Abstract
The benzyloxymethyl (BOM) group has been utilized widely in syntheses of a variety of natural and non-natural products. The BOM group is also one of few choices to protect uridine ureido nitrongen. However, hydrogenolytic cleavage of the BOM group of uridine derivatives has been unrealizably performed via heterogeneous conditions using Pd catalysts. One of the undesirable by-products formed by Pd-mediated hydrogenation conditions is the over-reduced product of which the C5–C6 double bond of the uracil moiety was saturated. To date, we have generated a wide range of uridine-containing antibacterial agents, where the BOM group has been utilized in their syntheses. In screening of deprotection conditions of the BOM group of uridine ureido nitrogen under Pd-mediated hydrogenation conditions, we realized that the addition of water to the iPrOH-based hydrogenation conditions can suppress the formation of over-reduced uridine derivatives and the addition of HCO2H (0.5%) dramatically improve the reaction rate. An optimized hydrogenation condition described here can be applicable to the BOM-deprotections of a wide range of uridine derivatives.
Keywords: Ureido nitrogen, Uridine, Benzyloxymethyl (BOM) group, Hydrogenolytic cleavage, Pd-C, MraY inhibitors
1. Introduction
To date, a large number of uridine-containing natural products that show significant biological activities have been isolated from the culture of Streptomyces spp.1
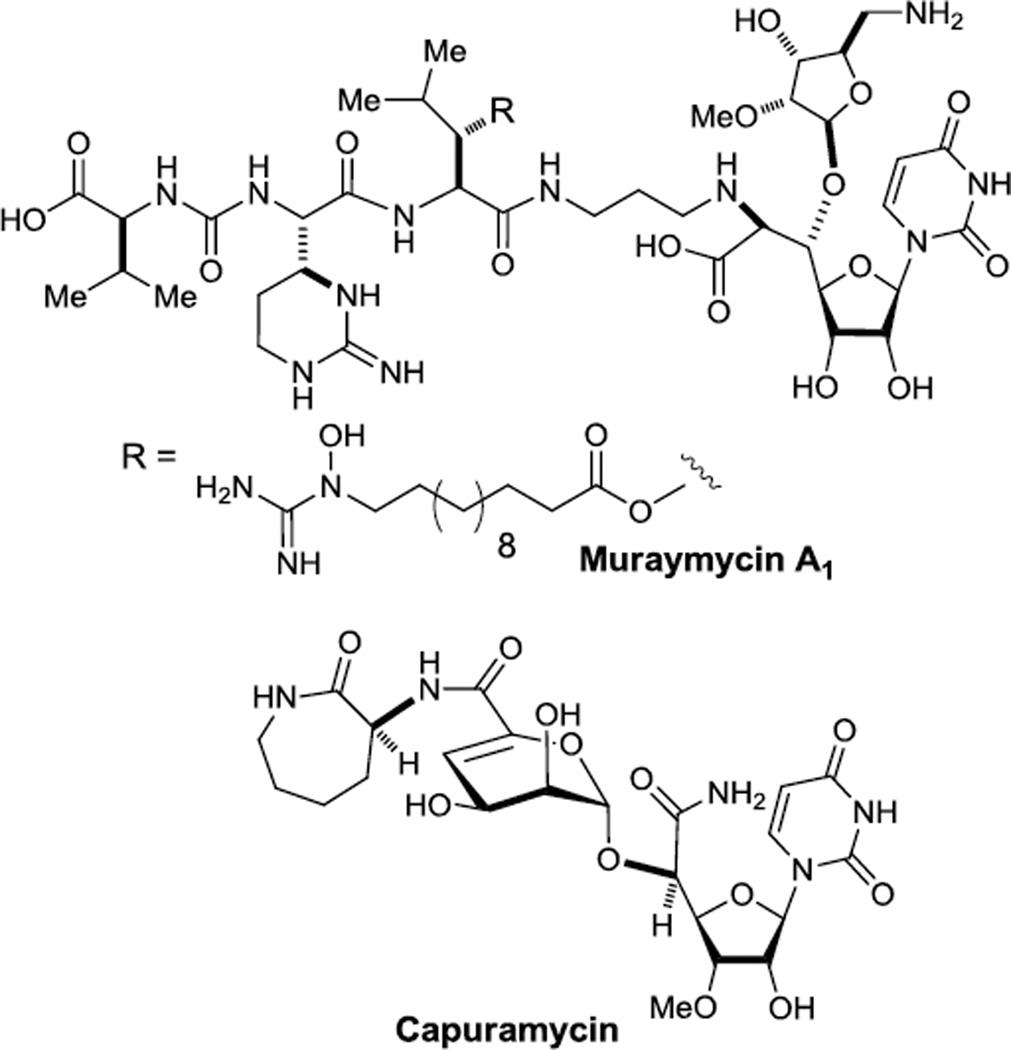
Uridine-containing antibiotics such as the muraymycins and capuramycin have gained great interest in the development of new antibacterial agents for multidrug (MDR)-bacterial infections.2 In our ongoing program of discovery of new MraY (catalyzing the biosynthesis of lipid I from UDP-N-acyl-Murpentapeptide) inhibitors for MDR-resistant Mycobacterium tuberculosis,3 we have generated a 200-membered library molecules based on the core structures of muraymycins and capuramycin (Figure 1). In their syntheses we utilized the benzyloxymethyl (BOM) group as a convenient protecting group for the uridine ureido nitrogen of the uracil moiety.4
Figure 1.
Muraymycin A1 and capuramycin.
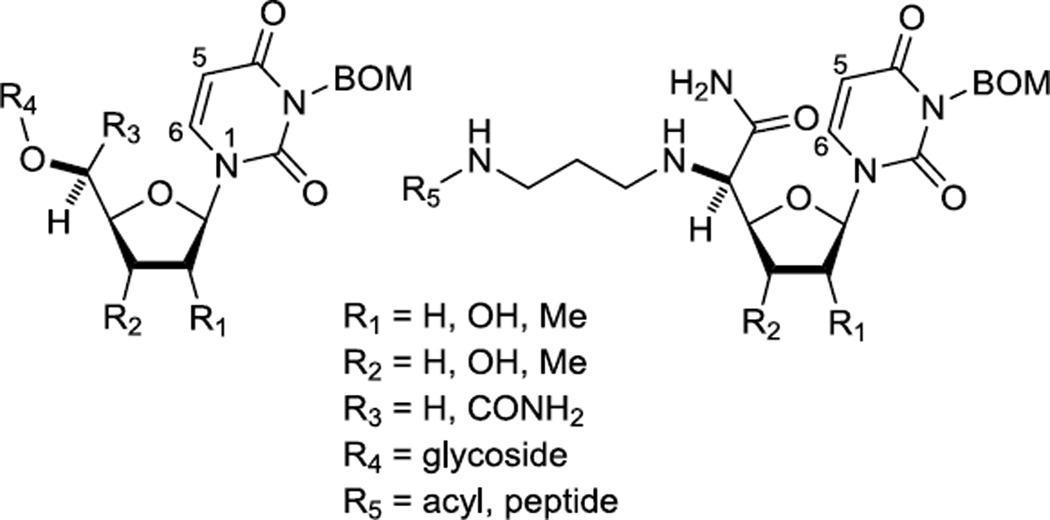
However, we encountered that success of deprotection of the BOM group of the uridine derivatives vary depending on the structure and physico-chemical properties of uridine derivatives. Chemical structures of representative key intermediates are shown in Figure 2. Various hydrogenation conditions for the deprotection of BOM protecting group have been reported in literatures using Pd catalysts in alcoholic solvents.5 Recently, Ducho and co-workers reported that in hydrogenolytic deprotection of the BOM group of the uridine derivative the over-reduction of the C5–C6 double of the uracil moiety could be minimized (~5%) by using Pd-black in MeOH in the presence of nBuNH2.6 However, in our studies addition of primary amines to suppress the over-reduction of the uracil double bond during deprotections of the BOM group resulted in generation of the desired product in capricious yields with significant amounts of the over-reduced product(s).7
Figure 2.
Representative structures of the key intermediates for the syntheses of MraY inhibitors.
In order to identify versatile hydrogenolytic deprotection conditions of the BOM group of uridine derivatives that do not produce the by-products (vide supra), we have screened hydrogenation conditions by varying Pd sources in a wide range of solvent systems. Herein, we report a simple but reliable hydrogenolytic condition that remove the BOM and Bn groups of uridine derivatives without affecting the C5–C6 double bond of the uracil moiety.
2. Results and discussion
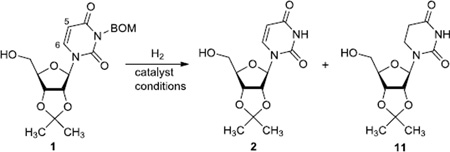
We have screened hydrogenolytic BOM-deprotection conditions with Pd-C, Pd black, Pd(OH)2, and PtO2 that convert 1 to 2 without contamination of the over-reduced product 11. A series of hydrogenation conditions examined are summarized in Table 1. Hydrogenation of 1 with 10% Pd-C in MeOH provided a mixture of 1 and 2 with predominantly 2 (entry 1). The same reaction with Pd-black, or Pd(OH)2 in MeOH resulted in the formation of a large amount of the over-reduced product 11 (entries 2 and 3). PtO2-Catalyzed hydrogenation reaction in MeOH gave 11 exclusively (entry 4). Significant solvent effect on the BOM deprotection with 10% Pd-C was observed. Reaction rate in EtOH or EtOAc or iPrOH were much slower than that in MeOH (entries 5–7). However, the 2/11 selectivity ratio was significantly improved in the reaction in EtOAc or iPrOH. It was found that the reaction in iPrOH-H2O (10/1) yielded the desired product 2 in 25% yield (>95% yield based on recovering 1) after 6h without contamination of 11 (entry 8). The conversion of 2 from 1 was slightly improved under medium pressure conditions (50 psi) (entry 9). In order to accelerate the Pd-C mediated hydrogenolytic cleavage of the BOM group of 1 in iPrOH-H2O (10/1), the effect of additives was investigated. All attempts with water tolerate Lewis acids such as CeCl3•7H2O and La(OTf)3 gave rise to the formation of the by-product 11 (data not shown in Table 1). The addition of HCO2NH4 to the reaction mixture did not noticeably improve the reaction rate, and resulted in the formation of 2 and 11 (80/20 ratio) in 90% yield after 12h (entry 10). It was found that the addition of (CF3)2CHOH was very effective in the selective BOM-deprotection of 1; under these conditions 1 could be converted to 2 in 90% yield after 12h (entry 11). Addition of AcOH (0.5%) was also effective in the selective deprotection of the BOM-group of 1 (entry 12), but the reaction was not completed in 6h. The Pd-C catalyzed hydrogenation in iPrOH-H2O (10/1) was dramatically accelerated by addition of HCO2H (0.5%); the reaction was completed within 6h to afford 2 in quantitative yield (entry 13). It was realized that under the conditions of entry 13, hydrogenolytic cleavage of the BOM group of 1 was completed even in 2h (entry 14). The reaction rate was increased under a medium pressure condition (50 psi) to yield 2 without contamination of the by-product 11 (entry 15).
Table 1.
Screening of conditions for hydrogenolytic cleavage of the BOM group of 1.
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | conditions | time (h) | ratio (2/11) | yield (%) |
| 1 | 10% Pd/C | MeOHa | 6 | 20/80 | >95 |
| 2 | Pd black | MeOHa | 6 | 10/90 | >95 |
| 3 | Pd(OH)2 | MeOHa | 6 | 5/95 | >95 |
| 4 | PtO2 | MeOHa | 6 | 0/100 | >95 |
| 5 | 10% Pd/C | EtOHa | 6 | 30/70 | 50 |
| 6 | 10% Pd/C | EtOAca | 6 | 95/5 | <10 |
| 7 | 10% Pd/C | iPrOHa | 6 | 95/5 | 25 |
| 8 | 10% Pd/C | iPrOH-H2O (10/1)a | 6 | 100/0 | 25 |
| 9 | 10% Pd/C | iPrOH-H2O (10/1) 50 psi | 6 | 100/0 | 30~40 |
| 10 | 10% Pd/C | iPrOH-H2O (10/1)a HCO2 NH4 (0.5%) | 12 | 80/20 | 90 |
| 11 | 10% Pd/C | iPrOH-H2O (10/1)a (CF3)2 CHOH (0.5%) | 12 | 100/0 | 90 |
| 12 | 10% Pd/C | iPrOH-H2O (10/1)a AcOH (0.5%) | 6 | 100/0 | 90 |
| 13 | 10% Pd/C | iPrOH-H2O (10/1)a HCO2H (0.5%) | 6 | 100/0 | >99 |
| 14 | 10% Pd/C | iPrOH-H2O (10/1)a HCO2H (0.5%) | 2 | 100/0 | >99 |
| 15 | 10% Pd/C | iPrOH-H2O (10/1) HCO2H (0.5%) 50 psi | 0.5 | 100/0 | >99 |
The reaction was conducted at 1atm H2 pressure.
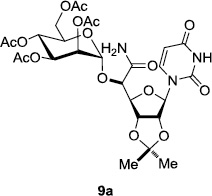
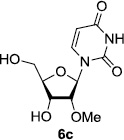
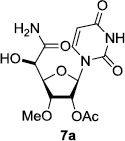
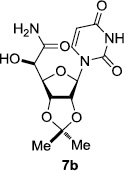
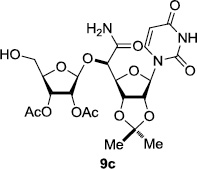
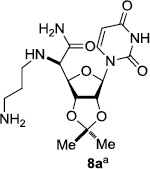
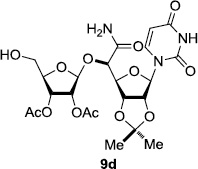
In order to obtain more insight into the optimized deprotection conditions (H2 (1atm), 10% Pd-C / iPrOH-H2O (10/1), HCO2H (0.5%)) for the BOM-protecting group using 1, we have applied these conditions to a wide variety of the BOM-protected uridine derivatives. Selected examples are summarized in Table 2. Simultaneous deprotections of the BOM and Bn groups of 1b and 1c using the conditions utilized in entries 1~3 in Table 1 resulted in the formation of the desired products 6b and 6c in very poor yields (0~20%) with the formation of a large amount of the over-reduced products in which the uracil moiety was saturated with H2. On the contrary, simultaneous hydrogenolytic cleavages of the BOM and Bn groups of 1b and 1c using the optimized conditions (H2 (1atm), 10% Pd-C / iPrOH-H2O (10/1), HCO2H (0.5%)) gave rise to the corresponding products 6b and 6c in quantitative yield (entries 1 and 2 in Table 2). Deprotections of the molecules, 2a and 2b, possessing the α-hydroxyamide functional group furnished the desired products 7a and 7b, respectively in quantitative yield within 3h. Significantly, the 1,3-diamino derivatives 3a and 3b could be converted to the corresponding BOM-deprotected diamine 8a and 8b, respectively, after a basic work-up to remove the HCO2H salts. On the other hand, hydrogenolytic deprotection of the BOM and Cbz groups of 3a with 10% Pd/C in MeOH resulted in an incomplete reaction mixture containing the BOM protected-8a in 90% yield even after prolonged reaction time. We have observed that conversion of the BOM-deptrotections are very for the BOM-protected uridines possessing free-amine in molecules. Thus, BOM-deprotection was not observed for 3a with 10% Pd/C in MeOH due to the fact that Cbz deprotection occurred prior to the BOM deprotection to form the free amine. However, a deactivation mechanism of the Pd catalyst by the complexation with the free amine is diminished in the Pd-mediated hydrogenation reactions by using a water containing solvent. Hydrogenolytic cleavage of the BOM group of the mannosides 4a using the optimized condition gave rise to 9a in 97% yield. In general, deprotections of the O-Cbz group require relatively long hydrogenation time even with the conditions of 10% Pd-C or Pd-back in MeOH in the presence of AcOH at 100 psi.8 The optimized conditions utilized in Table 2 could convert 4a to 9b in 95% yield within 12h without contamination of the over-reduced product. Similarly, the BOM group of the riboside 4c and the BOM and O-Cbz group of 4d could be efficiently deprotected to furnish 9c and 9d in high yield. The BOM- and N-Cbz-groups of the amino-ribosyl uridine 5a were deprotected via the optimized hydrogenolytic cleavage conditions (10% Pd-C in iPrOH-H2O (10/1) in the presence of HCO2H (0.5%)) to provide 10a in 97% within 3h. In our screening of hydrogenation conditions with Pd species for the simultaneous deprotections of 5a, the only conditions demonstrated in Table 2 provided the desired product 10a in high yield without affecting the uracil double bond.
Table 2.
Hydrogenolytic cleavage of the BOM group of uridine derivatives.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | starting material | product | reaction time (h) |
yield (%) |
entry | starting material | product | reaction time (h) |
yield (%) |
| 1 | 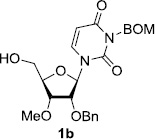 |
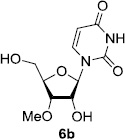 |
12 | >99 | 7 | 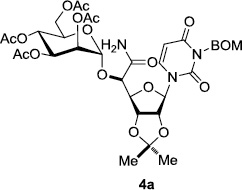 |
 |
3 | 97 |
| 2 | 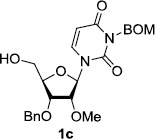 |
 |
12 | 95 | 8 | 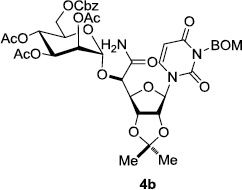 |
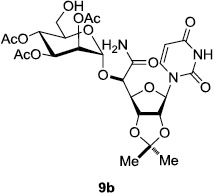 |
12 | 95 |
| 3 | 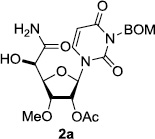 |
 |
3 | >99 | |||||
| 4 | 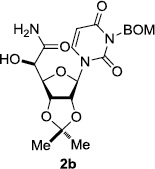 |
 |
3 | >99 | 9 | 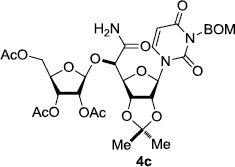 |
 |
3 | 97 |
| 5 | 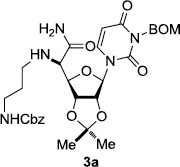 |
 |
3 | 92 | 10 | 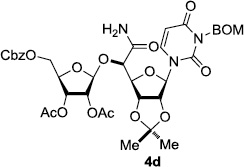 |
 |
12 | 97 |
| 6 | 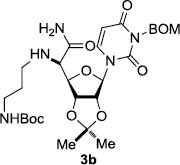 |
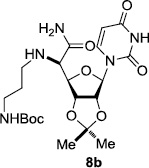 |
3 | >99 | 11 | 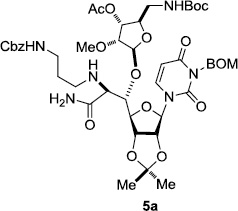 |
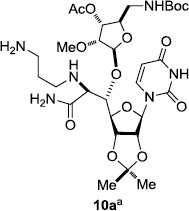 |
3 | 97 |
The product was basified to decomplex the HCO2H salt.
In summary, we have demonstrated hydrogenolytic cleavage reactions of the BOM group of a series of complex uridine derivatives with simple hydrogenation conditions (10% Pd-C in iPrOH-H2O (10/1) in the presence of HCO2H (0.5%) at 1 atm H2 pressure).9 Under these conditions, the BOM-protected uridines were cleanly converted to the corresponding deprotected uridines without formation of the by-products where the uracil C5–C6-double bond was saturated. Unlike the reported conditions to suppress the generation of the over-reduced uracil products by using amines,10 the components in the hydrogenation conditions reported here can readily be removed after the reaction by filtration followed by simple evaporation of all volatiles. Thus, an optimized hydrogenation condition reported here has a beneficial in systematic deprotections of the BOM group to generate a series of uridine derivatives. As we have demonstrated deprotections of the Bn, N-Cbz, and O-Cbz groups during the BOM-deprotection reactions, the hydrogenation conditions summarized in Table 2 should be applicable to debenzylations of a wide range of compounds possessing multiple benzyl groups in a single molecule.
Acknowledgments
The authors thank the National Institutes of Health (NIAID grant AI084411-02) and The University of Tennessee for generous financial supports. NMR data were obtained on instruments supported by the NIH Shared Instrumentation Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Yamaguchi H, Sato S, Yoshida S, Takeda K, Itoh M, Seto H, Otake N. J. Antibiot. 1986;39:1047–1053. doi: 10.7164/antibiotics.39.1047. [DOI] [PubMed] [Google Scholar]; (b) McDonald LA, Barbieri LR, carter GT, Lenoy E, Lotvin J, Peterson PJ, Siegel MM, Singh G, Williamson RT. J. Am. Chem. Soc. 2002;124:10260–10261. doi: 10.1021/ja017748h. [DOI] [PubMed] [Google Scholar]; (c) Muramatsu Y, Ishii MM, Inukai M. J. Antibiot. 2003;56:253–258. doi: 10.7164/antibiotics.56.253. [DOI] [PubMed] [Google Scholar]; (d) Igrashi M, Nakagawa M, Doi S, Hattori N, Naganwa H, Hamada M. J. Antibiot. 2003;56:580–583. doi: 10.7164/antibiotics.56.580. [DOI] [PubMed] [Google Scholar]; (e) Kimura K, Bugg TDH. Nat. Prod. Rep. 2003;20:252–273. doi: 10.1039/b202149h. [DOI] [PubMed] [Google Scholar]; (f) Bryskier A, Dini AC. Agents: Antibacter. Antifung. 2005:377–398. [Google Scholar]; (g) Timothy DH, Lloyd AJ, Roper DI. Infect. Disorders: Drug Targets. 2006;6:85–106. doi: 10.2174/187152606784112128. [DOI] [PubMed] [Google Scholar]; (h) Murakami R, Fujita Y, Kizuka M, Kagawa T, Muramatsu Y, Miyakoshi S, Takatsu T, Inukai M. J. Antibiot. 2007;60:690–695. doi: 10.1038/ja.2007.88. [DOI] [PubMed] [Google Scholar]; (i) Winn M, Goss RJM, Kimura K, Bugg TDH. Nat. Prod. Rep. 2010;27:279–304. doi: 10.1039/b816215h. [DOI] [PubMed] [Google Scholar]; (j) Guillemette C, Levesque E, Harvey M, Bellemare J, Menard V. Drug Metabo. Rev. 2010;42:24–44. doi: 10.3109/03602530903210682. [DOI] [PubMed] [Google Scholar]; (k) Yamamoto T, Koyama H, Kurajoh M, Shoji T, Tsutsumi Z, Moriwaki Y. Clinica Chimica Acta. 2011;412:1712–1724. doi: 10.1016/j.cca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 2.(a) Fang X, Gibbs BS, Coward JK. Bioorg. Med. Chem. Lett. 1995;5:2701–2706. [Google Scholar]; (b) Boojamra CG, Lemoine RC, Blais J, Vernier NG, Stein KA, Magon A, Chamberland S, Hecker SJ, Lee VJ. Bioorg. Med. Chem. Lett. 2003;13:3305–3309. doi: 10.1016/s0960-894x(03)00682-6. [DOI] [PubMed] [Google Scholar]; (c) Hotoda H, Furukawa M, Daigo M, Murayama K, Kaneko M, Muramatsu Y, Ishii MM, Miyakoshi S, Takatsu T, Inukai M, Kakuta M, Abe T, Harasaki T, Fukuoka T, Utsuic Y, Ohyac S. Bioorg. Med. Chem. Lett. 2003;13:2829–2832. doi: 10.1016/s0960-894x(03)00596-1. [DOI] [PubMed] [Google Scholar]; (d) Yamashita A, Norton E, Petersen PJ, Rasmussen BA, Singh G, Yang Y, Mansour TS, Ho DM. Bioorg. Med. Chem. Lett. 2003;13:3345–3350. doi: 10.1016/s0960-894x(03)00671-1. [DOI] [PubMed] [Google Scholar]; (e) Koga T, Fukuoka T, Harasaki T, Inoue H, Hotoda H, Kakuta M, Muramatsu Y, Yamamura N, Hoshi M, Hirota T. J. Antimicro. Chemother. 2004;54:755–760. doi: 10.1093/jac/dkh417. [DOI] [PubMed] [Google Scholar]; (f) Dini Christophe. Curr. Top. Med. Chem. 2005;5:1221–1236. doi: 10.2174/156802605774463042. [DOI] [PubMed] [Google Scholar]; (g) Reddy VM, Einck L, Nacy CA. Antimicro. Agents Chemother. 2008;52:719–721. doi: 10.1128/AAC.01469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ichikawa S. Chem. Pharm. Bull. 2008;56:1059–1072. doi: 10.1248/cpb.56.1059. [DOI] [PubMed] [Google Scholar]; (i) Dini C, Drochon N, Feteanu S, Guillot JC, Peixoto C, Aszodi J. Bioorg. Med. Chem. Lett. 2001;11:529–531. doi: 10.1016/s0960-894x(00)00715-0. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kurosu M, Li K, Crick DC. Org. Lett. 2009;11:2393–2397. doi: 10.1021/ol900458w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurosu M, Li K. J. Org. Chem. 2008;73:9767–9771. doi: 10.1021/jo801408x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kurosu M, Biswas K, Crick DC. Org. Lett. 2007;9:1141–1145. doi: 10.1021/ol070150f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kurosu M, Narayanasamy P, Crick DC. Heterocycles. 2007;72:339–352. [Google Scholar]; (e) Kurosu M, Mahapatra S, Narayanasamy P, Crick DC. Tetrahedron Lett. 2007;48:799–803. [Google Scholar]; (f) Wang Y, Kurosu M. Tetrahedron. 2012 doi: 10.1016/j.tet.2012.03.121. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Synthesis of uridine-containing natural products using the BOM-protecting group, see; Ichikawa S, Shuto S, Minakawa N, Matsuda A. J. Org. Chem. 1997;62:1368–1375. Fraser AS, Kawasaki AM, Jung ME, Muthiah M. Tetrahedron Lett. 2000;41:1523–1526. Prakash TP, Kawasaki AM, Lesnik EA, Owens SR, Manoharan M. Org. Lett. 2003;5:403–406. doi: 10.1021/ol027131k. Hirano S, Ichikawa S, Matsuda A. Angew. Chem. Int. Ed. 2005;44:1854–1856. doi: 10.1002/anie.200462439. Hirano S, Ichikawa S, Matsuda A. J. Org. Chem. 2007;72:9936–9946. doi: 10.1021/jo701699h. Kodama T, Matsuo C, Ori H, Miyoshi T, Obika S, Miyashita K, Imanishi T. Tetrahedron. 2009;41:2116–2123. Tanino T, Ichikawa S, Shiro M, Matsuda A. J. Org. Chem. 2010;75:1366–1377. doi: 10.1021/jo9027193. Jensen TB, Pasternak A, Madsen AS, Petersen M, Wengel J. Chem. Bio. Chem. 2011;12:1904–1911. doi: 10.1002/cbic.201100161.
- 5.(a) Connor DS, Klein GW, Taylor GN, Boeckman RK, Medwid JB. Org. Syn. 1972;52:C10–C19. [Google Scholar]; (b) Hanessian S, Kloss J, Sugawara T. J. Am. Chem. Soc. 1986;108:2758–2759. [Google Scholar]; (c) Goff DA, Harris RN, Bottaro JC, Bedford CD. J. Org. Chem. 1986;51:4711–4714. [Google Scholar]; (d) Kunz H, Waldman H. Protecting Groups. Chapter 3.1. In: Trost BM, Fleming I, editors. Comprehensive Organic Synthesis. Vol. 6. Oxford, United Kingdom: Pergamon Press; 1991. pp. 631–701. [Google Scholar]; (e) Prakash TP, Manoharan M, Kawasaki AM, Fraser AS, Lesnik EA, Sioufi N, Leeds JM, Teplova M, Egli M. Biochemistry. 2002;41:11642–11648. doi: 10.1021/bi020264t. [DOI] [PubMed] [Google Scholar]
- 6.Spork AP, Wiegmann D, Granitzka M, Stalke D, Ducho C. J. Org. Chem. 2011;76:10083–10098. doi: 10.1021/jo201935w. [DOI] [PubMed] [Google Scholar]
- 7.We screened a wide range of primary and secondary amines, and pyridines to suppress the over-reductions of the uracil double bond during hydrogenolytic deprotections of the BOM group of the uridine derivatives. Addition of amines to Pd-mediated hydrogenation conditions did not provide the desired BOM-deprotected products in a satisfactory yield.
- 8.(a) Daubert BF, King CG. J. Am. Chem. Soc. 1939;61:3328–3330. [Google Scholar]; (b) Bajwa JS. Tetrahedron Lett. 1992;33:2299–2302. [Google Scholar]; (c) Shue Y-K, Carrera GM, Tufano MD, Nadzan AM. J. Org. Chem. 1991;56:2107–2111. [Google Scholar]
- 9.General procedure for BOM deprotections: To a stirred solution of the BOM protected uridine derivative (1 eq.) in iPrOH:H2O (10:1) and HCO2H (0.5%) was added 10% Pd-C (10%) under N2. H2 gas was introduced to the reaction mixture using a double-folded balloon. Upon completion, the reaction mixture was filtered through a Celite pad and washed with EtOAc (or MeOH). All volatiles were evaporated in vacuo. When the HCO2H-amine salt was generated, the crude mixture was dissolved in CHCl3, and washed with aq. NaHCO3. The combined organic extracts were dried over Na2SO4 and evaporated in vacuo. Purification by a silica gel chromatography afforded the desired compound (yields were given in Table 1 and 2).
- 10.Reported amine additives for controlled-hydrogenation conditions with Pd species have relatively high boiling point.