Abstract
Unresolved questions remain concerning the derivation of the vagina with respect to the relative contributions from the Müllerian ducts, the urogenital sinus, and the Wolffian ducts. Recent molecular and cellular studies in rodents have opened up a large gap between the level of understanding of vaginal development in mice and understanding of human vaginal development, which is based on histology. To compare the findings in mice with human vaginal development and to address this gap, we analysed molecular characteristics of the urogenital sinus, Wolffian ducts, and Müllerian ducts in 8–14-week-old human specimens using immunohistochemical methods. The monoclonal antibodies used were directed against cytokeratin (CK) 14, CK19, vimentin, laminin, p63, E-cadherin, caspase-3, Ki67, HOX A13, and BMP-4. The immunohistochemical analysis revealed that, during weeks 8–9, the epithelium of the Müllerian ducts became positive for p63 as p63-positive cells that originated from the sinus epithelium reached the caudal tip of the fused Müllerian ducts via the Wolffian ducts. The lumen of the fused Müllerian ducts was closed by an epithelial plug that contained both vimentin-positive and vimentin-negative cells. Subsequently, the resulting epithelial tube enlarged by proliferation of basal p63-positive cells. The first signs of squamous differentiation were detected during week 14, with the appearance of CK14-positive cells. According to our results, all three components, namely, the urogenital sinus, Wolffian ducts, and Müllerian ducts, interacted during the formation of the human vagina. The sinus epithelium provided p63-positive cells, the Wollfian ducts acted as a ‘transporter’, and the Müllerian ducts contributed the guiding structure for the vaginal anlagen. Epithelial differentiation began at the end of the period studied and extended in a caudo-cranial direction. The present study is one of the first to provide up-to-date molecular correlates for human vaginal development that can be compared with the results of cell biological studies in rodents.
Keywords: cytokeratins, human prenatal development, p63, vagina, vimentin
Introduction
The development of the human vagina is a late incident in the process of organogenesis. It is an extremely complex developmental process, owing to the fact that the vagina is thought to arise from different primordia. At present, it is considered to have a dual origin (Sadler, 2004): the upper portion is thought to be derived from the Müllerian ducts (MDs; Mijsberg, 1924; Forsberg, 1973) and the lower portion from the urogenital sinus (Müller, 1830; Valentin, 1835; Koff, 1933; Bulmer, 1957), which provides tissue for the so-called vaginal plate. As a consequence, the upper portion is called the ‘Müllerian vagina’, whereas the lower portion is called the ‘sinus vagina’. A possible contribution of the residual portions of the Wolffian ducts (WDs) that are intercalated with the junction of the urogenital sinus and the MDs was assumed by earlier scientists (Mijsberg, 1924). A helper function of the WDs cannot be ruled out and is still under discussion (Mauch et al. 1985; Drews, 2007).
Most studies of the development of the human vagina are based on classical histological observations; only one study has also provided immunohistochemical data (Martens et al. 2007). It is obvious that, owing to methodical restrictions, studies on human specimens cannot be used to resolve the controversy concerning different concepts of vaginal development. Thus, animal models, especially rodents, are used to clarify the molecular mechanisms that underlie the origin and organogenesis of the vagina. In a recent review (Cai, 2009), the findings of such studies were summarized and compared with former morphological concepts. The results of this comparison provide evidence that both the ‘Müllerian’ and the ‘sinus’ vagina are formed by the MDs. In mice, the homeobox protein Hoxa13, which activates the bone morphogenetic protein 4 (BMP-4) promotor, plays an important role in vaginal differentiation. BMP-4 itself induces the expression of p63, which is a member of the p53 family, and the generation of stratified squamous epithelial cells. The new theory was supported by a cell line tracing experiment in which cells of the epithelia of the MDs, WDs, and urogenital sinus were labelled fluorescently in mouse embryos that were obtained by crossing tdTomato-EGFP transgenic mice with Cre-recombinase transgenic mice that expressed Cre recombinase in each type of epithelium (Kurita, 2010). The results showed that the lower vagina is formed via caudal growth of the ‘Müllerian vagina’, and that the vaginal tissue present in the adult is derived only from MD epithelium.
However, the morphology of the genital tract of rodents is different from that of the human female. We investigated the early development of the human vaginal anlagen (VA) in a complete series of human embryos and fetuses from week 8 to 14 postovulatory age (po; see O’Rahilly & Müller, 2010) using conventional histology and immunohistochemistry. To study the spatial and temporal arrangement of the developing epithelia in the human female vagina, the patterns of expression of several proteins were determined: two cytokeratins (CK14 and CK19) were used to detect vaginal and ectocervical squamous epithelia (Martens et al. 2007), and E-cadherin was used to identify the epithelial character of the MD and WD in general (Orvis & Behringer, 2007). To identify areas of apoptosis and proliferation in the epithelial linings, antibodies against caspase 3 and Ki67 were used, respectively. We were among the first researchers to describe the distribution of p63-positive cells, which are supposed to determine the fate of vaginal and uterine cells (Kurita et al. 2005), in early human specimens. To obtain information about the character of the MD and the WD, as well as the interaction of both ducts, the patterns of expression of vimentin and laminin were investigated, as performed in male human fetuses (Magro & Grasso, 1995). For the first time, HOX A13, which is expressed strongly in the developing mouse vagina, was studied in human prenatal samples by immunolabelling, and the presence of BMP-4, which is upregulated by HOX A13, was also analysed.
The study reported herein was designed to provide new insights into the origin and development of the early human vaginal epithelium and to compare data obtained from studies in mice with data gained from the investigation of human specimens.
Materials and methods
Materials
A total of 18 female embryos and fetuses, between weeks 8 and 14 po, were studied. The specimens were obtained from the collection of the Division of Clinical and Functional Anatomy, Innsbruck Medical University, or from legal abortions (according to Austrian law) collected by different gynaecologists, having received parental consent (number of specimens/week: 2/8th, 2/9th, 2/10th, 3/11th, 3/12th, 3/13th, and 5/14th). The specimens, which had already been investigated in other studies (Fritsch et al. 2007, 2010), showed no macroscopic abnormalities and were categorized according to their postovulatory age, which was based upon the crown–rump length (CRL) and external and internal morphology, or upon their estimated gynaecological age.
Tissue preparation and conventional histology
The specimens were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) . After dehydration and embedding in paraffin using a routine histological infiltration processor (Miles Scientific Inc., Naperville, IL, USA), the specimens were cut into 4-μm sections using a Microm ERGO Star Rotations microtome (Microm, Walldorf, Germany). The sections were mounted on Superfrost Plus microscope slides (Menzel, Braunschweig, Germany), dried overnight, dewaxed with xylene, and rehydrated in a graded alcohol series. Every 10th section of a series was stained with haematoxylin and eosin.
Immunohistochemistry
Immunostaining of the paraffin sections was performed using the Discovery XT automated staining system (Ventana Medical Systems, a member of the Roche group, Vienna, Austria). After drying, dewaxing and rehydration, antigen retrieval was initiated by heat-induced unmasking of the epitopes while the slides were immersed in citrate buffer. The slides were then incubated with selected monoclonal antibodies. After incubation for 1 h at 37 °C with primary antibody, a biotinylated secondary antibody (Ventana 760-4205, Vienna, Austria) was applied for 0.5 h at 37 °C. Subsequently, chromogenic detection with streptavidin horseradish peroxidase (SA-HRP) was performed using a diaminobenzidine (DAB) detection kit (Ventana 760-124, Vienna, Austria) in accordance with the manufacturer's instructions. To allow specific morphological analysis, counterstaining with haematoxylin and Bluing reagent was performed. After the staining procedure was completed, the specimens were dehydrated in series of ethanol and xylene, and mounted permanently using Cytoseal embedding medium (Microm, Walldorf).
The following antibodies were used: the anti-cytokeratin antibodies CK14 (ready to use; Novocastra, Newcastle upon Tyne, UK) and CK19 (at a dilution of 1 : 100; Thermo Fisher Scientific, Fremont, CA, USA), anti-E-cadherin (ready to use; Novocastra), anti-p63 (at a dilution of 1 : 100; Thermo Fisher Scientific), anti-laminin (ready to use; Biogenex, San Ramon, CA, USA), anti-vimentin (ready to use; Linaris, Wertheim-Bettingen, Germany), anti-Ki67 (ready to use; Linaris), anti-caspase-3 (at a dilution of 1 : 100; Sigma-Aldrich, St. Louis, MO, USA), anti-HOX A13 (at a dilution of 1 : 100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-BMP-4 (at a dilution of 1 : 50; Acris Antibodies GmbH, Herford, Germany).
Results
Weeks 8–10
At the beginning of the early period (the 8th week) of female urogenital development, fusion of the MDs took place, and the WDs were still visible. All ducts were open and had lumina. The epithelia of both the MD and the WD were positive for E-cadherin (not shown).
In the lower part of the genital cord, the MDs, which were lined by cuboidal cells, were in apposition to each other (Fig. 1A). Vimentin labelling distinguished the MDs clearly from the neighbouring WDs, which had an epithelium that was less cuboidal, i.e. flatter, whereas the WDs were strongly positive for vimentin, and the MDs were negative or only slightly positive (Fig. 1B). In contrast to the WDs, the caudal ends of the MDs did not open into the sinus (Fig. 1A). The caudal ends were separated from the Müllerian tubercle (an invagination caused by the blind tip of the MDs in the dorsal wall of the urogenital sinus) and its large cells, which stained only faintly with HE, by a mass of dark vimentin-positive mesodermal cells (Fig. 1A–D). Cells that were positive for p63 lined the basal layers of the sinus epithelium and the Müllerian tubercle and some were detected in the MDs. P63-positive cells also extended back into the openings of the WDs (Fig. 1C). Where the blind ends of the MDs were connected immediately to the Müllerian tubercle and/or to the WDs, interruptions of the basal lamina could be observed (Fig. 1D). These interruptions (gaps) might represent possible points of entry for p63-positive cells. In the middle part of the genital cord, the MDs could be seen to be fusing (Fig. 1E,H) and, laterally, the vimentin-positive WDs were immediately connected (Fig. 1F) to the fused MDs. At this level, the Wolffian epithelium was negative for p63 (Fig. 1G).
Fig. 1.
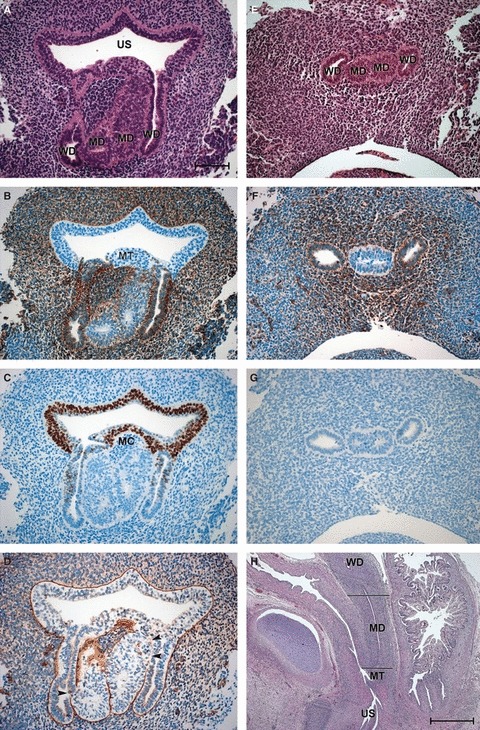
Sections of the vaginal anlagen (VA) of 8- and 10-week embryos. (A–D) Neighbouring transverse sections through the VA of an 8-week embryo at the level of the urogenital sinus. (A) HE staining; (B) vimentin; (C) p63; (D) laminin (arrowheads point to gaps in the basal lamina). (E–G) Neighbouring transverse sections through the VA of the same embryo at the level of the lower utero-vaginal canal. (E) HE staining; (F) vimentin; (G) p63. Bar: 100 μm. (H) Sagittal section through the VA of a 10-week fetus. The level of A–D is marked with the caudal line, whereas that of E–G is indicated by the cranial line. Bar: 1000 μm. MC, mesodermal cells; MD, Müllerian duct; MT, Müllerian tubercle; US, urogenital sinus; WD, Wolffian duct.
At the end of this early period (the 10th week), the caudal tips of the MDs had fused completely (Fig. 2A,B,D–F). The original epithelium of the MDs appeared to have been replaced by dark, basal p63-positive cells (Fig. 2B,E) and paler superficial cells. At the level of the openings of the WDs, p63-positive cells were found in the sinus epithelium, in the Müllerian tubercle, and in the WDs, from which they seemed to reach the Müllerian epithelium (Fig. 2E). Whereas apoptotic cells were found mainly in the WDs (Fig. 2F), labelling of vimentin was no longer restricted to the WDs but was also found in the MDs (Fig. 2C), especially in the middle portion of the VA where the lumen had started to close. Labelling of Ki67 (not shown) was seen in the VA as well as in the surrounding mesenchyme, and indicates a phase of proliferation. During the period of vaginal development described, no positive staining of HOX A13, BMP-4 or CK14 was detected in the MDs, WDs or VA.
Fig. 2.
Sections of the VA of 10- and 13-week female fetuses. (A–C) Sagittal sections through the utero-vaginal anlagen of a 10-week female fetus. (A) HE staining; (B) p63; (C) vimentin. Bar: 200 μm. (D–F) Neighbouring transverse sections through the VA and the WDs (we regret that cell detritus is found in the openings) of a 10-week female fetus at the level of the urogenital sinus. (D) HE staining; (E) p63; (F) caspase-3. Bar: 100 μm. (G) Sagittal section through the urogenital sinus of a 13-week female fetus, HOX A13. Bar: 100 μm. MD, Müllerian duct; US, urogenital sinus; WD, Wolffian duct.
Weeks 11–14
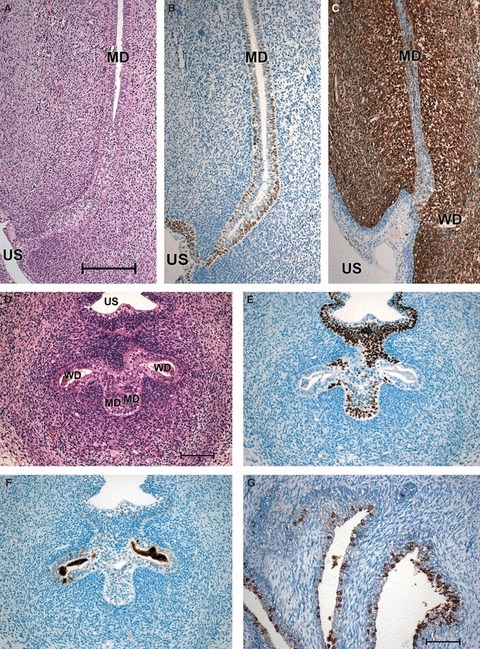
This period of female genital development was characterized by an enormous elongation of the VA, which was surrounded by a large number of sprouting vessels (Fig. 3A,B). The caudal region of the VA consisted of folded areas with lateral outgrowths and narrow portions (Fig. 3C) with dark, basal p63-positive cells (Fig. 3D). The lumen was filled by central pale cells that were often vimentin-positive (Fig. 3E). On the whole, the epithelium had a stratified character, and the cells were small and polygonal. Labelling of Ki67 was visible within the basal epithelium and the surrounding mesenchyme (Fig. 3F). In a few places, namely, above the urogenital sinus, remnants of the WDs were integrated into the vaginal folds (Fig. 3B–E). In transverse sections, the position and composition of the different cell types within the VA became even clearer: the basal cells were small, dark and positive for p63 (Fig. 3G,H) and they surrounded a mass of larger, paler cells that occluded the lumen. All the cells were enclosed by an uninterrupted basal lamina (Fig. 3I) and constituted the preliminary vaginal epithelium.
Fig. 3.
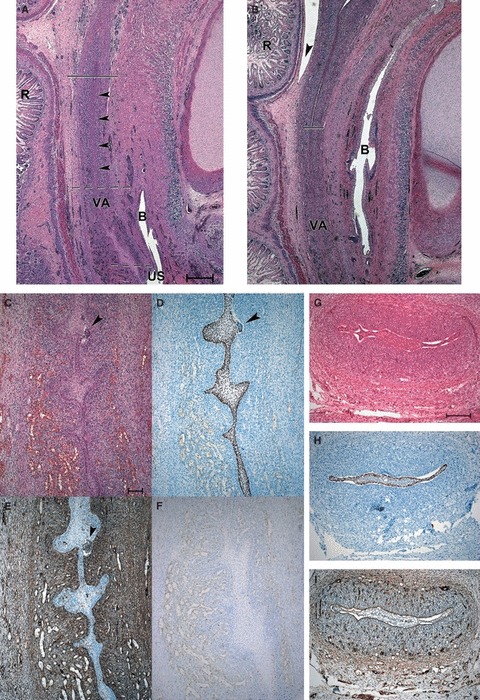
Sections of the VA of 13/14- and 14-week female fetuses. (A–B) Sagittal sections through the utero-vaginal anlagen of a 13/14-week female fetus. (A) HE staining showing the connection of the VA to the sinus; the striated lines mark the area of enlargement shown in C–F. The arrowheads point to the ribbon-like portion of the VA; the continuous line marks the border between the VA and the cervico-uterine portion. (B) HE staining showing the connection of the VA with the cervico-uterine portion (continuous line) and the peritoneal reflection (arrowhead). Bar: 500 μm. (C–F) Enlargements of the folded portion of the VA marked in A. (C) HE staining, a remnant of the WD is marked by an arrowhead. (D) p63, the WD remnant (arrowhead) is unlabelled. (E) vimentin labelling in luminar cells, the WD remnant (arrowhead) is strongly positive. (F) labelling of Ki67 in basal epithelial cells and in the surrounding mesenchyme. Bar: 100 μm. (G–I) Neighbouring transverse sections of the VA of a 14-week female fetus. (G) HE staining, the VA has folds and wings. The basal cells are dark and surround a group of paler cells in the midline. (H) p63; (I) laminin. Bar: 200 μm. B, bladder; R, rectum; US, urogenital sinus; VA, vaginal anlagen.
Cranial to the folded portion, the VA was a ribbon-like cord without a lumen (Fig. 3A). At a level beneath the peritoneal fold, just in front of the middle transverse rectal fold, the cranial end of the VA, where the epithelium became progressively less stratified, was demarcated clearly from the cervico-uterine portion of the MD. Here the character of the epithelium changed rapidly from small polygonal VA epithelial cells to columnar cervico-uterine cells (Fig. 4A). The latter stained strongly for p63 (Fig. 4A) and were also labelled positively for vimentin (Fig. 4B). Whereas the cells of the VA were slightly positive for CK19, those of the cervico-uterine portion were strongly positive (Fig. 4C). The VA showed the first signs of a squamous epithelial character, as represented by scattered CK14-positive basal cells (Fig. 4D). The occurrence of these cells stopped abruptly at the border between the VA and the cervico-uterine portion.
Fig. 4.
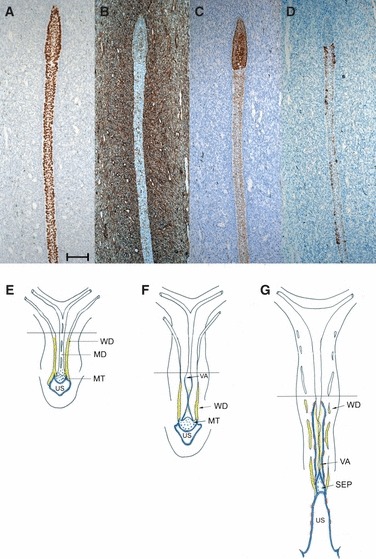
(A–D) Enlargements of neighbouring sagittal sections of a 13/14-week female fetus at the border between the VA and the cervico-uterine portion marked in Fig. 3A. (A) p63; (B) vimentin; (C) CK19; (D) CK14. Bar: 100 μm. (E–G) Schematic drawings (commonly used in the literature, redrawn after Koff, 1933) showing early vaginal development and the alterations in the distribution of vimentin = yellow, p63 = blue, and CK14 = red. The line indicates the level up to which we have presented our results. MD, Müllerian duct; MT, Müllerian tubercle; SEP, solid epithelial plug between VA and US; US, urogenital sinus; VA, vaginal anlagen; WD, Wolffian duct.
During the period of vaginal development described, no positive staining of HOX A13 or BMP-4 was found in the MDs, WDs or VA. However, the sinus epithelium was positive for HOX A13 (Fig. 2G), but not for BMP-4.
Discussion
The present study was undertaken to examine the molecular aspects of early development of the human vagina, within the limitations on research using human specimens. The results give a strong indication that all three primordia, namely, the urogenital sinus epithelium, WDs and MDs, interact during the early formation of the human vagina. Given that the conflicting ideas concerning the origin of the vagina have been highlighted and summarized thoroughly in different books (Gray & Skandalakis, 1972; O’Rahilly, 1977; Sadler, 2004) and papers (Koff, 1933; Bulmer, 1957; Cai, 2009; Kurita, 2010), we will emphasize our novel findings and compare them with the classical morphological descriptions of the development of the human vagina and the results of cell biological studies in mice.
Role of the urogenital sinus
One of the major points raised by Bulmer (1957) in his paper on vaginal development, which was based on the study of an uninterrupted series of human samples, was his belief that the vagina was formed from an upgrowth of differentiated epithelium from the dorsal wall of the sinus. He shared this opinion with Koff (1933) and Vilas (1932).
Our findings during the very early phase (8th week) of vaginal development showed clearly that the epithelium of the urogenital sinus was far more differentiated than that of the MDs and WDs at this stage. It was found to be a stratified epithelium with dark cells that were already positive for p63 (Fig. 4E); positive staining for p63 could not be detected in the downgrowing MDs or WDs, with the exception of the sinus region. At this level, where the WDs open into the sinus, and the distal parts of the MDs join and form a blind end, at a first glance, p63-positive cells do not reach the MDs. However, according to our findings, there is ample evidence that p63-positive cells from the sinus may reach the openings of the WDs, and direct contact of the cells with openings of the basal lamina between the MDs and WDs leads these cells to the tip of the MDs, where they start to spread. One week later (week 9), sections from the same level show that, at its base, the distal portion of the MD is full of p63-positive cells (Fig. 4F). Our findings with regard to the distribution of p63-positive cells during early vaginal development are in accordance with those of Kurita (2010), who showed that, during the early stages of development in mice, the lower vagina forms via the proliferation of p63-positive cells at the caudal end of the MDs. In an earlier paper, the same group (Kurita et al. 2005) compared the findings in mice with those from human samples, but they were not able to find parallels during the early stages owing to the restricted number and ages of their human specimens.
It is likely that the differentiation of the VA starts at the end of the period that we studied, because CK14-positive cells were found to be scattered throughout the length of the VA at this point (week 14; Fig. 4G). Cells that are positive for CK14 are characteristic of squamous differentiation. They are found in the adult human vagina, where they also express both bcl-2 and p63 (Kurita & Cunha, 2001). We assume that the CK14-positive cells that we identified are these basal keratinocytes of the vagina, and that their differentiation might be guided by the p63-positive cells from the sinus epithelium. Similarly, in p63−/− mice, the columnar epithelium persists in the lower female genital tract, no squamous differentiation occurs, and the urinary system lacks urothelial differentiation (Ince et al. 2002).
Role of the WDs
In 1933, Koff was of the opinion that Mijsberg (1924), who had concluded that the lower third of the vagina is formed by the WDs, had erred in the interpretation of his observations. During the early development of mice, a helper function of the WDs in the downgrowth of the MDs has been hypothesized (Mauch et al. 1985; Drews, 2007). An essential point of this hypothesis is that the MDs migrate downwards within the basal lamina of the WDs. This has been corroborated recently by molecular studies in mice (Orvis & Behringer, 2007), in which the WDs were found to give essential signals to the MDs and to guide them to the urogenital sinus. The data in mice were obtained at earlier stages of development than those of our human specimens. Nonetheless, they give hints that might explain our own results. There is no doubt that the WD cells do not contribute to the MDs, in mice or in humans. However, there is considerable evidence that, during the first stage of vaginal development, cells from the urogenital sinus use the openings of the WDs (Bulmer, 1957; Wartenberg, 1985) and the common or open basal lamina with the MDs as a means of transport to reach the MD. Our observation is in accordance with the thorough histological investigation and findings of Bulmer (1957). However, until now there has been no evidence from experimental studies to support our supposition. After the WDs have completed their functions of guidance and transport they start to regress (Mauch et al. 1985). We were not the first to detect the labelling of vimentin in the WD epithelium (Viebahn et al. 1987) and, furthermore, we found the WDs and MDs to be labelled differently with respect to vimentin during the early stages of development. These different patterns of intermediate filaments in WDs and MDs might also be a hint that WDs do not make a cellular contribution to the MDs. In the male fetus, Magro & Grasso (1995) described positive staining for vimentin in the MD and the disappearance of staining for vimentin at the beginning of MD regression. However, our findings for WDs in females do not agree with those of Magro & Grasso (1995) in males.
Role of MDs
It was shown recently that epithelial differentiation and regionalization in the hindgut has a cranio-caudal course and is initialized by endodermal cells (Fritsch et al. 2007). At first glance, this seems to differ from differentiation of the vaginal epithelium which, according to our results and those of previous studies (Koff, 1933; Bulmer, 1957; Forsberg, 1973; Orvis & Behringer, 2007; Kurita, 2010), runs in a caudo-cranial direction (Fig. 4E–G) and is probably initialized by cells or a cellular stimulus that is derived from the sinus, which itself is of endodermal origin. We can conclude this from our conventional histological sections as well as from the immunostaining of p63 and vimentin. One major reason for this phenomenon (a cellular stimulus derived from the sinus epithelium) has been revealed recently by Orvis & Behringer (2007), who pointed to the epithelial character of the early WDs, in contrast to that of the early MDs, which they considered to be mesoepithelial. Thus, we conclude that the MDs need an epithelial input/stimulus from the epithelium of the sinus in order to initiate cellular change from mesoepithelium to epithelium, and that they might need a ‘transporter’, i.e. the WDs. It becomes evident that, in comparison with anorectal development, early vaginal development is influenced by endodermal cells. It is probable that the epithelial input or stimulus consists of the p63-positive and CK14-positive cells that were found to ascend the VA. During this stimulus, the lumen of the fused MDs is closed by an epithelial plaque of pale MD cells that have acquired positive staining for vimentin. The cellular presence of the intermediate filament protein vimentin might be necessary for the closure, and this points to a distinct functional status of the pale MD cells.
In mice, HOX a13 has been reported to upregulate BMP-4 in the descending MD and to be responsible for cellular proliferation in the MD (Cai, 2009). During the period of development investigated in the present study, no positive immunostaining for HOX A13 or BMP-4 could be observed in MD cells; however, staining for HOX A13 and BMP-4 was present in the urogenital sinus. Therefore, we suspect that expression of HOX A13 and BMP-4 occurs during a later period of vaginal differentiation than that analysed in the present study, as shown already in mice (Masse et al. 2009).
Thus, there is no doubt that the main role of the fused MDs is to provide the guiding structure for the development of the human vagina. This is in agreement with the classical descriptions (Koff, 1933; Bulmer, 1957), as well as the recent findings of Kurita (2010) and Cai (2009). The squamous character of the vaginal epithelium, and thus the starting point for the process of epithelial change, was indicated first in week 14 of development by the presence of CK14-positive cells, which were restricted to the vaginal portion of the utero-vaginal anlagen and were not found in the cervico-uterine portion. On the basis of our results, we can only speculate about the nature of the border between the vaginal and cervico-uterine portions, which is situated just beneath the peritoneal fold and on a level with the middle transverse fold of the rectum, i.e. at the location of the future vaginal fornices. We suppose that, up to this point, the vaginal portion of the MDs is created by the MDs in close association with the WDs, but above this point the MDs develop on their own without influence from the WDs (Fig. 4E–G).
Further epithelial differentiation of the female genital tract and the question of whether the sinus-derived epithelium in the MDs is replaced by Müllerian cells during later stages (Kurita, 2010) will be the subject of a subsequent study.
Acknowledgments
We thank Claudia Siemon and Prof. Paul Debbage for careful reading and correction of the manuscript and Romed Hoermann for helpful suggestions and support with the Figures. The excellent support of Dr Wolf in the conservation of the human samples is gratefully acknowledged.
Author contributions
Helga Fritsch was responsible for the concept of the study, the acquisition of data, data interpretation, and writing of the manuscript. Nadia Adam started the data acquisition and was responsible for the critical revision of the study. Elisabeth Richter was responsible for the improvement of the technical procedures and the choice of markers.
References
- Bulmer D. The development of the human vagina. J Anat. 1957;91:490–509. [PMC free article] [PubMed] [Google Scholar]
- Cai Y. Revisting old vaginal topics: conversion of the Müllerian vagina and origin of the ‘sinus’ vagina. Int J Dev Biol. 2009;53:925–934. doi: 10.1387/ijdb.082846yc. [DOI] [PubMed] [Google Scholar]
- Drews U. Helper function of the Wolffian ducts and role of androgens in the development of the vagina. Sex Dev. 2007;1:100–110. doi: 10.1159/000100031. [DOI] [PubMed] [Google Scholar]
- Forsberg JG. Cervicovaginal epithelium: its origin and development. Am J Obstet Gynecol. 1973;115:1025–1043. doi: 10.1016/0002-9378(73)90687-x. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Aigner F, Ludwikowski B, et al. Epithelial and muscular regionalization of the human developing anorectum. Anat Rec. 2007;290:1449–1458. doi: 10.1002/ar.20589. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Zehm S, Illig R, et al. New insights into the development and differentiation of human anorectal epithelia. Are there clinical consequences? Int J Colorectal Dis. 2010;25:1231–1242. doi: 10.1007/s00384-010-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SW, Skandalakis JE. Embryology for Surgeons: The Embryological Basis for the Treatment of Congenital Defects. Philadelphia: Saunders; 1972. The female reproductive tract; pp. 633–643. [Google Scholar]
- Ince TA, Cviko AP, Quade BJ, et al. P 63 coordinates anogenital modeling and epithelial cell differentiation in the developing female urogenital tract. Am J Pathol. 2002;161:1111–1117. doi: 10.1016/S0002-9440(10)64387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff AK. Development of the vagina in the human fetus. Contrib Embryol. 1933;24:59–91. [PubMed] [Google Scholar]
- Kurita T. Developmental origin of vaginal epithelium. Differentiation. 2010;80:99–105. doi: 10.1016/j.diff.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cunha GR. Roles of p63 in differentiation of Müllerian duct epithelial cells. Ann NY Acad Sci. 2001;948:9–12. doi: 10.1111/j.1749-6632.2001.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, et al. Differential expression of p63 isoforms in female reproductive organs. Mech Dev. 2005;122:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Magro G, Grasso S. Expression of cytokeratins, Vimentin and basement membrane components in human fetal male müllerian duct and perimüllerian mesenchyme. Acta Histochem. 1995;97:13–18. doi: 10.1016/S0065-1281(11)80202-3. [DOI] [PubMed] [Google Scholar]
- Martens JE, Smedts F, van Muyden RC, et al. Reserve cells in human uterine cervical epithelium are derived from müllerian epithelium at midgestational age. Int J Gynecol Pathol. 2007;26:463–468. doi: 10.1097/pgp.0b013e31803c7c18. [DOI] [PubMed] [Google Scholar]
- Masse J, Watrin T, Laurent A, et al. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol. 2009;53:411–424. doi: 10.1387/ijdb.082680jm. [DOI] [PubMed] [Google Scholar]
- Mauch RB, Thiedemann KU, Drews U. The vagina is formed by down growth of Wolffian and Müllerian ducts. Anat Embryol. 1985;172:75–87. doi: 10.1007/BF00318946. [DOI] [PubMed] [Google Scholar]
- Mijsberg W. Über die Entwicklung der Vagina, des Hymen und des Sinus urogenitalis beim Menschen. Z Anat Entw-Gesch. 1924;74:684–760. [Google Scholar]
- Müller J. Bildungsgeschichte der Genitalien. Düsseldorf: Arnz; 1830. [Google Scholar]
- O’Rahilly R. The development of the vagina in the human. In: Blandau RJ, Bergsma D, editors. Morphogenesis and Malformation of the Genital System. Birth Defects. New York: Alan R. Liss; 1977. pp. 123–136. Original articles series 13. [Google Scholar]
- O’Rahilly R, Müller F. Developmental stages in human embryos. Revised and new measurements. Cells Tissues Organs. 2010;192:73–84. doi: 10.1159/000289817. [DOI] [PubMed] [Google Scholar]
- Orvis GD, Behringer RR. Cellular mechanisms of Müllerian duct formations in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler TW. Urogenital System. Langman′s Medical Embryology. Baltimore: Lippincott Williams & Wilkins; 2004. pp. 321–362. [Google Scholar]
- Valentin G. Handbuch der Entwicklungsgeschichte des Menschen mit vergleichender Rücksicht der Entwicklung der Säugetiere und Vögel. Berlin: Rücker; 1835. pp. 322–408. [Google Scholar]
- Viebahn C, Lane EB, Ramaekers FC. The mesonephric (wollfian) and paramesonephric (müllerian) ducts of golden hamsters express different intermediate-filament proteins during development. Differentiation. 1987;34:175–188. doi: 10.1111/j.1432-0436.1987.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Vilas E. Über die Entwicklung der menschlichen Scheide. Z Anat Entwickl Gesch. 1932;98:262–292. [Google Scholar]
- Wartenberg H. Morphological studies on the role of the periductal stroma in the regression of the human male Müllerian duct. Anat Embryol. 1985;171:311–323. doi: 10.1007/BF00347020. [DOI] [PubMed] [Google Scholar]


