Abstract
The objective of this study was to explore the efficacy of combination therapy with citalopram plus omega-3 fatty acids versus citalopram plus placebo (olive oil) in the initial treatment of individuals with Major Depressive Disorder (MDD). We hypothesized that combination therapy would not only lead to greater efficacy, but a more rapid onset of therapeutic response.
Forty-two subjects participated in this nine week randomized, masked, placebo-controlled study of combination therapy (2 grams containing a blend of 900mg EPA, 200mg DHA, and 100mg other omega-3 fatty acids twice daily plus citalopram) versus monotherapy (2 grams olive oil per day plus citalopram) treatment of MDD.
The combination therapy demonstrated significantly greater improvement in Hamilton Depression Rating scales scores beginning at week 4 (t = −2.48, df 38, p = 0.018) and continuing throughout the rest of the study (F = 7.32, df 1,177, p = 0.008).
Combination therapy was more effective than monotherapy in decreasing signs and symptoms of MDD during the 8 weeks of active treatment, however, combination therapy did not seem to enhance the speed of the initial antidepressant response. These findings suggest that there may be an advantage to combining omega-3 fatty acids with a selective serotonin uptake inhibitor in the initial treatment of individuals with MDD. A larger definitive study is warranted.
Keywords: Major Depressive Disorder, Treatment, Omega-3 fatty acids, Antidepressant, Combined therapy
Introduction
Major depressive disorder (MDD) is the most prevalent Axis I disorder and one of the leading causes of disability in the United States and worldwide.1, 2 The estimated lifetime incidence of MDD in adults in the US is more than 15%.3 In 2000, the economic burden of depressive disorders in the US was an estimated $83.1 billion, 30% of which was attributable to direct medical expenses.4
Although pharmacotherapy is the mainstay of treatment for depression, an adequate response to a single antidepressant medication, classically measured as an attenuation of 50% or more in the severity ofdepressive symptoms, only occurs in about 50%-75% of patients with a first trial.5-7 Furthermore, remission rates are even less and do not usually exceed 30% with any single agent.5-7 Preliminary data suggest that the combination treatments with two antidepressant agents were as well tolerated as monotherapy and may double the likelihood of remission compared with use of a single medication.8
Despite the availability of many antidepressants on the market, the search for a naturally occurring, inexpensive, safe, and effective agent continues. One such possibility is the use of n-3 omega polyunsaturated fatty acids (PUFAs) for treatment of depression. A link between omega-3 fatty acids and mood disorders is suggested by some studies showing a lower incidence of depression among populations with a diet rich in omega-3 fatty acids,9 although this issue remains controversial.10 Furthermore, lower plasma levels of omega-3 fatty acids have been reported in mood disorder patients compared to healthy controls.11-16 Recent meta-analysis17 confirmed that depression is associated with lower levels of total PUFAs and both types of omega-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The magnitude of differences in the plasma levels of EPA between depressed and non-depressed subjects becomes larger when analyses are restricted to studies that used the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for MDD diagnosis, suggesting that the omega-3 PUFA abnormalities were more significant in homogeneous groups of subjects with MDD. Clinical depression can be accompanied by low levels of omega-3 PUFAs in RBC, plasma and, as more found recently, brain tissue.18
Although there are multiple clinical trials supporting the efficacy of EPA and DHA in depressive disorders, 19 many issues remain unresolved. Most data support efficacy of EPA, while efficacy of DHA remains doubtful.20, 21 Also, a recently completed study22 evaluated EPA/DHA combinations, both as monotherapy and as a supplement to ongoing antidepressant treatment. It was determined that while both monotherapy and supplementation with omega-3 PUFA showed only weak efficacy signals, when administered to patients with MDD (without comorbid anxiety disorders), supplementation treatment appeared to be more efficacious and resulted in an effect size of 0.27. When studied in MDD patients with comorbid diabetes, EPA/DHA supplementation proved to be ineffective.23 Still, it seems that supplementation of ongoing antidepressant treatment with omega-3 PUFA in uncomplicated MDD appears promising.
This study addressed two primary questions:
Does omega-3 PUFA supplementation of citalopram lead to greater efficacy than antidepressant monotherapy?
Does omega-3 PUFA supplementation of citalopram lead to acceleration of antidepressant response as evidenced by statistically significant differences between the treatment groups at week 2 of treatment?
Methods
Subjects
This study was approved by the Institutional Review Board of Cedars-Sinai Medical Center. Potential study subjects were recruited via local advertisement or by physician referral from the greater Los Angeles area and underwent a preliminary telephone screening within the Department of Psychiatry and Biobehavioral Sciences. Recruited subjects were between 18 to 65 years of age who met DSM-IV criteria for current major depression by Structured Clinical Interview for DSM Disorders (SCID) and had a 21 item Hamilton Depression Rating Scale (HAM-D)24 score greater than 17. Women of childbearing potential were required to use effective contraception. Exclusion criteria were: (1) diagnosis of psychotic disorders including psychotic depression and bipolar disorders; (2) current drug or alcohol abuse or dependence, or history of drug or alcohol abuse or dependence within the previous six months; (3) unstable medical or neurological conditions that were likely to interfere with the treatment of depression; (4) history of allergy to citalopram or omega-3 fatty acids, finfish or shellfish; (5) history of failure of response to adequate trial of citalopram; (6) history of seizure disorder; (7) pregnancy; (8) need for concomitant therapy with psychotropic medications including antidepressants other than citalopram or neuroleptics; (9) active suicidal ideation or other safety concerns; (10) exposure to treatment with fluoxetine or monoamine oxidase inhibitors (MAOIs) in the previous two months; (11) being on anticoagulant therapy; and, (12) a dietary intake greater than 3.0g total omega-3 per day at baseline as assessed by a three-day food diary evaluated by a certified nutritionist.
Treatment / Study Design
All study participants were initially enrolled into a one week, single-blind, placebo run-in phase. At the end of the run-in phase, subjects still qualifying for inclusion with a HAM-D score greater than 17 were block randomized by sex to receive citalopram (20 mg/day). Half the subjects also received omega-3 and the other half placebo. Subjects were instructed to take citalopram in the morning and two omega-3 or placebo capsules twice daily with meals. Each omega-3 capsule contained 450 mg EPA, 100 mg DHA, and 50 mg other omega-3 fatty acids, whereas each placebo capsule contained 1000 mg of olive oil. Both placebo and active drug were obtained from Nordic Naturals. All subjects remained on citalopram (20 mg/day) until week 4 of the study, after which the dose could be increased to 40 mg/day if HAM-D scores had decreased less than 25% and side effects were not a concern, as is the standard of care for treatment with citalopram. Subjects were asked to avoid any major dietary changes throughout the duration of the study.
Assessments
Study participants were assessed by a study psychiatrist at each study visit (baseline, randomization, and study weeks 2, 4, 6 and 8) that included evaluation with the 21-item HAM-D. As secondary measures of efficacy, the Beck Depression Inventory (BDI)25 and the Montgomery-Asberg Depression Rating Scale (MADRS),26 Clinical Global Impression Scale (CGI) and Patient’s Global Improvement Scale (PGI) were also performed.27 The Treatment Emergent Symptoms Scale (TESS) was used to record side-effects.27 Protocol adherence was determined by pill counts and by assessment of citalopram blood levels. Samples were collected at baseline and study termination for determination of plasma c-reactive protein and 24-hour urinary cortisol levels.
Statistical Analysis
All data were inspected graphically and the Kolmogorov-Smirnov test was used to test whether continuous data followed a normal distribution prior to analysis. Continuous data failing tests of normality were log-transformed prior to further analysis. Where appropriate, both parametric (Chi-square tests, Student’s t-test) and non-parametric (Fisher Exact Tests, Wilcoxon rank sum tests) were used to compare the demographic, psychiatric and medical characteristics of the two treatment groups at baseline and to identify differences in citalopram dosing, protocol completion, adverse events, and adverse effects.
Analysis of efficacy was conducted according to the intent-to-treat principle.28 A mixed-effects linear regression model with ante-dependence covariance structure tested the primary hypothesis of whether the course of depression, as measured by HAM-D scores, differed between the treatment conditions over time (group x time interaction), while controlling for the changes in citalopram dosing over time (dose x time interaction). Subjects were classified as achieving full remission (HAM-D ≤ 7), partial remission (8 ≤ HAM-D ≤ 15, with HAM-D reduction of ≥ 50%), partial improvement (8 ≤ HAM-D < 15, with HAM-D reduction of ≤ 50% but ≥ 25%), and no improvement (≤ 25% reduction in HAM-D scores).29 The distribution of the number of subjects meeting these criteria for improvement was compared across treatment groups with a Wilcoxon Rank Sum Test. Secondary measures of efficacy (BDI, MADRS) were also analyzed with mixed model linear regression techniques. All analyses were performed using SAS v9.1 software. Data are reported as means ± standard deviation (SD) or frequency and percent.
Based on previous studies performed by those in our group,30 we determined that a sample size between 18 to 26 subjects completing the study per group had 80% power to detect a decrease in HAM-D scores with an effect size ranging between 0.8 to 1.0 at the 0.05 significance level.
Results
Seventy-five individuals were assessed for eligibility and 45 met entry criteria and were enrolled. After the one week placebo run-in period, 42 subjects continued to meet entry criteria and were randomized to the two study arms. In the citalopram + omega-3 treatment group, of the 20 initial subjects, two subjects were terminated and excluded from analysis due to previously undisclosed exclusion criteria (alcohol abuse, bipolar disorder) and one subject was lost to follow up. Among the 22 subjects randomized to the citalopram + placebo treatment group, two subjects withdrew specifically because of lack of efficacy, while five were lost to follow-up. There was no statistical difference in the drop-out rates between the two treatment groups (p = 0.118). There was also no association of drop-out rates by severity of depression as measured by HAM-D scores or by any demographic measure.
Study participants were an average of 40.5 ± 10.2 years old with 15% experiencing a first episode of MDD and an average current episode length of 21.8 ± 24.3 months. The average HAM-D score at baseline was 25.3 ± 4.4. Baseline demographic, medical and depression history measures were similar between the two study groups. The only significant difference between the two study groups at baseline was a higher MADRS score in the placebo-treated group (27.5 ± 6.3) than the omega-3 treated group (24.2 ± 3.7) (t = −2.1,35df, p = 0.048).
There was no difference in the dosing regimen of citalopram between the two treatment arms. At study week 4, citalopram dosing was increased from the initial 20 mg/day to 40 mg/day in 11 of 18 subjects (61%) in the omega-3 treated group, and in 9 of 18 subjects (50%) in the placebo-treated group (, p = 0.502). At study week 6, 14/18 (78%) in the omega-3 treated group and 10/17 (59%) in the placebo treated group were receiving citalopram (40 mg/day) (, p = 0.227) .
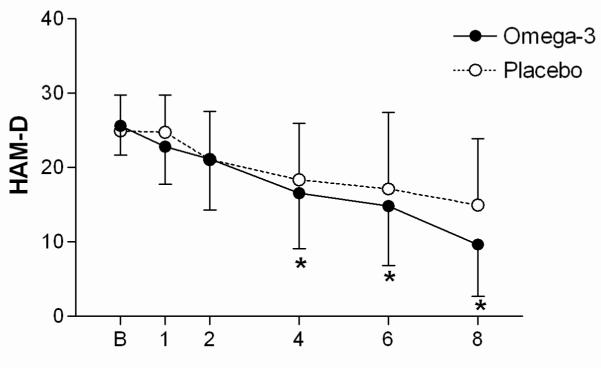
In the intent-to-treat analysis, and after covarying for citalopram dose prescribed, there was significantly greater improvement over time in HAM-D scores among subjects in the omega-3 treated group than in the placebo-treated group (group x time interaction, , p = 0.008) (Figure 1). Significant differences were noted between the treatment groups at study week 4 (t = −2.48,38df , p = 0.018) , week 6 (t = −2.83,38df , p = 0.007) , and at study completion (t = −2.92,38df , p = 0.006) . Furthermore, there was statistically significant improvement in the omega-3 treated group over the placebo treated group in terms of remission status (p = 0.018) (Table 1). There were trends for a group x time interaction for BDI scores (, p = 0.171) and MADRS scores (after covarying for differences at baseline, , p = 0.124). We observed no changes in plasma CRP or 24 hour urinary cortisol levels over the eight weeks of study (data not shown.)
Figure 1.
HAM-D measures of depressive symptoms for subjects treated with citalopram + placebo or citalopram + omega-3 supplements over the 8 weeks of study, mean ± standard deviation (* p < 0.05, computed via regression modeling).
Table 1.
Categorical improvement rates across both treatment groups.
| Citalopram+Omega-3 (n=18) |
Citalopram+Placebo (n=22) |
p-value* | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Full remission | 8 | (44) | 4 | (18) | |
| Partial remission | 3 | (17) | 3 | (14) | 0.018 |
| Partial improvement | 5 | (28) | 4 | (18) | |
| No improvement | 2 | (11) | 11 | (50) | |
Overall p-value computed via Wilcoxon rank sum test.
Based on detectable blood levels of citalopram, adherence to the treatment protocol was 89% in the omega-3 treatment group and 100% in the placebo-treated group. Analysis of only adherent subjects found no notable differences in these overall findings. The most frequently reported side effects were largely gastro-intestinal in nature such as nausea, diarrhea, indigestion, and constipation with 6/18 (33%) in the omega-3 group reporting such adverse events and 4/22 (18%) in the placebo-treated group. Less than 5% of subjects in either group reported other side effects such as headache, sedation, or sexual dysfunction. There were no significant adverse events observed.
Discussion
The current study showed that omega-3 augmentation of citalopram treatment produced a significantly greater reduction in HAM-D scores as compared to citalopram treatment alone. Statistically significant differences between treatment groups in HAM-D scores were detected beginning at week 4 of treatment, as well as at weeks 6 and 8. In contrast, there was no evidence of acceleration of antidepressant response, as there were no differences in HAM-D scores between treatment groups at week 2 of treatment. However, it should be noted that this study was powered to detect a 1.0 standard deviation difference between the groups so that an accelerated response, but of smaller magnitude, might not have been discernable at week 2.
This study confirms results by Lesperance et al.,22 Peet and Horrobin,31 and Nemets32 that showed efficacy of omega-3 supplementation of antidepressant treatment in subjects with uncomplicated MDD. The significant methodological difference between the current study and those cited above was that in the current study supplementation with omega-3 PUFA was started simultaneously with citalopram treatment. In prior studies, the fatty acids were added after the subjects had been taking the antidepressant for varying lengths of time. Nonetheless, both augmentation approaches produced similar results.
Mechanisms through which omega-3 fatty acids may be efficacious are related to inflammatory processes that have been linked to depression. Omega-3 fatty acids appear to decrease the production of inflammatory eicosanoids from arachidonic acid. There is also an effect of omega-3 fatty acids on brain-derived neurotrophic factor, which encourages synaptic plasticity, provides neuroprotection, and enhances neurotransmission.34 A third possible mechanism by which omega-3 fatty acids may modulate depression is by maintaining membrane integrity and fluidity.35 Support for involvement of omega-3 fatty acids in receptor functioning, neurotransmitter levels, and the metabolism of monamines implicated in depression has been provided by animal studies.9, 33
The data support the concept that omega-3 fatty acid augmentation of the SSRI citalopram is a beneficial and safe strategy for enhancing therapeutic response in non-treatment resistant depression. The logical development of this line of research would be to confirm these results across different classes of antidepressants and in larger studies that, if positive, will make omega-3 fatty acid augmentation eligible for wide clinical application. The limitations of the current study are the modest sample size and the short duration of the treatment period. The lack of effect of treatment on the two biological measures (c-reactive protein and urinary free cortisol levels) is unexpected, but it is a common occurrence in exploratory studies. Again, this may reflect the heterogeneity of the sample, its modest size, and the fact we are looking for an added effect beyond what is observed with standard antidepressant therapy.
In summary, our data suggest that initiation of treatment with an SSRI and PUFA simultaneously is advantageous in terms of efficacy when compared with treatment with SSRI as a monotherapy. The safety profile of such combination treatment is very favorable.
Acknowledgments
This study was funded through the National Institutes of Health’s National Center for Complementary and Alternative Medicine (R21-AT001077) and the National Center for Research Resources (M01-RR000425).
Footnotes
Author Disclosure Statement:
Drs. Gertsik and Ms. Bresee have no financial conflicts of interest to report. At the time of the study, but not currently, Dr. Poland received research support from GlaxoSmithKline and had stock worth <$5,000 in Pfizer. Dr. Rapaport serves a consultant to Wyeth; the National Institute of Mental Health; Dainippon-Sumitomo; Brain Cells, Inc.; Astellas Pharma; Quintiles; Pfizer; and Takeda Pharmaceuticals.
Literature Cited
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Alonso J, Angermeyer MC, Bernert S, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;109(s420):21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy SH, Eisfeld BS, Meyer JH, et al. Antidepressants in clinical practice: limitations of assessment methods and drug response. Hum Psychopharmacol. 2001;16:105–114. doi: 10.1002/hup.189. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 7.Nemeroff CB, Entsuah R, Benattia I, et al. Comprehensive analysis of remission (COMPARE) with venlafaxine versus SSRIs. Biol Psychiatry. 2008;63:424–434. doi: 10.1016/j.biopsych.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167:281–288. doi: 10.1176/appi.ajp.2009.09020186. [DOI] [PubMed] [Google Scholar]
- 9.Hibbeln JR, Salem N., Jr. Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Hakkarainen R, Partonen T, Haukka J, et al. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry. 2004;161:567–569. doi: 10.1176/appi.ajp.161.3.567. [DOI] [PubMed] [Google Scholar]
- 11.Adams PB, Lawson S, Sanigorski A, et al. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(Suppl):S157–161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 12.Tiemeier H, van Tuijl HR, Hofman A, et al. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Maes M, Smith R, Christophe A, et al. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, Christophe A, Delanghe J, et al. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 15.Edwards R, Peet M, Shay J, et al. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 16.Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:311–318. doi: 10.1054/plef.2002.0435. [DOI] [PubMed] [Google Scholar]
- 17.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 18.McNamara RK. DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J Nutr. 2010;140:864–868. doi: 10.3945/jn.109.113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 20.Marangell LB, Martinez JM, Zboyan HA, et al. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160:996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 21.Mischoulon D, Best-Popescu C, Laposata M, et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol. 2008;18:639–645. doi: 10.1016/j.euroneuro.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Lesperance F, Frasure-Smith N, St-Andre E, et al. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry. 2010 doi: 10.4088/JCP.10m05966blu. ePub June15. [DOI] [PubMed] [Google Scholar]
- 23.Bot M, Pouwer F, Assies J, et al. Eicosapentaenoic acid as an add-on to antidepressant medication for co-morbid major depression in patients with diabetes mellitus: A randomized, double-blind placebo-controlled study. J Affect Disord. 2010;126:282–286. doi: 10.1016/j.jad.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT. Depression Inventory. Center for Cognitive Therapy; Philadelphia: 1978. [Google Scholar]
- 26.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 27.Guy W. ECDEU assessment manual for psychopharmacology. U. S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; Rockville, Md.: 1976. [Google Scholar]
- 28.Mallinckrodt CH, Sanger TM, Dube S, et al. Assessing and interpreting treatment effects in longitudinal clinical trials with missing data. Biol Psychiatry. 2003;53:754–760. doi: 10.1016/s0006-3223(02)01867-x. [DOI] [PubMed] [Google Scholar]
- 29.Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):5–9. [PubMed] [Google Scholar]
- 30.Ott GE, Rao U, Nuccio I, et al. Effect of bupropion-SR on REM sleep: relationship to antidepressant response. Psychopharmacology (Berl) 2002;165:29–36. doi: 10.1007/s00213-002-1165-4. [DOI] [PubMed] [Google Scholar]
- 31.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 32.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 33.Logan AC. Neurobehavioral aspects of omega-3 fatty acids: possible mechanisms and therapeutic value in major depression. Altern Med Rev. 2003;8:410–425. [PubMed] [Google Scholar]
- 34.Ikemoto A, Nitta A, Furukawa S, et al. Dietary n-3 fatty acid deficiency decreases nerve growth factor content in rat hippocampus. Neurosci Lett. 2000;285:99–102. doi: 10.1016/s0304-3940(00)01035-1. [DOI] [PubMed] [Google Scholar]
- 35.Yehuda S, Rabinovitz S, Carasso RL, et al. Fatty acids and brain peptides. Peptides. 1998;19:407–419. doi: 10.1016/s0196-9781(97)00295-7. [DOI] [PubMed] [Google Scholar]