.102.23.2861. [DOI] [PubMed] [Google Scholar]
- Shinoka T, Breuer C. Tissue-engineered blood vessels in pediatric cardiac surgery. Yale J Biol Med. 2008;81(4):161–166. [PMC free article] [PubMed] [Google Scholar]
- Breuer CK. The development and translation of the tissue-engineered vascular graft. J Pediatr Surg. 2011;46(1):8–17. doi: 10.1016/j.jpedsurg.2010.09.058. [DOI] [PubMed] [Google Scholar]