Abstract
We evaluated variable patterns of pudendal nerve (PN) stimuli for reflex bladder excitation. Reflex activation of the bladder has been demonstrated previously with 20–33 Hz continuous stimulation of PN afferents. Neuronal circuits accessed by afferent mediated pathways may respond better to physiological patterned stimuli than continuous stimulation. Unilateral PN nerve cuffs were placed in neurologically intact male cats. PN stimulation (0.5–100 Hz) was performed under isovolumetric conditions at bladder volumes up to the occurrence of distension evoked reflex contractions. Stimulus evoked reflex bladder contractions were elicited in eight cats. Across all experiments, bursting of 2–10 pulses at 100–200 Hz repeated at continuous stimulation frequencies evoked significantly larger bladder responses than continuous (single pulse) stimulation (52.0 ± 44.5%). Bladder excitation was also effective at 1 Hz continuous stimuli, which is lower than typically reported. Variable patterned pulse bursting resulted in greater evoked reflex bladder pressures and increased the potential stimulation parameter space for effective bladder excitation. Improved bladder excitation should increase the efficacy of neuroprostheses for bladder control.
Keywords: Functional electrical stimulation (FES), neuroprosthesis, pudendal nerve, stimulation pattern, urinary system
I. Introduction
FOLLOWING spinal cord injury or other neurological disorders, loss of lower urinary tract control may occur. The resultant impact upon quality of life includes health problems and severe medical costs [1]–[3]. Typical management strategies for bladder care include medication, catheterization, and surgery [3]–[6]. These methods, when successful, can often have undesirable side effects such as bladder and urinary tract infections [3], [5], [6]. In individuals with spinal cord injury, a Brindley sacral root stimulator may be implanted to provide bladder control [4], [7], [8]. However, it is an invasive procedure which does not lead to physiologic voiding patterns [7] and usually requires a dorsal rhizotomy [4], [9] which results in loss of reflex erection and defecation [4], [5].
The pudendal nerve (PN) contains afferent fibers which initiate reflex bladder voiding and continence reflexes [10]–[21]. Nerve mapping has shown that afferent fibers in the pudendal nerve trace to the sacral roots used most often for sacral root stimulation [22], [23]. This reflex activation of the bladder travels through two systems: reflex circuitry contained within the sacral spinal cord and within the parasympathetic pelvic ganglion.
The use of peripheral nerve stimulation for bladder emptying offers advantages over sacral root stimulation. A surgical procedure to access the PN can be done in less time than for a sacral root electrode [13], with sufficient space for a multielectrode nerve cuff [24]. Access to the PN is also feasible with placement of a tined lead [13], [25] or needle electrode [17]. A rhizotomy is unnecessary, when using PN afferent stimulation to evoke bladder contractions [18], [26]. Furthermore, minimally invasive intraurethral stimulation to excite the bladder, activating urethral afferent fibers which feed into the pudendal nerve, has been clinically shown to be a convenient diagnostic tool for an implanted device [27], [28]. One current disadvantage of PN afferent stimulation, however, is that voiding is not as efficient as the use of sacral root stimulation following a rhizotomy [26]. Improving PN-evoked bladder contractions may reduce this deficiency.
Research has shown that applying continuous stimulation at 20-40 Hz to the PN can activate reflex voiding pathways in cats [12], [14]–[18], [26]. Animal research on PN stimulation has indicated success at eliciting contractions for bladder levels both below and above volumes which result in distension evoked contractions, although these studies focused on trials performed at bladder levels above distension evoked volumes [14]–[16], [26]. The basis for reflex pathways responding to 20-40 Hz stimulation is not fully understood, although it has been hypothesized to relate to peak firing rates during fluid flow through the urethra [14], indicating that improved stimulus patterns may be possible.
Many sensory neurons employ structured temporal codes to transfer information [29], [30]. Bursting of action potentials in neurons has been observed in a variety of settings, in mammalian spinal cord neurons [31], intestinal neurons [32], and electroreceptors [33]. Furthermore, bursting has been shown in nonmicturition related afferents of the PN [34]–[36] but has not yet been indicated in urethral afferents. The intraburst frequency, the burst duration and the interburst interval can all convey information about the input stimulus. There are indications that, in many situations, bursts of action potentials transfer signals more reliably because transmitter release is facilitated [37]. Enhanced responses to burst stimulus has been reported in the rat submandibular glands [38] and in colonic vasculature [39]. Both of these issues are pertinent in afferent stimulation evoked bladder response. Neuronal bursting may specifically mimic afferent signals to the sacral centers, which could increase outputs to the pelvic ganglion, enhancing transmitter release to the second order neurons.
The purpose of this study was to evaluate PN stimulus patterns for reflex bladder excitation that imitate neuronal bursting activity, at functional isovolumetric bladder volumes. Matching neuronal signaling processes may result in improved efficacy of PN evoked bladder contractions, which will enhance the performance of neuroprosthetics for restoration of bladder function.
II. Methods
A. Animal Model
This was a prospective study utilizing mature, neurologically and urologically intact, purpose-bred male cats. All animal studies were approved by the Case Western Reserve University Institutional Animal Care and Use Committee prior to initiation.
B. Experimental Preparation
Animals were initially sedated with an intramuscular injection of ketamine (35 mg/kg). Anesthesia was induced (65 mg/kg) and maintained (15 mg/kg) intravenously with a dose of α-chloralose. During surgical preparation, anesthesia was augmented with isoflurane (0.25%–2%). In five cats, buprenorphine (0.01 mg/kg) was administered every 12 h to ensure analgesia. Vitals were monitored (V9204 Advisor Monitor, Surgivet, Waukesha, WI) for the duration of the experiment. A port for fluid infusion was introduced into a cephalic vein and connected to an IV pump (Flo-Gard 6200, Baxter Healthcare, Deerfield, IL) with a glucose-saline drip. The bladder was exposed via a midventral line incision. A dual-lumen 6 Fr catheter (DLC-6D, Life-Tech, Stafford, TX) was placed through the dome of the bladder and held in place with a purse-string suture. Bladder pressure was recorded through one lumen of the bladder catheter, which was connected to a disposable pressure transducer (Deltran I, Utah Medical Products, Midvale, UT). Bladder infusion of room temperature saline through the other lumen of the bladder catheter was performed using a syringe pump (Genie, Kent Scientific, Torrington, CT).
The pudendal nerve was accessed via a postero-lateral gluteal approach on one side. A tripolar silicone nerve cuff (0.5 mm × 3 mm contact size; 1 mm intercontact distances) was placed on the pudendal nerve trunk proximal to the branching location of the distal rectal and deep perineal branches. Prior to initiation of experimental procedures, the cat was positioned prone on a foam platform, arranged such that the hips and upper torso were supported with the abdomen hanging freely.
C. Experimental Setup
Bladder pressure transducer outputs and TTL output signals from the stimulator (Pulsar 6bp-as, FHC, Bowdoinham, ME) were sampled at 100 Hz by a custom designed LabVIEW (version 7.1, National Instruments, Austin, TX) display, after being conditioned by a strain gauge and a feed-through module (NI SCC-SG24 & NI SCC-FT01, National Instruments), respectively, in a signal conditioning connecting block (NI SC-2345, National Instruments) connected to a DAQ-card (NI DAQ 6024E, National Instruments). Custom-designed electronics were used to extend the duration of each TTL pulse prior to the DAQ-card to eliminate under-sampling. The LabVIEW display provided real-time data viewing and stored data in text files on a laptop (Inspiron 8600, Dell, Round Rock, TX). Pressure and stimulus data were also written to strip chart paper with a chart recorder (TA11, Gould Instrument Services, Valley View, OH).
Starting at 50 μA, single, cathodic, current-controlled, 100-μs biphasic pulses were applied to the PN cuff to determine the threshold for fiber activation. The current level was incremented in 10–50 μA steps until visual activation of the external anal sphincter was observed. The threshold current for this motor activation of the pudendal nerve was defined in each experiment as the PNTH.
D. Stimulus Definitions
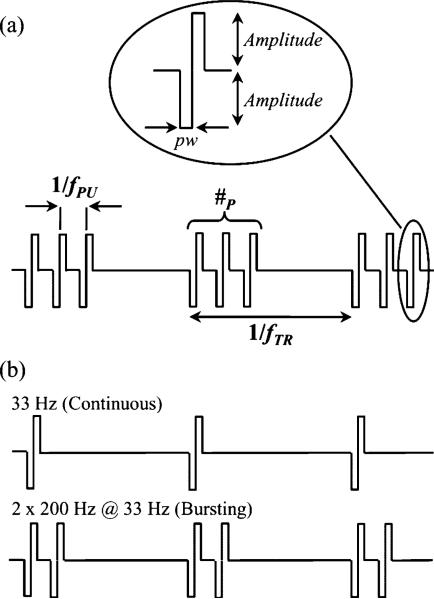
Stimuli for reflex bladder activation were given using cathode-leading, current-controlled balanced biphasic pulses with a fixed pulse width (pw) of 100 μs. Stimulus patterns (see Fig. 1) were arranged such that one or more pulses (#P) were repeated at a pulse frequency (fPU, in a manner similar to bursting. A train of pulses was repeated at a train rate frequency (fTR, which is analogous to the single pulse continuous frequency used by others. Stimulus durations (tSTIM) were approximately 20 s, varying between 15 and 60 s. Stimulus amplitudes were set at 2–3 times the PNTH. The stimulus pattern nomenclature used in these experiments is A#P x B fPU @ fTR. In this manner, 5 #P at 200 Hz fPU repeated every 1 s is represented as 5 × 200 Hz @ 1 Hz. Equivalent representations of a continuous 33 Hz train of pulses are 33 × 33 Hz @ 1 Hz and 1 × 33 Hz @ 33 Hz.
Fig. 1.
(a) Schematic representation of variable “burst” stimulus pattern. Nomenclature: pw = pulse width; #P = number of pulses; fPU = pulse frequency; fTR = train rate frequency. (b) Example traces for continuous (e.g., 33 Hz) and burst (e.g., 2 × 200 Hz @ 33 Hz) stimulation.
E. Experimental Protocol
Upon completion of the surgical prep, a cystometrogram was performed to examine the excitability of the bladder, filling at 0.5–2 mL/min. During filling, at 1–5 mL increments, the PN was stimulated to evaluate whether reflex evoked contractions could be elicited. Throughout this initial excitability evaluation, the stimulus pattern was alternated between a continuous 33 Hz train and a low fTR pulse bursting (e.g., 5 × 200 Hz @ 1 Hz). The bladder volume was recorded for the first reflex contraction elicited by PN stimulation (VSTIM)and for the first distension evoked contraction (VTH). Stimulus evoked contractions were defined as bladder pressure increases above 15 cm H2O from baseline and reaching a plateau by the end of the applied stimulus. Distension evoked contractions were defined in the absence of stimulation as bladder contractions evoking 15 cm H2O above baseline for at least 10 s.
At or above VSTIM, the bladder was maintained near isovolumetric either via a 3.5 or a 5 Fr catheter occluding the urethra or by replacing any volume voided after each trial. If the bladder passed beyond VTH into an over excitable state, due to added fluid from urine generation, the bladder was emptied to reset the system. The time duration of a bladder fill cycle in which the bladder was infused with saline and then eventually emptied was tracked. The urine generation rate for each fill cycle time duration was estimated based on the net volume removed beyond the infused amount. The bladder volume for a given stimulation trial was estimated using the net infused volume and urine generated assuming a constant urine rate per fill cycle.
Stimulation trials were performed with two objectives: evaluate continuous pulse trains in the frequency range from 0.5 to 100 Hz, focusing on 0.5, 1, 5, 10, 20, 33, and 50 Hz, and to evaluate bursting of pulses in the same continuous frequency range (fTR), using 1—25 #P repeated at 33–1000 Hz fPU, focusing on 1, 5, and 10 #P at 100 and 200 Hz fPU. In five experiments, balanced and randomized predetermined sets of parameter combinations were utilized, to increase the rigor of the study. In two experiments, #P was varied among 1, 5, and 10, fPU was varied between 100 and 200 Hz, and fTR was varied among 0.5, 1, and 3 Hz. In three experiments, #P was varied between 1 and 5 fPU for kept constant at 200 Hz while fTR was varied among 0.5, 1, 5, 10, 20, 33, and 50 Hz. Any repeat of these balanced sets, whether within an experiment or across experiments, was rerandomized.
F. Data Analysis
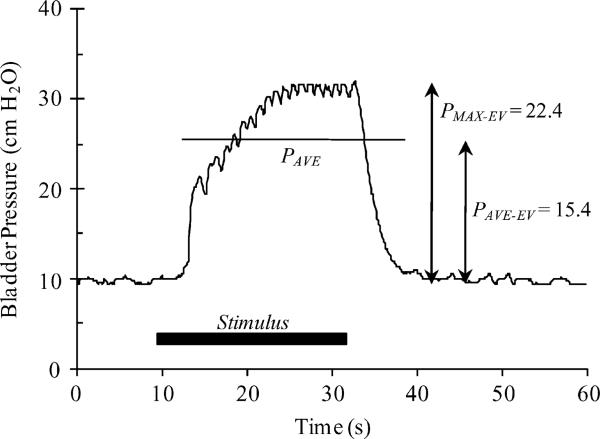
A sample evoked bladder contraction and measured parameters are shown in Fig. 2. During all trials in which the bladder was excitable, each applied stimuli and the corresponding bladder response were examined. The baseline pressure prior to initiation of the stimulus was defined by the 3 s average (PBASE–AVE) and standard deviation (PBASE–SD) of the bladder pressure. After the start of the stimulus, the evoked bladder pressures were analyzed while they exceeded an evaluation threshold (EVALTH), determined by PBASE–AVE + 3 × PBASE–SD. During the pressure evaluation period, the average (PAVE) and maximum (PMAX) pressures were recorded. Average (PAVE–EV) and maximum (PMAX–EV) evoked pressures were calculated as the difference between PAVE and PBASE–AVE and PMAX and PBASE–AVE, respectively.
Fig. 2.
Example of stimulus evoked bladder contraction, for a low-frequency bursting stimulus pattern (5 × 200 Hz @ 1 Hz). Measured parameters: PBASE–AVE = 3 s pressure average prior to stimulus initiation; PBASE–SD = 3 s pressure standard deviation prior to stimulus initiation; EVALTH = PBASE–AVE + 3 · PBASE–SD; PAVE = av-erage pressure while above EVALTH(duration noted by horizontal line in figure); PMAX = maximum pressure while above EVALTH; PAVE–EV PAVE – PBASE–AVE; PMAX–EV = PMAX – PBASE–AVE.
The data recorded during each experiment was analyzed using Excel (Office 2007, Microsoft, Redmond, WA) and JMP 6 (SAS, Cary, NC). Maximum and average evoked pressures were accumulated for each stimulus pattern within and across experiments. Each animal was categorized as a lower or a higher continuous frequency (fTR) responder using the responses for 1 and 33 Hz continuous stimuli. The linear trendline slope fit to the average PAVE–EV at 1 Hz and 33 Hz fTR for #P of 1 was used to differentiate between groups. A negative fTR – PAVE–EV slope for an experiment corresponded to lower stimulus frequencies exciting the bladder at greater levels than higher frequencies, resulting in that cat being categorized as a lower frequency responder. A positive fTR – PAVE–EV slope for an experiment corresponded to higher frequencies exciting the bladder at greater levels than lower frequencies, resulting in that cat being categorized as a higher frequency responder. Across experiments, blocks of trials from the two predetermined parameter set combinations were separately combined and analyzed with a multifactor main effects ANOVA after screening for interactions, with #P, fPU and fTR as factors as well as tSTIM and the bladder volume. Additionally, all trials performed under excitable conditions, and subgroups of lower frequency responders and higher frequency responders, were analyzed in the same manner with an ANOVA. Student's t-test was used for comparison testing of pooled groups of trials. The t-test for two dependent samples was used for comparison testing of subgroups within experiments pooled across experiments. For evaluations of statistical significance, α was set to 0.05.
III. Results
A. Animals
PN reflex excitability was evaluated in thirteen male cats (4.2 ± 0.7 kg, 3.6–5.6 kg; 1.7 ± 0.3 years old, 0.7–2.0 years old). The nerve cuff was placed on the PN such that both the deep perineal and caudal rectal branches were included. In two cats, the nerve cuff was placed closer to the spinal cord, such that the urethral sensory nerves were also included. Across all experiments, the average PNTH was 273 ± 117 μA (90–500 μA). In eight experiments (three cats on buprenorphine), bladder contractions were evoked by afferent PN stimulation. In the remaining experiments, afferent PN excitation modulated bladder responses but did not initiate contractions. Only the results from the eight fully excitable cats are presented here.
B. Excitable Bladder Volume
Stimulus evoked contractions were obtained below VTH in all cats. The average VSTIM was 28.1 ± 17.2 mL (22.6 mL median). Of the 47 PN stimulus responsive bladder fill cycles, 85.1% (n = 40) had VSTIM below VTH. In six fill cycles (12.8%), VSTIM was equal to VTH. In one (2.1%) cycle, VSTIM was above VTH (102.3%). Across experiments, for bladder fill cycles in which both PN stimulation evoked and distension evoked contractions occurred (23 cycles), the average VSTIM/VTH ratio was 81.0 ± 23.7% (median = 88.0%). If cycles without the occurrence of a distension evoked contraction are included in the analysis, by using the maximum infused volume for VTH, then the average VSTIM/VTH ratio across experiments was 84.2 ± 10.7% (median = 83.1%).
C. Responses to Continuous Stimulation
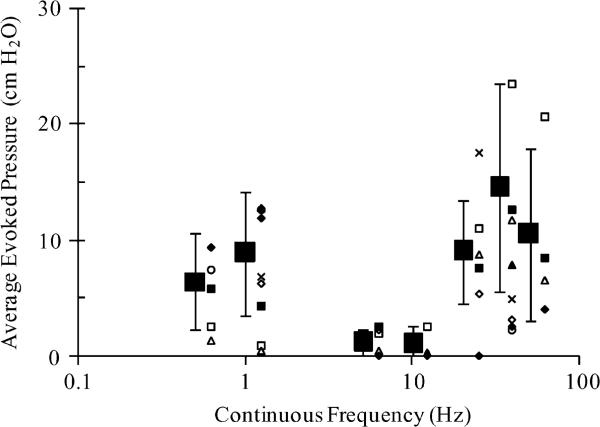
For continuous, single pulse stimulation, evoked bladder responses were averaged across all cats. Pooled PAVE–EV and PMAX–EV results across all trials performed at key frequencies are given in Table I. PAVE–EV results across experiments and experimental averages are shown in Fig. 3. Bladder excitation for 5 Hz stimuli was significantly lower (p < 0.05) than that at 0.5, 1, 20, and 50 Hz. In addition, comparing results at 5 Hz to results at 33 Hz and comparing 10 Hz results to all other frequencies except for 5 Hz indicated significant difference at α < 0.10. Limited trials at other continuous frequencies gave lower evoked pressures for frequencies between and outside the two peaks seen in Fig. 3 while matching excitability within the second peak (nonexcitable frequencies: 2, 3, 60, 75, and 100 Hz; excitable frequencies: 25 and 40 Hz). Accumulation of PMAX–EV results across stimulus trials indicated the same trends as for PAVE–EV. For simplicity, only PAVE–EV data are presented.
TABLE I.
Evoked Pressures for Single Pulse Continuous Frequencies
| fTR (Hz) | PAVE-EV (cm H2O) Average ± S.D. | PMAX-EV (cm H2O) Average ± S.D. | Trials (Cats) |
|---|---|---|---|
| 0.5 | 6.4 ± 2.2 | 11.9 ± 7.6 | 22 (5) |
| 1 | 8.9 ± 5.4 | 15.1 ± 8.5 | 30 (8) |
| 5 | 1.1 ± 1.1 | 1.9 ± 1.4 | 9 (5) |
| 10 | 1.0 ± 1.7 | 1.7 ± 2.1 | 9 (3) |
| 20 | 9.0 ± 4.5 | 17.0 ± 8.3 | 18 (6) |
| 33 | 14.5 ± 9.0 | 27.9 ± 17.3 | 109 (8) |
| 50 | 10.5 ± 7.4 | 17.9 ± 13.3 | 11 (4) |
Fig. 3.
Effective continuous stimulation frequencies for bladder activation. Average evoked pressures (PAVE–EV) at 0.5, 1, 5, 10, 20, 33, and 50 Hz train rate frequencies (fTR), for one pulse (#P). Large squares indicate results averaged across all trials performed, with pooled standard deviations as error bars. Smaller icons at each frequency represent averages from individual experiments.
Bladder contractions were evoked by stimulation at both lower (0.5–1 Hz) and higher (20–50 Hz) continuous frequencies. Five cats (including both cats with PN cuff placements that contained urethral sensory fibers) were categorized as lower frequency responders (average fTR – PAVE–EV slope = –0.31 ± 0.17) and three were categorized as higher frequency responders (average fTR – PAVE–EV slope = 0.87 ± 0.43). These two groups were significantly different from each other (p < 0.05). In the lower frequency responding cats, 1 Hz continuous stimuli evoked significantly greater PAVE–EV than 33 Hz continuous stimuli (11.0 ± 3.8 cm H2O, 4.2 ± 3.6 cm H2O, p < 0.001). In the higher frequency responding cats, 33 Hz continuous stimuli evoked significantly greater PAVE–EV than 1 Hz continuous stimuli (16.9 ± 8.1 cm H2O, 1.7 ± 3.2 cm H2O, p < 0.01).
D. Responses to Pulse Bursting
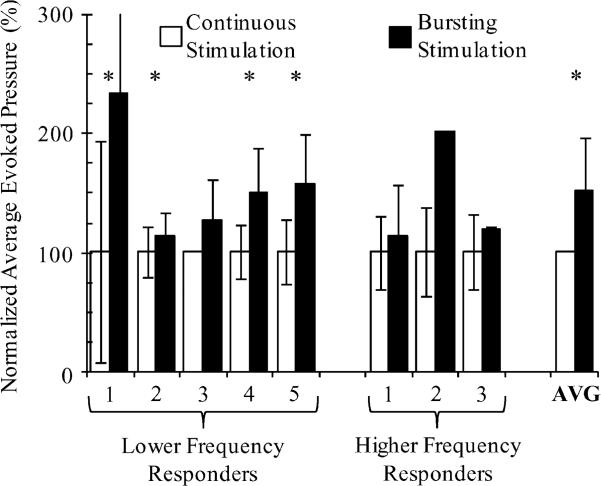
The use of variable patterned pulse bursting resulted in increased evoked pressures over single pulse continuous stimulation in all experiments (Fig. 4). The best burst patterned stimulus provided an average increase of 52.0 ± 44.5% (p < 0.004) over the dominant single pulse continuous stimulation frequency (1 Hz or 33 Hz). Lower frequency responders increased 56.4 ± 46.6% (p < 0.008) and higher frequency responders increased 44.6 ± 49.6% (p > 0.10, nonsignificant due to small n of three experiments) from single pulse stimuli to the most favorable bursting patterned stimuli. For two experiments, the absence of error bars in Fig. 4 indicates that only a single trial was run for a stimulus parameter combination.
Fig. 4.
Pulse bursting increased evoked pressure over continuous single pulse stimuli in every experiment. The multipulse stimulus parameter combination in each experiment that resulted in the largest evoked pressure was used for comparison. In each experiment, the average and standard deviation (error bars) of the average evoked pressure for single and multiple pulse stimulus patterns are normalized to the mean average evoked pressure for the single pulse stimulus pattern. Continuous stimulation frequencies were 1 Hz for lower frequency responders and 33 Hz for higher frequency responders. Bursting stimulation patterns were 5 × 100/200 Hz @ 1 Hz for lower frequency responders 1, 2, 3, and 5, and 10 × 100/200 @ 1 Hz for lower frequency responder 4. Bursting stimulation patterns were 2 × 200 Hz @ 20, 25, and 33 Hz for higher frequency responders 1, 2, and 3, respectively. Asterisks indicate experiments in which average pressures were significantly different (p < 0.05), using student's t-test. The evoked pressure increase average across experiments was also significantly different (p < 0:005), according to a t-test for two dependent samples. The standard deviation for bursting stimulation in lower frequency responder #1 was 123%.
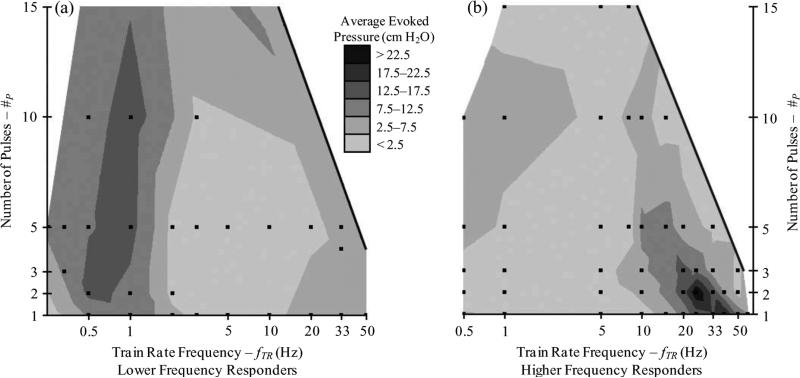
The effect of the train rate frequency (fTR) and number of pulses (#P) on evoked bladder pressures (PAVE–EV) is shown in Fig. 5. Regions of improved PAVE–EV are evident on the surface plots for both lower and higher frequency responding animals. The largest evoked pressures occurred for bursting stimulation patterns with greater than one pulse, as opposed to the commonly used single pulse, continuous stimulation patterns. Pulse frequencies (fPU) used in Fig. 5 were 100 and 200 Hz for lower frequency responders and 200 Hz for higher frequency responders.
Fig. 5.
Contour plots of average evoked pressure (PAVE–EV) against number of pulses (#P) and train rate frequency (fTR), for (a) lower frequency responding and (b) higher frequency responding experiments. Preferred ranges indicate that the addition of pulses results in greater evoked pressures than that evoked by continuous frequency stimulation at #P = 1. The dots indicate locations of stimulus parameter combinations that were included; 280 trials across 27 stimulus parameter combinations in (a) and 293 trials across 47 stimulus parameter combinations in (b). The region outside the solid line indicates the limit of the parameter space where adding pulses leads to a continuous stimulation frequency, equal to or greater than the pulse frequency (fPU), for a pulse frequency of 200 Hz.
The most effective bursting stimulus patterns in lower frequency responding cats were consistent, leading to a robust increase in the evoked pressure over the nominal single pulse stimuli. The best bursting stimulus patterns in lower frequency responders were 5/10 × 100/200 Hz @ 1 Hz, depending on the cat. Across all trials with these parameters, significantly greater PAVE–EV than 1 Hz single pulse continuous stimuli was evoked (14.3 ± 4.7 cm H2O, 11.0 ± 3.8 cm H2O, p < 0.002). Without pooling, 5 and 10 pulses × 100/200 Hz @ 1 Hz waveforms evoked significantly greater PAVE–EV than 1 Hz continuous stimuli (14.2 ± 4.8 cm H2O, p < 0.004; 15.4 ± 4.0 cm H2O, p < 0.003) while not being significantly different from each other (p > 0.35).
The most effective bursting stimulus patterns were not consistent in higher frequency responding cats, leading to a moderate increase in the evoked pressure over the nominal single pulse stimuli. In higher frequency responding cats, a bursting pattern could always be selected to generate greater pressures than the nominal single pulse stimuli, but not at a regular fTR. The best bursting stimulus patterns were 2 × 200 Hz @ 20/25/33 Hz, depending on the cat. Across all trials with 2 #P and 20, 25, and 33 Hz fTR, significantly greater PAVE–EV than 33 Hz single pulse continuous stimuli was not evoked (16.6 ± 7.0 cm H2O, 16.9 ± 8.0 cm H2O, p > 0.90). If only the best bursting patterned stimulus from each higher frequency responding cat is pooled together using parameters given in Fig. 4, for comparison to 33 Hz single pulse continuous stimuli, a greater but not significant difference was observed (20.2 ± 8.4 cm H2O, 16.9 ± 8.0 cm H2O, p > 0.30).
Although increasing #P initially improved PAVE–EV, further increasing #P led to a fall-off of the evoked bladder pressure response (Fig. 5). This decrease was observed before stimulation became essentially continuous, the limit of the parameter space. The evoked bladder pressure fell to about 25% of the maximum PAVE–EV when the number of pulses was 51.8 ± 9.3% (higher frequency responders: for 10, 20, 33, and 50 Hz fTR) and 17.6 ± 9.8% (lower frequency responders: for 0.5 and 1 Hz fTR) of the amount that would result in a continuous stimulus at a fPU of 200 Hz.
The fPU space was not thoroughly explored, however fPU of 100 and 200 Hz (#P = 5, fTR = 1 Hz) were not significantly different (15.6 ± 3.9 cm H2O, 13.7 ± 5.0 cm H2O, p > 0.10) from each other. For lower frequency responding animals, variations to fPU indicated regions of low and high evoked responses, with approximately 75 Hz separating the two regions. Pulse frequencies of 33 and 50 Hz were significantly lower than both 100 and 200 Hz (p < 0.02) but were not significantly different from each other (p > 0.60), for #P = 5, fTR = 1 Hz. In two cats, limited trials of fPU at 500 and 1000 Hz were not different than 100 and 200 Hz (p >, 0.35), for #P = 5, fTR = 1 Hz.
E. ANOVA Analyses
Across all data pooled together and data subgroups of the lower and higher frequency responders and pooled trials of the balanced sets, most significant experimental factors were consistent. Any variations found in the analyses were expected based on the balanced set designs or shifts in viewpoint due to analyzing a fraction of the overall data set. The train rate frequency (fTR) was always a significant factor (p < 0.05). The number of pulses (#P) was only significant (p < 0.05) in the ANOVA analysis of the pooled data (p > 0.05 otherwise). This was expected as subgroups of the pooled data allowed for nonlinear interactions between fTR and #P to balance out the effect of pulse bursting at a few #P values. The pulse frequency (fPU) followed a similar pattern of testing significance when limited trials at extreme values, as described above, had an increased effect depending on the data subset being analyzed (p < 0.05 only for lower frequency responder group). Other factors, such as tSTIM and the bladder volume, were consistently significant (p < 0.001, p < 0.05). Thus, using the randomized pre-determined sets or all data pooled together did not change the conclusions of this work that pulse bursting results in greater evoked bladder pressures.
IV. Discussion
In all cats, bursting of PN stimuli evoked greater reflex bladder contractions than continuous single pulse stimuli. Whereas the higher frequency responding cats had similar optimal single pulse stimuli (20 Hz, 33 Hz) as that reported elsewhere [14]–[17], evidence of responsiveness to low frequency (0.5–1 Hz) PN stimuli was also obtained. The use of low frequency stimuli with pulse bursting while operating at lower bladder volumes may result in improved efficacy of clinically implemented neuroprostheses for restoration of bladder function.
A. Responses to Pulse Bursting
The variable patterned stimuli used here are analogous to action potential burst timings, with #P related to the number of spikes, fPU to the intraburst frequency, and 1/fTR to the inter-burst interval. Neural recordings have indicated action potential patterns similar to the stimuli used in this study, including hippocampal spike timings (2-5 × ~ 200 Hz @ 40 Hz) [37] and the firing rates of neurons near intact intestinal mucosa responding to mucosal stimulation (3.8 ± 0.3 × 39 ± Hz) [32]. PN afferents have shown bursts of 4–16 spikes in response to scrotal temperature changes [36], bursting (8–10 spikes up to 400 Hz) initiated by mechanostimulation of the vaginal wall [34], and action potential firing rates increasing up to 400 Hz in reaction to clitoral tapping [35]. Comparable variable patterned stimuli have been used for nonbladder related afferent fiber activation, such as for improved release of “brain-derived neurotrophic factor” in the dorsal horn when using bursting (4 × 100 Hz @ 2 Hz) in comparison to constant frequency stimuli [40], enhanced reciprocal inhibition between ankle flexor and extensor muscles (10 pulses × 100 Hz @ 0.67 Hz) while continuous stimulation was ineffective [41], activation of visceral pain responses in the bowel (5 × 200 Hz @ 2 Hz) [42], and reduction of soleus muscle spasticity in hemiplegics by superficial peroneal stimulation (5 × 250 Hz @ 0.3/0.7/2/4 Hz) [43]. Bursting patterned stimulation may increase recruitment of bladder activating post-ganglionic pelvic nerve fibers as has been shown to occur for increased frequency of preganglionic stimulation [44]. The occur-rence of burst patterns during sensory stimuli and their effectiveness in synaptic transmission strongly suggests a possible role in evoking reflex bladder contractions. Our results in exploring burst pattern parameters (#P and fTR) support this role.
The present study defines the effective bursting parameter regions for afferent mediated bladder excitation. At least two studies have used bursting stimulation for bladder excitation. A 3 × 100 Hz @ 1 Hz patterned stimulus has been used to test reflex bladder discharges [12]. The use of 3 × 300 Hz @ 0.3 Hz and 3 × 300 Hz @ 3 Hz patterned stimuli were included with the use of continuous 10 Hz, 20 Hz, and 50 Hz stimulation for PN-evoked excitation of the bladder in decerebrate and spinalized cats [11]. Whereas those studies alluded to the possibility of using lower frequency stimuli coupled with bursting of pulses for reflex bladder activation, they did not evaluate a wider spectrum of parameters as was done here. These examples are consistent with our results in evoking bladder contractions using pulse bursting and they used stimulus parameters near the most effective lower frequency range observed in this study (Fig. 5).
The use of pulse bursting increases the potential parameter space for generating bladder contractions with PN stimuli. This extra dimension allows for optimal stimulus patterns for reflex bladder excitation to be explored, which may lead to improved voiding efficiencies closer to that obtained when using sacral stimulation following dorsal rhizotomy. However, the increased parameter space is not unlimited. Combined increases in #P and fTR result in continuous or near-continuous stimuli that can approach or exceed fPU, which are not effective. Responsiveness of the system to these patterned stimuli, though, falls off well before the total pulses per second become fPU, (Fig. 5).
B. Lower Frequency Excitation
Most PN-afferent bladder excitation studies have focused on the use of 20–33 Hz continuous stimuli [12], [14]–[18], [26]. The higher frequency responding cats had similar effective single pulse stimuli (20 Hz, 33 Hz). This study provides evidence of responsiveness to low frequency (0.5–1 Hz) PN stimuli, though less than that evoked by higher frequency stimuli. Although 2 Hz has been used for reflex bladder excitation via intraurethral stimulation in cats [27] and humans [27], [28], PN excitation has not focused on lower frequencies. In general, PN stimulation with continuous stimulation frequencies below 10 Hz are thought to primarily induce continence reflexes, not bladder excitation [15], [17], [18]. Researchers have performed limited trials of PN stimulation at lower frequencies, suggesting the potential to evoke contractions at 0.5 Hz [16], 1 Hz [10], 2 Hz [14], and below 10 Hz [17]. In the present work, lower frequency responders were effectively activated by continuous single pulse 0.5 and 1 Hz stimuli. Limited trials outside that range indicated a quick reduction in excitability (1.25 Hz excitable; 0.25 Hz and 2 Hz not consistently excitable; 3 Hz inhibitory) (Fig. 5).
In lower frequency responding cats, 5 and 10 #P repeated at 1 Hz fTR were consistently the best burst stimuli. However, the optimal fTR for burst patterned stimuli was not consistent in higher frequency responding cats, with pooled results not significantly greater than for single pulse 33 Hz continuous stimuli. The lack of a consistent best fTR is due to the broader range of higher frequencies. The variability in optimal frequencies (20–33 Hz) in higher frequency responding cats is consistent with the variability in preferred frequencies observed by different research groups (20 Hz [16], [18] versus 33 Hz [14], [15], [17]).
The lack of a consistent dominant frequency range across all experiments has been reported elsewhere [17]. Other research has indicated excitation for both lower and higher frequency PN stimulation but has not discussed whether the preferred frequency was consistent across experiments [14], [16]. The preferential excitation of the PN reflex pathway using lower or higher frequency stimulation patterns among experiments and the varying evoked pressure levels are not well understood but may be linked to the activation of different afferent fiber classes within the PN [17], anatomical variations among PNs [17], and varying effects of the anesthetic agents, surgical procedures, and other experimental procedures performed.
C. Clinical Relevance
Pulse bursting increases the potential parameter space for bladder contractions evoked by PN stimuli, thereby providing a wider range of utility for clinical applications. As the optimal stimulus parameters for humans are unknown [28], having a larger parameter set to choose from, both for bursting and low frequency stimuli, may increase the prospect of clinical effectiveness and will yield more customization options for individual neuroprosthetic users. Although the magnitude of the evoked pressure increases with bursting seen here was not large (3.3 cm H2O in both lower frequency and higher frequency responders), the percentage increase seen (30% and 19.5%, respectively) may have a clinical impact.
For higher frequency responders, using an intermittent on/off pattern for a bursting patterned stimuli may result in greater bladder drive and more effective voiding than simple intermittent trains of continuous stimuli. The effective stimulus duty cycle for low frequency bursting (#P · fTR/fPU = 2.5-10% for 5 × 200 Hz @ 1 Hz and 10 × 100 Hz @ 1 Hz) is well below that used in intermittent PN stimulation for voiding (77%–83%) [26] and sacral root motor drive (33%–67%) [7], [8], [26]. The smaller duty cycles and lower aggregate stimulation levels from lower frequency stimulus bursting may reduce the potential for habituation, which can limit the effectiveness of afferent-mediated approaches. The extended stimulus off periods in bursting stimulation allow more time for voiding and may lead to more physiological voiding patterns by allowing a nearly continuous stream of urine. The potential for improved voiding may allow PN stimulation to reach voiding efficiencies obtained by sacral stimulation after dorsal rhizotomy. Additionally, stimulator power requirements are decreased for lower frequency bursting when compared to the use of continuous stimulation or bursting at higher frequencies due to the lower number of total pulses per second.
As has been discussed elsewhere [15], [16], [18], PN stimulation for bladder excitation may be coupled with inhibitory PN stimulation such that continence could be induced until a time that a neuroprosthetic user was ready to empty their bladder. This bladder neuroprosthetic will be particularly useful for an individual with spinal cord injury who has a hyperreflexive bladder that contracts at low volumes. After reducing bladder contractions by using an inhibitory stimulus for continence, the use of a pulse bursting stimulus may provide improved bladder excitation during the voiding phase of bladder control. Future translational studies should include voiding trials, tests of efficacy after spinal transection and evaluations in clinical settings.
D. Bladder Volume
A minimum bladder volume is necessary before either dis-tension-evoked (VTH) or stimulation-evoked (VSTIM) bladder contractions can be elicited [15], [21], [28]. The majority of PN afferent stimulation studies that indicated the ability to evoke reflex contractions below VTH have focused on trials above VTH [14]–[16], operating at VSTIM/VTH of 125%–200% [14]–[16], [26]. The present work indicated that consistent, effective PN stimulation for reflex excitation of the bladder at functional (below “full”) volumes is feasible. Ratios of VSTIM/VTH of 66 ± 17% [14], 78 ± % [15], less than 70% [16], and 65 ± 17% [45] have been observed, using slightly different threshold criteria. The present work utilized bladder volumes below VTH the majority of the time, with an average VSTIM/VTH ratio of 81.0 ± 23.7%. This VSTIM/VTH ratio is likely overestimated due to a more relaxed bladder contraction threshold definition. Extending the 24 fill cycles that did not result in a distension evoked contraction to the point where VTH was reached would have resulted in a lower VSTIM/VTH ratio. Focusing research on reflex bladder excitation at volumes below that resulting in distension evoked contractions will give greater autonomy to a potential neuroprosthetic user, providing a greater bladder volume margin in which a user would be able to empty their bladder before undesired contractions could lead to incontinence.
E. Study Limitations
The unbalanced evaluation of stimulus parameters across experiments was a limitation of this study. In each experiment, if preferential responsiveness to lower or higher fTR stimuli was observed then the parameter space in that region was tested further. Aside from the limited use of factorial designs, a complete evaluation of the parameter space in all cats was not performed. However, as is indicated in the results, ANOVA analyses of the randomized predetermined sets and the overall data set indicated similar significant factors for cases where partitioning the data by taking a data subset did not distort the analysis. This indicates that the data was not unreasonably biased in any direction.
Several factors may have contributed to the minimal excitability seen in the five cats that were excluded, including possible shifts in spinal reflexes or damage to lower urinary tract innervation due to surgical procedures and other experimental protocols performed, the possibility of bypassing a tight VSTIM/VTH range for evoking contractions, or possible anesthetic interference [46]. It may also be possible that some cats do not respond to neuromodulation for bladder control using afferent pathways. Clinical trials investigating the use of neuromodulation for nonfunctional bladders have indicated a subset of their subjects (20%) that were nonresponders [13].
V. Conclusion
Variable or bursting stimulus patterns evoke greater bladder contractions than that elicited by single pulse continuous stimuli. Bursting increases the effective stimulation parameter space for generating bladder contractions. This work also provides evidence that PN-evoked bladder contractions can be elicited with lower frequency stimuli than typically used and that consistent reflex bladder excitation at functional bladder volumes is feasible. Improved bladder excitation should increase the efficacy of PN afferent-based neuroprostheses for restoration of bladder function.
Acknowledgment
The authors thank T. Goetz, A. Boger, T. Mariano, and M. Stone for their assistance with experimental setup and preparation.
This work was supported in part by the Department of Veterans Affairs RR&D 3675R, NIH HD40298, DK077089, AR07505, and by the Department of Education under Grant P200A040207.
Biography
 Tim M. Bruns (M’02–S’05) received the B.S. degree with honors in electrical engineering and a minor in bioengineering from the University of Illinois, Urbana-Champaign, in 2000, and the M.S. degree in bioengineering from Arizona State University, Tempe, in 2001. He is currently working toward the Ph.D. degree in the Biomedical Engineering Department at Case Western Reserve University, Cleveland, OH.
Tim M. Bruns (M’02–S’05) received the B.S. degree with honors in electrical engineering and a minor in bioengineering from the University of Illinois, Urbana-Champaign, in 2000, and the M.S. degree in bioengineering from Arizona State University, Tempe, in 2001. He is currently working toward the Ph.D. degree in the Biomedical Engineering Department at Case Western Reserve University, Cleveland, OH.
He was a Principal Systems Engineer with Baxter Healthcare Corporation, Round Lake, IL, from 2002 to 2005. His is currently a Biomedical Engineer at the Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, OH. His research interests are in the use of functional electrical stimulation and neuroprostheses to restore function, including clinical applications of afferent stimulation for bladder control and investigation of spinal cord reflex pathways.
Mr. Bruns is a member of the Biomedical Engineering Society. While with Baxter Healthcare, he was a recipient of the Alyx team Duckbutt Award.
Narendra Bhadra received graduate medical and postgraduate orthopedic degrees from University of Calcutta, India, in 1978 and 1983, respectively, and the Ph.D. degree in bioengineering from Case Western Reserve University, Cleveland, OH, in 2001. He completed his residency and postgraduate training at Calcutta Medical College and University College of Medicine, Calcutta.
He was employed as Medical Officer and Clinical Tutor in the Department of Surgery, Medical College, Calcutta, India and as Research Scientist in the Bioengineering Center, Jadavpur University, India. He was an Assistant Professor in the National Institute for Orthopedic Handicapped in India and has worked as Staff Scientist at Axon Engineering Inc., Cleveland, OH, on developing an implantable spinal stimulation system. He is currently a Researcher at the Neural Engineering Center, Department of Biomedical Engineering at Case Western Reserve University, Cleveland, OH, and Biomedical Engineer at the Louis Stokes Cleveland Veterans Affairs Medical Center, OH. His principal research interests are in design of neural stimulation electrodes for functional electrical stimulation and the development of neuroprostheses and use of feedback control for lower urinary tract rehabilitation.
 Kenneth J. Gustafson (M’07) received the B.S.E., M.S., and Ph.D. degrees in bioengineering from Arizona State University, Tempe, in 1991, 1995, and 1997, respectively. He completed a research fellowship in 1999 in the Department of Cardiac Surgery, California Pacific Medical Center, San Francisco, CA.
Kenneth J. Gustafson (M’07) received the B.S.E., M.S., and Ph.D. degrees in bioengineering from Arizona State University, Tempe, in 1991, 1995, and 1997, respectively. He completed a research fellowship in 1999 in the Department of Cardiac Surgery, California Pacific Medical Center, San Francisco, CA.
He is currently an Assistant Professor in the Departments of Biomedical Engineering and Urology, Case Western Reserve University, Cleveland, OH, a Principal Investigator in the Cleveland FES Center, and a Research Scientist at the Louis Stokes Cleveland Veterans Affairs Medical Center. His research interests are in neural engineering and include the development and clinical application of neural prostheses to restore pelvic functions, neurophysiology and neural control of pelvic functions, and skeletal muscle power harnessing.
Dr. Gustafson received an Excellence in Neural Engineering Award from the IEEE Engineering in Medicine and Biology Society and the Biomedical Engineering Society, in 2002.
REFERENCES
- 1.Shingleton WB, Bodner DR. The development of urologic complications in relationship to bladder pressure in spinal cord injured patients. J. Amer. Paraplegia Soc. 1993;16:14–17. doi: 10.1080/01952307.1993.11735878. [DOI] [PubMed] [Google Scholar]
- 2.Creasey GH, Dahlberg JE. Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Arch. Phys. Med. Rehabil. 2001;82:1520–1525. doi: 10.1053/apmr.2001.25912. [DOI] [PubMed] [Google Scholar]
- 3.Burns AS, Rivas DA, Ditunno JF. The management of neurogenic bladder and sexual dysfunction after spinal cord injury. Spine. 2001;26:S129–S136. doi: 10.1097/00007632-200112151-00022. [DOI] [PubMed] [Google Scholar]
- 4.Creasey GH, Grill JH, Korsten M, Betz HSU,R, Anderson R, Walter J. An implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: A multicenter trial. Arch. Phys. Med. Rehabil. 2001;82(11):1512–1519. doi: 10.1053/apmr.2001.25911. [DOI] [PubMed] [Google Scholar]
- 5.Gaunt RA, Prochazka A. Control of urinary bladder function with devices: Successes and failures. Prog. Brain Res. 2006;152:163–194. doi: 10.1016/S0079-6123(05)52011-9. [DOI] [PubMed] [Google Scholar]
- 6.Tai C, Roppolo JR, de Groat WC. Spinal reflex control of micturition after spinal cord injury. Restor Neurol. Neurosci. 2006;24:69–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Brindley GS. An implant to empty the bladder or close the urethra. J. Neurol. Neurosurg. Psychiatry. 1977;40:358–369. doi: 10.1136/jnnp.40.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brindley GS, Polkey CE, Rushton DN. Sacral anterior root stimulators for bladder control in paraplegia. Paraplegia. 1982;20:365–381. doi: 10.1038/sc.1982.65. [DOI] [PubMed] [Google Scholar]
- 9.Grunewald V, Bhadra N, Creasey GH, Mortimer JT. Functional conditions of micturition induced by selective sacral anterior root stimulation: Experimental results in a canine animal model. World J. Urol. 1998;16:329–336. doi: 10.1007/s003450050076. [DOI] [PubMed] [Google Scholar]
- 10.Mazieres L, Jiang CH, Lindstrom S. Bladder paraympathetic response to electrical stimulation of urethral afferents in the cat. Neurourol. Urodyn. 1997;16:471–472. [Google Scholar]
- 11.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci. Lett. 1998;244:137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 12.Jiang CH, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J. Physiol. 1999;517:599–605. doi: 10.1111/j.1469-7793.1999.0599t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: A prospective, single-blinded, randomized, crossover trial. Neurourol. Urodyn. 2005;24:643–647. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 14.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J. Neurophysiol. 2005;93:2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 15.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J. Physiol. 2006;577:115–126. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp. Neurol. 2006;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Yoo PB, Grill WM. Minimally-invasive electrical stimulation of the pudendal nerve: A pre-clinical study for neural control of the lower urinary tract. Neurourol. Urodyn. 2007;26:562–569. doi: 10.1002/nau.20376. [DOI] [PubMed] [Google Scholar]
- 18.Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol. Urodyn. 2007;26:570–577. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 19.Vodusek DB, Light JK, Libby JM. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol. Urodyn. 1986;5:381–389. [Google Scholar]
- 20.Lee YH, Creasey GH, Lim H, Song J, Song K, Kim J. Detrusor and blood pressure responses to dorsal penile nerve stimulation during hyperreflexic contraction of the bladder in patients with cervical cord injury. Arch. Phys. Med. Rehabil. 2003;84:136–140. doi: 10.1053/apmr.2003.50075. [DOI] [PubMed] [Google Scholar]
- 21.Grill WM, Craggs MD, Foreman RD, Ludlow CL, Buller JL. Emerging clinical applications of electrical stimulation: Opportunities for restoration of function. J. Rehabil. Res. Develop. 2001;38:641–653. [PubMed] [Google Scholar]
- 22.Huang JC, Deletis V, Vodusek DB, Abbott R. Preservation of pudendal afferents in sacral rhizotomies. Neurosurgery. 1997;41:411–415. doi: 10.1097/00006123-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Vodusek DB. Anatomy and neurocontrol of the pelvic floor. Digestion. 2004;69:87–92. doi: 10.1159/000077874. [DOI] [PubMed] [Google Scholar]
- 24.Gustafson KJ, Zelkovic PF, Feng AH, Draper CE, Bodner DR, Grill WM. Fascicular anatomy and surgical access of the human pudendal nerve. World J. Urol. 2005;23:411–418. doi: 10.1007/s00345-005-0032-4. [DOI] [PubMed] [Google Scholar]
- 25.Spinelli M, Malaguti S, Giardiello G, Lazzeri M, Tarantola J, Van Den Hombergh U. A new minimally invasive procedure for pudendal nerve stimulation to treat neurogenic bladder: Description of the method and preliminary data. Neurourol. Urodyn. 2005;24:305–309. doi: 10.1002/nau.20118. [DOI] [PubMed] [Google Scholar]
- 26.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J. Neural Eng. 2006;3:43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson KJ, Creasey GH, Grill WM. A catheter based method to activate urethral sensory nerve fibers. J. Urol. 2003;170:126–129. doi: 10.1097/01.ju.0000070821.87785.14. [DOI] [PubMed] [Google Scholar]
- 28.Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci. Lett. 2004;360:9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Gerstner W, Kreiter AK, Markram H, Herz AVM. Neural codes: Firing rates and beyound. Proc. Nat. Acad. Sci. USA. 1997;94:12740–12741. doi: 10.1073/pnas.94.24.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krahe R, Gabbiani F. Burst firing in sensory systems. Nat. Rev. Neurosci. 2004;5:13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- 31.Dekhuijzen AJ, Bagust J. Analysis of neural bursting: Non-rhythmic and rhythmic activity in isolated spinal cord. J. Neurosci. Methods. 1996;67:141–147. [PubMed] [Google Scholar]
- 32.Bertrand PP. Bursts of recurrent excitation in the activation of intrinsic sensory neurons of the intestine. Neuroscience. 2004;128:51–63. doi: 10.1016/j.neuroscience.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Scheich H, Bullock TH, Hamstra RH., Jr. Coding properties of two classes of afferent nerve fibers: High-Frequency electroreceptors in the electric fish, eigenmannia. J. Neurophysiol. 1973;36:39–60. doi: 10.1152/jn.1973.36.1.39. [DOI] [PubMed] [Google Scholar]
- 34.Cueva-Rolon R, Munoz-Martinez EJ, Delgado-Lezama R, Raya JG. The cat pudendal nerve: Afferent fibers responding to mechanical stimulation of the perineal skin, the vagina or the uterine cervix. Brain Res. 1994;655:1–6. doi: 10.1016/0006-8993(94)91589-x. [DOI] [PubMed] [Google Scholar]
- 35.Kawatani M, Tanowitz M, de Groat WC. Morphological and electrophysiological analysis of the peripheral and central afferent pathways from the clitoris of the cat. Brain Res. 1994;646:26–36. doi: 10.1016/0006-8993(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 36.Pierau FK, Torrey P, Carpenter DO. Afferent new fiber activity responding to temperature changes of scrotal skin of the rat. J. Neurophysiol. 1975;38:601–612. doi: 10.1152/jn.1975.38.3.601. [DOI] [PubMed] [Google Scholar]
- 37.Lisman JE. Bursts as a unit of neural information: Making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- 38.Tobin G. Presynaptic muscarinic receptor mechanisms and submandibular responses to stimulation of the parasympathetic innervation in bursts in rats. Auton. Neurosci. 2002;99:111–118. doi: 10.1016/s1566-0702(02)00094-2. [DOI] [PubMed] [Google Scholar]
- 39.Edwards AV, Andersson PO, Jarhult J, Bloom SR. Studies of the importance of the pattern of autonomic stimulation in relation to alimentary effectors. Q. J. Exp. Physiol. 1984;69:607–614. doi: 10.1113/expphysiol.1984.sp002847. [DOI] [PubMed] [Google Scholar]
- 40.Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JC, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J. Neurosci. 2001;21:4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez MA, Field-Fote EC, Floeter MK. Patterned sensory stimulation induces plasticity in reciprocal ia inhibition in humans. J. Neurosci. 2003;23:2014–2018. doi: 10.1523/JNEUROSCI.23-06-02014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossel P, Drewes AM, Petersen P, Nielsen J, Arendt-Nielsen L. Pain produced by electric stimulation of the rectum in patients with irritable bowel syndrome: Further evidence of visceral hyperalgesia. Scand. J. Gastroenterol. 1999;34:1001–1006. doi: 10.1080/003655299750025101. [DOI] [PubMed] [Google Scholar]
- 43.Fujita K, Kataoka S, Kamada Y, KaShimosugi Y, Takahashi A. Soleus spasticity reduction by repeated burst stimulation. 4th Annu. Conf. Int. Functional Electr. Stimulation Soc.; Sendai, Japan. 1999.pp. 21–31. [Google Scholar]
- 44.DeGroat WC, Saum WR. Synaptic transmission in parasym-pathetic ganglia in the urinary bladder of the cat. J. Physiol. 1976;256:137–158. doi: 10.1113/jphysiol.1976.sp011316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robain G, Combrisson H, Mazieres L. Bladder response to urethral flow in the awake ewe. Neurourol. Urodyn. 2001;20:641–649. doi: 10.1002/nau.1014. [DOI] [PubMed] [Google Scholar]
- 46.Rudy DC, Downie JW, McAndrew JD. Alpha-Chloralose alters autonomic reflex function of the lower urinary tract. Am. J. Physiol. 1991;261:R1560–7. doi: 10.1152/ajpregu.1991.261.6.R1560. [DOI] [PubMed] [Google Scholar]