Abstract
Dapivirine, a non-nucleoside reverse transcriptase inhibitor, is a potent and promising anti-HIV molecule. It is currently being investigated for use as a vaginal microbicide in two dosage forms, a semi-solid gel and a silicone elastomer ring. Quick-dissolving films are promising and attractive dosage forms that may provide an alternative platform for the vaginal delivery of microbicide drug candidates. Vaginal films may provide advantages such as discreet use, no product leakage during use, lack of requirement for an applicator for insertion, rapid drug release and minimal packaging and reduced wastage. Within this study the in vitro bioactivity of dapivirine as compared to the NNRTI UC781 was further established and a quick dissolve film was developed for vaginal application of dapivirine for prevention of HIV infection. The developed film was characterized with respect to its physical and chemical attributes including water content, mechanical strength, drug release profile, permeability, compatibility with lactobacilli and bioactivity. The anti-HIV activity of the formulated dapivirine film was confirmed in in vitro and ex vivo models. Importantly the physical and chemical properties of the film as well as its bioactivity were maintained for a period of 18 months. In conclusion, a vaginal film containing dapivirine was developed and characterized. The film was shown to prevent HIV-1 infection in vitro and ex vivo and have acceptable characteristics which make this film a promising candidate for testing as vaginal microbicide.
Keywords: Dapivirine, TMC120, UC781, Microbicides, Thin Films, HIV prevention, Vaginal Drug Delivery
Introduction
During 2009, 2.6 million people became infected with HIV with 1.8 million deaths resulting in that year from AIDS related cases (1). Prevention strategies of HIV infection, such as condom use, did not prove to be effective as their use may be restricted by social and cultural normality. Thus a method for prevention of HIV transmission is required to alter the negative impact of this epidemic. Since heterosexual transmission is the major pathway of HIV transmission, prevention methods such as microbicide products may provide an important tool in combating the spread of infection. Microbicide products are being developed for use vaginally or rectally to prevent sexual transmission of HIV-1 (2).
Microbicides can be classified by their mode of action into non-specific and HIV-specific. The HIV-specific microbicides include entry, fusion, integrase, or reverse transcriptase inhibitors (3, 4). In clinical trials, microbicide candidate products such as nonoxynol-9, Carraguard and PRO2000 failed either due to damage to the vaginal epithelium resulting in increased infection in women using the product or lack of efficacy (5–7). In 2010, the Center for AIDS Program of Research in South Africa (CAPRISA) 004 trial evaluated the effectiveness and safety of a 1% tenofovir vaginal gel product for the prevention of HIV-1 sexual transmission in women (8). Tenofovir is an adenosine nucleotide reverse transcriptase inhibitor (NRTI) that blocks HIV-1 transmission by inhibiting the reverse transcription of the viral RNA. It was shown by in vitro and ex vivo testing that 1% tenofovir vaginal gel has the potential to be a topical microbicide (9). The results from the CAPRISA 004 trial showed that 1% tenofovir gel administered in a coitally related fashion reduced HIV-1 infection incidents by an estimated 39% (8). The success of tenofovir gel underscores the potential use of antiretroviral agents (ARVs) as topical microbicides.
One class of ARVs is the non-nucleoside reverse transcriptase inhibitors (NNRTI). NNRTIs are being evaluated as microbicide drug candidates. The first example of protection using in vitro models with NNRTI’s was with UC781, a potent anti-HIV drug candidate with hydrophobic nature (10–13). Another NNRTI being developed as a topical microbicide product is dapivirine (4[4-[(2,4,6-trimethylphenyl)amino-2-pyrimidinyl]amino]benzonitrile) or (TMC120). Data shows that dapivirine has a favorable toxicity profile and potent anti-HIV activity against a panel of HIV-1 isolates and a wide range of NNRTI-resistant isolates (14). Dapivirine has been evaluated in vaginal gel and intravaginal ring (IVR) dosage forms (15–20). A dual chamber model of female cervical mucosa was used to test the ability of dapivirine gel to block HIV-1 infection of sub-epithelial targets and confirmed activity at sub-micromolar concentrations (21). In addition, dapivirine gel was shown to be successful in preventing HIV-1 vaginal transmission by cell associated HIV in a humanized severe combined immunodeficient (hu-SCID) mouse model (20). A study by Nuttal et al. showed the tendency of dapivirine to accumulate in vaginal and cervical tissues for >24 h which supports its development as a microbicide for once daily application. On the other hand, for non daily applications, IVRs have become the focus as controlled drug delivery systems. Both reservoir and matrix type rings containing dapivirine were developed. A study conducted by Malcolm et al. (19) using the core type silicone elastomer demonstrated in in vitro drug release studies zero-order release of dapivirine from the ring for 71 days with about 140 μg dapivirine released daily.
Clinically, several phase I and I/II studies evaluated safety and pharmacokinetics (PK) of dapivirine gel and ring products. Pharmacokinetic studies conducted for dapivirine vaginal gel formulation indicated safety and showed a low systemic absorption of dapivirine (22–25). Intravaginal rings containing dapivirine were evaluated in two Phase 1 studies for safety and PK over a seven day application period and showed safety and effective delivery of dapivirine to the lower genital tract. Systemic absorption of dapivirine delivered from the ring was found to be low with levels <50 pg/ml (26). In a separate Phase 1 study which compared the two types of dapivirine IVRs, reservoir and matrix rings, 28 day use was well tolerated and did not induce any serious or severe adverse events. (27). Collectively, the clinical data presented to date for dapivirine in these two dosage forms show dapivirine to be safe with adequate pharmacokinetics.
As microbicides are intended to prevent HIV-1 sexual transmission, user acceptability and compliance become important for the success of microbicides to prevent infection. Therefore, it is likely that several dosage forms with different attributes will be needed to provide women with more choices which will likely result in improved adherence. In turn this will ultimately lead to greater efficacy. The use of quick-dissolving thin films for drug delivery has become widely implemented for oral drug delivery. Thin films offer an alternative dosage form for delivery of microbicide drug candidates to the vagina. A thin film is a polymeric solid dosage form. The advancement in polymer science has provided thin films with the flexibility to meet the demands and requirements for use as drug delivery systems. Thin films dissolve when placed on a mucosal surface to release the active agent. These films offer fast and accurate dose administration and can be administered without an applicator. Additionally, their rapid dissolving nature ensures quick release once applied. Other advantages of vaginal films include of discreet use and decreased product volume (28, 29). There is no introduction of extra fluids with administration of vaginal films, which means no product leakage upon use. Additionally, from a product development stand point thin films are reproducible dosage forms that can be used to stabilize drugs that are susceptible to degradation in aqueous environments. Finally, several published acceptability studies that incorporated vaginal films showed films are preferred by women over other vaginal formulations, such as gels, foams, and suppositories (30–32).
Vaginal films currently marketed include the Vaginal Contraceptive Film (VCF) products the VCF and the vaginal lubricating film. Vaginal films have also been evaluated as delivery system for other drugs such as Itraconazole (ITR) for treatment of Vaginal Candidiasis (33), Clindamycin Phosphate for treatment of bacterial vaginosis (BV) (34). In the microbicide field, vaginal films have been used as a delivery system for nonoxynol-9 which was the first microbicide candidate evaluated in clinical trials (35). Other examples for vaginal films used in microbicide research include cellulose acetate 1,2-benzenedicarboxylate (CAP) (36) and polystyrene sulfonate (PSS) (37). The aim of this study was to develop, characterize and assess lactobacillus compatibility and the potential for efficacy of a vaginal film containing the NNRTI dapivirine for vaginal use to prevent HIV-1 sexual transmission. Comparative studies were carried out with drug substance and film formulation to demonstrate that the formulation process did not result in significant loss of antiviral activity, a factor of significant importance in microbicide development.
Materials and Methods
Dapivirine was provided by the International Partnership for Microbicides (IPM). Dapivirine is a white micronized powder with molecular weight of 329.4 and pKa of 5.8. It is water insoluble (LogP = 5.27 at pH 9) and has a 2.5 mg/ml solubility in propylene glycol. UC781 is also a water insoluble NNRTI. Given that the potent anti-HIV activity of UC781 has been well established (12, 38) it was used as a control in in vitro bioactivity studies.
Dapivirine In vitro anti-HIV activity
Single HIV-1 Replication Cycle Assays Using P4R5 Indicator Cells
Single HIV-1 replication cycle assays were carried out using P4R5 indicator cells (obtained from Dr. J. Mellors, University of Pittsburgh). P4R5 cells express CD4, CXCR4 and CCR5 as well as a β-galactosidase reporter gene under the control of the HIV LTR promoter. Cells were seeded in 96-well microplates (5×103 cells per well) and maintained in DMEM/10% FBS supplemented with puromycin (0.5 μg/ml). To assess direct antiviral activity of test substances, P4R5 cells were exposed to varying concentrations of the drug or drug formulation, and then immediately inoculated with HIV-1 in the continued presence of the drug. The extent of infection was assessed after 48h using a fluorescence-based β-galactosidase detection assay using 4-methyllumbelliferyl-β-D-galactopyranoside as previously described (39).
To assess the protective effect of the drugs, P4R5 cells were exposed to varying concentrations of the drug or drug formulation for 18h. The medium was removed and then the cells were extensively washed and inoculated with virus in the absence of exogenous drug. The extent of infection was assessed after 48h as described above.
MT-2 Cell Line
Multiple round HIV-1 Infection (Virus Spread) Assays using MT-2 Cell Lymphoblastoid Cells
Multiple-round viral replication (virus spread) was assessed using MT-2 cells cultured in 96-well microplates (6.5×104 cells/well). To assess direct antiviral activity of test substances, cells were exposed to varying concentrations of the drug or drug formulation, and then immediately inoculated with HIV-1 (25 ng HIV-1 p24/well) in the continued presence of the drug. The extent of infection was evaluated four days after virus addition by microscopic observation of HIV-1 induced syncytium formation as described (12, 39).
To assess the protective effect of the drugs or drug formulations, uninfected MT-2 cells were incubated with various concentrations of UC781 or dapivirine for 18 hours. Exogenous drug was then removed by extensive washing of the cells with culture medium. The washed cells were infected with equivalent infectious doses of HIV-1 (25 ng HIV-1 p24/well). The extent of viral infection was determined four days later by microscopic observation of HIV-1 induced syncytium formation and validated by measuring the level of HIV-1 p24 antigen in the cell-free culture supernatants (12, 39).
H9+ Human T-cell Line
H9+ cells are cells that are persistently infected with HIV-1 (contain stably-integrated HIV-1 proviral DNA) but which do not undergo HIV-induced cytopathic effects (12). Test compounds were dissolved in DMSO (10 mM stock solution). For experiments, a small volume of the DMSO stock was rapidly mixed with a large volume (1000x) of the aqueous medium to give a well dispersed aqueous solution/dispersion of the drug in aqueous medium which was then used in the experiments. To evaluate the ability of the compounds to prevent cell to cell transmission of HIV-1, H9+ cells were incubated for 18 hrs in the absence or presence of 1 μM or 10 μM of test compound. The cells were then washed extensively with culture medium to remove exogenous drug and then were co-cultured with uninfected MT-2 cells at a ratio of 300:1 MT-2 to H9+ cells. The extent of viral infection of the MT-2 cells was determined after three days of co-culture by visual comparison of CPE relative to cells that were not treated with either compound.
To determine the ability of test compound to attenuate the infectivity of the virus produced by H9+ cells, the cells were incubated in the absence or the presence of the drug for 18h and then washed extensively as described above. The washed cells were then cultured in drug free medium for 24h and the virus produced during this period was isolated by centrifugal removal of the cells. Aliquots of the cell-free culture supernatants containing equal amounts of virus (based on p24) were used to infect MT-2 cells, and the level of infection was determined by visual examination of CPE at day 4 post-infection.
Dapivirine-Excipient Compatibility
As part of film formulation manufacturing, dapivirine was mixed with the polymer solution (which contains all excipients). So it was necessary to establish a profile of dapivirine compatibility with the excipient mixture. Weighed amounts of dapivirine were placed in glass vials. 320 μl propylene glycol was added to each vial. Polymer solution (1.68 ml) composed of 0.0567 g polyvinyl alcohol (PVA), 0.024g hydroxypropyl methyl cellulose (HPMC) 4000, and 0.0384g polyethylene glycol (PEG) 8000 was added to each vial and vortexed to mix dapivirine well with the excipients. The vials were sealed under nitrogen and protected from light using aluminum foil. The vials were placed in conditions of 30°C/65% relative humidity (RH), 40°C/75% RH or 50°C. At predetermined time points two vials were removed from each storage condition for drug quantitation. Dapivirine drug content was determined using a UPLC method. The method utilizes a Waters Acquity BEH C18 1.7μ 2.1×50mm. The mobile phase used was (A) 0.08% TFA in water (B) 0.05% TFA in Acetonitrile (ACN).
Dapivirine Film Formulation
The film formulation was composed of polyvinyl alcohol (PVA), hydroxypropyl methyl cellulose (HPMC) 4000 cp, polyethylene glycol 8000 (PEG), propylene glycol, and glycerin. Solvent casting methods were used for film manufacture. Briefly, an aqueous film solution containing the excipients and dapivirine (in a dispersed state) was prepared. The solution was cast onto a polyester substrate attached to the hot surface of an automatic film applicator (Elcometer® 4340) using either a 4″ or 8″ doctor blade. The film sheet was allowed to dry for 20 minutes before it was removed from the substrate. Once film sheets were obtained they were cut using a die press into 1″ × 2″ individual unit doses.
Physical and Chemical Characterization
Dapivirine films weight, thickness and appearance were recorded. Tensile strength as a measure of films strength was assessed using a texture analyzer (TA-XT.Plus) connected to a data acquisition and analysis software. Tensile strength was calculated using the following equation: Tensile strength = Force (N)/Cross sectional area of the film (cm2). Residual water content of the films was measured using Karl-Fisher apparatus (Metrohm, 758 KFD Titrino). Film disintegration testing was conducted by submerging a film in 3 ml water and mixing by rotation using an orbital shaker. Disintegration was monitored visually as the time for complete film structural loss. For determination of dapivirine drug content, films were dissolved in a 50% ACN solution and heated for 5 minutes at 50°C. An aliquot was taken from the solution and analyzed using the previously described UPLC method. Dissolution of dapivirine films was evaluated using a class IV USP apparatus (SOTAX CP7) using a 60 ml reservoir and a flow rate of 16 ml/min. The dissolution media used was distilled water containing 1% Cremophor. Dapivirine solubility in the dissolution media is 40 μg/ml. Film dissolution was monitored under sink conditions for 60 minutes and dapivirine was detected by UV spectrometer at 290 nm.
Stability Assessment
Dapivirine films were held for 18 months at 30°C/65% RH and for 6 months at 40°C/75% RH. Testing of the films was conducted at predetermined time points. At each time points weight, thickness, appearance, tensile strength, water content, and drug content were tested. In addition, disintegration, dissolution, Lactobacillus compatibility and in vitro anti-HIV activity (TZM-bl cell based model) were tested at specific periods throughout the stability protocol.
Compatibility with Lactobacillus
The standard microbicide safety test was used as previously described (40, 41) to assess Lactobacillus compatibility with dapivirine vaginal film. Briefly, bacterial suspensions were prepared in ACES buffer and the films were then dissolved and mixed in the suspensions. The suspensions were then incubated for 30 min at 37°C. Samples were taken at zero time and again after 30 min. Viability was determined by standard plate count. A sample passes if the reduction in viability was < log10.
Dapivirine Tissue Permeability
Human excised cervical tissue was obtained from University of Pittsburgh Health Sciences Tissue Bank under IRB Protocol PRO09110431. Tissue samples were from healthy volunteers undergoing routine hysterectomy for non-cervical issues. A section of each tissue was retained for histological evaluation. Excess stromal tissue was removed and the epithelial layer isolated using a Thomas-Stadie Riggs tissue slicer by a longitudinal slice through the entire tissue. Tissue thickness was measured by placing the tissue between two pre-measured slides. Tissue permeability studies were preformed in a Franz cell system (PermeGear, Nazareth, PA). Isolated epithelial sections of the tissue were placed between the donor and receptor compartments of the Franz cell with the epithelial side of the tissue oriented toward the donor compartment. The tissue-loaded Franz cells are maintained in an environment at 37°C throughout the experiment. The receptor was filled with 10% PEG 300 in Krebs buffer (pH 4.0). Dapivirine solubilized in the receptor medium (100 μg/ml) or dapivirine film (sufficient weight of film to obtain 100 μg/ml) solubilized in receptor medium (400 μl) was added to the donor chamber. At predetermined time points, 200 μl was removed from the receptor chamber and replaced with the same volume of fresh medium. The collected samples were analyzed for drug content using previously described UPLC methods.
Dapivirine film in vitro anti-HIV activity
HIV-RT and Cell-Based HIV-1 Replication Assay
Weighed pieces of dapivirine films were dissolved in either DMEM (for cell-based assays) or 1X RT-buffer (for in vitro RT assays) at 1:10 w/v. Multiple dilutions of the starting solutions were then prepared and used. For the RT assay, the ability of each film dilution to inhibit the RNA-dependent DNA polymerase activity of HIV-1 RT was determined using poly(rA)-oligo(dT) and [3H-TTP] as previously described (13). Cell-based single HIV-1 replication cycle assays for direct antiviral activity of the film formulations used P4R5 indicators cells as described above. TZM-bl Assay
Anti-HIV activity testing using TZM-bl assay was performed as previously described (42). Dapivirine films or placebo films were dissolved in 2 ml of saline. Ten-fold serial dilutions up to 1:107 of the original 2 ml sample of the dapivirine film were made. The placebo film was handled in a similar manner. As a control, the drug substance was used. Dapivirine was dissolved in DMSO and serial dilutions beginning at 1000 nM were made using the TZM-bl medium (DMEM with 10% BSA supplemented with antibiotics). The respective dilutions were added in triplicate to plated TZM-bl cells. HIV-1BaL was added next and the assay was cultured for 48 hours. Infection was detected by adding BrightGlo (Promega) a chemiluminescent developer of luciferase to each well. Control wells comprising cells alone or cells with HIV-1 only served as background and maximal luciferase activity, respectively. Efficacy was calculated as %Inhibition of infection by the following formula:
The effective dose at 50% was calculated using GraphPad Prism software.
Dapivirine film ex vivo anti-HIV activity
Polarized Explant Model (PEM)
Freshly obtained cervical tissue was incubated in a solution consisting of equal amounts of Penicillin-Streptomycin solution and Fungizone (Mediatech, Inc, Manassas, VA) for 10 minutes at room temperature, then rinsed three times with RPMI media prior to use. Defined pieces of tissue were clamped mechanically between two Neoflon cell chambers. The receptor chamber was filled with RPMI/10% serum/1% Penicillin-Streptomycin solution. One fourth of a dapivirine film (1.25 mg/film) was weighed and dissolved in 400 μl of RPMI, and this solution was added on top of the tissue in the donor chamber. The tissue was then incubated with this RPMI-film preparation for 1 h. After incubation, the film solution was removed from the epithelial surface of the tissue, and the surface was rinsed several times with RAFT medium to remove any residual film. A total of 400 μl consisting of RAFT medium/2 μg/ml PHA-M, virus inoculum, and 14C-PEG 4000 (tissue integrity tracer) was added to the donor chamber, and the tissue was incubated for 4h to allow HIV infection/transmission to occur. The virus used was the dual-tropic HIV-1 89.6. After 4h, aliquots of both donor and receptor chamber medium were taken and reserved for further analysis (scintillation spectrometry, qRT-PCR of HIV-1 RNA, infectivity).
Cervical Explant Model
In this model both toxicity and anti-HIV-1 activity were assessed and set up as previously described (9, 43). Briefly, explants were placed with the luminal side up in a transwell. The edges around the explants were sealed with Matrigel™ (BD Biosciences, San Jose, CA). The explants were maintained with the luminal surface at the air-liquid interface. The lamina propria was immersed in medium. The explants were set-up in duplicate for each treatment. For toxicity testing, dapivirine or placebo film were dissolved in 2 ml of culture medium and 100 μl were applied to the apical side of the explants for 18 hours. The next day, explants were washed and viability was evaluated using the MTT assay and histology. For anti-HIV activity, 100 μl of dissolved dapivirine or placebo film were mixed with 5×105 tissue culture 50% infectious dose (TCID50) of HIV-1BaL and added to the apical side of the explants. Eighteen hours after application the explants were washed and fresh medium was applied to the basolateral compartment. Every 3 to 4 days over a 3 week period, supernatant was collected and stored at −80°C for HIV-1 p24 analysis and fresh medium was added back. Stored supernatants were tested for HIV-1 replication using an HIV-1 p24gag ELISA (Perkin Elmer Life and Analytical Sciences, Inc., Waltham, MA). End of study explants were fixed in 10% buffered formalin (Fisher Scientific, Pittsburgh, PA) and processed by routine paraffin embedding for histology to determine product efficacy by immunohistochemical (IHC) analysis for HIV-1 p24 antigen. For toxicity evaluation, slides were stained with hematoxylin and eosin (Sigma Chemical Co., St Louis, MO). For the IHC assays all reagents and wash solutions were from the DAKO Corp, Carpentaria, CA. De-paraffinized, rehydrated sections were digested in 0.1 mg/ml Proteinase K for 15 min, washed, and incubated at room temperature for 1 hour with anti-p24 (dilution 1:6). Visualization was achieved with the DAKO Corp.’s LSAB-2 kit with the universal alkaline phosphatase system. Sections were counterstained in Mayer’s hematoxylin. Negative controls were tissue sections from each explant incubated with normal mouse ascitic fluid.
Results
In vitro anti-HIV activity evaluation
Dapivirine anti-HIV activity in P4R5 indicator cells
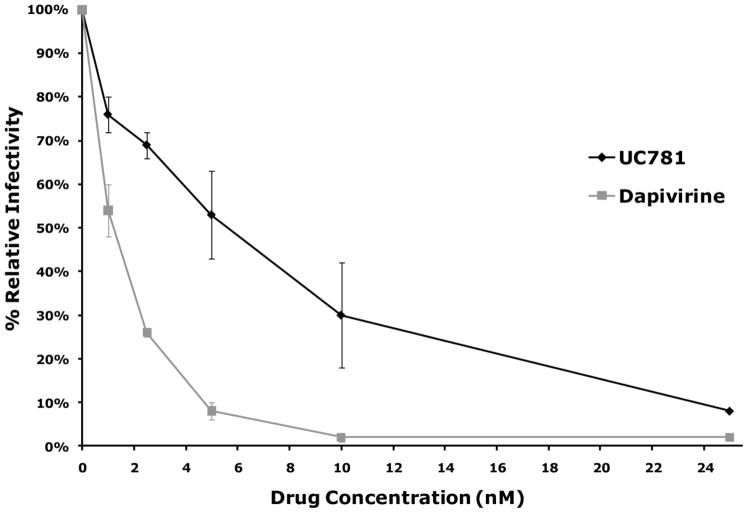
The 50% effective concentration (EC50) for dapivirine against HIV–1 was determined by infecting HeLa derived P4R5 indicator cells. Dapivirine was compared to another NNRTI (UC781) whose potent anti-HIV activity has been well established (12, 38). The EC50 for UC781 and dapivirine in this model were found to be 5 nM and 1 nM, respectively (Figure 1-A).
Figure 1.
(A) P4R5 cells were exposed to varying concentrations of dapivirine or UC781, and then immediately inoculated with HIV-1 in the continued presence of the drug. The extent of infection was assessed after 48h using a fluorescence-based β-galactosidase detection assay using 4-methyllumbelliferyl-β-D-galactopyranoside. Dapivirine showed greater potency than UC781 in single HIV-1 replication cycle assays using the P4R5 indicator cell line. Dapivirine EC50 is 1 nM and UC781 EC50 is 5 nM. (B) P4R5 cells were exposed to varying concentrations of dapivirine or UC781 for 18h. The medium was removed and the cells were extensively washed and inoculated with HIV-1 virus in the absence of exogenous drug. The extent of infection was assessed after 48h. Both dapivirine and UC781 were able to establish a protective barrier to infection in P4R5 cells following drug pretreatment and subsequent exposure to HIV-1 in the absence of exogenous drug. Dapivirine showed greater potency than UC781 in this context.
To determine whether or not dapivirine can serve as a protective barrier to prevent subsequent infection of cells by HIV–1 in the absence of exogenous drug, P4R5 indicator cells were first exposed to various concentrations of either UC781 or dapivirine. The cells were then washed free of exogenous drug and then exposed to HIV-1. Results showed that dapivirine was able to provide a protective barrier against subsequent HIV–1 infection (Figure 1-B).
Dapivirine anti-HIV activity in MT-2 cells
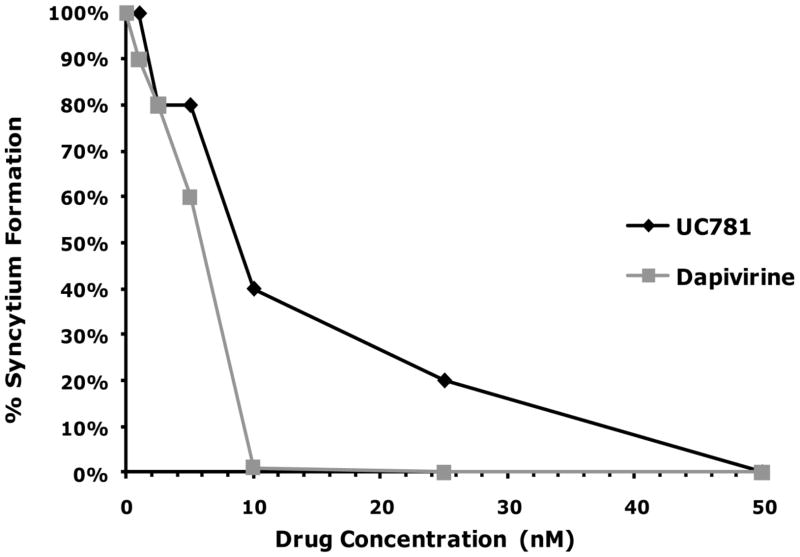
To corroborate the dose response curve data observed with P4R5 cells, MT-2 cells were incubated with virus in absence or presence of various concentrations of dapivirine or UC781. Dapivirine was found to have an EC50 value of 5 nM in this model as compared to UC781 which had an EC50 value of 10 nM at day 4 post-infection. No syncytium formation was observed in the presence of ≥ 50 nM of either drug. From these data it is shown that dapivirine has slightly greater enhanced activity over UC781. This finding parallels results obtained from the P4R5 cell dose response curves (Figure 2-A).
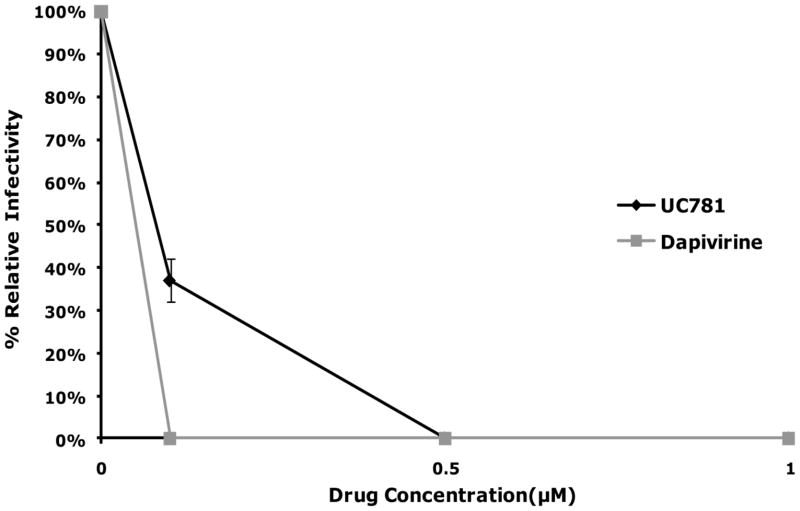
Figure 2.
(A) MT-2 cells were exposed to varying concentrations of dapivirine or UC781, and then immediately inoculated with HIV-1 (25 ng HIV-1 p24/well) in the continued presence of the drug. The extent of infection was evaluated four days after virus addition by microscopic observation of HIV-1 induced syncytium formation. Dapivirine showed greater direct antiviral potency than UC781. Dapivirine EC50 is 5 nM and UC781 EC50 is 10 nM. (B) Uninfected MT-2 cells were incubated with various concentrations of dapivirine or UC781 for 18 hours. Exogenous drug was removed by extensive washing of the cells with culture medium. The washed cells were infected with equivalent infectious doses of HIV-1 (25 ng HIV-1 p24/well). The extent of viral infection was determined four days later by microscopic observation of HIV-1 induced syncytium formation and validated by measuring the level of HIV-1 p24 antigen in the cell-free culture supernatants. Both dapivirine and UC781 were able to establish a protective barrier to infection in MT-2 cells following drug pretreatment and subsequent exposure to HIV-1 in the absence of exogenous drug. Dapivirine again showed greater potency than UC781.
The effect of pre-incubation with either dapivirine or UC781 on subsequent HIV infection was also determined in an MT-2 cell based model. Supernatant from MT-2 cultures that were pretreated with 1 μM of dapivirine resulted in 100 fold less p24 antigen generation compared to control MT-2 cultures that were not pre-incubated with dapivirine. A similar reduction in the amount of p24 antigen present in the MT-2 cultures pretreated with 5 to 10 μM of UC781 was observed (Figure 2-B). The relative levels of CPE observed visually were reflective of the p24 results. These results indicate that dapivirine was able to provide an effective barrier against subsequent infection with HIV-1 after the removal of exogenous drug. This observation is similar to results previously noted with UC781 (11).
Dapivirine anti-HIV activity in human H9+ cell line
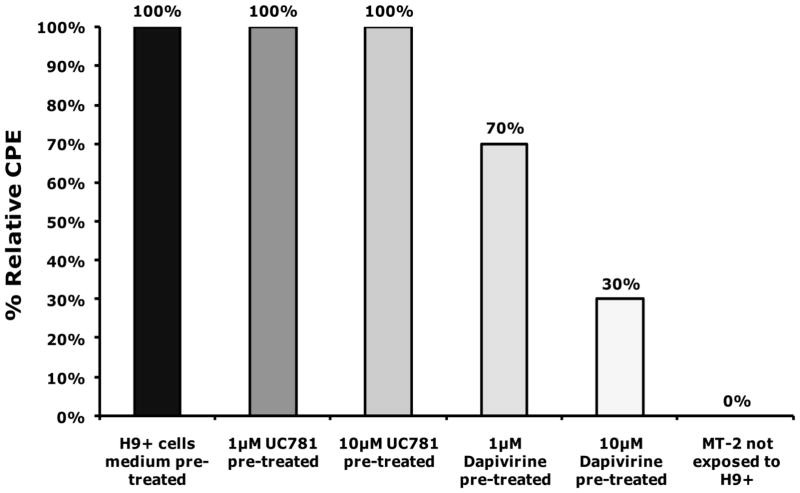
The ability of dapivirine to prevent cell to cell transmission of HIV-1 was tested in H9+ cells. As indicated in (Figure 3), the culture of MT-2 cells that were exposed to H9+ cells that were pretreated with 10 μM of dapivirine showed a 70% reduction in CPE levels compared to MT-2 cells exposed to H9+ cells that were not pretreated. This data implies that dapivirine is able to inhibit cell-to-cell transmission of HIV-1.
Figure 3.
H9+ cells were incubated for 18 hrs in the absence or presence of 1 μM or 10 μM of dapivirine or UC781. The cells were washed extensively with culture medium to remove exogenous drug and then were co-cultured with uninfected MT-2 cells. The extent of viral infection of the MT-2 cells was determined after three days of co-culture by visual comparison of CPE relative to cells that were not treated with either compound. Dapivirine pre-treatment of MT-2/H9+ cell co-cultures resulted in 70% reduction in CPE levels compared to control MT-2/H9+ co-cultures. Dapivirine can inhibit HIV-1 cell to cell transmission.
Additionally the ability of dapivirine to attenuate the infectivity of virus produced by H9+ cells was tested. Incubation of persistently infected H9+ cells with 1 μM of dapivirine resulted in a 50% reduction of infectivity of virus subsequently produced by these treated cells when cultured in the absence of exogenous drug (data not shown). Virus produced from H9+ cells exposed to 10 μM of dapivirine as described in the methods was essentially non-infectious. This is approximately one order of magnitude greater than that required to inactivate isolated virions alone. These results indicate that dapivirine is able to reduce the infectivity of HIV virions produced from persistently infected H9+ cells that had been previously exposed to dapivirine.
Dapivirine-Excipient Compatibility Study
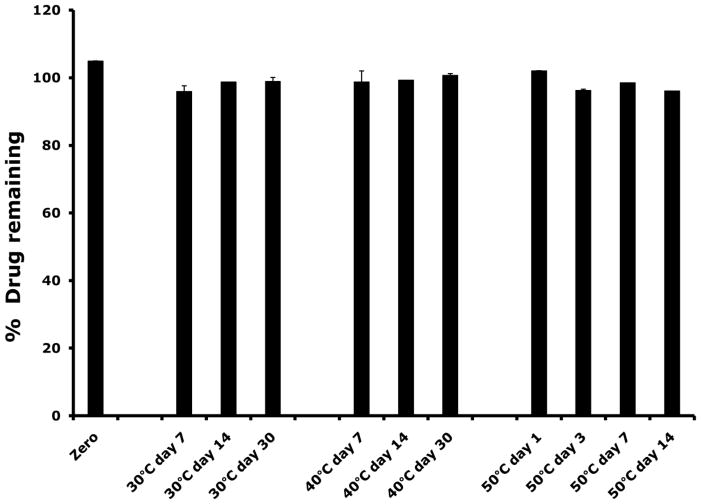
To design a film formulation for dapivirine vaginal delivery, a compatibility profile of dapivirine with the excipients to be used in the film formulation was performed. Incubation of dapivirine with the excipient mixture (propylene glycol, PVA, HPMC, and PEG8000) at different conditions did not result in drug loss. Stability of dapivirine in the excipient mixture was an indication of dapivirine-excipient compatibility (Figure 4).
Figure 4.
Dapivirine was mixed with the film excipient mixture (composed of propylene glycol PVA, HPMC, and PEG8000) and stored at 30°C/65% relative humidity (RH), 40°C/75% RH or 50°C. Dapivirine drug content was determined using a UPLC method. No loss of dapivirine was observed after 14 days storage in the three storage conditions.
Development and evaluation of dapivirine vaginal film
Formulation
Dapivirine was formulated in a polyvinyl alcohol (PVA) based vaginal film. PVA constituted 38.3% (w/w) of the film. Other excipients in the formulation included 19.1% hydroxylpropyl methyl cellulose (HPMC), 25.5% polyethylene glycol (PEG) 8000, 7.9% glycerin and 7.9% propylene glycol. The films were made using solvent cast technique. The target loading dose for the film was 1.25 mg dapivirine per film. Dapivirine film containing 0.5 mg dapivirine was also made to evaluate the impact of dosing level on film properties. These dosing levels were chosen based on clinical studies with dapivirine gel products. Phase I studies using dapivirine gels at concentrations of 0.01%, 0.02% and 0.05% have been conducted (22–25). 2.5 mL of the gel was administered in these studies. Consequently the administered dose corresponds to 0.25, 0.5 and 1.25 mg dapivirine, respectively. Comparing dosing level with that which is reported for the vaginal ring, a clinical study which evaluated both reservoir and matrix ring types utilized a dapivirine loading level of 25 mg (27). Results from this study showed that the reservoir ring had on average a residual amount of dapivirine of 24.33 mg after 28 days of vaginal application. The matrix ring had on average a residual amount of dapivirine of 14.51 mg after 28 days of vaginal application. Considering that both rings exhibit zero-order release, the calculated amount delivered of dapivirine would be 24 μg/day (reservoir ring) and 375 μg/day (matrix ring). Based on a single daily application of the film dosage form, the film delivers a significantly higher amount of dapivirine as compared to the ring used in previous studies.
Physical and Chemical Characterization
Dapivirine films were soft, flexible and translucent. Individual films units were made 1″ × 2″ in size were 70 μm thick and weighed 90 mg on average. The films were designed to dissolve rapidly upon exposure to an aqueous environment (< 10 minutes). Table 1 shows dapivirine film characteristics with both dosing levels 1.25 mg and 0.5 mg. Dosing level was found not to alter film properties.
Table 1.
Dapivirine film physical and chemical characteristics
| Parameter | 1.25 mg Dapivirine Film | 0.5 mg Dapivirine Film |
|---|---|---|
| Weight (mg) | 91.23 ± 4.93 (n = 6) | 94.7 ± 10.01 (n =14) |
| Thickness (mm) | 0.073 ± 0.005 (n = 6) | 0.063 ± 0.007 (n = 14) |
| Disintegration (min) | 6.33 ± 0.577 (n = 3) | 4.33 ± 0.58 (n = 3) |
| Water content % (w/w) | 1.38 ± 0.17 (n = 3) | 2.4 ± 0.19 (n = 3) |
| Tensile strength (N/cm2) | 538.24 ± 57.17 (n = 3) | 777.59 ± 19.99 (n = 3) |
| Drug Content % (w/w) | 1.4 ± 0.03 (n = 42) | 0.546 ± 0.016 (n = 5) |
All values presented as mean ± SD
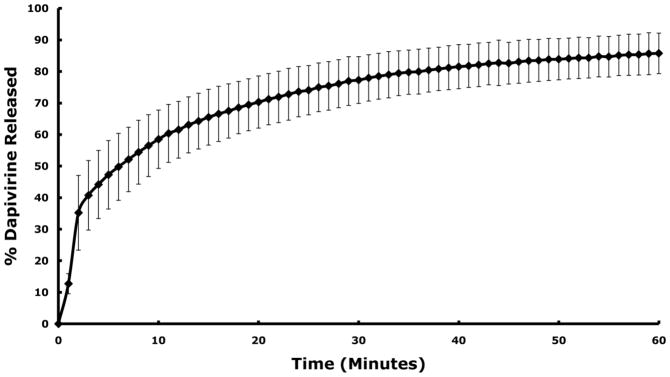
To establish the rapid release of drug from the film matrix, dapivirine film dissolution was evaluated in a class IV USP dissolution apparatus. Results showed that greater than 50% of the loaded dapivirine was released from the film within 10 minutes (Figure 5).
Figure 5.
Dapivirine film (1.25 mg/film) dissolution was tested in standard class IV USP method. The medium used was a 1% Cremophor aqueous solution. Dapivirine was rapidly released from the film. 50% of dapivirine was released from the film in less than 10 minutes. Data presented as mean ± SD (n = 3).
Stability Assessment
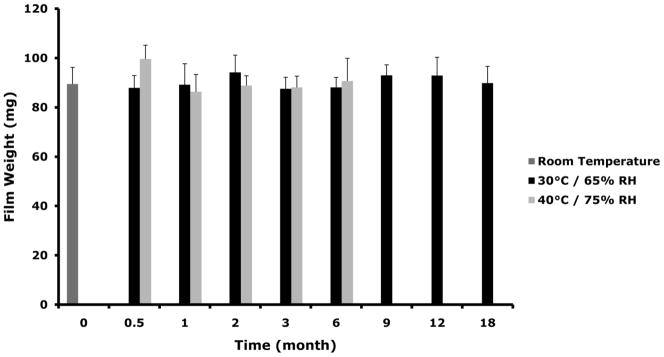
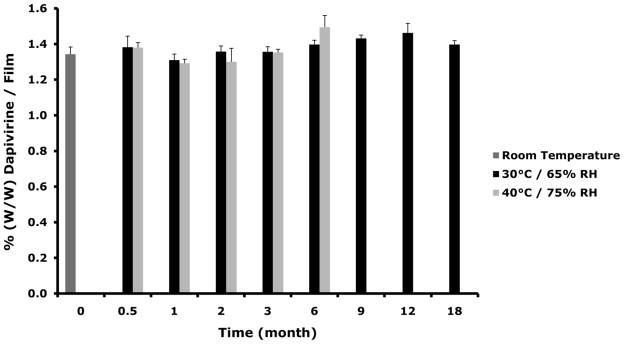
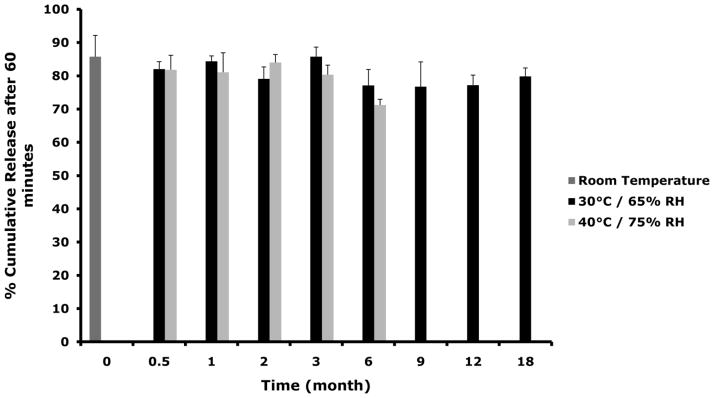
Dapivirine film (1.25 mg/film) was stored at 30°C/65% RH and 40°C/75% RH for stability assessment. Based on the physical and chemical assessments conducted dapivirine film was shown to be stable for a period of 18 months when held at 30°C/65% RH and for at least 6 months when held at accelerated conditions (40°C/75% RH). Figure 6 shows film weights, drug content, and dissolution obtained for each condition over the stability time period.
Figure 6.
Dapivirine film (1.25 mg/film) was stored for 18 month in 30°C/65% RH and for 6 in 40°C/75% RH. Several physical and chemical characteristics were monitored over the time course of the stability study including weight, drug and dissolution. (A) No change was observed in dapivirine film weight over the time course of stability testing. Data presented as mean ± SD. (B) Dapivirine film amount remained stable in the film at the different conditions tested. Data presented as mean ± SD. (C) Four films were tested per each time point. Dissolution testing over the study period showed no change in release values (cumulative release of dapivirine after 60 minutes). Data presented as mean ± SD. Three films were tested per each time point.
Compatibility with Lactobacillus
Dapivirine film compatibility with Lactobacillus was assessed using the Standard Microbicide Safety Test (SMST). Dapivirine film did not induce any deteriorating effects on several strains of Lactobacillus as no loss of bacterial viability was observed (Table 2). Furthermore, compatibility with lactobacillus was monitored throughout the stability assessment period. In the Lactobacillus film compatibility testing the breakdown into toxic products was not detected over time since loss of viability was not observed over the 18 month period of stability testing.
Table 2.
Evaluation of dapivirine film compatibility with Lactobacillus
| Lactobacillus strain tested | Log Difference (T30 minute Plate Count − T0 minute Plate Count) |
|---|---|
| L. crispatus ATCC 33197 | 0.245 |
| L. jensenii ATCC 25258 | 0.0587 |
| L. jensenii LBP 28Ab | 0.158 |
Legend: Results present are the averages of all experiments using the organisms. A positive value indicates there was a slight increase in the viability. These organisms were tested at different time intervals. The films passed all safety tests; the standard for passing the test is there cannot be a loss of viability ≥ 1 log10.
Dapivirine permeability in human excised cervical tissue
The permeability of dapivirine into human excised cervical tissue was studied for both film formulated and unformulated dapivirine in a Franz cell model. Calculated permeability parameters are shown in (Table 3). Dapivirine as drug substance or film formulated was able to permeate through the epithelium of excised human cervical tissue under the experimental conditions described. There was no significant difference observed between permeability coefficients obtained for formulated or unformulated dapivirine.
Table 3.
Calculated permeability parameters of dapivirine drug substance
| Tissue # | 1.25 mg Dapivirine film | Dapivirine drug substance | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| 1 | 1.66E-06 | 6.22E-07 | 3.66E-06 | 9.76E-07 |
| 2 | 2.19E-06 | 2.81E-07 | 2.1E-06 | 1.78E-06 |
| 3 | 1.15E-06 | 2.53E-07 | 3.04E-06 | 8.29E-07 |
Legend:
Apparent permeability values Papp (cm/sec) for dapivirine drug substance and 1.25 mg dapivirine film. For each tissue sample, permeability testing was done in triplicate. Values presented as mean ± SD.
Dapivirine Film anti-HIV activity
In Vitro Assessment of anti-HIV activity
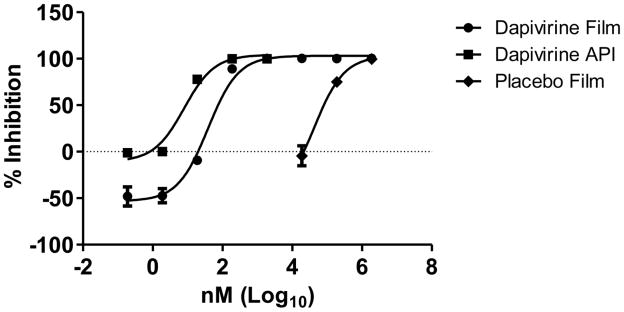
Dapivirine film (1.25 mg/film) was tested for anti-HIV activity in the TZM-bl assay. Dapivirine film had an EC50 of 38.7 nM which was similar to the API (EC50 of 7.9 nM) in this assay (Figure 7). Dapivirine film maintained anti-HIV activity over 18 months when held at 30°C/65% RH and for at least 6 months when held at accelerated conditions (40°C/75%). The placebo film had minimal anti-HIV activity which returned to baseline activity after a 1:100 dilution. This activity observed in the placebo film is more than likely due to the presence of the polymer excipients. No cellular toxicity was observed at any time point over the stability period as tested in the TZM-bl assay (data not shown).
Figure 7.
Dapivirine and placebo films were dissolved in 2 ml saline and serial dilutions made. The active pharmaceutical ingredient (API) was dissolved in DMSO and serial dilutions made. The dilutions were applied to the TZM-bl cells in triplicate with HIV-1BaL. After 48 hours, HIV-1 infection was measured by adding BrightGlo (Promega) to the plates and reading for luminescent activity. The data show the API with an IC50 of 7.9 nM and the Dapivirine film with an IC50 of 38.3 nM. Minimal anti-HIV-1 activity was associated with the film.
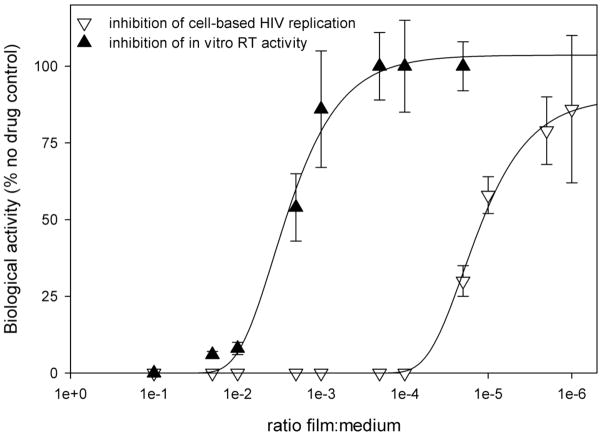
Furthermore, dapivirine film (0.5 mg/film) was tested for its ability to inhibit HIV-RT and cell-based HIV-1 replication in vitro. Inhibition of cell-based HIV-1 replication by dapivirine film (0.5 mg/film) is over 200-fold more potent than inhibition of RT activity (Figure 8). This observation is probably due to the hydrophobic nature of dapivirine.
Figure 8.
Weighed pieces of dapivirine films were dissolved in either DMEM (for cell-based assays) or 1X RT-buffer (for in vitro RT assays) at 1:10 w/v. Multiple dilutions of the starting solutions were then prepared and used. For the RT assay, inhibition of the RNA-dependent DNA polymerase activity of HIV-1 RT was determined using poly(rA)-oligo(dT) and [3H-TTP]. Cell-based single HIV-1 replication cycle assays for direct antiviral activity of the film formulations used P4R5 indicators cells as described above. Inhibition of cell-based HIV-1 replication by dapivirine film (0.5 mg/film) is over 200-fold more potent than inhibition of RT activity.
Ex Vivo Assessment of anti-HIV activity
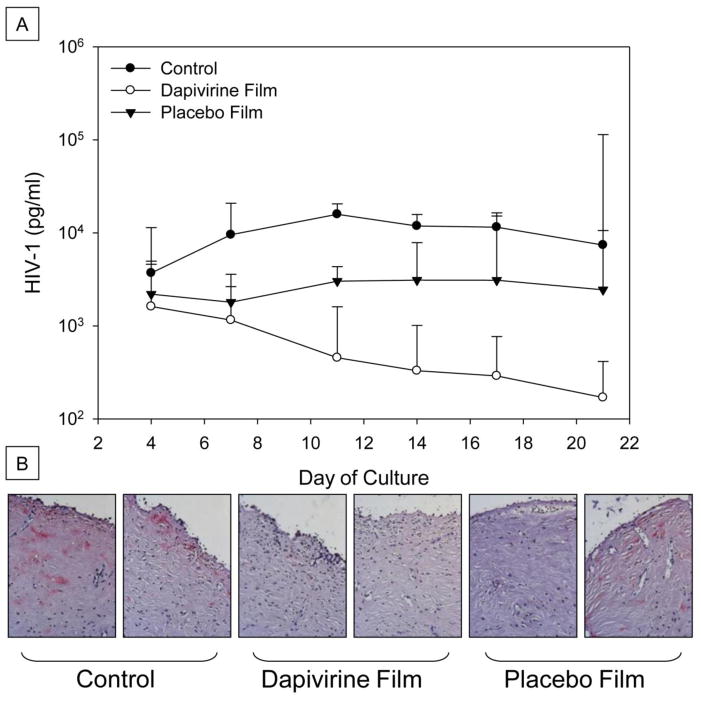
The bioactivity of dapivirine film was tested in an excised human cervical tissue explant model. Tissues were treated with 100 μl of film containing 1.25 mg dapivirine film dissolved in 2 ml of medium, 100 μl of placebo film dissolved in 2 ml of medium, or left untreated (control). Overall, both dapivirine and placebo film showed bioactivity with a reduction in HIV-1 p24 levels (Figure 9-A). However, all of the explants exposed to the Dapivirine film were protected as compared to 6 of 8 explants protected by the placebo film. These were confirmed by immunohistochemical staining for HIV-1 p24 in tissues (Figure 9-B). These data support the TZM-bl data showing undiluted placebo film has some anti-HIV-1 activity.
Figure 9.
Dapivirine and placebo films were dissolved in 2 ml of medium and 100 μl was applied to the apical surface of the designated explants while HIV-1 was added to all of the explants. After an overnight culture, the explants were washed and followed for 21 days. Every 3 to 4 days, supernatant was collected and fresh medium replenished. The supernatant was tested for HIV-1 p24 levels measured over the course of 21 days (A). Dapivirine film inhibited HIV-1 infection in 8/8 explants tested. Placebo film inhibited HIV-1 infection in 6/8 explants tested. At study endpoint, the tissues were fixed and immnunohistochemistry was performed for HIV-1 p24 expressing cells (B). Representative histology pictures of tissues after 21 day of infection studies. No infection of either dapivirine film-treated explants while one of the two placebo film-treated explants showed HIV-1 infection. Both control explants were infected with HIV-1.
The ability of dapivirine film (1.25 mg/film) to block HIV-1 transmission was also tested in a polarized explant model (PEM). In this model, a piece of cervical tissue is clamped between two chambers mechanically. In this model dapivirine film blocked HIV-1 transmission. The number of HIV-1 copies as measured by qRT-PCR in the receptor compartment was below detection limits indicating blocking of HIV-1 transmission in the explant tissue (Table 4)
Table 4.
Effects of 1.25 mg Dapivirine film on HIV-1 transmission in the polarized explant model (PEM)
| PEM Sample | HIV donor (copies/ml) | HIV receptor (copies/ml) | HIV explants culture (copies/ml) | 14PEG donor (total cmp) | 14PEG receptor (total cmp) |
|---|---|---|---|---|---|
| No Treatment | 2 × 108 | 9.2 × 102 | 1.19 × 105 | 636632 | 100 |
| 1.25 mg Dapivirine Film | 2 × 108 | Below Detection | Below Detection | 941416 | Below Background |
Discussion
Confirmation of the anti-HIV activity of dapivirine was evaluated in several in vitro cell based assays and provided further support of dapivirine’s potency. Additionally, these studies demonstrated a memory or protective effect for dapivirine in that cells pretreated with drug are protected from subsequent infection in the absence of exogenous drug. This observation is important in that it establishes a potential for use of dapivirine in a coitally independent manner.
In the three models that were tested (P4R5, MT-2, and TZM-bl), the potent activity of dapivirine was confirmed to have an EC50 value between 1–10 nM. This value is similar to those previously obtained using other systems (14). Since the role of HIV free virus or cell associated virus has not been fully elucidated for HIV transmission, it is important to establish the ability of a microbicide drug candidate to prevent infection by both cell free virus and cell associated virus. The ability of dapivirine to block cell to cell transmission of HIV-1 was determined in human H9+ cells. Pretreatment of H9+ cells with dapivirine reduced CPE formation (an indicator of viral infection) by 70% as compared to untreated cells. Additionally, pretreated H9+ cells were allowed to incubate with virus for 24 hours in the absence of dapivirine, the produced virus was then used to attempt to infect MT-2 cells. Virus produced from H9+ cells exposed to dapivirine had decreased infectivity.
Development of any drug product requires incorporation of excipients for successful formulation. It is important to establish that dapivirine does not have any incompatibility with the excipients of choice in the formulation. For this reason, a dapivirine excipient compatibility study was conducted with those excipients identified for utilization in the quick dissolve film formulation. Demonstration of the ability to maintain dapivirine stability in the presence of the excipient mixture was established. Once compatibility was established, a final optimized vaginal film formulation containing dapivirine was developed. Typically, a film formulation is composed of a polymer(s), a plasticizer to provide flexibility to the film, a humectant to enhance soft texture, and a disintegrant to provide rapid film disintegration once in touch with fluids (44). The polymer of choice for the dapivirine film formulation was polyvinyl alcohol (PVA). PVA is the polymer base which is used in several currently marketed vaginal film products. Co-polymers were evaluated for their ability to improve film properties. It was found that the cellulose derivative HPMC when used in combination with PVA provided optimal film attributes. Propylene glycol was used to facilitate the dispersion of dapivirine in the polymeric solution. Given the hydrophilic nature of the polymer base (PVA) and the hydrophobic nature of dapivirine, the drug exists as a dispersion in the film product. Sedimentation studies were carried out to ensure adequate dispersion of dapivirine in the polymeric solution through the film product manufacturing process. Polymer concentration was optimized to provide adequate viscosity to maintain the dapivirine dispersion. Sorbitol, propylene glycol, glycerin and combinations of these were evaluated as plasticizers for the film formulation. It was found that use of glycerin enhanced film flexibility, mechanical properties and also enhanced film softness. Since rapid disintegration was targeted it was necessary to include a disintegrant (PEG 8000) in the film formulation. Film manufacture involved pouring an 8″ film sheet which was then cut into individual 1″ × 2″ pieces. Content uniformity studies were conducted to ensure adequate distribution of drug was achieved both across the film sheet as well as within each individual film.
To ensure rapid delivery of the dapivirine from the film dosage form, film disintegration rate was set at less than 10 minutes. Both disintegration and dissolution studies confirmed rapid drug release from the film formulation. However, it should be noted that due to the hydrophobic nature of dapivirine it was necessary to include Cremophor in the dissolution medium to maintain sink conditions throughout the dissolution experiment. To ensure film structural stability over time, the amount of residual water in the final film should be kept as low as possible. For the developed dapivirine film, water content remained at a level which was less than 5% throughout the stability assessment over an 18 month period. Tensile strength was monitored to confirm structural stability. Results of the stability study established a minimal shelf life of 18 months for the dapivirine film formulation.
Because dapivirine works by interrupting the viral cycle at the replication step, it is desirable that dapivirine enter the tissue to reach the target cells which lie in the sub-epithelial layers. The stratified multilayer vaginal epithelium may represent a barrier for dapivirine permeation. For this reason, the permeability of dapivirine in both the formulated and unformulated state was evaluated using excised cervical tissue in a Franz-cell model to establish the permeability profile for dapivirine. No significant differences were found between the apparent permeability coefficients obtained for unformulated dapivirine as compared to those obtained for film formulated dapivirine. This data suggests that quick disintegration allows for the rapid release of dapivirine from the film resulting in permeability profiles similar to those for unformulated drug. These studies are important in that they establish the ability of dapivirine to permeate into the target tissue to be available for inactivation of HIV viral replication.
It is important for any dosage form to maintain drug’s activity. The anti-HIV activity of dapivirine films was tested in vitro in a HIV-RT assay, cell-based HIV-1 replication assay and TZM-bl assay. Inhibition of cell-based HIV-1 replication was over 200-fold more potent than inhibition of RT activity. This observation is probably due to the hydrophobic nature of dapivirine. Film dissolution leads to precipitation and substantial loss of bioactivity in pure aqueous media such as that used in the RT assay. However, cells provide a hydrophobic environment in the context of the plasma membrane which can aid in sequestration of bioactive drug thereby leading to substantially more potent inhibition in cell-based replication assays. Therefore, we concluded that a rapid cell-based replication assay is preferable to in vitro RT assays as a means of quality control for bioactivity for dapivirine film. It should be noted that the 1.25 mg dapivirine film anti-HIV activity was not tested in HIV-RT and cell-based HIV-1 replication assays. However, as stated in the results the 0.5 mg dapivirine film showed inhibition of HIV-1 in both assays. The 1.25 mg dapivirine film showed potent activity in the TZM-bl model. Therefore it is to be expected that the 1.25 mg dapivirine film would show potent inhibition of HIV-1 in the HIV-RT and cell based HIV replication assays. The TZM-bl assay also confirmed that dapivirine films maintained potent anti-HIV activity.
Although permeability studies showed that dapivirine was able to permeate target tissues, it is important to establish that this results in adequate anti-HIV activity. For this reason, the anti-HIV activity of dapivirine films was studied in ex vivo models using human cervical explant tissue. Two models were chosen for these studies. The first is the polarized explant model which has been thoroughly described in the literature (9, 43). This model uses a transwell system in which the tissue is sealed using matrigel establishing the polarization between the apical and basolateral compartments. A second model was used in which the tissue is sandwiched between a donor and receptor compartments providing a mechanical seal separating the two compartments. In this second model a more thorough barrier is formed, thus tissue permeation is more specifically confined to the exposed area. For this purpose we developed and validated a polarized explant model (PEM) that can be used to study HIV-1 infection of cervical tissue as well as the anti-HIV activity of microbicide product candidates. Dapivirine film was shown to exhibit inhibition of HIV-1 infection in both models used.
Topical microbicides should not exert any toxic or harmful effects on the natural defenses of the local environment. One of the important natural defenses in the vagina is the microflora, specifically lactobacillus. Dapivirine film compatibility with Lactobacillus was tested and no loss of bacterial viability or formation of toxic products was observed. Additionally, no cellular toxicity of the film has been detected in the TZM-bl cell model. These studies provide evidence of safety of the dapivirine film product.
The choice of a dosage form for microbicides is dictated by several factors such as chemical characteristics of the drug candidate and user acceptability and compliance. For topical vaginal microbicides, hydrogels have been the main dosage form being used as they have a history of use in female products and an established user acceptability profile. However, hydrogels are usually associated with leakage, messiness and requirement of an applicator for use. These factors may hinder the deployment of microbicides because of reduced user acceptability. Thus different dosage forms are required. Furthermore, socioeconomic factors as well as personal preference may play a role in dosage form choice for individual users. For these reasons, it is important to offer women choices with regard to dosage form type to ensure maximal user acceptance which will ultimately impact willingness to use the product and hence efficacy. One dosage form option for vaginal delivery of drug substances is a quick dissolving film. Quick dissolving films are a solid dosage form which has widely been used for oral delivery of drugs but have also been explored for vaginal delivery of drug substances. Quick dissolving films are easy to use, require no applicator, cause no messiness or leakage due to the small dose volume, and are inexpensive to manufacture. Given that microbicide products are specifically being targeted for undeveloped countries such as sub-Saharan Africa where the HIV-1 epidemic is the worst, the economic feasibility of a product is critical to its effectiveness.
Concluding Remarks
Dapivirine shows great promise as a microbicide drug candidate. Its high potency against HIV was confirmed within the studies presented. Additionally, its capacity to provide protection from HIV infection was further elucidated. Dapivirine can be successfully formulated as a quick dissolve vaginal film which provides rapid drug release, lacks toxicity to the innate microflora lactobacilli and maintains stability and bioactivity over 18 months. Given the potential advantage of the film dosage form, it is important to further evaluate this promising drug/delivery system in the clinic.
Acknowledgments
The work presented was supported through a grant from the International Partnership for Microbicides (IPM) and the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institute of Health (IPCP U19, AI082639). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. UNAIDS; 2010. [Google Scholar]
- 2.Garg AB, Nuttall J, Romano J. The future of HIV microbicides: challenges and opportunities. Antivir Chem Chemother. 2009;19(4):143–50. doi: 10.1177/095632020901900401. [DOI] [PubMed] [Google Scholar]
- 3.Zydowsky TM. Microbicides: chemistry, structure, and strategy. Curr Opin HIV AIDS. 2008 Sep;3(5):548–53. doi: 10.1097/COH.0b013e32830ab9dd. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007 Mar 3;369(9563):787–97. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- 5.Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005 May 1;39(1):1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- 6.van de Wijgert JH, Shattock RJ. Vaginal microbicides: moving ahead after an unexpected setback. AIDS. 2007 Nov 30;21(18):2369–76. doi: 10.1097/QAD.0b013e3282ef83fd. [DOI] [PubMed] [Google Scholar]
- 7.Moscicki AB. Vaginal microbicides: where are we and where are we going? J Infect Chemother. 2008 Oct;14(5):337–41. doi: 10.1007/s10156-008-0630-3. [DOI] [PubMed] [Google Scholar]
- 8.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, Cost M, Huang Y, Gai F, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS ONE. 2010;5(2):e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borkow G, Salomon H, Wainberg MA, Parniak MA. Attenuated infectivity of HIV type 1 from epithelial cells pretreated with a tight-binding nonnucleoside reverse transcriptase inhibitor. AIDS Res Hum Retroviruses. 2002 Jul 1;18(10):711–4. doi: 10.1089/088922202760072339. [DOI] [PubMed] [Google Scholar]
- 11.Motakis D, Parniak MA. A tight-binding mode of inhibition is essential for anti-human immunodeficiency virus type 1 virucidal activity of nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002 Jun;46(6):1851–6. doi: 10.1128/AAC.46.6.1851-1856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hossain MM, Parniak MA. In vitro microbicidal activity of the nonnucleoside reverse transcriptase inhibitor (NNRTI) UC781 against NNRTI-resistant human immunodeficiency virus type 1. J Virol. 2006 May;80(9):4440–6. doi: 10.1128/JVI.80.9.4440-4446.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Parniak MA, Isaacs CE, Hillier SL, Rohan LC. Characterization of cyclodextrin inclusion complexes of the anti-HIV non-nucleoside reverse transcriptase inhibitor UC781. AAPS J. 2008 Dec;10(4):606–13. doi: 10.1208/s12248-008-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher P, Harman S, Azijn H, Armanasco N, Manlow P, Perumal D, et al. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2009 Feb;53(2):487–95. doi: 10.1128/AAC.01156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena BB, Han YA, Fu D, Rathnam P, Singh M, Laurence J, et al. Sustained release of microbicides by newly engineered vaginal rings. AIDS. 2009 May 15;23(8):917–22. doi: 10.1097/QAD.0b013e32832af57c. [DOI] [PubMed] [Google Scholar]
- 16.Nuttall JP, Thake DC, Lewis MG, Ferkany JW, Romano JW, Mitchnick MA. Concentrations of dapivirine in the rhesus macaque and rabbit following once daily intravaginal administration of a gel formulation of [14C]dapivirine for 7 days. Antimicrob Agents Chemother. 2008 Mar;52(3):909–14. doi: 10.1128/AAC.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta KM, Pearce SM, Poursaid AE, Aliyar HA, Tresco PA, Mitchnik MA, et al. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J Pharm Sci. 2008 Oct;97(10):4228–39. doi: 10.1002/jps.21331. [DOI] [PubMed] [Google Scholar]
- 18.Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, McCullagh SD. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006 Nov 15;325(1–2):82–9. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005 Nov;56(5):954–6. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 20.Di Fabio S, Van Roey J, Giannini G, van den Mooter G, Spada M, Binelli A, et al. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS. 2003 Jul 25;17(11):1597–604. doi: 10.1097/00002030-200307250-00003. [DOI] [PubMed] [Google Scholar]
- 21.Van Herrewege Y, Michiels J, Waeytens A, De Boeck G, Salden E, Heyndrickx L, et al. A dual chamber model of female cervical mucosa for the study of HIV transmission and for the evaluation of candidate HIV microbicides. Antiviral Res. 2007 May;74(2):111–24. doi: 10.1016/j.antiviral.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Jespers VA, Van Roey JM, Beets GI, Buve AM. Dose-ranging phase 1 study of TMC120, a promising vaginal microbicide, in HIV-negative and HIV-positive female volunteers. J Acquir Immune Defic Syndr. 2007 Feb 1;44(2):154–8. doi: 10.1097/QAI.0b013e31802bb35f. [DOI] [PubMed] [Google Scholar]
- 23.Nel AM, Coplan P, Smythe SC, McCord K, Mitchnick M, Kaptur PE, et al. Pharmacokinetic Assessment of Dapivirine Vaginal Microbicide Gel in Healthy, HIV-Negative Women. AIDS Res Hum Retroviruses. 2010 Sep 21; doi: 10.1089/aid.2009.0227. [DOI] [PubMed] [Google Scholar]
- 24.Nel AM, Coplan P, van de Wijgert JH, Kapiga SH, von Mollendorf C, Geubbels E, et al. Safety, tolerability, and systemic absorption of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS. 2009 Jul 31;23(12):1531–8. doi: 10.1097/QAD.0b013e32832c413d. [DOI] [PubMed] [Google Scholar]
- 25.Nel AM, Smythe SC, Habibi S, Kaptur PE, Romano JW. Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs. Hydroxyethyl cellulose-based universal placebo gel. J Acquir Immune Defic Syndr. 2010 Oct 1;55(2):161–9. doi: 10.1097/QAI.0b013e3181e3293a. [DOI] [PubMed] [Google Scholar]
- 26.Romano J, Variano B, Coplan P, Van Roey J, Douville K, Rosenberg Z, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009 May;25(5):483–8. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 27.Nel A, Smythe S, Young K, Malcolm K, McCoy C, Rosenberg Z, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009 Aug 1;51(4):416–23. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 28.Rohan LC, Sassi AB. Vaginal Drug Delivery Systems for HIV Prevention. AAPS J. 2009 Feb 5; doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romano J, Malcolm RK, Garg S, Rohan LC, Kaptur PE. Microbicide delivery: formulation technologies and strategies. 2008. [DOI] [PubMed] [Google Scholar]
- 30.Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: Where have we been? Where are we going? J Womens Health Gend Based Med. 2001 Mar;10(2):163–73. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- 31.Raymond E, Alvarado G, Ledesma L, Diaz S, Bassol S, Morales E, et al. Acceptability of two spermicides in five countries. Contraception. 1999 Jul;60(1):45–50. doi: 10.1016/s0010-7824(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 32.Raymond EG, Chen PL, Condon S, Luoto J, Barnhart KT, Creinin MD, et al. Acceptability of five nonoxynol-9 spermicides. Contraception. 2005 Jun;71(6):438–42. doi: 10.1016/j.contraception.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobaria NB, Badhan AC, Mashru RC. A novel itraconazole bioadhesive film for vaginal delivery: design, optimization, and physicodynamic characterization. AAPS PharmSciTech. 2009;10(3):951–9. doi: 10.1208/s12249-009-9288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobaria N, Mashru R. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clindamycin phosphate. Pharm Dev Technol. 2009 Oct 20; doi: 10.3109/10837450903262058. [DOI] [PubMed] [Google Scholar]
- 35.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998 Aug 20;339(8):504–10. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 36.Neurath AR, Strick N, Li YY. Water dispersible microbicidal cellulose acetate phthalate film. BMC Infect Dis. 2003 Nov 14;3:27. doi: 10.1186/1471-2334-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg S, Vermani K, Garg A, Anderson RA, Rencher WB, Zaneveld LJ. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm Res. 2005 Apr;22(4):584–95. doi: 10.1007/s11095-005-2490-1. [DOI] [PubMed] [Google Scholar]
- 38.Zussman A, Lara L, Lara HH, Bentwich Z, Borkow G. Blocking of cell-free and cell-associated HIV-1 transmission through human cervix organ culture with UC781. AIDS. 2003 Mar 28;17(5):653–61. doi: 10.1097/00002030-200303280-00002. [DOI] [PubMed] [Google Scholar]
- 39.Abram ME, Sarafianos SG, Parniak MA. The mutation T477A in HIV-1 reverse transcriptase (RT) restores normal proteolytic processing of RT in virus with Gag-Pol mutated in the p51-RNH cleavage site. Retrovirology. 2010;7:6. doi: 10.1186/1742-4690-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moncla BJ, Hillier SL. Why nonoxynol-9 may have failed to prevent acquisition of Neisseria gonorrhoeae in clinical trials. Sex Transm Dis. 2005 Aug;32(8):491–4. doi: 10.1097/01.olq.0000170444.13666.e9. [DOI] [PubMed] [Google Scholar]
- 41.Moncla BJ, Pryke K, Isaacs CE. Killing of Neisseria gonorrhoeae, Streptococcus agalactiae (group B streptococcus), Haemophilus ducreyi, and vaginal Lactobacillus by 3-O-octyl-sn-glycerol. Antimicrob Agents Chemother. 2008 Apr;52(4):1577–9. doi: 10.1128/AAC.01023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002 Jun;46(6):1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cummins JE, Jr, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, et al. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007 May;51(5):1770–9. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hariharan M, Bogue BA. Orally dissolving film strips (ODFS): the final evolution of orally dissolving dosage forms. Drug Delivery Technology. 2009 Feb;9(2):24–9. [Google Scholar]