Abstract
In adults, the lateral pterygoid muscle (LPM) is usually divided into the upper and lower heads, between which the buccal nerve passes. Using sagittal or horizontal sections of 14 fetuses and seven embryos (five specimens at approximately 20–25 weeks; five at 14–16 weeks; four at 8 weeks; seven at 6–7 weeks), we examined the topographical relationship between the LPM and the buccal nerve. In large fetuses later than 15 weeks, the upper head of the LPM was clearly discriminated from the lower head. However, the upper head was much smaller than the lower head in the smaller fetuses. Thus, in the latter, the upper head was better described as an ‘anterior slip’ extending from the lower head or the major muscle mass to the anterior side of the buccal nerve. The postero-anterior nerve course seemed to be determined by a branch to the temporalis muscle (i.e. the anterior deep temporal nerve). At 8 weeks, the buccal nerve passed through the roof of the small, fan-like LPM. At 6–7 weeks, the LPM anlage was embedded between the temporobuccal nerve trunk and the inferior alveolar nerve. Therefore, parts of the LPM were likely to ‘leak’ out of slits between the origins of the mandibular nerve branches at 7–8 weeks, and seemed to grow in size during weeks 14–20 and extend anterosuperiorly along the infratemporal surface of the prominently developing greater wing of the sphenoid bone. Consequently, the topographical relationship between the LPM and the buccal nerve appeared to ‘change’ during fetal development due to delayed development of the upper head.
Keywords: buccal nerve, human fetus, lateral pterygoid muscle, sphenoid bone, temporalis muscle
Introduction
The human lateral pterygoid muscle (LPM) is composed of the upper and lower heads. However, whether these heads are functionally different still remains unclear (reviewed by White, 1985; Kineberg, 1991; Bertilsson & Ström, 1995). The buccal nerve usually passes between the two heads of the LPM (Fig. 1), but in which developmental process does the buccal nerve pass between them? According to Aziz et al. (1998), the two heads are most frequently supplied by a common nerve including the buccal nerve. Schumacher et al. (1976) and Akita et al. (2000) described these heads of the LPM as being supplied by multiple nerve twigs from the mandibular nerve root. Foucart et al. (1998) suggested that the LPM is composed of five to six independent functional musculo-aponeurotic layers. Nevertheless, descriptions on the fetal LPM have been limited to the functional aspect in terms of temporomandibular joint movement (Öğőtcen-Toller & Juniper, 1993; Carranza et al. 2006). However, Akita et al. (2000) have postulated a model of fetal muscle cleavage including the LPM based on a detailed dissection study of adult cadavers.
Fig. 1.
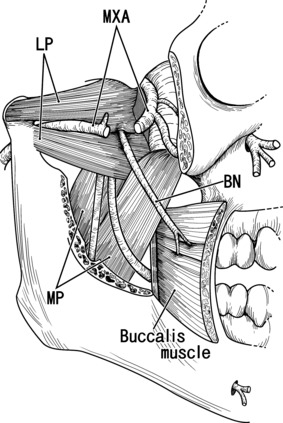
Buccal nerve passing between the upper and lower heads of the lateral pterygoid muscle: a diagram based on the adult anatomy. Lateral view of the right half of the head. The zygomatic arch, a part of the mandible, and the masseter muscle were removed to show the downward course of the buccal nerve (BN). In this case the maxillary artery (MXA; partly removed) also passes through the lateral pterygoid muscle (LP). MP, medial pterygoid muscle.
In the aforementioned context, Mérida-Velasco et al. (2001) noted that, even in fetuses, the buccal nerve often passes through the inferior part of the temporalis muscle. However, their major interest was focused on classification rather than the developmental sequence responsible for the morphology. For this reason, their materials were limited to specimens with a crown–rump length (CRL) exceeding 35 mm, or in which the topographical relationships had already become fixed. In the present study, therefore, our aim was to clarify (i) when and how the two heads of the LPM form in human fetuses, and (ii) when the buccal nerve forms its course between the two heads. The upper and lower heads are easily identified in frontal or sagittal sections because of their topography. Unfortunately, small specimens in the collection we used were generally cut horizontally. For this reason, we paid special attention to the correspondence between sagittal and horizontal sections of the fetal LPM. In addition, we also focused on the course of the buccal nerve through the temporalis muscle.
Materials and methods
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). Approval for the study was granted by the ethics committee of the university. We examined paraffin-embedded sections of 14 fetuses and seven embryos: five specimens with a CRL of 185–240 mm, or approximately 20–25 weeks, five with a CRL of 100–135 mm, or approximately 14–16 weeks, four with a CRL of 35–50, or approximately 8–9 weeks, and seven with a CRL of 20–27 mm, or approximately 6–7 weeks. All the specimens were part of the large collection kept at the Embryology Institute of the Universidad Complutense, Madrid, being the products of urgent abortion, miscarriages or ectopic pregnancies managed at the Department of Obstetrics of the University. The donated fetuses had been fixed in 10% v/v formalin solution and stocked in the same solution for more than 3 months. Specimens larger than 110 mm CRL were decalcified in 5% v/v nitric acid for 1–4 days at room temperature.
After routine procedures for paraffin-embedded histology, sections were cut sagittally (all five specimens at 20–25 weeks, three of five at 14–16 weeks, one of four at 8–9 weeks, and two of seven at 6–7 weeks) or horizontally (the other 10 specimens) at a thickness of 5–10 μm at intervals of 500 μm (large specimens at 20–25 weeks), 100 μm (at 14–16 weeks), or 20 μm (the other small specimens). The sagittal sections were prepared for one side of the head after division of the specimen along the midline. The horizontal planes were, more or less, oblique relative to the frontal plane because of flexure of the embryonic head and neck. Most sections were stained with hematoxylin and eosin (HE), while some were subjected to silver impregnation or azan staining.
Identification of fetal structures around the Meckel's and Reichert's cartilages was based on our recent studies (Rodríguez-Vázquez et al. 1992, 2006, 2011; Katori et al. 2011a,b; Osanai et al. 2011) of the areas overlapping with those of the present study, i.e. identifications started from the trigeminal ganglion and the mandibular nerve root.
Results
With regard to the topographical relationship between the buccal nerve and the LPM, for readers who are unfamiliar with fetal morphology, the present descriptions start from large fetuses, as they have more or less the same topographical anatomy as that in adults. Likewise, to provide a better understanding of the topography of each figure, we attempt to show not only the buccal nerves and LPM in the prospective masticatory space, but also major anatomical landmarks around the space, such as the oral wall (or buccinator muscle), eye, cochlea and pharyngotympanic tube. In all figures showing sagittal sections (Figs 2, 3, 5 and 6), the right-hand side of the figure corresponds to the anterior (or face) side of the head, and panels A, B, and so on are displayed from the medial to the lateral side. In figures showing horizontal sections (Figs 4, 7 and 8), the upper (or left-hand) side corresponds to the anterior (or lateral) side of the head.
Fig. 2.
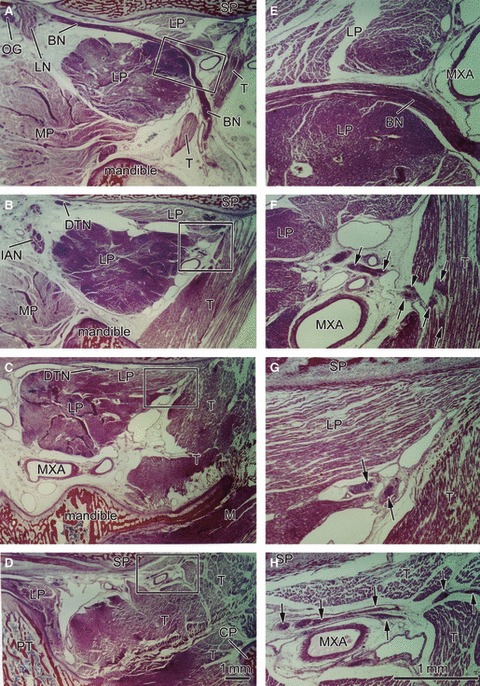
Sagittal sections of a 22-week-fetus (CRL 185 mm) showing a longitudinal course of the buccal nerve. Panel A is the most medial side of the figure, and panel D the most lateral side. The right-hand side of the figure corresponds to the anterior side of the head. Panels E–H are higher magnification views of squares in panels A–D, respectively. Intervals between panels are 4 mm (A–B), 7 mm (B–C) or 3 mm (C–D), respectively. The buccal nerve (BN) is longitudinally cut in panel A. The lower head of the lateral pterygoid muscle (LP), cut transversely, is larger than the upper head, most of which is cut longitudinally in the anterosuperior side of the nerve course. Both heads are attached in panel C. The BN also passes through the inferomedial part of the temporalis muscle (T). The anterior deep temporal nerve from the BN enters the T in panel F, whereas the other deep temporal nerves (DTN) end in panel H. These nerves are indicated by arrows in the higher magnification views (panels E–H). Dark staining in parts of the lateral pterygoid and temporalis muscles appears to be due to postmortem change different from other muscles. Panels A–D (or Panels E–H) have been prepared at the same magnification (scale bars in panels D and H). BN, buccal nerve; CP, coronoid process of the mandible; CTN, chorda tympani nerve; DTN, deep temporal nerves; IAN, inferior alveolar nerve; ICA, internal carotid artery; LN, lingual nerve; LP, lateral pterygoid muscle; MC, Meckel's cartilage; MN, mandibular nerve; Mn, masseter nerve; MP, medial pterygoid muscle; MXA, maxillary artery; MXN, maxillary nerve; OG, otic ganglion; PT, cartilaginous pterygoid part of the sphenoid (independent from the other parts); PTT, pharyngotympanic tube; RC, Reichert's cartilage; SP, sphenoid bone anlagen; T, temporalis muscle; ZA, zygomatic arch.
Fig. 3.
Sagittal sections of a 16-week-fetus (CRL 125 mm) showing that the upper head of the lateral pterygoid muscle is much smaller than the lower head. Panel A is the most medial side of the figure, whereas panel H is the most lateral side. The right-hand side of the figure corresponds to the anterior side of the head. Panel F is a higher magnification view of a square in panel E. Intervals between panels are 0.7 mm (A–B), 0.4 mm (B–C), 0.4 mm (C–D), 0.2 mm (D–E), 0.4 mm (E–G) or 1.2 mm (G–H), respectively. The upper and lower heads of the lateral pterygoid muscle (LP) are attached in panel A but they are separated by veins (panel B), by the buccal nerve (BN) and vein (panel C) or by the BN and maxillary artery (MXA; panels D, E and G). The BN issues from the anterior deep temporal nerve (arrows in panel F). In panel H, the BN makes an impression at the superolateral margin of the temporalis muscle (T). Asterisk in panels C–E indicate an intermediate muscle bundle between the T and masseter muscle (M). FA, facial artery; M, masseter muscle. BN, buccal nerve; CP, coronoid process of the mandible; CTN, chorda tympani nerve; DTN, deep temporal nerves; IAN, inferior alveolar nerve; ICA, internal carotid artery; LN, lingual nerve; LP, Lateral pterygoid muscle; MC, Meckel's cartilage; MN, mandibular nerve; Mn, masseter nerve; MP, medial pterygoid muscle; MXA, maxillary artery; MXN, maxillary nerve; OG, otic ganglion; PT, cartilaginous pterygoid part of the sphenoid (independent of the other parts); PTT, pharyngotympanic tube; RC, Reichert's cartilage; SP, sphenoid bone anlagen; T, temporalis muscle; ZA, zygomatic arch.
Fig. 5.
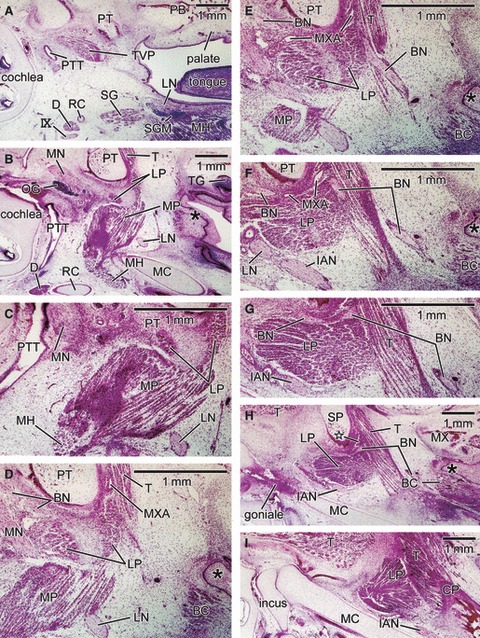
Sagittal sections of a 9-week-fetus (CRL 50 mm) showing a small upper head of the lateral pterygoid muscle in the upper side of the buccal nerve course. Panel A is the most medial side of the figure and panel I the most lateral side. The right-hand side of the figure corresponds to the anterior side of the head. Panels C and G are higher magnification views of panels B and H, respectively. In addition, panels D and E are also prepared at higher magnification to show the buccal nerve course (scale bars in each panel). Intervals between panels are 0.9 mm (A–B), 0.1 mm (B–D), 0.1 mm (D–E), 0.1 mm (E–F), 0.1 mm (F–H) or 0.3 mm (H–I), respectively. The medial margin of the lateral pterygoid muscle (LP) is identified as two small masses separated by a mesenchymal tissue (panels B and C). This mesenchymal tissue looks like an intramuscular tendon but disappears in the midportion of the muscle (panels D–F). As a whole, the muscle shows a fan-like shape (panel H and I). In panels G and H, the buccal nerve passed through a narrow space between the cartilaginous pterygoid part of the sphenoid and the lateral pterygoid muscle (LP). In the superior side of the nerve, a small fragment of the muscle is seen (star in panel H). Asterisk indicates a posterolateral end of the oral mucosa. BC, buccinators muscle; D, digastricus muscle posterior belly or the intermediate tendon; MH, mylohyoideus muscle; PB, palatine bone; SG, styloglossus muscle; SMG, submandibular gland; TG, teeth germ; TVP, tensor veli palatini muscle; IX, glossopharyngeal nerve. BN, buccal nerve; CP, coronoid process of the mandible; CTN, chorda tympani nerve; DTN, deep temporal nerves; IAN, inferior alveolar nerve; ICA, internal carotid artery; LN, lingual nerve; LP, lateral pterygoid muscle; MC, Meckel's cartilage; MN, mandibular nerve; Mn, masseter nerve; MP, medial pterygoid muscle; MXA, maxillary artery; MXN, maxillary nerve; OG, otic ganglion; PT, cartilaginous pterygoid part of the sphenoid (independent of the other parts); PTT, pharyngotympanic tube; RC, Reichert's cartilage; SP, sphenoid bone anlagen; T, temporalis muscle; ZA, zygomatic arch.
Fig. 6.
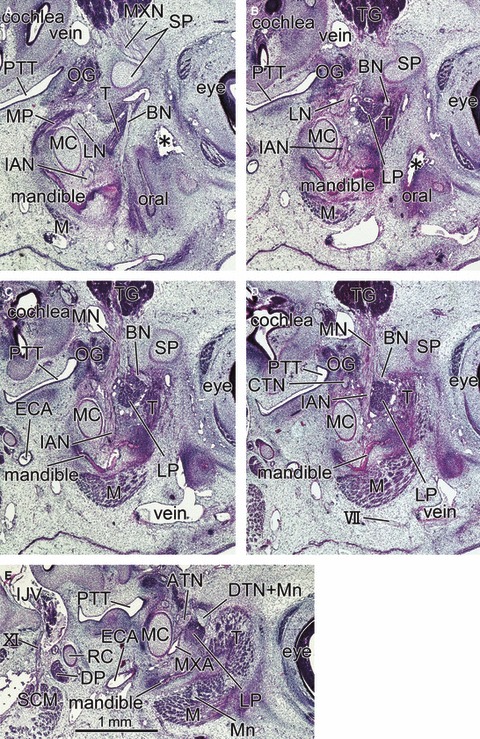
Sagittal sections of a 6-week-embryo (CRL 20.5 mm) showing the primitive lateral pterygoid muscle at a wedged position between the buccal nerve and inferior alveolar nerve. Panel A is the most medial side of the figure and panel E the most lateral side. The right-hand side of the figure corresponds to the anterior side of the head. All panels are prepared at the same magnification (scale bar in panel E). Intervals between panels are 0.3 mm (A–B), 0.15 mm (B–C), 0.2 mm (C–D) or 0.35 mm (D–E), respectively. In panel A, the buccal nerve (BN) runs inferiorly along the medial aspect of the temporalis muscle (T) toward the oral cavity (oral). In panels B–D, the nerve runs laterally along the superior margin of the lateral pterygoid muscle (LP). The mandible is in the early stage of development along the Meckel's cartilage (MC). The medial pterygoid muscle (MP) is located in the inferior side rather than medial side of the lateral muscle. Asterisk in panel B indicates an artefactual damage during the histological procedure. DP, digastricus muscle posterior belly; ECA, external carotid artery; IJV, internal jugular vein; M, masseter muscle; SCM, sternocleidomastoideus muscle; TG, trigeminal ganglion; VII, facial nerve; XI, accessory nerve. BN, buccal nerve; CP, coronoid process of the mandible; CTN, chorda tympani nerve; DTN, deep temporal nerves; IAN, inferior alveolar nerve; ICA, internal carotid artery; LN, lingual nerve; LP, lateral pterygoid muscle; MC, Meckel's cartilage; MN, mandibular nerve; Mn, masseter nerve; MP, medial pterygoid muscle; MXA, maxillary artery; MXN, maxillary nerve; OG, otic ganglion; PT, cartilaginous pterygoid part of the sphenoid (independent of the other parts); PTT, pharyngotympanic tube; RC, Reichert's cartilage; SP, sphenoid bone anlagen; T, temporalis muscle; ZA, zygomatic arch.
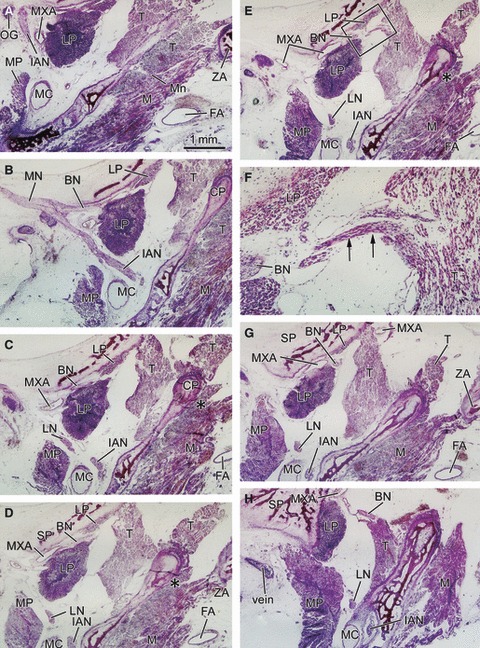
Fig. 4.
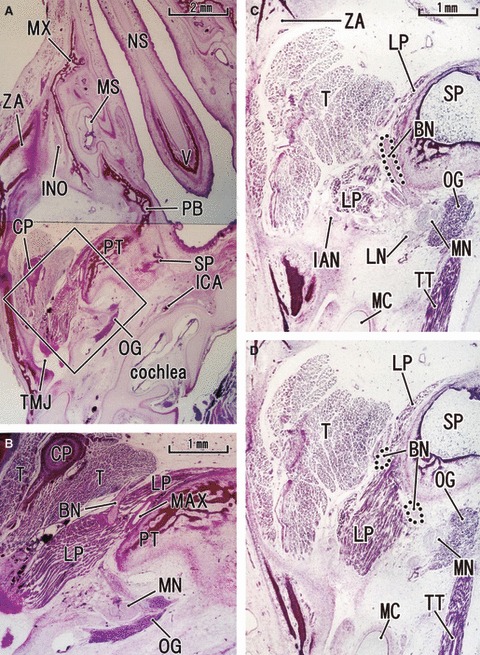
Anterior slip of the lateral pterygoid muscle seen in horizontal sections at 14–16 weeks of gestation. The upper side of the figure corresponds to the anterior side of the head and the left-hand side to the lateral side of the head. (A,B) A 16-week-fetus (CRL 135 mm). (C,D) A 14-week-fetus (CRL 105 mm). Panel B is a higher magnification view of a square in panel A. Panel C is 0.1 mm superior side of panel D. In panel B, the buccal nerve (BN) penetrates the lateral pterygoid muscle (LP) and passes through it. In panel C, the BN runs across the superior aspect of the muscle and along the sphenoid. In the anterior side of the nerve course, a slip of the muscle is seen extending along and around the sphenoid or pterygoid (panels C and D). However, there appears to be no difference in muscle fiber direction between the anterior and posterior parts of the muscle. In panel B, apart from the BN, the maxillary artery (MXA) runs along a narrow space between the pterygoid and lateral pterygoid muscle. Panels B–D are prepared at the same magnification (scale bar in panels A, B and C). INO, infraorbital nerve; MS, maxillary sinus; MX, maxilla; NS, nasal septum; PB, palatine bone; TMJ, temporomandibular joint and its disk; TT, tensor tympani muscle; V, vomer; BN, buccal nerve; CP, coronoid process of the mandible; CTN, chorda tympani nerve; DTN, deep temporal nerves; IAN, inferior alveolar nerve; ICA, internal carotid artery; LN, lingual nerve; LP, lateral pterygoid muscle; MC, Meckel's cartilage; MN, mandibular nerve; Mn, masseter nerve; MP, medial pterygoid muscle; MXA, maxillary artery; MXN, maxillary nerve; OG, otic ganglion; PT, cartilaginous pterygoid part of the sphenoid (independent of the other parts); PTT, pharyngotympanic tube; RC, Reichert's cartilage; SP, sphenoid bone anlagen; T, temporalis muscle; ZA, zygomatic arch.
Fig. 7.
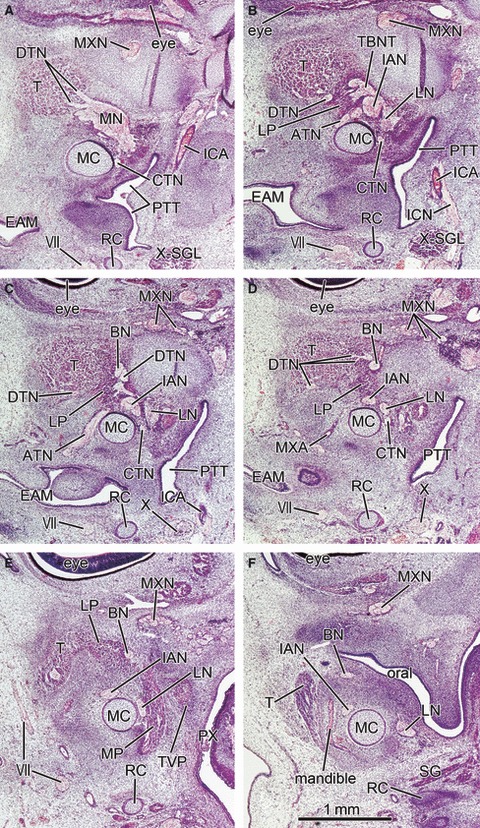
Lateral pterygoid muscle restricted at a wedged position between the buccal and inferior alveolar nerves in horizontal sections at 6 weeks (CRL 22 mm). Panel A is the most superior side of the figure and panel F the most inferior side. The upper side of the figure corresponds to the anterior side of the head and the left-hand side to the lateral side of the head. All panels are prepared at the same magnification (scale bar in panel F). Intervals between panels are 0.2 mm (A–B, B–C, C–D) and 0.3 mm (D–E, E–F), respectively. The deep temporal nerves (DTN; panel A) are thick and located in the superior side of the lateral pterygoid muscle anlage (LP) at a wedged position between the buccal and inferior alveolar nerves (BN, IAN; panels B–E). In panel B, the buccal nerve and the anterior deep temporal nerve form the temporobuccal nerve trunk (TBNT). The mandible is in the initial stage of development (panel F). Oral, oral cavity; ATN, auriculotemporal nerve; CTN, chorda tympani nerve; EAM, external auditory meatus; ICN, internal carotid nerve; PX, pharynx; SG, styloglossus muscle; SGL, spiral ganglion; TVP, tensor veli palatini muscle; VII, facial nerve; X, vagus nerve; X-SGL, superior ganglion of the vagus nerve. BN, buccal nerve; CP, coronoid process of the mandible; CTN, chorda tympani nerve; DTN, deep temporal nerves; IAN, inferior alveolar nerve; ICA, internal carotid artery; LN, lingual nerve; LP, lateral pterygoid muscle; MC, Meckel's cartilage; MN, mandibular nerve; Mn, masseter nerve; MP, medial pterygoid muscle; MXA, maxillary artery; MXN, maxillary nerve; OG, otic ganglion; PT, cartilaginous pterygoid part of the sphenoid (independent of the other parts); PTT, pharyngotympanic tube; RC, Reichert's cartilage; SP, sphenoid bone anlagen; T, temporalis muscle; ZA, zygomatic arch.
Fig. 8.
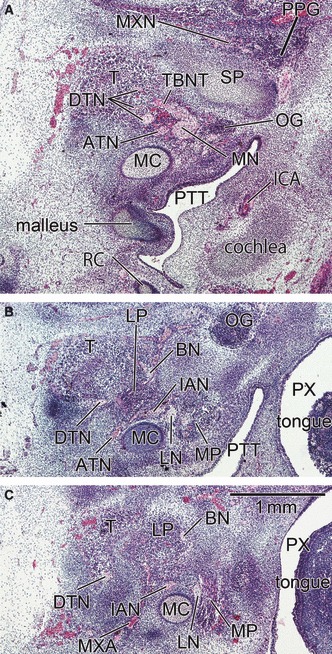
A mesenchymal condensing of the buccal and inferior alveolar nerves suggests the anlage of the lateral pterygoid muscle in horizontal sections at 6 weeks (CRL 21 mm). Panel A is the most superior side of the figure and panel C the most inferior side. The upper side of the figure corresponds to the anterior side of the head and the left-hand side to the lateral side of the head. All panels were prepared at the same magnification (scale bar in panel C). Intervals between panels are 0.3 mm (A–B) and 0.2 mm (B–C), respectively. The morphology shown in this figure appears the earliest stage in all figures. The deep temporal nerves (DTN; panel A) are thick and located in the superior side of the lateral pterygoid muscle anlage (LP). Parts of the DTN make a common trunk (TBNT) with the buccal nerve (BN). In panel B, the BN and the inferior alveolar nerve (IAN) sandwich the lateral pterygoid muscle anlage. ATN, auriculotemporal nerve; PPG, pterygopalatine ganglion; PX, pharynx. BN, buccal nerve; CP, coronoid process of the mandible; CTN, chorda tympani nerve; DTN, deep temporal nerves; IAN, inferior alveolar nerve; ICA, internal carotid artery; LN, lingual nerve; LP, lateral pterygoid muscle; MC, Meckel's cartilage; MN, mandibular nerve; Mn, masseter nerve; MP, medial pterygoid muscle; MXA, maxillary artery; MXN, maxillary nerve; OG, otic ganglion; PT, cartilaginous pterygoid part of the sphenoid (independent of the other parts); PTT, pharyngotympanic tube; RC, Reichert's cartilage; SP, sphenoid bone anlagen; T, temporalis muscle; ZA, zygomatic arch.
In sagittal sections of specimens later than 14 weeks, the upper head of the LPM was clearly discriminated from the lower head at the mediolateral level including the buccal nerve course (Figs 2 and 3). However, lateral to the nerve course, the two heads were attached and mutually continuous without any clear demarcation by loose connective tissue or veins. The upper head, consistently smaller than the lower head, was always located anterior to the latter. The difference in size of the LPM was more evident in specimens at 14–16 weeks than in those at 20–25 weeks. Thus, in horizontal sections at 14–16 weeks (Fig. 4), the upper head was better described as an ‘anterior slip’ extending from the lower head or the major muscle mass. The anterior slip, extending along and around the sphenoid and pterygoid, was located anterior to the postero-anterior course of the proximal part of the buccal nerve.
The buccal nerve issued from a branch to the temporalis muscle (i.e. the anterior deep temporal nerve; Figs 2F and 3EF) and, at and distal to the site of branching, the nerve changed direction from a postero-anterior straight course along the sphenoid to the antero-inferiorly curved course. The other, deep temporal nerves ran horizontally along the sphenoid on the lateral side of the buccal nerve course; when the LPM on the lateral side diminished in size, the nerves divided into thin twigs, including medially recurrent ones, and reached the temporalis muscle (Fig. 2CGH). In horizontal sections at 14–16 weeks (Fig. 4), the buccal nerve ran anteriorly along the lateral or posterior side of the anterior muscle lip (i.e. the upper head; see above). Figure 4C demonstrates the buccal nerve running along the superior aspect of the LPM, whereas the nerve penetrates the muscle belly at a slightly inferior site, as shown in Fig. 4D. The buccal nerve passed through the inferomedial part of the temporalis muscle in two of the 10 large specimens (Fig. 2). In seven of the other specimens, the buccal nerve made an impression on the temporalis muscle surface (Fig. 3H).
In contrast to the constant course of the buccal nerve, the maxillary artery penetrated, or did not penetrate, the LPM. Thus, the artery did not always accompany the buccal nerve, although the present observations of the arterial course in small specimens were insufficient. In 12 sides of 10 specimens larger than 100 mm CRL, we found three types of variations: (i) the artery penetrated the LPM together with the buccal nerve at four sites (Fig. 3DE); (ii) the artery penetrated the LPM but took a course different from the nerve, such as along the sphenoid, at three sites (Fig. 4B); (iii) the artery did not penetrate the LPM but passed below it to reach a space between the LPM and the temporalis muscle at five sites (Fig. 2C). A left/right difference in the arterial course was not evident in the limited number of specimens used for horizontal sections.
At 8 weeks, it became difficult to find a section including both the LPM and medial pterygoid muscle: major parts of the latter were located on the medial and inferior sides of the LPM and temporalis muscle (Fig. 5). The LPM was composed of a single, fan-like bundle of muscle fibers. The buccal nerve ran along or passed through the roof of the LPM. The upper head was more difficult to identify than in the larger specimens, corresponding to a small anteromedial part or slip of the fan-like muscle mass. As in the larger specimens, a point at which the buccal nerve course changed was seen in the anterior margin of the LPM (or a site of attachment between the LPM and the temporalis muscle; Fig. 5FG). In contrast to larger specimens, a topographical relationship between the buccal nerve and the temporalis muscle became clearly identifiable, due possibly to the small size of the inferiomedial part of the muscle: the nerve ran antero-inferiorly along the medial aspect of the temporalis muscle (Fig. 5E).
At 6–7 weeks, the morphology did not depend on embryo size but varied between specimens: for example, in accordance with muscle differentiation, a specimen with a CRL of 20.5 mm (Fig. 6) appeared to be at a much later stage than a 21-mm embryo (Fig. 8). Because development of the temporalis muscle was restricted around the prospective coronoid process of the mandible (Fig. 6), the buccal nerve course was clearly identifiable along the medial aspect of the muscle (Fig. 6A): this was the case for specimens at 8 weeks (see above). The primitive LPM was much smaller than the temporalis muscle, and located adjacent to the medio-inferior aspect of the latter (Figs 6 and 7). The sphenoid bone anlage was restricted to the area around the future round foramen: thus, the LPM appeared to be ‘floating’ without any attachment to hard tissues. The LPM anlage was located in a wedged position between the temporobuccal nerve trunk (a common trunk of the primitive buccal and temporalis nerves) and the inferior alveolar nerve (Fig. 6CD). The supplying nerves originated from the wedged positions between these two nerve origins (Figs 6C and 7C). This position corresponded to a site immediately inferior to the relatively thick origins of the mandibular nerve branches (Figs 6CD, 7B–D and 8B). Consequently, we were unable to identify the upper head of the LPM in the earliest group of specimens.
Discussion
According to the present observations, the temporalis muscle was well differentiated from the anlage at a stage earlier than, and more superiorly to, the LPM. The buccal nerve consistently contains, or forms, a common trunk with the anterior deep temporal nerve for the temporalis muscle innervation (Akita et al. 2000). The deep temporal nerves were as thick as the lingual nerve at 6–7 weeks. Therefore, in the initial stage of development, the anterior deep temporal nerve appeared to determine the postero-anterior, straight course of the buccal nerve. The other, deep temporal nerves also took a similar postero-anterior course along the sphenoid, possibly due to the high position of the target muscle. The buccal nerve seemed capable of turning in a direction inferior to the oral mucosa after loss of route guidance, and often made an impression on the temporalis muscle surface. This morphology suggested mechanical stress from the temporalis muscle on the buccal nerve because of muscle expansion. At a stage later than the initial determination of the buccal nerve course, the nerve seemed to be fixed to the sphenoid by the LPM itself, rather than by the anterior temporal nerve. Although a nerve may be too weak to make an impression to the muscle surface in adults, such morphology is not uncommon in fetal extremities, where a muscle is likely to form an aponeurosis facing the sciatic and other thick nerves (e.g. Nakamura et al. 2011), possibly because fetal nerves are thick and tough.
The present study demonstrated that the so-called upper head of the LPS was most likely to develop and grow at stages later than the major part of the muscle, or the so-called lower head. A part of the LPM anlage was likely to ‘leak’ out of slits between the origins of the mandibular nerve branches at 7–8 weeks. The delayed development and growth appeared to change the topographical relationship between the upper head and the buccal nerve. This hypothetical process is demonstrated schematically in Fig. 9. However, other than sites along the nerve course, the upper and lower heads of the LPM were almost continuous. The maxillary artery was also likely to separate these two heads when it penetrated the muscle (for details, see the second from last paragraph). The delayed but rapid growth from the small anterior muscle slip to the definite upper head might depend on development of the sphenoid bone greater wing in the mid-term fetus, although we did not measure the rate between stages. In fact, the anterior slip of the LPM, i.e. the suggested initial morphology of the upper head (Fig. 9BC), extended along and became attached to the greater wing of the developing sphenoid bone. Between parts of a single muscle, we know of few examples of a site-dependent difference in development and growth. Our limited knowledge extends to two muscles: (i) the courses of the diaphragm appear to develop later than the major, dome-like part (Hayashi et al. 2011); (ii) the posterior part of the levator ani muscle develops later than the anterior part (Niikura et al. 2010). However, these examples do not include a change in appearance of the topographical relationship between a muscle and a thick nerve penetrating it. If the delayed development and growth of part of a muscle is considered to be one of the strategies used in muscle morphogenesis, it is in clear contrast to another strategy, i.e. ‘build and scrap’, such as muscle cell death for elimination of certain fiber types (reviewed by McClearn et al. 1995).
Fig. 9.
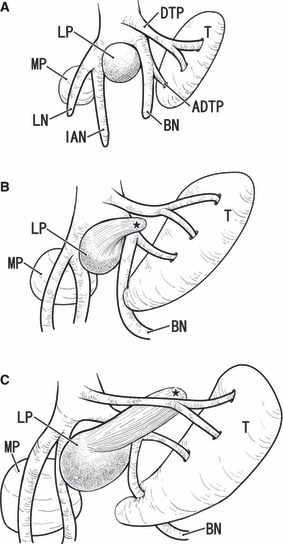
Schematic representation of a developmental change in the topographical relation between the lateral pterygoid muscle and buccal nerve. Viewed from the lateral side. The right-hand side of the figure corresponds to the anterior side of the head. In the early stage or at 6–7 weeks of gestation (panel A), the lateral pterygoid muscle anlage (LP) is restricted at a wedged position between the buccal nerve (BN) and the inferior alveolar nerve (IAN). The anlage of the temporalis muscle (T) is located superior to the lateral pterygoid muscle. In the intermediate stage (8–9 weeks; panel B), the buccal nerve changes direction at a branching site of the anterior deep temporal nerve (ADTP). The perspective upper head or the fetal anterior muscle slip of the lateral pterygoid muscle (black star) extends anterosuperiorly between the buccal nerve and the other deep temporal nerves (DTN). In the late stage (approximately 14–20 weeks; panel C), the upper head grows significantly and pushes the deep temporal nerve to the sphenoid bone. The buccal nerve may make an impression when the expanding temporalis muscle attacks the nerve (arrow in panel C). The actual difference in size of the muscles between panels is much more evident than it appears in the figures. LN, lingual nerve; MP, medial pterygoid muscle.
The LPM is supplied by multiple thin twigs from the mandibular nerve root (Akita et al. 2000), whereas the buccal nerve passes through the muscle to provide sensory innervation of the oral mucosa. The buccal nerve accompanies the anterior deep temporal nerve. Such an overall anatomical relationship between muscle and nerves seems to be consistent with the anatomy of the coracobrachialis muscle and musculocutaneous nerve: the muscle is innervated by multiple twigs from the brachial plexus (Woo et al. 2010), whereas the nerve passes through the muscle, supplies the arm flexor muscles, and finally takes a long course toward the hand for skin sensory innervation. Moreover, being similar to the buccal nerve, the course of the musculocutaneous nerve varies considerably between individuals, and sometimes does not penetrate the coracobrachialis muscle (Guerri-Guttenberg & Ingolotti, 2009; Remerand et al. 2010). The brachialis muscle, one of the flexors supplied by the musculocutaneous nerve, is usually also innervated by the radial nerve (Oh et al. 2009): this morphology may also be similar to that of the temporalis muscle, which is supplied by the buccal nerve and others. According to Koizumi and colleague (Koizumi, 1989; Koizumi & Sakai, 1995), in humans and three ape species the site in the coracobrachialis where the musculocutaneous nerve penetrates depends on the ratio of the segmental spinal nerve components (C5–7) supplying the coracobrachialis. However, Kawashima et al. (2007) later ruled out this cause–effect relationship in orangutans. The musculocutaneous nerve penetrates the coracobrachialis muscle in fetuses (not embryos), although the nerve component was not considered (Kwolczak-McGrath et al. 2008; Uysal et al. 2009; Kervancioglu et al. 2011). In addition, the characteristic topographical relationship between a muscle and the muscle-penetrating nerve is also known for the piriformis muscle and the peroneal nerve component of the sciatic nerve, for which segmental variations have been described in detail by Chiba and colleagues (Chiba, 1992; Chiba et al. 1994). The details of the head patterns of the LPM thus also may depend on the nerve morphology.
Finally, it is necessary to discuss variations in the heads of the adult LPM: 1-head, 2-head and 3-head patterns have been reported (Troiano, 1967; Naohara, 1989; Abe, 1992; Birou et al. 1992). However, the buccal nerve consistently penetrated the LPM, at least in the larger specimens we examined here. Although the vascular anatomy was somewhat beyond the scope of this study, we observed several variations in the topographical relationship between the fetal LPM and the maxillary artery, the latter penetrating or not penetrating the LPM. In addition to this artery, we observed a thick drainage vein of the sphenoid running through the LPM in association with the buccal nerve in one late-stage fetus (figures not shown). The LPM seems to carry two clearly divided heads when the artery and vein follow the same course as the buccal nerve (e.g. Fig. 3). With regard to how many heads the LPM carries, there may be a spectrum of variations depending on a combination of vascular and nerve variations. Moreover, in a case in which all three courses of the artery, vein and nerve are different because of the suspected unclear separation of muscle bundles by these structures, one researcher may identify the LPM as having a multiple-head pattern, whereas another researcher may identify a 1-head pattern. We consider that a fetal study of head(s) formation should be followed by a future detailed macroscopic study of the adult LPM and its associated vessels and nerves.
The major limitation of this study was that sagittal sections were not available for early-stage fetuses or embryos. A small upper head is difficult to identify in horizontal sections. Likewise, the margin between the developing LPM and the sphenoid was difficult to identify by HE staining, especially in the early stage. Desmin immunohistochemistry, if available, would clearly demonstrate the early stage of muscle insertion, as our group has proved recently (Abe et al. 2010).
Acknowledgments
This research was supported by Oral Health Science Center Grant hrc8 from Tokyo Dental College, and by a Project for Private Universities matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology), Japan, 2010–2012.
References
- Abe S. Investigations of the run and the attachment of the lateral pterygoid muscle in Japanese. Shikwa Gakuho. 1992;92:1349–1365. [Google Scholar]
- Abe SH, Rhee SK, Osonoi M, et al. Expression of intermediate filaments at muscle insertions of human fetuses. J Anat. 2010;217:167–173. doi: 10.1111/j.1469-7580.2010.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita K, Shimokawa T, Sato T. Positional relationships between the masticatory muscles and their innervating nerves with special reference to the lateral pterygoid and the midmedial and discotemporal muscle bundles of temporalis. J Anat. 2000;197:291–302. doi: 10.1046/j.1469-7580.2000.19720291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz MA, Cowie RJ, Skinner CE, et al. Are the two heads of the human lateral pterygoid separate muscles? A perspective based on their nerve supply. J Orofac Pain. 1998;12:226–239. [PubMed] [Google Scholar]
- Bertilsson O, Ström D. A literature survey of a hundred years of anatomic and functional lateral pterygoid muscle research. J Orofac Pain. 1995;9:17–23. [PubMed] [Google Scholar]
- Birou G, Garcier JM, Guillot M, et al. Corrélations de l’imagerie TDM et IRM du muscle ptérygoidïen latéral. Ann Radiol. 1992;35:198–203. [PubMed] [Google Scholar]
- Carranza ML, Carda C, Simbrón A, et al. Morphology of the lateral pterygoid muscle associated to the mandibular condyle in the human prenatal stage. Acta Odontol Latinoam. 2006;19:29–36. [PubMed] [Google Scholar]
- Chiba S. Multiple positional relationships of nerves arising from the sacral plexus to the piriformis muscle in humans. Kaibougaku Zasshi. 1992;67:691–724. [PubMed] [Google Scholar]
- Chiba S, Ishibashi Y, Kasai T. Perforation of dorsal branches of the sacral nerve plexus through the piriformis muscle and its relation to changes of segmental arrangements of the vertebral column and others. Kaibougaku Zasshi. 1994;69:281–305. [PubMed] [Google Scholar]
- Foucart JM, Girin JP, Carpentier P. Innervation of the human lateral pterygoid muscle. Surg Radiol Anat. 1998;20:185–189. doi: 10.1007/BF01628893. [DOI] [PubMed] [Google Scholar]
- Guerri-Guttenberg RA, Ingolotti M. Classifying musculocutaneous nerve variations. Clin Anat. 2009;22:671–683. doi: 10.1002/ca.20828. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Rodríguez-Vázquez JF, Cho BH, et al. Pleuroperitoneal canal closure and fetal adrenal gland. Anat Rec. 2011;294:633–644. doi: 10.1002/ar.21351. [DOI] [PubMed] [Google Scholar]
- Katori Y, Rodriguez-Vazquez JF, Kawase T, et al. Early fetal development of hard tissue pulleys for the human obliquus superior and tensor veli palatini muscles. Ann Anat. 2011a;193:127–133. doi: 10.1016/j.aanat.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Katori Y, Kim JH, Rodriguez-Vazquez JF, et al. Early fetal development of the intermediate tendon of the digastricus and omohyoideus muscles: a critical difference in histogenesis. Clin Anat. 2011b;24:843–852. doi: 10.1002/ca.21182. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Yoshitomi S, Sasaki H. Nerve fiber tracing of branches to the coracobrachialis muscle in a Bornean orangutan (Pongo pygmaeus pygmaeus) Anat Histol Embryol. 2007;36:19–23. doi: 10.1111/j.1439-0264.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- Kervancioglu P, Orhan M, Kikinc N. Patterns of motor branching of the musculocutaneous nerve in human fetuses and clinical signification. Clin Anat. 2011;24:168–178. doi: 10.1002/ca.21095. [DOI] [PubMed] [Google Scholar]
- Kineberg I. The lateral pterygoid muscle: some anatomical, physiological and clinical considerations. Ann R Australas Coll Dent Surg. 1991;11:96–108. [PubMed] [Google Scholar]
- Koizumi M. A morphological study on the coracobrachialis muscle. Kaibougaku Zasshi. 1989;64:18–35. [PubMed] [Google Scholar]
- Koizumi M, Sakai T. The nerve supply to coracobrachialis in apes. J Anat. 1995;186:395–403. [PMC free article] [PubMed] [Google Scholar]
- Kwolczak-McGrath A, Kolesnik A, Ciszek B. Anatomy of branches of the musculocutaneous nerve to the biceps and brachialis in human fetuses. Clin Anat. 2008;21:142–146. doi: 10.1002/ca.20583. [DOI] [PubMed] [Google Scholar]
- McClearn D, Medville R, Noden D. Muscle cell death during the development of head and neck muscles in the chick embryo. Dev Biol. 1995;202:365–377. doi: 10.1002/aja.1002020406. [DOI] [PubMed] [Google Scholar]
- Mérida-Velasco JR, Rodríguez-Vázquez JF, De La Cuadra C, et al. The course of the buccal nerve: relationships with the temporalis muscle during the prenatal period. J Anat. 2001;198:423–429. doi: 10.1046/j.1469-7580.2001.19840423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Suzuki D, Murakami G, et al. Human fetal anatomy of the posterior semimembranosus complex at the knee with special reference to the gastrocnemio-semimembranosus bursa. Knee. 2011;18:271–277. doi: 10.1016/j.knee.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Naohara H. The macroscopic and microscopic study of the human lateral pterygoid muscle. Tsurumi Shigaku. 1989;15:1–26. [PubMed] [Google Scholar]
- Niikura H, Jin ZW, Cho BH, et al. Human fetal anatomy of the coccygeal attachments of the levator ani muscle. Clin Anat. 2010;23:566–574. doi: 10.1002/ca.20983. [DOI] [PubMed] [Google Scholar]
- Öğőtcen-Toller M, Juniper RP. The embryologic development of the human lateral pterygoid muscle and its relationships with the temporomandibular joint disc and Meckel's cartilage. J Oral Maxillofac Surg. 1993;51:772–778. doi: 10.1016/s0278-2391(10)80420-3. [DOI] [PubMed] [Google Scholar]
- Oh CS, Won HS, Lee KS, et al. Origin of the radial nerve branch innervating the brachialis muscle. Clin Anat. 2009;22:495–499. doi: 10.1002/ca.20788. [DOI] [PubMed] [Google Scholar]
- Osanai H, Abe S, Rodríguez-Vázquez JF, et al. Human orbital muscle: a new point of view from the fetal development of extraocular connective tissue. Invest Ophthalmol Vis Sci. 2011;52:1501–1506. doi: 10.1167/iovs.10-6013. [DOI] [PubMed] [Google Scholar]
- Remerand F, Laulan J, Couvret C, et al. Is the musculocutaneous nerve really in the coracobrachialis muscle when performing an axillary block? An ultrasound study. Anesth Analg. 2010;110:1729–1734. doi: 10.1213/ANE.0b013e3181dc25c8. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF, Mérida-Velasco JR, Jiménez-Collado J. Development of the human sphenomandibular ligament. Anat Rec. 1992;233:453–460. doi: 10.1002/ar.1092330312. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF, Mérida-Velasco JR, Verdugo-López S, et al. Morphogenesis of the second pharyngeal arch cartilage (Reichert's cartilage) in human embryos. J Anat. 2006;208:179–189. doi: 10.1111/j.1469-7580.2006.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Vázquez JF, Murakami G, Verdugo-López S, et al. Closure of the middle ear with special references to development of the tegmen tympani of the temporal bone. J Anat. 2011;218:690–698. doi: 10.1111/j.1469-7580.2011.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher GH, Lau H, Freund E, et al. Zur Topographie der muskulären Nerven Ausbreitungen. 9. Kaumuskeln. M. pterygoideus medialis und lateralis verschiedener Kautypen vertreter. Anat Anz. 1976;139:71–87. [PubMed] [Google Scholar]
- Troiano MF. New concept of the insertion of the lateral pterygoid muscle. J Oral Surg. 1967;25:337–340. [PubMed] [Google Scholar]
- Uysal II, Karabulut AK, Bőyőkmumcu M, et al. The course and variations of the branches of the musculocutaneous nerve in human fetuses. Clin Anat. 2009;22:337–345. doi: 10.1002/ca.20734. [DOI] [PubMed] [Google Scholar]
- White LW. The lateral pterygoid muscle: fact and fiction. J Clin Orthod. 1985;19:584–587. [PubMed] [Google Scholar]
- Woo JS, Shin C, Hur MS, et al. Spinal origins of the nerve branches innervating the coracobrachialis muscle: clinical implications. Surg Radiol Anat. 2010;32:659–662. doi: 10.1007/s00276-010-0665-x. [DOI] [PubMed] [Google Scholar]


