Abstract
Nearly forty years ago, social psychologists began applying the information processing framework of cognitive psychology to the question of how humans understand and represent knowledge about themselves and others. This approach gave rise to the immensely successful field of social cognition and fundamentally changed the way in which social psychological phenomena are studied. More recently, social scientists of many stripes have turned to the methods of cognitive neuroscience to understand the neural basis of social cognition. A pervasive finding from this research is that social knowledge, be it about one's self or of others, is represented in the medial prefrontal cortex. This review focuses on the social cognitive neuroscience of self and person knowledge in the medial prefrontal cortex. We begin with a brief historical overview of social cognition, followed by a review of recent and influential research on the brain basis of self and person knowledge. In the latter half of this review we discuss the role of familiarity and similarity in person perception and of spontaneous processes in self and other referential cognition. Throughout, we discuss the myriad ways in which the social cognitive neuroscience approach has provided new insights into the nature and structure of self and person knowledge.
Social cognition, broadly defined, is concerned with the cognitive processes and mental representations that organize and influence how people perceive both themselves and others. Although social psychologists have always followed a largely cognitive approach (1-4), it was not until the 1980s that the study of social cognition became the prevailing paradigm in experimental social psychology (5-6). This shift in perspective was primarily the result of a larger cognitive revolution that was taking place in psychology and which made available a new set of methods that could be adapted to the study of social knowledge. In the early 1990s, a similar exchange of ideas and methods took place as cognitive scientists adopted neuroscientific tools to further their understanding of cognition, thus heralding the discipline of cognitive neuroscience. More recently, social scientists of many stripes have turned to using the methods of cognitive neuroscience to study the neural basis of social cognition. This new field of social cognitive neuroscience has developed at a rapid pace, so that, as of 2012, there are well over 150 laboratories around the globe actively investigating the neural basis of social cognition using a wide array of methodologies (e.g. neuroimaging, psychophysiology and, neuropsychology). Since its inception one of the central preoccupations of social cognitive neuroscience has been to understand how the brain enables people to understand both their own mind as well as that of others.
In this review, we focus on how self and person knowledge is represented in the brain. We begin with a historical overview of social cognition research on self and person knowledge, followed by a review of current and influential social cognitive neuroscience research. Recent neuroscience research has shown evidence of shared representations between self and intimately known others, thus in the latter half of this review, we discuss the role of intimacy, familiarity and similarity in person perception. In addition, we also highlight some recent directions that the field has taken, such as the study of self-referential processing with respect to past and future selves, viewing oneself and others in a positive light (i.e., self-enhancement) and finally, the study of spontaneous and implicit processes in self and other-referential cognition. Throughout this review we draw links between modern day neuroscientific research and classic work in social cognition, highlighting how each serves to enrich the other.
A Brief Overview of Social Cognition Research on Self and Others
From the beginning social psychology has studied the mechanisms by which social behavior is guided by people's subjective perceptions of themselves and others. Although the terminology of cognitive psychology did not enter into social psychological discourse until the cognitive revolution of the 1960s and 1970s, early social psychological theories nevertheless posited mental processes that stand between perception and social behavior (2, 4, 7-8, for a review see 9). For instance, early social psychologists relied on the study of mental processes to describe how people form impressions of others (7), understand the causal relationships between people's intentions and their behavior (2) or resolve dissonance between contradictory beliefs (1). The emergence of cognitive psychology in the 1960s and 70s brought with it a number of new tools and theories with which to study social phenomenon. Given that social psychologists were already favorably inclined towards a cognitive approach, the assimilation of ideas from cognitive psychology occurred rapidly (5, 10-15) leading to a surge of research on the cognitive processes and mental representations that organize and influence people's perceptions of themselves and of others. In the following sections we review classic and influential social cognition research as it pertains to the representation of self and person knowledge, for a more contemporary review of social cognition, the reader is referred to the WIRES review of Social Cognition (16).
Self-knowledge
Everyday conversation is filled with hundreds of mundane examples of people talking about themselves and describing minute facets of their personalities and beliefs to others (e.g., “I think that's funny”; “I loathe waiting in line”; “Call me cheap, but I still prefer Bollinger to Dom Perignon”). People's conception of themselves and their personalities are thought to arise through a process of self-perception, whereby individuals makes attributions about their own personality from observation and memory (17). Research on the self in social cognition is principally concerned with the structure of people's mental representation of themselves and in how this representation influences their perception of others. For instance, personality traits deemed central to people's mental representations of the self (i.e. their self-schema) are more chronically accessible (18), and, consequently, influence the attributions people make about others' behavior (19-23). In a similar vein, when individuals are asked to judge the similarity of other people they default to using their own self as a reference point (24-25).
One of the earliest attempts to examine the cognitive implications of having a mental representation of the self was research on memory for information when considered with reference to themselves (i.e. self-referential processing). For instance, early research showed that judging personality traits according to whether or not they apply to oneself improves subsequent recall of these words as compared to thinking about the semantic meaning of the words (26-27) or judging whether or not the traits are applicable to other persons (28-30, for a review see 31). These findings led to considerable debate over the special status of the self-concept. Some interpreted these data as evidence that the self is a special “superordinate schema” with privileged access to memory (27, 32-34) whereas others have argued that there is nothing special about the self (35-36), and that the self-referential memory advantage is instead an artifact of the self's elaborately organized representation which encourages a greater “depth-of-processing” (14-15). Instrumental in settling this debate was the status of the mnemonic advantage afforded to information processed with reference to close friends and family members. Early on, it was noted that encoding information generated by a significant other (versus information associated with a stranger) increases memory recall (37-38). The theory that the self-reference effect in memory was primarily an artifact of the self-schema being more organized and elaborated implies that the memory advantage for self-referential processing would be reduced when compared to judging traits with respect to an intimately known person (e.g., a parent or best-friend), rather than a stranger. Indeed, when comparing recall for information encoded with reference to the self and with reference to a close other, the self-referential memory advantage was found to be either reduced, or, in some cases, to disappear completely (28-30, 32-33). Interestingly, it appears that it is intimacy, and not simply familiarity, that is the key factor in promoting increased recall as highly familiar others who are not personally known to the subject (e.g., political figures, movie actors) do not elicit the same memory advantage as a close and intimate friend (31). Although the initial explanation for this effect was that information about close friends, like information about the self, is habitually processed in an intricate and organized manner, an alternate interpretation proposed by Aron and colleagues (39-40) is that people incorporate aspects of close others into their self-schema, resulting in a tendency to confuse one's own traits with those of a close friend, and vice versa (41).
The findings from the studies outlined above describe some of the structural features of an individual's representation of their own self but says little about the accuracy of these representations and whether they are subject to bias. Research on self-evaluation has shown that individuals frequently suffer from a positivity bias (42) such that they view themselves as being better-than-average and as being in possession of more positive traits (43-44). This self-enhancement effect also occurs when people evaluate their performance on a task, frequently judging themselves to be better than they actually are (e.g. 45). Interestingly, this pattern appears to carry over to evaluations of intimately known others. For example, when asked to evaluate a romantic partner, people rate their partner as better-than-average compared to less well-known others (46). This also extends to the attributions individuals make about other people's behaviors, such that they tend to make more situational attributions for close friends and partners, but rely on dispositional explanations for less intimately known others (47).
Person knowledge
There is more to becoming acquainted with someone than learning their thoughts on the best champagnes for which to toast a graduating student (it's Veuve Clicquot by the way). The act of getting to know someone new invariably involves going beyond the minutia of their likes and dislikes in order to extract some underlying sense of the type of person they are. Starting with early work on impression formation, it has been argued that people are driven to arrive at a coherent and unified understanding of other people's behavior (7). Fritz Heider, prefiguring many later attribution theories, described humans as having a fundamental need to predict and understand the behavior of others (2). In a similar vein, Jones & Davis (48) posited that people are inherently biased towards inferring intent in other people's actions and that this tendency naturally leads them to make dispositional trait attributions to explain their behavior (49). The idea that traits are the primary building blocks of attributions is supported by research in social cognition, which demonstrates that trait-level descriptions are one of the principal ways in which person knowledge is organized (34, 50-53).
Much like judging traits with reference to the self, forming impressions of others has been shown to increase recall of person information over and above a simple memorization strategy (54-55). This finding was initially interpreted as evidence that, like the self-reference effect, forming impressions of people organizes behavioral information in memory around trait inferences, leading to a greater depth of memory encoding (54, 56). Since this early work, there have been conflicting theories as to how behavioral information is organized in memory. Some researchers suggested that behavioral information is stored with, and organized around, trait concepts (54, 57-58) whereas others have argued that behavior and trait information are stored separately, such that a person can recall trait information about another person without necessarily remembering the behaviors that support these characterizations (59). Additional evidence for this last conjecture comes in the form of neuropsychological case studies of patients with severe anterograde amnesia (for a recent review see 60). Study of these patients has shown that their trait knowledge of themselves (61-62) and others (63) is preserved despite severe impairments in the ability to recall episodic memories, including any episode or behavior that might exemplify the traits in question.
What happens when an individual forms an impression of someone who appears similar to people that they already know well? Recent work by Anderson and colleagues shows that when people form an impression of someone who is similar to a close friend or family member, they tend to exaggerate the similarities and assume that this new person shares many of the same traits as their close friend (64-66). This “transference” of traits, from significant other to unknown stranger, is thought to arise due to the chronic accessibility of people's mental representation of significant others (65). In short, people who are superficially similar to close others will activate the representation of that close other, which in turn, increases the likelihood that traits normally ascribed to a close friend will be transferred to the unknown person.
An enduring dichotomy in impression formation has been the juxtaposition of explicit and implicit (i.e. spontaneous) impression formation (67). Across several studies, Uleman and colleagues have shown that people readily make trait inferences in the absence of an explicit impression formation goal (53, 68-70). For instance, when people observe trait-implying behaviors, they spontaneously infer a dispositional trait which is then falsely remembered on a subsequent memory test. Until relatively recently, it was thought that spontaneously inferred traits were only associated with behaviors, and not with the actor performing the behaviors. Thus, implicit impressions were not considered to be true impressions of persons (71). However, recent work has shown that spontaneously inferred traits are, in fact, linked to an actor's face (72), and that an actor's face alone is sufficient to cue false recognition of trait words (68, 70). Taken together, this research shows that, even in the absence of an explicit intent to form an impression, people will spontaneously infer traits whenever they observe another engaging in meaningful behavior.
The Neural Representation of Self and Others
The functional neuroanatomy of social cognition has been studied from a multiplicity of theoretical and methodological viewpoints. Some researchers have approached the topic by focusing on the perceptual systems involved in representing social stimuli (i.e., faces and bodies (73-75), emotional expressions (76) and biological motion (77) whereas others have investigated higher-level social cognitive processes, including empathy for others' pain (78-79) reasoning about others' beliefs (80-81), experiencing social rejection (82-84) or regulating social behaviors (85-86). In the following section, we focus specifically on research investigating the neural representation of knowledge about one's self and others. Although reflecting on self and others recruits regions throughout the brain (87-88), a consistently identified area of convergence has been the medial prefrontal cortex (MPFC) (89-90), noted for its involvement in social cognition across a variety of domains (91). Other regions that have been associated with thinking about oneself or others are: the posterior cingulate cortex, anterior temporal lobes and the temporoparietal junction (TPJ) (92-93). In addition, viewing social stimuli, be it faces or complex social scenes, often recruits inferotemporal regions involved in visual perception, especially those involved in representing the visual features of other people such as the lateral fusiform and superior temporal sulcus (74, 94). However, when it comes to the representation of abstract and trait knowledge about self and others, the MPFC stands apart in that it is the most commonly implicated region (95) and is often the only area to dissociate between multiple features of social targets, such as familiarity and similarity.
Throughout this discussion, a distinction is made between ventral (VMPFC) and dorsal (DMPFC) regions of the MPFC, the former being commonly implicated in self-referential cognition and the latter being more commonly associated with impression formation and thinking about the mental states of others (Figure 1). Although the definitions of VMPFC and DMPFC in this context is primarily functional, cytoarchitectonic boundaries have been roughly demarcated. VMPFC is generally restricted to Brodmann's area 10, whereas, in this context, DMPFC incorporates Brodmann's areas 8 and 9 and is anterior and ventral to the dorsal medial prefrontal regions implicated in cognitive control and conflict monitoring (for a review see 96). The following sections review both MPFC sub regions as they pertain to self- and other-referential cognition. For the sake of clarity, a functional distinction is made between these regions such that VMPFC is primarily discussed in terms of self-referential cognition and DMPFC in terms of thinking about others. However, it is important to note at the outset that there is often significant overlap between the MPFC regions recruited by these two processes such that the contribution of VMPFC and DMPFC to self and other-referential processing may be more akin to a gradient than a true functional dissociation (97), an idea which we will return to later in the review.
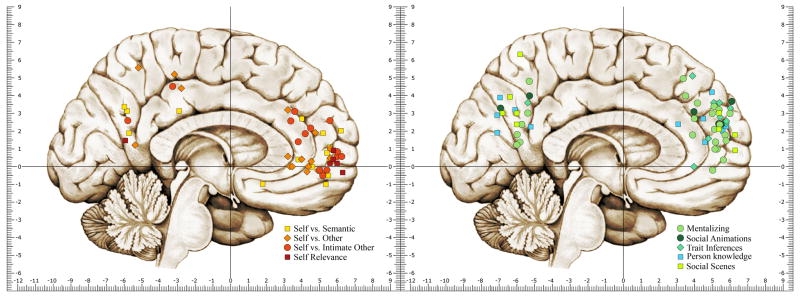
Figure. 1.
A functional distinction between ventral medial prefrontal cortex (VMPFC) and dorsal medial prefrontal cortex (DMPFC) is suggested by neuroimaging research implicating VMPFC in the representation of semantic knowledge about the self and DMPFC in the representation of social knowledge about others. (A). Location of foci of peak activation in the medial prefrontal cortex and posterior cingulate cortex from studies of self-referential cognition. (B). Location of activation foci for studies of explicit trait attribution and impression formation tasks as well as spontaneous recruitment of MPFC when viewing intimately familiar faces, social animations and social scenes. Coordinates (Y,Z) are in Montreal Neurological Institute (MNI) stereotaxic space and are constrained along the left-right plane according to a volume defined by |X| < 15, Y > 30, -10 < Z < 55 and projected onto the medial surface of the brain. Coordinates originally reported in Talairach atlas space were converted to MNI stereotaxic space according to a non-linear registration of the Talairach atlas to MNI space (204).
The Neural Basis of Self-Knowledge
By the 1990s, research on the cognitive basis of the self-referential memory effect arrived at something of an impasse (98-99). By and large, researchers were convinced that the memory advantage that occurs when thinking about information with reference to the self was due to a depth-of-processing effect (35-36). As knowledge of the brain systems involved in learning and memory improved, it became apparent that, in neuroscientific terms, what the depth-of-processing explanation for the self-referential effect would predict is that processing information with reference to the self would lead to increased activity in regions involved in semantic memory encoding, specifically the left inferior prefrontal cortex (100-101). Alternatively, if processing information with reference to the self did in fact invoke a unique “superordinate schema” (27) then it would be expected that different regions of the brain would be involved.
Initially, three studies examined brain activity during self-referential memory encoding. The first experiment, using Positron Emission Tomography, examined brain activity in 8 individuals as they judged whether trait words were applicable to themselves, a familiar other person, or when they considered the semantic meaning of the words. Although MPFC activity differentiated self-referential from semantic judgments, the study failed to find any brain region that differentiated self and other-referential processing (102). In light of the small sample size and failure to replicate the standard self-referential memory advantage, Kelley and colleagues revisited this question (103). In a comparatively large sample of participants, they found that the VMPFC distinguished between both self and other-referential processing as well as self and semantic processing (103), a finding which was independently corroborated by a study which compared self-referential processing of trait words to general semantic knowledge (104). One issue with these findings is that the involvement of VMPFC in self-referential cognition does not necessarily indicate that this region is responsible for the memory advantage that occurs when making self-referential judgments. This missing piece of the puzzle came later, when Macrae and colleagues (105) investigated whether activity in VMPFC during self-referential encoding is associated with subsequent memory recall. The results of this study demonstrated that the magnitude of VMPFC activity during self-referential encoding was predictive of which traits were subsequently remembered best. These findings provided evidence that self-referential processing is special in so far that its memory advantage is not simply reducible to a deeper form of semantic encoding (87, 98). Moreover, these initial studies served to show that the MPFC is involved in self-referential cognition more generally and opened the door to experiments that move beyond self-referential memory encoding and focus instead on the nature of self-referential cognition itself.
The earliest attempts to examine potential moderators of self-referential cognition examined the role of emotional valence in self-referential thinking (106-108). As noted by Moran and colleagues (108), one of the confounds associated with this type of research is that positive traits are more likely to be judged as self-descriptive than negative traits (e.g., 42), making it difficult to tease apart emotional valence from self-relevance. In order to correct for this, Moran and colleagues employed a factorial design, in which self-relevance was crossed with emotional valence during a trait judgment task. Confirming prior work suggesting that VMPFC is sensitive to self-relevance (105), but not emotional valence (106), they found that VMPFC was indeed uniquely sensitive to self-relevance (108, see also 105, 109). Conversely, emotional valence was associated with activity in an area of the ventral anterior cingulate cortex that did not differentiate between high and low self-relevant traits (108). Such a finding would be expected if VMPFC was primarily involved in representing psychological knowledge about the self. Moreover this result dovetails nicely with earlier research in social cognition showing that the self-referential memory effect is sensitive to self-relevance (27) but not emotional valence (29).
Upon identifying the role of VMPFC in self-referential cognition, it was only a matter of time before researchers returned to the question of whether the neural representation of close others is similar to that for oneself. Early research in social cognition provided evidence that memory recall for traits judged with reference to close others is similar to those processed with reference to self (27-29, 31-33). Functional neuroimaging studies have so far obtained conflicting results, with some studies showing that thinking about self and close others leads to an MPFC response that is intermediate between that for self and that for familiar –but not close- others (110-112) and other studies finding no difference in MPFC response when comparing close and familiar others (113). Although there are more studies than these that have examined trait judgments for close friends and significant others they either lack an unknown person for comparison (114-117) or did not directly compare self and intimate others to unknown others (118-119) rendering it difficult to conclusively assess VMPFC's role in thinking about close others. At present, it appears that the preponderance of neuroscientific evidence is in line with behavioral findings in social cognition, converging on the theory that the neural representation of close others in the VMPFC lies somewhere between self and unknown others (110-112). If true, these results would lend support to the notion that close others are incorporated into one's self-schema (39-40, 110-112) and may help explain why people occasionally confuse their friends' traits for their own (41).
Recent developments in the neuroscience of self-referential cognition have seen researchers turn to the question of the VMPFC's involvement in thinking about oneself in the past and imagining oneself in the future (115, 120-122). This new line of research has been influenced by recent social psychological findings indicating that people introspect about their past self (i.e. “think about yourself when you were a senior in high school”) in much the same way that they reflect on other people. For example, individuals are more likely to overemphasize dispositional factors when explaining past behavior or thinking about future behavior (123-124). They are also more likely to recall autobiographical events - and imagine future ones - from a third- person perspective (124-125). Similarly, when participants are asked to make costly decisions (e.g., “how much time are willing to devote to tutoring a peer?”) about their present self, their future self or another person, their answers for their future self are more similar to those for other people than they are to the answers given for their present self (126). Taken together, these findings suggest that people often fail to fully consider how future events will impact their own future behavior, in much the same way that they occasionally fail to consider the impact of situational constraints on the current behavior of others (e.g., 48-49, 127-128). D'Argembeau and colleagues (115, 120) further investigated this dissociation of past/future self from present self in two studies in which thinking about one's present self was contrasted with thinking about oneself in the past, one's expected future self and other people. In line with the behavioral findings mentioned above, VMPFC was preferentially recruited for self-referential cognition invoking one's present self, but failed to differentiate between past self, future self and others (115, 120). Interestingly, when participants are asked to envision a future event that is related to their personal goals, such as a college graduation, VMPFC shows increased activity compared to future events that are not goal related (129). This suggests that envisioning future events that are relevant to one's personal goals elicits a representation of self that is comparable to that when thinking about the self in the present and richer than when considering future events that are not goal related (122).
Two recent studies have examined how the failure to fully consider one's future self impacts decisions to delay immediate rewards in favor of larger future ones, a phenomenon called temporal discounting (130). Using a trait-judgment task, Ersner-Hershfield and colleagues (121) found that the rostral anterior cingulate, contiguous with VMPFC, showed greater activity for present vs. future self, the magnitude of which predicted individual differences in the willingness to delay an immediate monetary reward (121). In a related study, Mitchell and colleagues (122) took a different tack and used an affective forecasting task in which participants judged how much they, or an unknown person, would enjoy a series of pleasurable activities tomorrow versus a year from now (122). Judgments with reference to the present-self (compared to future-self) recruited theVMPFC and the difference in VMPFC activity between present and future selves predicted subjects' willingness to wait several weeks for a larger monetary reward en lieu of accepting an immediate but smaller one (122). Of particular interest is the fact that the authors were able to show that this effect was specific to self-referential cognition as differences in VMPFC activity when making judgments about a present and future other was unrelated to temporal discounting (122). Taken together, these findings suggest that shortsighted decisions may be explained by people's failure to adequately simulate the cost that their future selves will have to pay, in effect, treating them like an altogether different person.
Another direction that research on self-referential processing has recently taken is the study of self-enhancement during self-evaluation and social comparisons (131). As reviewed earlier, individuals commonly view themselves as possessing more positive traits and judge themselves as being better-than-average when compared to peers (42-44). Social cognitive neuroscience research on this topic has shown that, although the VMPFC is involved in making self-evaluations generally, accuracy of self-evaluations is instead related to activity in the orbitofrontal cortex (OFC). For instance, when individual make overconfident judgments about their performance on a task, or when they compare their own personality to that of an average peer, activity in the OFC is inversely related to the degree to which they show self-enhancement (132-133). That is to say, the more activity in OFC during self-evaluations and social comparisons, the less bias people showed towards making self-enhancing comparisons. Interestingly, this effect also obtains for close others (i.e., romantic partners) who, like the self, are frequently judged to be better-than-average (134). Together these studies suggest that while the VMPFC is generally involved in self-evaluations, the degree to which these evaluations are biased towards unrealistically positive or overconfident judgments is related instead to activity in regions of the OFC.
The Neural Basis of Person Perception and Impression Formation
Surprisingly, research on the neural basis of person perception did not begin as a natural extension of the cognitive work on impression formation reviewed earlier. Instead, it was researchers seeking to understand the peculiar social deficits in neurodevelopmental disorders such as autism and Asperger's syndrome that first began studying the perception of other's mental states. These early studies were principally concerned with the neural substrates of the ability to infer the mental states of others, something for which patients with autism had shown a severe deficit (135-136). In the first two studies to examine this phenomenon, it was found that the DMPFC, anterior temporal poles, posterior cingulate, and temporoparietal junction exhibited greater activity when participants read stories which required subjects to attribute covert mental states to characters (137) or when participants judged whether a historical figure would have knowledge of everyday objects (138). The process of attributing mental states to others was termed mentalizing, a label which has stuck, leading to some confusion of terminology between research in social cognitive neuroscience and earlier work in social cognition which has traditionally referred to the process of understanding another person's behavior and intentions by the terms attribution, impression formation, or simply, person perception.
Since this early work, several studies have shown that the DMPFC and other areas associated with mentalizing are implicated in a wide range of tasks which all in some way involve thinking about the intentions and mental states of others (95). For instance, these regions exhibit increased activity when participants view animations depicting agentic behavior (139-142), when reading stories or viewing cartoons in which characters maintain a false belief about an event (81, 143-145), when making accurate inferences about another's emotions (146), and when viewing scenes of social interactions (147-149) or deception (150).
In an early review of the mentalizing literature, Gallagher and Frith (93) suggested that, of the many regions thought to be involved in mentalizing, the DMPFC was chiefly responsible for representing mental states, whereas other regions subserve a more general role in visual processing or episodic recollection depending on the stimulus modality and task demands. This conclusion was derived primarily from the results of two neuroimaging studies which showed that the DMPFC was the only region which differentiated between playing a computer game with an ostensibly real player compared to knowingly playing against the computer (although in reality everyone was always playing against the computer) (151-152). This conjecture concerning the dominant role of DMPFC in mentalizing was challenged in a study by Saxe and Wexler (145), who found that the temporoparietal junction was selectively involved in false belief reasoning, while activity in DMPFC generalized beyond false-belief reasoning and was involved in contemplating social information about a person more generally.
Recently, it has been argued that the DMPFC is particularly sensitive to conditions in which an individual is required to form social inferences based on minimal information (91, 153). For instance, when participants reason about uncertain beliefs, or attempt to infer someone's personality and preferences, the DMPFC shows a greater response relative to tasks that call for inferences about unambiguous beliefs and preferences (e.g. Erin always sits in the back of the class. Where does Erin like to sit?). On the other hand, the TPJ was shown to be primarily sensitive to reasoning about beliefs, compared to preferences, irrespective of their certainty (153). The results of this study suggest that the DMPFC is involved in abstracting person knowledge from others' behavior, whereas the TPJ may be more important for on-line inferences concerning what others believe about a particular situation.
In an earlier section, we reviewed research in social cognition that demonstrated a mnemonic advantage for impression formation over other strategies (54-56). The general explanation for this effect was analogous to that for self-referential memory in that impression formation was thought to promote a deeper form of encoding compared to other strategies (55). Neuroscientific evidence against this explanation came from a study by Mitchell and colleagues (154) demonstrating that semantic knowledge of psychological traits is primarily represented in the DMPFC, suggesting that like self-referential cognition, the neural representation of person knowledge is distinct from that for non-social forms of knowledge (154). Subsequent studies went on to demonstrate that activity in DMPFC when forming impressions of others (155), or when learning to associate person knowledge with faces (156), was predictive of later memory for person knowledge. Thus, like the self-referential memory effect, the mnemonic advantage afforded by impression formation appears to be not simply a depth-of-processing effect but is due to recruitment an entirely different system of brain regions specialized for social cognition (91). This work was extended by studies showing that the DMPFC is particularly sensitive to impressions formed based on psychological traits (157) and personality descriptions (158) and shows increased activity when impressions are formed based on contextual information compared to impressions formed based on non-verbal behavior (159). In addition, the DMPFC is also involved in forming spontaneous trait inferences. For example, when people read statements that communicate specific information about a personality trait (i.e., trait diagnostic) versus statements that do not (e.g., “He photocopied the article”), the DMPFC shows increased activity for trait diagnostic statements in the absence of explicit instructions to form an impression of the person (160-161).
Interestingly, as reviewed in the previous section, most studies of self-referential cognition use a familiar other (e.g., President Bush or a friend) as a control condition. Given the research reviewed above, one would expect that thinking about another person's traits would lead to increased DMPFC activity. Strangely, this result is seldom reported in the literature on self-referential cognition. Of the studies that directly compare other-referential cognition to self many studies failed to find any response in DMPFC for other-referential vs. self-referential cognition (113, 117-118, 162) whereas other studies instead find either greater DMPFC activity for self-, compared to other-, referential cognition (110, 114-116), or no difference between thinking about self and others (119). Only Pfeifer and colleagues (163) found greater DMPFC for other-referential cognition compared to self. Interestingly, when examining studies that report the contrast of other-referential judgments compared to semantic judgments, we find, as expected, greater activity in DMPFC (102, 117, 119).
Part of the variability across studies may be explained by the possibility that DMPFC is recruited when making trait judgments irrespective of the target (e.g., 119). This conjecture was demonstrated in a recent study in which individuals made trait and appearance based judgments for self, an intimately known other and a familiar, but not personally known, other. Although VMPFC showed the standard self-referential effect of greater activity for trait or appearance judgments of self compared to others, the DMPFC instead showed a different pattern –it exhibited greater activation for trait judgments than for appearance judgments, but did not discriminate between self and all other targets (111). This suggests that while VMPFC is sensitive to self-relevance, DMPFC appears to instead be sensitive to the domain of the task (traits vs. appearance) but does not clearly dissociate between self and other (although see 97).
The Role of Familiarity, Similarity and Intimacy in Self-Referential Cognition and Person Perception
As noted above, within the domain of self-referential cognition, a number of studies show that thinking about close others leads to a pattern of VMPFC activity that is somewhere in between the activity pattern exhibited when thinking about oneself and thinking about others (110-112). Similarly, studies on mentalizing have shown that forming impressions of similar others recruits approximately the same VMPFC region as thinking about oneself (114, 164-166). Across these disparate studies, there are three dimensions along which person perception can be said to operate: 1. Intimacy –a category reserved for significant others (e.g.,, close friends and family); 2. Familiarity –which describes people with whom we are familiar (e.g., acquaintances, movie actors and politicians), but for whom we do not possess intimate person knowledge; and finally, 3. Similarity –which describes our perception of another's similarity to ourselves, applicable both to close others and to relative strangers.
As mentioned previously, social cognitive research has shown that memory for traits processed with reference to an intimate other is intermediate between that for self and that for familiar, but not personally known, others (28-30, 32-33). Consistent with this observation, studies have shown that VMPFC activity when thinking about intimate others is also in between that obtained when thinking about oneself or unfamiliar others (110-112). Interestingly, compared to faces of familiar or unknown others, viewing faces of close others (e.g., friends and family) primarily yields activity centered more dorsally in DMPFC rather than VMPFC (167-168). One likely explanation for these divergent findings is that in the work of Gobbini and colleagues (167-168), participants were not explicitly required to reflect on the traits of others, but may have done so spontaneously for faces that they knew intimately. Such a conjecture is in accord with research on spontaneous social cognition (see the following section), which has generally implicated the DMPFC in the spontaneous representation of others' mental states.
Familiar others, such as famous public figures, are people for whom an individual has some personal knowledge, but are not intimately familiar with. At present, this dimension of person perception has received little attention in social cognitive neuroscience research; familiar others are generally used as a comparison for self-referential processing, but rarely have they been examined in their own right (i.e., by contrasting familiar with unfamiliar persons). An exception to this is research examining the neural basis of perceiving faces for which individuals have recently acquired some some amount of person knowledge (169-170). In these two studies, perceiving faces previously associated with person knowledge resulted in increased activity in DMPFC compared to novel (170) or perceptually familiar faces (169). These findings are compatible with the research reviewed above on viewing faces of close others (e.g., 167-168) and further suggest that DMPFC is involved in spontaneously representing person knowledge for intimate others, for whom we already posses extensive person knowledge, and for unknown others who have been rendered familiar through their association with dispositional information.
The third dimension along which person perception can occur is similarity. In general, research has shown that thinking about similar others activates a similar VMPFC region as thinking about oneself (114, 165-166). A particularly elegant demonstration of this made use of the phenomenon of repetition suppression, in which activity in a region is attenuated upon successive executions of the same cognitive operation, thereby allowing investigators to determine the sensitivity of a given region to certain features of a stimulus. Jenkins and colleagues (164) showed that VMPFC activity was suppressed when self-referential judgments were followed by judgments of a similar other, but was not suppressed when followed by judgments of a dissimilar other (164). These findings go beyond evidence of overlapping activity for self and similar others within VMPFC and suggest that the same neuronal population is in use when thinking about a similar other and when thinking about oneself. Interestingly, a recent study failed to observe an effect of similarity on activity in VMPFC (110). Across three experiments it was found that, although activity in this region dissociated close friends from unknown others, it failed to differentiate between similar and dissimilar friends or between similar and dissimilar others (110). Thus it is suggested that VMPFC is responding primarily to interpersonal closeness rather than similarity per se. As mentioned by the authors, one possible reason for the failure to find an effect of similarity may be that the similarity manipulation used in this study is not as extreme as that in the prior studies mentioned above which tended to operationalize similarity in terms of political beliefs (114, 164-166).
The distinctions between intimacy, familiarity and similarity suggest meaningful differences in the cognitive processes subserved by VMPFC and DMPFC. Findings from the studies reviewed above suggest that the VMPFC is sensitive to the overlap between self and close others (110-112) as well as self and similar others (114, 164-166), though generally only in tasks which require explicit self-reflection. The DMPFC, on the other hand, is primarily involved in representing person knowledge and mental states and, accordingly, does not distinguish between social targets based on intimacy (111) or similarity (165, although, see 166) in explicit trait attribution tasks. However, when recall of traits is cued, such as when viewing the face of an intimately known other (167-170), then DMPFC appears to be involved in the retrieval of person knowledge.
Spontaneous Social Cognition
Most of the research on the brain basis of social cognition has examined the neural underpinnings of explicit judgments about the self and others. However, real-world social cognition generally occurs spontaneously (53, 67, 171). Within the domain of self-referential cognition, three studies of implicit social cognition have recently emerged: the first demonstrates that VMPFC is spontaneously recruited for highly self-relevant biographical material (109), the second shows that the VMPFC tracks ownership of objects (172) and the third finds that VMPFC exhibits greater activation when viewing images representing identity categories for which an individual is highly schematic (173). In the first of these, Moran and colleagues employed an oddball paradigm, in which subjects were tasked with picking out the oddball in a series of sequentially presented words. Embedded in these words were biographical items of personal importance to the subject (i.e., the name of their street or the name of their family dog; (109). A comparison of these items to non self-relevant items revealed that the VMPFC was spontaneously activated upon reading biographical information. In the second study, participants were assigned imaginary ownership of a series of objects which could either belong to them or to another person. In this study, the authors found that a region of VMPFC that was shown to respond during self-referential trait judgments also responded more to objects which the participants “owned” (172). Finally, in the third study, Rameson and colleagues recruited subjects who identified highly with either athletics or science (i.e., self-schematic; (18). When subjects viewed photographs related to their respective self-schemas, they showed greater spontaneous recruitment of VMPFC for images that matched their self-schema (173). These studies suggest that VMPFC is sensitive to highly self-relevant information, ownership of objects and highly schematic information, all in the absence of explicit instructions to make a self-referential judgment.
Given the studies reviewed above, it might be expected that viewing one's own face would recruit VMPFC in much the same way that viewing autobiographical information does (e.g. (109)). Interestingly, research on viewing one's own face (i.e. self-face recognition) seldom reports activity anywhere in the medial prefrontal cortex (174-175 although see 176), suggesting that viewing one's own face, unlike viewing the faces of others, is not enough to elicit spontaneous social cognition about onself (for a similar argument see 177).
Within the domain of person perception, the study of spontaneous processes in social cognition has a much longer history. One of the first behavioral examples of spontaneous mental state attribution dates back to early work of Heider and Simmel (178), in which people were asked to describe the motion of simple animations of geometric shapes. In one condition, the movement paths of the shapes created the illusion that the objects were interacting with one another. When viewing these “social” animations, participants spontaneously constructed complex stories to describe what was occurring, frequently describing a rivalry among competing suitors who were attempting to win over the affections of a small circle. Inspired by this early work, Castelli and colleagues (140) showed that when participants view similar social animations they spontaneously recruit the DMPFC. A number of studies have subsequently replicated this finding using similar social animations (139, 141) or animations whose movements can be construed as intentional, or unintentional, depending on the surrounding context (142). Likewise, studies have shown that people spontaneously recruit DMPFC when viewing stimuli that strongly promote mentalizing (i.e., stories and scenes depicting social interactions). For instance, DMPFC is engaged when viewing short films depicting social interactions (147), when thinking about the thoughts and intentions of virtual characters while playing a video game (179), and is the only region sensitive to changes in characters when reading stories (180). Recently, the role of individual differences in the spontaneous recruitment of DMPFC was examined using natural social interaction scenes (148). In this study, participants who varied on a self-report measure of empathy viewed a series of social and non-social scenes while performing a simple categorization task. DMPFC was selectively engaged during social scene processing, and the magnitude of its activation was correlated with individual differences in empathizing. This preferential activation in DMPFC to social interaction scenes shares similarities to the work mentioned above in which DMPFC was spontaneous recruited when viewing the faces of close and intimate others (167-168) and when viewing faces which had been previously paired with person knowledge (169-170) (Figure 2). Taken together, these findings suggest that DMPFC is involved, not only in spontaneously retrieving person knowledge, but also in spontaneously extracting person knowledge when viewing social interactions.
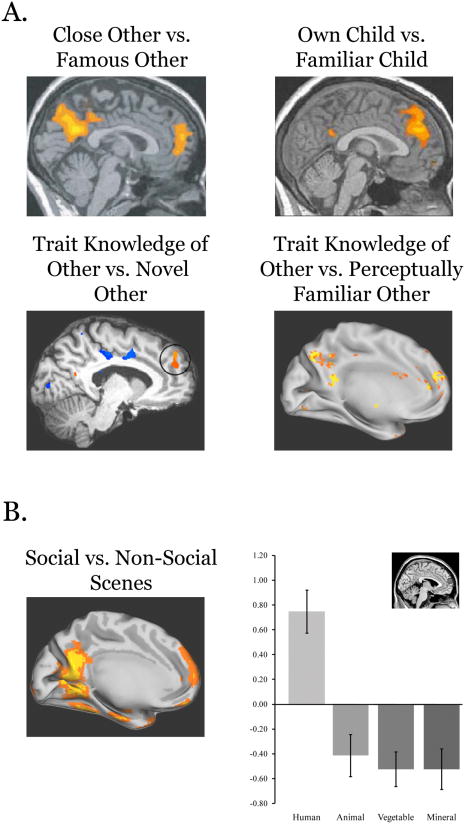
Figure. 2.
(A). The dorsal medial prefrontal cortex is spontaneously recruited when viewing faces of intimately known others and when viewing faces which have become associated with person knowledge through prior learning (167-170). (B) DMPFC is also spontaneously recruited when viewing complex social interaction between unknown people compared to non-social categories (i.e. animal, vegetable or mineral categories) (148). The similarities between these studies suggests that DMPFC is involved not only in the spontaneous retrieval of person knowledge when viewing intimately known others (170) but is also involved in spontaneously extracting person knowledge when viewing unfamiliar people engaged in social interactions (148).
The research on spontenaous social cogniton outlined above suggests that people automatically attempt to decipher the minds of others whenver they are presented with social stimuli. In many ways, these modern neuroscientific accounts bring us back full circle to the early work of Heider (2) who posited that people are constantly forming causal theories of others' behavior in order to predict what they'll do next. As we've seen, the methods of social cognitve neuroscience can be used to measure these spontaneous social cognitve processes, a feat which can be difficult to conduct in behavioral experiments. Moreover, with a deeper understanding of the features of social material that drive activity in the MPFC, it may eventually be possible to use activity in this region to uncover the specific contents of natural person perception, such as the precise trait that is being inferred during spontaneous trait inferences. Such ventures are already under way in other domains of cognitive neuroscience and we touch on this briefly in the next section.
Cognitive Processes and Representational Content in Social Cognitive Neuroscience
Social cognitive neuroscience has principally focused on the cognitive processes involved in thinking about the self and others. As we've just seen, many studies have examined whether thinking about particular social targets (i.e., others or, close friends) recruits similar brain regions, and by association similar cognitive processes, as thinking about the self (111-112, 114, 165). Social cognition has, however, traditionally emphasized not only the cognitive processes involved in thinking about people, but also the structure of the cognitive representations (i.e., schemas) implicit in different forms of social knowledge. For example, early research on implicit personality theory focused on the multidimensional structure of people's idiosyncratic representations of traits in order to explain individual differences in person perception (181-184).
Recent advances in neuroimaging methodology have begun to focus on the informational content contained in distributed patterns of activity. New methods, such as multivariate pattern analysis (MVPA), have revealed surprising new information about how the brain represents visual categories, such as faces (185-186) and the semantic meaning of nouns (187-188). One form of MVPA focuses specifically on the similarity between neural representations of different classes of stimuli (190). This analysis of representational similarity has revealed striking correspondence between the neural representation of visual objects in humans and monkeys (190) and shown neural evidence suggesting that the representation of different animal species follows an animacy gradient with primates and insect at opposite ends (191). Interestingly, many of the techniques used in representational similarity analysis of functional neuroimaging data (e.g., multidimensional scaling and clustering analysis) were also common in early investigations into the structure of social knowledge that took place in the 1960s and 1970s (e.g., (184, 192)). (for a review, see (193)). Although, these novel analytical tools have yet to be applied to the domain of social cognitive neuroscience, the next few years will undoubtedly see a surge of studies investigating the neural representation of social knowledge within the MPFC. Findings from such experiments promise to shed new light on the nature of the neural representation of social information, as well as serve to directly link neural data with extant theories concerning the structure of mental representations of self and others.
Is The Medial Prefrontal Cortex Critical for Social Cognition?
Throughout this review we focused specifically on the role of MPFC in self and other referential cognition. Although other regions may be called upon when thinking about oneself or forming an impression of others, we've argued that the MPFC is of central importance in representing self and person knowledge, particularly at the level of abstract psychological traits. For example, recent research offers evidence that brain regions outside the MPFC may have an important influence in shaping the way in which we construe our own traits as well as how we form impressions of others. As reviewed above, studies on motivated self-evaluations show that, although the MPFC is involved in self-evaluations generally, the accuracy these self-evaluations is related to activity in the OFC (132-133). Within the domain of impression formation, it has been shown that although the DMPFC is involved in abstracting traits and forming impressions, other regions, such as the amygdala are sensitive to the evaluative diagnosticity of information during first impressions (194) and may be involved in modulating DMPFC during the acquisition of person knowledge, increasing the strength with which social information is paired with different social targets (156).
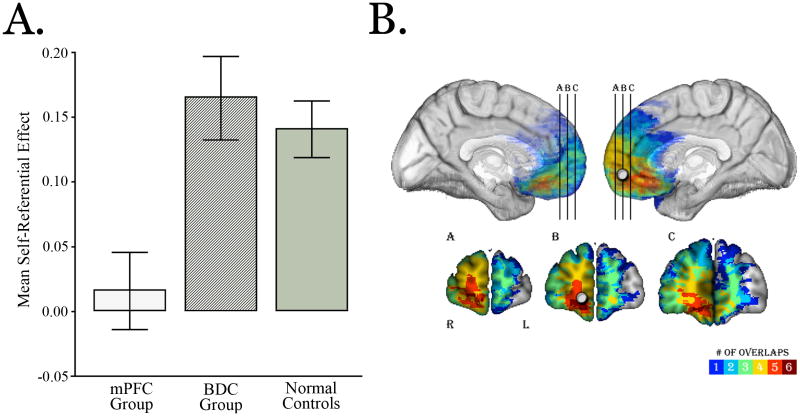
Research on atypical populations and patients with damage to the MPFC are important in demonstrating the critical role of this region self and other referential cognition. Studies of patients with autism suggest functional abnormalities in the representation of self (195) and of others (139) as evidenced by reduced recruitment of VMPFC when considering traits with reference to self (195) and reduced recruitment of DMPFC when viewing Heider and Simmel animations designed to illicit mental state inferences (139). Additional evidence comes from patients with damage to the medial prefrontal cortex. In a study of 4 patients with extensive damage to the VMPFC and orbitofrontal cortex, researchers found that these patients exhibited deficient self-monitoring of behavior, frequently violating social norms during an interpersonal interaction (196). Interestingly, these patients only became aware of their inappropriate behavior after being a shown a video tape of themselves, suggesting that, though they are aware of social norms, they nevertheless have difficulty monitoring their own behavior to avoid violating these norms. Finally, a recent study of patients with damage to VMPFC offers convincing evidence of the critical role of this region in self-referential processing. In this study, the authors selected six patients with VMPFC damage such that the region of maximal lesion overlap across patients was centered near the area that differentiated between self and other-referential judgments in the Kelley et al study (103) (Figure 3). These patients, along with a group of non-VMPFC patients and healthy controls, performed a standard self-referential encoding task (197). Compared to both the non-VMPFC patients and healthy controls, patients with VMPFC damage failed to show the standard self-referential memory advantage (Figure 3). Although it is difficult to say whether the VMPFC is uniquely specialized for self-referential processing, the evidence does suggest that the VMPFC is critical for both self-knowledge and self-monitoring.
Figure. 3.
(A) Compared to healthy control subjects and patients with damage outside the ventromedial prefrontal cortex (VMPFC), patients with VMPFC damage showed no evidence of the self-referential memory advantage (197). (B). Patients were selected so that the region of maximum lesion overlap was centered on the VMPFC region reported in Kelley and colleagues (103) which demonstrated greater activity during self referential processing. The gray dot represents the location of the region of interest from the Kelley et al study (MNI coordinates: 10,52,2).
Are The Ventral and Dorsal Regions of The MPFC Differentially Involved in Represeting Self and Person Knowledge?
It's been suggested that, although the VMPFC is frequently recruited during self-referential cognition, it is by no means selective for this process (198) as it also shows an increased response when thinking about close others and during mentalizing (199). Despite this overlap, the peak activation coordinates of studies in self and other referential cognition (Figure 1) suggest that the more ventral aspect of MPFC (Z = 0) is primarily engaged by self-referential tasks, whereas the more dorsal aspect (Z > 20) is involved in forming impressions of others, inferring their mental states and judging the relevance of personality traits of others (and occasionally also for self, see (111). A recent meta-analysis by Denny, Kober, Wager and Ochsner offers quantitative evidence of differential involvement of the MPFC in self and other-referential cognition (97). Using multilevel kernel density analysis (200) Denny and colleagues performed a meta-analysis of brain activation foci from studies involving self or other-related judgments (though not necessarily restricted to social or person knowledge). Direct comparison of self and other-related judgments revealed significantly more studies implicating the VMPFC for self-related judgments and, conversely, more studies implicating the DMPFC for other-related judgments. Moreover this distinction was further qualified by the results of a logistic regression analysis on MPFC activation coordinates from 67 studies showing that studies involving self or other judgments followed a ventral-dorsal gradient such that ventral regions showed a greater probability of studies involving self-referential cognition whereas dorsal regions had a greater probability of studies involving other-referential cognition (97). The findings from this meta-analysis offer evidence that, despite significant overlap, self and other-referential judgments are separable in the MPFC.
What then can we say about the function of the VMPFC and DMPFC? The evidence of a functional gradient in MPFC suggests some division of labor within the MPFC that maps onto different cognitive processes that are putatively engaged during self or other-referential cognition. One speculative hypothesis is that VMPFC is involved in simulating the mental states of others with reference to oneself (164), whereas more dorsal regions are involved in abstracting (148, 158, 161, 169), retrieving (167-168) and learning social knowledge (156). More recently, it's been suggested that the VMPFC is involved in ascribing meaning to a wide range of stimuli, both social and non-social, and that it its role in self-referential cognition is primarily because information about the self tends to be highly meaningful to individuals (201). In sum, our understanding of the precise computations carried out by the VMPC and DMPFC, and their relation to other regions involved in social cognition, is far from complete and will likely remain a topic of future research for some time to come.
Conclusions
Social knowledge, be it about the self or others, has traditionally been conceptualized as being no different than non-social knowledge. It has often been suggested that social cognition operates on the same principles as non-social cognition and that it is only the subject matter that differs (5, 14). However, findings from social cognitive neuroscience have challenged this view, demonstrating that knowledge about self and others is represented in fundamentally different brain regions than non-social knowledge (98, 103, 154).
In this review, we examined research on the role of the MPFC in representing self and person knowledge. Recent theories of MPFC function have suggested that the MPFC subserves reasoning about inherently ambiguous or counterfactual information (91), simulating future events and reconstructing past ones (202) ascribing personal meaning to information (201) and is involved in continuously generating predictions of people and events in order to guide behavior (203). What these theories share is a view of MPFC as being crucial for constructing inferences about phenomena that are, by their very nature, imprecise and often impossible to verify. Long ago Heider posited that people have a fundamental need to predict the future and control events (2). This drive pushes people to seek out explanations for others' behavior. Social cognitive neuroscience has identified an important role for the MPFC in representing knowledge about ourselves and about others. This combined font of information allows us to understand and predict the behavior of strangers, despite having limited knowledge of who they are and what they are like. As social cognitive neuroscience enters its second decade, we predict that the application of new methodologies, and new analytic techniques, will allow social scientists to investigate the content of people's mental representations and to track how these change over time, such as when familiarity matures into intimacy.
Acknowledgments
We thank Bryan Denny and Carissa Philippi for helpful discussion.
Writing of this manuscript was facilitated by an NIMH grant (59282) to TFH.
Contributor Information
Dylan D. Wagner, Department of Psychological and Brain Sciences, Center for Cognitive Neuroscience, Dartmouth College, Hanover, New Hampshire
James V. Haxby, Department of Psychological and Brain Sciences, Center for Cognitive Neuroscience, Dartmouth College, Hanover, New Hampshire
Todd F. Heatherton, Department of Psychological and Brain Sciences, Center for Cognitive Neuroscience, Dartmouth College, Hanover, New Hampshire
References
- 1.Festinger L. A Theory of Cognitive Dissonance. Stanford University Press; Stanford: 1957. [Google Scholar]
- 2.Heider F. The Psychology of Interpersonal Relations. Wiley; New York: 1958. [Google Scholar]
- 3.Heider F. On Social Cognition. American Psychologist. 1967;22(1):25–31. doi: 10.1037/h0024299. [DOI] [PubMed] [Google Scholar]
- 4.Lewin K. In: Field theory in social science; selected theoretical papers. C D, editor. Harper & Row; New York: 1951. [Google Scholar]
- 5.Fiske ST, Taylor SE. Social Cognition. Random House; New York: 1984. [Google Scholar]
- 6.Markus H, Zajonc RB. The cognitive perspective in social psychology. In: Lindzey G, Aronson E, editors. The Handbook of Social Psychology. Random House; New York: 1985. pp. 137–230. [Google Scholar]
- 7.Asch SE. Forming impressions of personality. The Journal of Abnormal and Social Psychology. 1946;41(3):258–290. doi: 10.1037/h0055756. [DOI] [PubMed] [Google Scholar]
- 8.Asch SE. Social Psychology. Prentice-Hall; New York: 1952. [Google Scholar]
- 9.Zajonc RB. Cognition and social cognition: A historical perspective. In: Festinger L, editor. Retrospections on social psychology. Oxford University Press; New York: 1980. [Google Scholar]
- 10.Carroll JS, Payne JW. Cognition and Social Behavior. Erlbaum; Hillsdale: 1976. [Google Scholar]
- 11.Landman J, Manis M. Social Cognition: Some Historical and Theoretical Perspectives. In: Leonard B, editor. Advances in Experimental Social Psychology. Academic Press; 1983. pp. 49–123. [Google Scholar]
- 12.Taylor SE. The interface of cognitive and social psychology. In: Harvey J, editor. Cognition, social behavior, and the environment. Lawrence Erlbaum; Hillsdale: 1981. [Google Scholar]
- 13.Wyer RS, Jr, Srull TK. Human cognition in its social context. Psychol Rev. 1986;93(3):322–359. [PubMed] [Google Scholar]
- 14.Klein SB, Kihlstrom JF. Elaboration, Organization, and the Self-Reference Effect in Memory. Journal of Experimental Psychology: General. 1986;115(1):26–38. doi: 10.1037//0096-3445.115.1.26. [DOI] [PubMed] [Google Scholar]
- 15.Klein SB, Loftus J. The Nature of Self-Referent Encoding: The Contributions of Elaborative and Organizational Processes. Journal of Personality and Social Psychology. 1988;55(1):5–11. [Google Scholar]
- 16.Bodenhausen GV, Todd AR. Social cognition. Wiley Interdisciplinary Reviews: Cognitive Science. 2010;1(2):160–171. doi: 10.1002/wcs.28. [DOI] [PubMed] [Google Scholar]
- 17.Bem DJ. Self-Perception Theory. In: Leonard B, editor. Advances in Experimental Social Psychology. Academic Press; 1972. pp. 1–62. [Google Scholar]
- 18.Markus H. Self-schemata and processing information about the self. Journal of Personality and Social Psychology. 1977;35(2):63–78. [Google Scholar]
- 19.Bargh JA, Bond RN, Lombardi WJ, Tota ME. The Additive Nature of Chronic and Temporary Sources of Construct Accessibility. Journal of Personality and Social Psychology. 1986;50(5):869–878. [Google Scholar]
- 20.Bargh JA, Thein RD. Individual Construct Accessibility, Person Memory, and the Recall-Judgment Link. The Case of Information Overload. Journal of Personality and Social Psychology. 1985;49(5):1129–1146. [Google Scholar]
- 21.Fong GT, Markus H. Self-schema and judgment about others. Social Cognition. 1982;1(3):191–204. [Google Scholar]
- 22.Higgins ET, King GA, Mavin GH. Individual construct accessibility and subjective impressions and recall. Journal of Personality and Social Psychology. 1982;43(1):35–47. [Google Scholar]
- 23.Markus H, Smith J, Moreland RL. Role of the self-concept in the perception of others. Journal of Personality and Social Psychology. 1985;49(6):1494–1512. [Google Scholar]
- 24.Catrambone R, Beike D, Niedenthal P. Is the self-concept a habitual referent in judgments of similarity? Psychological Science. 1996;7(3):158–163. [Google Scholar]
- 25.Srull TK, Gaelick L. General principles and individual differences in the self as a habitual reference point: An examination of self-other judgments of similarity. Social Cognition. 1983;2(2):108–121. [Google Scholar]
- 26.Hull JG, Levy AS. The organizational functions of the self: An alternative to the Duval and Wicklund Model of self-awareness. Journal of Personality and Social Psychology. 1979;37(5):756–768. [Google Scholar]
- 27.Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35(9):677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- 28.Bower GH, Gilligan SG. Remembering information related to one's self. Journal of Research in Personality. 1979;13(4):420–432. [Google Scholar]
- 29.Ferguson TJ, Rule BG, Carlson D. Memory for personally relevant information. Journal of Personality and Social Psychology. 1983;44(2):251–261. [Google Scholar]
- 30.Kuiper NA, Rogers TB. Encoding of personal information: Self-other differences. Journal of Personality and Social Psychology. 1979;37(4):499–514. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- 31.Symons CS, Johnson BT. The self-reference effect in memory: A meta-analysis. Psychological Bulletin. 1997;121(3):371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- 32.Keenan JM, Baillet SD. Memory for personally and socially significant events. Attention and Performance. 1980;8:651–669. [Google Scholar]
- 33.Lord CG. Schemas and images as memory aids: Two modes of processing social information. Journal of Personality and Social Psychology. 1980;38(2):257–269. [Google Scholar]
- 34.Maki RH, McCaul KD. The effects of self-reference versus other reference on the recall of traits and nouns. Bulletin of the Psychonomic Society. 1985;23:169–172. [Google Scholar]
- 35.Greenwald AG, Banaji MR. The Self as a Memory System: Powerful, but Ordinary. Journal of Personality and Social Psychology. 1989;57(1):41–54. [Google Scholar]
- 36.Kihlstrom JF, Klein SB. Handbook of social cognition, Vol 1: Basic processes. Lawrence Erlbaum; Hillsdale: 1994. The self as a knowledge structure; pp. 153–208. [Google Scholar]
- 37.Brenner M. The next-in-line effect. Journal of Verbal Learning and Verbal Behavior. 1973;12(3):320–323. [Google Scholar]
- 38.Brenner M. Unpublished doctoral dissertation. University of Michigan; 1976. Memory and interpersonal relations. [Google Scholar]
- 39.Aron A, Aron EN, Smollan D. Inclusion of Other in the Self Scale and the Structure of Interpersonal Closeness. Journal of Personality and Social Psychology. 1992;63(4):596–612. [Google Scholar]
- 40.Aron A, Aron EN, Tudor M, Nelson G. Close Relationships as Including Other in the Self. Journal of Personality and Social Psychology. 1991;60(2):241–253. [Google Scholar]
- 41.Mashek DJ, Aron A, Boncimino M. Confusions of self with close others. Personality and Social Psychology Bulletin. 2003;29(3):382–392. doi: 10.1177/0146167202250220. [DOI] [PubMed] [Google Scholar]
- 42.Taylor SE, Brown JD. Illusion and Well-Being: A Social Psychological Perspective on Mental Health. Psychological Bulletin. 1988;103(2):193–210. [PubMed] [Google Scholar]
- 43.Brown JD. Evaluations of Self and Others: Self-Enhancement Biases in Social Judgments. Social Cognition. 1986;4(4):353–376. [Google Scholar]
- 44.Dunning D, Meyerowitz JA, Holzberg AD. Ambiguity and self-evaluation: The role of idiosyncratic trait definitions in self-serving assessments of ability. Journal of Personality and Social Psychology. 1989;57(6):1082–1090. [Google Scholar]
- 45.Kruger J, Dunning D. Unskilled and unaware of it: How difficulties in recognizing one's own incompetence lead to inflated self-assessments. Journal of Personality and Social Psychology. 1999;77(6):1121–1134. doi: 10.1037//0022-3514.77.6.1121. [DOI] [PubMed] [Google Scholar]
- 46.Murray SL, Holmes JG, Griffin DW. The self-fulfilling nature of positive illusions in romantic relationships: love is not blind, but prescient. J Pers Soc Psychol. 1996;71(6):1155–1180. doi: 10.1037//0022-3514.71.6.1155. [DOI] [PubMed] [Google Scholar]
- 47.Taylor SE, Koivumaki JH. The perception of self and others: acquaintanceship, affect, and actor-observer differences. J Pers Soc Psychol. 1976;33(4):403–408. doi: 10.1037//0022-3514.33.4.403. [DOI] [PubMed] [Google Scholar]
- 48.Jones EE, Davis KE. From Acts To Dispositions The Attribution Process In Person Perception. In: Leonard B, editor. Advances in Experimental Social Psychology. Academic Press; 1966. pp. 219–266. [Google Scholar]
- 49.Jones EE, Harris VA. The attribution of attitudes. Journal of Experimental Social Psychology. 1967;3(1):1–24. [Google Scholar]
- 50.Gordon SE, Wyer RS. Person Memory: Category-Set-Size Effects on the Recall of a Person's Behaviors. Journal of Personality and Social Psychology. 1987;53(4):648–662. [Google Scholar]
- 51.Klein SB, Loftus J. Behavioral Experience and Trait Judgments about the Self. Personality and Social Psychology Bulletin. 1993;19(6):740–745. [Google Scholar]
- 52.Klein SB, Loftus J, Trafton JG, Fuhrman RW. Use of Exemplars and Abstractions in Trait Judgments: A Model of Trait Knowledge About the Self and Others. Journal of Personality and Social Psychology. 1992;63(5):739–753. [Google Scholar]
- 53.Winter L, Uleman JS. When are social judgments made? Evidence for the spontaneousness of trait inferences. Journal of Personality and Social Psychology. 1984;47(2):237–252. doi: 10.1037//0022-3514.47.2.237. [DOI] [PubMed] [Google Scholar]
- 54.Hamilton DL, Katz LB, Leirer VO. Cognitive representation of personality impressions: Organizational processes in first impression formation. Journal of Personality and Social Psychology. 1980;39(6):1050–1063. [Google Scholar]
- 55.Hamilton DL, Katz LB, Leirer VO. Organizational processes in impression formation. Person Memory: The Cognitive Basis of Social Perception. 1980:121–153. [Google Scholar]
- 56.Hamilton DL. Cognitive representations of persons. Social Cognition: The Ontario Symposium. 1981;1:135–159. [Google Scholar]
- 57.Srull TK, Wyer RS., Jr Person Memory and Judgment. Psychological Review. 1989;96(1):58–82. doi: 10.1037/0033-295x.96.1.58. [DOI] [PubMed] [Google Scholar]
- 58.Wyer RS, Jr, Bodenhausen GV, Srull TK. The cognitive representation of persons and groups and its effect on recall and recognition memory. Journal of Experimental Social Psychology. 1984;20(5):445–469. [Google Scholar]
- 59.Klein SB, Loftus J. The role of abstract and exemplar-based knowledge in self-judgments: Implications for a cognitive model of the self. Advances in Social Cognition. 1990;3:131–139. [Google Scholar]
- 60.Klein SB, Lax ML. The unanticipated resilience of trait self-knowledge in the face of neural damage. Memory. 2010;18(8):918–948. doi: 10.1080/09658211.2010.524651. [DOI] [PubMed] [Google Scholar]
- 61.Klein SB, Loftus J, Kihlstrom JF. Self-knowledge of an amnesic patient: Toward a neuropsychology of personality and social psychology. Journal of Experimental Psychology: General. 1996;125:250–260. doi: 10.1037//0096-3445.125.3.250. [DOI] [PubMed] [Google Scholar]
- 62.Tulving E. Self-knowledge of an amnesic individual is represented abstractly. In: Srull TK, Wyer RS Jr, editors. The mental representation of trait and autobiographical knowledge about the self. Erlbaum; Hillsdale, NJ: 1993. [Google Scholar]
- 63.Johnson MK, Kim JK, Risse G. Do Alcoholic Korsakoff's Syndrome Patients Acquire Affective Reactions? Journal of Experimental Psychology: Learning, Memory and Cognition. 1985;11(1):22–36. doi: 10.1037//0278-7393.11.1.22. [DOI] [PubMed] [Google Scholar]
- 64.Andersen SM, Chen S. The relational self: An interpersonal social-cognitive theory. Psychological Review. 2002;109(4):619–645. doi: 10.1037/0033-295x.109.4.619. [DOI] [PubMed] [Google Scholar]
- 65.Andersen SM, Glassman NS, Chen S, Cole SW. Transference in Social Perception: The Role of Chronic Accessibility in Significant- Other Representations Journal of Personality and Social Psychology. 1995;69(1):41–57. doi: 10.1037//0022-3514.69.1.41. [DOI] [PubMed] [Google Scholar]
- 66.Chen S, Andersen SM. Relationships from the Past in the Present: Significant-Other Representations and Transference in Interpersonal Life. In: Mark PZ, editor. Advances in Experimental Social Psychology. Academic Press; 1999. pp. 123–190. [Google Scholar]
- 67.Uleman JS. Spontaneous versus intentional inferences in impression formation. In: Chaiken S, Trope Y, editors. Dual-process theories in social psychology. Guilford; New York: 1999. pp. 141–160. [Google Scholar]
- 68.Todorov A, Uleman JS. Spontaneous trait inferences are bound to actors' faces: Evidence from a false recognition paradigm. Journal of Personality and Social Psychology. 2002;83(5):1051–1065. [PubMed] [Google Scholar]
- 69.Todorov A, Uleman JS. The efficiency of binding spontaneous trait inferences to actors' faces. Journal of Experimental Social Psychology. 2003;39(6):549–562. [Google Scholar]
- 70.Todorov A, Uleman JS. The person reference process in spontaneous trait inferences. Journal of Personality and Social Psychology. 2004;87(4):482–493. doi: 10.1037/0022-3514.87.4.482. [DOI] [PubMed] [Google Scholar]
- 71.Bargh JA. The Four Horsemen of automaticity: Awareness, efficiency, intention, and control in social cognition. In: Wyer RS Jr, Srull TK, editors. Handbook of social cognition. Erlbaum; Hillsdale: 1994. [Google Scholar]
- 72.Carlston DE, Skowronski JJ. Savings in the Relearning of Trait Information as Evidence for Spontaneous Inference Generation. Journal of Personality and Social Psychology. 1994;66(5):840–856. [Google Scholar]
- 73.Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293(5539):2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- 74.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45(1):32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 75.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 76.Whalen PJ, Davis FC, Oler JA, Kim H, Kim JM, Neta M. Human amygdala responses to facial expressions of emotion. In: Phelps EA, Whalen PJ, editors. The Human Amygdala. Guilford Press; New York: 2009. [Google Scholar]
- 77.Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):435–445. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 79.Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- 80.Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annu Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- 81.Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 82.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 83.Powers KE, Wagner DD, Norris CJ, Heatherton TF. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9(8):1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- 85.Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci. 2011 doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wagner DD, Heatherton TF. Giving in to temptation: The emerging cognitive neuroscience of self-regulatory failure. In: Baumeister RF, Vohs KD, editors. The Handbook of Self-Regulation. 2nd. Guilford Press; New York: 2010. [Google Scholar]
- 87.Heatherton TF. Neuroscience of self and self-regulation. Annu Rev Psychol. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell JP, Heatherton TF. Components of a social brain. In: Gazzaniga MS, editor. Cognitive Neurosciences IV. MIT Press; Cambridge, MA: 2009. pp. 951–958. [Google Scholar]
- 89.Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 90.Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- 91.Mitchell JP. Social psychology as a natural kind. Trends Cogn Sci. 2009;13(6):246–251. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 94.Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- 95.Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30(3):829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Overwalle F. A dissociation between social mentalizing and general reasoning. NeuroImage. 2011;54(2):1589–1599. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 97.Denny BT, Kober H, Wager TD, Ochsner K. A Meta-Analysis of Functional Neuroimaging Studies of Self and Other Judgments Reveals a Spatial Gradient for Mentalizing in Medial Prefrontal Cortex. Journal of Cognitive Neuroscience. doi: 10.1162/jocn_a_00233. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heatherton TF, Macrae CN, Kelley WM. What the Social Brain Sciences Can Tell Us about the Self. Current Directions in Psychological Science. 2004;13(5):190–193. [Google Scholar]
- 99.Macrae CN, Kelley WM, Heatherton TF. A self less ordinary: The medial prefrontal cortex and you. In: Gazzaniga MS, editor. Cognitive Neurosciences III. MIT Press; Cambridge, MA: 2004. pp. 1067–1076. [Google Scholar]
- 100.Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nature Neuroscience. 1999;2(4):311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- 101.Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281(5380):1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 102.Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, et al. In search of the self: A positron emission tomography study. Psychological Science. 1999;10(1):26–34. [Google Scholar]
- 103.Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 104.Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(Pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 105.Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- 106.Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160(11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- 107.Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, et al. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22(4):1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 108.Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci. 2006;18(9):1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- 109.Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Soc Neurosci. 2009;4(3):197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krienen FM, Tu PC, Buckner RL. Clan mentality: evidence that the medial prefrontal cortex responds to close others. J Neurosci. 2010;30(41):13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moran JM, Lee SM, Gabrieli JDE. Dissociable Neural Systems Supporting Knowledge about Human Character and Appearance in Ourselves and Others. Journal of Cognitive Neuroscience. 2010:1–9. doi: 10.1162/jocn.2010.21580. [DOI] [PubMed] [Google Scholar]
- 112.Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self-representation. Neuroimage. 2007;34(3):1310–1316. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 113.Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50(3):1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- 115.D'Argembeau A, Feyers D, Majerus S, Collette F, Van der Linden M, et al. Self-reflection across time: cortical midline structures differentiate between present and past selves. Soc Cogn Affect Neurosci. 2008;3(3):244–252. doi: 10.1093/scan/nsn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- 117.Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 118.Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 119.Vanderwal T, Hunyadi E, Grupe DW, Connors CM, Schultz RT. Self, mother and abstract other: an fMRI study of reflective social processing. Neuroimage. 2008;41(4):1437–1446. doi: 10.1016/j.neuroimage.2008.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.D'Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Salmon E. Modulation of medial prefrontal and inferior parietal cortices when thinking about past, present, and future selves. Soc Neurosci. 2010;5(2):187–200. doi: 10.1080/17470910903233562. [DOI] [PubMed] [Google Scholar]
- 121.Ersner-Hershfield H, Wimmer GE, Knutson B. Saving for the future self: neural measures of future self-continuity predict temporal discounting. Soc Cogn Affect Neurosci. 2009;4(1):85–92. doi: 10.1093/scan/nsn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitchell JP, Schirmer J, Ames DL, Gilbert DT. Medial prefrontal cortex predicts intertemporal choice. J Cogn Neurosci. 2011;23(4):857–866. doi: 10.1162/jocn.2010.21479. [DOI] [PubMed] [Google Scholar]
- 123.Nussbaum S, Trope Y, Liberman N. Creeping Dispositionism: The Temporal Dynamics of Behavior Prediction. Journal of Personality and Social Psychology. 2003;84(3):485–497. [PubMed] [Google Scholar]
- 124.Pronin E, Ross L. Temporal differences in trait self-ascription: when the self is seen as an other. J Pers Soc Psychol. 2006;90(2):197–209. doi: 10.1037/0022-3514.90.2.197. [DOI] [PubMed] [Google Scholar]
- 125.Libby LK, Eibach RP. Looking back in time: self-concept change affects visual perspective in autobiographical memory. J Pers Soc Psychol. 2002;82(2):167–179. [PubMed] [Google Scholar]
- 126.Pronin E, Olivola CY, Kennedy KA. Doing unto future selves as you would do unto others: psychological distance and decision making. Pers Soc Psychol Bull. 2008;34(2):224–236. doi: 10.1177/0146167207310023. [DOI] [PubMed] [Google Scholar]
- 127.Trope Y, Liberman N. Temporal Construal. Psychological Review. 2003;110(3):403–421. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- 128.Gilbert DT, Malone PS. The correspondence bias. Psychol Bull. 1995;117(1):21–38. doi: 10.1037/0033-2909.117.1.21. [DOI] [PubMed] [Google Scholar]
- 129.D'Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, et al. The neural basis of personal goal processing when envisioning future events. Journal of Cognitive Neuroscience. 2010;22(8):1701–1713. doi: 10.1162/jocn.2009.21314. [DOI] [PubMed] [Google Scholar]
- 130.Chapman GB. Temporal discounting and utility for health and money. Journal of Experimental Psychology: Learning Memory and Cognition. 1996;22(3):771–791. doi: 10.1037//0278-7393.22.3.771. [DOI] [PubMed] [Google Scholar]
- 131.Beer J. This time with motivation: The implications of social neuroscience for research on motivated self- and other-perception (and vice versa) Motivation and Emotion. 2012;36(1):38–45. [Google Scholar]
- 132.Beer JS, Hughes BL. Neural systems of social comparison and the “above-average” effect. NeuroImage. 2010;49(3):2671–2679. doi: 10.1016/j.neuroimage.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 133.Beer JS, Lombardo MV, Bhanji JP. Roles of medial prefrontal cortex and orbitofrontal cortex in self-evaluation. Journal of Cognitive Neuroscience. 2010;22(9):2108–2119. doi: 10.1162/jocn.2009.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hughes BL, Beer JS. Orbitofrontal Cortex and Anterior Cingulate Cortex Are Modulated by Motivated Social Cognition. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr213. [DOI] [PubMed] [Google Scholar]
- 135.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 136.Happe FGE. An advanced test of theory of mind: Understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. Journal of Autism and Developmental Disorders. 1994;24(2):129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- 137.Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57(2):109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- 138.Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. NeuroReport. 1995;6(13):1741–1746. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 139.Castelli F, Frith C, Happé F, Frith U. Autism, asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 140.Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 141.Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci. 2007;19(11):1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- 142.Wheatley T, Milleville SC, Martin A. Understanding animate agents: distinct roles for the social network and mirror system. Psychol Sci. 2007;18(6):469–474. doi: 10.1111/j.1467-9280.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 143.Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: An fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 144.Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18(2):262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- 145.Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43(10):1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 146.Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proc Natl Acad Sci U S A. 2009;106(27):11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, et al. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21(3):1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 148.Wagner DD, Kelley WM, Heatherton TF. Individual Differences in the Spontaneous Recruitment of Brain Regions Supporting Mental State Understanding When Viewing Natural Social Scenes. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wolf I, Dziobek I, Heekeren HR. Neural correlates of social cognition in naturalistic settings: a model-free analysis approach. Neuroimage. 2010;49(1):894–904. doi: 10.1016/j.neuroimage.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 150.Grezes J, Frith CD, Passingham RE. Inferring false beliefs from the actions of oneself and others: an fMRI study. Neuroimage. 2004;21(2):744–750. doi: 10.1016/S1053-8119(03)00665-7. [DOI] [PubMed] [Google Scholar]
- 151.Gallagher HL, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. Neuroimage. 2002;16(3):814–821. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- 152.McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proc Natl Acad Sci U S A. 2001;98(20):11832–11835. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jenkins AC, Mitchell JP. Mentalizing under uncertainty: dissociated neural responses to ambiguous and unambiguous mental state inferences. Cereb Cortex. 2010;20(2):404–410. doi: 10.1093/cercor/bhp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci U S A. 2002;99(23):15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mitchell JP, Macrae CN, Banaji MR. Encoding-specific effects of social cognition on the neural correlates of subsequent memory. J Neurosci. 2004;24(21):4912–4917. doi: 10.1523/JNEUROSCI.0481-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baron SG, Gobbini MI, Engell AD, Todorov A. Amygdala and dorsomedial prefrontal cortex responses to appearance-based and behavior-based person impressions. Social Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. Neuroimage. 2005;28(4):757–762. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 158.Mitchell JP, Neil Macrae C, Banaji MR. Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26(1):251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 159.Zaki J, Hennigan K, Weber J, Ochsner KN. Social cognitive conflict resolution: contributions of domain-general and domain-specific neural systems. J Neurosci. 2010;30(25):8481–8488. doi: 10.1523/JNEUROSCI.0382-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ma N, Vandekerckhove M, Van Overwalle F, Seurinck R, Fias W. Spontaneous and intentional trait inferences recruit a common mentalizing network to a different degree: Spontaneous inferences activate only its core areas. Soc Neurosci. 2010:1–16. doi: 10.1080/17470919.2010.485884. [DOI] [PubMed] [Google Scholar]
- 161.Mitchell JP, Cloutier J, Banaji MR, Macrae CN. Medial prefrontal dissociations during processing of trait diagnostic and nondiagnostic person information. Soc Cogn Affect Neurosci. 2006;1(1):49–55. doi: 10.1093/scan/nsl007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Powell LJ, Macrae CN, Cloutier J, Metcalfe J, Mitchell JP. Dissociable neural substrates for agentic versus conceptual representations of self. J Cogn Neurosci. 2010;22(10):2186–2197. doi: 10.1162/jocn.2009.21368. [DOI] [PubMed] [Google Scholar]
- 163.Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. J Cogn Neurosci. 2007;19(8):1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jenkins AC, Macrae CN, Mitchell JP. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proc Natl Acad Sci U S A. 2008;105(11):4507–4512. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- 166.Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 167.Gobbini MI, Leibenluft E, Santiago N, Haxby JV. Social and emotional attachment in the neural representation of faces. Neuroimage. 2004;22(4):1628–1635. doi: 10.1016/j.neuroimage.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 168.Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56(4):225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 169.Cloutier J, Kelley WM, Heatherton TF. The influence of perceptual and knowledge-based familiarity on the neural substrates of face perception. Soc Neurosci. 2010:1–13. doi: 10.1080/17470911003693622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Todorov A, Gobbini MI, Evans KK, Haxby JV. Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia. 2007;45(1):163–173. doi: 10.1016/j.neuropsychologia.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 171.Zaki J, Ochsner K. The need for a cognitive neuroscience of naturalistic social cognition. Ann N Y Acad Sci. 2009;1167:16–30. doi: 10.1111/j.1749-6632.2009.04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim K, Johnson MK. Extended self: medial prefrontal activity during transient association of self and objects. Social Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rameson LT, Satpute AB, Lieberman MD. The neural correlates of implicit and explicit self-relevant processing. Neuroimage. 2010;50(2):701–708. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- 174.Keenan JP, Wheeler MA, Gallup GG, Jr, Pascual-Leone A. Self-recognition and the right prefrontal cortex. Trends in Cognitive Sciences. 2000;4(9):338–344. doi: 10.1016/s1364-6613(00)01521-7. [DOI] [PubMed] [Google Scholar]
- 175.Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere an event-related fMRI study. NeuroImage. 2005;25(3):926–935. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 176.Kircher TTJ, Senior C, Phillips ML, Benson PJ, Bullmore ET, et al. Towards a functional neuroanatomy of self processing: effects of faces and words. Cognitive Brain Research. 2000;10(1-2):133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- 177.Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 178.Heider F, Simmel M. An Experimental Study of Apparent Behavior. The American Journal of Psychology. 1944;57(2):243–259. [Google Scholar]
- 179.Spiers HJ, Maguire EA. Spontaneous mentalizing during an interactive real world task: an fMRI study. Neuropsychologia. 2006;44(10):1674–1682. doi: 10.1016/j.neuropsychologia.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 180.Speer NK, Reynolds JR, Swallow KM, Zacks JM. Reading stories activates neural representations of visual and motor experiences. Psychol Sci. 2009;20(8):989–999. doi: 10.1111/j.1467-9280.2009.02397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cohen CE, Ebbesen EB. Observational goals and schema activation: A theoretical framework for behavior perception. Journal of Experimental Social Psychology. 1979;15(4):305–329. [Google Scholar]
- 182.Critcher CR, Dunning D. Egocentric pattern projection: How implicit personality theories recapitulate the geography of the self. Journal of Personality and Social Psychology. 2009;97(1):1–16. doi: 10.1037/a0015670. [DOI] [PubMed] [Google Scholar]
- 183.Rosenberg S, Sedlak A. Structural Representations of Implicit Personality Theory. In: Leonard B, editor. Advances in Experimental Social Psychology. Academic Press; 1972. pp. 235–297. [Google Scholar]
- 184.Wiggins JS. Personality Structure. Annual Review of Psychology. 1968;19(1):293–350. doi: 10.1146/annurev.ps.19.020168.001453. [DOI] [PubMed] [Google Scholar]
- 185.Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 186.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10(9):424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 187.Mitchell TM, Shinkareva SV, Carlson A, Chang KM, Malave VL, et al. Predicting Human Brain Activity Associated with the Meanings of Nouns. Science. 2008;320(5880):1191–1195. doi: 10.1126/science.1152876. [DOI] [PubMed] [Google Scholar]
- 188.Shinkareva SV, Malave VL, Mason RA, Mitchell TM, Just MA. Commonality of neural representations of words and pictures. NeuroImage. 2011;54(3):2418–2425. doi: 10.1016/j.neuroimage.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 189.Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis - connecting the branches of systems neuroscience. Front Syst Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kriegeskorte N, Mur M, Ruff DA, Kiani R, Bodurka J, et al. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron. 2008;60(6):1126–1141. doi: 10.1016/j.neuron.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Connolly AC, Guntupalli JS, Gors J, Hanke M, Halchenko YO, et al. The representation of biological classes in the human brain. J Neurosci. 2012;32(8):2608–2618. doi: 10.1523/JNEUROSCI.5547-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rosenberg S, Nelson C, Vivekananthan PS. A multidimensional apporach to the structure of personality impressions. Journal of Personality and Social Psychology. 1968;9(4):283–294. doi: 10.1037/h0026086. [DOI] [PubMed] [Google Scholar]
- 193.Jones LE. Multidimensional Models of Social Perception, Cognition and Behavior. Applied Psychological Measurement. 1983;7(4):451–472. [Google Scholar]
- 194.Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat Neurosci. 2009;12(4):508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- 195.Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, et al. Atypical neural self-representation in autism. Brain: A Journal of Neurology. 2010;133(Pt 2):611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- 196.Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience. 2006;18(6):871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- 197.Philippi CL, Duff MC, Denburg NL, Tranel D, Rudrauf D. Medial pFC Damage Abolishes the Self-reference. Effect Journal of Cognitive Neuroscience. 2011:1–7. doi: 10.1162/jocn_a_00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gillihan SJ, Farah MJ. Is Self Special? A Critical Review of Evidence From Experimental Psychology and Cognitive Neuroscience. Psychological Bulletin. 2005;131(1):76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- 199.Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review. 2009;116(1):252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- 200.Kober H, Wager TD. Meta-analysis of neuroimaging data. 2. Vol. 1. Wiley Interdisciplinary Reviews: Cognitive Science; 2010. pp. 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 203.Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1235–1243. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage. 2008;42(2):717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading/Resources
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Fiske ST. Thinking Is for Doing: Portraits of Social Cognition From Daguerreotype to Laserphoto. Journal of Personality and Social Psychology. 1992;63(6):877–889. doi: 10.1037//0022-3514.63.6.877. [DOI] [PubMed] [Google Scholar]
- Special Issue: Social Neuroscience and Its Contribution to Social Psychological Theory. Social Cognition. 2010;(28):663–757. [Google Scholar]
- Wegner DM, Vallacher RR. Implicit Psychology: An Introduction to Social Cognition. Oxford University Press; New York: 1977. [Google Scholar]
- Todorov A, Fiske S, Prentice D. Social Neuroscience: Toward Understanding the Underpinnings of the Social Mind. Oxford University Press; New York: 2011. [Google Scholar]