Abstract
Transcatheter aortic valve replacement (TAVR) is a new technology that recently has been shown to improve survival and quality of life in patients with severe symptomatic aortic stenosis who are not surgical candidates [1]. The development and design of transcatheter valves has been ongoing for the past 20 years, and TAVR has now been approved by the FDA as a treatment for aortic stenosis in patients who are not surgical candidates. In the United States, there are currently two transcatheter valves available: the Edwards Sapien Valve and the Medtronic CoreValve. While similar in some design elements, they also have characteristic differences that affect both the mechanism of delivery as well as performance in patients. This review aims to take a closer look at the development of this new technology, review the published clinical results, and look toward the future of transcatheter valve therapeutics and the challenges therein.
Keywords: transcatheter aortic valve replacement, TAVR, TAVI, Medtronic CoreValve, Edwards Sapien Valve
Introduction
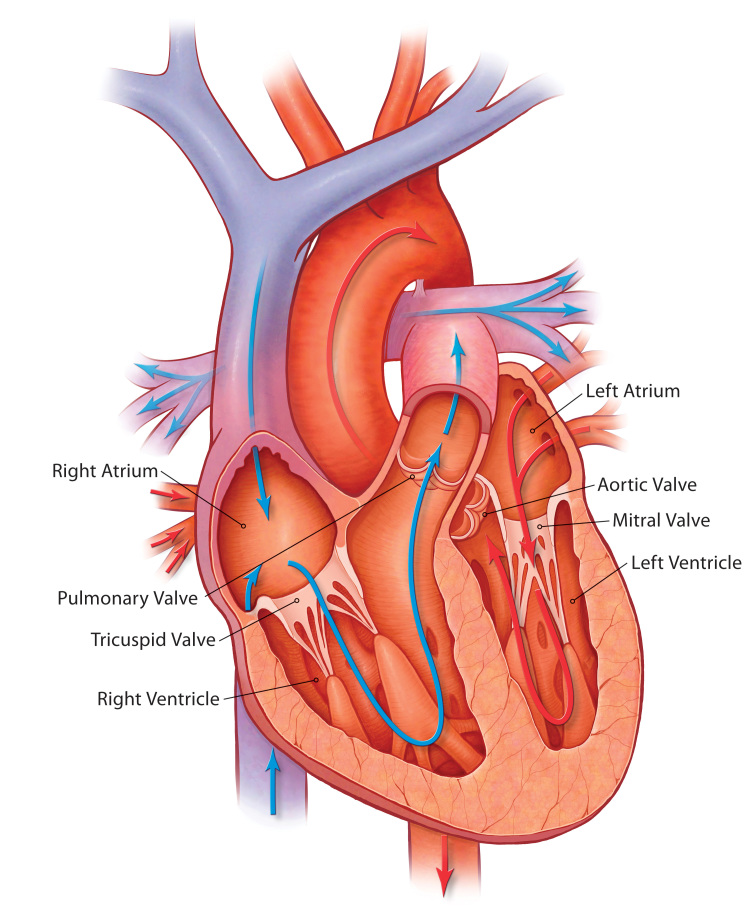
The aortic valve is one of four valves in the human heart (Figure 1). It is located between the left ventricle and aorta and in 99 percent of individuals is trileaflet in structure (in 1 percent of cases it can be bicuspid or unicuspid). During left ventricular systole (contraction), the pressure in the left ventricle increases until it rises just above the systolic pressure in the aorta. At this point in systole, the aortic valve opens and blood exits the left ventricle into the systemic circulation via the aorta. Thereafter, during left ventricular diastole (relaxation), the pressure in the left ventricle drops, and the pressure in the aorta forces the aortic valve back into its closed position. There are two primary disease processes that can affect the aortic valve: aortic insufficiency and aortic stenosis. In aortic insufficiency, also referred to as aortic regurgitation, the aortic valve is incompetent or leaky, and blood flows back into the left ventricle from the aorta during diastole. In aortic stenosis, the valve fails to open fully, thereby creating a systolic pressure gradient between the left ventricle and aorta. Both of these disease processes can contribute to progressive left ventricular dysfunction and symptomatic heart failure, adversely affecting patient morbidity and mortality.
Figure 1.
Anatomy of the human heart demonstrating the four chambers (left and right atria, and left and right ventricles) and the four main valves (mitral, tricuspid, aortic, and pulmonary). The aortic valve is located between the left ventricle and aorta. (Image provided by Medtronic, Inc.)
Aortic stenosis is the most frequent type of valvular heart disease in Western countries, with a prevalence of 2 percent in people over the age of 65, 3 percent in people over age 75, and 4 percent percent in people over age 85 [2,3]. The disease is characterized by a long latency period during which patients remain symptom free, followed by a rapid decline in functional status and life expectancy after the appearance of symptoms. The three classic symptoms that aortic stenosis may manifest are angina, syncope, and heart failure [4]. Medical treatment for severe symptomatic aortic stenosis is not effective, and without aortic valve replacement, the rate of mortality is approximately 25 percent at 1 year and 50 percent at 2 years [5,6]. We are entering an era in medicine in which our patient population is older, and as a result, the prevalence of aortic stenosis is increasing. For many of these older patients, surgical aortic valve replacement (SAVR) is a high-risk procedure with significant morbidity and mortality. Due to these risks, as well as other important factors including depressed left ventricular function and patient preference, in clinical practice, 30 to 40 percent of patients with symptomatic severe aortic stenosis do not undergo surgery [7-9]. To meet the medical needs of this population, a new technology has emerged over the past decade and is now being put into clinical practice: transcatheter aortic valve replacement. This technology has had significant impacts throughout the heath care field with the creation of a new biotechnology industry around transcatheter valves, the creation of multidisciplinary “heart teams” within clinical practice, and the construction of hybrid procedure rooms with both cath lab and operating room capabilities.
This review will focus on transcatheter aortic valve replacement (TAVR1), a new technology for the treatment of aortic stenosis in high and extreme risk surgical patients.
History of TAVR
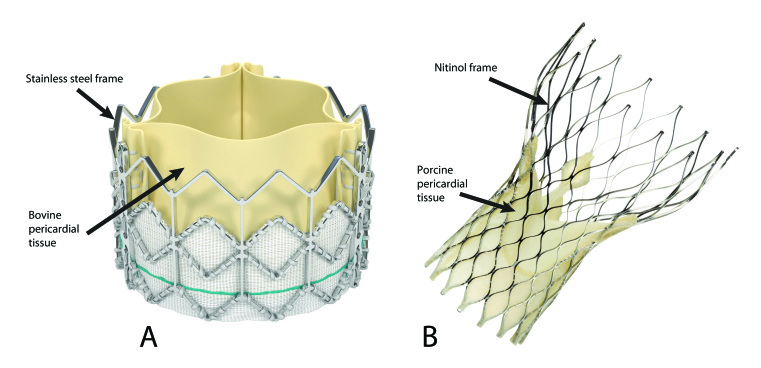
The first transcatheter aortic valve implantations performed in animals were conducted in 1992 by Dr. Andersen and colleagues [10]. It would be another 10 years before Dr. Alan Cribier performed the first in-man TAVR procedure in France in 2002 [11]. In this first case, Dr. Cribier and his colleagues implanted a percutaneous heart valve that consisted of three bovine pericardial leaflets mounted within a tubular stainless steel balloon-expandable stent. Prior to implantation, the stent-valve was securely crimped onto a balloon catheter, which was subsequently advanced across the aortic valve. The balloon was then rapidly inflated and deflated, resulting in successful deployment of the first transcatheter aortic valve in man [11]. Since that first patient in 2002, there has been a rapid growth within the field of structural heart disease and transcatheter valve therapeutics. Five years after Cribier’s first case, TAVR was approved for use in Europe, and since then more than 40,000 patients have been treated worldwide with this technique, which is now indicated as a treatment strategy for both non-surgical patients and patients in high surgical risk groups [1,12-14]. The research and development of transcatheter valves has evolved rapidly as a result of these early successes, and there are currently at least eight valves in commercial development. In the United States, two of these valves are available: the balloon expandable Edwards Sapien prosthesis (Edward Lifesciences Inc: Irvine, CA; Figure 2a) and the Medtronic self-expanding CoreValve ReValving System (Medtronic Inc: Minneapolis, MN; Figure 2b).
Figure 2.
A. Fully expanded Edwards Sapien Valve with its stainless steel frame and trileaflet construction made from bovine pericardial tissue. (Published with permission from Edward Lifesciences Inc: Irvine, CA.) B. Fully expanded Medtronic CoreValve with its nitinol frame and trileaflet construction made from porcine pericardial tissue. (Image provided by Medtronic, Inc. In the U.S., the Medtronic CoreValve® System is available for investigational purposes only.)
Design of Transcatheter Aortic Valves
Both the Edwards-Sapien Valve and the Medtronic CoreValve are designed to function through a mechanism similar to a normally functioning human tricuspid aortic valve. However, while both are trileaftlet in design with a metallic framework for support, their construction as well as preparation and delivery have significant differences. The integrated Edwards-Sapien transcatheter heart valve system is comprised of bovine pericardial tissue made from three identical sections of bovine pericardium that have been preserved in buffered glutaraldehyde to enable crosslinking of the tissue while preserving flexibility and strength. The valve tissue is affixed to a radiopaque stainless steel stent frame within a fabric cuff at its inflow aspect and to attachment bars on the commissural posts at its outflow aspect using polytetrafluoroethylene (PTFE) sutures to create a unidirectional trileaftlet tissue valve. The tissue used for the Sapien valve is identical to that used on the popular Carpentier-Edwards Perimount bioprosthetic valve used for SAVR. Prior to delivery, the valve is tightly compressed using a crimping mechanism onto a balloon catheter. The compressed device is then inserted into a tip-deflecting catheter delivery system. Prior to delivery, aortic balloon valvuloplasty is performed, and thereafter the Sapien transcatheter heart valve system is advanced up the aorta and placed across the native aortic valve. The balloon with the attached valve is then rapidly inflated and deflated, expanding and releasing the Sapien valve. The newly functioning valve is passively secured to the underlying native leaflets and to the aortic annulus as a result of this delivery. There are two sizes of the Sapien valve currently available, and they are 23mm and 26mm in diameter. The 23mm valve requires a 22-French sheath, and the 26mm valve requires a 24-French sheath for delivery [15].
In contrast to the Sapien valve, the CoreValve is a self-expanding valve with a Nitinol frame. Although the first generation CoreValve (first implanted in humans in 2004) was also made from bovine pericardium with an intra-annular valve function similar to that of the Sapien valve, the current generation CoreValve is made from porcine (not bovine) pericardium. The choice of porcine pericardium for the CoreValve may be due in part to suggested benefits that include diminished tissue thickness (thus allowing for a smaller sheath size), higher tensile strength, and tolerance to bending, as well as less tissue elongation providing for more consistent valve leaflet coaptation [16].
The choice of nitinol as opposed to stainless steel gives the CoreValve system the ability to be loaded onto a catheter delivery system that does not require a balloon and enables the valve to be gradually deployed in stages. The unique properties of nitinol, a metal alloy of nickel and titanium, were first described by William Buehler and Frederick Wang during research at the Naval Ordnance Laboratory in 1962 [17]. Nitinol exhibits what is known as a martensitic transformation, which allows it to undergo a reversible, solid state transformation. As a result, at warmer temperatures (including body temperature), nitinol forms a very strong, primitive cubic crystal structure referred to as austenite with a high radial strength. At colder temperatures, however, such as when placed in an ice water bath, nitinol transforms into a complex monoclinic crystal structure known as martensite. At this lower temperature, nitinol exhibits the property of superelasticity, giving it 10 to 30 times the elasticity of ordinary metal, enabling the CoreValve metal stent frame to be tightly compressed within the small delivery sheath required for the TAVR procedure. Lastly, nitinol exhibits the property of shape memory such that the shape of the higher temperature austenite is “remembered” despite the deformed shape that occurs at the lower temperature, and thus, when it is deployed in the warmer temperature of the body, it regains its original configuration.
Clinical Trials with TAVR
While both the Edwards Sapien and the Medtronic CoreValve have been available commercially in Europe for several years, in the United States it has been only recently that the Edwards Sapien valve has become available for use outside of clinical trials (the CoreValve is still only available through clinical trials in the United States). U.S. Food and Drug Administration (FDA) approval for the Edwards Sapien valve, which occurred in November 2011, came on the heels of the recent publications of the PARTNER study [1,12]. In the PARTNER study, there were two cohorts of patients. Cohort A involved 699 patients with severe symptomatic aortic stenosis who were at high risk for surgical complications or death on the basis of comorbidities with a predicted risk of death of at least 15 percent at 30 days after the procedure. In cohort A, patients were randomized in a 1:1 fashion to either SAVR or TAVR. Cohort B involved 358 patients with severe symptomatic aortic stenosis who were not candidates for surgery due to coexisting conditions that would be associated with a predicted probability of 50 percent or more of either death or significant morbidity 30 days after surgery. In cohort B, patients were randomized in a 1:1 fashion to either standard medical therapy (including aortic valvuloplasty) or TAVR. For both cohorts, two cardiac surgeons had to evaluate the patient and agree that each patient was either not suitable for surgery or was at high risk for surgical complications based on the above criteria.
In cohort A (Table 1), TAVR and SAVR were found to be equivalent in overall mortality at 1 year with a rate of death from any cause of 24.2 percent following the TAVR procedure and 26.8 percent following SAVR. Although not statistically significant (p = 0.07), the rate of major stroke following the TAVR procedure was 5.1 percent vs. 2.4 percent following SAVR. There was a significant difference in major vascular complications that favored SAVR (11.5 percent TAVR vs. 3.5 percent SAVR), while conversely, there was less major bleeding in TAVR (14.7 percent TAVR vs. 25.7 percent SAVR) and a trend toward a decreased incidence of new onset of atrial fibrillation (12.1 percent TAVR and 17.1 percent SAVR) [12].
Table 1. Clinical outcomes in high-risk patients from the PARTNER Trial (cohort A) randomized to either TAVR or SAVR.
| Outcome | Transcatheter AVR (TAVR) N=348 | Surgical AVR (SAVR) N=351 | P-Value |
| 30-day Mortality | 12 (3.4%) | 22 (6.5%) | 0.07 |
| 1-year Mortality | 84 (24.2%) | 89 (26.8%) | 0.44 |
| Major Vascular Complications | 38 (11.0%) | 11 (3.2%) | <0.001 |
| New Atrial Fibrillation | 42 (12.1%) | 60 (17.1%) | 0.07 |
| New Pacemaker | 19 (5.7%) | 16 (5.0%) | 0.68 |
| Major Stroke at 1-year | 17 (5.1%) | 8 (2.4%) | 0.07 |
| Major Bleed at 1-year | 49 (14.7%) | 85 (25.7%) | <0.001 |
In cohort B (Table 2), the results of which were published 8 months prior to cohort A, TAVR was found to be superior to standard therapy in regard to death from any cause (30.7 percent TAVR vs. 49.7 percent from standard therapy) and repeat hospitalization (40 percent TAVR vs. 79 percent standard therapy) at 1 year. In addition, TAVR was associated with sustained improvement in New York Heart Association (NYHA) class at 30 days, 6 months, and 1 year. At one year, however, TAVR was associated with an increased risk of major stroke (7.8 percent vs. 3.9 percent), major bleeding (22.3 percent vs. 11.2 percent), and vascular complications (16.8 percent vs. 2.2 percent) [1]. In cohort B, patients in the standard therapy arm were treated both medically and with aortic balloon valvuloplasty if clinically indicated, with 37 percent of patients in the standard therapy arm undergoing aortic balloon valvuloplasty. Based on the results from cohort B of the PARTNER study showing a dramatic improvement in 1 year mortality, in November 2011, the FDA approved TAVR for patients with symptomatic severe aortic stenosis who are not surgical candidates.
Table 2. Clinical outcomes in extreme-risk patients from the PARTNER Trial (cohort B) randomized to either TAVR or standard therapy.
| Outcome | Transcatheter AVR (TAVR) N=179 | Standard Therapy N=179 | P-Value |
| 30-day Mortality | 9 (5%) | 5 (2.8%) | 0.41 |
| 1-year Mortality | 55 (30.7%) | 89 (49.7%) | <0.001 |
| Major Vascular Complications | 29 (16.2%) | 2 (1.1%) | <0.001 |
| New Atrial Fibrillation | 1 (0.6%) | 3 (1.7%) | 0.62 |
| New Pacemaker | 8 (4.5%) | 14 (4.8%) | 0.27 |
| Major Stroke at 1-year | 14 (7.8%) | 7 (3.9%) | 0.18 |
| Major Bleed at 1-year | 40 (22.3%) | 20 (11.2%) | 0.007 |
The CoreValve Pivotal U.S. Clinical Trial is ongoing, and thus, the data available for this device come from smaller European studies and other registries. Three-year data from the Italian CoreValve registry of 181 patients were recently published [18]. Similar to the PARTNER study population, all procedures were approved for use in patients considered at high risk for surgery. All-cause mortality was 23.6 percent at 1 year, similar to 1-year mortality in PARTNER A of 24.2 percent (Table 3). As of January 2012, enrollment in the extreme risk surgical arm of CoreValve U.S. Pivotal Trial had been completed, and it is expected that enrollment for the high risk arm will be completed by the fall of 2012.
Table 3. Comparison of clinical outcomes in patients from the Italian CoreValve Registry and cohort A of the PARTNER trial [12,18].
Comparing the Two Valves
While the outcomes in terms of survival and symptom improvement are similar between comparable patient groups who undergo TAVR with either the CoreValve or Edwards Sapien Valve, there are notable differences in complications seen in patients treated with the two valves (Table 3). One such difference between the CoreValve and Sapien Valve is the need for permanent pacemaker placement (PPM) after the procedure. The incidence of PPM placement after CoreValve ranges from 12.1 percent in the Italian registry to up to 39 percent in other registries [19-22]. In contrast, the incidence of need for PPM in the PARTNER study with the Sapien Valve was 5.7 percent and 4.5 percent in groups A and B respectively, which was not different from those patients treated either medically or surgically [1,12]. The increased incidence of PPM placement after the CoreValve procedure is due in part to the fact that the frame of the CoreValve extends below the aortic annulus and thus lays adjacent to the left bundle branch. As a result, the His bundle may be adversely affected during the expansion of the prosthesis due to the high radial force of the self-expanding nitinol frame of the CoreValve [19].
While the structure and design of the CoreValve leads to an increased need for PPM placement, it also allows for the valve to be more easily delivered and repositioned during delivery. In contrast to the Sapien Valve, which requires either a large 22 French or 24 French sheath for delivery, the CoreValve is able to be delivered through an 18 French sheath. Since the anatomy of many patients cannot accept a 22 or 24F sheath, the CoreValve can thus be used in many patients who otherwise would not have been able to tolerate the size of the Sapien delivery system. As a result of the larger sheath size for delivery, there is an associated increase in vascular complications seen with the Sapien Valve (Table 3). The primary vascular access for sheath placement and subsequent delivery of both the CoreValve and Sapien valve is via the femoral artery; however, due to the large sheath sizes required, alternative access sites are being investigated. These include direct left ventricular apical access for the Sapien Valve and both subclavian and direct aortic access for the CoreValve. The outcomes from these alternative access sites as compared to femoral access have not been fully evaluated; however, data from studies, including the STACCATO trial that compared the transapical approach for TAVR with the Sapien valve against conventional SAVR, has raised concerns about these alternative approaches [23].
The ability to adjust the positioning of the valve during delivery gives the CoreValve a distinct advantage over the Sapien Valve. Due to the self-expanding nature of the Nitinol frame of the CoreValve, the CoreValve can be deployed in stages allowing for subtle adjustments in position during the deployment phases (Figure 3). In contrast, the Sapien Valve is rapidly deployed with a single balloon expansion that does not allow for repositioning either during or after deployment (Figure 4).
Figure 3.
Stepwise desheathing deployment mechanism of Medtronic CoreValve system. A shows the valve completely collapsed within the delivery sheath. B and C show the gradual expansion of the nitinol framed valve as the delivery sheath is slowly retracted. During this stage of deployment, the valve is still attached to the delivery catheter and can be repositioned or even completely recaptured and removed. D shows the final release of the valve from the delivery sheath. With the valve now fully released from the sheath, it can no longer be easily adjusted. (Image provided by Medtronic, Inc. In the U.S., the Medtronic CoreValve® System is available for investigational purposes only.)
Figure 4.
Rapid balloon deployment mechanism of Edwards Sapien system. A shows the crimped valve loaded onto the balloon delivery catheter. B shows the valve being deployed by rapid balloon inflation. With deflation of the balloon, the valve maintains its configuration and the balloon catheter can then be removed leaving behind the fully functioning Sapien Valve. (Published with permission from Edward Lifesciences Inc: Irvine, CA.)
Conclusions
In the design, development, and now clinical application of both the Edwards Sapien Valve and Medtronic CoreValve, we are witness to a new era in the field of valvular heart disease. However, despite the advances within this arena, there remain significant hurdles to overcome. These include addressing the increase in stroke observed in the PARTNER study, the aforementioned increased incidence in permanent pacemaker placement for patients getting the CoreValve, the ability to reposition if needed, as well as issues of alternative access in patients whose femoral anatomy cannot accommodate the large sheath sizes required for the transfemoral approach. Significant research and product development is currently ongoing to addresses each of these issues, including the use of cerebral protection devices similar to those used in carotid procedures, the use of smaller devices, and the design of retrievable devices, as well as alternative access techniques including subclavian, apical, and direct aortic access. There are no fewer than six other transcatheter valve types currently being evaluated, each of which employs unique features aimed to improve the design and function of this technology. Lastly, as modifications and improvements are made to the design, the medical field must continue to adapt and develop in response to this new technology. New recommendations and expert consensus documents are being developed to help guide the use of this new technology [24].
We are no longer in an era in which the old boundaries between cardiac specialties apply. To obtain the best outcome for the patient, there must be a true collaboration between the cardiothoracic surgeon and interventional cardiologist, not only in evaluation of the patient but in the procedure itself. Just as with the development of the valve design, the development of the “heart valve team” will take time but is just as critical for the success of this technology.
Acknowledgments
I would like to thank Dr. Michael Cleman, Dr. Craig Thompson, Dr. Alexandra Lansky, Dr. John Elefteriades, Dr. Sabet Hashim, and Dr. Arnar Geirsson.
Abbreviations
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
- PPM
permanent pacemaker
- NYHA
New York Heart Association
- FDA
Food and Drug Administration
- PTFE
polytetrafluoroethylene
Disclaimer
Dr. Forrest is a co-investigator for the Medtronic CoreValve US Pivotal Trial and implanting physician for the Edwards Sapien Transcatheter Heart Valve System. He has no financial interest/arrangement or affiliation with any organizations that could result in a conflict of interest in the context of the subject of this review.
Footnotes
1Transcatheter aortic valve replacement (TAVR) and transcatheter aortic valve implantation (TAVI) are used interchangeably throughout the literature and refer to the same procedure.
References
- Leon MB. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- Iung B. et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Stewart BF. et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- Ross J Jr., Braunwald E. Aortic stenosis. Circulation. 1968;38(1 Suppl):61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- Otto CM. Timing of aortic valve surgery. Heart. 2000;84(2):211–218. doi: 10.1136/heart.84.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardin JM. et al. Aortic stenosis: can severity be reliably estimated noninvasively? Chest. 1980;77(2):130–131. doi: 10.1378/chest.77.2.130. [DOI] [PubMed] [Google Scholar]
- Iung B. et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26(24):2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- Bouma BJ. et al. Variability in treatment advice for elderly patients with aortic stenosis: a nationwide survey in The Netherlands. Heart. 2001;85(2):196–201. doi: 10.1136/heart.85.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellikka PA, Sundt TM. Management of asymptomatic severe aortic stenosis: is aortic valve replacement indicated? Nat Clin Pract Cardiovasc Med. 2008;5(10):608–609. doi: 10.1038/ncpcardio1304. [DOI] [PubMed] [Google Scholar]
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J. 1992;13(5):704–708. doi: 10.1093/oxfordjournals.eurheartj.a060238. [DOI] [PubMed] [Google Scholar]
- Cribier A. et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- Smith CR. et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- Webb J, Cribier A. Percutaneous transarterial aortic valve implantation: what do we know? Eur Heart J. 2011;32(2):140–147. doi: 10.1093/eurheartj/ehq453. [DOI] [PubMed] [Google Scholar]
- Vahanian A. et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2008;29(11):1463–1470. doi: 10.1093/eurheartj/ehn183. [DOI] [PubMed] [Google Scholar]
- The Edwards SAPIEN THV Transcatheter Heart Valve System for Patients With Severe Aortic Stenosis Who Are Not Candidates for Conventional Open-Heart Aortic Valve Replacement Surgery. Briefing Document for the FDA Circulatory Systems Device Panel Advisory Committee [Internet] 2011. Jul 20, Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM262935.pdf .
- Liao K. et al. Bovine pericardium versus porcine aortic valve: comparison of tissue biological properties as prosthetic valves. Artif Organs. 1992;16(4):361–365. doi: 10.1111/j.1525-1594.1992.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Metallurgy: The Alloy That Remembers. TIME Magazine. 1968 Sept 13; [Google Scholar]
- Ussia GP. et al. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J. doi: 10.1093/eurheartj/ehr491. Epub 2012 Jan 12. [DOI] [PubMed] [Google Scholar]
- Calvi V. et al. Incidence rate and predictors of permanent pacemaker implantation after transcatheter aortic valve implantation with self-expanding CoreValve prosthesis. J Interv Card Electrophysiol. doi: 10.1007/s10840-011-9634-5. Epub 2011 Nov 25. [DOI] [PubMed] [Google Scholar]
- Calvi V. et al. Early conduction disorders following percutaneous aortic valve replacement. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S126–S130. doi: 10.1111/j.1540-8159.2008.02298.x. [DOI] [PubMed] [Google Scholar]
- Jilaihawi H. et al. Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis. Am Heart J. 2009;157(5):860–866. doi: 10.1016/j.ahj.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Piazza N. et al. Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. JACC Cardiovasc Interv. 2008;1(3):310–316. doi: 10.1016/j.jcin.2008.04.007. [DOI] [PubMed] [Google Scholar]
- O’Riordan M. STACCATO: Transapical TAVI in surgery-eligible patients stopped due to adverse events [Internet] 2011. Nov 10, Available from: http://www.theheart.org/article/1307437.do .
- Holmes DR Jr.. et al. 2012 ACCF/AATS/SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2012;59(13):1200–1254. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]