Abstract
The ideal cancer treatment would specifically target cancer cells yet have minimal or no adverse effects on normal somatic cells. Telomerase, the ribonucleoprotein reverse transcriptase that maintains the ends of human chromosome, is an attractive cancer therapeutic target for exactly this reason [1]. Telomerase is expressed in more than 85% of cancer cells, making it a nearly universal cancer marker, while the majority of normal somatic cells are telomerase negative. Telomerase activity confers limitless replicative potential to cancer cells, a hallmark of cancer which must be attained for the continued growth that characterizes almost all advanced neoplasms [2]. In this review we will summarize the role of telomeres and telomerase in cancer cells, and how properties of telomerase are being exploited to create targeted cancer therapies including telomerase inhibitors, telomerase-targeted immunotherapies and telomerase-driven virotherapies. A frank and balanced assessment of the current state of telomerase inhibitors with caveats and potential limitations will be included.
Keywords: Senescence, Telomeres, Virotherapy, Immunotherapy
1. Telomere structure and function
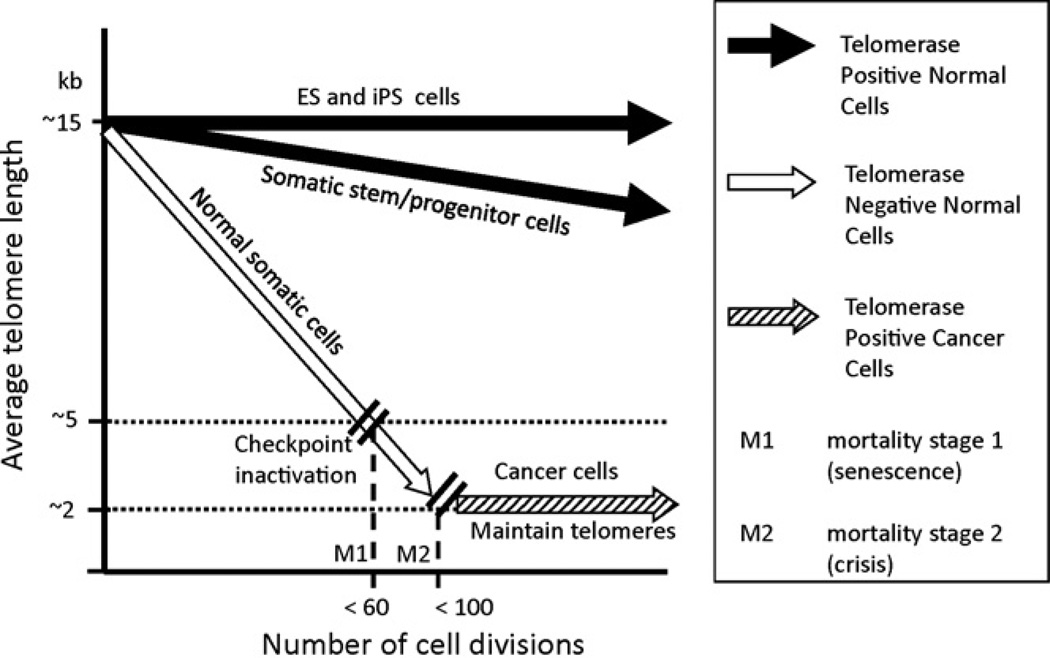
Human telomeres consist of tandem double stranded hexameric DNA repeats (TTAGGGn) at the ends of each chromosome [3], with a 3′ G-rich single-stranded overhang. The ends of telomeres form a lariat-like structure called a t-loop [4], which is postulated to be formed by strand invasion of the 3′ single strand overhang into the preceding double stranded telomeric DNA that is then stabilized by bound telomeric proteins [5]. A six-protein complex known as “shelterin” has remarkable specificity for telomeres and some members of the complex specifically bind telomeric DNA. The shelterin component proteins function to maintain telomere length, promote t-loop formation, recruit telomerase to telomeric ends, and to protect the ends of chromosomes from being recognized as DNA damage [6–8]. In cells that lack telomerase, telomeres shorten with each cell division due to incomplete replication of the lagging strand, oxidative damage, and telomere processing events that are yet to be fully understood [9–11]. After a finite number of cell divisions, cells lacking telomerase enter a state of growth arrest called replicative senescence. Telomere attrition likely leads to cellular senescence because of a disruption of their ability to protect chromosome ends from DNA damage and repair mechanisms. Functional telomeres must evade both ATM and ATR checkpoint pathways, both of which respond to DNA double strand breaks at telomeres [12–15]. In normal cells that lack telomerase, telomere attrition leads to critically short telomeres which trigger a localized DNA damage response [12,16–19] and p53-mediated cell cycle arrest [12,16,18,20,21]. It is possible that telomeres reach a critically short length that is no longer competent to assemble into the t-loop structure, and that even a few “uncapped” telomeres in a cell is sufficient to initiate replicative senescence [17]. However, cells that have acquired an inactivation of common cell cycle checkpoint proteins in the p53 and p16/Rb pathways are able to continue dividing (termed extended lifespan) and continue to lose telomeric sequences with each division [19,22]. These cells ultimately reach a crisis stage [23,24] which is marked by genomic instability due to chromosome end fusions and increased aneuploidy, and leads to p53-independent apoptosis [25,26]. Cancer cells which are able to maintain their telomeres must escape this crisis stage in order to continue to proliferate without limits. Escape from crisis is a rare event in human cells and thus senescence (also called M1) and crisis (M2) likely serve a potent anti-cancer mechanisms in long-lived species such as humans (Fig. 1) [23,24,27]. While telomerase expression is the mechanism for telomere maintenance in the vast majority of cancers there is at least one other documented telomere maintenance mechanism termed ALT (alternative lengthening of telomeres), which permits cells to escape crisis by DNA recombination [28–32].
Fig. 1.
Comparison of telomere lengths to cell divisions: human embryonic stem cells (ES) and perhaps induced pluripotent stem cells (iPS) have robust telomerase activity and may completely maintain telomere lengths. Somatic stem cells/progenitors that transiently express telomerase or only express moderate amounts of telomerase partially, but not completely, maintain telomeres. With increased age and cell divisions, telomeres continue to shorten in these cells. Normal somatic cells which do not express telomerase have the greatest rate of telomere shortening with each cell division. Cancer cells which reactivate or upregulate telomerase fully maintain telomeres but generally at greatly reduced lengths.
2. Telomerase
Telomerase activity is not detected in most somatic cells, with the exception of some adult pluripotent stem cells, proliferative cells of renewal tissues, and male germline cells [33]. However, telomerase activity is nearly universal in human cancer cell lines, and is found in about 85–90% of primary tumors [29]. Telomerase activity counteracts progressive telomere shortening during cellular replication by synthesizing new telomeric DNA repeats at the chromosomal termini [34]. The human telomerase holoenzyme core components are a catalytic reverse transcriptase called hTERT (human telomerase reverse transcriptase) [35,36], and an associated template RNA called hTR or hTERC (human Telomerase RNA) [36–39]. hTR is ubiquitously expressed in all human cells, and telomerase activity is limited by the expression of hTERT, which is only found in cells with detectable telomerase activity [40,41]. Telomerase extends telomeres by binding to the 3′ single stranded overhang at the telomere end, catalyzing the addition of a single telomere repeat, and translocating to the new terminus. This processive cycle continues until the holoenzyme dissociates from the telomere [42,43]. In addition to the two core components of the telomerase holoenzyme complex, there are many confirmed and putative telomerase-associating proteins. The size of the human telomerase holoenzyme is approximately 1.5 mDa, which is significantly larger than the expected mass of a single hTR and hTERT (approximately 280 kD) [44]. A recent purification strategy was designed to isolate only catalytically active telomerase, revealing that only one additional component, dyskerin, was required for in vitro telomerase activity [45]. While other telomerase-associated proteins may not be necessary for catalytic activity, they may play a role in holoenzyme stability, recruitment or regulation.
Telomerase levels must be limiting for cells to maintain normal telomere length homeostasis, demonstrated by the continued elongation of telomeres when both hTERT and hTR are overexpressed in human primary and cancer cell lines [46]. Telomerase levels are regulated at every level of protein and RNA processing, as well as at the level of complex assembly and subcellular localization [42,43]. Telomerase activity at the telomere is also regulated at the level of telomerase recruitment to the telomere. While the exact mechanism of telomerase recruitment is still not fully known, it is likely part of a negative feedback loop created by shelterin proteins bound at the telomere that serve as negative regulators of telomerase extension of telomeres (reviewed in [8,47]). The prevailing model of shelterin-mediated telomere length regulation is that longer telomeres recruit more shelterin complex, which in turn limits future telomerase elongation. This negative feedback loop is thought to be responsible for the stable telomere length found in cancer cells, and is likely responsible for at least partially maintaining telomere length homeostasis in germ cells and other stem-like cells in which telomerase is active [8,48]. Thus, there is likely to be mechanisms regulating telomerase recruitment or processivity depending on whether cells are at equilibrium conditions versus non-equilibrium conditions.
Telomerase activity is detected and measured using a PCR-based assay called the Telomere Repeat Amplification Protocol, or TRAP [29,49,50]. The TRAP assay is very sensitive, and can detect telomerase activity from a wide range of sample sizes, from small tissue biopsies to a just a few cancer cells [51]. PCR-amplified telomerase reaction products from the TRAP assay can be semi-quantitated by normalizing the signal to an internal standard (ITAS) that accounts for variability in PCR efficiency. TRAP analysis of a large panel of primary normal versus tumor samples, as well as mortal versus immortalized cell lines, revealed that telomerase activity is restricted in normal human somatic cells but is activated in cancer and immortalized cells [29]. The approximately 10% of cancer cells that do not upregulate telomerase have been found either to be “mortal” tumor cells or to maintain their telomeres through a telomere recombination pathway called ALT (alternative lengthening of telomeres) [52]. The ability of cancer cells to replicate indefinitely through telomerase activation or ALT pathway activation has been called one of the hallmarks of cancer [2]. Indeed, tumors which have short telomeres and no detectable telomerase activity are self-limiting, and almost always regress spontaneously [53,54]. These results indicate that a telomere maintenance mechanism is necessary for tumor progression, and suggest that telomerase is an attractive target for selective cancer therapies. We will discuss three independent approaches to target telomerase expressing cells, their current status, and limitations to their use.
3. Telomerase inhibition
A fundamental quality of cancer cells is their capacity to replicate without limits, which is achieved by telomerase-mediated telomere maintenance in the majority of advanced tumors [19,55]. Thus, telomerase inhibitors have the potential to be used as a selective anti-cancer therapy which disrupts the replicative capacity of telomerase-positive cancer cells [2]. Normal somatic cells which do not utilize telomerase activity to maintain telomere length would be largely unaffected, as normal cells which are telomerase competent almost always have longer telomeres compared to telomerase-positive cancer cells [33]. This may provide a therapeutic window where tumor cells could be driven to apoptosis before normal telomerase competent cells are adversely affected. However, this generally indicates that inhibitors of telomerase will not be well suited without tumor debulking. The template region of the telomerase RNA (hTR) offers an accessible substrate for direct enzymatic inhibition using oligonucleotide-based small molecule inhibitors [56–71]. While much still needs to be learned regarding the conformation, composition and recruitment of the catalytically active telomerase holoenzyme, it is certain that the template region of hTR must be exposed and accessible in order to synthesize de novo telomere repeats. Thus, oligonucleotides that can hybridize to the 11-base hTR template region act as competitive telomerase inhibitors (not antisense targeting messenger RNA). One such compound that has been developed is GRN163L, currently known as Imetelstat, is a lipidated N3′–P5′ thio-phosphoramidate 13-mer. The thio-phosphoramidate backbone causes the oligonucleotide to be water soluble, acid stable, nuclease resistant and to form stable RNA duplexes [61,64,72–74]. The 5′ palmitoyl moiety of GRN163L causes the compound to be lipid soluble, allowing for cellular uptake without the use of lipophilic carriers and improving telomerase inhibition [64,65,68]. The sequence of GRN163L (5′-palmitate-TAGGGTTAGACAA-NH2-3′) targets a 13 nucleotide region of hTR, preventing it from forming an active complex with hTERT.
The effects of GRN163L have been investigated in a number of cancer cell lines and mouse xenograft models. Chronic exposure to GRN163L has been shown to inhibit telomerase and cause telomere shortening in cancer cell lines derived from diverse origins, including tumors of the brain, breast, bladder, liver, lung, prostate and stomach [56,61,66,68,75–78]. GRN163L-induced telomere shortening initiates cellular crisis caused by chromosomal fusions, anaphase bridges and subsequent apoptosis [61]. In mice with human tumor xenografts, GRN163L was well-tolerated and induced telomerase inhibition in doses ranging from 5 mg/kg to 1000 mg/kg [61]. Xenograft models showed that GRN163L works to inhibit tumor growth, prevent growth of metastases, and sensitizes tumors to conventional chemotherapy agents [61,68]. GRN163L also is able to cross the blood-brain barrier to target glioblastoma xenograft tumors, supporting the further study of using telomerase inhibition alone or in combination with conventional therapies in glioblastoma patients [77]. GRN163L has already completed several Phase I trials in patients with chronic lymphocytic leukemia and solid tumors such as breast cancer and non small cell lung cancer [79]. These Phase I trials showed that intravenously infused GRN163L has excellent bioavailability, pharmacokinetics and tolerability, and after dose-escalation studies, a dose of 9.4 mg/kg was chosen for Phase II clinical trials. Phase II trials of GRN163L alone, in combination with other chemotherapy and targeted drugs, or in a maintenance setting after standard chemotherapy are now being conducted for patients with NSCLC, advanced breast cancer, chronic leukemia, essential thrombocythemia and multiple myeloma (Table 1).
Table 1.
List of ongoing human clinical trials using a variety of approaches to targeting telomerase.
| Trial identifier | Condition | Intervention | Phase |
|---|---|---|---|
| NCT00124189 | Chronic lymphoproliferative diseases | Imetelstat (GRN163L) | I |
| NCT01242930 | Multiple myeloma | Imetelstat (GRN163L) | II |
| NCT01256762 | Locally recurrent or metastatic breast cancer | Imetelstat (GRN163L) + bevacizumab + paclitaxel | II |
| NCT01137968 | Non-small cell lung cancer | Imetelstat (GRN163L) + bevacizumab | II |
| NCT01265927 | Breast neoplasms | Imetelstat (GRN163L) + trastuzumab | I |
| NCT01243073 | Essential thrombocythemia | Imetelstat (GRN163L) | II |
| NCT00021164 | Melanoma adult solid tumor | Aldesleukin + incomplete Freund’s adjuvant + telomerase 540–548 peptide vaccine | II |
| NCT00069940 | Brain and central nervous system tumors; gastrointestinal stromal tumor; sarcoma | Telomerase 540–548 peptide vaccine + sargramostim | I |
| NCT00509457 | Carcinoma, non-small-cell lung | GV 1001 telomerase peptide | |
| NCT01247623 | Malignant melanoma | GV 1001 telomerase peptide + temozolomide | I, II |
| NCT00061035 | Prostatic neoplasms | Anti-telomerase transgenic lymphocyte immunization vaccine (TLI) | I |
| NCT00197912 | Advanced melanoma | Tumor antigen loaded autologous dendritic cells | I, II |
| NCT00925314 | Stage III melanoma | Anti-telomerase transgenic lymphocyte immunization | II |
| NCT00079157 | Breast cancer | Incomplete Freund’s adjuvant + telomerase 540–548 peptide vaccine + sargramostim | I |
| NCT00425360 | Pancreatic cancer | Sargramostim + telomerase peptide vaccine GV1001 + Capecitabine + gemcitabine | III |
| NCT00573495 | Breast cancer | hTERT/survivin multi-peptide vaccine | I |
| NCT00197860 | Advanced renal cell carcinoma | Tumor antigen loaded autologous dendritic cells | I, II |
| NCT00510133 | Acute myelogenous leukemia | GRNVAC1 | II |
| NCT01153113 | Metastatic prostate cancer | GRNVAC1 | I, II |
Because telomerase inhibitors may require a period of treatment to produce telomeres short enough to trigger cancer cell death, telomerase inhibition therapy may be most effective when used in conjunction with conventional chemotherapies, radiation or other targeted therapeutics such as angiogenic inhibitors. The long lag phase between initiating telomerase inhibition and induction of cancer cell death may allow for increasing the tumor mass, and is likely to be inefficient at reducing tumor bulk as an individual treatment. In addition, there may be some unknown off-target effects of Imetelstat. In contrast, conventional chemotherapy approaches result in immediate tumor mass reduction without effecting telomere length causing tumors to often develop treatment resistance and eventually there is recurrence of disease (Fig. 2). Using a telomerase inhibitor such as GRN163L in combination with conventional cancer therapies should cause progressive telomere shortening in cancer cells that are not initially susceptible to combination treatment, theoretically leading to a more durable response and decreased disease recurrence (Fig. 2). Telomerase inhibitor therapy may also be used as a maintenance therapy following conventional chemotherapy, radiation or surgery to extend survival with reduced side effects of long-term treatment (Fig. 2). Yet another potential use for telomerase inhibitors is as chemotherapy and/or a radiation sensitizing agent, enabling the efficient use of lower doses of chemotherapy drugs and radiation.
Fig. 2.
Comparisons of chemotherapy alone to combining chemotherapy with a telomerase inhibitor. Due to some toxicities in combining therapies in phase I clinical trials, a phase II trial is in progress using the telomerase inhibitor, Imetelstat, in a maintenance setting following standard chemotherapy.
An important factor in whether telomerase inhibitors such as GRN163L are able to limit cancer recurrence and relapse is whether the drug is able to target residual stem-like cancer cells. A cancer stem cell is defined as one that can self-renew, initiate tumor formation and regenerate all of the cell types found in a tumor [80]. There is increasing evidence that rare cancer stem cell populations which are refractory to conventional treatments are responsible for initiation and recurrence in a variety of hematologic and solid tumors [81–83]. While conventional therapies may not target these cancer stem cells, progressive telomere shortening induced by combination or maintenance treatment with telomerase inhibitors would potentially impair their self-renewal properties. Recent studies have shown that telomerase inhibitors target cancer stem cell populations in multiple myeloma, prostate, brain, breast and pancreatic cancer [77,78,84–86]. Cancer stem cell populations were reduced in size [78,85,86], had reduced proliferation [84], shortened telomeres [78,86], and impaired ability to form characteristic free-floating spherical colonies [77,85] after treatment with the GRN163L telomerase inhibitor. These studies indicate that telomerase inhibition does disrupt cancer stem cell self renewal, and supports the hypothesis that telomerase inhibition will be a viable maintenance treatment to decrease disease recurrence following other therapies.
4. Telomerase-targeted immunotherapy
Several telomerase-based immunotherapy strategies have been developed and many are in advanced clinical trials, making this a rapidly-progressing field of anti-telomerase cancer therapy [87–101]. Telomerase is an attractive target antigen for cancer immunotherapy because it is expressed almost universally in human cancers and is functionally required to sustain malignant tumor long-term growth [87]. In brief, anti-telomerase immunotherapy sensitizes immune cells to tumor cells expressing hTERT peptides as surface antigens via the human leukocyte antigen (HLA) class I pathway [102]. This causes an expansion of telomerase-specific CD8+ cytotoxic T lymphocytes (CTLs), directing the patient’s own immune system to target and kill telomerase positive tumor cells [101,103]. The immune response can be induced by exposure to antigen presenting cells that either overexpress immunogenic hTERT fragments [92] or have been pulsed with immunogenic hTERT peptides. To date, 26 different hTERT peptides have been utilized to elicit an anti-telomerase immune response [88,97,101,102,104–111]. Vaccination studies using the I540–548 peptide have produced functional anti-tumor responses in prostate, breast and melanoma patients [89,96,112]. The hTERT E611–626 peptide is a promiscuous HLA class II epitope [91] that can be administered without HLA typing patients, making it a good candidate to stimulate CD4+ T lymphocytes and generate a general (perhaps universal) cancer vaccine. This peptide is available as an injectable vaccine, GV1001. GV1001 has been shown to induce hTERT-specific T cell responses in Phase I/II clinical trials for non-small cell lung cancer [91] and non-resectable pancreatic carcinoma [113]. GV1001 is currently in a randomized Phase III clinical trial in patients with locally advanced or metastatic pancreatic cancer (ClinicalTrials.gov Identifier: NCT00425360) with results expected in 2012. With objective responses in the randomized trial, GV1001 could become the first approved anti-telomerase based cancer therapy. Despite promising earlier trials with GV1001, a recent small trial with GV1001 did not show clinical efficacy. In this study none of six patients with cutaneous T cell lymphomas showed positive responses and one patient even had progressive disease leading the trial to end early (J Dermatological Science 62 (2011) 75–83). So as with all peptide immunotherapies, it is still too early to know if this will become an approved therapy for cancer.
Another anti-telomerase vaccine, GRNVAC1, utilizes autologous mobilized immature dendritic cells isolated from the patient’s own circulating blood which are then transduced ex vivo with mRNAs encoding a near full-length hTERT protein. These primed dendritic cells display a multitude of hTERT fragments and are then matured ex vivo and returned back to patients to elicit a polyclonal anti-hTERT T cell response [92]. GRNVAC1 has completed Phase II clinical trials in patients with acute myelogenous leukemia and metastatic prostate cancer, and the vaccine has thus far proven to be well-tolerated and produce ahTERT-specific immune response in a significant fraction of patients [personal communications]. While the ex vivo immunotherapy trials may be more effective compared to peptide immunotherapies, they are quite expensive trials and require much more regulatory surveillance.
In summary, the current results of these anti-telomerase cancer immunotherapy clinical trials are encouraging. Multiple vaccine strategies are being developed and tested in patients with melanoma, lung, prostate, breast, and pancreatic cancers [89,91,92,96,99,112,113], and these trials have generally produced a specific immune response against hTERT positive tumor cells. While telomerase is expressed in some normal tissues [33,114,115], no patients have exhibited serious adverse effects (such as autoimmune disease or bone marrow depletion) indicative of an immune response against normal cells. One explanation is that normal cells express very low levels of hTERT [115], making them poor targets relative to tumor cells with high levels of hTERT expression. Another possibility is that hTERT peptides are differentially expressed on the surface of tumor and normal cells. Further studies are needed to better understand the regulation of the degradation, processing and presentation of hTERT antigens on tumor and normal cells. Nevertheless, current results indicate that induction of hTERT-specific immune responses is safe and well-tolerated and the use of anti-telomerase immunotherapies in cancer treatment regimens is promising. Additional studies are needed to address the processing, presentation and response elicited by hTERT epitopes, and future clinical studies are needed as well as the ones in progress that should help clarify the effect of telomerase-targeted immunotherapies on tumor regression.
5. Telomerase-directed viral therapy
While the RNA component of human telomerase is ubiquitously expressed in all human cells, the hTERT promoter is very tightly regulated and telomerase activity is generally not detected (e.g. the hTERT promoter is silent) in most normal cells [42,116,117]. Telomerase positive tumor cells can thus be directly targeted by introducing suicide genes or oncolytic viruses driven by the hTERT promoter. One approach that has been reported involves using the hTERT promoter to drive the expression of a pro-apoptotic “suicide” gene (such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [118] or caspase 6 [119]). The hTERT-driven suicide gene is then introduced to a tumor where expression of the suicide gene in telomerase-positive cells induces apoptosis. In an alternate version of this telomerase-driven gene therapy, tumors are provided with ahTERT-driven prodrug-activating enzyme [120–124], which results in apoptosis of telomerase-positive cells when administered in conjunction with a prodrug that is metabolized into a toxin.
A second strategy which utilizes the specificity of the hTERT promoter directs the replication of a lytic adenovirus in telomerase-positive cells [125]. The modified hTERT promoter driven adenovirus only expresses the viral proteins E1A and E1B, required for viral replication, when present in telomerase-positive cells [126–131]. Pre-clinical studies showed selective targeting and effective cytolysis of telomerase positive cells derived from a variety of human cancer cell lines, while normal telomerase-negative cells showed reduced viral replication and cytotoxicity. Furthermore, mouse xenograft studies showed that telomerase-specific viral therapy reduced the size of xenograft tumor, retarded tumor growth and extended survival [126–131]. Following direct injection of the adenoviral vector into the xenograft tumor, viral particles were found replicating in distant tumor tissues, suggesting that the viral particles can circulate in blood and lymph to target distant metastases [125,127]. These results encouraged the design of a Phase I clinical trial using the OBP-301 virus, also known as Telomelysin [132]. This study enrolled sixteen patients with a variety of advanced solid tumors, and determined that a single intratumoral injection of Telomelysin was well tolerated at all doses and generated anti-tumor activity [132]. Based on these results, Phase II trials of Telomelysin are expected, and second-generation viruses that combine telomerase targeted viral replication and suicide gene expression are being developed [133–135]. The use of telomerase-specific viral therapy in combination with irradiation and chemotherapy such as paclitaxel, cisplatin and gemcitabine is also being examined [136–139]. These studies should determine if oncolytic viruses have potential as a broad-spectrum cancer therapy. However, it should be noted that most previously attempted adenoviral-based cancer therapies have failed to provide robust tumor responses.
6. Concluding remarks
Telomerase is considered an almost universal target for human cancers since telomerase-mediated telomere maintenance is the mechanism employed by a vast majority of cancer cells to enable limitless proliferation. In this review, we discussed three separate approaches to specifically target telomerase-expressing cancer cells, and clinical trials are currently underway to establish the utility of these strategies as cancer therapies (Table 1). Current and ongoing results suggest that telomerase-based therapies have an increasing potential to become an important component of future cancer treatment regimens.
Acknowledgements
NCI SPORE P50 CA70907 and CA127297 (J.W.S.) and a DOD BCRP predoctoral award (C.M.B.) are acknowledged. While there are no conflicts of interest, J.W.S. and W.E.W. were former SAB members of Geron Corporation.
Abbreviations
- ALT
alternative lengthening of telomeres
- hTERT
human telomerase reverse transcriptase
- hTR
human telomerase RNA
- TRAP
telomere repeat amplification protocol
- CTL
cytotoxic T lymphocytes
- NSCLC
non small cell lung cancer
- HLA
human leukocyte antigen
Footnotes
Conflict of interest
The authors declare there is no conflict of interest.
References
- 1.Shay JW, Wright WE. Telomerase: a target for cancer therapeutics. Cancer Cell. 2002;2:257–265. doi: 10.1016/s1535-6108(02)00159-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. U. S. A. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 5.Greider CW. Telomeres do D-loop-T-loop. Cell. 1999;97:419–422. doi: 10.1016/s0092-8674(00)80750-3. [DOI] [PubMed] [Google Scholar]
- 6.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 7.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 8.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 9.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat. Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 10.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 11.Shore D, Bianchi A. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J. 2009;28:2309–2322. doi: 10.1038/emboj.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 13.Silverman J, Takai H, Buonomo SB, Eisenhaber F, de Lange T. Human Rif1, ortholog of a yeast telomeric protein, is regulated by ATM and 53BP1 and functions in the S-phase checkpoint. Genes Dev. 2004;18:2108–2119. doi: 10.1101/gad.1216004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol. Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 15.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 16.Fagagna FdAd, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 17.Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol. Biol. Cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- 19.Shay JW, Wright WE. Telomerase activity in human cancer. Curr. Opin. Oncol. 1996;8:66–71. doi: 10.1097/00001622-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 20.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 21.Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 22.Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp. Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 23.Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp. Gerontol. 1992;27:383–389. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- 24.Wright WE, Pereira-Smith OM, Shay JW. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol. Cell. Biol. 1989;9:3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macera-Bloch L, Houghton J, Lenahan M, Jha KK, Ozer HL. Termination of lifespan of SV40-transformed human fibroblasts in crisis is due to apoptosis. J. Cell. Physiol. 2002;190:332–344. doi: 10.1002/jcp.10062. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Mar V, Zhou W, Harrington L, Robinson MO. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- 28.Shay JW, Wright WE, Brasiskyte D, Van der Haegen BA. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene. 1993;8:1407–1413. [PubMed] [Google Scholar]
- 29.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 30.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur. J. Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 32.Reddel RR. Alternative lengthening of telomeres, telomerase, and cancer. Cancer Lett. 2003;194:155–162. doi: 10.1016/s0304-3835(02)00702-4. [DOI] [PubMed] [Google Scholar]
- 33.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–579. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- 35.Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 37.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 38.Harrington L, Zhou W, McPhail T, Oulton R, Yeung DS, Mar V, Bass MB, Robinson MO. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11:3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 40.Ducrest AL, Szutorisz H, Lingner J, Nabholz M. Regulation of the human telomerase reverse transcriptase gene. Oncogene. 2002;21:541–552. doi: 10.1038/sj.onc.1205081. [DOI] [PubMed] [Google Scholar]
- 41.Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558–1561. [PubMed] [Google Scholar]
- 42.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol. Mol. Biol. Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. (table of contents). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelleher C, Teixeira MT, Forstemann K, Lingner J. Telomerase: biochemical considerations for enzyme and substrate. Trends Biochem. Sci. 2002;27:572–579. doi: 10.1016/s0968-0004(02)02206-5. [DOI] [PubMed] [Google Scholar]
- 44.Schnapp G, Rodi HP, Rettig WJ, Schnapp A, Damm K. One-step affinity purification protocol for human telomerase. Nucleic Acids Res. 1998;26:3311–3313. doi: 10.1093/nar/26.13.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 46.Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006;25:565–574. doi: 10.1038/sj.emboj.7600952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Boeck G, Forsyth RG, Praet M, Hogendoorn PC. Telomere-associated proteins: cross-talk between telomere maintenance and telomere-lengthening mechanisms. J. Pathol. 2009;217:327–344. doi: 10.1002/path.2500. [DOI] [PubMed] [Google Scholar]
- 48.Forsyth NR, Wright WE, Shay JW. Telomerase and differentiation in multi-cellular organisms: turn it off, turn it on, and turn it off again. Differentiation. 2002;69:188–197. doi: 10.1046/j.1432-0436.2002.690412.x. [DOI] [PubMed] [Google Scholar]
- 49.Herbert BS, Hochreiter AE, Wright WE, Shay JW. Nonradioactive detection of telomerase activity using the telomeric repeat amplification protocol. Nat. Protoc. 2006;1:1583–1590. doi: 10.1038/nprot.2006.239. [DOI] [PubMed] [Google Scholar]
- 50.Wright WE, Shay JW, Piatyszek MA. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995;23:3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hess JL, Highsmith WE., Jr Telomerase detection in body fluids. Clin. Chem. 2002;48:18–24. [PubMed] [Google Scholar]
- 52.Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 53.Henson JD, Neumann AA, Yeager TR, Reddel RR. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21:598–610. doi: 10.1038/sj.onc.1205058. [DOI] [PubMed] [Google Scholar]
- 54.Hiyama E, Hiyama K, Yokoyama T, Matsuura Y, Piatyszek MA, Shay JW. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat. Med. 1995;1:249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- 55.Ouellette MM, Choi KH. Encyclopedia of Life Sciences. Chichester, UK: John Wiley & Sons Ltd.; 2007. Telomeres and telomerase in ageing and cancer. ( http://www.els.net/). [Google Scholar]
- 56.Dikmen ZG, Wright WE, Shay JW, Gryaznov SM. Telomerase targeted oligonucleotide thio-phosphoramidates in T24-luc bladder cancer cells. J. Cell. Biochem. 2008;104:444–452. doi: 10.1002/jcb.21635. [DOI] [PubMed] [Google Scholar]
- 57.Shay JW, Wright WE. Mechanism-based combination telomerase inhibition therapy. Cancer Cell. 2005;7:1–2. doi: 10.1016/j.ccr.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 58.White LK, Wright WE, Shay JW. Telomerase inhibitors. Trends Biotechnol. 2001;19:114–120. doi: 10.1016/s0167-7799(00)01541-9. [DOI] [PubMed] [Google Scholar]
- 59.Hamilton SE, Pitts AE, Katipally RR, Jia X, Rutter JP, Davies BA, Shay JW, Wright WE, Corey DR. Identification of determinants for inhibitor binding within the RNA active site of human telomerase using PNA scanning. Biochemistry. 1997;36:11873–11880. doi: 10.1021/bi970438k. [DOI] [PubMed] [Google Scholar]
- 60.Norton JC, Piatyszek MA, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase activity by peptide nucleic acids. Nat. Biotechnol. 1996;14:615–619. doi: 10.1038/nbt0596-615. [DOI] [PubMed] [Google Scholar]
- 61.Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, Wright WE, Shay JW. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65:7866–7873. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
- 62.Gryaznov SM, Jackson S, Dikmen G, Harley C, Herbert BS, Wright WE, Shay JW. Oligonucleotide conjugate GRN163L targeting human telomerase as potential anticancer and antimetastatic agent. Nucleosides Nucleotides Nucleic Acids. 2007;26:1577–1579. doi: 10.1080/15257770701547271. [DOI] [PubMed] [Google Scholar]
- 63.Jackson SR, Zhu CH, Paulson V, Watkins L, Dikmen ZG, Gryaznov SM, Wright WE, Shay JW. Antiadhesive effects of GRN163L—an oligonucleotide N3′ → P5′ thio-phosphoramidate targeting telomerase. Cancer Res. 2007;67:1121–1129. doi: 10.1158/0008-5472.CAN-06-2306. [DOI] [PubMed] [Google Scholar]
- 64.Gellert GC, Dikmen ZG, Wright WE, Gryaznov S, Shay JW. Effects of a novel telomerase inhibitor, GRN163L, in human breast cancer. Breast Cancer Res. Treat. 2006;96:73–81. doi: 10.1007/s10549-005-9043-5. [DOI] [PubMed] [Google Scholar]
- 65.Herbert BS, Gellert GC, Hochreiter A, Pongracz K, Wright WE, Zielinska D, Chin AC, Harley CB, Shay JW, Gryaznov SM. Lipid modification of GRN163, an N3′ → P5′ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene. 2005;24:5262–5268. doi: 10.1038/sj.onc.1208760. [DOI] [PubMed] [Google Scholar]
- 66.Hochreiter AE, Xiao H, Goldblatt EM, Gryaznov SM, Miller KD, Badve S, Sledge GW, Herbert BS. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin. Cancer Res. 2006;12:3184–3192. doi: 10.1158/1078-0432.CCR-05-2760. [DOI] [PubMed] [Google Scholar]
- 67.Gomez-Millan J, Goldblatt EM, Gryaznov SM, Mendonca MS, Herbert BS. Specific telomere dysfunction induced by GRN163L increases radiation sensitivity in breast cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:897–905. doi: 10.1016/j.ijrobp.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 68.Djojosubroto MW, Chin AC, Go N, Schaetzlein S, Manns MP, Gryaznov S, Harley CB, Rudolph KL. Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology. 2005;42:1127–1136. doi: 10.1002/hep.20822. [DOI] [PubMed] [Google Scholar]
- 69.Ozawa T, Gryaznov SM, Hu LJ, Pongracz K, Santos RA, Bollen AW, Lamborn KR, Deen DF. Antitumor effects of specific telomerase inhibitor GRN163 in human glioblastoma xenografts. Neuro-oncol. 2004;6:218–226. doi: 10.1215/S1152851704000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang ES, Wu K, Chin AC, Chen-Kiang S, Pongracz K, Gryaznov S, Moore MA. Telomerase inhibition with an oligonucleotide telomerase template antagonist: in vitro and in vivo studies in multiple myeloma and lymphoma. Blood. 2004;103:258–266. doi: 10.1182/blood-2003-02-0546. [DOI] [PubMed] [Google Scholar]
- 71.Akiyama M, Hideshima T, Shammas MA, Hayashi T, Hamasaki M, Tai YT, Richardson P, Gryaznov S, Munshi NC, Anderson KC. Effects of oligonucleotide N3′ → P5′ thio-phosphoramidate (GRN163) targeting telomerase RNA in human multiple myeloma cells. Cancer Res. 2003;63:6187–6194. [PubMed] [Google Scholar]
- 72.Herbert BS, Pongracz K, Shay JW, Gryaznov SM, Shea-Herbert B. Oligonucleotide N3′ → P5′ phosphoramidates as efficient telomerase inhibitors. Oncogene. 2002;21:638–642. doi: 10.1038/sj.onc.1205064. [DOI] [PubMed] [Google Scholar]
- 73.Asai A, Oshima Y, Yamamoto Y, Uochi TA, Kusaka H, Akinaga S, Yamashita Y, Pongracz K, Pruzan R, Wunder E, Piatyszek M, Li S, Chin AC, Harley CB, Gryaznov S. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res. 2003;63:3931–3939. [PubMed] [Google Scholar]
- 74.Gryaznov S, Pongracz K, Matray T, Schultz R, Pruzan R, Aimi J, Chin A, Harley C, Shea-Herbert B, Shay J, Oshima Y, Asai A, Yamashita Y. Telomerase inhibitors–oligonucleotide phosphoramidates as potential therapeutic agents. Nucleosides Nucleotides Nucleic Acids. 2001;20:401–410. doi: 10.1081/NCN-100002314. [DOI] [PubMed] [Google Scholar]
- 75.Shammas MA, Qazi A, Batchu RB, Bertheau RC, Wong JY, Rao MY, Prasad M, Chanda D, Ponnazhagan S, Anderson KC, Steffes CP, Munshi NC, De Vivo I, Beer DG, Gryaznov S, Weaver DW, Goyal RK. Telomere maintenance in laser capture microdissection-purified Barrett’s adenocarcinoma cells and effect of telomerase inhibition in vivo. Clin. Cancer Res. 2008;14:4971–4980. doi: 10.1158/1078-0432.CCR-08-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shammas MA, Koley H, Bertheau RC, Neri P, Fulciniti M, Tassone P, Blotta S, Protopopov A, Mitsiades C, Batchu RB, Anderson KC, Chin A, Gryaznov S, Munshi NC. Telomerase inhibitor GRN163L inhibits myeloma cell growth in vitro and in vivo. Leukemia. 2008;22:1410–1418. doi: 10.1038/leu.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marian CO, Cho SK, McEllin BM, Maher EA, Hatanpaa KJ, Madden CJ, Mickey BE, Wright WE, Shay JW, Bachoo RM. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin. Cancer Res. 2010;16:154–163. doi: 10.1158/1078-0432.CCR-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marian CO, Wright WE, Shay JW. The effects of telomerase inhibition on prostate tumor-initiating cells. Int. J. Cancer. 2010;127:321–331. doi: 10.1002/ijc.25043. [DOI] [PubMed] [Google Scholar]
- 79.Molckovsky A, Siu LL. First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J. Hematol. Oncol. 2008;1:20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 81.Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87–98. doi: 10.3233/bd-2007-26108. [DOI] [PubMed] [Google Scholar]
- 82.Farnie G, Clarke RB. Breast stem cells and cancer. Ernst Schering Found. Symp. Proc. 2006;5:141–153. doi: 10.1007/2789_2007_049. [DOI] [PubMed] [Google Scholar]
- 83.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Brennan SK, Wang Q, Tressler R, Harley C, Go N, Bassett E, Huff CA, Jones RJ, Matsui W. Telomerase inhibition targets clonogenic multiple myeloma cells through telomere length-dependent and independent mechanisms. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joseph I, Tressler R, Bassett E, Harley C, Buseman CM, Pattamatta P, Wright WE, Shay JW, Go NF. The telomerase inhibitor imetelstat depletes cancer stem cells in breast and pancreatic cancer cell lines. Cancer Res. 2010;70:9494–9504. doi: 10.1158/0008-5472.CAN-10-0233. [DOI] [PubMed] [Google Scholar]
- 86.Castelo-Branco P, Zhang C, Lipman T, Fujitani M, Hansford L, Clarke I, Harley CB, Tressler R, Malkin D, Walker E, Kaplan DR, Dirks P, Tabori U. Neural tumor-initiating cells have distinct telomere maintenance and can be safely targeted for telomerase inhibition. Clin. Cancer Res. 2011;17:111–121. doi: 10.1158/1078-0432.CCR-10-2075. [DOI] [PubMed] [Google Scholar]
- 87.Vonderheide RH. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene. 2002;21:674–679. doi: 10.1038/sj.onc.1205074. [DOI] [PubMed] [Google Scholar]
- 88.Vonderheide RH, Anderson KS, Hahn WC, Butler MO, Schultze JL, Nadler LM. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin. Cancer Res. 2001;7:3343–3348. [PubMed] [Google Scholar]
- 89.Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, Stephans KF, Masutomi K, Loda M, Xia Z, Anderson KS, Hahn WC, Nadler LM. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin. Cancer Res. 2004;10:828–839. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]
- 90.Vonderheide RH, Schultze JL, Anderson KS, Maecker B, Butler MO, Xia Z, Kuroda MJ, von Bergwelt-Baildon MS, Bedor MM, Hoar KM, Schnipper DR, Brooks MW, Letvin NL, Stephans KF, Wucherpfennig KW, Hahn WC, Nadler LM. Equivalent induction of telomerase-specific cytotoxic T lymphocytes from tumor-bearing patients and healthy individuals. Cancer Res. 2001;61:8366–8370. [PubMed] [Google Scholar]
- 91.Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S, Moller M, Eriksen JA, Gaudernack G. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2006;55:1553–1564. doi: 10.1007/s00262-006-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E, Vieweg J. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J. Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 93.Carpenter EL, Vonderheide RH. Telomerase-based immunotherapy of cancer. Expert Opin. Biol. Ther. 2006;6:1031–1039. doi: 10.1517/14712598.6.10.1031. [DOI] [PubMed] [Google Scholar]
- 94.Vonderheide RH. Universal tumor antigens for cancer vaccination: targeting telomerase for immunoprevention. Discov. Med. 2007;7:103–108. [PubMed] [Google Scholar]
- 95.Danet-Desnoyers GA, Luongo JL, Bonnet DA, Domchek SM, Vonderheide RH. Telomerase vaccination has no detectable effect on SCID-repopulating and colony-forming activities in the bone marrow of cancer patients. Exp. Hematol. 2005;33:1275–1280. doi: 10.1016/j.exphem.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 96.Domchek SM, Recio A, Mick R, Clark CE, Carpenter EL, Fox KR, DeMichele A, Schuchter LM, Leibowitz MS, Wexler MH, Vance BA, Beatty GL, Veloso E, Feldman MD, Vonderheide RH. Telomerase-specific T-cell immunity in breast cancer: effect of vaccination on tumor immunosurveillance. Cancer Res. 2007;67:10546–10555. doi: 10.1158/0008-5472.CAN-07-2765. [DOI] [PubMed] [Google Scholar]
- 97.Minev B, Hipp J, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4796–4801. doi: 10.1073/pnas.070560797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat. Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 99.Nava-Parada P, Emens LA. GV-1001, an injectable telomerase peptide vaccine for the treatment of solid cancers. Curr. Opin. Mol. Ther. 2007;9:490–497. [PubMed] [Google Scholar]
- 100.Mavroudis D, Bolonakis I, Cornet S, Myllaki G, Kanellou P, Kotsakis A, Galanis A, Nikoloudi I, Spyropoulou M, Menez J, Miconnet I, Niniraki M, Cordopatis P, Kosmatopoulos K, Georgoulias V. A phase I study of the optimized cryptic peptide TERT(572y) in patients with advanced malignancies. Oncology. 2006;70:306–314. doi: 10.1159/000096252. [DOI] [PubMed] [Google Scholar]
- 101.Liu J-P, Chen W, Schwarer AP, Li H. Telomerase in cancer immunotherapy. Biochim. Biophys. Acta. 2010;1805:35–42. doi: 10.1016/j.bbcan.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 102.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 103.Vonderheide RH. Prospects and challenges of building a cancer vaccine targeting telomerase. Biochimie. 2008;90:173–180. doi: 10.1016/j.biochi.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arai J, Yasukawa M, Ohminami H, Kakimoto M, Hasegawa A, Fujita S. Identification of human telomerase reverse transcriptase-derived peptides that induce HLA-A24-restricted antileukemia cytotoxic T lymphocytes. Blood. 2001;97:2903–2907. doi: 10.1182/blood.v97.9.2903. [DOI] [PubMed] [Google Scholar]
- 105.Hernandez J, Garcia-Pons F, Lone YC, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Identification of a human telomerase reverse transcriptase peptide of low affinity for HLA A2.1 that induces cytotoxic T lymphocytes and mediates lysis of tumor cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12275–12280. doi: 10.1073/pnas.182418399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scardino A, Gross DA, Alves P, Schultze JL, Graff-Dubois S, Faure O, Tourdot S, Chouaib S, Nadler LM, Lemonnier FA, Vonderheide RH, Cardoso AA, Kosmatopoulos K. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J. Immunol. 2002;168:5900–5906. doi: 10.4049/jimmunol.168.11.5900. [DOI] [PubMed] [Google Scholar]
- 107.Schroers R, Huang XF, Hammer J, Zhang J, Chen SY. Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-helper cells. Cancer Res. 2002;62:2600–2605. [PubMed] [Google Scholar]
- 108.Schroers R, Shen L, Rollins L, Rooney CM, Slawin K, Sonderstrup G, Huang XF, Chen SY. Human telomerase reverse transcriptase-specific T-helper responses induced by promiscuous major histocompatibility complex class II-restricted epitopes. Clin. Cancer Res. 2003;9:4743–4755. [PubMed] [Google Scholar]
- 109.Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, Faure O, Guillaume P, Firat H, Chouaib S, Lemonnier FA, Davoust J, Miconnet I, Vonderheide RH, Kosmatopoulos K. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J. Clin. Invest. 2004;113:425–433. doi: 10.1172/JCI19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thorn M, Wang M, Kloverpris H, Schmidt EG, Fomsgaard A, Wenandy L, Berntsen A, Brunak S, Buus S, Claesson MH. Identification of a new hTERT-derived HLA-A*0201 restricted, naturally processed CTL epitope. Cancer Immunol. Immunother. 2007;56:1755–1763. doi: 10.1007/s00262-007-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cortez-Gonzalez X, Sidney J, Adotevi O, Sette A, Millard F, Lemonnier F, Langlade-Demoyen P, Zanetti M. Immunogenic HLA-B7-restricted peptides of hTRT. Int. Immunol. 2006;18:1707–1718. doi: 10.1093/intimm/dxl105. [DOI] [PubMed] [Google Scholar]
- 112.Wenandy L, Sørensen RB, Sengeløv L, Svane IM, thor Straten P, Andersen MH. The immunogenicity of the hTERT540-548 peptide in cancer. Clin. Cancer Res. 2008;14:4–7. doi: 10.1158/1078-0432.CCR-07-4590. [DOI] [PubMed] [Google Scholar]
- 113.Bernhardt SL, Gjertsen MK, Trachsel S, Moller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with nonresectable pancreatic cancer: a dose escalating phase I/II study. Br. J. Cancer. 2006;95:1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uchida N, Otsuka T, Shigematsu H, Maeda M, Sugio Y, Itoh Y, Niho Y. Differential gene expression of human telomerase-associated protein hTERT and TEP1 in human hematopoietic cells. Leuk. Res. 1999;23:1127–1132. doi: 10.1016/s0145-2126(99)00149-6. [DOI] [PubMed] [Google Scholar]
- 115.Tahara H, Yasui W, Tahara E, Fujimoto J, Ito K, Tamai K, Nakayama J, Ishikawa F, Ide T. Immuno-histochemical detection of human telomerase catalytic component, hTERT, in human colorectal tumor and non-tumor tissue sections. Oncogene. 1999;18:1561–1567. doi: 10.1038/sj.onc.1202458. [DOI] [PubMed] [Google Scholar]
- 116.Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 117.Wick M, Zubov D, Hagen G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT) Gene. 1999;232:97–106. doi: 10.1016/s0378-1119(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 118.Katz MH, Spivack DE, Takimoto S, Fang B, Burton DW, Moossa AR, Hoffman RM, Bouvet M. Gene therapy of pancreatic cancer with green fluorescent protein and tumor necrosis factor-related apoptosis-inducing ligand fusion gene expression driven by a human telomerase reverse transcriptase promoter. Ann. Surg. Oncol. 2003;10:762–772. doi: 10.1245/aso.2003.01.021. [DOI] [PubMed] [Google Scholar]
- 119.Komata T, Kondo Y, Kanzawa T, Hirohata S, Koga S, Sumiyoshi H, Srinivasula SM, Barna BP, Germano IM, Takakura M, Inoue M, Alnemri ES, Shay JW, Kyo S, Kondo S. Treatment of malignant glioma cells with the transfer of constitutively active caspase-6 using the human telomerase catalytic subunit (human telomerase reverse transcriptase) gene promoter. Cancer Res. 2001;61:5796–5802. [PubMed] [Google Scholar]
- 120.Liu J, Zou WG, Lang MF, Luo J, Sun LY, Wang XN, Qian QJ, Liu XY. Cancer-specific killing by the CD suicide gene using the human telomerase reverse transcriptase promoter. Int. J. Oncol. 2002;21:661–666. [PubMed] [Google Scholar]
- 121.Majumdar AS, Hughes DE, Lichtsteiner SP, Wang Z, Lebkowski JS, Vasserot AP. The telomerase reverse transcriptase promoter drives efficacious tumor suicide gene therapy while preventing hepatotoxicity encountered with constitutive promoters. Gene Ther. 2001;8:568–578. doi: 10.1038/sj.gt.3301421. [DOI] [PubMed] [Google Scholar]
- 122.Plumb JA, Bilsland A, Kakani R, Zhao J, Glasspool RM, Knox RJ, Evans TR, Keith WN. Telomerase-specific suicide gene therapy vectors expressing bacterial nitroreductase sensitize human cancer cells to the pro-drug CB1954. Oncogene. 2001;20:7797–7803. doi: 10.1038/sj.onc.1204954. [DOI] [PubMed] [Google Scholar]
- 123.Schepelmann S, Ogilvie LM, Hedley D, Friedlos F, Martin J, Scanlon I, Chen P, Marais R, Springer CJ. Suicide gene therapy of human colon carcinoma xenografts using an armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer Res. 2007;67:4949–4955. doi: 10.1158/0008-5472.CAN-07-0297. [DOI] [PubMed] [Google Scholar]
- 124.Zhou JH, Tang B, Liu XL, He DW, Yang DT. hTERT-targeted E. coli purine nucleoside phosphorylase gene/6-methylpurine deoxyribose therapy for pancreatic cancer. Chin. Med. J. (Engl.) 2007;120:1348–1352. [PubMed] [Google Scholar]
- 125.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Irving J, Wang Z, Powell S, O’Sullivan C, Mok M, Murphy B, Cardoza L, Lebkowski JS, Majumdar AS. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Ther. 2004;11:174–185. doi: 10.1038/sj.cgt.7700666. [DOI] [PubMed] [Google Scholar]
- 127.Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, Taki M, Kyo S, Tanaka N, Fujiwara T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004;10:285–292. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- 128.Lanson NA, Jr, Friedlander PL, Schwarzenberger P, Kolls JK, Wang G. Replication of an adenoviral vector controlled by the human telomerase reverse transcriptase promoter causes tumor-selective tumor lysis. Cancer Res. 2003;63:7936–7941. [PubMed] [Google Scholar]
- 129.Wirth T, Zender L, Schulte B, Mundt B, Plentz R, Rudolph KL, Manns M, Kubicka S, Kuhnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181–3188. [PubMed] [Google Scholar]
- 130.Fujita K, Kimura M, Kondo N, Sakakibara A, Sano D, Ishiguro Y, Tsukuda M. Anti-tumor effects of telomelysin for head and neck squamous cell carcinoma. Oncol. Rep. 2008;20:1363–1368. [PubMed] [Google Scholar]
- 131.Nakajima O, Ichimaru D, Urata Y, Fujiwara T, Horibe T, Kohno M, Kawakami K. Use of telomelysin (OBP-301) in mouse xenografts of human head and neck cancer. Oncol. Rep. 2009;22:1039–1043. doi: 10.3892/or_00000533. [DOI] [PubMed] [Google Scholar]
- 132.Nemunaitis J, Tong AW, Nemunaitis M, Senzer N, Phadke AP, Bedell C, Adams N, Zhang YA, Maples PB, Chen S, Pappen B, Burke J, Ichimaru D, Urata Y, Fujiwara T. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol. Ther. 2010;18:429–434. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hioki M, Kagawa S, Fujiwara T, Sakai R, Kojima T, Watanabe Y, Hashimoto Y, Uno F, Tanaka N. Combination of oncolytic adenovirotherapy and Bax gene therapy in human cancer xenografted models. Potential merits and hurdles for combination therapy. Int. J. Cancer. 2008;122:2628–2633. doi: 10.1002/ijc.23438. [DOI] [PubMed] [Google Scholar]
- 134.Liao Z, Huang C, Zhou F, Xiong J, Bao J, Zhang H, Sun W, Xie C, Zhou Y. Radiation enhances suicide gene therapy in radioresistant laryngeal squamous cell carcinoma via activation of a tumor-specific promoter. Cancer Lett. 2009;283:20–28. doi: 10.1016/j.canlet.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 135.Zhang JF, Wei F, Wang HP, Li HM, Qiu W, Ren PK, Chen XF, Huang Q. Potent anti-tumor activity of telomerase-dependent and HSV-TK armed oncolytic adenovirus for non-small cell lung cancer in vitro and in vivo. J. Exp. Clin. Cancer Res. 2010;29:52. doi: 10.1186/1756-9966-29-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuroda S, Fujiwara T, Shirakawa Y, Yamasaki Y, Yano S, Uno F, Tazawa H, Hashimoto Y, Watanabe Y, Noma K, Urata Y, Kagawa S. Telomerase-dependent oncolytic adenovirus sensitizes human cancer cells to ionizing radiation via inhibition of DNA repair machinery. Cancer Res. 2010;70:9339–9348. doi: 10.1158/0008-5472.CAN-10-2333. [DOI] [PubMed] [Google Scholar]
- 137.Kondo N, Tsukuda M, Kimura M, Fujita K, Sakakibara A, Takahashi H, Ishiguro Y, Toth G, Matsuda H. Antitumor effects of telomelysin in combination with paclitaxel or cisplatin on head and neck squamous cell carcinoma. Oncol. Rep. 2010;23:355–363. [PubMed] [Google Scholar]
- 138.Takakura M, Nakamura M, Kyo S, Hashimoto M, Mori N, Ikoma T, Mizumoto Y, Fujiwara T, Urata Y, Inoue M. Intraperitoneal administration of telomerase-specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther. 2010;17:11–19. doi: 10.1038/cgt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu D, Kojima T, Ouchi M, Kuroda S, Watanabe Y, Hashimoto Y, Onimatsu H, Urata Y, Fujiwara T. Preclinical evaluation of synergistic effect of telomerase-specific oncolytic virotherapy and gemcitabine for human lung cancer. Mol. Cancer Ther. 2009;8:980–987. doi: 10.1158/1535-7163.MCT-08-0901. [DOI] [PubMed] [Google Scholar]