Abstract
Methods for detection and quantitation of hydroethidine (HE) and its oxidation products by HPLC analysis are described. Synthetic methods for preparations of authentic standards (2-hydroxyethidium and diethidium) are provided. Potential applications of the HPLC methods to chemical and biological systems are discussed. Specific examples of chromatograms obtained using UV-Vis absorption, fluorescence, electrochemical and mass spectrometry detectors are provided. The development of a dual electrochemical and fluorescence detection methodology and its applications are described. The HPLC-based method enables analyses of HE and its oxidation products such as ethidium and the dimeric products of HE. Ramifications of HPLC measurements of HE and its oxidation products in the detection and quantitation of 2-hydroxyethidium, the diagnostic marker product of superoxide and HE, in the intracellular milieu are discussed. Similarly, mitochondria-targeted HE conjugated to a triphenylphosphonium group (Mito-HE or Mito-SOX) also forms oxidation products (dimers of Mito-HE and Mito-E+) that can affect the detection and quantitation of 2-hydroxy-mito-ethidium, the diagnostic marker product of Mito-HE and superoxide in mitochondria.
Introduction
Hydroethidine (HE, Figure 1), also known as dihydroethidium (DHE), is a fluorogenic probe that has been used for almost 20 years in the detection of intracellular superoxide radical anion (O2•−). The red fluorescent product of HE reaction with superoxide has long been assumed to be the two-electron oxidation product, ethidium (E+). However, recent publications in well defined model systems suggest that 2-hydroxyethidium (2-OH-E+) is the sole reaction product of HE and O2•− [1–3]. Nonetheless, both fluorescent products (2-OH-E+ and E+) are typically formed in cells under oxidative conditions in different ratios. In general, E+ has been detected at a much higher level than 2-OH-E+ [2–4]. Due to their spectral overlap, it is extremely difficult to distinguish between the two products in biological systems using the fluorescence spectroscopy. Attempts to resolve the spectra and quantify selectively the product, 2-OH-E+, have been reported [5,6]. The quantitation of 2-OH-E+ using the fluorescence spectroscopy can also be confounded by its intracellular distribution such as binding to DNA and differential partitioning due to different hydrophobicity and/or pH, factors that influence the fluorescence quantum yield and position of the maxima of excitation/emission bands [4]. Increasing evidence suggests that intracellular quantitation of superoxide, based on fluorescence measurements in cellular systems, is inadequate and potentially erroneous [2,4,7,8]. Therefore, the detection and quantitation of 2-OH-E+ by HPLC separation of the fluorescent products in cell and tissue extracts has become the method of choice, with over 30 papers published in the past 4 years [1–5,7–33]. There are several modalities with which HE oxidation products can be detected. Although the fluorescence detection remains a favorite choice for quantitation of 2-OH-E+, the UV-Vis absorption, electrochemical or MS detection methods enable measurements of less fluorescent and non-fluorescent products of HE at a higher degree of sensitivity. These alternate detection methods have made it possible to detect and quantitate radical-mediated oxidative dimeric products of HE lacking fluorescence under the excitation/emission setup optimized for 2-OH-E+ [4]. Recently a mitochondria-targeted analog of HE (Mito-HE, MitoSOX™ Red, Figure 1) has been synthesized and is being widely used for the quantitation of mitochondria-derived superoxide [5,6]. Despite differences in the intracellular distribution of HE and Mito-HE, their chemical reactivity in redox reactions is very similar (Figure 2) [5,8]. Thus, we will focus in this review on the experiments utilizing HE with the understanding that most of the discussion concerning the utility and limitation of HE-based assay is also applicable to Mito-HE.
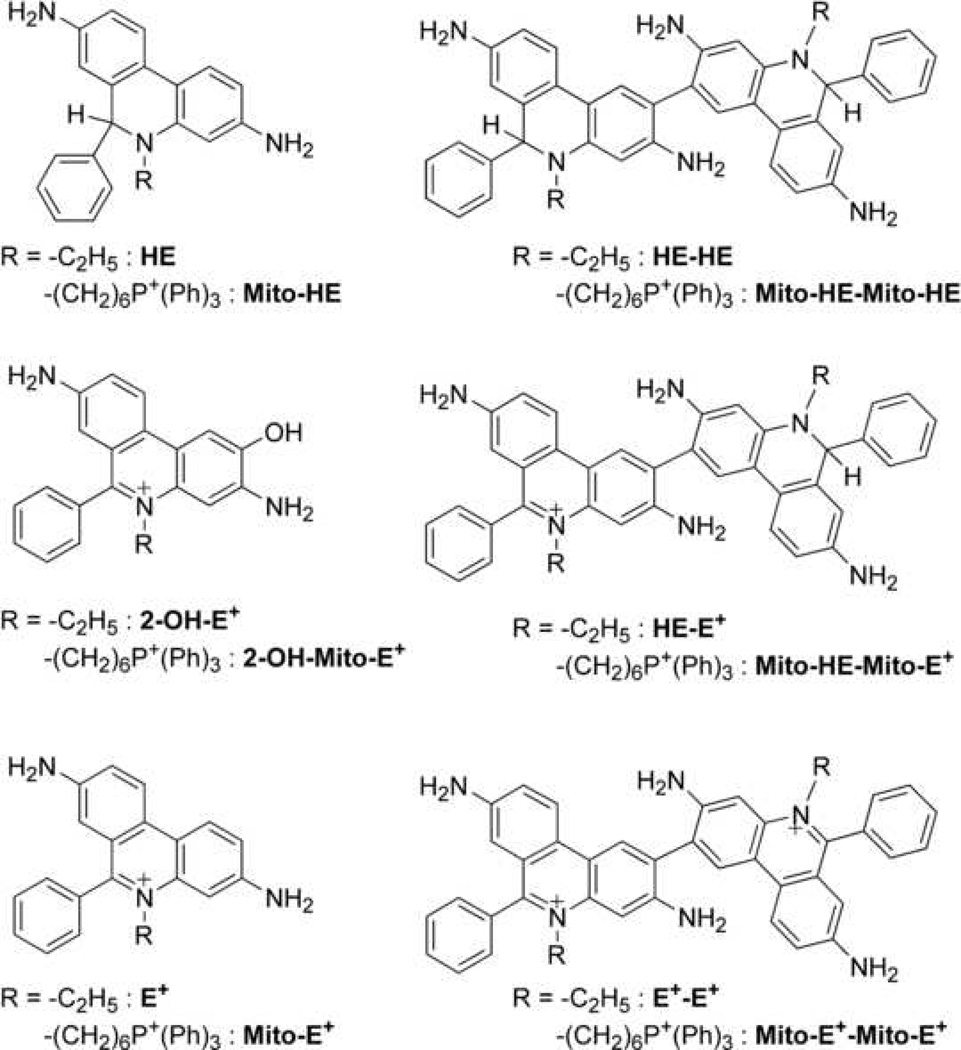
Figure 1.
Structures of HE and Mito-HE and their oxidation products.
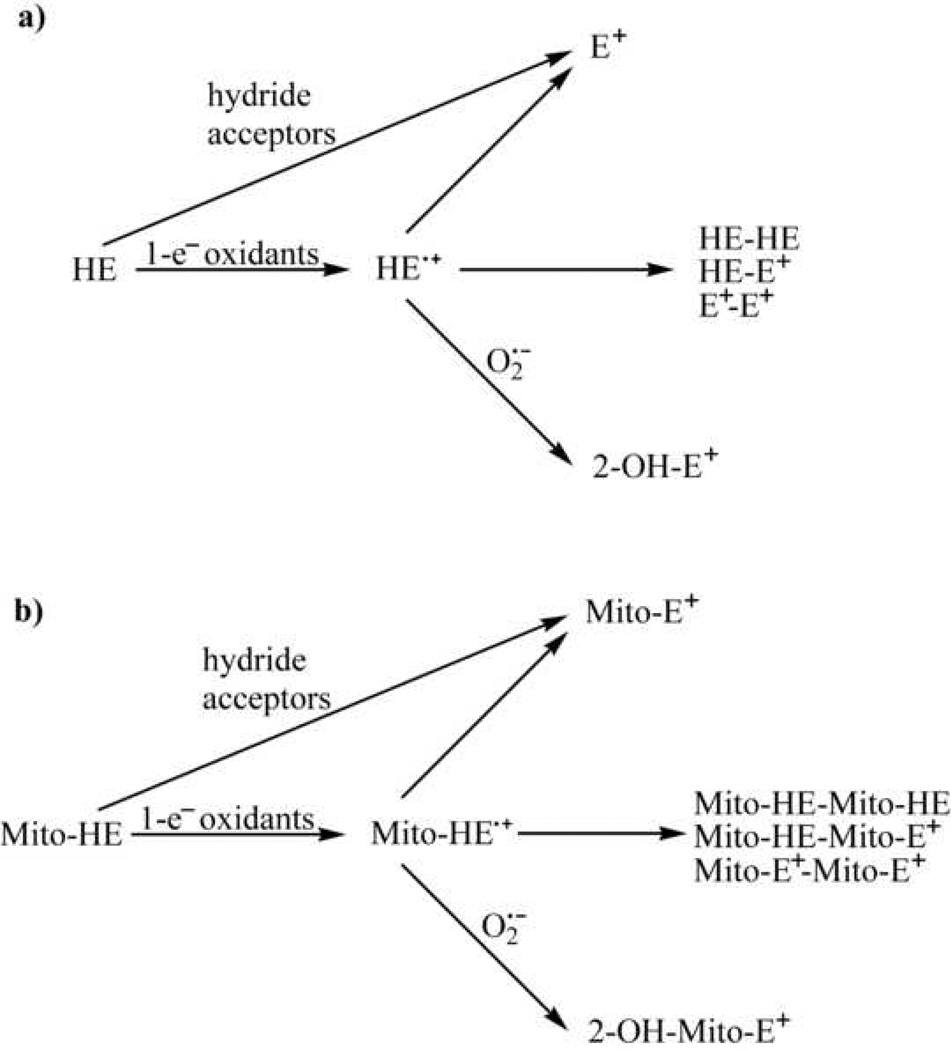
Figure 2.
Oxidation pathways of HE (a) and Mito-HE (b).
Principles
As both monomeric (2-OH-E+ and E+) and dimeric (HE-HE, HE-E+ and E+-E+) products of HE oxidation can be detected in extracts from cells incubated with HE, it is essential to understand their mechanisms of formation, taking into consideration the various oxidant-induced pathways of HE consumption.
The measurement of superoxide radical anion using the HE probe is based on its exclusive conversion to 2-OH-E+ that is highly specific for detecting superoxide in biological systems [2,4,34,35]. It was shown that the conversion of HE into 2-OH-E+ initially involves a one-electron oxidation of HE to the radical cation. However, this initial reaction step is induced by other one-electron oxidants, as has been shown in the case of hydroxyl radical (•OH) and dibromine radical anion (Br2•−), and therefore, is not particularly specific for superoxide (Figure 2) [10]. What makes this reaction product unique for superoxide is the subsequent step, i.e., the superoxide reaction with the HE radical species (radical cation or neutral radical) or Mito-HE radical, leading to formation of the final product, 2-hydroxyethidium or 2-hydroxy-mito-ethidium [10,20]. In the absence of superoxide, other one-electron oxidants can convert HE and Mito-HE into several dimeric products (e.g., HE-HE, HE-E+ and E+-E+ or their mitochondria-targeted analogs) [8]. As in the case of ferricyanide and ferricytochrome c, both dimeric products and E+ have been detected as the products, it is likely that the intermediate HE radical species can dismutate to form ethidium. Ethidium and mito-ethidium can be also formed in a process of direct hydride transfer from HE or Mito-HE to the appropriate hydride acceptor [10] without involving any radical intermediate.
On the basis of our current understanding of the chemistry of HE and Mito-HE, we can conclude that 2-OH-E+ and 2-OH-Mito-E+ formation in cells and tissues incubated with HE or Mito-HE should arise from the reaction with superoxide. On the other hand, the formation of dimeric products of HE or Mito-HE is indicative of the reaction with one-electron oxidants that oxidize HE or Mito-HE to its radical form. Formation of E+ and Mito-E+ is less informative, however. Both E+ and Mito-E+ can be formed during oxidation of HE or Mito-HE enzymatically, by hydride transfer or by a one-electron oxidation mechanism. While the exact nature of the one-electron oxidant cannot be derived from the structure of the dimeric product, it may include heme proteins (for example peroxidases and cytochrome c), peroxynitrite-derived oxidants or iron-derived oxidants [8,10,36]. In some cases, however, the exact nature of the oxidant could be established using selective inhibitors of the specific oxidation pathways.
The relatively high chemical reactivity of HE and Mito-HE, while enabling the detection of several oxidants, may be also a limiting factor in the detection of superoxide. High reactivity of the probe together with a high concentration of the co-oxidant generated inside the cells will lead to low steady-state intracellular levels of HE. Under this condition, HE can’t effectively compete with SOD (the rate constants of the reaction with superoxide are ca. 2 × 109 M−1s−1 for SOD [37] and ca. 2 × 106 M−1s−1 for HE [10] and ca. 4 × 106 M−1s−1 for Mito-HE [5]). While intracellular HE concentration is determined by the rate of the probe influx and the rate of HE consumption, agents capable of interfering with at least one of those factors may seriously compromise the ability of the probe to compete for superoxide and other oxidants. Therefore, monitoring intracellular concentration of HE or Mito-HE in each experiment is crucial and changes in HE or Mito-HE concentration should be taken into account for proper interpretation of the experimental results obtained for HE dimer, Mito-HE dimer or 2-OH-E+ and 2-OHMito-E+.
Materials
Hydroethidine (Fluka, cat. no. 37291)
Mito-HE (MitoSOX™ Red, Invitrogen, cat. no. M36008)
DMSO (Sigma, D2650)
Dulbecco’s PBS (Sigma, D8537)
Triton X-100 (Sigma-Aldrich, cat. no. T9284)
Ethidium bromide (Fluka, cat. no. 46065)
Perchloric acid, 70% (GFS Chemicals, item no. 67)
Methanol (Sigma-Aldrich, cat. no. 34860)
Ethanol (AAPER)
Phosphoric acid, 85% (J.T. Baker, cat. no. 0260-02)
Potassium phosphate, monobasic (Sigma-Aldrich, cat. no. P0662)
Potassium phosphate, dibasic (Fluka, cat. no. 60356)
DTPA (Fluka, cat. no. 32319)
Potassium bromide (Sigma-Aldrich, cat. no. 243418)
1-Butanol (Sigma-Aldrich, cat. no. 34867)
Acetonitrile (Sigma-Aldrich, cat. no. 34851)
Trifluoroacetic acid (Thermo Scientific, cat. no. 28903)
Formic acid (Riedel-de Haën, cat. no. 27001)
Potassium ferricyanide (Sigma-Aldrich, cat. no. 244023)
Potassium nitrosodisulfonate (Aldrich, cat. no. 220930)
Tetrachloro-1,4-benzoquinone (Fluka, cat. no. 23280)
Bradford reagent (Sigma-Aldrich, cat. no. B6916) or other reagent for determination of protein concentration in cell lysate
Bovine serum albumin (Sigma, cat. no. A8806)
Protocol
Synthesis of 2-hydroxyethidium (2–3 days)
2-Hydroxyethidium is synthesized by reacting HE with nitrosodisulfonate (NDS) using two moles of NDS per one mole of HE [20]. For a large scale synthesis, HE can be synthesized by reduction of E+ as described previously [20,38].
Prepare 0.5 M phosphate buffer pH 7.4.
Prepare 1.0 mM DTPA in water.
Prepare 10 ml of 1 mM NDS in 50 mM phosphate pH 7.4, 100 µM DTPA. The concentration of NDS should be determined by spectrophotometry using the extinction coefficients values of 1690 M−1cm−1 (at 248 nm) and 20.8 M−1cm−1 (at 545 nm) [20].
Prepare 20 mM HE in DMSO. The concentration should be determined by spectrophotometry using the extinction coefficients values of 1.8 · 104 M−1cm−1 (at 265 nm) and 9.75 · 103 M−1cm−1 (at 345 nm) [20]. For that purpose to the quartz cuvette containing 998 µl aqueous solution of 50 mM phosphate buffer pH 7.4 and 100 µM DTPA add 2.5 µl of 20 mM HE in DMSO, mix by inversion and collect the spectrum. Subtract the spectrum of the corresponding mixture with added pure DMSO instead of HE stock solution and determine the absorbance values at 265 and 345 nm for calculating the HE concentration.
-
To the glass vial add:
1 ml of 0.5 M phosphate buffer, pH 7.4
1 ml of 1 mM DTPA
6 ml of water
2 ml of 1 mM NDS
Mix the solution and add 50 µl of 20 mM solution of HE in DMSO.
Incubate the mixture at room temperature for 1 h.
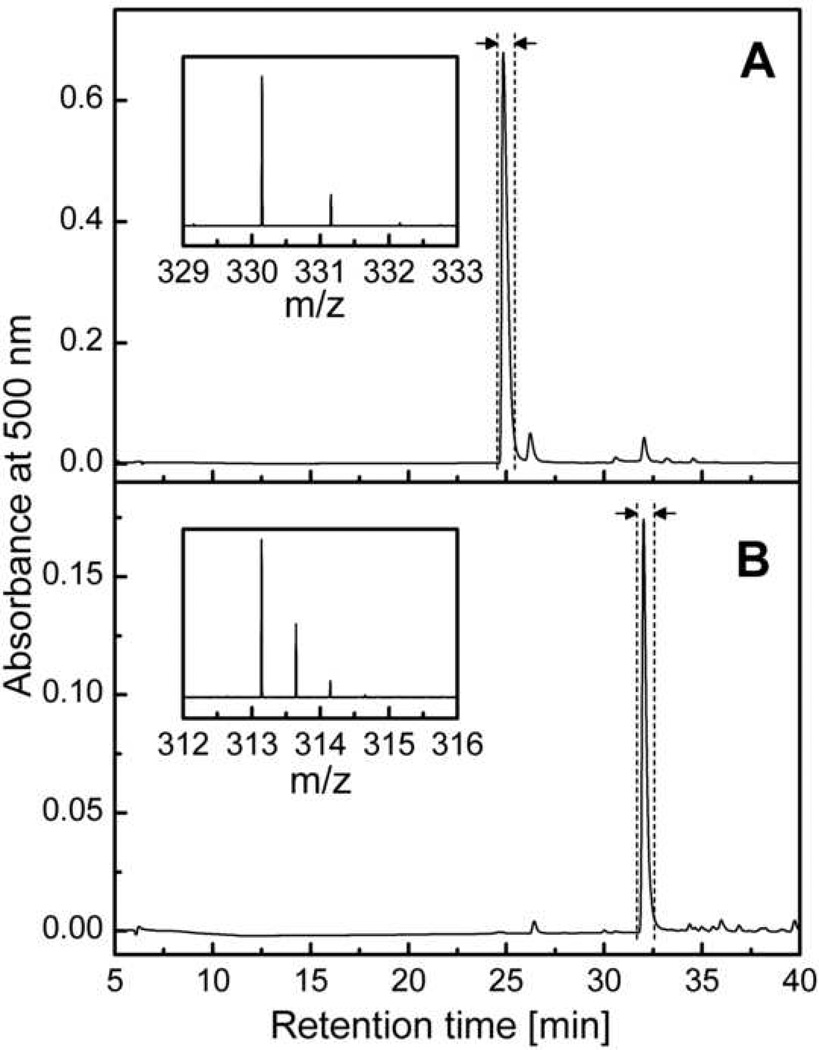
The reaction mixture contains 2-hydroxyethidium as the major product with a small amount of ethidium as a contaminating by-product (Figure 3A). The purification of the crude 2-hydroxyethidium can be achieved by silica-gel low-pressure chromatography, using the C18 solid phase extraction cartridges or by HPLC. The methodology for 2- hydroxyethidium purification has been described previously [4,20]. Below is a description of the HPLC method of purification of 2-hydroxyethidium. The products of HE oxidation are separated on a semi-preparative C18 column (Beckmann Ultrasphere, 250 mm × 10 mm, 5 µm), using a gradient of acetonitrile/water mixture containing 0.1% TFA under the flow rate of 2.4 ml/min (Table 1). Under the HPLC conditions shown in Figure 3A, the 2-hydroxyethidium was found to elute at approximately 25 min after the injection. The fraction collected should be analyzed by mass spectrometry to confirm the identity of the pure product (see inset in Figure 3A, m/z = 330.16, the intervals between the isotopic peaks should be 1.0 indicating a z value of 1). Please note that the fraction collected is 2-hydroxyethidium dissolved in a mixture of water and acetonitrile containing 0.1% TFA. One can lyophilize the fractions collected to obtain the salt of 2-hydroxyethidium cation and trifluoroacetate anion. The concentration of 2-OH-E+ in water at pH 7.4 is determined by spectrophotometry using the absorbance at 470 nm and the extinction coefficient of 1.2 ·104 M−1cm−1 [7].
Figure 3.
HPLC chromatograms collected during purification of 2-OH-E+ (A) and E+-E+ (B) on semi-preparative column Beckmann Ultrasphere (250 mm × 10 mm) using the gradient described in Table 1 and a flow rate of 2.4 ml/min. The vertical dashed lines and arrows mark the beginning and end of the fraction collection. Insets show the MS spectra of the collected fractions.
Table 1.
The gradient elution conditions used for purification of the oxidation products of HE and Mito-HE by semi-preparative HPLC.
| Time [min] |
0.1% TFA in water |
0.1% TFA in MeCN |
|
|---|---|---|---|
| HE | 0 | 90% | 10% |
| 46 | 30% | 70% | |
| 50 | 0% | 100% | |
| 55 | 0% | 100% | |
| 60 | 90% | 10% | |
| Mito-HE | 0 | 60% | 40% |
| 30 | 45% | 55% | |
| 40 | 0% | 100% | |
| 50 | 0% | 100% | |
| 55 | 60% | 40% | |
Synthesis of 2-hydroxy-mito-ethidium (2–3 days)
2-Hydroxy-mito-ethidium can be synthesized by a similar protocol as described above for 2-hydroxyethidium using NDS [4,5,8] except for the following changes:
In step 4, due to the availability of Mito-HE in vials containing 50 µg of the compound, the concentration of Mito-HE in DMSO should be ca. 5 mM. The concentration should be determined by spectrophotometry in a similar way as for HE, but using the extinction coefficients values of 1.6 · 104 M−1cm−1 (at 267 nm) and 7.5 · 103 M−1cm−1 (at 355 nm) [4].
In step 5, instead of 50 µl of 20 mM HE, use 200 µl of 5 mM Mito-HE.
As in the case of HE, there is a small contamination of the product by non-hydroxylated cation (Mito-E+) and the product should be purified by HPLC. For that purpose the reaction mixture is initially extracted with n-BuOH and the organic extract dried as follows:
Transfer the reaction mixture into two 15 ml tubes (5 ml per tube).
Add ca. 20 mg of KBr to each tube.
Add 3 ml of n-BuOH to each tube, close the tubes and vortex for 1 min.
Leave the tubes for 10 min or centrifuge the tubes (3 min at 1,000 g) to obtain separated layers of aqueous (lower) and organic (upper) phases.
Transfer the upper (organic) layer carefully to a new glass vial (combine the extracts from both tubes) using a glass Pasteur pipette.
Repeat the steps 3–4 two more times and combine all the extracts together.
Evaporate n-BuOH under air or nitrogen flux or using rotary evaporator with the temperature set at 40 °C.
Redissolve the dry residue in 1 ml of ethanol and dilute with 9 ml of water containing 0.1% TFA. 2-Hydroxy-mito-ethidium is separated from mito-ethidium using the same HPLC conditions as described for 2-hydroxyethidium purification, but using a different gradient (Table 1). The fraction collected should be analyzed by mass spectrometry to confirm the identity of the pure product (m/z = 326.66, the intervals between the isotopic peaks should be 0.5 indicating a z value of 2; deprotonated form: m/z = 646.30, the intervals between the isotopic peaks should be 1.0 indicating a z value of 1). The final product can be lyophilized and stored at 4 °C as a trifluoroacetate salt. The concentration of 2-OH-Mito-E+ after dissolving in water can be determined at pH 7.4 by spectrophotometry using the absorbance at 478 nm and the extinction coefficient of 9.4 ·103 M−1cm−1 [5].
Synthesis of mito-ethidium (2–3 days)
Mito-ethidium can by synthesized by reacting Mito-HE with chloranil (tetrachloro-1,4-benzoquinone) [4,8] as follows:
Prepare 10 ml of 2 mM solution of chloranil in methanol.
Prepare 5 mM solution of Mito-HE in DMSO.
Prepare 0.5 M solution of phosphate buffer pH 7.4.
Prepare 1.0 mM solution of DTPA in water.
-
To the glass vial add:
1.3 ml of 0.5 M phosphate buffer, pH 7.4
1.3 ml of 1 mM DTPA
10 ml of water
33 µl of 2 mM chloranil in MeOH
Mix the solution and add 131.6 µl of 5 mM HE in DMSO.
Incubate the mixture at room temperature for 4 h.
This reaction mixture contains mito-ethidium as a major product. Mito-E+ can be extracted from the reaction mixture using n-BuOH and purified by HPLC by the same method as described above for 2-OH-Mito-E+. The identity of the pure product should be confirmed by mass spectrometry (m/z = 315.66, the intervals between the isotopic peaks should be 0.5 indicating a z value of 2; deprotonated form: m/z = 630.31, the intervals between the isotopic peaks should be 1.0 indicating a z value of 1). The obtained product can be lyophilized and stored at 4 °C as the trifluoroacetate salt. The concentration of Mito-E+ after dissolving in water can be determined at pH 7.4 by spectrophotometry using the absorbance at 488 nm and the extinction coefficient of 5.8 ·103 M−1cm−1 [5].
Synthesis of diethidium (2–3 days)
The synthesis of diethidium is based on the oxidation of hydroethidine by potassium ferricyanide [8] as follows:
Prepare 10 ml of 1 M solution of K3Fe(CN)6 in water.
Prepare 20 mM solution of HE in DMSO.
Prepare 0.5 M solution of phosphate buffer pH 7.4.
Prepare 1.0 mM solution of DTPA in water.
-
To the glass vial add:
1 ml of 0.5 M phosphate buffer, pH 7.4
1 ml of 1 mM DTPA
8 ml of 1 M K3Fe(CN)6
Mix the solution and add 50 µl of 20 mM HE in DMSO.
Incubate the mixture at room temperature for 1 h.
The reaction mixture thus obtained contains diethidium as a major product along with other oxidation products. The pure form of diethidium can be isolated using a HPLC equipped with a fraction collector and a semi-preparative column. However, due to the high concentration of K3Fe(CN)6 in the reaction mixture, it is desirable to extract the product from the reaction mixture containing the ferricyanide so as to avoid precipitation of ferricyanide inside the HPLC column. The extraction can be carried out using n-butanol (n-BuOH) as described above for the preparation of 2-hydroxy-mito-ethidium.
The procedure for purification of diethidium by HPLC is similar to the one described above for 2-hydroxyethidium (Table 1), except for its retention time (Figure 3B). The identity of the collected compound should be confirmed by MS analysis (see inset in Figure 3B, m/z = 313.15, the intervals between the isotopic peaks should be 0.5 indicating z value of 2). As with 2-OH-E+, the collected fractions can be lyophilized to obtain the solid salt of diethidium trifluoroacetate. After dissolving in the aqueous solution of phosphate buffer (pH 7.4), the concentration of diethidium can be determined by spectrophotometry using an extinction coefficient of 1.2 · 104 M−1cm−1 at 497 nm.
Synthesis of di-mito-ethidium (2–3 days)
The synthesis of di-mito-ethidium (Mito-E+-Mito-E+) is based on the oxidation of Mito-HE by potassium ferricyanide [8] as follows:
Prepare 10 mM solution of K3Fe(CN)6 in water.
Prepare 5 mM solution of Mito-HE in DMSO.
Prepare 0.5 M solution of phosphate buffer pH 7.4.
Prepare 1.0 mM solution of DTPA in water.
-
To the glass vial add:
1 ml of 0.5 M phosphate buffer, pH 7.4
1 ml of 1 mM DTPA
8 ml of water
150 µl of 10 mM K3Fe(CN)6
Mix the solution and add 100 µl of 5 mM Mito-HE in DMSO.
Incubate the mixture at room temperature for 15 min.
The reaction mixture thus obtained contains di-mito-ethidium as a major product along with other oxidation products. Mito-E+-Mito-E+ can be extracted from the reaction mixture using n-BuOH and purified by HPLC by the same method as described above for 2-OH-Mito-E+. The identity of the pure product should be confirmed by mass spectrometry analysis (m/z = 315.15, the intervals between the isotopic peaks should be 0.25 indicating a z value of 4; deprotonated form: m/z = 419.87, the intervals between the isotopic peaks should be 0.33 indicating a z value of 3). The product can be lyophilized and stored at 4 °C as the trifluoroacetate salt.
Cell experiment (2–4 hours)
Incubate BAEC or other cells of interest in medium containing 10 µM HE for 20 – 60 minutes.
Stop the incubation by replacing the medium with equivalent volume of ice-cold DPBS.
Remove DPBS and scrape the cells in 1 ml of ice-cold DPBS.
Transfer the cells suspension into an Eppendorf tube (1.5– 2 ml) and place the tube on ice.
Centrifuge the cell suspension to pellet the cells: 5 min × 1000 g at 4 °C.
Remove the supernatants and freeze the cell pellets and store at or below −80 °C or immediately process the samples for HPLC analysis.
Preparation of the sample for HPLC analysis (4–5 hours)
Add 150–200 µl of 0.1% Triton X-100 in DPBS to the tube with the cell pellet.
Lyse the cells by aspirating and dispensing back of the suspension using the insulin syringe with a needle. Repeat the step 10 times.
Transfer 2–10 µl (depending on the expected protein concentration and protein assay used) of the cell lysate into an empty tube for the determination of the protein concentration in the cell lysate. For each sample the protein concentration should be determined at least twice to check the experimental reproducibility.
Transfer 100 µl of the cell lysate to the tube containing 100 µl of 0.2 M solution of HClO4 in MeOH, vortex the mixture and place on ice. Leave the mixture on ice for 1–2 hrs to allow protein precipitation.
After incubating on ice centrifuge the samples for 30 min at 20,000 g at 4 °C.
Transfer 100 µl of the supernatant into a tube containing 100 µl of 1 M solution of potassium phosphate buffer pH 2.6 and vortex.
Centrifuge the samples for 15 min at 20,000 g at 4 °C.
Transfer the supernatant into HPLC vial equipped with 200-µl conical insert, seal the vial and place the sample back on ice.
Place the samples in the refrigerated autosampler and run the analyses.
HPLC analysis (60–95 min per sample)
The separation of HE and its oxidation products by HPLC is based on the consecutive elution of the analytes using a gradient of acidified acetonitrile/water mixture from low (mobile phase A) to high (mobile phase B) concentration of acetonitrile. The conditions of the HPLC analyses, as routinely used in the authors’ laboratory are shown in Table 2 for HE and Table 3 for Mito-HE.
Table 2.
The instrumental setup for the analysis of HE and its oxidation products by HPLC with different detectors.
| UV-Vis absorption and fluorescence detection |
Electrochemical detection |
MS detection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mobile phase A | 0.1% TFA in water | 50 mM potassium phosphate buffer pH 2.6; 10% MeCN; water | 0.1% formic acid in water | |||||||
| Mobile phase B | 0.1% TFA in MeCN | 50 mM potassium phosphate buffer pH 2.6; 60% MeCN; water | 0.1% formic acid in MeCN | |||||||
| Column | Kromasil C18 250 mm × 4.6 mm 5 µm, 100 Å | Synergi Polar RP 250 mm × 4.6 mm 4 µm, 80 Å | Synergi Polar RP 250 mm × 2.0 mm 4 µm, 80 Å | |||||||
| Flow rate | 0.5 ml/min | 0.5 ml/min | 0.1 ml/min | |||||||
| Typical injection volume | 50 µl | 50 µl | 20 µl | |||||||
| Gradient | Time [min] |
A [%] |
B [%] |
Time [min] |
A [%] |
B [%] |
Time [min] |
A [%] |
B [%] |
|
| 0 | 90 | 10 | 0 | 60 | 40 | 0 | 80 | 20 | ||
| 46 | 30 | 70 | 30 | 0 | 100 | 60 | 0 | 100 | ||
| 47 | 0 | 100 | 40 | 0 | 100 | 70 | 0 | 100 | ||
| 55 | 0 | 100 | 45 | 60 | 40 | 75 | 80 | 20 | ||
| 60 | 90 | 10 | 60 | 60 | 40 | 95 | 80 | 20 | ||
| 75 | 90 | 10 | ||||||||
| Detector settings | UV-Vis absorption (diode array) detector: 220, 242, 290, 370, 500 nm | Potentials on coulometric electrodes: 0, 200, 280, 365, 400, 450, 500, 600 mV | Total ionic current collected over the range of m/z values of 100 – 1000 Positive ions detection mode. |
|||||||
| Fluorescence detector: | ||||||||||
| Time [min] |
λexc [nm] |
λemi(1) [nm] |
λemi(2) [nm] |
|||||||
| In case of dual detection the potentials on the electrodes are: 0, 220, 320, 450, 500, 600 and the fluorescence parameters are as follows: 0–17 min − λexc = 358 nm, λemi = 440 nm; 17–45 min − λexc = 490 nm, λemi = 565 nm. | ||||||||||
| 0–25 | 358 | 440 | 450 | |||||||
| 25–60 | 490 | 567 | 596 | |||||||
Table 3.
The instrumental setup for the analysis of Mito-HE and its oxidation products by HPLC with different detectors.
| UV-Vis absorption and fluorescence detection |
Electrochemical detection |
|||||
|---|---|---|---|---|---|---|
| Mobile phase A | 0.1% TFA in water | 50 mM potassium phosphate buffer pH 2.6; 10% MeCN; water | ||||
| Mobile phase B | 0.1% TFA in MeCN | 50 mM potassium phosphate buffer pH 2.6; 60% MeCN; water | ||||
| Column | Kromasil C18 250 mm × 4.6 mm 5 µm, 100 Å | Synergi Polar RP 250 mm × 4.6 mm 4 µm, 80 Å | ||||
| Flow rate | 0.5 ml/min | 0.5 ml/min | ||||
| Typical injection volume | 50 µl | 50 µl | ||||
| Gradient | Time [min] |
A [%] |
B [%] |
Time [min] |
A [%] |
B [%] |
| 0 | 80 | 20 | 0 | 35 | 65 | |
| 10 | 55 | 45 | 30 | 0 | 100 | |
| 30 | 45 | 55 | 50 | 0 | 100 | |
| 40 | 0 | 100 | 55 | 35 | 65 | |
| 46 | 0 | 100 | 70 | 35 | 65 | |
| 50 | 80 | 20 | ||||
| 65 | 80 | 20 | ||||
| Detector settings | UV-Vis absorption (diode array) detector: 220, 270, 290, 365, 500 nm | Potentials on coulometric electrodes: 0, 200, 280, 365, 400, 450, 500, 600 mV | ||||
| Fluorescence detector: λexc = 510 nm, λemi = 595 nm | ||||||
a) HPLC with absorbance/fluorescence detection (HPLC-Abs/Fl)
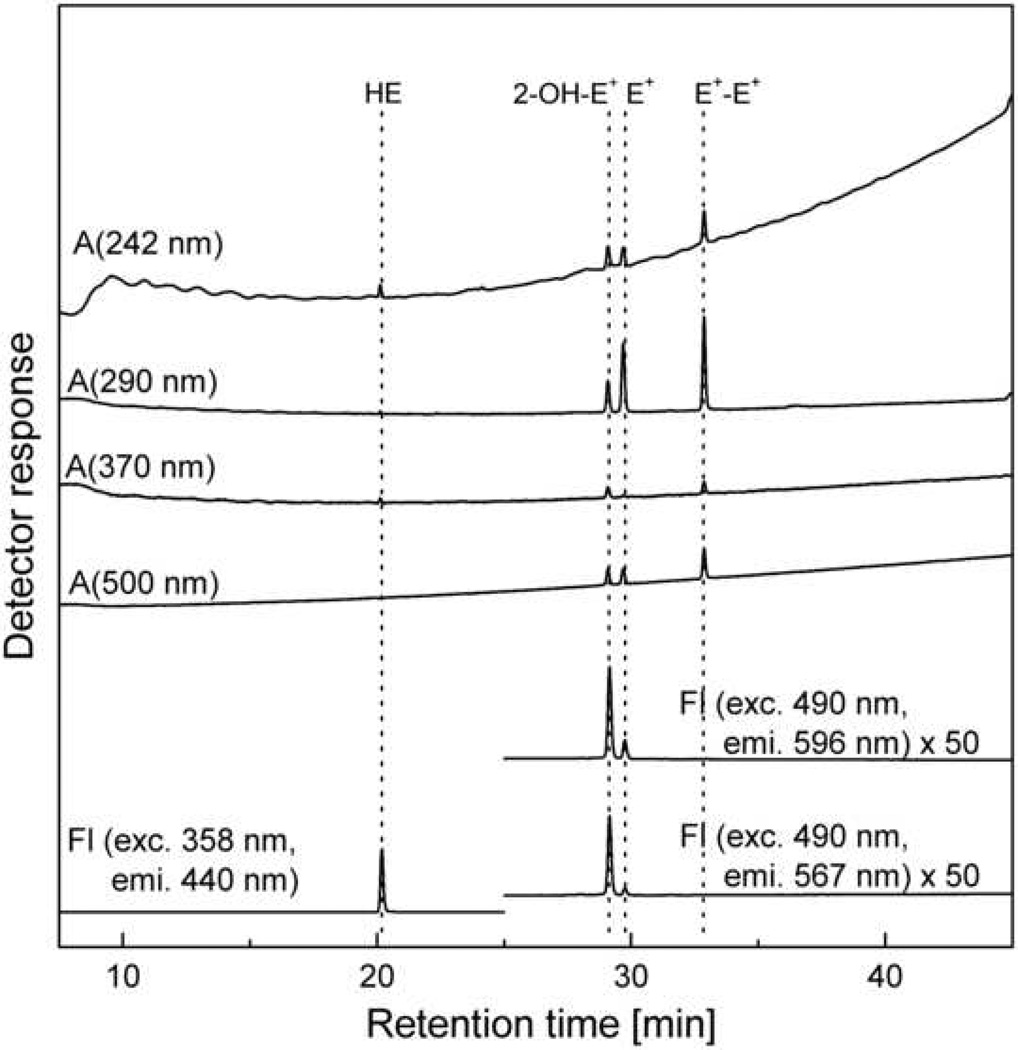
The chromatogram of the mixture of HE, 2-OH-E+, E+ and E+-E+ obtained using the HPLC system equipped with both UV-Vis absorption and fluorescence detectors is shown in Figure 4 and for Mito-HE and its oxidation products in Figure 7A. The setup of HPLC with an UV-Vis absorption and fluorescence detection has been published elsewhere [2,8,20]. The detectors are configured in a series such that the eluent flows initially through the absorption detector and then through the fluorescence detector. Here we show that by taking advantage of the wavelength programming capability of modern fluorescence detectors, one can detect HE as well as the 2-OH-E+ and E+ with a high degree of sensitivity. Under the conditions used (Fig. 4), the signal intensity of HE is nearly 2 orders of magnitude higher than those of 2-OH-E+ and E+ (Figure 4). The fluorescence parameters for 2-hydroxyethidium were modified slightly in order to improve the sensitivity of detection.
Figure 4.
Chromatogram (HPLC-Abs/Fl) of the mixture of HE, 2-OH-E+, E+ and E+-E+ (1 µM each) in aqueous solution of 0.1 M potassium phosphate pH 2.6 containing 25% MeOH. All conditions are as shown in Table 2 except the injection volume was decreased to 5 µl to avoid saturation of the fluorescence detector due to HE signal. The intensity of fluorescence signal of 2-OH-E+ and E+ (25 – 45 min) has been multiplied by 50 to appear on the same intensity scale as the HE fluorescence signal.
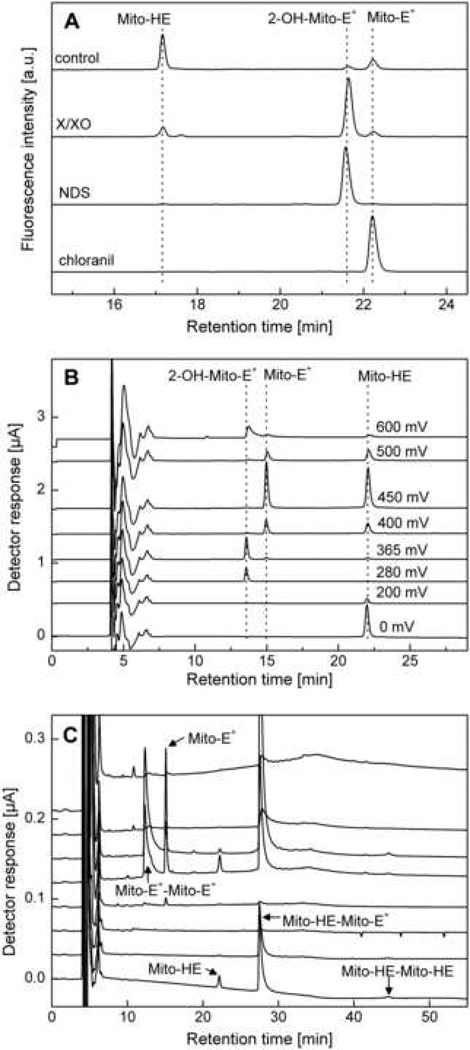
Figure 7.
HPLC analyses of Mito-HE and its oxidation products. (A) HPLC-absorbance/fluorescence analysis (as described in Table 3) of products formed from Mito-HE (50 µM) incubated without any additive (control) or with xanthine (1 mM)/xanthine oxidase (2 mU/ml) (X/XO) or nitrosodisulfonate (NDS, 100 µM) or chloranil (25 µM) for 1 h in phosphate buffer (50 mM, pH 7.4) containing DTPA (0.1 mM). Samples were analyzed following dilution (1:1) with ice-cold phosphate buffer (1 M, pH 2.6). (B) HPLC-EC chromatogram of a mixture of Mito-HE, 2-OH-Mito-E+ and Mito-E+ (ca. 1 µM each) in a phosphate buffer pH 2.6 (saturated solution) containing 25% (by vol.) methanol. (C) HPLC-EC chromatogram of a mixture of Mito-HE (50 µM) and potassium ferricyanide (100 µM) incubated for 15 min in phosphate buffer (50 mM, pH 7.4) containing DTPA (0.1 mM). Prior to analysis, the sample was diluted (1:9) with ice-cold phosphate buffer (1 M, pH 2.6). (Figure adapted from ref. [8])
The mobile phase for the HPLC system with UV-Vis absorption and fluorescence detectors is prepared as follows:
Mobile phase A: Mix 1 l of water with 1 ml of TFA.
Mobile phase B: Mix 1 l of acetonitrile (MeCN) with 1 ml of TFA.
b) HPLC with electrochemical detection (HPLC-EC)
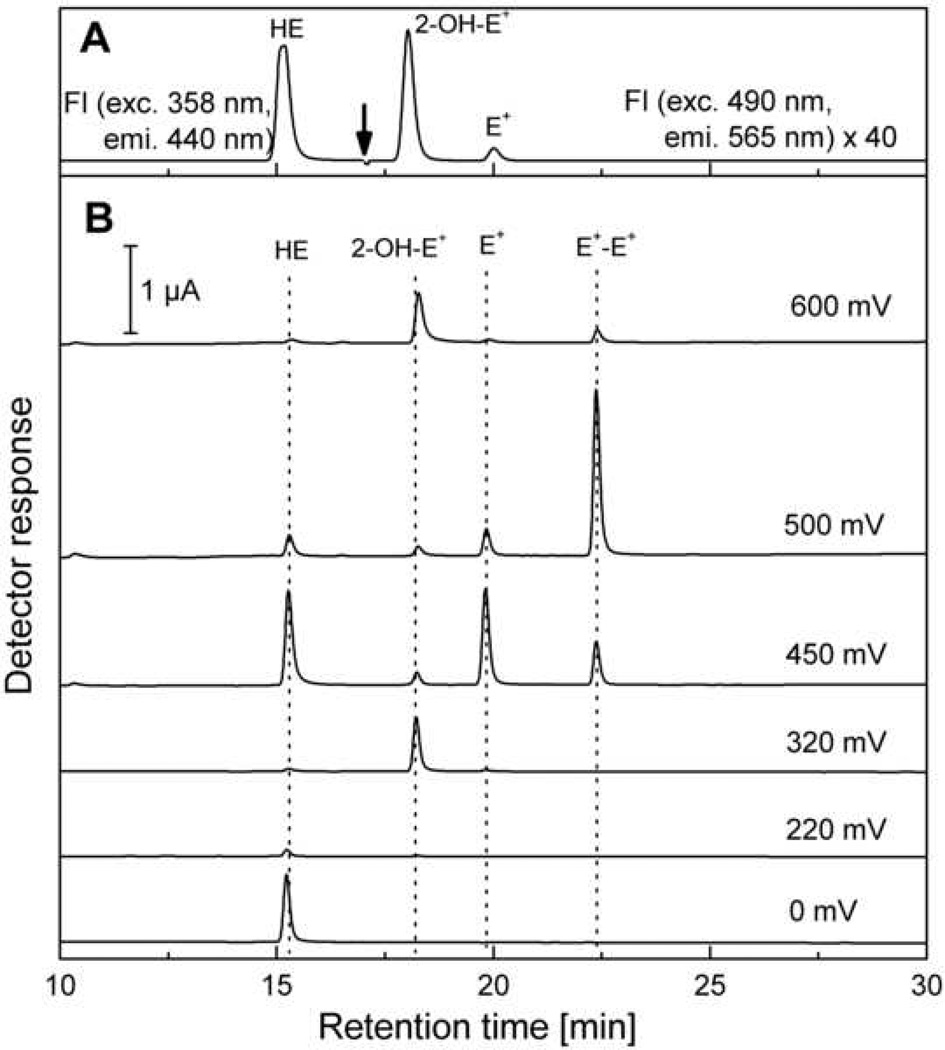
Figure 5 shows the chromatogram of an equimolar mixture of HE, 2-OH-E+, E+ and E+-E+ obtained using the HPLC system equipped with electrochemical (coulometric) and fluorescence detectors. The HPLC-EC chromatogram of an equimolar mixture of Mito-HE, 2-OH-Mito-E+ and Mito-E+ is shown in Figure 7B and of the reaction mixture of Mito-HE with potassium ferricyanide in Figure 7C. The HPLC-EC instrument setup is shown in Table 2 for single electrochemical [7,8,10] as well as for dual electrochemical/fluorescence options. Due to the destructive nature of the coulometric detection (i.e., 100% of the analyte undergoes oxidation), the fluorescence detector cannot be placed in a series with electrochemical detectors. Instead, the flow leaving from the column is split into two detectors. As discussed above, the fluorescence detector can be programmed so that both HE and 2-OH-E+/E+ can be detected with high sensitivity. One of the advantages of dual detection is that even with co-eluting materials, there is a high likelihood that their electrochemical and fluorescence properties are different. By the same token, obtaining the same values of the concentration of analytes using both modes of detection is a very good indicator of purity of the eluting compound. In addition, the dual detection enables the quantitation of several analytes in a single HPLC run. For example, we were able to monitor doxorubicin uptake using the fluorescence detector from cells treated with HE and doxorubicin (unpublished data).
Figure 5.
HPLC-EC/FL chromatogram of the mixture of standards (as desribed for the Figure 4). The chromatographic conditions are the same as described in Table 2 for HPLC with dual electrochemical/fluorescence detection. Panel A shows the fluorescence signal and the arrow indicates the time at which the fluorescence detector settings have been changed. The intensity of fluorescence signal of 2-OH-E+ and E+ (17 – 30 min) has been multiplied by 40 to appear on the same intensity scale as HE fluorescence signal. Panel B shows the signals detected on CoulArray electrochemical detector.
With regard to the HPLC with electrochemical detection technique, the water used for preparing the mobile phase should first be passed through a C18 cartridge (for example Alltech Prevail SPE cartridge) to remove the traces of organic contaminants that can be present even after purification using a typical Millipore or an equivalent system. In all the procedures described below for HPLC-EC, the same purification method should be employed for water preparation. Instead of TFA the acidic phosphate buffer is used in the mobile phase for HPLC-EC. Described below is the procedure for preparation of the mobile phase for HPLC-EC system.
0.2 M phosphate buffer pH 2.6. To the 1 l volumetric flask add:
19.05 g KH2PO4
4.1 ml H3PO4 (85%)
Water (prepared as described above) up to 1 l
Filter the solution using vacuum filtering system and 0.2 µm filter.
Check the pH of the solution after dilution to obtain 50 mM phosphate buffer. The pH value should be within the range 2.5 – 2.7.
Mobile phase A: To the 1 l volumetric flask add:
250 ml 0.2 M phosphate buffer pH 2.6
500 ml water
100 ml acetonitrile
Add water up to 1 l
Filter the solution using vacuum filtering system and 0.2 µm filter. Keep the mobile phase refrigerated until use.
Mobile phase B: To the 1 l volumetric flask add:
250 ml 0.2 M phosphate buffer pH 2.6
140 ml water
600 ml acetonitrile
Add water up to 1 l
Filter the solution using vacuum filtering system and 0.2 µm filter. Keep the mobile phase refrigerated until use.
c) HPLC with MS detection (HPLC-MS)
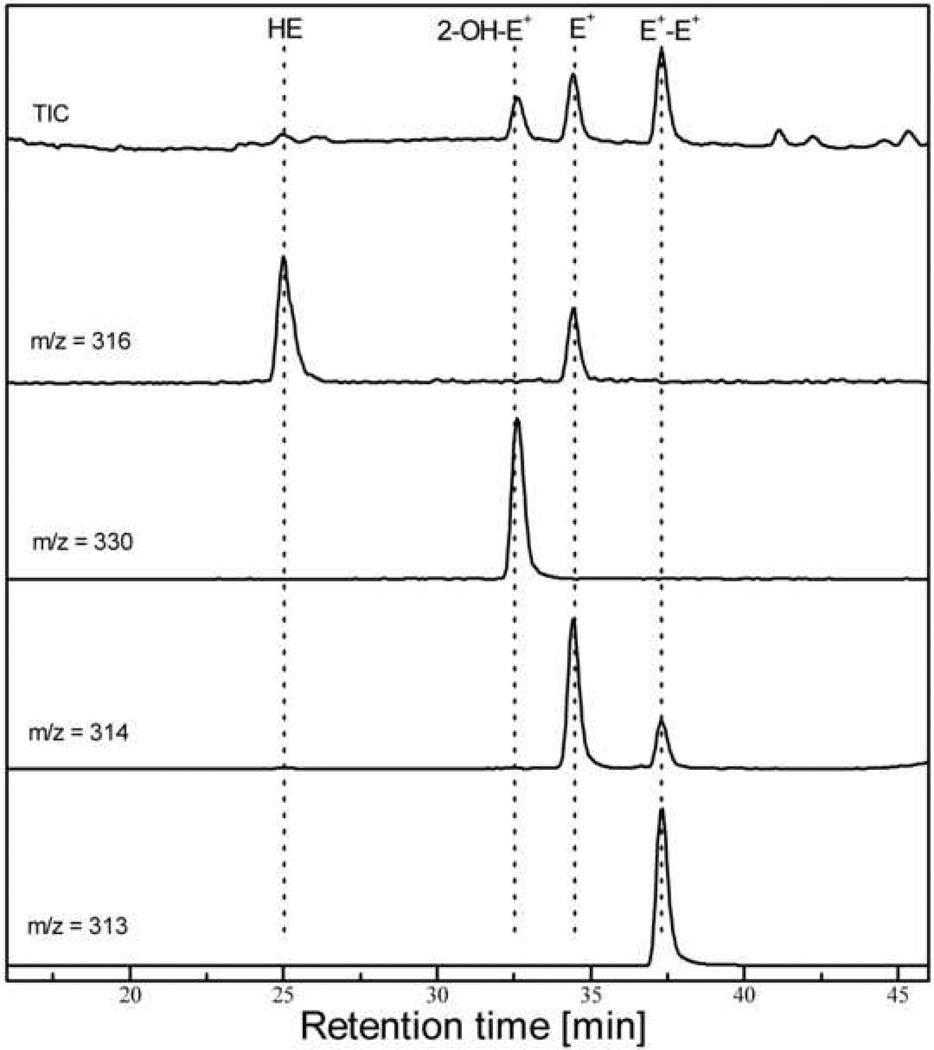
The HPLC-MS chromatogram of the mixture of HE, 2-OH-E+, E+ and E+-E+ is shown in Figure 6. As mass spectrometry detection is based on the mass to charge ratio (m/z), the HPLC-MS system offers a high selectivity, as compared to other detection methods. The mobile phase for HPLC-MS should be prepared as follows:
Mobile phase A: Mix 1 l of water with 1 ml of formic acid
Mobile phase B: Mix 1 l of acetonitrile (MeCN) with 1 ml of formic acid
Figure 6.
HPLC-MS chromatogram of the mixture of standards (as described for the Figure 4). The chromatographic conditions are the same as described in Table 2. The traces at specific m/z values have been extracted from total ionic current (TIC) trace using the software provided with HPLC-MS system.
Calculations and expected results
HPLC-EC analysis of the BAEC lysate
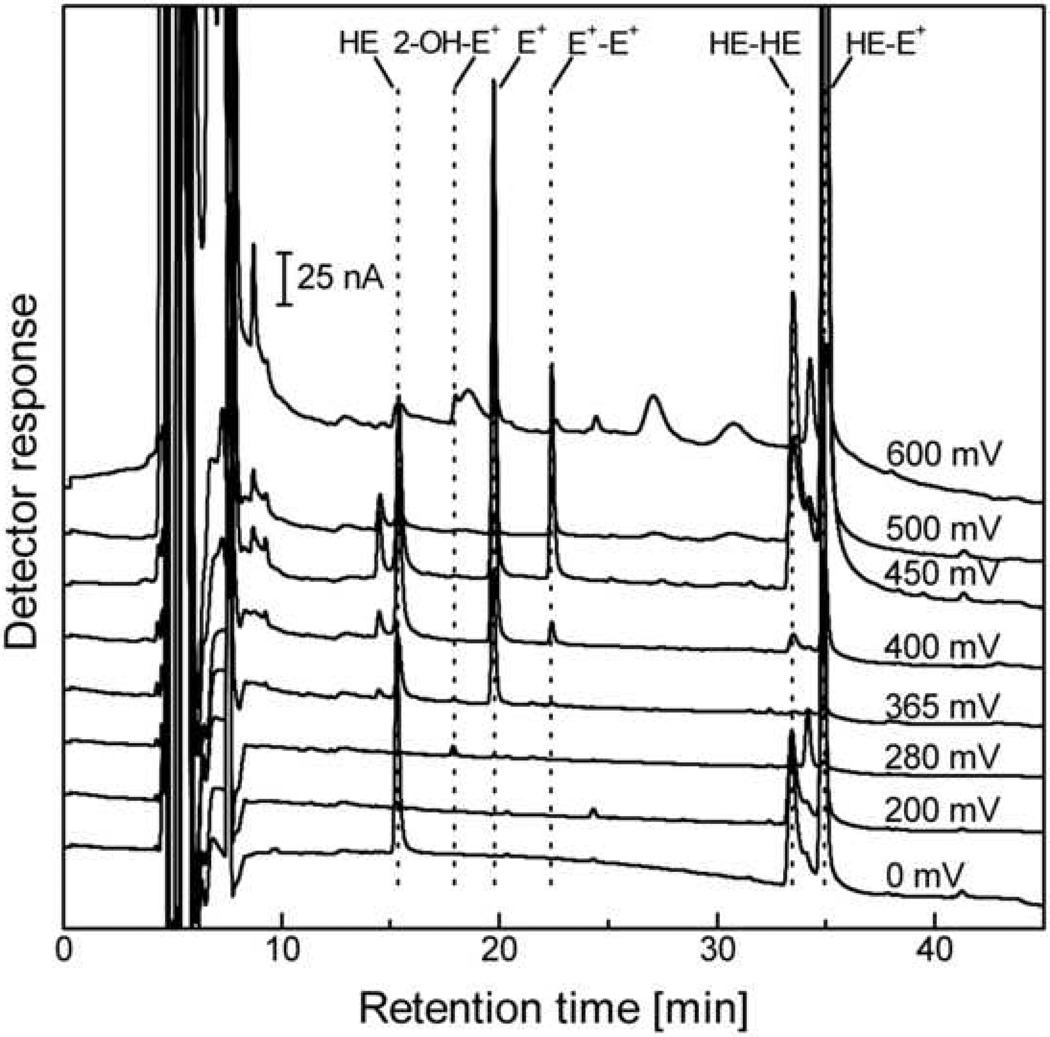
The HPLC-EC chromatogram obtained from bovine aortic endothelial cells (BAEC) incubated with HE is shown in Figure 8. The major peaks in the chromatogram are due to ethidium and the dimeric products, while the peak of 2-OH-E+ is the smallest one. This indicates that most of HE is oxidized via multiple pathways intracellularly.
Figure 8.
HPLC-EC chromatogram of the extract from BAEC incubated at 37 °C for 20 min with 10 µM HE in DMEM containing 2% FBS. Before HPLC analysis the sample has been processed as described in the text.
Calculations
The steps used for quantitation of the analytes in the cell lysates are described below (Table 4) using the data shown in Figure 8. For quantitation purposes, the area of the peaks (column 3) corresponding to the analytes of interest (column 1) are measured using the software provided with the HPLC system. Based on the known dependence of the peak areas on the concentration of the analytes, the concentration of the analytes in the sample taken for HPLC analysis is calculated (column 5). The concentration of the analytes in the cell lysates is calculated by multiplying the concentration shown in column 5 by 4 which takes into account the dilutions made during sample processing. As the numbers of cells in different samples may vary, the results are normalized to the protein concentration in the cell lysates (column 7). The amount of the sample per mg of protein is shown in column 8. Because of the lack of availability of pure standards of HE-HE and HE-E+, these peak areas can be directly normalized to the protein concentration in cell lysates and compared between different treatments.
Table 4.
Example of the calculations of the amount of HE and its oxidation products in the BAEC lysate, based on the chromatogram shown in Figure 8.
| analyte | Retention time [min] |
Potentials used for quantitation [mV] |
Sum of peak areas [µC] |
Conc. in HPLC vial [µM] |
Conc. in cell lysate [µM] |
Conc. of protein in cell lysate [mg/ml] |
Amount of the analyte per mg of protein [nmol/mg] |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| HE | 15.3 | 0, 200 | 1.65 | 0.20 | 0.81 | 3.6 | 0.22 |
| 2-OH-E+ | 17.9 | 200, 280, 365 | 0.089 | 0.0089 | 0.036 | 3.6 | 0.010 |
| E+ | 19.8 | 280, 365, 400, 450, 500 | 7.09 | 0.43 | 1.72 | 3.6 | 0.48 |
| E+-E+ | 22.4 | 365, 400, 450, 500 | 1.98 | 0.066 | 0.26 | 3.6 | 0.073 |
| HE-HE | 33.4 | 0, 200, 280 | 3.77 | 3.6 | 1.05a | ||
| HE-E+ | 34.9 | 0, 200, 280 | 6.60 | 3.6 | 1.83a | ||
for HE-HE and HE-E+ instead of concentrations in the cell lysate, the peak areas (column 4) have been normalized to the protein concentration and expressed in the units of µC·ml·mg−1.
Caveats
Changes in intracellular HE concentration
Due to the competition between HE or Mito-HE and other intracellular scavengers of superoxide (mainly, SOD), the yield of 2-hydroxyethidium or 2-hydroxy-mito-ethidium is dependent not only on the rate of superoxide production, but also on the ratio of the concentrations of HE or Mito-HE and other scavengers. Thus, changes in HE or Mito-HE and/or other scavengers will alter the yield of 2-OH-E+ or 2-OH-Mito-E+ even at constant rate of superoxide production. The same logic is applicable to formation of other oxidative products of HE or Mito-HE in cells as well. Therefore, any measurements of HE or Mito-HE products (2-OH-E+, E+ or HE dimers and 2-OH-Mito-E+, Mito-E+ or Mito-HE dimers) in response to a specific treatment should always be accompanied by simultaneous monitoring of HE or Mito-HE uptake into cells.
Extraction methods
To recover HE and its oxidation products from cells and tissues, different extraction methods have been proposed [2–4,11,19]. While the authors previously used the extraction method based on mixing (1:1) of the cell lysate with acidic methanol, it is now apparent that there exist other methods, using pure methanol or acetonitrile, for improved yield, depending on the cell or tissue type. Thus, it is preferred to use several extraction methods and ultimately choose the one that gives the best yield of the analytes without causing HE degradation during sample processing. Due to different physico-chemical properties of mitochondria-targeted analog of HE, the experiment with Mito-HE may require different extraction method than HE to obtain similar recovery of the analytes. Note: the authors previously observed that the extraction with n-BuOH causes a partial oxidation of HE lowering the yield of HE and artifactually increasing the concentration of the oxidation products determined in cell lysates, as compared to extraction with methanol or acetonitrile.
Photo-oxidation of HE
Exposure of HE to light can induce modification of HE forming oxidation products (e.g., 2-hydroxyethidium and ethidium) [7,11]. Light-induced oxidation of HE to E+ may also be catalyzed by 2-OH-E+ [7]. Therefore, exposure of samples containing HE to light should be minimized. If samples containing HE and 2-OH-E+ are exposed to light, E+ formation occurs that is proportional to the concentration of 2-OH-E+. Thus, while E+ is not a direct product of the reaction of HE with superoxide, it can be increased under conditions generating superoxide (e.g., xanthine/xanthine oxidase) in the presence of HE and light [7]. It is likely that Mito-HE will undergo a similar type of photochemical oxidation.
Interaction of HE with SOD mimetics
The propensity of HE to undergo oxidation may limit the range of agents used to stimulate or inhibit production of superoxide or other ROS because these agents may be able to directly react with HE leading to misinterpretations. It has been shown that manganese porphyrin, Mn(III)TBAP (manganese (III) tetrakis (4-benzoic acid)porphyrin chloride), a widely used as SOD mimetic, can directly oxidize HE leading to decreased formation of 2-hydroxyethidium, a superoxide-specific product [7]. Thus, the expected decrease in 2-hydroxyethidium after incubation of the cells with Mn(III)TBAP could arise from HE depletion in cells in a process unrelated to superoxide formation. Indeed, it has been recently reported that Mn(III)TBAP does not possess an SOD-like activity due to its unfavorable redox properties [39]. The possibility of a direct reaction between HE and the antioxidant reagents should be taken into consideration for proper interpretation of results.
Interaction of HE and Mito-HE with heme proteins
HE and Mito-HE have been also reported to react with various heme proteins, including cytochrome c, horseradish peroxidase (HRP), myoglobin, hemoglobin and mitochondrial respiratory complex IV [8,11,36,40–43]. In most cases the heme proteins can catalyze the oxidation of HE in the presence of H2O2. H2O2 alone does not oxidize HE or Mito-HE. The major products of those reactions catalysed by heme/H2O2 reactions are dimeric products of HE oxidation (HE-HE, HE-E+ and E+-E+) and ethidium cation [8]. Similar reactivity has been also observed in the case of Mito-HE and several dimeric products have been detected. As the rate constant of the reaction of HE and Mito-HE with cytochrome c is relatively high (104 – 105 M−1s−1 [8]), the consumption of HE and Mito-HE by heme proteins may significantly affect the availability of the probes for other oxidants, especially in mitochondria, due to high concentration of cytochrome c within the mitochondrial intermembrane compartment. These reactions will seriously hamper the use of HE and Mito-HE in the detection of superoxide. Thus, monitoring intracellular HE and Mito-HE along with their oxidation products including E+ or Mito-E+ and the dimers is essential for proper interpretation of results.
Binding of HE and Mito-HE to the vial walls
An additional confounding factor in HE-based assay of superoxide and oxidants is the binding of HE and its oxidation products to the vial walls [4]. This can significantly affect the quantification of 2-hydroxyethidium and HE dimers. Due to their physico-chemical properties the binding extent can be an even bigger problem in the case of Mito-HE and its oxidation products [4]. The binding process can be inhibited by acidification of the solution and by using a suitable organic solvent. However, higher content of organic solvent in the samples may affect the retention of HE and its products on the reversed-phase HPLC column. Thus, the amount of organic solvent should be optimized to protect the analytes from binding to the vial walls while at the same time not affect their retention on the HPLC column. In our laboratory the extent of binding is usually minimized by keeping the samples for HPLC analysis under acidic conditions (pH 2.6) and in the presence of 25% methanol.
Recently, new fluorescent probes for detecting intracellular hydrogen peroxide have been developed [44–47]. It is critical to develop a HPLC-based experimental approach similar to that described in this study for rigorous interpretation of cellular detection and quantitation of hydrogen peroxide using these probes.
The knowledge of the chemical reactivity (including the rate constants) of the probes along with their intracellular concentration is essential for any quantitative analyses of products derived from such probes.
Acknowledgements
The authors thank all present and past collaborators, whose names are given in the references for their various contributions to the development of the method presented. This work was supported by National Institutes of Health Grants HL073056, HL063119 and NS40494.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors do not declare any conflict of interest.
Reference List
- 1.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am. J. Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 4.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 5.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat. Protoc. 2008;3:941–947. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- 7.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. The confounding effects of light, sonication, and Mn(III)TBAP on quantitation of superoxide using hydroethidine. Free Radic. Biol. Med. 2006;41:1050–1057. doi: 10.1016/j.freeradbiomed.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic. Biol. Med. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitsett J, Picklo MJ, Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler. Thromb. Vasc. Biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 10.Zielonka J, Sarna T, Roberts JE, Wishart JF, Kalyanaraman B. Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch. Biochem. Biophys. 2006;456:39–47. doi: 10.1016/j.abb.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes DC, Wosniak J, Jr, Pescatore LA, Bertoline MA, Liberman M, Laurindo FR, Santos CX. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am. J. Physiol Cell Physiol. 2007;292:C413–C422. doi: 10.1152/ajpcell.00188.2006. [DOI] [PubMed] [Google Scholar]
- 12.Clavreul N, Bachschmid MM, Hou X, Shi C, Idrizovic A, Ido Y, Pimentel D, Cohen RA. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2006;26:2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 13.Aitken RJ, Wingate JK, De Iuliis GN, Koppers AJ, McLaughlin EA. Cis-unsaturated fatty acids stimulate reactive oxygen species generation and lipid peroxidation in human spermatozoa. J. Clin. Endocrinol. Metab. 2006;91:4154–4163. doi: 10.1210/jc.2006-1309. [DOI] [PubMed] [Google Scholar]
- 14.Slane BG, ykin-Burns N, Smith BJ, Kalen AL, Goswami PC, Domann FE, Spitz DR. Mutation of succinate dehydrogenase subunit C results in increased O2.-, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- 15.Whitsett J, Martasek P, Zhao H, Schauer DW, Hatakeyama K, Kalyanaraman B, Vasquez-Vivar J. Endothelial cell superoxide anion radical generation is not dependent on endothelial nitric oxide synthase-serine 1179 phosphorylation and endothelial nitric oxide synthase dimer/monomer distribution. Free Radic. Biol. Med. 2006;40:2056–2068. doi: 10.1016/j.freeradbiomed.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Al-Enezi KS, Alkhalaf M, Benov LT. Glycolaldehyde induces growth inhibition and oxidative stress in human breast cancer cells. Free Radic. Biol. Med. 2006;40:1144–1151. doi: 10.1016/j.freeradbiomed.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 17.De Iuliis GN, Wingate JK, Koppers AJ, McLaughlin EA, Aitken RJ. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J. Clin. Endocrinol. Metab. 2006;91:1968–1975. doi: 10.1210/jc.2005-2711. [DOI] [PubMed] [Google Scholar]
- 18.Hao L, Nishimura T, Wo H, Fernandez-Patron C. Vascular responses to alpha1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2006;26:819–825. doi: 10.1161/01.ATV.0000204344.90301.7c. [DOI] [PubMed] [Google Scholar]
- 19.Georgiou CD, Papapostolou I, Patsoukis N, Tsegenidis T, Sideris T. An ultrasensitive fluorescent assay for the in vivo quantification of superoxide radical in organisms. Anal. Biochem. 2005;347:144–151. doi: 10.1016/j.ab.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Zielonka J, Zhao H, Xu Y, Kalyanaraman B. Mechanistic similarities between oxidation of hydroethidine by Fremy's salt and superoxide: stopped-flow optical and EPR studies. Free Radic. Biol. Med. 2005;39:853–863. doi: 10.1016/j.freeradbiomed.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Shang T, Kotamraju S, Zhao H, Kalivendi SV, Hillard CJ, Kalyanaraman B. Sepiapterin attenuates 1-methyl-4-phenylpyridinium-induced apoptosis in neuroblastoma cells transfected with neuronal NOS: role of tetrahydrobiopterin, nitric oxide, and proteasome activation. Free Radic. Biol. Med. 2005;39:1059–1074. doi: 10.1016/j.freeradbiomed.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Huh J, Liepins A, Zielonka J, Andrekopoulos C, Kalyanaraman B, Sorokin A. Cyclooxygenase 2 rescues LNCaP prostate cancer cells from sanguinarine-induced apoptosis by a mechanism involving inhibition of nitric oxide synthase activity. Cancer Res. 2006;66:3726–3736. doi: 10.1158/0008-5472.CAN-05-4033. [DOI] [PubMed] [Google Scholar]
- 23.Nath KA, d'Uscio LV, Juncos JP, Croatt AJ, Manriquez MC, Pittock ST, Katusic ZS. An analysis of the DOCA-salt model of hypertension in HO-1−/− mice and the Gunn rat. Am. J. Physiol Heart Circ. Physiol. 2007;293:H333–H342. doi: 10.1152/ajpheart.00870.2006. [DOI] [PubMed] [Google Scholar]
- 24.Ceravolo GS, Fernandes L, Munhoz CD, Fernandes DC, Tostes RC, Laurindo FR, Scavone C, Fortes ZB, Carvalho MH. Angiotensin II chronic infusion induces B1 receptor expression in aorta of rats. Hypertension. 2007;50:756–761. doi: 10.1161/HYPERTENSIONAHA.107.094706. [DOI] [PubMed] [Google Scholar]
- 25.Rossary A, Arab K, Steghens JP. Polyunsaturated fatty acids modulate NOX 4 anion superoxide production in human fibroblasts. Biochem. J. 2007;406:77–83. doi: 10.1042/BJ20061009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray JP, Heck DE, Mishin V, Smith PJ, Hong JY, Thiruchelvam M, Cory-Slechta DA, Laskin DL, Laskin JD. Paraquat increases cyanide-insensitive respiration in murine lung epithelial cells by activating an NAD(P)H:paraquat oxidoreductase: identification of the enzyme as thioredoxin reductase. J. Biol. Chem. 2007;282:7939–7949. doi: 10.1074/jbc.M611817200. [DOI] [PubMed] [Google Scholar]
- 27.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid. Redox. Signal. 2007;9:1825–1836. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 28.Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2007;27:762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- 29.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel P, Schuhmacher S, Kienhofer J, Muller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Munzel T, Burkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc. Res. 2008 doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurindo FR, Fernandes DC, Santos CX. Assessment of superoxide production and NADPH oxidase activity by HPLC analysis of dihydroethidium oxidation products. Methods Enzymol. 2008;441:237–260. doi: 10.1016/S0076-6879(08)01213-5. [DOI] [PubMed] [Google Scholar]
- 33.Vasquez-Vivar J, Whitsett J, Ionova I, Konorev E, Zielonka J, Kalyanaraman B, Shi Y, Pieper GM. Cytokines and lipopolysaccharides induce inducible nitric oxide synthase but not enzyme activity in adult rat cardiomyocytes. Free Radic. Biol. Med. 2008 doi: 10.1016/j.freeradbiomed.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic. Biol. Med. 1998;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein S, Fridovich I, Czapski G. Kinetic properties of Cu,Zn-superoxide dismutase as a function of metal content--order restored. Free Radic. Biol. Med. 2006;41:937–941. doi: 10.1016/j.freeradbiomed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Thomas G, Roques B. Proton magnetic resonance studies of ethidium bromide and its sodium borohydride reduced derivative. FEBS Letters. 1972;26:169–175. [Google Scholar]
- 39.Reboucas JS, Spasojevic I, Batinic-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J. Biol. Inorg. Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 40.Palazzolo-Ballance AM, Suquet C, Hurst JK. Pathways for intracellular generation of oxidants and tyrosine nitration by a macrophage cell line. Biochemistry. 2007;46:7536–7548. doi: 10.1021/bi700123s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patsoukis N, Papapostolou I, Georgiou CD. Interference of non-specific peroxidases in the fluorescence detection of superoxide radical by hydroethidine oxidation: a new assay for H2O2. Anal. Bioanal. Chem. 2005;381:1065–1072. doi: 10.1007/s00216-004-2999-x. [DOI] [PubMed] [Google Scholar]
- 42.Papapostolou I, Patsoukis N, Georgiou CD. The fluorescence detection of superoxide radical using hydroethidine could be complicated by the presence of heme proteins. Anal. Biochem. 2004;332:290–298. doi: 10.1016/j.ab.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2', 7'-dichlorofluorescin. J. Leukoc. Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- 44.Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J. Am. Chem. Soc. 2005;127:16652–16659. doi: 10.1021/ja054474f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller EW, Chang CJ. Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr. Opin. Chem. Biol. 2007;11:620–625. doi: 10.1016/j.cbpa.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang MC, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am. Chem. Soc. 2004;126:15392–15393. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickinson BC, Chang CJ. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 2008;130:11561. doi: 10.1021/ja802355u. [DOI] [PMC free article] [PubMed] [Google Scholar]