Abstract
While large grazers can often be excluded effectively from algal aquaculture operations, smaller herbivores such as small crustaceans and gastropods may be more difficult to control. The susceptibility of three Gracilaria species to herbivores was evaluated in multiple-choice experiments with the amphipod Ampithoe ramondi and the crab Acanthonyx lunulatus. Both mesograzers are common along the Mediterranean coast of Israel. When given a choice, the amphipod preferred to consume Gracilaria lemaneiformis significantly more than either G. conferta or G. cornea. The crab, however, consumed equivalent amounts of G. lemaneiformis and G. conferta, but did not consume G. cornea. Organic content of these algae, an important feeding cue for some mesograzers, could not account for these differences. We further assessed the susceptibility of a candidate species for aquaculture, G. lemaneiformis, against local algae, including common epiphytes. When given a choice of four algae, amphipods preferred the green alga Ulva lactuca over Jania rubens. However, consumption of U. lactuca was equivalent to those of G. lemaneiformis and Padina pavonica. In contrast, the crab showed a marked and significant preference for G. lemaneiformis above any of the other three algae offered. Our results suggest that G. cornea is more resistant to herbivory from common mesograzers and that, contrary to expectations, mixed cultures or epiphyte growth on G. lemaneiformis cannot reduce damage to this commercially appealing alga if small herbivores are capable of recruiting into culture ponds. Mixed cultures may be beneficial when culturing other Gracilaria species.
Keywords: Acanthonyx lunulatus, Ampithoe ramondi, Gracilaria, herbivore choice, mesograzers
1. Introduction
Herbivore damage of cultured seaweeds can lead to dramatic decreases in biomass, which translate to reduced yields per effort and concomitant monetary losses (Buschmann et al., 2001; Friedlander, 2008). While large herbivores may be excluded from aquaculture operations by simple mechanical devices such as cages or fences, smaller herbivores that are millimeters to a few centimeters long can pass through these barriers or recruit as larvae into culture ponds, causing significant losses in cultivated algal biomass. When excluding mesograzers is not feasible, algal traits that reduce herbivore damage on the crop alga become important to the commercial success of the aquaculture operation.
Mesograzers such as amphipods, isopods, polychaetes, and snails have all been recorded as pests in cultivated seaweeds (Nicotri, 1977; Shacklock and Croft, 1981; Buschmann et al., 1997, 2001; Smit et al., 2003; Hansen et al., 2006). The effect of these smaller herbivores, however, is variable. Some mesograzers are indirectly beneficial to the cultivated algal host because they preferentially consume epiphytes (Shacklock and Doyle, 1983; Brawley and Fei, 1987; Anderson et al., 1998) but these benefits can be overwhelmed quickly by the negative effects of grazing on the aquacultured host when mesograzer densities are too high causing alternative food sources to become rare (Shacklock and Doyle, 1983; Smit el al., 2003). The effects of micrograzers such as endoparasitic copepods are less well understood, but these can also have devastating effects on algal aquaculture (Friedlander et al., 1996; Friedlander, 2008).
A number of studies have tested the effectiveness of various methods to control mesograzer and micrograzer pests (Friedlander et al., 1996; Smit et al., 2003; Hansen et al. 2006). All these studies have focused on ways of maintaining herbivore populations at a minimum by directly killing these consumers (e.g., dipping algae in freshwater or passing thin flat algae through rollers). An alternative way of enhancing algal production yields under potential herbivore damage is to select species or strains of algae which have herbivore resistance. Resistance may also be acquired by association of the crop alga with another species that is more attractive to herbivores or reduces the ability of herbivores to locate the preferred host. These approaches are common practices in agriculture (Vandermeer, 1989; Shelton and Badenes-Perez, 2006; Cook et al., 2007; Radcliffe et al., 2009), but they have not been widely applied to seaweed aquaculture.
In this study, we assess the susceptibility of three species of Gracilaria to local mesograzers that can recruit into cultivation ponds in Israel. We also compare the susceptibility of a widely-cultured target species, G. lemaneiformis, to that of local seaweeds that the herbivores encounter readily in the field. The outcome of these studies can be used to select species or combinations of species that can be used to enhance the productivity of Gracilaria aquaculture facilities.
2. Materials and methods
The gammaridean amphipod Ampithoe ramondi (Audouin) and the majid crab Acanthonyx lunulatus (Risso) were selected due to their abundance on intertidal algae. Animals were collected from Jania rubens (Linnaeus) Lamouroux, Padina pavonica (Linnaeus) Thivy, and Ulva lactuca Linnaeus at the vicinity of the Israel Oceanographic and Limnological Research laboratory in Haifa (32°49′0″N 34°59′0″E). Gracilaria conferta (Schousboe ex Montagne) J. et G. Feldmann, G. cornea J. Agardh (wild phenotype), and G. lemaneiformis (Bory) Dawson Acleto et Foldvik (= Gracilariopsis lemaneiformis) were obtained from aquaculture ponds, in which they had been cultured for several years (Levy and Friedlander, 1994; Friedlander, 2001; Friedlander et al., 2001). Specific details of the cultivation of these Gracilaria species in Israel, including growth conditions and impact of small grazers, are found in Friedlander (2008).
Because Gracilaria contains no known chemical defenses against herbivores (Paul et al., 2001; also various chapters in Amsler, 2009), nutritional quality is likely an important cue for mesograzer feeding on this algal genus. We measured ash-free dry mass (AFDM) in thalli of G. conferta, G. cornea, and G. lemaneiformis (n=10) as an approximation for overall nutrient content in the algae. Most studies present nutrient content data as proportions of dry mass. However, animals perceive nutrients as a function of wet mass or volume (reward per bite). Therefore, AFDM data herein are presented as both percent of dry mass (which allows comparisons with other published works) and wet mass (which is how herbivores perceive algae) (Cruz-Rivera and Hay 2001). To determine wet to dry mass ratios algal pieces were dried on absorbent paper, placed on pre-weighed aluminum dishes and weighed (N=10). After drying at 60 °C for approximately 5 days, algae were weighed again. Ash content was measured by burning the dried algae in a furnace at 450 °C for 12 h, after which the mass of ash was obtained.
To assess the relative susceptibility of Gracilaria species to mesograzers, two sets of multiple-choice feeding assays with Ampithoe ramondi and Acanthonyx lunulatus were conducted. The first set of these experiments compared the susceptibility of Gracilaria conferta, G. cornea, and G. lemaneiformis by offering pieces of all three algae simultaneously to amphipods or crabs. Treatment replicates consisted of small plastic containers to which fresh seawater, grazers and algae were added. Controls for autogenic changes in algal mass (Peterson & Renaud 1989) were interspersed among treatments and had similar masses of algae in fresh seawater, but no grazers. A weighed piece of each of the three Gracilaria species (ca. 150–250 mg) was placed in each replicate or control container (n = 10 for both amphipods and crabs). Either three adult A. ramondi or one A. lunulatus were added to the treatments and allowed to feed for a maximum of three days. Upon termination of these multiple-choice assays, all mesograzers were removed, changes in algal mass were measured, and amount eaten was calculated after correcting for mass changes unrelated to consumption (Peterson & Renaud 1989, Cronin & Hay 1996).
A second set of multiple choice assays was conducted using the same general protocols as above. In these assays the palatability of a target species, Gracilaria lemaneiformis, was compared to that of local algae that mesograzers normally encounter, including epiphytes (e.g., Ulva spp., Friedlander, 2008). Thus, these experiments offered amphipods or crabs a simultaneous choice of four algae: the foreign cultivated species Gracilaria lemaneiformis, and the three local algae Jania rubens, Padina pavonica, and Ulva lactuca. Sample sizes and calculations of consumption were as explained above. Because of the differences in the density of different algae, weights of pieces ranged between 200–400 mg, however, the area covered by these algal portions in the dishes was visually approximated so that animals would have similar probability of encountering all algae as they moved in the experimental replicates. For amphipods, five A. ramondi per replicate were used. One or two A. lunulatus per replicate were used depending on their size.
Statistical comparisons of AFDM among Gracilaria species were performed with one-way ANOVA, followed by Tukey-Kramer post hoc tests. Because diet treatments in multiple-choice experiments are not independent from each other, parametric analyses are not appropriate. For the multiple-choice feeding assays, comparisons were made using Friedman’s tests followed by the appropriate non-parametric post hoc comparisons (Conover 1999). This test allows for comparing multiple non-independent treatments as long as replication equals or exceeds the number of treatments compared (Stachowicz and Hay, 1999; Cruz-Rivera and Hay, 2001; Sotka and Hay, 2002; Cruz-Rivera and Paul, 2006)
3. Results
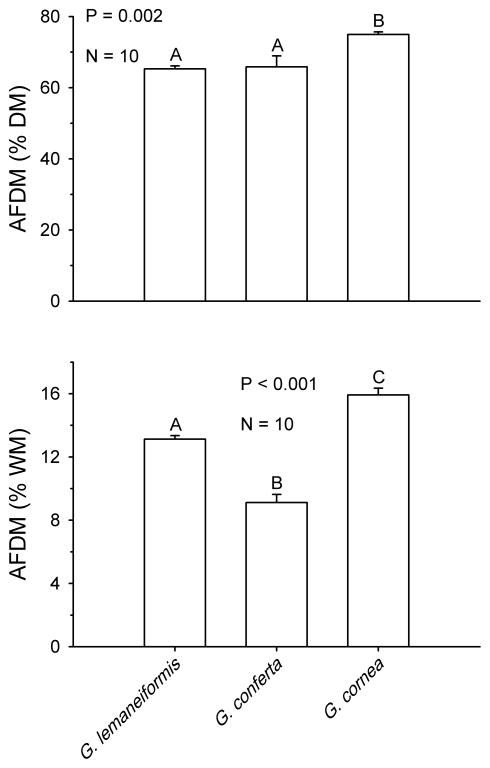
There were significant differences in the organic content of the three Gracilaria species used in this study both by dry mass (P=0.002, one-way ANOVA, Fig 1 top) and wet mass (P<0.001, one-way ANOVA, Fig 1 bottom). By dry mass, G. cornea had the highest percentage of AFDM; with G. lemaneiformis and G. conferta having approximately 12–13% less AFDM (Fig. 1, top). Differences in organic content by wet mass, which are more accurate describing how herbivores perceive their foods, were more marked. By wet mass, G. cornea still had the highest percent of AFDM/WM, followed by G. lemaneiformis. However, G. conferta had the lowest organic content of all three species (18% less than G. lemaneiformis and 43% less than G. cornea). Differences by wet mass were statistically significant when all three Gracilaria species were compared (Fig. 1 bottom).
Fig. 1.
Content of ash-free dry mass in three species of Gracilaria expressed as percent of dry mass (top panel) and wet mass (bottom panel). Bars represent means +1 SE. Analyses and significant groupings are from one-way ANOVA followed by SNK tests.
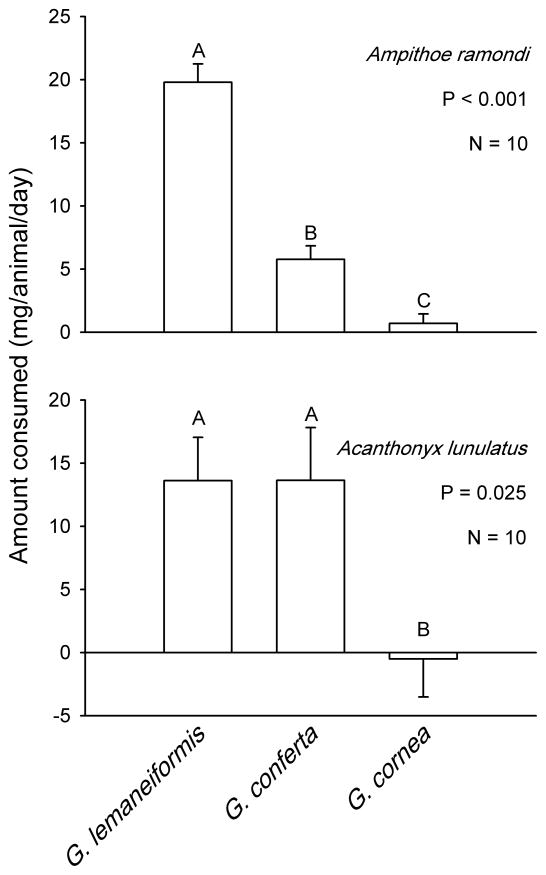
When the amphipod Ampithoe ramondi was offered a choice among three Gracilaria species used in aquaculture, a strong preference for G. lemaneiformis was exhibited (P<0.001, Friedman’s test, Fig. 2 top). Consumption of G. conferta was only 29% of that of G. lemaneiformis, while consumption of G. cornea was only 4% of that of G. lemaneiformis. The crab Acanthonyx lunulatus also feed selectively on these algae (P=0.025, Friedman’s test, Fig. 2 bottom), however, consumption of G. lemaneiformis and G. conferta was equivalent. Crabs did not consume significant amounts of G. cornea, which tended to grow in the presence of the consumers during the experiment (Fig. 2, bottom).
Fig. 2.
Feeding preferences of the amphipod Ampithoe ramondi (top) and the majid crab Acanthonyx lunulatus (bottom) on three Gracilaria species offered simultaneously. Bars represent means +1 SE. Analyses and significant groupings are from non-parametric Friedman’s tests followed by the appropriate pair-wise comparisons. Negative numbers indicate net growth in the presence of consumers.
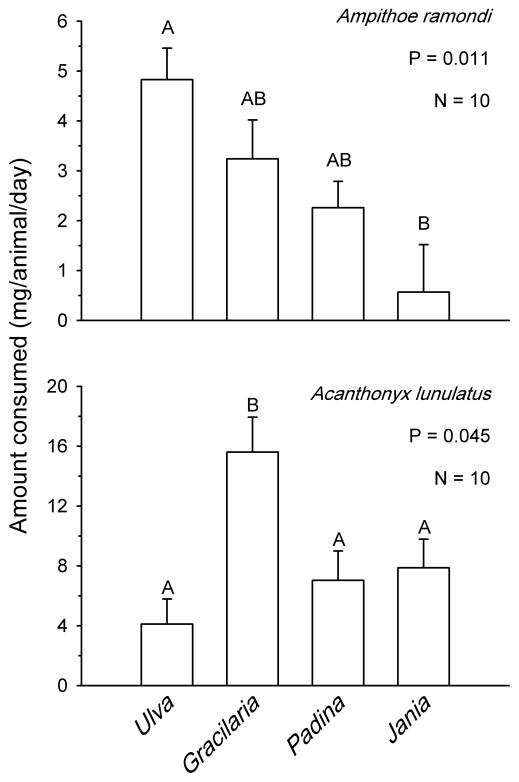
When the palatability of four algae was compared simultaneously (Fig. 3), the cultivated alga Gracilaria lemaneiformis was consumed as much or more than any of the other three algae available to mesograzers. For A. ramondi, significant differences in consumption could be detected (P=0.011, Friedman’s test, Fig. 3 top), however, post hoc analyses showed that these differences in consumption only occurred between Ulva lactuca and Jania rubens. Consumption of U. lactuca, G. lemaneiformis, and Padina pavonica was equivalent for this amphipod. The crab Acanthonyx lunulatus showed a clear and significant preference for G. lemaneiformis above all other algae offered (P=0.045, Friedman’s test, Fig. 3 bottom). Crabs consumed U. lactuca, P. pavonica and J. rubens in equivalent quantities.
Fig. 3.
Feeding preferences of amphipods (top) and crabs (bottom) fed simultaneously on aquacultured Gracilaria lemaneiformis and three common algal species from Israel. Bars represent means +1 SE. Analyses and significant groupings are from non-parametric Friedman’s tests followed by the appropriate pair-wise comparisons.
There were apparent differences in overall algal consumption per animal when the two multiple choice experiments were compared (Figs. 2 and 3). For example, A. ramondi individuals consumed about half the total amount of algae when four algae were present than when only Gracilaria species were available. Because our animals were field collected at different times, and only used once during each assay, slight differences in size, genetic makeup, nutritional or physiological state of the consumers could account for this (in addition to likely differences in nutrient content when all algae are compared). Our experiments were not designed to test for these variables, and thus we can only speculate about these differences at this point.
4. Discussion
Our data demonstrate that susceptibility to mesograzers in cultivated Gracilaria is dependent on both the species of alga and of grazer. For example, Gracilaria conferta was a lower preference food for amphipods, but a preferred food for crabs, when tested against two other Gracilaria species (Fig. 2). Nevertheless, G. cornea emerged as a grazing resistant species to both species of mesograzers tested. Preferences of amphipods and crabs on these algae were not related to the nutritional content of the algae. The least susceptible species, G. cornea, was also the one with the highest content of AFDM (per dry or wet mass), whereas the preferred G. lemaneiformis had intermediate amounts. Previous studies have shown that AFDM can influence feeding behavior in both marine amphipods and crabs (Stachowicz and Hay, 1999; Cruz-Rivera and Hay, 2001; Sotka and Hay, 2002). However, this algal trait could be more important in monoculture conditions, as it affects feeding rates through compensatory feeding on less nutritious algae (Cruz-Rivera and Hay, 2000; 2001). Our study does not preclude the possibility that other more specific nutritional cues (e.g., amino acids) could influence feeding by these consumers, however, organic content is often correlated with other nutritional traits and is known to affect feeding in both mesograzers and macrograzers (Neighbors and Horn, 1991; Hay et al. 1994, Stachowicz and Hay, 1999; Cruz-Rivera and Hay, 2001; Sotka and Hay, 2002). For example, high ash content coincided with low preference, but also with variations in carbohydrates and lipids, in an analysis of nutritional traits in 22 algae and one seagrass eaten or not eaten by two fishes (Neighbors and Horn, 1991).
In the case of the two grazers studied here, the mechanics of feeding related to algal structure might have been more important than choices based on organic content alone. Both G. lemaneiformis and G. conferta are finely branched species that can be readily cut by the chelae of A. lunulatus. In contrast, G. cornea is wider and more coarsely branched, making it more difficult for crabs to manipulate this alga. Amphipods such as A. ramondi have slicing mouthparts that can cut through the surface of algae and operate at a finer scale than crabs. Thus, branching pattern is less of a mechanical constraint for feeding in these animals.
Other traits of Gracilaria may potentially affect consumer choice as well. Agar cell walls of Gracilaria are presumably degraded by mesograzer consumers. Bacterial degradation of Gracilaria was shown to cause an oxygen burst and an increase in reactive oxygen species (ROS) including hydrogen peroxide, and CHBr3, which led to the elimination of associated bacteria. Various Gracilaria species differed in the magnitude of this effect (Weinberger and Friedlander, 2000; Weinberger, 2007). This could similarly lead to differences in deterrence toward mesograzers and consequently different levels of Gracilaria resistance to herbivores. While intraspecific variation in algal susceptibility may occur as a function of algal life history, in most cases these differences are most noticeable in algae with heteromorphic life histories (Lubchenco and Cubit, 1980; Dethier, 1981; Littler and Littler, 1983). Few cases of differential grazing in algae with isomorphic life histories are known (Buschmann and Santelices, 1987; Luxoro and Santelices 1989; Thornber et al., 2006). Cultivated Gracilaria are often sterile, thus reducing the potential that crops may be differently susceptible to mesograzers depending on reproductive condition or life stage.
Although G. lemaneiformis is cultivated in Canada, China, Japan, Mexico and Peru, (Zemke-White and Ohno, 1999; Yang et al., 2006) it appears to be a poor candidate for cultivation in the Mediterranean under conditions that cannot effectively control mesograzer recruitment on the algae. For both consumers tested, and compared to congeneric and non-congeneric species, G. lemaneiformis remained a preferred food (Figs. 2 and 3), even in the presence of Ulva lactuca – one of the most common epiphytes in this area, including Gracilaria cultivation ponds (Friedlander et al., 2001; Friedlander, 2008). Previous studies have also shown that this alga is also more susceptible to epiphytes than other Gracilaria species (Friedlander et al., 2001). In contrast, G. cornea is less susceptible to both grazers (Fig. 2) and epiphytes (Friedlander et al., 2001).
Mesograzer and micrograzer infestations in aquaculture facilities can lead to significant losses of the cultivated biomass (Buschmann et al., 2001; Friedlander, 2008). While a number of control methods have been tested against diverse animals (Friedlander et al., 1996; Shacklock and Croft, 1981; Smit et al, 2003; Hansen et al., 2006), the taxonomic and physiological diversity of consumers and their hosts precludes any single method from being universally applicable. For example, biocontrol using fishes may be effective against crustaceans (Friedlander et al., 1996), but not against shelled gastropods (Shacklock and Croft, 1981) or species that eventually reach an escape in size (Smit et al., 2003). Mechanical control by passing algae through rollers is effective against taxonomically diverse mesograzers (Shacklock and Croft, 1981), but its use depends on the morphology of the cultured algae. Submersion in freshwater may also be effective, but its application depends on the tolerances of both the grazers and the cultured algae to sudden changes in salinity (Smit et al., 2003; Hansen et al., 2006).
Selecting plant species or varieties that are herbivore resistant is a common practice in agricultural systems (Radcliffe et al., 2009). In aquaculture, screening for algae has largely focused instead on selecting species or strains that show fast growth under culture conditions (e.g., Levy and Friedlander, 1994), and on increasing the yield and quality of desired products (Buschmann et al., 2001; Friedlander, 2008). The use of “trap crops” and “push-pull” strategies in agriculture is also common (Vandermeer, 1989; Shelton and Badenes-Perez, 2006; Cook et al., 2007; Radcliffe et al., 2009). In these approaches, more attractive plants are used to lure herbivores away from the main crop plants; the “traps” can then be treated to eliminate herbivores. Alternatively, non-crop plants may repel pests from the target crops. Neither practice is regularly used in the culture of algae. Our data suggest that Ulva lactuca, Padina pavonica, or even some Gracilaria species may reduce the damage by some grazers on potential target species if used in dual culture. The success of this approach, however, will depend on the careful selection of co-cultured species, the balance between competitive interactions between cultured algae, and on the dynamics of algal-grazer interactions. We propose that the exploration of algal susceptibility to herbivores be integrated into preliminary screening programs selecting algal strains or species for cultivation.
Highlights.
Mesograzers can cause significant losses in aquacultured Gracilaria.
G. cornea is more resistant to mesograzers than G. conferta or G. lemaneiformis.
G. lemaneiformis was consumed more than, or as much as, epiphytes and other algae.
Mixed culturing may increase yield of only some Gracilaria species by reducing herbivory on them.
Acknowledgments
Funding for this work was provided by a grant from the Israeli Ministry of Industry and Commerce to M. Friedlander. Partial funding for E. Cruz-Rivera was provided by a postdoctoral supplement to NIH grant CA 53001 (J. Horwitz, P.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsler CD. Algal chemical ecology. 1. Springer-Verlag; Berlin: 2009. [Google Scholar]
- Anderson BC, Smit AJ, Bolton JJ. Differential grazing effects by isopods on Gracilaria gracilaris and epiphytic Ceramium diaphanum in suspended raft culture. Aquaculture. 1998;169:99–109. [Google Scholar]
- Brawley SH, Fei XG. Studies of mesoherbivory in aquaria and an unbarricaded mariculture farm on the Chinese coast. J Phycol. 1987;23:614–623. [Google Scholar]
- Buschmann AH, Briganti F, Retamales CA. Intertidal cultivation of Gracilaria chilensis (Rhodophyta) in southern Chile: long term invertebrate abundance patterns. Aquaculture. 1997;156:269–278. [Google Scholar]
- Buschmann AH, Correa JA, Westermeier R, Hernández-González MC, Norambuena R. Red algal farming in Chile: a review. Aquaculture. 2001;194:203–220. [Google Scholar]
- Buschmann AH, Santelices B. Micrograzers and spore release in Iridaea laminarioides Bory (Rhodophyta:Gigartinales) J Exp Mar Biol Ecol. 1987;108:171–179. [Google Scholar]
- Conover WJ. Wiley series in probability and statistics: applied probability and statistics section. 3. Wiley; New York: 1999. Practical nonparametric statistics. [Google Scholar]
- Cook SM, Khan ZR, Pickett JA. The use of push-pull strategies in integrated pest management. Annu Rev Entomol. 2007;52:375–400. doi: 10.1146/annurev.ento.52.110405.091407. [DOI] [PubMed] [Google Scholar]
- Cronin G, Hay ME. Induction of seaweed chemical defenses by amphipod grazing. Ecology. 1996;77:2287–2301. [Google Scholar]
- Cruz-Rivera E, Hay ME. Can quantity replace quality? Food choice compensatory feeding, and fitness of marine mesograzers. Ecology. 2000;81:201–219. [Google Scholar]
- Cruz-Rivera E, Hay ME. Macroalgal traits and the feeding and fitness of an herbivorous amphipod: the roles of selectivity, mixing, and compensation. Mar Ecol Prog Ser. 2001;218:249–266. [Google Scholar]
- Cruz-Rivera E, Paul VJ. Feeding by coral reef mesograzers: Algae or cyanobacteria? Coral Reefs. 2006;25:617–627. [Google Scholar]
- Dethier MN. Heteromorphic algal life histories: the seasonal pattern and response to herbivory of the brown crust, Ralfsia californica. Oecologia. 1981;49:333–339. doi: 10.1007/BF00347594. [DOI] [PubMed] [Google Scholar]
- Friedlander M. Inorganic nutrition in pond cultivated Gracilaria conferta (Rhodophyta): nitrogen, phosphate and sulfate. J Appl Phycol. 2001;13:279–286. [Google Scholar]
- Friedlander M. Israeli R & D activities in seaweed cultivation. Israel J Plant Sci. 2008;56:15–28. [Google Scholar]
- Friedlander M, Kashman Y, Weinberger F, Dawes CJ. Gracilaria and its epiphytes: 4. The response of two Gracilaria species to Ulva lactuca in a bacteria-limited environment. J Appl Phycol. 2001;13:501–507. [Google Scholar]
- Friedlander M, Weintraub N, Freedman A, Sheer J, Snovsky Z, Shapiro J, Kissil GW. Fish as potential biocontrollers of Gracilaria culture. Aquaculture. 1996;145:113–118. [Google Scholar]
- Hansen JP, Robertson-Andersson D, Troell M. Control of the herbivorous gastropod Fissurella mutabilis (Sow.) in a land-based integrated abalone–seaweed culture. Aquaculture. 2006;255:384–388. [Google Scholar]
- Hay ME, Kappel QE, Fenical W. Synergisms in plant defenses against herbivores: interactions of chemistry, calcification, and plant quality. Ecology. 1994;75:1714–1726. [Google Scholar]
- Levy I, Friedlander M. Seasonal growth activity of local and foreign gracilarioid strains in Israel. J Appl Phycol. 1994;6:447–454. [Google Scholar]
- Littler MM, Littler DS. Heteromorphic life-history strategies in the brown alga Scytosiphon lomentaria (Lyngb.) Link. J Phycol. 1983;19:425–431. [Google Scholar]
- Lubchenco J, Cubit J. Heteromorphic life histories of certain marine algae as an adaptation to variation in herbivory. Ecology. 1980;61:676–687. [Google Scholar]
- Luxoro C, Santelices B. Additional evidence for ecological differences among isomorphic reproductive phases of Iridaea laminarioides (Rhodophyta: Gigartinales) J Phycol. 1989;25:206–212. [Google Scholar]
- Neighbors MA, Horn MH. Nutritional quality of macrophytes eaten and not eaten by two temperate-zone herbivorous fishes: a multivariate comparison. Mar Biol. 1991;108:471–476. [Google Scholar]
- Nicotri ME. The impact of crustacean herbivores on cultured seaweed populations. Aquaculture. 1977;12 (2):127–136. [Google Scholar]
- Paul VJ, Cruz-Rivera E, Thacker RW. Chemical mediation of macroalgal-herbivore interactions: ecological and evolutionary perspectives. In: McClintock J, Baker B, editors. Marine Chemical Ecology. CRC Press, LLC; 2001. pp. 227–265. [Google Scholar]
- Peterson CH, Renaud PE. Analysis of feeding preference experiments. Oecologia. 1989;80:82–86. doi: 10.1007/BF00789935. [DOI] [PubMed] [Google Scholar]
- Radcliffe EB, Hutchison WD, Cancelado RE, editors. Integrated Pest Management: Concepts, Tactics, Strategies and Case Studies. Cambridge University Press; Cambridge, UK: 2009. [Google Scholar]
- Shacklock PF, Croft GB. Effect of grazers on Chondrus crispus in culture. Aquaculture. 1981;22:331–342. [Google Scholar]
- Shacklock PF, Doyle RW. Control of epiphytes in seaweed cultures using grazers. Aquaculture. 1983;31:141–151. [Google Scholar]
- Shelton AM, Badenes-Perez FR. Concepts and applications of trap cropping in pest management. Annu Rev Entomol. 2006;51:285–308. doi: 10.1146/annurev.ento.51.110104.150959. [DOI] [PubMed] [Google Scholar]
- Smit AJ, Fourie AM, Robertson BL, du Preez DR. Control of the herbivorous isopod Paridotea reticulata in Gracilaria gracilis tank cultures. Aquaculture. 2003;217:385–396. [Google Scholar]
- Sotka EE, Hay ME. Geographic variation among herbivore populations in tolerance for a chemically rich seaweed. Ecology. 2002;83:2721–2735. [Google Scholar]
- Stachowicz JJ, Hay ME. Reduced mobility is associated with compensatory feeding and increased diet breadth of marine crabs. Mar Ecol Prog Ser. 1999;188:169–178. [Google Scholar]
- Thornber C, Stachowicz JJ, Gaines S. Tissue type matters: selective herbivory on different life history stages of an isomorphic alga. Ecology. 2006;87:2255–2263. doi: 10.1890/0012-9658(2006)87[2255:ttmsho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Vandermeer J. The Ecology of Intercropping. Cambridge Univ. Press; New York: 1989. [Google Scholar]
- Weinberger F. Pathogen-Induced Defense and Innate Immunity in Macroalgae. Biol Bull. 2007;213:290–302. doi: 10.2307/25066646. [DOI] [PubMed] [Google Scholar]
- Weinberger F, Friedlander M. Response of Gracilaria conferta (Rhodophyta) to oligoagars results in defense against agar-degrading epiphytes. J Phycol. 2000;36:1079–1086. [Google Scholar]
- Yang YF, Fei XG, Song JM, Hu HY, Wang JC, Chung IK. Growth of Gracilaria lemaneiformis under different cultivation conditions and its effects on nutrient removal in Chinese coastal waters. Aquaculture. 2006;254:248–255. [Google Scholar]
- Zemke-White WL, Ohno M. World seaweed utilization: an end-century summary. J Appl Phycol. 1999;11:369–376. [Google Scholar]