Abstract
In this study, we show that boronates, a class of synthetic organic compounds, react rapidly and stoichiometrically with peroxynitrite (ONOO−) to form stable hydroxy derivatives as major products. Using stopped-flow kinetic technique, we measured the second order rate constants for the reaction with ONOO−, hypochlorous acid (HOCl), and hydrogen peroxide (H2O2), and found that ONOO− reacts with 4-acetylphenylboronic acid nearly a million times (k = 1.6 × 106 M−1 s−1) faster than H2O2 (k = 2.2 M −1 s−1) and over two hundred times faster than HOCl (k = 6.2 × 103 M−1 s−1). Nitric oxide (•NO) and superoxide (O •2−) together, but not alone, oxidized boronates to the same phenolic products. Similar reaction profiles were obtained with other boronates. Results from this study will likely help develop a novel class of fluorescent probes for detection and imaging of ONOO− formed in cellular and cell-free systems.
Keywords: Boronic acids, Boronates, Peroxynitrite, Probes, Kinetics, Stopped flow, HPLC
Introduction
Peroxynitrite/peroxynitrous acid (ONOO−/ONOOH), a potent oxidant and nitrating agent formed from the diffusion-controlled reaction between nitric oxide (•NO) and superoxide (O •2−) anion, has been implicated as a key pathophysiological intermediate in various diseases, including acute and chronic inflammatory processes, ischemia-reperfusion, and neurodegenerative disorders [1-4]. Despite two decades of intense research, methodologies to directly detect and quantitate ONOO− in cellular and biological systems are still lacking [5,6]. As reiterated in recent reviews, development of new probes and techniques for direct detection of ONOO−, a key reactive intermediate in redox biology, is essential for understanding the role of reactive nitrogen species (RNS) in the biological milieu [7]. Most existing detection methods of ONOO− are based on the reaction of hydroxyl radical (•OH) and nitrogen dioxide radical (•NO2), which from the radical pair cage, •NO2 …•OH with small organic molecules such as tyrosine, tryptophan, salicylic acid, and dihydrorhodamine [8-10]. Peroxynitrite reacts directly with thiols, methionine, ebselen, porphyrins, and carbonyl compounds [11-13]; yet these reactions have not been employed for ONOO− detection. The role of ONOO− has been questioned with regard to protein tyrosyl nitration [14,15]. Myeloperoxidase (MPO) / hydrogen peroxide (H2O2)-catalyzed oxidation of nitrite anion also could result in protein tyrosyl nitration [14,16]. Thus, probes that form a characteristic product by reacting rapidly and directly with ONOO−, rather than with its radical intermediates, •NO2 and •OH, are critically needed.
Toward this end, a new class of fluorescent probes containing a boronate structure has been developed for selective detection of H2O2 [17,18]. This assay was based on the conversion of weakly fluorescent arylboronates into strongly fluorescent phenolic products. While the reaction of •NO and O •2− with boronates was tested individually, the possibility of oxidation of boronates by ONOO− was not investigated [19,20]. In this study, we report that boronates (Figure 1) react directly and rapidly with ONOO−. This forms the corresponding hydroxyl derivatives, phenols, as major products. The rate constant determined for this reaction is several orders of magnitude greater than for H2O2 or hypochlorous acid (HOCl). Potential implications for imaging of ONOO− in cells using boronate-containing fluorescence probes are discussed.
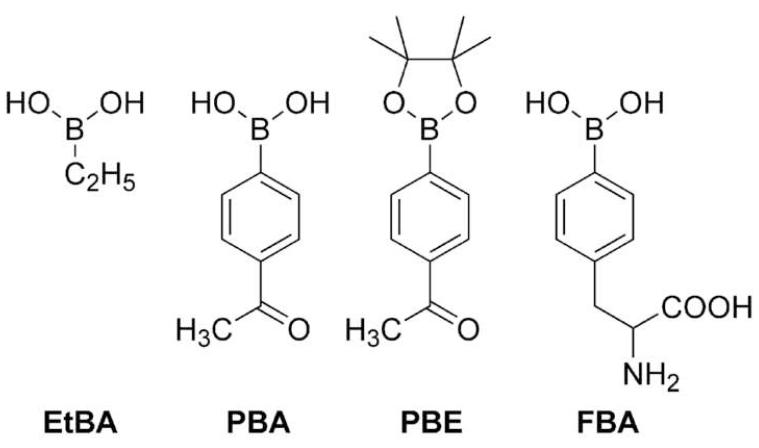
Figure 1. Chemical structures of boronates used in this study.
Materials and Methods
Materials
FBA was obtained from Ryscor Science, and other boronic compounds were from Boron Molecular. XO and SOD were from Roche Diagnostics, CAT was from Boehringer Mannheim, MPO was from Calbiochem, and all other reagents (of highest purity available) were from Sigma. All solutions were prepared using deionised water (Millipore Milli-Q system). ONOO− was prepared by reacting nitrite with H2O2, according to the published procedure [21]. The concentration of ONOO− in alkaline aqueous solutions (pH>12) was determined by measuring the absorbance at 302 nm (ε = 1670 M−1cm−1).
Determination of O2•− and •NO fluxes
•NO fluxes were determined from the measured rate of the decomposition of PAPA-NONOate by following the decrease of its characteristic absorbance at 250 nm ( = 8 · 103 M−1cm−1). This rate was multiplied by a factor of two to get the rate of •NO release (assuming that two molecules of •NO are released from one molecule of PAPA-NONOate). Superoxide flux was determined by monitoring the cytochrome c reduction following the increase in absorbance at 550 nm (using an extinction coefficient of 2.1 · 104 M−1 cm−1).
Oxidation of boronic compounds induced by ONOO−, H2O2, HOCl, O2•−, and MPO/H2O2
Typically, boronic acids and esters (100 μM) were incubated with MPO (15 nM), H2O2 (50 μM) and NaNO2 (500 μM) or NaCl (0.15 M) in a phosphate buffer (100 mM, pH 7.4) containing DTPA (10 μM) at room temperature for 30 min. Reactions were terminated by adding CAT (100 U/ml) and directly analyzed by HPLC. In other experiments, boronic compounds (100 μM) were incubated with XO, X (200 μM) and PAPA-NONOate (30 μM) in a phosphate buffer (100 mM, pH 7.4) solution containing DTPA (10 μM) at room temperature for 15 min. Reaction mixture contained either CAT (100 U/ml) and/or SOD enzyme (0.5 mg/ml). Finally, in some experiments, boronic compounds (100 μM) were rapidly mixed with ONOO− (10 – 250 μM), hypochlorous acid (10 – 250 μM) or H2O2 (10 – 250 μM) in a phosphate buffer (100 mM, pH 7.4). With ONOO− and HOCl, reaction mixtures were incubated at room temperature for 10 min, and then analyzed by HPLC. With H2O2, samples were incubated for several hours prior to HPLC analysis. To investigate the effect of carbon dioxide (CO2) on the yield of the phenolic product of the reaction of boronates with ONOO−, we have carried out the reaction in the presence of sodium bicarbonate (NaHCO3). Bicarbonate anion is known to exist at neutral pH in the equilibrium with CO2 and based on the dissociation constant of carbonic acid (pKa = 6.35), we have calculated the concentration of CO2 at pH 7.4 to be 2.2 mM in the equilibrium with 25 mM HCO3− anions. To ensure the solutions reached this equilibrium, peroxynitrite was added 10 – 20 min after preparing the solutions.
HPLC and UV-Vis analyses of oxidation products
HPLC experiments were performed using an Agilent 1100 system. For detecting boronic compounds and their oxidation products, typically 10 μl of sample was injected into the HPLC system equipped with a C18 column (Alltech, Kromasil, 250 mm × 4.6 mm, 5 μm, 80 Å) that was equilibrated with 5% CH3CN [containing 0.1% (v/v) trifluoroacetic acid (TFA)] in 0.1% TFA aqueous solution. Compounds were eluted during a linear increase in acetonitrile fraction from 5 to 100% over 60 min (using a flow rate of 0.5 ml/min). For the quantification of FBA and the products of its reaction with ONOO−, typically 50 μl of sample was injected into the HPLC system equipped with a Synergi Hydro-RP column (Phenomenex, 250 × 4.6 mm, 4 m) that was equilibrated with aqueous solution of TFA (0.1%). Over the first 20 min after injection, the CH3CN fraction was increased from 0% to 5% and from 5% to 20% over the next 15 min. The flow rate was set at 1 ml/min. The UV-Vis absorption spectra were collected using an Agilent 8453 spectrophotometer.
Stopped-flow measurements
Stopped-flow kinetic experiments were performed on an Applied Photophysics 18MX stopped-flow spectrophotometer equipped with photomultiplier (PM) for absorption measurements [22]. The thermostatted cell (25 °C) with a 10-mm optical pathway was used for kinetic measurements. Typically, reactions were performed under pseudo first-order conditions (greater than tenfold excess boronate over ONOO− at boronate concentrations ranging from 100 M to 1 mM).
Kinetic analysis
The kinetic traces were fitted to a single-exponential function corresponding to the first-order kinetics. Results from fitting of at least three kinetic traces were averaged to obtain the first-order rate constant. From the slopes of the plots of the observed first-order rate constants versus the concentration of the reactant, the second-order rate constants were obtained.
Results
Kinetics of oxidation of boronates
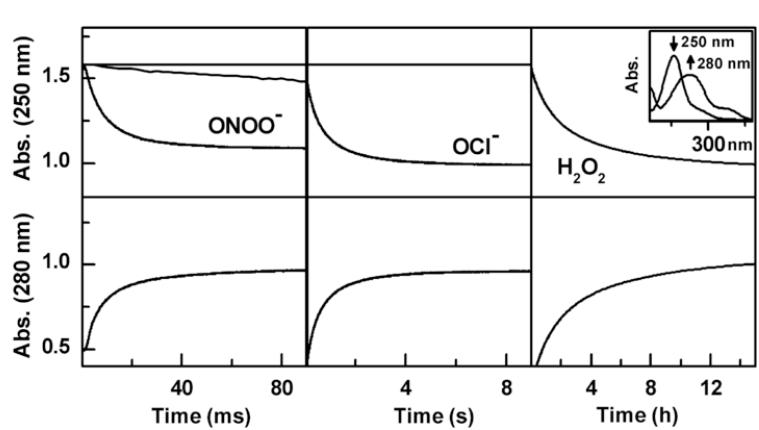
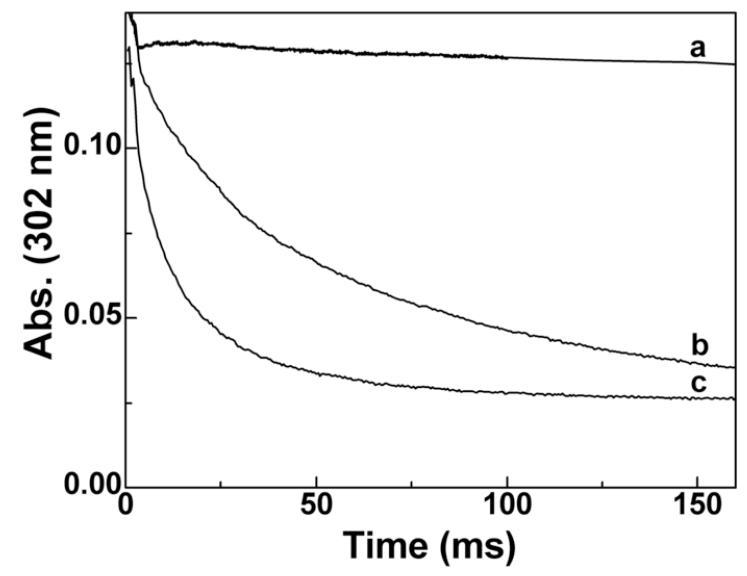
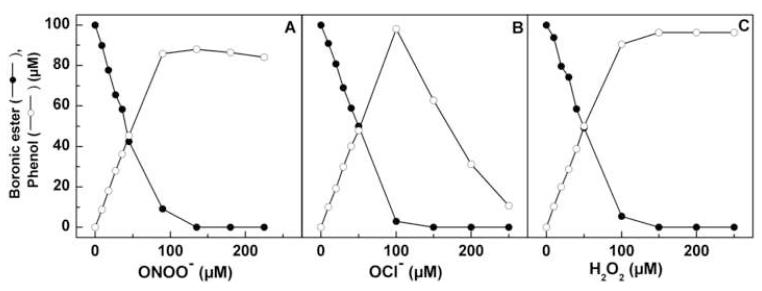
The reaction kinetics for boronate oxidation by ONOO−, HOCl, and H2O2 were investigated under pseudo first-order conditions, using excess of either boronate in the case ONOO− or oxidants HOCl and H2O2. The rate constants for the reactions of selected aromatic and aliphatic boronic compounds are shown in Table 1. Remarkably, ONOO− reacts with boronates as fast as it reacts with ebselen. Figure 2 shows the comparative kinetic traces of ONOO−, H2O2, and HOCl with 4-acetylphenylboronic pinacolate ester (PBE). As shown, the reaction of H2O2 with boronates is much slower (over a period of hours under the experimental conditions used), as compared with ONOO−, which takes place at a millisecond time scale. Reaction between boronates and HOCl occurred over several seconds. This is one of the fastest reactions of ONOO− with small molecular weight organic molecules. The rate constant between aliphatic boronate (ethylboronic acid, EtBA) and ONOO−, obtained by monitoring the decomposition of ONOO− at 302 nm [21] (Fig. 3) was an order of magnitude lower than for aromatic analogs (Table 1).
Table 1.
The apparent second-order rate constants of the reaction of boronates with ONOO—, HOCl, and H2O2 at pH 7.4.
| Second-order rate constant, k [M−1 s−1] |
|||
|---|---|---|---|
| Boronate | Peroxynitrite | Hypochlorous acid |
Hydrogen peroxide |
| EtBA | (2.8±0.2) × 105 | (1.2±0.2) × 104 | -a |
| PBA | (1.6±0.3) × 106 | (6.2±0.3) × 103 | 2.2±0.1 |
| PBE | (1.2±0.3) × 106 | (5.7±0.3) × 103 | 2.2±0.1 |
| FBA | (1.4±0.3) × 106 | (2.7±0.3) × 104 | 2.5±0.1 |
not measured
Figure 2. Kinetics of the phenyl boronic ester reactions with ONOO−, hypochlorite, and H2O2.
The decay kinetics of PBE were monitored at 250 nm and formation of 4′-hydroxyacetophenone was monitored at 280 nm. Incubations contained 100 μM PBE and 100 μM oxidant (after mixing) in phosphate buffer (pH 7.4, 100 mM) containing DTPA (10 μM). Measurements were made at room temperature. (Inset) shows the absorption spectra of PBE ( max = 250 nm) and 4′-hydroxyacetophenone ( max = 280 nm).
Figure 3.
Decay kinetics of ONOO− at 25 oC in phosphate buffer (pH 7.4, 250 mM) (a) in the absence of boronates, (b) in the presence of EtBA (100 μM), and (c) in the presence of PBA (100 μM).
Quantitation of products of boronate oxidation: Stoichiometric analyses
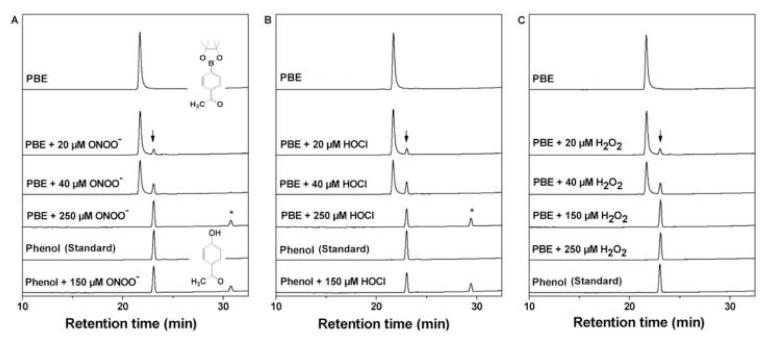
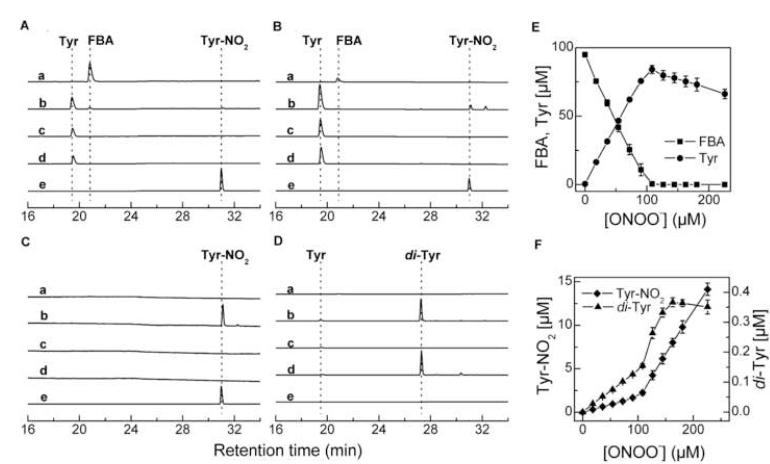
The oxidation of 4-acetylphenylboronic ester by ONOO−, H2O2, and HOCl was monitored by following the substrate consumption and the formation of corresponding 4′-hydroxyacetophenone using the HPLC technique (Fig. 4). As shown in Figure 4 (left), PBE eluted at 21.9 min. With increasing concentration of ONOO−, the intensity due to PBE peak decreased, and another peak eluted at 23.2 min (marked by a downward arrow) appeared. This new peak coincided with that of the 4′-hydroxyacetophenone. With further increase in ONOO− (250 μM), another peak eluting at 32 min appeared. This is also formed from 4′-hydroxyacetophenone and ONOO− (marked by an asterisk). This peak has been assigned to the nitrated phenol, as it coeluted with the authentic standard of 3′-nitro-4′-hydroxyacetophenone.
Figure 4. HPLC analyses of products formed from reaction between PBE and ONOO−, HOCl, and H2O2.
(A-C) Incubation mixtures consisted of 100 μM PBE in phosphate buffer (pH 7.4, 100 mM) containing DTPA (10 μM) and oxidants at the indicated concentrations. PBE and the corresponding phenol, 4′-hydroxyacetophenone, were detected using the HPLC/UV detection at 250 nm, respectively. Asterisks (*) in (A) and (C) indicate nitrated and chlorinated tyrosyl products. In the case of HOCl experiments, DTPA was omitted.
Figure 4 (middle) shows the HPLC chromatograms of reaction mixtures of PBE with HOCl. With increasing HOCl concentration, PBE was oxidized to the corresponding phenol (marked by a downward arrow). In the presence of increasing HOCl concentration, an additional peak, attributable to the chlorinated product (marked by an asterisk), was detected. Addition of taurine to the reaction mixture inhibited the reaction, indicating that HOCl, but not chloramine formed in the reaction of HOCl with taurine, is able to convert boronic compounds into phenols. Figure 4 (right) shows the HPLC chromatograms of the reaction mixtures containing PBE and H2O2. Similar to ONOO− and HOCl, H2O2 oxidized PBE to 4′-hydroxyacetophenone. However, in contrast to ONOO− and HOCl, with increasing concentrations of H2O2 there was no further oxidation of the phenol formed during the reaction. Figure 5 shows the substrate depletion and product formation during titration of PBE with ONOO−, HOCl, and H2O2. It is evident that PBE reacts with all three oxidants stoichiometrically, with one molecule of the boronate consumed per one molecule of the oxidant. However, while in the case of HOCl and H2O2, the maximal yield of the phenol was close to 100%, the yield obtained with ONOO− reached 80-85%. Increasing the concentration of ONOO− decreased the yield of phenol. Similar results were obtained with phenylalanine boronic acid (FBA) (Fig. 6). Tyrosine was formed as a major product during oxidation of FBA by ONOO− and nitrotyrosine and dityrosine were formed even in the presence of excess FBA, when the reaction of ONOO− with the corresponding phenol (i.e., tyrosine) is not very probable. This indicates that a small fraction (< 20%) of the adduct of ONOO− to the boronic compound decays via free radical pathways. The exact nature of the reactions occurring during the decomposition of the adduct is currently under investigation.
Figure 5. Relationship between substrate depletion and product formation from reaction of the boronate with oxidants.
(A-C) Experimental conditions are essentially the same as in Fig. 3. After a 15-min (ONOO-, HOCl) or 24 h (H2O2) incubation period, reaction mixtures were analyzed by HPLC/UV method. PBE was detected at 250 nm and 4′-hydroxyacetophenone at 280 nm.
Figure 6. HPLC chromatograms of the reaction between phenylalanine boronic acid (FBA) and ONOO−.
(A-C): Absorption traces recorded at 220 nm (A), 280 nm (B) and 350 nm (C); (D): Fluorescence traces (excitation at 284 nm, emission at 410 nm). (a) FBA (250 μM); (b) FBA (250 μM) + ONOO− (200 μM); (c) Tyr (100 μM); (d) Tyr (100 μM) + HRP (10 nM) + H2O2 (100 μM); (e) Tyr-NO2 (100 μM). All samples have been incubated for 15 min at room temperature in aqueous solution containing phosphate buffer (0.1 M, pH 7.4) and DTPA (10 μM). To fit the scale, traces e have been scaled down by a factor of 0.1 (panel B) and 0.03 (panel C). (E) and (F), the dependences of the phenylalanine boronate and nitrotyrosine concentrations in reaction mixtures on the amount of ONOO− added to the solution of FBA (100 μM).
Oxidation of boronates in xanthine (X) /xanthine oxidase (XO) and PAPA-NONOate systems
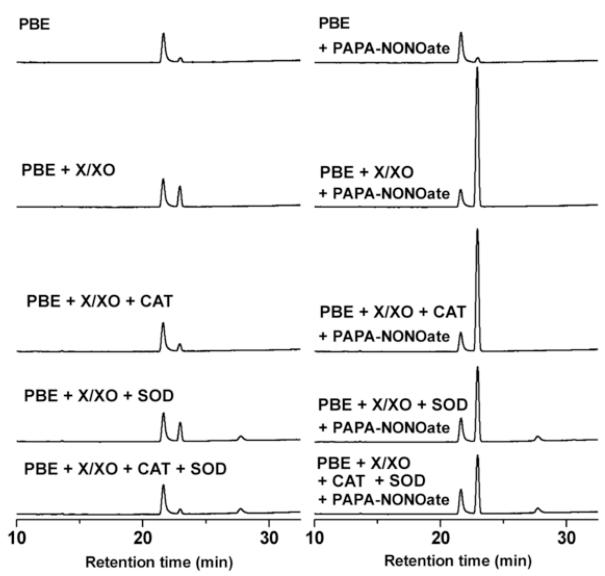
The oxidation of PBE was investigated in the presence of O •2− and •NO. To this end, PBE (100 μM) was incubated with X (200 μM), XO (10 mU/ml) in phosphate buffer (100 mM, pH 7.4) containing DTPA (10 μM) for 15 min at room temperature, and products were analyzed by HPLC (Fig. 7, left panel). In parallel, similar incubations were performed but in the presence of a •NO donor, PAPA-NONOate (100 μM), and products were analyzed by HPLC (Fig. 7, right panel). In the absence of PAPA-NONOate, there was a modest conversion of PBE to 4′-hydroxyacetophenone, which was sensitive to CAT, but not to the superoxide dismutase (SOD) addition (Fig. 7, left panel), indicating that H2O2, but not O •2− was responsible for oxidation of PBE. In contrast, addition of PAPA-NONOate to the incubation mixtures containing PBE and X/XO significantly enhanced the formation of 4′-hydroxyacetophenone, which was inhibited (=50%) in the presence of high concentrations of SOD but not CAT (Fig. 7, right panel). This suggests that ONOO− formed from •NO and O •2− is responsible for oxidation of PBE to the corresponding phenol. In the X/XO/PAPA-NONOate system (generating equal fluxes of •NO and O •2−) the amount of PBE consumed was nearly equal to the amount of ONOO− that was expected to be produced during the incubation, and the 4′-hydroxyacetophenone was formed with about 90% yield (compared to the amount of PBE consumed).
Figure 7. HPLC analyses of products formed from X/XO/PAPA-NONO-ate-dependent oxidation of PBE.
Incubation mixtures included 100 μM PBE and X (200 μM)/XO in phosphate buffer (pH 7.4, 100 mM) containing (10 μM DTPA) in the presence or absence of PAPA-NONOate (100 μM). After a 15-min incubation at room temperature, products were analyzed as described for Fig. 3. Where indicated, CAT (100 U/ml) and SOD (0.5 mg/ml) were added. O •2− and •NO fluxes (2.6 μM/min each) were determined as described in Materials and Methods.
PBE and 4′-hydroxyacetophenone were detected at 280 nm.
GSH and CO2 effects on boronate oxidation by ONOO−
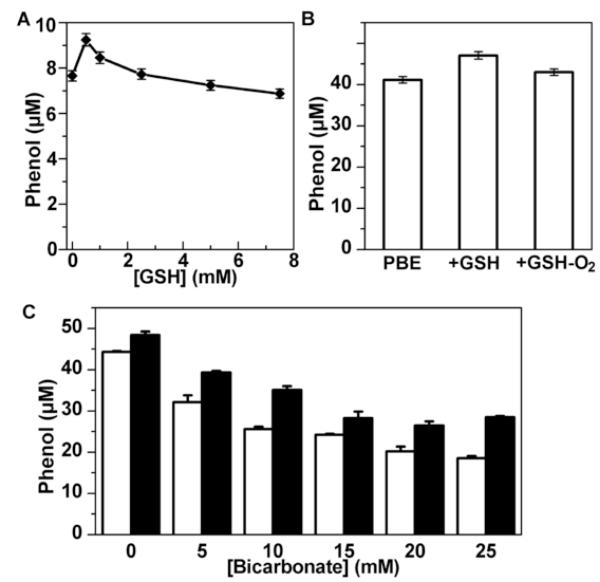
As ONOO−-dependent reactions are often altered in the presence of biologically relevant reductants (e.g., glutathione, GSH) and HCO3− [23-25], we investigated the effects of varying the levels of GSH and HCO3−on the extent of ONOO−-mediated oxidation of boronate, PBE. Figure 8A shows the effect of increasing concentrations of GSH on 4′-hydroxyacetophenone formation in incubation mixtures containing PBE in phosphate buffer (100 mM, pH 7.4) and DTPA (10 μM). In the presence of relatively low concentrations of GSH (1 mM or less), there was enhanced formation of 4′-hydroxyacetophenone. With increasing GSH concentration, the yield of the phenolic product decreased; however, even in the presence of 6-7 mM GSH, ONOO− reacted with PBE forming 4′-hydroxyacetophenone, although the yield gradually decreased. The decrease in the yield of 4′-hydroxyacetophenone may be explained in terms of the competition between PBE and GSH for ONOO−. The reason for an increase in phenol yield at lower concentrations of GSH is less clear at present. When incubations were carried out in N2-purged solutions, this increase in product formation (at low GSH) was largely abolished (Fig. 8B). This finding suggests that oxidant(s) derived from the adduct of ONOO− with PBE can react with GSH and form a reactive intermediate in the presence of oxygen that may increase the yield of phenol.
Figure 8. The effects of glutathione and HCO3− on boronate oxidation by ONOO−.
(A) Incubation mixtures contained PBE (50 μM), ONOO− (10 μM), and GSH at concentrations as indicated in phosphate buffer (pH 7.4, 100 mM) containing DTPA (10 μM). (B) Relative concentrations of 4′-hydroxyacetophenone formed in incubation mixtures containing PBE (100 μM), GSH (500 μM), and ONOO− (50 μM, slow infusion 10 μM/min) in phosphate buffer (pH 7.4, 100 mM) containing DTPA (10 μM). The product was measured 15 min after the addition of ONOO−. (C) PBE (100 μM) was mixed with ONOO− (50 μM) in a phosphate buffer (pH 7.4, 100 mM) containing DTPA (10 μM) and HCO3− (open bars) or GSH (500 μM) and HCO3− (solid bars)
HCO3− is another ubiquitous component in cells. As ONOO− reacts with carbon dioxide (CO2) forming an intermediate, nitrosoperoxycarbonate anion (ONOOCO2−, with a half-life < 1 ms), that decomposes to •NO2 and carbonate radical anion (CO •3−) [26,27], the effects of varying concentrations of HCO3−and GSH on boronate oxidation were investigated. There was a concentration-dependent decrease in the extent of PBE oxidation to 4′-hydroxyacetophenone by ONOO− in the presence of HCO3− (Fig. 8C). The addition of GSH (500 μM) to the above incubation mixture increased the yield of 4′-hydroxyacetophenone. This increase was again dependent on the presence of oxygen, indicating that GSH-derived oxidation products may induce additional product formation from boronate. Overall, these results suggest that even in the presence of GSH and HCO3−, ONOO− can convert boronates into corresponding phenols.
Oxidation of boronates by MPO/H2O2 system
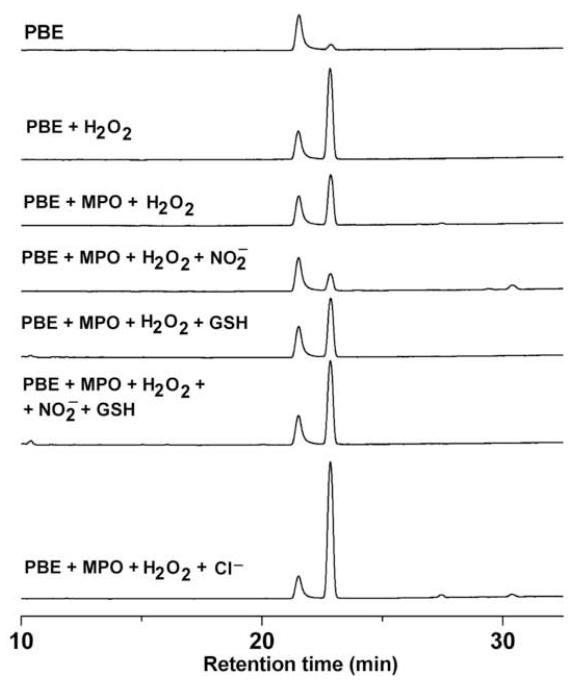
To investigate whether other reactive nitrogen species (e.g., nitrogen dioxide, •NO2) oxidize boronate in the same way as does ONOO−, we used the MPO and H2O2 system. PBE was incubated with MPO, H2O2, and nitrite anion (NO2−) in phosphate buffer (50 mM, pH 7.4) containing DTPA (10 μM). Unlike ONOO−, the oxidant (•NO2) generated from MPO, H2O2, and NO2− did not increase 4′-hydroxyacetophenone formation. The yield of the phenolic product was, however, enhanced in the presence of GSH (Fig. 9). This is tentatively attributed to oxidation of boronate by GSH/•NO2/O2-derived oxidants. Although •NO2 by itself is not responsible for oxidation of PBE to the corresponding phenol, GSH accelerates the conversion of PBE to 4′-hydroxyacetophenone. In the presence of chloride anion, there was increased formation of phenol due to HOCl-mediated oxidation of boronate.
Figure 9. HPLC analyses of products formed during MPO/H2O2-mediated oxidation of boronates.
Incubation mixtures contained PBE (100 μM), MPO (15 nM), H2O2 (50 μM) and other agents, NO2− (500 μM), NaCl (0.15 M ), or GSH (500 μM), as indicated in phosphate buffer (pH 7.4, 100 mM) with DTPA (10 μM). Reaction was initiated by the addition of H2O2.
Products were measured 15 min after adding H2O2.
Discussion
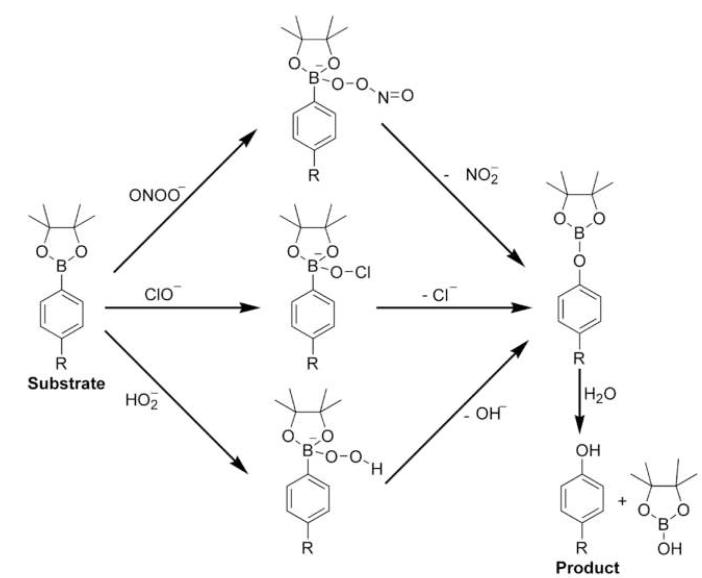
Here we have shown that ONOO− reacts directly with boronic compounds yielding the corresponding hydroxyl derivatives (phenols or alcohols) as final products. As compared to reactivity between ONOO− and other small organic molecules, the bimolecular rate constant (k = 106 M−1 s−1) measured for the reaction between ONOO− and boronates is very high. This high reactivity of boronic compounds toward ONOO−, as compared with other oxidants, makes them attractive candidates as potential traps and fluorescent probes for cellular imaging of ONOO−. From product analyses and substrate consumption studies, we conclude that boronates react with ONOO− at a 1:1 stoichiometry, yielding the corresponding phenol as a major product (80-85%) and possibly free radical intermediates and radical-derived products as minor products (<20%). We propose the reaction scheme in which the initial reaction occurs with the formation of the ONOO− adduct to the boronic compound that subsequently decomposes predominantly via a nonradical pathway forming the phenolic product (Fig. 10). HOCl and H2O2 react stoichiometrically with boronates yielding the corresponding phenol (Fig. 10), although at much slower rates as compared to ONOO− (Table 1).
Figure 10. Proposed mechanism of oxidation of boronates by ONOO−, HOCl, and H2O2.
Studies with X/XO and NONO-ates indicate that neither O •2− nor •NO react with boronates. We verified that nitrogen dioxide alone doesn’t convert the boronic compounds into phenols. ONOO−-mediated oxidation of PBE to 4′-hydroxyacetophenone was enhanced in the presence of low concentrations of GSH, and moderately attenuated at high concentrations of GSH (Fig. 8). The GSH-mediated increase in 4′-hydroxyacetophenone formation was oxygen-dependent and CAT-independent. This suggests that the reactive species derived from glutathionyl radical and molecular oxygen, but not glutathionyl radical per se, can oxidize boronic compounds to the corresponding phenols. The lack of the CAT effect also excludes the possible involvement of H2O2 that could be produced via the reaction of glutathiyl radical (GS•) radical with glutathiolate anion (GS−), with formation of glutathione disulfide radical anion (GSSG•−) and subsequent oxygen reduction leading to O •2− radical anion that dismutates to H2O2. Enhanced production of 4′-hydroxyacetophenone from PBE was also observed in incubations containing HCO −and GSH, possibly due to formation of GS• 3 radical.
Boronates react with ONOO− nearly a million times faster than with H2O2. The boronic acid group, wherein the boron atom is sp2-hybridized, is a very strong electrophile, and its reaction with a powerful nucleophile, ONOO−, is energetically favored. Nearly four decades ago, Keith and Powell reported that the decomposition of ONOO− was increased in borate buffer, which was attributed to a transperoxidation reaction between ONOO− and borate, forming a peroxyborate intermediate [28]. Recently, it was shown that peroxymonophosphate reacts with boronates much faster than does H2O2 [29]. In the case of oxidants studied in this paper, reaction timescales ranged from milliseconds (for ONOO−), seconds (for OCl−), and hours (for hydroperoxide anion, HOO−) (Fig. 1, Table 1). At physiological pHs (=7.4), the percentage of HOO− is 0.005% as compared to ONOO− (83%) and OCl− (46%). This is consistent with the pKa’s of H2O2 (11.7), ONOOH (6.7), and HOCl (7.47). This may partially explain the differences in the observed second-order rate constants for these species with boronates. Although HOCl reacts with boronates a hundred to a thousand times slower than ONOO−, this reaction may still be viable, because of its increased chemical stability. However, due to more rapid electrophilic reactions of HOCl with endogenous amines and thiols, it is rather unlikely that the boronic compounds can effectively compete for HOCl in the cellular systems. Moreover, the specific scavengers of HOCl (e.g., taurine) can be used to identify the nature of intracellular oxidant responsible for boronate oxidation.
The conversion of phenylboronic esters by ONOO−, HOCl, and H2O2 to the corresponding phenolic product is shown in Figure 10. The initial step of the reaction involves a bimolecular collision between the electrophilic boronate and the nucleophilic anion (ONOO−, OCl−, HOO−). With H2O2 and HOCl, nearly a 100% conversion of the boronate to the corresponding phenolic product occurs (Fig. 5). Additional products were not formed with H2O2; however, as shown in Figure 5, higher concentrations of HOCl caused a rapid decrease in the levels of phenolic product. This is attributed to formation of the corresponding chlorinated phenol. In the case of ONOO−, the yield of the phenolic product was about 85% (Fig. 5). The published reports on the reaction between ONOO− and carbonyl compounds and carbon dioxide indicate that the adducts formed decompose by a nonradical and radical pathway [13,30]. It is likely that a similar radical-mediated minor decomposition pathway occurs for the boronate/ONOO− adduct. Formation of dityrosyl type products strongly implicates a role for radical-mediated decomposition. We are currently investigating the radical-mediated minor pathway in detail. We believe that the proposed reaction pathway between boronates and ONOO−, OCl−, and HOO− is quite general, and should be applicable to many other boronates. The rapid direct reaction between arylboronates and ONOO−, as compared to H2O2, coupled with a nearly stoichiometric conversion of the adduct into phenolic product, make boronate-based fluorescent probes ideal candidates for cellular imaging of ONOO−. Potential boronate-based fluorescence probes, some of which have already been reported in the literature [20,31,32], are shown in Figure S1. It is likely that fluorescein-based boronates will be eminently suitable for detection and imaging of cellular ONOO− due to their high fluorescent quantum yields and suitable excitation/emission characteristics.
In conclusion, we have shown that several oxidants (ONOO−, HOCl, H2O2) react stoichiometrically at different rates with boronates, a class of organic compounds, which can be incorporated into several fluorescent probes, to generate the corresponding phenols. The present study opens up new possibilities for quantitative detection and inhibition of ONOO− in cells by using boronate-containing probes in biological systems.
Supplementary Material
Acknowledgements
This work was performed with the help of a grant funded by NHLBI (HL063119).
Abbreviations
- CAT
catalase
- CO2
carbon dioxide
- DTPA
diethylenetriaminepentaacetic acid
- EtBA
ethylboronic acid
- FBA
4-phenylalanine boronic acid
- GS•
glutathiyl radical
- GS−
glutathiolate anion
- GSH
glutathione
- GSSG•−
glutathione disulfide radical anion
- H2O2
hydrogen peroxide
- HCO3−
bicarbonate anion
- HOCl
hypochlorous acid
- HOO−
hydroperoxide anion
- MPO
myeloperoxidase
- •NO
nitric oxide
- •NO2
nitrogen dioxide radical
- NO2−
nitrite anion
- O2•−
superoxide radical anion
- OCl−
hypochlorite anion
- •OH
hydroxyl radical
- ONOO−
peroxynitrite
- ONOOCO2−
nitrosoperoxycarbonate anion
- ONOOH
peroxynitrous acid
- PAPA-NONOate
(Z)-1-[N-(3-aminopropyl)-N-(n-propyl)amino]diazen-1-ium-1,2-diolate
- PBA
4-acetylphenylboronic acid
- PBE
4-acetylphenylboronic acid pinacol ester
- SOD
superoxide dismutase
- X
xanthine
- XO
xanthine oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schopfer FJ, Baker PR, Freeman BA. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response. Trends Biochem. Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- [5].Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem. Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- [6].Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- [7].Radi R. Peroxynitrite and reactive nitrogen species: the contribution of ABB in two decades of research. Arch. Biochem. Biophys. 2009;484:111–113. doi: 10.1016/j.abb.2009.03.012. [DOI] [PubMed] [Google Scholar]
- [8].Augusto O, Gatti RM, Radi R. Spin-trapping studies of peroxynitrite decomposition and of 3-morpholinosydnonimine N-ethylcarbamide autooxidation: direct evidence for metal-independent formation of free radical intermediates. Arch. Biochem. Biophys. 1994;310:118–125. doi: 10.1006/abbi.1994.1147. [DOI] [PubMed] [Google Scholar]
- [9].Kaur H, Whiteman M, Halliwell B. Peroxynitrite-dependent aromatic hydroxylation and nitration of salicylate and phenylalanine. Is hydroxyl radical involved. Free Radic. Res. 1997;26:71–82. doi: 10.3109/10715769709097786. [DOI] [PubMed] [Google Scholar]
- [10].Wardman P. Methods to measure the reactivity of peroxynitrite-derived oxidants toward reduced fluoresceins and rhodamines. Methods Enzymol. 2008;441:261–282. doi: 10.1016/S0076-6879(08)01214-7. [DOI] [PubMed] [Google Scholar]
- [11].Lee J, Hunt JA, Groves JT. Manganese Porphyrins as Redox-Coupled Peroxynitrite Reductases. Journal of the American Chemical Society. 1998;120:6053–6061. [Google Scholar]
- [12].Masumoto H, Sies H. The reaction of ebselen with peroxynitrite. Chem. Res. Toxicol. 1996;9:262–267. doi: 10.1021/tx950115u. [DOI] [PubMed] [Google Scholar]
- [13].Uppu RM, Winston GW, Pryor WA. Reactions of peroxynitrite with aldehydes as probes for the reactive intermediates responsible for biological nitration. Chem. Res. Toxicol. 1997;10:1331–1337. doi: 10.1021/tx970056f. [DOI] [PubMed] [Google Scholar]
- [14].Palazzolo-Ballance AM, Suquet C, Hurst JK. Pathways for intracellular generation of oxidants and tyrosine nitration by a macrophage cell line. Biochemistry. 2007;46:7536–7548. doi: 10.1021/bi700123s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in cytokine-activated murine macrophages. Involvement of a peroxidase/nitrite pathway rather than peroxynitrite. J. Biol. Chem. 2001;276:34051–34058. doi: 10.1074/jbc.M100585200. [DOI] [PubMed] [Google Scholar]
- [16].Sampson JB, Ye Y, Rosen H, Beckman JS. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch. Biochem. Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- [17].Miller EW, Chang CJ. Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr. Opin. Chem. Biol. 2007;11:620–625. doi: 10.1016/j.cbpa.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao W. Lighting up H2O2: the molecule that is a “necessary evil” in the cell. Angew. Chem. Int. Ed Engl. 2009;48:3022–3024. doi: 10.1002/anie.200805651. [DOI] [PubMed] [Google Scholar]
- [19].Chang MC, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am. Chem. Soc. 2004;126:15392–15393. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 2007;3:263–267. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- [21].Kissner R, Beckman JS, Koppenol WH. Peroxynitrite studied by stopped-flow spectroscopy. Methods Enzymol. 1999;301:342–352. doi: 10.1016/s0076-6879(99)01098-8. [DOI] [PubMed] [Google Scholar]
- [22].Radi R. Kinetic analysis of reactivity of peroxynitrite with biomolecules. Methods Enzymol. 1996;269:354–366. doi: 10.1016/s0076-6879(96)69036-3. [DOI] [PubMed] [Google Scholar]
- [23].Bonini MG, Augusto O. Carbon dioxide stimulates the production of thiyl, sulfinyl, and disulfide radical anion from thiol oxidation by peroxynitrite. J. Biol. Chem. 2001;276:9749–9754. doi: 10.1074/jbc.M008456200. [DOI] [PubMed] [Google Scholar]
- [24].Lemercier JN, Padmaja S, Cueto R, Squadrito GL, Uppu RM, Pryor WA. Carbon dioxide modulation of hydroxylation and nitration of phenol by peroxynitrite. Arch. Biochem. Biophys. 1997;345:160–170. doi: 10.1006/abbi.1997.0240. [DOI] [PubMed] [Google Scholar]
- [25].Lymar SV, Jiang Q, Hurst JK. Mechanism of carbon dioxide-catalyzed oxidation of tyrosine by peroxynitrite. Biochemistry. 1996;35:7855–7861. doi: 10.1021/bi960331h. [DOI] [PubMed] [Google Scholar]
- [26].Lymar SV, Hurst JK. Carbon dioxide: physiological catalyst for peroxynitrite-mediated cellular damage or cellular protectant? Chem. Res. Toxicol. 1996;9:845–850. doi: 10.1021/tx960046z. [DOI] [PubMed] [Google Scholar]
- [27].Zhang H, Squadrito GL, Pryor WA. The mechanism of the peroxynitrite-carbon dioxide reaction probed using tyrosine. Nitric. Oxide. 1997;1:301–307. doi: 10.1006/niox.1997.0130. [DOI] [PubMed] [Google Scholar]
- [28].Keith WG, Powell RE. Kinetics of decomposition of peroxynitrous acid. J Chem Soc A. 1969:90. [Google Scholar]
- [29].LaButti JN, Gates KS. Biologically relevant chemical properties of peroxymonophosphate (=O3POOH) Bioorg. Med. Chem. Lett. 2009;19:218–221. doi: 10.1016/j.bmcl.2008.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Merenyi G, Lind J, Goldstein S. The rate of homolysis of adducts of peroxynitrite to the C=O double bond. J. Am. Chem. Soc. 2002;124:40–48. doi: 10.1021/ja011799x. [DOI] [PubMed] [Google Scholar]
- [31].DiCesare N, Lakowicz JR. Chalcone-analogue fluorescent probes for saccharides signaling using the boronic acid group. Tetrahedron Letters. 2002;43:2615–2618. doi: 10.1016/s0040-4039(02)00312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Du L, Li M, Zheng S, Wang B. Rational Design of a Fluorescent Hydrogen Peroxide Probe Based on the Umbelliferone Fluorophore. Tetrahedron Lett. 2008;49:3045–3048. doi: 10.1016/j.tetlet.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.