Abstract
We have used particulate silver coating on stainless steel to prevent in vivo bacterial infection. Stainless steel is commonly used as an implant material for fracture management. The antimicrobial use of silver has been well documented and studied, therefore the novelty of this research is the use of a particulate coating as well as facing the real world challenges of a fracture repair implant. The variable parameters for applying the coating were time of deposition, silver solution concentration, voltage applied, heat treatment temperature between 400 to 500 °C and time. The resultant coating is shown to be non-toxic to human osteoblasts using an MTT assay for proliferation and SEM images for morphology. In vitro silver release studies of various treatments were done using simulated body fluid. The bactericidal effects were tested by challenging the coatings with P. aeruginosa in a bioreactor and compared against uncoated stainless steel. A 13-fold reduction in bacteria was observed at 24 hours and proved to be statistically significant.
Keywords: Silver, Antimicrobial, Stainless Steel Implants, Fracture Management
1. Introduction
Every year there are an estimated 50 million road traffic accidents [1, 2]. The resulting injuries are prone to infection because they can occur in remote locations and often involve an open wound being exposed for extended periods of time [3–5]. By the time the patient is brought to the hospital, infection is often a great concern. Even under the best surgical conditions, infection is still a concern. Since 90% of all road traffic injuries occur in developing nations [6–8], there is often a greater risk of infection as ideal surgical circumstances are not always possible.
The use of antibiotics to prevent infections when incorporated onto or into orthopedic devices or wound care products has met with limited success over the years. Limited success has also been seen with non-antibiotic remedies such as chlorohexidine, nitrofurazone, PVP-I, mafenide and mupirocin. Historically, amino-glycoside antibiotics (tobramycin, amikacin, gentamicin) were highly effective, and their original selection was based on the fact that most patients had never been treated with these rather toxic parenterals, as opposed to tetracyclines, semi-synthetic penicillins, cephalosporins, quinolones and macrolides--antibiotics prescribed for practically every patient who ever had either an upper respiratory or urinary tract infection. The thinking was that such little-used antibiotics would not present a threat of the development of drug resistance. This theory was incorrect. As a replacement, one of the remarkable transition elements, Silver, has come into wide use especially for topical treatment. The effectiveness of silver revolves around its low propensity to select for resistance, its broad spectrum of activity and its high chemotherapeutic ratio (toxic dose divided by effective dose). Silver is biocidal in the ionic form and, unlike many antibiotics, has at least six different mechanisms of action [9–13]. Silver has the added benefit of being highly toxic to microorganisms with a relatively low toxicity to human tissues [14–17].
Stainless steel has long been a standard material used for fracture management implants due to its mechanical properties, corrosion resistance and low cost. In particular, it is a common material used in cases of fracture repair, such as those caused by road traffic accidents. Therefore, it could be beneficial to apply an antimicrobial coating to stainless steel to address the risk of infection from open wounds and surgical conditions. The antimicrobial agent used was ionic silver.
The objective of this research is to develop a silver coating that will be adherent to the stainless steel implants and offer an antimicrobial surface to prevent infection. This coating should operate within the constraint that the silver release should not be toxic to the surrounding tissue. To accomplish this, it is hypothesized that electrochemical routes can be used to deposit particulate Ag+ on stainless steel which can be used on fracture management implants to prevent infection. This approach was chosen because it is cost effective and easy to use. Also, it can be incorporated with current manufacturing processes for a finished product before sterilization. There is a knowledge gap that exists concerning how a particulate coating can be applied. Film based silver coatings have been developed and are currently being used in industry. However, particulate based silver coatings are not yet fully explored. A particulate coating has the advantage that it does not completely cover the surface, which leaves the substrate material exposed and retains the substrate’s properties. The challenge is to address how particles can be adhered to the surface such that the coating will remain viable even after implantation.
The novelty of this research is that we address the challenges of the real world. Developing an adhesion mechanism that can be applied after final machining without degrading the product is essential to the utility of this method. This research is also novel because we employ a particulate based silver coating as opposed to the more common film based coatings. We have shown that a particulate silver coating can be applied to fracture management devices, survive realistic situational challenges and still release silver above the minimal inhibitory concentration.
To better understand the electro-deposition process that can yield adherent silver to a SS substrate with an appropriate load of Ag+ ions, a broad range of experiments have been performed. Parameters such as solution concentration of silver nitrate during electrodeposition, deposition current and time, and post deposition heat treatment times and temperatures have been experimented with towards a viable silver coating approach. After the coating’s optimization, bacteria cultures were examined to see the antimicrobial effect of the treatment and in vitro Ag+ release studies were performed. In addition, human osteoblast cell culture was performed to ensure that the coating did not adversely affect human cell attachment and proliferation.
2. Materials and Methods
2.1 Sample Preparation
FDA approved stainless steel (316L) nails from the Surgical Implant Generation Network (SIGN, WA) designed for the repair of long bone fractures were used in this study. It should be noted that multiple studies were performed during the course of this research and a wide range of process parameters were investigated. Samples were first cleaned with DI water and acetone prior to electrodeposition of silver. Electrodeposition was performed using an aqueous solution of AgNO3 with concentration between 0.001–0.5 M with platinum as the anode material. The deposition was carried out by applying a DC current (0.05–0.25 Amperes) between 2 and 7 minutes at room temperature. SEM images were taken to verify and categorize the deposition of silver. It was observed that the as-deposited coating would not adhere strongly enough to the SS to survive handling, sterilization and surgical procedures. Therefore, post deposition heat treatments were designed and performed. The coated samples were heat treated in a vertical tube furnace in air atmosphere between 300 and 600° C for 2 to 20 minutes and allowed to cool naturally at room temperature. Finally, the samples were sprayed with DI water and wiped with a towel to remove any loose silver attached to the surface.
2.2 Silver Release Rate
In order to simulate how much silver would be released into the body upon implantation, a simulated body fluid (SBF) was prepared keeping the ionic concentration the same as blood plasma according to the method presented by Kokubo et al. [18, 19]. The SBF was adjusted to a pH of 7.38 and maintained at a temperature of 37° C for the duration of the study. Each sample was placed in a sterile vial with 10mL of SBF. At each time point the solution was replaced with fresh SBF. Upon completion, the solution was analyzed for Ag+ content using an atomic absorption spectrophotometer (AAS). The measurements were completed using a Shimadzu AA-6800 Atomic Absorption Spectrophotometer (Shimadzu, Kyoto, Japan). The samples were tested in “Flame Mode” using air and acetylene (C2H2) fuel, and data collection was carried out using Shimadzu WizAArd software. To calibrate the machine, standards of 0.5, 2.5 and 5μg/mL were created with ultra-pure water by diluting known concentration samples (High-Purity Standards, Charleston, SC, USA). During testing, a pre-spray time of 30 seconds and an integration time of 10 seconds were used.
2.3 Antimicrobial Activity
To test how effective the coating would be against bacteria that are a common threat in surgical situations, silver coated SS samples were challenged with pseudomonas aeruginosa. Samples were ultrasonically cleaned and rinsed with DI water and acetone before coating. They were coated with an electrodeposition technique and heat treated as outlined above. A stock of p. aeruginosa was incubated for 24 hours in growth media prior to injecting it into the prepared bioreactor. The bioreactor used was a 1 L beaker that has a custom airtight lid which holds columns that extend down into the beaker. These inert columns hold the samples with set-screws such that they can all be subject to similar, laminar flow. The bioreactor setup can be seen in Figure 1. All components used were sterilized using an autoclave prior to use. Once the samples were fixed onto the columns and growth media was added, cell stock was injected in the amount of 10% by volume of the growth media in the bioreactor. The media was kept in motion by using a cross stir rod on a stir plate. Air was also slowly bubbled into the media and was allowed to escape via a filtered outlet. After 24 hours, the samples were removed from the bioreactor, scraped and rinsed into test tubes using a buffer solution. The solution was then mixed using a homogenizer to break up cell clusters and ensure uniformity. Next a serial dilution was carried out, and each dilution was plated onto agar plates. Each dilution was a 1:10 dilution and 20μL per dilution was pipetted 10 times into each of three sections per agar plate. The plates were then incubated for 24 hours and cell counting was performed.
Figure 1.
Bioreactor setup. Multiple samples or controls can be tested by placing disc samples in the circular cutouts. They are held in place with set screws. This setup allows for air supply and outgoing tubes. Once samples were in place and growth media was added, the bioreactor was sealed and bacteria were injected at the top. Smaller diameter samples required an adapter cuff seen in dark grey (right).
2.4 Human Osteoblast Cell Interaction
The effect of Ag+ coating on human osteoblast cells was studied using an established hFOB cell line 1.19 (ATCC, Manassas, VA). All samples were sterilized by placing them under UV light in a fume hood for 20 minutes. A base medium of 1:1 mixture of Ham’s F12 Medium and Dulbecco’s Modified Eagle’s Medium (DMEM/F12, Sigma, St. Louis, MO), with 2.5mML-glutamine (without phenol red) was used. The medium was supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 0.3 mg ml−1 G418 (Sigma). The well plates were then incubated in a controlled atmosphere of 5% CO2 and 95% air at 37°C. Cell media was changed once every two days.
At three and seven days, samples were taken and prepared for cell proliferation study. Proliferation was measured using an MTT assay. MTT solution (5mg/mL) was prepared by dissolving MTT in phosphate-buffered saline (PBS), filtered and sterilized. 10% MTT solution was added to each well and allowed to incubate for two hours to allow formazan formation. An MTT solubilization solution made of 10% Triton X-100, 0.1 N HCl and isopropanol was then added to dissolve the formazan crystals and the mixture was centrifuged to ensure that other material would not interfere with the optical density measurements. 0.2mL of the supernatant was placed in a new well plate and optical density was measured at 570 nm using a microplate reader. This was performed in triplicate for each sample.
At three and seven day points, cell morphology was also examined. Samples were separated from media and rinsed with 0.1 M PBS. Samples were then fixed with 2% paraformaldehyde / 2% gluteraldehyde in 0.1 M cacodylate buffer overnight at 4°C after rinsing with 0.1 M cacodylate buffer. Samples were then fixed in 2% osmium tetroxide (OsO4) for two hours at room temperature. Samples were then dehydrated using an ethanol series and hexamethyl-disilazane (HMDS) drying protocol. Dried samples were gold sputter coated and examined with a Hitachi s-570 field emission SEM.
2.5 Contact Angle and Surface Energy
The static contact angles of sessile drops of liquids on polished controls and coated samples were measured using a face contact angle set-up equipped with a camera (VCA Optima, AST Products Inc., MA, USA). Flat disk samples were cut from the nail cross section were used to facilitate the accuracy of testing the material’s surface energy. The disks were polished to ensure consistent surface roughness between samples prior to deposition. For surface energy calculations deionized water and one apolar liquid, diiodomethane, were used. The liquid surface tension and contact angle were used to calculate the surface energy [20]. In Fowek’s equation below, θ is the contact angle of liquid L and solid S, γd is the dispersion component of the surface energy and γp is the polar component.
3. Results
3.1 Silver Deposition
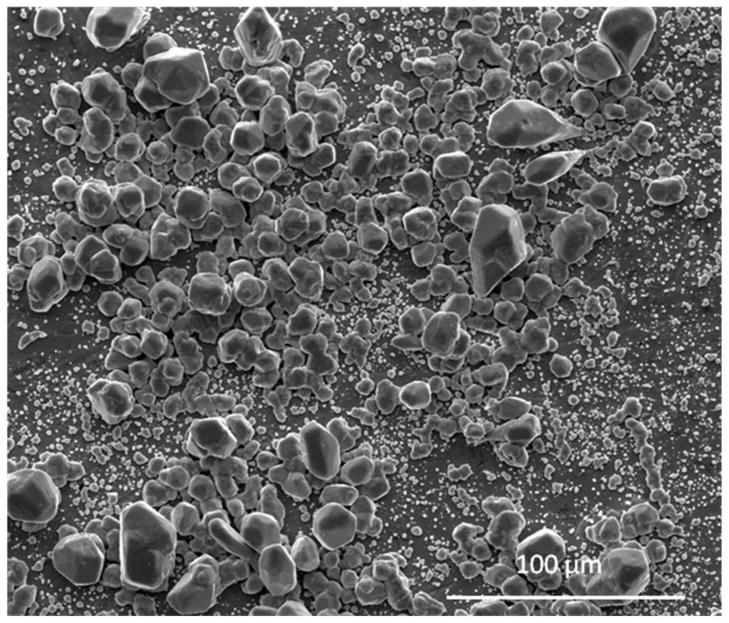
The setup allowed for many parameters to be controlled and varied accordingly. For optimization, a balance of solution concentration, applied current and deposition time were required. Preliminary results showed that silver was being deposited over the entire surface and Figure 2 shows that there is a good range of particle sizes. The electro-deposition resulted in a bimodal distribution of particles, with the large particles mostly between 10 and 20 microns. The majority of the smaller particles were less than one micron in size. To address the question of adherence, adhesive tape was applied to a section of a coated sample and removed to observe how much silver would remain. Figure 3 shows that the tape removed most of the loose particles from the surface.
Figure 2.
SEM images of electro-deposited silver particles. There is a bi-modal distribution of particles. While the majority of the smaller particles are less than one micron in size, large particles are mostly between 10 and 20 microns.
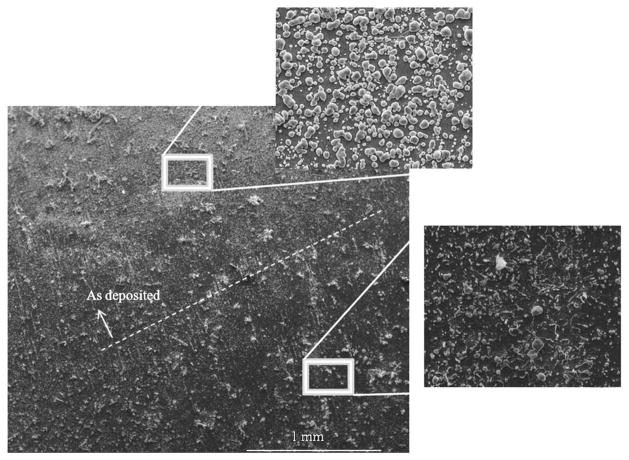
Figure 3.
Below the dashed line silver has been removed and above is “as deposited.” The tape test removed most of the loose particles from the surface.
3.2 Silver Release Rate
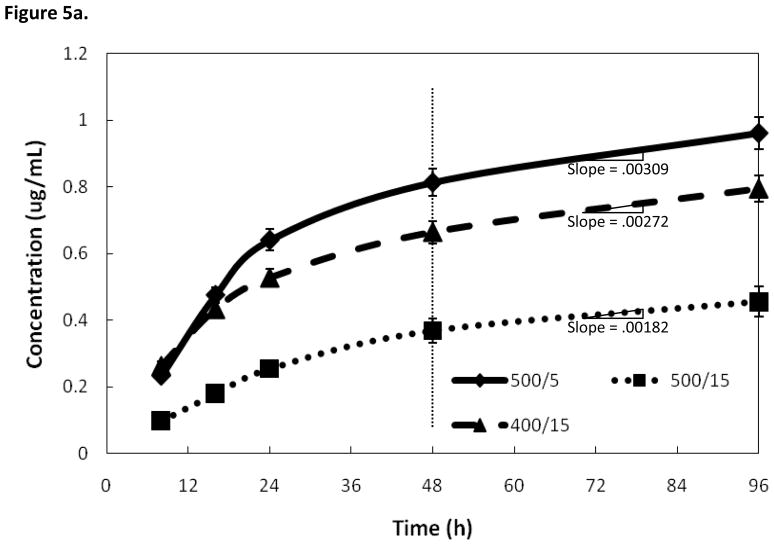
The low adhesion issue of silver particles was addressed by a post deposition heat treatment. The heat treatment time and temperature were varied to find an optimal set of parameters. Temperature was varied between 300 and 600° C and heat treatment time was varied between 2 and 20 minutes in order to find an optimal treatment to increase adhesion of silver particles. To simplify the treatment specifications, the parameters will be listed as heat treatment temperature/heat treatment time i.e. a sample heat treated at 500° C for 5 minutes will be referred to as 500°/5 from now on. SEM images were taken of a sample before and after heat treatment to observe its effects. Figure 4 shows that there was no noticeable morphological difference in heat treated samples compared to non samples prior to heat treatment. Grain sizes, grain boundaries, coating thickness and coverage appear unaffected by heat treatment. Figure 5a shows three samples coated with the same deposition regime, but with different heat treatments. Of the three samples, the 500°/15 heat treatment showed the lowest silver release. This can be explained by too much adhesion of the particles. The next highest release was seen with the 400°/15 heat treatment. The highest release profile was seen with the 500°/5 heat treatment was a compromise of higher temperature and time. The largest difference between the treatments can be seen within the first 24–48 hours. After 48 hours, all three of the heat treatments provided similar release rates. The rate of release was reduced after 48 hours, but the samples continued to release silver for the duration of the study.
Figure 4.
Sample before (left) and after (right) heat treatment of 400° for 15 minutes. There is no significant change in the coating thickness, grain sizes or grain boundaries.
Figure 5.
Figure 5a. Effects on silver release rate of varying heat treatment parameters. All three coatings underwent identical deposition parameters for 3.5 minutes. The main difference between the coatings is their release rate for the first 24–48 hours. After 48 hours the release rates are similar.
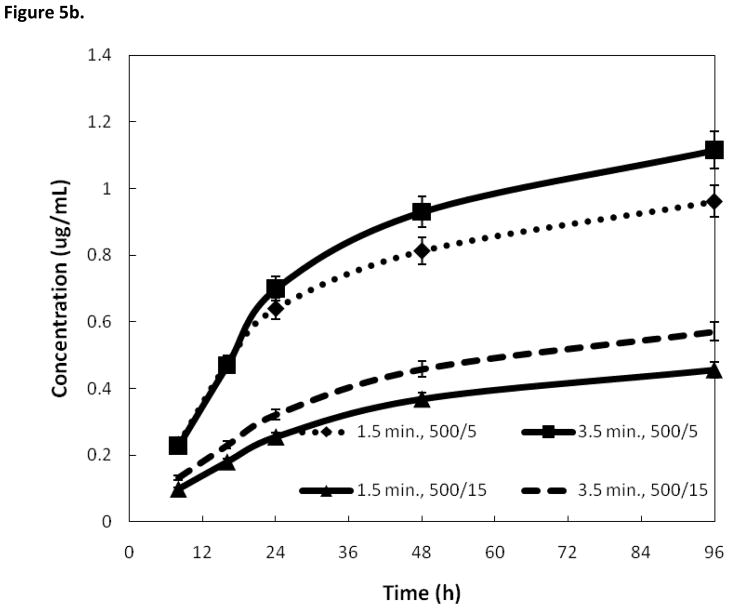
Figure 5b. Effects on silver release rate of varying duration of electrodeposition. In each pair, the longer deposition time showed a higher release rate. However, the differences in heat treatments showed to have a larger effect than deposition time.
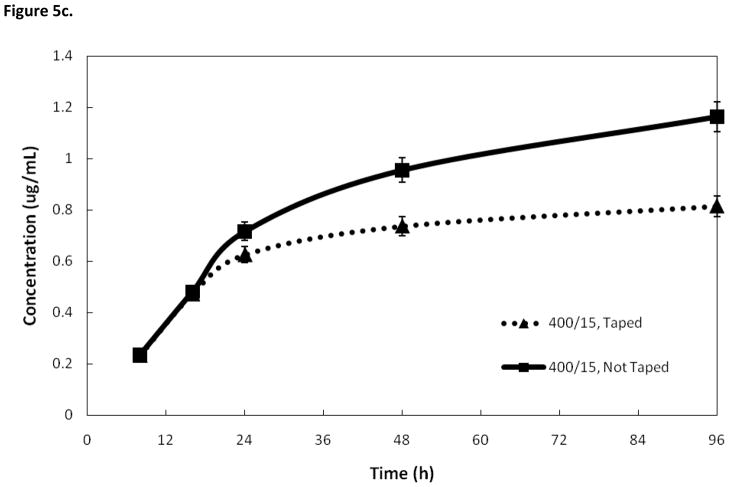
Figure 5c. Effects on silver release rate of removing loose silver with tape. Removing loose silver particles with tape lowers the amount of silver released. Samples prepared with a 3.5 minute deposition time.
Figure 5b compares the silver release rates of two different coating methods where the only difference is the duration of deposition. The first set of samples was prepared with a deposition time of 1.5 minutes, and heat treatment of 500°/5. The second set of samples was prepared with a deposition time of 3.5 minutes and heat treatment of 500°/5. Another pair of samples was prepared with deposition times of 1.5 and 3.5 minutes, both having a heat treatment of 400°/15. In both deposition treatments, the sample with a longer deposition time had a higher silver release rate. However, the deposition time had less of an affect on silver release than the heat treatment conditions. Figure 5c shows the AAS results of an attempt to simulate how much silver would be released in real-world conditions if the nails were handled before implantation. Samples were prepared in duplicate and then one was pressed with tape to remove loose silver and then both were put through an SBF trial and analyzed using AAS. The sample that was taped had a lower silver release rate. Treatment consisted of a deposition time of 3.5 minutes, followed by a heat treatment of 500°/15.
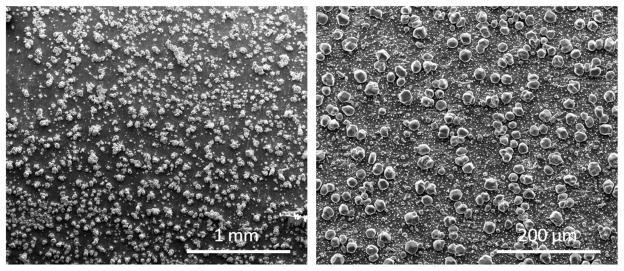
It was also of interest how the coating would withstand sterilization and related handling. Samples were prepared using a 2.5 minute deposition time with heat treatment of 500°/10. The rigorous sterilization process involved three steps: first the sample was steam autoclaved at 270° C for 15 minutes. Next it went through passivation by a solution (pH 2) at 60° C for 10 minutes. Finally it was placed in an ultrasonic cleaner for 15 minutes. Figure 6 shows SEM images of the surfaces of as processed and sterilized samples. The sterilization process visibly reduced the amount of silver particles present on the surface.
Figure 6.
SEM images for comparison of as processes vs sterilized samples. The sterilization process involved three steps: first the sample was steam autoclaved at 270° C for 15 minutes. Next it went through passivation by a solution (pH 2) at 60° C for 10 minutes. Finally it was palced in an ultrasonic cleaner for 15 minutes. Samples were prepared using a 2.5 minute deposition time with heat treatment of 500°/10.
3.3 Antimicrobial Efficacy
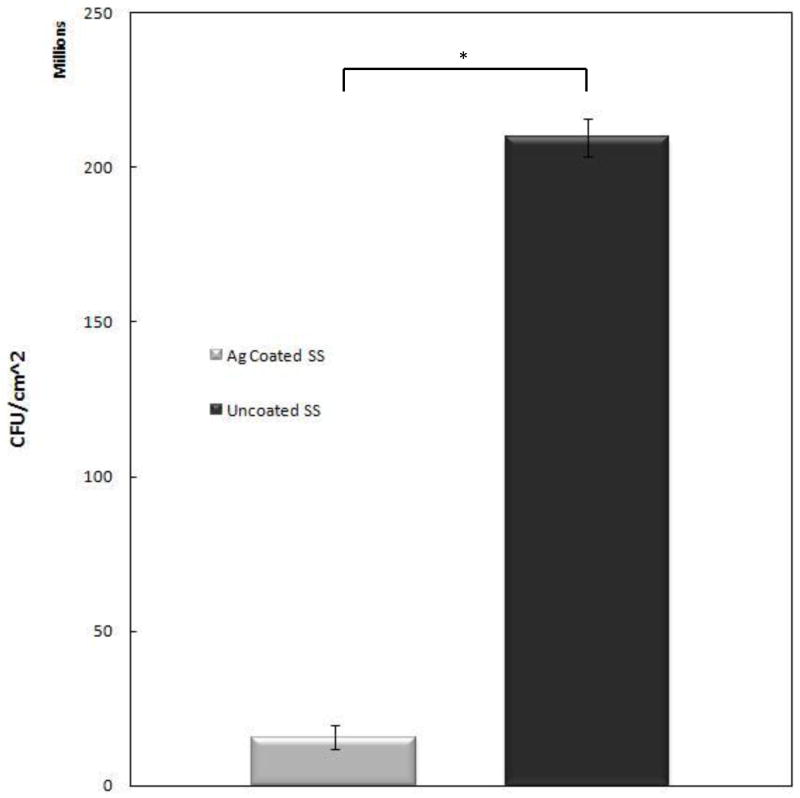
Antimicrobial effects of the silver coating were examined after 24 hours. P. aeruginosa was used because it is estimated that over 80% of infections associated with metallic implants are caused by gram negative bacteria [21]. The antimicrobial test results are shown in Figure 7 and are presented as an average of the samples, which had identical coatings versus an average of controls, which had no coating. The Ag+ coated samples showed more than a 13-fold decrease in bacteria compared to the non-coated control samples over a period of only 24 hours. The samples for this study were prepared using a deposition time of 2.5 minutes with a post deposition heat treatment of 500°/2.5, which showed to be a robust coating that could survive handling and still release silver.
Figure 7.
Silver coated samples and uncoated controls challenged with Pseudomonas aeruginosa results after 24 hours in bioreactor. Performed in triplicate (p <0.001). The coated samples for this study were prepared using a deposition time of 2.5 minutes. Post deposition they were heat treated at 500°/2.5.
3.4 Osteoblast Cell Interaction
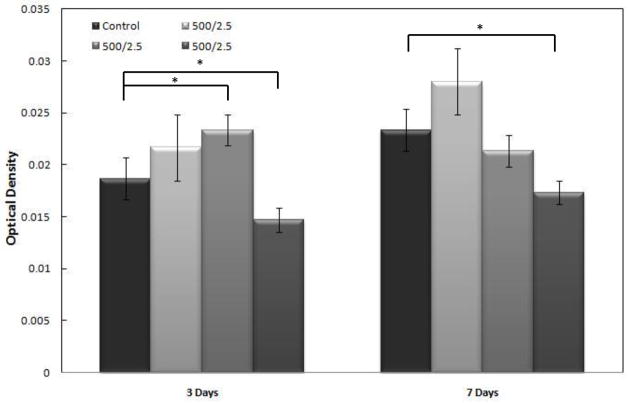
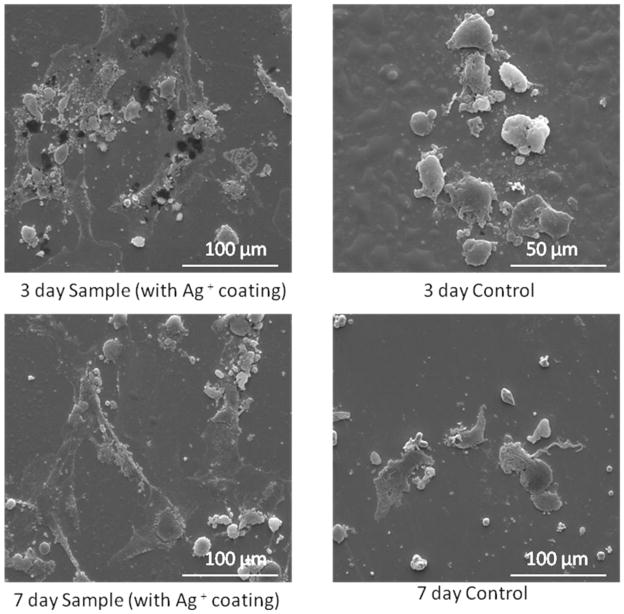
Samples were taken after three and seven days of interaction with hFOB cells. Because this treatment is designed for antimicrobial use, the purpose of studying its affects on osteoblasts is to ensure that Ag+ deposition is not harmful to human tissues. Figure 8 shows MTT assay results of identically prepared samples compared to a non-coated stainless steel control. The samples with a silver coating on average had as many, if not more, active cells as the control. The samples for this study, as well as cell morphology were all prepared using a 2.5 minute deposition time. Samples were all heat treated at 500° C for 2.5 minutes. Cell proliferation was used as a measure of cytotoxicity via the MTT assay. Reductase enzymes in active cells reduce the MTT to purple formazan and a solubilization solution is added to dissolve the formazan crystals into a solution so that the absorbance can be measured with a spectrophotometer. Therefore, higher numbers means there were more active cells present to reduce the MTT, showing that the silver coating was no more toxic to human osteoblasts than a non coated SS substrate. Three and seven day samples were examined under SEM for cell morphology. The SEM images seen in Figure 9 show that on both the three and seven day silver coated samples, the cells are wider, have expanded more and are attaching better than on the control samples without any coating.
Figure 8.
Optical density of MTT assay from osteoblast study. Coated samples were prepared with a deposition time of 2.5 minutes and a heat treatment of 2.5 minutes at 500° C (*p<0.05). Three day sample mean=0.0199, three day control mean=0.01867. Seven day sample mean=0.0222, seven day control mean=0.0233.
Figure 9.
SEM observations of samples and controls subjected to osteoblast study. Coated samples were prepared with a deposition time of 2.5 minutes and a heat treatment of 500°/2.5.
Figure 10 shows the surface energy of four coated and non-coated samples. All samples were prepared using a deposition time of 2.5 minutes. The samples are compared to averaged controls, which have comparable surface energy to literature values [22, 23]. The first sample was heat treated at 400°/15. The second sample had the same heat treatment, but post heat treatment adhesive tape was applied to the surface and removed. Samples 3 and 4 were heat treated at 500°/5. Tape was then used on sample 4 to remove any loose silver particles. The surface energy of all the samples is higher than the surface energy of the control. The silver coating effectively increases the wettability of the surface, making the test liquids have a lower contact angle. This translates into a higher surface energy.
Figure 10.
Surface energy based on contact angle of samples vs. averaged controls. All samples were prepared using a deposition time of 2.5 minutes. The first sample was heat treated at 400°/15. The second sample had the same heat treatment, but post heat treatment adhesive tape was applied to the surface and removed. Samples 3 and 4 were heat treated at 500°/5. Tape was then used on sample 4 to remove any loose silver particles. All samples showed a significant difference compared to the control (p<0.05).
4. Discussion
Silver’s antimicrobial properties are now being used on catheters, medical devices, wound dressings, clothing and everyday items such as grocery carts and door handles [24–26]. The FDA has already approved some silver coated devices for clinical use. Silver has been used for centuries to keep water clean and for other sanitary purposes, but recently it is receiving a lot of attention due to the rise in antibiotic resistant bacteria. Silver is a counter to this because it works via many ways, making bacterial resistance very rare.
In the recent past, silver has been used in film coatings and on other types of surfaces. For example, in our own previous work [10, 11], silver has been applied to a titanium surface with nanotubes, or onto a surface coated with tricalcium phosphate (TCP). Nanotubes, TCP or other surface modifications serve to roughen the surface, which naturally enhances the adhesion of silver. In contrast, this work deposited Ag+ particles onto a smooth, machined SS surface where adhesion is a big concern. Electrodeposition is a cost effective method that can easily be incorporated into most manufacturing processes. By using electrodeposition, silver particles of size varying from the nano to the micro scale can be deposited on the substrate surface. This is advantageous because silver acts in numerous ways and having a range of particle sizes can ensure the most appropriate particle size for the method is present. However, the particles are poorly bonded to the surface. Because of this poor adhesion, the process was adapted to include a post deposition heat treatment step.
Optimization of heat treatment was necessary because higher temperature and time caused a decrease in silver release due to the ions being fixed to the substrate too strongly. Conversely, insufficient heat treatment would not provide enough adhesion for the coating to survive sterilization procedures or normal handling during surgery. A balance was necessary between time and temperature; if temperature was increased then time should be decreased to prevent excessive bonding. If temperature was decreased then time should be increased to ensure that bonding is sufficient. To compensate for the fact that some silver will be removed in normal handling, a precaution is to initially deposit more silver than is necessary. Having more silver on the surface during heat treatment will bond more silver to the surface, allowing the desired amount to remain when the loose silver is wiped off. This results in a more robust coating that can deliver the desired amount of silver while reducing the amount that is lost during handling or sterilization. If a high temperature and time were used, heavy discoloration of the substrate was observed. Balancing heat treatment temperature and time was also necessary to prevent this. Though discoloration of the substrate would not affect its performance, it is not a desirable modification. Heat treatment could have been performed in an argon atmosphere, but in order to keep the processing simple and reduce costs, it was better to use a moderate temperature. Effects of the heat treatment were examined to see if there was a significant difference. Using a Rockwell hardness tester a full length nail control sample measured 30.0Rc at the proximal end of the nail, 28.5Rc mid-shaft and 29.5Rc at the distal end. The treated sample measured 31.0Rc at the proximal end, 29.5Rc mid-shaft and 29.5Rc at the distal end. The difference falls within the ±2 acceptable variance as per specifications. A dimensional inspection confirmed that there was no distortion to the finished product caused by the coating and heat treatment process.
Biofilms are a major reason for infection to occur and are especially concerning on medical devices [39]. Once a biofilm has formed, it is difficult to remove and can necessitate the removal of the medical device. Therefore, the focus of this silver coating is to prevent biofilm formation. This is addressed by the coating of silver particles. A secondary benefit can be achieved through the release of silver ions, but for there to be a safe benefit the silver concentration must be low enough such that it is not toxic to human tissues. Although silver is known for its antimicrobial activity, a Minimum Inhibitory Concentration (MIC) is required for the silver to be fully effective, but too high of a concentration can be cytotoxic. The MIC varies for each bacterium depending on factors such as cell wall thickness and composition but a general concentration range for inhibition is 0.3–20 μg/mL. For bactericidal effects, a minimum concentration of 2–20μg/mL is necessary [14, 24, 27, 28]. Attention is required when choosing the optimal concentration because it has been suggested that concentrations higher than 10μg/mL can be toxic to some human cells [25, 28]. With the main goal being prevention of biofilm formation, this research has been shown to provide a statistically significant reduction in bacteria (p < 0.001). Silver has been used for its antimicrobial properties for many years, and it was shown to be quite effective against p. aeruginosa in this trial. A 13-fold reduction in colony forming units within only 24 hours was observed. This effectiveness agrees with other publications that have used silver as an antimicrobial agent [10–11, 29–30]. It also operates within safe silver concentrations and has been shown to achieve concentrations of 0.5–1.2 mg/L when applied to stainless steel and with a post deposition heat treatment. Measures have also been taken to make this antimicrobial treatment more robust in order to withstand scenarios such as sterilization, which could result in a reduced concentration.
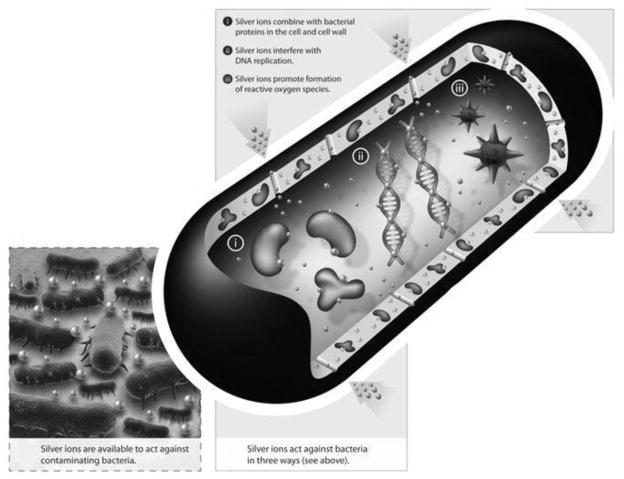
The antimicrobial pathways used by this coating have not been identified, and all the details of how Ag+ works are not entirely known, but there a few hypotheses. Overall Ag+ ions are very active within the body and inactivate bacteria via a number of pathways. Figure 11 is a graphical demonstration of how silver affects bacteria. One pathway is that with silver present, the bacteria will no longer replicate. This is because when the Ag+ ion enters the cell, a precautionary reaction happens to the DNA molecules. The DNA changes to a condensed form and loses its replication abilities. These effects have been observed in both gram positive and gram negative bacteria [31–33]. Another antibacterial pathway of silver is to interact with sulfur and phosphorus containing compounds. The sulfur containing thiol group is an important group for protein activity within the cell, and they contribute to enzymatic activity. Silver combines the SH groups of the proteins, which leads to their inactivation [33–36]. In addition, the bacteria membranes are known to include sulfur containing proteins and silver can bind to those sites. This could explain changes in morphology of the cell membrane that have been observed and would also explain the disruption to respiration. Yet another pathway that silver can impede bacteria is for it to interact with ribosomes and thereby suppresses the expression of enzymes and proteins necessary for ATP production [37]. Specifically, it has been suggested that the ribosomal subunit S2 is inhibited due to silver activity. This denatures the ribosome and impairs its functions. Specifically, the TCA cycle and electron transport chain are disrupted, which halts ATP production leading to ATP deficiencies in the cell [37]. Though it is not clear from our research which of these pathways are active, it is clear that silver addition significantly reduced p. aeruginosa activity on the SS surface.
Figure 11.
Antimicrobial pathways of how silver ions work within the bacteria. Shown are silver’s interaction with the cell wall, interaction with ribosomes, the denaturation of DNA and the formation of reactive oxygen species [36].
Cytotoxicity was examined using an established protocol for an MTT assay [10, 11]. The silver coated samples did not show any toxicity effect and in some cases cell proliferation on silver coated samples was greater than on stainless steel controls. The improved proliferation can be explained by the higher surface energy of the silver coated samples. The coating improved wettability, lowering the contact angle which raised surface energy and increased cell attachment and proliferation. This effect has been seen in previous work [20].
5. Conclusions
An antimicrobial coating could greatly reduce the risk of infection in many surgeries and open wound injury scenarios. Because stainless steel is a widely used implant material for fracture management, using a silver coating on SS is a very practical application. With the rise of antibiotic resistance, silver provides a viable antimicrobial agent that can be applied directly onto implants. This treatment also has the advantage of acting at the site of possible infection. This paper has expanded on the understanding of applying a coating using silver nitrate and post heat treatment of stainless steel. This coating has been shown to be effective against bacteria while being non-threatening to human tissues. Further studies could be useful to determine the in vivo utility of this coating. Our findings can also be useful for optimizing other coating setups and applying similar coatings onto other substrate materials.
Research highlights.
Processing of particulate silver coating that are strongly adherent on SS surface
Optimized the amount of silver that is sufficient to reduce bacterial colonization but non-toxic to human bone tissue
The adhesion strength of silver was sufficient to survive industrial sterilization steps used for fracture management devices
Acknowledgments
The authors would like to acknowledge the financial support from the National Science Foundation under the grant no. CMMI-0728348 and the National Institute of Health under the grant no. EB-ROI-007351 from the NBIB. The authors gratefully acknowledge experimental help from Dr. Haluk Beyenal for his assistance with bacterial testing. We would also like to acknowledge the experimental help from Dr. Mangal Roy for his SEM work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts I. War on the roads. BMJ. 2002;324:1107–8. doi: 10.1136/bmj.324.7346.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nantulya V, Reich M. The neglected epidemic: road traffic injuries in developing countries. BJM. 2002;324:1139–41. doi: 10.1136/bmj.324.7346.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustilo R, Merkow R, Templeman D. The Management of Open Fractures. J Bone Joint Surg Am. 1990;72:299–304. [PubMed] [Google Scholar]
- 4.Dellinger E, Miller S, Wertz M, Grypma M, Droppert B, Anderson P. Risk of Infection After Open Fracture of the Arm or Leg. Arch Surg. 1988;123(11):1320–27. doi: 10.1001/archsurg.1988.01400350034004. [DOI] [PubMed] [Google Scholar]
- 5.Newman J. The Prevention of Infection in open Fractures. J Antimic Chemo. 1983;11:391–92. doi: 10.1093/jac/11.5.391. [DOI] [PubMed] [Google Scholar]
- 6.Ghaffar A, Hyder A, Bishai D, Morrow R. Interventions for control of road traffic injuries: review of effectiveness literature. J Pak Med Assoc. 2002;52(2):69–73. [PubMed] [Google Scholar]
- 7.Krug E, Sharma G, Lozano R. The Global Burden of Injuries. Am J Pub Health. 2000;90(4):523–6. doi: 10.2105/ajph.90.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mock C. Injury in the Developing World. West J Med. 2001;175(6):372–4. doi: 10.1136/ewjm.175.6.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendriks J, Van Horn J, Van Der Mei H, Busscher H. Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection. Biomat. 2004;25:545. doi: 10.1016/s0142-9612(03)00554-4. [DOI] [PubMed] [Google Scholar]
- 10.Roy M, Bandyopadhyay A, Bose S. In vitro antimicrobial and biological properties of laser assisted tricalcium phosphate coating. Mat Sci Eng, C. 2009:10–1016. doi: 10.1016/j.msec.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das K, Bose S, Bandyopadhyay A, Karandikar A, Gibbins B. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. J of Biomedical Materials Research Applied Biomaterials. 2008;87B:455–60. doi: 10.1002/jbm.b.31125. [DOI] [PubMed] [Google Scholar]
- 12.Campocciaa D, Montanaroa L, Arciola C. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–2339. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Wright J, Lam K, Burrell R. Wound management in an era of increasing bacterial antibiotic resistance: a role for topical silver treatment. Am J Infect Control. 1998;26(6):572–7. doi: 10.1053/ic.1998.v26.a93527. [DOI] [PubMed] [Google Scholar]
- 14.Schierholz J, Lucas L, Rump A, Pulverer G. Efficacy of silver coated medical devices. J Hosp Inf. 1998;40:257–62. doi: 10.1016/s0195-6701(98)90301-2. [DOI] [PubMed] [Google Scholar]
- 15.Gadd G, Laurence O, Briscoe P, Trevors J. Silver accumulation in Pseudomonas stutzeri AG 259. Bio Metals. 1989;2:168–173. doi: 10.1007/BF01142556. [DOI] [PubMed] [Google Scholar]
- 16.Wahlberg J. Percutaneous toxicity of metal compounds. Arch Environ Health. 1989;11:201–203. doi: 10.1080/00039896.1965.10664198. [DOI] [PubMed] [Google Scholar]
- 17.Williams R, Williams D. Albumin adsorption on metal surfaces. Biomat. 1988;9:206. doi: 10.1016/0142-9612(88)90085-3. [DOI] [PubMed] [Google Scholar]
- 18.Kokubo T, Kushitani H, Sakka S, Kitsugi, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Matl Res. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 19.Ohtsuki C, Kokubo T, Neo M, Kotani S, Yamamuro T, Nakamura T, Bando Y. Bone Bonding Mechanism of sintered b-3CaO P2O5. Phosph Res Bull. 1991;1:191–196. [Google Scholar]
- 20.Das K, Bose S, Bandyopadhyay A. Surface modifications and cell materials interactions with anodized Ti. Acta Bio. 2007;3:573–585. doi: 10.1016/j.actbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Kunin C. Detection, Prevention and Management of Urinary Tract Infection. 4. Philadelphia, PA: Lea & Febiger; 1987. pp. 245–288. [Google Scholar]
- 22.Hao L, Lasrence J, Li L. The wettability modification of bio-grade stainless steel in contact with simulated physiological liquids by means of laser irradiation. App Surface Sci. 2005;247:453–457. [Google Scholar]
- 23.Hallab N, Bundy K, O’Connor K, Moses R, Jacobs J. Evaluation of polymeric biomaterial surface energy and surface roughness characteristics for direct cell adhesion. Tissue Eng. 2001;7:1. doi: 10.1089/107632700300003297. [DOI] [PubMed] [Google Scholar]
- 24.Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimic Chem. 2007 doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- 25.Monteiro D, et al. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Intl J Antimic Ag. 2009;34:103–110. doi: 10.1016/j.ijantimicag.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 26. [accessed 1-12-11]; http://www.biocote.com.
- 27.Petica A, Gavriliu S, Lungua M, Burunteaa N, Panzarub C. Colloidal silver solutions with antimicrobial properties. MSE B. 2008;152:22–27. [Google Scholar]
- 28.Hidalgo E, Dominguez C. Study of cytotoxicity mechanisms of silver nitrate in human dermal fibroblasts. Tox Letters. 1998;98:169–79. doi: 10.1016/s0378-4274(98)00114-3. [DOI] [PubMed] [Google Scholar]
- 29.Esteban-Tejeda L, Malpartida F, Esteban-Cubillo A, Pecharroman C, Moya JS. Antibacterial and antifungal activity of a soda-lime glass containing copper nanoparticles. Nanotechnology. 2009;20:505701. doi: 10.1088/0957-4484/20/50/505701. [DOI] [PubMed] [Google Scholar]
- 30.Panacek A, Kolar M, Vecerova R, Prucek R, Soukupova J, Krystof V, Hamal P, Zboril R, Kvitek L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30:6333–6340. doi: 10.1016/j.biomaterials.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 31.Balazs D, et al. Inhibition of bacterial adhesion on PVC endotracheal tubes by RF-oxygen glow discharge, sodium hydroxide and silver nitrate treatments. Biomaterials. 2004;25:2139–51. doi: 10.1016/j.biomaterials.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 32.Mahendra R, Alka Y, Aniket G. Silver nanoparticles as a new generation of antimicrobials. Biotech Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Feng Q, Wu J, Chen G, Cui F, Kim T, Kim J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J W&S. 2000;52:662–8. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Morones J, Elechiguerra J, Camacho A, Holt K, Kouri J, Ramirez J, Yacaman M. The bactericidal effect of silver nanoparticles. Nanotech. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T. Mode of Bactericidal Action of Silver Zeolite and Its Comparison with That of Silver Nitrate. Appl Environ Microbiol. 2003;69:4278–81. doi: 10.1128/AEM.69.7.4278-4281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta A, Maynes M, Silver S. Effects of Halides on Plasmid-Mediated Silver Resistance inEscherichia coli. Appl Environ Microbiol. 1998;64:5042–5. doi: 10.1128/aem.64.12.5042-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanaka M, Hara K, Kudo J. Bactericidal Actions of a Silver Ion Solution on Escherichia coli, Studied by Energy-Filtering Transmission Electron Microscopy and Proteomic Analysis. App Env Micr. 2005;71(11):7589–93. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]