SUMMARY
The germ cells of Caenorhabditis elegans serve as a useful model to study the balance between proliferation and differentiation within the context of development and changing environmental signals experienced by the animal. Germ cells adjacent to a stem cell niche in the distal region of the gonad retain the capacity to divide during adulthood, making them unique from other cells in the organism. We will highlight recent advances in our understanding of mechanisms that control proliferation, as well as the signaling pathways involved in promoting mitosis at the expense of differentiation.
INTRODUCTION
Development of all multicellular organisms requires the balance between proliferation and differentiation of cells. Within the germ line, this balance influences the reproductive fitness of an individual, as proliferation ensures continued presence of gamete precursors while differentiation leads to gamete production or apoptosis. To ensure species fitness, the gonads of many animals maintain a mitotically active pool of germline stem cells (GSCs). The GSCs of the transparent nematode Caenorhabditis elegans are especially amenable to the study of proliferation, since the traits that make C. elegans particularly tractable for research are combined with the fact that GSCs are unique from other cells for their capacity to continually proliferate during adulthood. With these experimental advantages, many advances have been made in our understanding of how germ cell proliferation is regulated during development. Recent studies have demonstrated that germ cell proliferation is coordinated with somatic development by interweaving environmental influences and somatic cell guidance with intrinsic regulation of germ cell proliferation. Here, we will highlight these new findings and incorporate them with the standard view of how cell proliferation is regulated by a stem cell niche.
OVERVIEW OF C. ELEGANS GERMLINE DEVELOPMENT
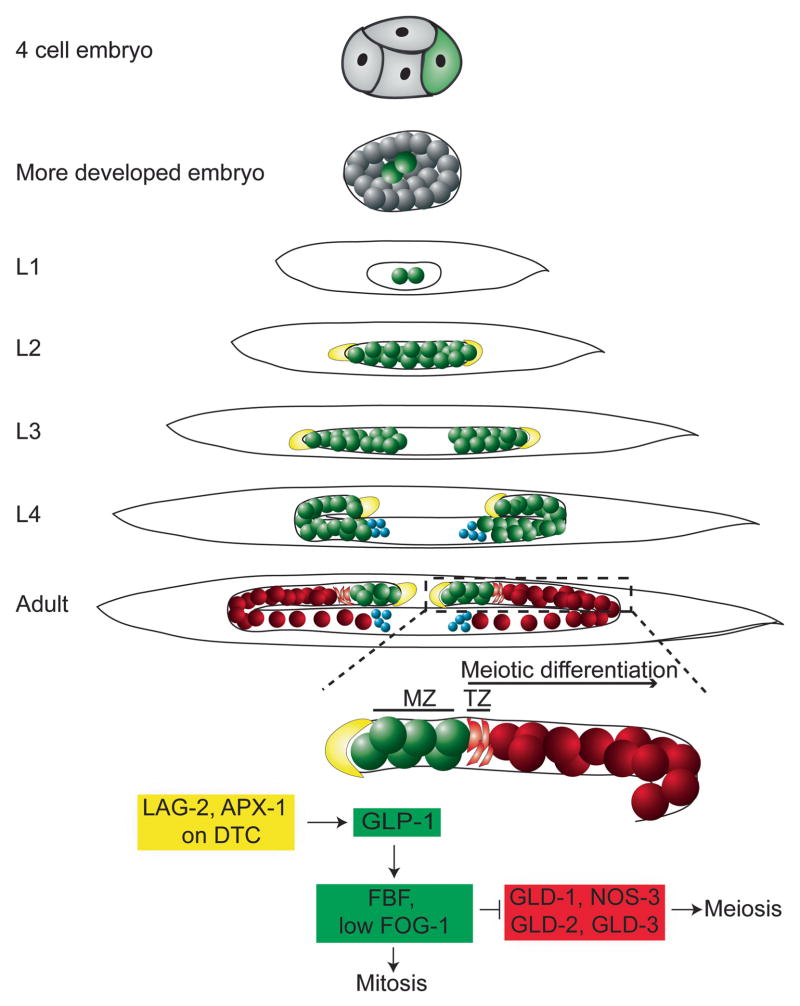
Germ cells are set aside early during embryogenesis in C. elegans [Fig. 1 and reviewed by Kimble and Crittenden (2005, 2007)]. The embryonic P lineage divides only four times post-fertilization before specifying P4 as the sole ancestor of all germ cells in the animal. P4 divides once to form two primordial germ cells (PGCs), Z2 and Z3, during embryogenesis. Z2 and Z3 remain mitotically quiescent during the remainder of embryogenesis, but begin to divide after about midway through the first larval stage (L1). The cells continue proliferating as the gonad expands during the L2 and L3 larval stages. The somatic distal tip cell (DTC) of each gonad arm, which guides distal growth of the germ line, begins to turn towards the middle of the animal during the transition between L3 and L4. Soon after entry into L3, cells furthest from the DTC initiate meiosis. Meiosis differs significantly from mitosis in that it directs recombination between chromosomes and also includes an extra round of DNA reduction that results in haploid cells. Meiosis is closely followed by gametogenesis, such that sperm are produced at the L4 stage and oocytes in the adult. Germ cells within the adult gonad arm thus have a polarized and progressive arrangement, in which proliferating cells are maintained near the DTC, and more differentiated cells are positioned proximally.
Figure 1.
Germline development in the C. elegans hermaphrodite. Germ cells are indicated by colored spheres while the distal tip cell (DTC) is depicted in yellow. Germline stem cells (GSCs) in the mitotic cell cycle within the mitotic zone (MZ) are diagrammed in green, cells entering meiosis in the transition zone (TZ) are diagramed as crescents. Cells in spermatogenic meiosis are colored blue and cells in oogenic meiosis are colored red. The germ line is set aside during the very first cell divisions of the embryo, and divides into Z2 and Z3, the precursors of all germ cells of the animal, before hatching. Z2 and Z3 proliferate in the expanding gonad during L2 and split into two gonad arms during L3. The distal tip cell begins to direct gonad migration inward, and meiosis is initiated in proximal populations of each gonad arm during L3. These early meiotic cells develop as sperm in L4. In young adults, the proliferating cells entering meiosis differentiate into oocytes in the proximal arms of each gonad, where they sit adjacent to mature spermatids. A region of the one adult gonad arm is outlined in a dashed line and enlarged below the diagram, along with a greatly simplified model of the intrinsic GLP-1 signaling that promotes mitosis. Delta ligands LAG-2 (and subsequently APX-1) in the DTC drive GLP-1 signaling in the GSCs within the MZ. At the transition zone (TZ), loss of GLP-1 signaling leads to reduced FBF and consequently elevated GLD-1, NOS-2, GLD-2, and GLD-3 proteins and subsequent entry into a meiotic differentiation pathway.
In the adult hermaphrodite germ line, the region containing proliferating germ cells is known as the “mitotic zone” (MZ). Based on various estimates, between 200 and 250 germ cells are mitotically active within the MZ, which extends approximately 19–22 cell diameters from the distal tip of the gonad (Hansen et al., 2004; Lamont et al., 2004). Intriguingly, these “cells” are actually a syncytium. However, as each nucleus does have its own associated cytoplasm, and appears to behave autonomously (Hall et al., 1999; Crittenden et al., 2006), use of the term “germ cell” is the convention in the field and will be used in this review as well. All cells within the MZ are actively cycling (Crittenden et al., 1994, 2006), though at slightly different average rates depending on their position relative to the distal tip (Maciejowski et al., 2006). Nuclei in M phase are relatively scarce, as just 1.36–4.3% of all nuclei between 3 and 10 cell diameters from the DTC are in pro-metaphase, metaphase, or anaphase (Crittenden et al., 2006; Maciejowski et al., 2006)]. Therefore, the bulk of the life of proliferating germ cells is spent in interphase. Calculations based upon several sources (Crittenden et al., 2006; Maciejowski et al., 2006; Jaramillo-Lambert et al., 2007) have led to an estimation that the mitotic cells must double their populations over three times to maintain the final germ cell number in adult gonad arms (Hubbard, 2007). Given the rarity of mitotic divisions and the difficulty of generating visual markers in GSCs, cell cycle regulation has not yet been extensively studied in this system. Rather, the GSCs have better served as a model to understand how a stem cell population is created and maintained, as well as how environmental signals can trigger proliferative changes to groups of cells.
INTRINSIC REGULATION OF GERM CELL PROLIFERATION
With the extensive literature and many excellent reviews that have been written focusing on the pathways in germ cells that regulate proliferation and differentiation (e.g., Hubbard 2007; Kimble and Crittenden, 2007), we have chosen to keep our discussion of these events relatively brief and simple, and focus instead on some of the newer studies that add to our understanding of intrinsic germ cell proliferation.
Control of Germ Cell Proliferation by Notch signaling
The importance of the DTC in regulating proliferation of the GSCs was established by early studies of the C. elegans germ line. Cell ablation experiments determined that the DTC is necessary and sufficient for promotion of proliferation or prevention of meiotic differentiation of adjacent GSCs (Kimble and White, 1981). Signaling from the DTC to the GSCs is dependent upon the canonical Notch signaling pathway, as loss-of-function mutation of either the Delta/LAG-2 ligand expressed by the DTC or the Notch/GLP-1 receptor on the GSCs mimic effects of DTC ablation (Austin and Kimble, 1987; Kimble and Crittenden, 2005; Table 1). The Notch family member GLP-1 was identified as the receptor on the surface of GSCs that responds to the non -autonomous signals from the DTC via the LAG-2 ligand (Austin and Kimble, 1987; Crittenden et al., 1994). Additionally, tumorous germ lines filled with mitotic germ cells are found in animals with gain-of-function alleles of glp-1, indicating that glp-1 signaling is sufficient to drive cellular proliferation (Berry et al., 1997; Pepper et al., 2003). In wild-type animals, the entry into meiosis is triggered concomitant with a decrease in Notch signaling, likely due to increasing distance from the DTC (Kimble and White, 1981; Austin and Kimble, 1987; Seydoux et al., 1990). Signaling between the DTC and the adjacent GSCs begins with expression within the DTC of LAG-2, which is driven by the basic helix-loop-helix transcription factor HLH-2/E/Daughterless needed for DTC specification (Chesney et al., 2009). Proper presentation of Delta/LAG-2 by the DTC to the GSCs is likely to involve many trafficking molecules, including the Bro-1-domain protein EGO-2 and EPN-1/epsin, which probably aid in vesicle targeting and cargo selection, respectively (Tian et al., 2004; Liu and Maine, 2007). After L3, the DTC expresses a second GLP-1 ligand, APX-1, which contributes redundantly to promote Notch signaling and GSC proliferation (Nadarajan et al., 2009).
TABLE 1.
Summary of Genes Involved in Regulating GSC Proliferation
| Pathway | Protein | Ortholog/Domain | Function in germ cell proliferation |
|---|---|---|---|
| Notch | GLP-1 | Notch | Germ cell receptor required for proliferation |
| LAG-2/APX-1 | Delta | Somatic niche cell ligands for GLP-1 | |
| LAG-1 | CSL | Transcription regulator that mediates GLP-1 signal | |
| SEL-8 | Mastermind | Transcription co-regulator with LAG-1 | |
| SEL-10 | E3 ubiquitin ligase | Potential regulator of GLP-1 proteolysis | |
| EGO-2 | Bro1 | Promotes LAG-2/APX-1 expression | |
| EPN-1 | Epsin | Endocytic adaptor that promotes LAG-2/APX-1 | |
| Cell cycle | CEP-1 | p53 | Transcription factor that induces cell cycle arrest |
| VRK-1 | VRK1 kinase | Promotes proliferation in germ cells | |
| CKI-1/2 | p27 CDK inhibitor | Blocks cell cycle progression | |
| PHG-1 | Gas1 | Required for cell cycle arrest | |
| PTC-1 | Patched | Receptor required for germ cell proliferation | |
| NOS-1/-2 | Nanos | Required for larval germ cell survival/proliferation | |
| Mitosis versus meiosis decision | FBF-1/-2 | Pumilio/Puf | Required for germ cell proliferation |
| GLD-1 | RNA-binding protein | Pro-meiotic translation inhibitor | |
| GLD-2 | polyA polymerase | Promotes entry into meiosis | |
| GLD-3 | BicC | Promotes entry into meiosis | |
| FOG-1 | poly A tail-binding | Promotes larval germ cell proliferation when low | |
| NOS-3 | Nanos | Required for germ cell proliferation | |
| Insulin signaling | DAF-2 | Insulin receptor | Promotes larval germ cell proliferation |
| DAF-16 | FOXO TF | Inhibits larval germ cell proliferation | |
| INS-3/-33 | Insulin | Signaling peptides that promote proliferation | |
| AGE-1 | PI3 kinase | Promotes L1 larval germ cell proliferation | |
| AKT-1 | PKB kinase | Promotes L1 larval germ cell proliferation | |
| DAF-18 | PTEN | Inhibits early larval germ cell proliferation | |
| Miscellaneous | AAK-2 | AMPK2 | Inhibits early larval germ cell proliferation |
| DAF-7 | TGF-beta | Promotes early larval germ cell proliferation | |
| PAR-4 | LKB1 kinase | Inhibits early larval germ cell proliferation | |
| NHR-49 | Nuclear receptor | Triggers onset of ARD and inhibits proliferation |
Based on work in other systems, GLP-1 binding to the LAG-2/APX-1 ligand likely triggers cleavage of intracellular residues of GLP-1, resulting in internalization of the C-terminus that interacts with LAG-1, a DNA-binding CSL protein, and SEL-8, a transcriptional activator, to form a ternary complex able to activate transcription (Christensen et al., 1996; Doyle et al., 2000; Petcherski and Kimble, 2000). The regulation of ligand expression and presentation, molecular mechanisms of GLP-1/Notch internalization and subsequent modifications, as well as the stoichiometry of the complex that interacts with DNA, are all subjects that remain to be elucidated. Loss of any of the components of the Notch signaling pathway drastically reduces proliferation of the GSCs but does not impair meiosis, while ectopic activation of Notch signaling results in excess proliferation and a tumorous germline phenotype. Therefore, Notch signaling is the primary binary switch that regulates the balance between mitosis and meiosis in the germ line. However, whether GLP-1/Notch signaling directly promotes mitosis or prevents meiosis—or perhaps is involved in regulating both processes—has been difficult to discern, as mitosis and meiosis are mutually opposing in this system.
Although several proteins are known to act downstream of GLP-1/Notch signaling, whether most are direct targets of GLP-1 transcriptional regulation is not established. In several other systems, the identification of Notch transcriptional targets has been aided by the recognition of DNA sequences bound by CSL. CSL is a transcriptional regulator that actively represses target promoters by recruiting repressive chromatin modifiers, but then functions as an activator when internalized GLP-1/Notch associates with CSL (Kopan and Ilagan, 2009). However, in C. elegans the absence of the CSL factor LAG-1 does not result in a tumorous germ line (Qiao et al., 1995), suggesting that targets of GLP-1 regulation are kept in a ‘permissive’ rather than “repressive” state, and consistent with the possibility that GLP-1 acts to promote mitosis rather than repress meiosis. Currently, little is known about which components of Notch signaling are true targets for direct transcriptional activation by GLP-1 in the GSCs. However, identification of the conserved DNA sequence bound by LAG-1 provided the potential to recognize candidate genes likely to be direct GLP-1 targets, including lag-1, lin-12, and glp-1 itself (Christensen et al., 1996).
The potential positive feedback loop suggested by putative LAG-1 binding sites upstream of the glp-1 locus (Christensen et al., 1996) could balance rapid turnover of GLP-1 protein levels, as GLP-1 appears to be internalized within a few cell divisions (Crittenden et al., 1994). In mammalian cells, the CDK8 kinase phosphorylates the PEST domain of intracellular Notch during target transcriptional activation and targets it for proteasomal degradation (Fryer et al., 2004). In C. elegans, loss of the E3-ubiquitin ligase SEL-10 enhances germline over-proliferation in a weak glp-1(gf) mutant background, suggesting that SEL-10 may target intracellular GLP-1 to the proteasome for degradation (Pepper et al., 2003a). It seems clear that proteasomal activity is indeed likely to regulate the entry into meiosis, since abrogation of several components of the proteasome enhance GSC proliferation in weak glp-1(gf) backgrounds (Macdonald et al., 2008). However, whether reduction of both sel-10 and proteasomal function causes the tumorous GSC proliferation that one might expect from ectopic GLP-1-signaling cannot be assessed, due to mis-regulation of other targets of SEL-10 that cause an early phenotype (Macdonald et al., 2008). Whether SEL-10 is the only regulator of intracellular GLP-1 degradation, and how SEL-10 regulates GLP-1 independently of its PEST domain, remains an open question.
The pivotal role of GLP-1/Notch signaling in regulating proliferation in the GSCs validates the importance of continued research into this pathway. Biochemical analysis of molecular modifications and transcriptional targets of GLP-1 will be particularly useful for our understanding of regulation of mitosis in GSCs.
Downstream Regulators of the Mitotic/Meiotic Entry Decision
In addition to the rate of cell division, the decision to enter meiosis also influences the size of the MZ in the adult. Our discussion will therefore highlight some of the mechanisms by which meiosis is suppressed within the MZ. We refer the reader to more extensive reviews of the regulation of meiotic entry for more detailed analysis of differentiation within the germ line (Kimble and Crittenden, 2007; Byrd and Kimble, 2009; Racher and Hansen, 2010). GLP-1/Notch signaling within the distal GSCs feeds into a regulatory network of RNA-binding proteins that mediate the decision between self-renewal and meiotic differentiation. One of the few known targets of GLP-1 transcriptional activity is fbf-2, which encodes an RNA-binding PUF family protein. FBF-2 acts redundantly with FBF-1 to promote self-renewal and prevent meiotic entry (Crittenden et al., 2002; Lamont et al., 2004). As FBF-1 and FBF-2 are nearly identical RNA-binding proteins enriched within the distal proliferative zone of the germ line, they are often collectively described as FBF. FBF represses translation of many target mRNAs, including transcripts for two key RNA-binding proteins that promote meiotic entry: GLD-1 and GLD-3 (Eckmann et al., 2004; Suh et al., 2009). Interestingly, FBF also represses translation of the fog-1 transcript, a homolog of the cytoplasmic polyadenylation element-binding protein (Jin et al., 2001) that acts at low levels to promote proliferation but activates spermatogenesis at high levels (Thompson et al., 2005). Maintaining low levels of FOG-1 expression is especially important for GSC proliferation during larval development and is likewise necessary for the proliferation of GSCs in a glp-1(gf); fbf-1(lf) tumor (Thompson et al., 2005), suggesting that low FOG-1 levels act redundantly with FBF in certain situations to increase cellular divisions. However, low FOG-1 levels alone are unable to drive extended periods of GSC proliferation, indicating that FBF is the primary driver of GSC self-renewal in response to GLP-1/Notch signaling (Thompson et al., 2005).
Entry into meiosis is regulated chiefly by two pathways that act redundantly to control RNA targets. The RNA translational repressor GLD-1 and the Nanos homolog NOS -3 coordinate one pathway of meiotic entry, whereas the second pathway is regulated by the poly (A) polymerase GLD-2 and translational activator GLD-3 proteins (Kadyk and Kimble, 1998; Crittenden et al., 2002; Eckmann et al., 2004; Hansen et al., 2004). FBF within proliferating cells represses the translation of both gld-1 and gld-3 mRNAs (Crittenden et al., 2002; Eckmann et al., 2004), indicating that FBF inhibits meiosis by preventing the function of both of these pathways. Ectopic proliferation of GSCs is observed when meiotic progression fails, as when both the GLD-1/NOS-3 and GLD-2/GLD-3 meiotic pathways are blocked [reviewed by Hansen and Schedl (2006)]. Surprisingly, a gld-1; fbf-1 fbf-2 triple mutant germ line is also tumorous (Crittenden et al., 2002), suggesting that FBF acts within the GLD-2/GLD-3 pathway to promote meiosis and leading to the hypothesis that perhaps low levels of FBF bound to GLD-2 or GLD-3 can modulate its activity to aid the transition into meiosis (Eckmann et al., 2004). This transition is likely to begin within the nuclei in the more proximal cells of the MZ, which are usually in pre-meiotic S-phase, suggesting a possible functional link (Jaramillo-Lambert et al., 2007). Pre-meiotic S phase is considerably slower than mitotic S phase and the processes are regulated by distinct controls, indicating that pre-meiotic S phase nuclei have actually entered meiosis (Jaramillo-Lambert et al., 2007). However, as there are no known markers to distinguish pre-meiotic from mitotic S-phases [nuclei of both phases are recognized by antibodies to REC-8 under certain fixation conditions (Hansen et al., 2004)], nuclei are usually considered mitotic until they adopt the morphologically distinct crescent shape of paired and synapsed homologous chromosomes of leptotene and zygotene stages of meiosis. How the cells decide to switch into pre-meiotic S phase is unclear, but as this is the first indication of differentiation, this decision is likely to rely on the same signals that regulate meiotic entry.
Regulation of proliferation in the C. elegans germ line depends greatly upon proteins that modulate RNA activity. Not only are FBF and FOG-1 RNA-binding proteins that promote proliferation, the pivotal players of meiotic entry (NOS-3, GLD-1, GLD-2, and GLD-3) also regulate their targets at the RNA level. Pro-meiotic transcripts may also be regulated by RNA splicing, as reduction of several pre-mRNA splicing factors increases proliferation at the expense of meiosis in a glp-1(gf) background (Mantina et al., 2009; Kerins et al., 2010). An overarching theme of proliferation regulation in the C. elegans germ line is therefore that key proteins modulate target RNA transcripts to achieve significant control with relatively few proteins but also to gain the capacity to respond to stimuli rapidly without the need for transcription or protein modifications or trafficking.
EXTRINSIC REGULATION OF GERM CELL PROLIFERATION
Proliferation of GSCs in the C. elegans germ line is also regulated as a response to cues from conditions external to the GSCs, including the stage of development, the availability of nutrients and potentially harmful stresses in the environment. The GSCs respond to these signals in different ways, depending largely upon the developmental stage of the animal. The distinctive regulatory pathways that function during larval development to regulate GSC proliferation have been recently reviewed (Korta and Hubbard, 2010), so they will be described only briefly here. Significantly, glp-1(gf) mutants do not have excessive or precocious GSC proliferation during embryonic PGC quiescence (Berry et al., 1997), dauer (Narbonne and Roy, 2006), or after ablation of the sheath/spermatheca precursor cell (SS; McCarter et al., 1997), suggesting that extrinsic regulatory mechanisms can override GLP-1/Notch promotion of proliferation. Conversely, the germ line of glp-1(null) mutants undergo one or two rounds of division to ultimately produce an average of 20 sperm, indicating that at least the first few divisions are independent of GLP-1 signaling requirements (Austin and Kimble, 1987). Therefore, although GLP-1/Notch signaling is pivotal for maintenance of a population of proliferative GSCs in the adult germ line, other pathways are additionally needed for proper germ cell proliferation during early larval development.
Somatic Cell Influence on Germ Cell Proliferation During Larval Development
Germ cell proliferation is coordinated with development of the adjacent somatic gonad structures. During normal larval development, GSC proliferation causes an exponential increase in mitotic nuclei during L3, which plateaus during L4 and adulthood. The radical increase in proliferation during L3 is dependent upon GLP-1 activation by the redundant LAG-2 and APX-1 ligands present on the DTC (Nadarajan et al., 2009), signals from the most proximal sheath cell (Sh1; Killian and Hubbard, 2005), sufficient production of ribosomes (Kudron and Reinke, 2008), and an insulin-mediated signal (IIR signaling; Michaelson et al., 2010). At least two significant changes occur to the somatic structure of the gonad during L3 that affect GSC proliferation. First, APX-1 becomes expressed in the DTC and acts partially redundantly with LAG-2 to activate GLP-1-dependent GSC proliferation (Nadarajan et al., 2009). Additionally, the distal-most pair of sheath cells, the Sh1 cells, are born during mid-L3 and cover the pre-meiotic proximal regions of the MZ until late-L4, by which point expansion of the GSCs and continued distal migration of the DTC increases the distance between the DTC and the Sh1 cells. Therefore, the Sh1 cells envelop pre-meiotic undifferentiated nuclei during the period of most rapid proliferation: mid-L3 through the L4/adult molt. Ablation of Sh1 causes a reduced rate of GSC proliferation, indicating a role for Sh1 cells in promoting GSC proliferation (Killian and Hubbard, 2005). How do the Sh1 cells encourage proliferation of the underlying GSCs during L3 and early L4? Optimal ribosome biogenesis within the Sh1 cells is likely to be especially important for the signals that promote proliferation of GSCs during L3 and L4 (Killian and Hubbard, 2004; Voutev et al., 2006). Ribosome biogenesis is also crucial within the GSCs themselves, as mutations that affect rRNA levels have drastic effects upon GSC proliferation during larval development (Kudron and Reinke, 2008). Additionally, optimal environmental conditions during L3 and L4 induce the canonical DAF-2/Insulin-IGF-like receptor (IIR) signaling pathway within the germ line, promoting cell cycle progression by inhibiting activity of the transcription factor DAF-16/FOXO (Michaelson et al., 2010). Two insulin-like IIR ligands, INS-3, and INS-33, act upstream of the GSC DAF-2/IIR signaling; however, INS-3 and INS-33 expressions have not been detected in the somatic gonad and the loss of proliferation caused by ablation of the SS cells is not mediated by DAF-16 (Michaelson et al., 2010). These findings indicate that the IIR signaling pathway is not the sole signal from the Sh1 cells to promote GSC proliferation. Therefore, the signal(s) from the Sh1 cell that promotes larval GSC proliferation remains elusive.
The signals that promote the proliferation of GSCs originate adjacent to the MZ of the germ line, limiting proliferation to this region and permitting differentiation and gametogen-esis in the proximal arms of gonads. However, in mutant conditions in which germ cells proliferate more proximally [due to either insufficient distance from the DTC (Seydoux et al., 1990; Killian and Hubbard, 2004) or increased GLP-1 signaling (Pepper et al., 2003b)], undifferentiated GSCs may come into contact with newly born proximal Sh2-5/spermatheca cells and receive proliferative signals that induce a proximal tumor (Killian and Hubbard, 2005). These observations led to the model that proximal sheath cells send proliferative signals under normal conditions, but these are not obeyed by differentiated cells that have begun the meiotic pathway. Further investigation of proximal tumors indicated that the promoters of genes encoding candidate GLP-1 ligands such as apx-1 and arg-1 are active in the most proximal sheath cells (Sh4 and Sh5), and thus likely activate GLP-1 activity in undifferentiated germ cells to induce ectopic proliferation (McGovern et al., 2009). Depending on the developmental stage of the animal, the dependence of proximal tumors on signals from the proximal sheath cells can vary, suggesting that the context of the reception of the proliferative signals is especially important (Killian and Hubbard, 2005).
The Influence of Genotoxic Stress on GSC Proliferation
The differentiation status of a germ cell is likewise important for how it will respond to genotoxic stress. In germ cells that have initiated meiosis, DNA damage results in the activation of the CEP-1/p53 transcription factor and induction of CED-3/Caspase-dependent apoptosis [reviewed by Gartner et al. (2008)]. In contrast, DNA damage in proliferative GSCs results in a CEP-1/p53-dependent cell cycle arrest (Derry et al., 2007). The inhibition of CEP-1 activity in the absence of damage is achieved in part by a kinase, VRK-1; loss of VRK-1 activity results in a CEP-1-dependent decrease in GSC proliferation (Waters et al., 2010). However, whether CEP-1 is a direct target of VRK-1 phosphorylation is unclear. Significantly, unlike the p53-mediated cell cycle arrest achieved by cki-1-2/p21 activity in mammalian cells, in C. elegans GSCs the inhibition of proliferation in response to DNA damage and CEP-1 activity is mediated by phg-1/Gas1 (Derry et al., 2007). The mechanism by which PHG-1, whose transcription may be activated by CEP-1 (Derry et al., 2007), could achieve proliferation inhibition is unclear; however, Gas1 is a GPI-linked β-1-3-glucanosyltransferase that acts as part of a complex on the plasma membrane to inhibit proliferation (Ruaro et al., 2000), but is also predicted to physically bind nuclear p53 (Ruaro et al., 1997). Mammalian Gas1 has been shown to promote Sonic hedgehog (Shh) signaling during mammalian development [reviewed by Kang et al. (2007)], but although the PTC-1/Patched receptor is expressed on proliferating GSCs in C. elegans, no ortholog of Shh has been identified in C. elegans and ptc-1 mutants do not have ectopic GSC proliferation (Kuwabara et al., 2000). Therefore, the etiology of a PHG-1-dependent cell cycle arrest is ambiguous and may prove an exciting avenue for research into DNA-damage induced GSC proliferation regulation in C. elegans.
Nutrition Influences GSC Proliferation at Multiple Developmental Stages
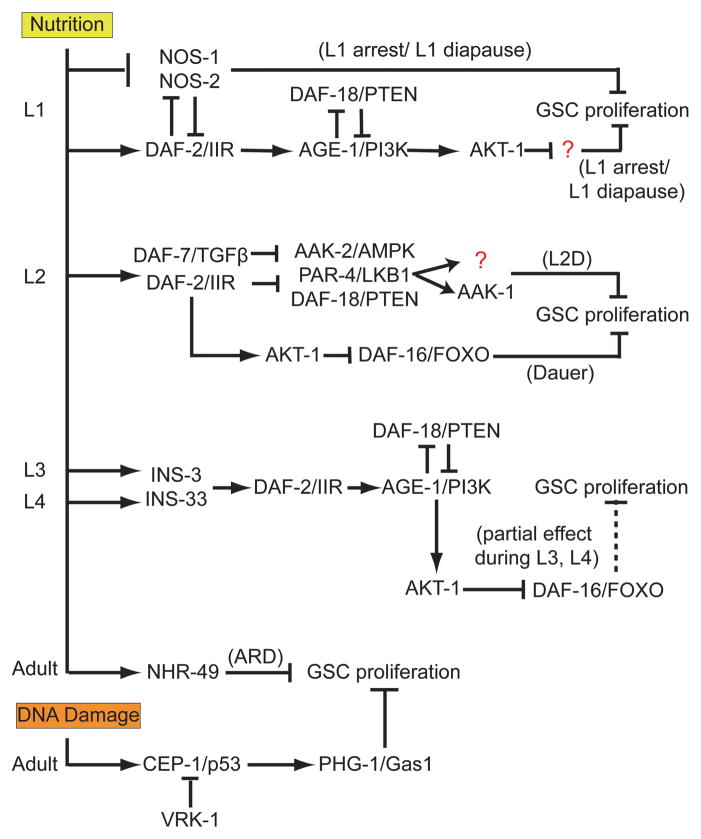
In addition to genotoxic stress, C. elegans germ cells are particularly sensitive to nutritional levels in the environment. Low availability of nutrients can instigate a cease in proliferation to await more favorable conditions. It is noteworthy that there is no cell division of PGCs during embryogenesis, when the egg is essentially impervious to environmental influences. Rather, only after animals hatch and environmental conditions can be assessed is the decision made regarding whether to invest resources into germ cell proliferation. Starvation conditions sensed upon hatching, at the entry into L2, and at the end of L4 can trigger the entry into periods of proliferative quiescence known as L1 diapause (“L1 arrest”), dauer, and adult reproductive diapause (ARD), respectively (Baugh and Sternberg, 2006; Fielen-bach and Antebi, 2008; Angelo and Van Gilst, 2009). The loss of signaling from the DAF-2/Insulin-IGF-like receptor (IIR) pathway is involved in prohibiting continued division of the germ cells, though the developmental context of the starvation conditions results in the use of different downstream components of the pathway to mediate the proliferative arrest (Fig. 2).
Figure 2.
Environmental regulation of GSC proliferation at developmental stages of the gonad. During L1, starvation conditions reduce canonical DAF-2/IIR signaling that depends upon both AGE-1/PI3K and AKT-1 but is independent of DAF-16/FOXO, resulting in loss of GSC proliferation (Fukuyama et al., 2006). Persistent NOS-1 and NOS-2 activity during starvation conditions also prevent GSC proliferation during L1 arrest (Subramaniam and Seydoux, 1999), although whether DAF-2/IIR signaling regulates NOS-1 and NOS-2 is unknown. During the establishment of dauer during L2D, DAF-7/TGFβ and DAF-2/IIR signaling is reduced, resulting in the activation of DAF-18/PTEN, AAK-2/AMPK, and PAR-4/LKB1. PAR-4/LKB-1 activates AAK-1 and at least one other target which collectively prevent GSC proliferation. After dauer is well established, DAF-16/FOXO is necessary to maintain the control of GSC proliferation (Narbonne and Roy, 2006). During L3 and L4, nutritional availability is potentially mediatedby INS-3 and INS-33 ligands activating DAF-2/IIR signaling using the canonical pathway to promote optimal GSC proliferation (Michaelson et al., 2010). If starvation is sensed during late L4, NHR-49 steroid signaling induces ARD by undetermined pathways to prevent GSC proliferation (Angelo and Van Gilst, 2009). Adult GSCs will also cease to proliferate in response to DNA damage in a CEP-1/p53 and PHG-1/Gas-1 dependent manner (Derry et al., 2007) that is held in check by VRK-1 activity during periods with low genotoxic stress (Waters et al., 2010). [Parts of this diagram are modified from (Fukuyama et al., 2006) and (Narbonne and Roy, 2006).]
The DAF-2/Insulin-IGF-like receptor (IIR) signaling pathway senses nutrient conditions to control the onset of an “L1 diapause,” also known as an “L1 starvation arrest,” during which all cells of the animals cease to divide, including the PGCs Z2 and Z3 (Baugh and Sternberg, 2006); however, IIR signaling does not regulate PGC proliferation using the canonical IIR pathway, as loss of the known transcription factor regulated by IIR signaling, DAF-16(FOXO), does not result in PGC proliferation during starvation conditions (Fukuyama et al., 2006). The factor(s) responsible for inhibiting PGC proliferation during L1 diapause remain unknown, although it is dependent upon the IIR-agonist DAF-18/PTEN preventing the AGE-1/PI-3 and AKT-1/PKB kinase activities normally activated by DAF-2/IIR (Fukuyama et al., 2006). Additionally, in the absence of food, NOS-1 and NOS-2 repress transcription, the release of which is correlated with the subsequent proliferation of the PGCs Z2 and Z3 in mid-L1 in a GLP-1-dependent manner (Subramaniam and Seydoux, 1999). It would be interesting to see whether NOS-1 and NOS-2 are regulated by DAF-2/IIR signaling to prevent inappropriate precocious PGC proliferation. Alternatively, NOS-1 and NOS-2 may act in a pathway parallel to DAF-18/PTEN to prevent GSC proliferation when nutrition is not available. Also, GLP-1 is needed for the normal proliferation of Z2 and Z3 during L1 (Subramaniam and Seydoux, 1999); it would be informative to determine whether GLP-1 activity is necessary or sufficient for the extra germ cell divisions triggered by the loss of nos-1 and nos-2 during L1 diapause. These questions would address how NOS-1/NOS-2 regulation and lack of IIR signaling are coordinated to result in the prevention of PGC division during L1 starvation.
At the end of L1, if environmental conditions are unfavorable, the animal will enter an extended L2 stage (L2d) in preparation for dauer, a developmentally arrested condition in which the energetically expensive process of GSCs proliferation is inhibited [recently reviewed by Fielenbach and Antebi (2008)]. Despite appropriate LAG-2 and GLP-1 expression in distal regions of dauer gonads, GSCs arrest in interphase and fail to proliferate, even in glp-1(gf) mutant backgrounds (Narbonne and Roy, 2006). These observations suggest that dauer conditions induce factors that act either downstream of or in parallel to GLP-1 signals to inhibit mitotic proliferation. These factors appear to reduce both DAF-7/TGF-β and DAF-2/IIR signaling, which results in inhibition of GSC proliferation; this inhibition is dependent upon DAF-18/PTEN and AAK-2/AMPK activity and loss of AKT-1/PKB activity (Narbonne and Roy, 2006). The PAR-4/LKB1 kinase is required for activation of AAK-2, but as par-4(lf) enhances the defects of GSC regulation of aak-2(lf) mutants, other targets of PAR-4 responsible for maximum GSC proliferation inhibition remain unidentified (Narbonne and Roy, 2006). Surprisingly, the canonical DAF-2 signaling pathway transcription factor DAF-16(FOXO) appears to be dispensable for GSC proliferative control until dauer has been well established, indicating that the target of IIR-dependent AKT-2 activity that acts during dauer to prevent GSC proliferation is likewise unidentified. Lastly, AAK-2 functions cell autonomously to repress GSC proliferation (Narbonne and Roy, 2006), but how somatic signals are transmitted to activate AAK-2 during L2d is also an open question. Therefore, lack of nutrients indicated by reduced DAF-7/TGF-β and DAF-2/IIR signaling result in a loss of GSC proliferation, though our understanding of the mechanism of regulation of the germ line remains incomplete.
As we have discussed, GSCs cease to proliferate in crowded and nutritionally starved conditions that trigger L1 diapause and dauer stages. Recently, an additional period of GSC quiescence has been identified: ARD, which is triggered when crowded populations of worms are removed from food during mid-L4 (Angelo and Van Gilst, 2009). The starvation-sensing nuclear receptor NHR-49 triggers ARD entry, resulting in apoptosis of all meiotic cells and all but a small pool of GSCs in the distal region of each gonad arm (Angelo and Van Gilst, 2009). Upon reintroduction of food, both GSC proliferation and meiosis are re-initiated to create gonads that are grossly normal (Angelo and Van Gilst, 2009), though whether the canonical DAF-2/IIR signaling pathway is involved has not been established. The ability of the animal to not only halt the energetically expensive process of GSC proliferation during times of starvation but also to harvest nutrients from the germ line as needed suggests a remarkable plasticity to harsh environmental conditions. It is noteworthy that the energy that is saved when the germ line of an animal is lost can be used to extend lifespan; this effect is dependent upon steroid hormone signals transmitted by the somatic gonad cells (Yamawaki et al., 2010). The discovery of ARD indicates that in times of significant energy shortage, the germ line can be harvested for energy required for survival via apoptosis and engulfment of the germ cells; the communication of environmental conditions to trigger the effect on the germ line is similarly dependent upon steroid signaling (Angelo and Van Gilst, 2009). The small pools of GSCs that are retained within the germ line of ARD animals might be protected by GLP-1 signaling activated by LAG-2 expression in the DTC, though this should be tested by checking whether more germ cells are protected during ARD conditions in a glp-1(gf) background. Future research will undoubtedly clarify which pathways and somatic gonad cells are involved in both the response of the germ line to ARD conditions, as well as to signals that indicate the exit from ARD that permits the reconstitution of the germ line.
CONCLUSIONS AND OPEN QUESTIONS
Investigations into GSC proliferation during different stages of development indicate that the germ line is highly sensitive to both stressful conditions that cause a decrease in proliferation and interactions with somatic cells that promote proliferation. Differences in signaling pathways that mediate the response of the GSCs to changes in the environment vary slightly, suggesting that the context of the GSC response is particularly important. The continued communication between the GSCs and the somatic gonad is pivotal to decisions of whether to continue to proliferate during the entire life of the animal. Understanding how C. elegans GSC regulate proliferation in the dynamic environment of a developing animal complements our understanding of cell proliferation regulation in other systems, highlighting that context of signal reception is critical to whether a cell will choose to divide. The dynamic clues that GSCs receive during development suggest a complicated network of interactions that regulate GSC proliferation. The observations that GSCs are mitotically quiescent early in development (embryogenesis), that initiation of mitosis is initially independent of levels of GLP-1 activity, and that environmental stresses can regulate proliferation of GSCs regardless of position of the DTCs, suggest that although GLP-1/Notch signaling remains a pivotal pathway to promote GSC proliferation, other pathways also contribute to regulation of germ cell proliferation. In conclusion, it seems likely that both instructive and permissive signals cooperate to promote GSC proliferation, which in turn regulates fitness of the individual.
Recent investigations that have highlighted how GSC proliferation regulation varies depending on environmental and developmental contexts has greatly improved our understanding of cellular proliferation within a multicellular organism. Nonetheless, several key questions remain incompletely answered. Particularly interesting will be investigations of how favorable nutritional and energetic conditions are signaled to the germ cells throughout the development of the animal, as well as how the germ cells interpret those signals. The GLP-1/Notch signaling pathway is crucial for optimal proliferation of the GSCs, but surprisingly little is known about the molecular regulation of GLP-1 signaling, or about the direct genomic targets of GLP -1/Notch activity. Multiple potentially overlapping mechanisms use RNA regulation to control the balance between mitosis and meiosis, though much remains unexplored about not only the targets of regulation, but also which mechanisms are responsible for each overarching effect. The answers to these questions will help to paint a more complete picture of the regulation of cellular proliferation within the context of a developing multicellular organism.
Acknowledgments
The authors apologize to those whose work was not cited due to space constraints. Related work in the Reinke lab is supported by Connecticut Stem Cell Reseach Grants Program and the March of Dimes.
Abbreviations
- GSC
germ cells
- DTC
distal tip cell
- MZ
mitotic zone
- PGC
primordial germ cell
- SS
sheath/spermathecal cell
- IIR
insulin/IGF-like receptor
- ARD
adult reproductive diapause
References
- Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol. 2006;16:780–785. doi: 10.1016/j.cub.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Berry LW, Westlund B, Schedl T. Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development. 1997;124:925–936. doi: 10.1242/dev.124.4.925. [DOI] [PubMed] [Google Scholar]
- Byrd DT, Kimble J. Scratching the niche that controls Caenorhabditis elegans germline stem cells. Semin Cell Dev Biol. 2009;20:1107–1113. doi: 10.1016/j.semcdb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney MA, Lam N, Morgan DE, Phillips BT, Kimble J. C. elegans HLH-2/E/Daughterless controls key regulatory cells during gonadogenesis. Dev Biol. 2009;331:14–25. doi: 10.1016/j.ydbio.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S, Kodoyianni V, Bosenberg M, Friedman L, Kimble J. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H) Development. 1996;122:1373–1383. doi: 10.1242/dev.122.5.1373. [DOI] [PubMed] [Google Scholar]
- Crittenden SL, Troemel ER, Evans TC, Kimble J. GLP-1 is localized to the mitotic region of the C. elegans germ line. Development. 1994;120:2901–2911. doi: 10.1242/dev.120.10.2901. [DOI] [PubMed] [Google Scholar]
- Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417:660–663. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- Crittenden SL, Leonhard KA, Byrd DT, Kimble J. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol Biol Cell. 2006;17:3051–3061. doi: 10.1091/mbc.E06-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry WB, Bierings R, van Iersel M, Satkunendran T, Reinke V, Rothman JH. Regulation of developmental rate and germ cell proliferation in Caenorhabditis elegans by the p53 gene network. Cell Death Differ. 2007;14:662–670. doi: 10.1038/sj.cdd.4402075. [DOI] [PubMed] [Google Scholar]
- Doyle TG, Wen C, Greenwald I. SEL-8, a nuclear protein required for LIN-12 and GLP-1 signaling in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2000;97:7877–7881. doi: 10.1073/pnas.97.14.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann CR, Crittenden SL, Suh N, Kimble J. GLD-3 and control of the mitosis/meiosis decision in the germline of Caenorhabditis elegans. Genetics. 2004;168:147–160. doi: 10.1534/genetics.104.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell. 2004;16:509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Fukuyama M, Rougvie AE, Rothman JH. C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr Biol. 2006;16:773–779. doi: 10.1016/j.cub.2006.02.073. [DOI] [PubMed] [Google Scholar]
- Gartner A, Boag PR, Blackwell TK. Germline survival and apoptosis. WormBook; 2008. pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D, Schedl T. The regulatory network controlling the proliferation-meiotic entry decision in the Caenorhabditis elegans germ line. Curr Top Dev Biol. 2006;76:185–215. doi: 10.1016/S0070-2153(06)76006-9. [DOI] [PubMed] [Google Scholar]
- Hansen D, Hubbard EJ, Schedl T. Multi-pathway control of the proliferation versus meiotic development decision in the Caenorhabditis elegans germline. Dev Biol. 2004;268:342–357. doi: 10.1016/j.ydbio.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Hubbard EJ. Caenorhabditis elegans germ line: A model for stem cell biology. Dev Dyn. 2007;236:3343–3357. doi: 10.1002/dvdy.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Lambert A, Ellefson M, Villeneuve AM, Engebrecht J. Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev Biol. 2007;308:206–221. doi: 10.1016/j.ydbio.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Jin SW, Kimble J, Ellis RE. Regulation of cell fate in Caenorhabditis elegans by a novel cytoplasmic polyadenylation element binding protein. Dev Biol. 2001;229:537–553. doi: 10.1006/dbio.2000.9993. [DOI] [PubMed] [Google Scholar]
- Kadyk LC, Kimble J. Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development. 1998;125:1803–1813. doi: 10.1242/dev.125.10.1803. [DOI] [PubMed] [Google Scholar]
- Kang JS, Zhang W, Krauss RS. Hedgehog signaling: Cooking with Gas1. Sci STKE. 2007;2007:e50. doi: 10.1126/stke.4032007pe50. [DOI] [PubMed] [Google Scholar]
- Kerins JA, Hanazawa M, Dorsett M, Schedl T. PRP-17 and the pre-mRNA splicing pathway are preferentially required for the proliferation versus meiotic development decision and germ-line sex determination in Caenorhabditis elegans. Dev Dyn. 2010;239:1555–1572. doi: 10.1002/dvdy.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian DJ, Hubbard EJ. C. elegans pro-1 activity is required for soma/germline interactions that influence proliferation and differentiation in the germ line. Development. 2004;131:1267–1278. doi: 10.1242/dev.01002. [DOI] [PubMed] [Google Scholar]
- Killian DJ, Hubbard EJ. Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev Biol. 2005;279:322–335. doi: 10.1016/j.ydbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Germline proliferation and its control. WormBook; 2005. pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korta DZ, Hubbard EJ. Soma-germline interactions that influence germline proliferation in Caenorhabditis elegans. Dev Dyn. 2010;239:1449–1459. doi: 10.1002/dvdy.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudron MM, Reinke V. C. elegans nucleostemin is required for larval growth and germline stem cell division. PLoS Genet. 2008;4:e1000181. doi: 10.1371/journal.pgen.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara PE, Lee MH, Schedl T, Jefferis GS. A C. elegans patched gene, ptc-1, functions in germ-line cytokinesis. Genes Dev. 2000;14:1933–1944. [PMC free article] [PubMed] [Google Scholar]
- Lamont LB, Crittenden SL, Bernstein D, Wickens M, Kimble J. FBF-1 and FBF-2 regulate the size of the mitotic region in the C. elegans germline. Dev Cell. 2004;7:697–707. doi: 10.1016/j.devcel.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Liu Y, Maine EM. The Bro1-domain protein, EGO-2, promotes Notch signaling in Caenorhabditis elegans. Genetics. 2007;176:2265–2277. doi: 10.1534/genetics.107.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald LD, Knox A, Hansen D. Proteasomal regulation of the proliferation vs. meiotic entry decision in the Caenorhabditis elegans germ line. Genetics. 2008;180:905–920. doi: 10.1534/genetics.108.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J, Ugel N, Mishra B, Isopi M, Hubbard EJ. Quantitative analysis of germline mitosis in adult C. elegans. Dev Biol. 2006;292:142–151. doi: 10.1016/j.ydbio.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Mango SE, Maine EM, Kimble J. Carboxy-terminal truncation activates glp-1 protein to specify vulval fates in Caenorhabditis elegans. Nature. 1991;352:811–815. doi: 10.1038/352811a0. [DOI] [PubMed] [Google Scholar]
- Mantina P, MacDonald L, Kulaga A, Zhao L, Hansen D. A mutation in teg-4, which encodes a protein homologous to the SAP130 pre-mRNA splicing factor, disrupts the balance between proliferation and differentiation in the C. elegans germ line. Mech Dev. 2009;126:417–429. doi: 10.1016/j.mod.2009.01.006. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: Multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev Biol. 1997;181:121–143. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJ. A “latent niche” mechanism for tumor initiation. Proc Natl Acad Sci USA. 2009;106:11617–11622. doi: 10.1073/pnas.0903768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137:671–680. doi: 10.1242/dev.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajan S, Govindan JA, McGovern M, Hubbard EJ, Greenstein D. MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans. Development. 2009;136:2223–2234. doi: 10.1242/dev.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne P, Roy R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development. 2006;133:611–619. doi: 10.1242/dev.02232. [DOI] [PubMed] [Google Scholar]
- Pepper AS, Killian DJ, Hubbard EJ. Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics. 2003a;163:115–132. doi: 10.1093/genetics/163.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper AS, Lo TW, Killian DJ, Hall DH, Hubbard EJ. The establishment of Caenorhabditis elegans germline pattern is controlled by overlapping proximal and distal somatic gonad signals. Dev Biol. 2003b;259:336–350. doi: 10.1016/s0012-1606(03)00203-3. [DOI] [PubMed] [Google Scholar]
- Petcherski AG, Kimble J. LAG-3 is a putative transcriptional activator in the C. elegans Notch pathway. Nature. 2000;405:364–368. doi: 10.1038/35012645. [DOI] [PubMed] [Google Scholar]
- Qiao L, Lissemore JL, Shu P, Smardon A, Gelber MB, Maine EM. Enhancers of glp-1, a gene required for cell-signaling in Caenorhabditis elegans, define a set of genes required for germline development. Genetics. 1995;141:551–569. doi: 10.1093/genetics/141.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racher H, Hansen D. Translational control in the C. elegans hermaphrodite germ line. Genome. 2010;53:83–102. doi: 10.1139/g09-090. [DOI] [PubMed] [Google Scholar]
- Ruaro EM, Collavin L, Del Sal G, Haffner R, Oren M, Levine AJ, Schneider C. A proline-rich motif in p53 is required for transactivation-independent growth arrest as induced by Gas1. Proc Natl Acad Sci USA. 1997;94:4675–4680. doi: 10.1073/pnas.94.9.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruaro ME, Stebel M, Vatta P, Marzinotto S, Schneider C. Analysis of the domain requirement in Gas1 growth suppressing activity. FEBS Lett. 2000;481:159–163. doi: 10.1016/s0014-5793(00)02005-6. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Schedl T, Greenwald I. Cell-cell interactions prevent a potential inductive interaction between soma and germline in C. elegans. Cell. 1990;61:939–951. doi: 10.1016/0092-8674(90)90060-r. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Seydoux G. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans. Development. 1999;126:4861–4871. doi: 10.1242/dev.126.21.4861. [DOI] [PubMed] [Google Scholar]
- Suh N, Crittenden SL, Goldstrohm A, Hook B, Thompson B, Wickens M, Kimble J. FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics. 2009;181:1249–1260. doi: 10.1534/genetics.108.099440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BE, Bernstein DS, Bachorik JL, Petcherski AG, Wickens M, Kimble J. Dose-dependent control of proliferation and sperm specification by FOG-1/CPEB. Development. 2005;132:3471–3481. doi: 10.1242/dev.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development. 2004;131:5807–5815. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- Voutev R, Killian DJ, Ahn JH, Hubbard EJ. Alterations in ribosome biogenesis cause specific defects in C. elegans hermaphrodite gonadogenesis. Dev Biol. 2006;298:45–58. doi: 10.1016/j.ydbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Waters K, Yang AZ, Reinke V. Genome-wide analysis of germ cell proliferation in C. elegans identifies VRK-1 as a key regulator of CEP-1/p53. Dev Biol. 2010;344:1011–1025. doi: 10.1016/j.ydbio.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki TM, Berman JR, Suchanek-Kavipurapu M, McCormick M, Maria Gaglia M, Lee SJ, Kenyon C. The somatic reproductive tissues of C. elegans promote longevity through steroid hormone signaling. PLoS Biol. 2010:8. doi: 10.1371/journal.pbio.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]