Abstract
Objective
To determine if the prevalence of mental disorders and related factors increase as advanced cancer patients get closer to death.
Method
Baseline, cross-sectional data from 289 patients who were assessed prior to their death as part of a multi-site, longitudinal, prospective cohort study of advanced cancer patients. Major Depressive Disorder, Generalized Anxiety Disorder, Panic Disorder, and Posttraumatic Stress Disorder were assessed using the Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I). Other factors examined included existential well-being, patient grief about their illness, physical symptom burden, terminal illness acknowledgment, peacefulness, and the wish to live or die.
Results
Closeness to death was not associated with higher rates of mental disorders. Patients closer to death exhibited increased existential distress and physical symptom burden, were more likely to acknowledge being terminally ill, and were more likely to report an increased wish to die.
Conclusion
Results do not provide support for the common clinical assumption that the prevalence of depressive and anxiety disorders increases as death nears. However, patients' level of physical distress, acknowledgment of terminal illness, and wish to die, possibly reflecting acceptance of dying, increased as death approached. Longitudinal studies are needed to confirm individual changes in rates of mental disorder as patients approach death.
Keywords: advanced cancer, oncology, mental disorders, existential distress, proximity to death, terminal illness awareness
Introduction
As death approaches, it is common for patients with terminal illness to experience an exacerbation in the number and severity of their physical and psychosocial stressors. These stressors can include growing frailty with debilitating physical symptoms, loss of independence, decreased social connectedness, increased existential distress, demoralization [1], death anxiety, conservation-withdrawal [2, 3], and preparatory-grief [4]. The progression of illness and associated stressors may remind patients of their terminal health status, increase their existential distress, and heighten their risk for developing psychiatric disorders. Given this, many clinicians working in palliative care settings have speculated that rates of mental disorders increase as death approaches; however, we were unable to locate a direct empirical examination of this hypothesis.
Earlier, methodologically-limited studies of cancer patients suggested an association between depression and poorer functioning [9–12]. However, these investigations did not focus on patients at the end of life. More recently, Akechi and colleagues studied psychiatric morbidity among Japanese terminally ill cancer patients and demonstrated an association between having advanced cancer and the presence of an adjustment or depressive disorder [13–15]. Akechi et al. also examined the clinical course of psychiatric disorders among advanced cancer patients using only two assessment points [13, 15]. In one longitudinal study report, they offered descriptions of the clinical course observed among patients (e.g., approximately 71% of patients who met criteria for major depressive disorder at baseline no longer met criteria at follow-up), but did not test whether rates of depression or other mental disorders at baseline were significantly different from those at follow-up [14]. Whether the Japanese experience is generalizable to an American population is unclear.
In contrast to findings suggesting a relationship between disease progression and increased mental health problems, Kissane et al. [16] found that similar prevalence rates of DSM-IV mental disorder diagnoses when comparing early stage to advanced breast cancer patients. This comparison was made, however, using baseline assessments from two separate psychosocial intervention clinical trials targeting cohorts of early stage and advanced breast cancer patients, respectively [16]. We have not identified prior research that has specifically examined whether or not prevalence rates of depressive or anxiety disorders increase as death approaches in a single patient sample.
Our understanding of how rates of psychiatric conditions change over time within the context of cancer is complicated in part by the inconsistent findings of previous epidemiological studies [5–8]. While researchers often attribute the variation in point prevalence estimates to the use of different assessment methods [5, 7], the mixed results may also be due to the fact that studies have compared patients who were at different time points in the course of their illness trajectory. Many psychiatric epidemiology investigations of patients diagnosed with a terminal illness have grouped participants into broad categories, including palliative care, terminally ill, advanced cancer, and end-of-life, which do not adequately capture the potential diversity of such samples. Patients vary dramatically along dimensions of physical functioning and quality of life as their disease progresses, and thus within-sample heterogeneity may explain why prior studies examining prevalence estimates of psychosocial factors have reported discrepant results.
Grabsch et al. [17] emphasized the importance of understanding the epidemiology of psychiatric morbidity among advanced cancer patients to minimize the frequency of clinicians neglecting cases that warrant attention. Existential distress at the end of life is often considered normative, which may result in clinicians overlooking or undertreating psychological symptoms [18]. Furthermore, clinical depression may be overlooked because of the difficulty differentiating it from existential distress [6, 18]. In fact, prior studies have suggested that clinicians underestimate the level of depression among more severely depressed cancer patients [19, 20]. This may be particularly problematic when providers are working with advanced cancer patients, as poor detection of psychopathology and low referrals rates have been found [18, 21]. If proximity to death is indeed a risk factor for mental illness, then this might indicate that providers should enhance efforts to detect symptoms of psychopathology such as depression as disease advances so that the appropriate interventions or referrals may be made. If, on the other hand, prevalence rates of mental disorders are not associated with closeness to death, then this would suggest that practitioners may be able to diagnose and treat mental disorders as they do in any other context. Observed psychopathology would not be considered normative.
In the current study, we were interested in examining rates of psychiatric disorders at various stages of disease progression (measured by proximity to death at the time of evaluation) in order to test the hypothesis that the prevalence of these disorders increases as patients approach death. Given the unavailability of data with repeated mental health assessments in end-of-life research, we used what is arguably the best available data for the purpose: baseline assessments of a patient cohort recruited for the Coping with Cancer (CwC) study. The CwC is a multi-site, prospective, longitudinal investigation of an ethnically and geographically diverse sample of advanced cancer patients and their caregivers designed to answer questions about diagnosable depressive and anxiety disorders among advanced stage cancer patients and their caregivers. A previous publication on data from the CwC demonstrated that approximately 12% of patients met criteria for at least one major psychiatric condition at baseline [22].
In the present cross-sectional study, we examined the rates of mental disorders among patients to determine whether the prevalence estimates varied in groups that were categorized by temporal closeness to death, ranging from 0 to greater than 6 months. Results from analyses were adjusted for physical symptom burden, a potentially important confound [23, 24], in order to isolate the mental disturbance from that attributable to underlying disease. We also investigated other psychological factors that might be expected to change in intensity as death approaches and that have previously been associated with psychiatric morbidity among patients, such as existential distress and patients' cognitive and emotional acceptance of their terminal health status [25]. Prior studies have suggested that patients' awareness of their terminal prognosis (i.e., cognitive acceptance) tends to increase as they approach death [26, 27]. Prigerson [28] found that terminally ill patients who acknowledge death (i.e., terminal illness acknowledgment [TIA]) are more likely to receive palliative care without further aggressive interventions. Patients exhibiting TIA are also more likely to engage in advance care planning [25] has also been associated with increased advance care planning [25]. While cognitive acceptance in the absence of emotional acceptance may be associated with greater distress, when both TIA and a sense of peacefulness are present, caregivers have reported improved quality of death in patients' last week of life [25]. Examination of these additional influential factors represents a novel contribution to this literature.
METHOD
Study Group and Design
The participants in this cross-sectional investigation were 289 advanced cancer patients who were recruited as part of the CwC study, a multi-institutional longitudinal evaluation (National Institutes of Health [NIH] grants MH63892, CA106370) of the prevalence of mental illness and patterns of mental health service utilization in advanced cancer patients and their primary informal (nonpaid) caregivers. This report focuses on baseline data from patients for whom date of death data was available; patients who were still alive at the time of these analyses were not included.
Participants were recruited between August 1, 2002 and May 25, 2007 from the Yale Cancer Center (CT), the Veterans Affairs Connecticut Healthcare System Comprehensive Cancer Clinics (CT), Memorial Sloan-Kettering Cancer Center (NY), and the Parkland Hospital Palliative Care Service (TX). Inclusion criteria for the study were: 1) diagnosis of advanced cancer (presence of distant metastasis and failure of first-line chemotherapy); 2) diagnosis at a participating site; 3) age 20 years or older; 4) identified unpaid, informal caregiver; and 5) adequate stamina to complete the interview. Patients were excluded when they met criteria for dementia or delirium using the neurobehavioral cognitive status examination [29] or could not speak English or Spanish.
Interviewers from each site participated in an initial 2-day training session followed by annual review training sessions conducted by research staff at Yale University. They were required to achieve a high standard of accuracy and reliability based on concordance with the Yale Project Director's rating of the Structured Clinical Interview for the DSM-IV [30] diagnoses (target kappa > 0.85). Patient interviews took 45 minutes to complete on average. Patients who declined participation were asked to complete a brief questionnaire regarding their reasons for refusal, amount of emotional and physical distress, and demographics. All study protocol and contact documents were reviewed and approved by the human subjects committee at Yale University and at each of the participating institutions.
Of the 927 patients who were approached for participation and confirmed to be eligible for the CwC, 287 (31%) declined participation. The most common reasons for nonparticipation included “not interested” (n = 120), “caregiver refuses” (n = 37), and “too upset” (n = 28). Nonparticipants were more likely to be white (chi-square = 9.96; p = 0.04) and to report greater distress on a 5-point Likert scale whose extremes ranged from 1 (“minimal/nonexistent”) to 5 (“distraught”; mean score of 3.8 vs. 2.8; p < 0.0001). There were no significant differences between nonparticipants and participants in gender, age, or education. CwC participants who were not included in the current study were those who were still alive at the time of our analyses (n = 351). When compared to participants in the current study, these survivors were more educated (mean 13.4 vs. 12.4 years; p = 0.002); and were more likely to have health insurance (chi-square = 67.74; p < 0.0001); to be white (chi-square = 23.91; p < 0.0001); Catholic, Protestant, or Jewish (chi-square = 35.65; p < 0.0001); and to be married (chi-square = 14.31; p < 0.0008). Survivors were not significantly different from participants in the present study with respect to gender, age, or the presence of any SCID-I diagnosis assessed or psychotropic medication used at baseline.
Measures
Patients provided demographic information, including gender, age, ethnicity, education level, religion, marital status, and health insurance status. The Zubrod Performance Scale [31] and the Karnofsky Performance Status Scale [32–34] were used to assess performance status, and the Charlson Comorbidity Index [35] was used to assess severity of illness based on multiple organ failure. Physical symptom burden was assessed using a composite of three items from the McGill Quality of Life Questionnaire [36], a reliable quality of life measure validated for use with palliative care populations. Patients were asked: 1) to indicate whether any of 12 listed physical symptoms were present over the past two days, 2) to rate the extent to which any physical symptoms that they had experienced over the past two days bothered them using an 11-point Likert scale ranging from 0 = “Did not bother me at all” to 10 = “Bothered me tremendously,” and 3) to rate how they have felt over the past two days on an 11-point Likert scale ranging from 0 = “Physically terrible” to 10 = “Physically well.”
Patients' closeness to death was calculated using the recorded date of death. First, we first computed the number of days between the baseline interview and the recorded date of death. Next, we converted closeness to death into an ordinal variable that was measured in months rather than days because of the common clinical practice of physicians and preference of patients to discuss life expectancy using rougher estimates. After examining the distribution of this variable, we found that the majority of patients were assessed within six months of their death. We then created seven “closeness to death” temporal intervals, which were defined (in the number of days from death) as follows: 0 to 30 days (n = 46), 31 to 60 days (n = 37), 61 to 90 days (n = 39), 91 to 120 days (n = 26), 121 to 150 days (n = 17), 151 to 180 days (n = 21), and 181 to 1377 days (n = 103) from death.
The CwC investigation conducted comprehensive evaluations of physical health and psychosocial functioning, including structured clinical interviews using the Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I) [30] to assess mental disorders. These included major depressive disorder (MDD), generalized anxiety disorder (GAD), panic disorder (PD), and posttraumatic stress disorder (PTSD). Reliability and validity for the SCID-I has been established [30, 37, 38]. Patients only completed the full SCID-I modules assessing disorders for which they screened positive.
The rater-administered version of the Inventory of Complicated Grief-Revised (ICG-R) [39] was used to diagnose Complicated Grief, now called Prolonged Grief Disorder (PGD) [40], the diagnostic criteria of which were determined and applied later in the course of the CwC. The PGD criteria were modified so that patients were asked to evaluate symptoms related to the loss of their healthy self due to cancer (rather than due to interpersonal loss) and did not include the duration criterion.
Mental disorder diagnoses were analyzed as dichotomous outcomes (i.e., met criteria versus did not meet criteria for a diagnosis). MDD was also evaluated using the Endicott symptom substitution method because symptom endorsement among advanced cancer patients could be a consequence of physical disease and/or treatment [41]. The Endicott method involves modifying symptom criteria such that weight loss is substituted with depressed appearance, insomnia with social withdrawal or decreased talkativeness, loss of energy with brooding, self-pity, or pessimism, and poor concentration with lack of reactivity/cannot be cheered up [41].
Self-report data about psychotropic medication use (i.e., antidepressants, anxiolytics, and/or antipsychotics) were extracted from a questionnaire assessing mental health service utilization [22, 42] among patients and caregivers and was coded as a dichotomous variable.
Patients' self-reported terminal illness acknowledgment (TIA) was assessed with the question, “How would you describe your current health status?” Response options were as follows: 1 = “Relatively healthy”; 2 = “Seriously but not terminally ill”; and 3 = “Seriously and terminally ill.” TIA was coded as a dichotomous variable; it was considered present among those patients who endorsed response option 3 (“Seriously and terminally ill”) and absent among those who selected 1 or 2 [28].
Patients reported to what extent they felt “deep inner peace or harmony” on a six-point Likert scale, where 1 = “Many times a day,” and 6 = “Never or almost never.” This item was extracted from the NIA/Fetzer Multidimensional Measure of Religiousness/Spirituality for Use in Health Research [43], which was included as an assessment of general spiritual experiences. The median response for the study sample was 3 (“most days”). Responses of 3 (“most days”) or greater were coded as 1 (“peaceful”); otherwise responses were coded as 2. A one-item measure of peacefulness has been demonstrated to have a strong association with a variety of spirituality constructs, including faith, purpose, and emotional and spiritual well-being [44]. Peaceful awareness was considered present among patients coded as both peaceful and aware (i.e., for whom TIA was present) and absent among patients who were peaceful but unaware, aware but not at peace, or neither at peace nor aware. Existential well-being was assessed more generally using the existential subscale of the McGill Quality of Life Questionnaire [36].
Patients also completed the 13-item Yale Evaluation of Suicidality (YES) scale, which has been demonstrated as a valid and reliable measure of suicidality [45]. For the current study, we examined responses to the first two items, which asked patients to evaluate the strength of their wish to live and the strength of their wish to die in light of their current circumstances. These two single items were rated on a 4-point scale ranging from 1 = “Strong” to 4 = “Have none.” We coded responses of a “strong” and “moderate” wish to live/die as present and “weak” and “no wish to live/die” as absent.
Data Analysis
Participant characteristics were determined using descriptive statistics. Logistic regression models were constructed to determine whether the following factors were significantly associated with closeness to death based on the temporal bins described in the Methods section: 1) each of the four psychiatric diagnoses, 2) at least one of the four psychiatric diagnoses, 3) use of psychotropic medications, 4) TIA, 5) peacefulness, 6) peaceful awareness, 7) patient grief, 8) wish to live, and 9) wish to die. Linear regression was conducted to predict mean physical symptom burden and existential well-being. Colinearity was assessed by cross-tabulations and plots of the analyzed covariates. Physical symptom burden was selected as a covariate in the adjusted regression analyses due to its significant association with closeness to death. Data were analyzed with the SAS System for Windows v. 8.2 (SAS Institute, Inc., Cary, NC) with two-tailed statistical tests.
RESULTS
Participant Characteristics
The majority of patients were white (63%), male (56%), of relatively good health status (76% with Zubrod Score ≤ 2, which corresponds to patients being ambulatory at least 50% of their waking hours), insured (80%), and were on average at least high school educated. The median age of the study participants was 60 years old. See Table 1 for a summary of patient characteristics. A variety of primary cancer sites were represented among participants, including lung (20%), colon (15%), breast (9%), and pancreatic (8%) cancers.
Table 1.
Patient Characteristics at Time of Assessment
| Characteristic | Total Patient Sample Na = 289 | With Mental Disorder Diagnosis Na = 30 | Without Mental Disorder Diagnosis Na = 259 | t or χ2 b | p |
|---|---|---|---|---|---|
| Gender; N (%) | |||||
| Male | 160 (56) | 16 (6) | 136 (49) | 0.04 | 0.8393 |
| Female | 127 (44) | 14 (5) | 110 (40) | ||
| Age, years; mean (SD) | 58.7 (12.0) | 54.3 (13.2) | 59.1 (11.8) | 2.07* | 0.0396 |
| Race/Ethnicity; N (%) | |||||
| White, non-Hispanic | 182 (63) | 20 (7) | 153 (55) | 0.16c | 0.9250 |
| Black, non-Hispanic | 52 (18) | 5 (2) | 47 (17) | ||
| Hispanic | 48 (17) | 5 (2) | 41 (15) | ||
| Asian | 3 (1) | 3 (1) | 0 (0) | ||
| Other | 2 (1) | 2 (1) | 0 (0) | ||
| Education, years; mean (SD) | 12.4 (4.1) | 11.4 (4.4) | 12.1 (3.9) | 1.02 | 0.3088 |
| Religion; N (%) | |||||
| Catholic | 110 (38) | 15 (6) | 87 (32) | 6.29c | 0.2791 |
| Protestant | 51 (18) | 4 (1) | 45 (17) | ||
| Baptist | 42 (16) | 6 (2) | 36 (13) | ||
| Pentecostal | 8 (3) | 2 (1) | 6 (2) | ||
| Jewish | 7 (2) | 1 (0.5) | 5 (2) | ||
| Muslim | 2 (1) | 0 (0) | 2 (1) | ||
| Other | 55 (19) | 2 (1) | 49 (18) | ||
| None | 13 (5) | 0 (0) | 12 (4) | ||
| Marital Status; N (%) | |||||
| Married | 158 (55) | 18 (7) | 115 (45) | 1.27 | 0.5300 |
| Karnofsky Score; mean (SD) | 62.7 (17.6) | 60.7 (16.5) | 63.5 (16.9) | 0.81 | 0.4190 |
| Zubrod Performance Scale Score; mean (SD) | 1.8 (0.9) | 1.9 (0.9) | 1.8 (0.9) | −0.34 | 0.7328 |
| Charlson Comorbidity Index; mean (SD) | 8.2 (2.6) | 7.7 (1.8) | 8.3 (2.7) | 0.98 | 0.3298 |
| Patient survival from baseline (days); mean (SD) | 193 (212) | 197 (217) | 173 (190) | 0.54 | 0.5904 |
| Health Insurance N (%) | |||||
| Insured | 157 (55) | 14 (5) | 121 (48) | 0.01 | 0.9223 |
| Uninsured | 129 (45) | 12 (5) | 108 (42) |
Note.
p < 0.05.
p < 0.01.
p < 0.001.
Sample sizes vary due to missing data; 278 of the 289 patients in this study had SCID-I data available.
Test of comparison between patients with and without mental disorder diagnosis.
Comparison did not include categories with zero frequencies.
Mental Disorders
Overall, 10.8% (n = 30) of the 278 deceased patients with available SCID-I data in this sample met criteria for at least one of the four psychiatric diagnoses (MDD, GAD, PD, and PTSD) assessed. The prevalence rates of psychiatric morbidity in the current sample were comparable to those previously reported on data from the CwC [22]. Prevalence rates of SCID-I diagnoses by cancer type are detailed in Table 2. Patients with lung, colon, pancreatic, and breast cancers exhibited the highest rates of mental disorders overall. There were no significant differences in the type of cancer among patients who were diagnosed with at least one SCID-I diagnosis.
Table 2.
Prevalence Rates of Mental Disorders by Cancer Site
| Cancer Sitea | At Least One SCID-I Diagnosisb (n = 274) | χ2 (df)c | p | Generalized Anxiety Disorder (n = 273) | SCID Depression (n = 272) | Panic Disorder (n = 271) | Posttraumatic Stress Disorder (n = 273) |
|---|---|---|---|---|---|---|---|
| Lung | 2.6 % (7/274) | 0.17 (1) | 0.68 | 0.4% (1/273) | 1.1% (n = 3/272) | 0.7% (2/271) | 1.1% (3/273) |
| Colon | 1.8% (5/274) | 0.02 (1) | 0.88 | 0.0% (2/273) | 0.7% (2/272) | 0.7% (2/271) | 0.4% (1/273) |
| Pancreatic | 1.5% (4/274) | 1.28 (1) | 0.26 | 0.4% (1/273) | 1.5% (n = 4/272) | 0.7% (2/271) | 0.4% (1/273) |
| Breast | 1.1% (3/274) | 0.06 (1) | 0.80 | 0.4% (1/273) | 1.1% (n = 3/272) | 0.0% (0/271) | 0.4% (1/273) |
| Stomach | 0.4% (1/274) | 0.02 (1) | 0.89 | 0.0% (0/273) | 0.0% (0/272) | 0.4% (1/271) | 0.0% (0/273) |
| Esophageal | 0.4% (1/274) | 0.08 (1) | 0.77 | 0.4% (1/273) | 0.4% (1/272) | 0.0% (0/271) | 0.4% (1/273) |
| Brain | 0.4% (1/274) | 0.43 (1) | 0.51 | 0.0% (0/273) | 0.0% (0/272) | 0.4% (1/271) | 0.0% (0/273) |
| Other Cancer | 0.4% (1/274) | 0.16 (1) | 0.69 | 0.0% (0/273) | 0.4% (1/272) | 0.0% (0/271) | 0.0% (0/273) |
| Gallbladder | 0.0% (0/274) | 0.63 (1) | 0.43 | 0.0% (0/273) | 0.0% (n = 0/272) | 0.0% (0/271) | 0.0% (0/273) |
Note.
Sample sizes vary due to missing SCID-I and/or cancer site data.
Refers to presence of at least one of four SCID diagnoses, not including depression diagnosed using Endicott substitution method.
Tests comparing Cancer Site and At Least One SCID-I Diagnosis conducted. Cell counts comparing each cancer site and individual SCID-I diagnoses were too small to estimate stable, reliable differences between the subgroups. df = degrees of freedom.
Patients who currently met criteria for at least one of the SCID-I diagnoses assessed were significantly younger than those patients without psychiatric morbidity, but there were no other significant differences in demographic or medical characteristics between these groups (all p's > 0.10; see Table 1).
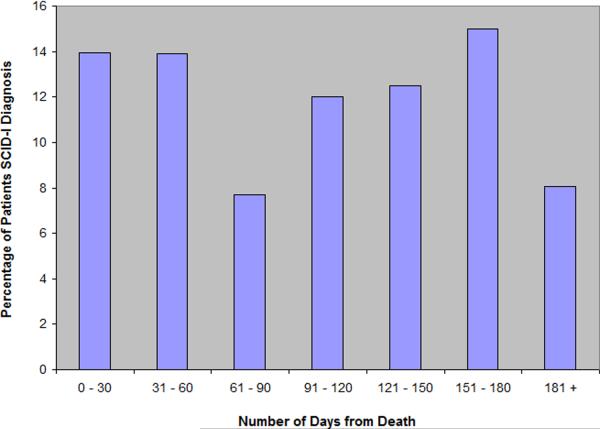
Percentages of patients who met criteria for at least one mental disorder are graphically illustrated in Figure 1. Rates of mental illness among patients did not significantly increase or decrease as time to death approached. That is, closeness to death did not predict the presence of MDD, GAD, PD, PTSD, or the presence of at least one of these disorders (all p's > 0.10; see Table 3). There similarly was not a significant association between psychotropic medication use and patients' proximity to death (p > 0.10).
Figure 1. Percentages of Patients with at Least One Depressive or Anxiety Disorder.
Note. The bar representing the percentage of patients assessed 181 + days includes patients who died between 181 and 1377 from their baseline assessment (n = 103). This temporal bin is disproportionately large when compared to the other six temporal bins (each 30 days).
Table 3.
Associations between Closeness to Death and Mental Disorders among Patients
| Diagnosisa | Unadjusted OR (95% CI) | p value | Adjusted OR (95% CI)b | p value | ||
|---|---|---|---|---|---|---|
| Generalized Anxiety Disorder | 1.003 | (1.727 – 0.383) | 0.9877 | 1.217 | (0.852 – 1.739) | 0.2801 |
| SCID Depression | 0.958 | (0.793 – 1.159) | 0.6607 | 1.060 | (0.863 – 1.303) | 0.5784 |
| Endicott Depression | 0.924 | (0.753 – 1.134) | 0.4477 | 1.022 | (0.815 – 1.283) | 0.8479 |
| Panic Disorder | 1.023 | (0.767 – 1.363) | 0.8793 | 1.085 | (0.800 – 1.471) | 0.5999 |
| Posttraumatic Stress Disorder | 0.829 | (0.616 – 1.115) | 0.2146 | 0.955 | (0.696 – 1.309) | 0.7748 |
| At Least One of the Above Diagnosesc | 0.926 | (0.787 – 1.090) | 0.3559 | 1.016 | (0.852 – 1.212) | 0.8565 |
Note.
Binary outcomes indicating the presence or absence of current diagnosis.
Logistic regression analyses adjusted for patients' physical symptom burden.
Refers to presence of at least one of four SCID diagnoses, not including depression diagnosed using Endicott substitution method.
Although the tests of association between mental disorders and closeness to death were nonsignificant, we conducted post-hoc analyses in order to further characterize those patients close to death who did meet criteria for a SCID-I diagnosis. Of the 30 patients who met criteria for at least one SCID-I diagnosis, over one-third (36.7%) were within two months or less of dying. Therefore, we divided patients into two groups: “closer to death” (assessed two months or less from their death; n = 79) and “less close to death” (assessed more than two months from their death; n = 199). Of the 79 patients in the closer to death group, 11 (13.9% of closer to death group; 4.0% of total sample) met criteria for a SCID-I diagnosis. Of the 199 patients who were further from death, 19 (9.6% of less close to death group; 5.8% of total sample) met criteria for a SCID-I diagnosis, but these groups did not significantly differ.
Patients closer to death who carried a SCID-I diagnosis were characterized by conducting separate logistic regressions predicting the presence of at least one mental disorder diagnosis in order to determine if there was a significant interaction between each patient characteristic or outcome variable of interest and the dichotomous closeness to death variable. We found that patients who met criteria for at least one SCID-I diagnosis and who were close to death were significantly younger, had lower scores on the Charlson Comorbidity Index, were less likely to use psychotropic medication, and were less likely to acknowledge their terminal health status or to be peacefully aware of their diagnosis than patients who did not meet criteria for a mental disorder diagnosis and were close to death (p`s < 0.05). Significant main effects of close to death status were observed for each of these variables, as well (all p's < 0.02). Statistically significant results are summarized in Table 4.
Table 4.
Prediction of the Presence of a Mental Disorder Diagnosis by Patient Variables and Close to Death Status
| Variable | Closer to Deatha | Further from Deatha | Closer to Death × Patient Characteristic Interaction Effect | p | ||
|---|---|---|---|---|---|---|
| With Dx (N = 11) | Without Dx (N = 68) | With Dx (N = 19) | Without Dx (N = 180) | |||
| OR (95% CI) | ||||||
| Age, years; mean (SD) | 45.9 (14.1) | 61.2 (12.3) | 59.1 (10.1) | 58.5 (11.5) | 0.91 (0.85 – 0.98)** | 0.008 |
| Charlson Comorbidity Index; mean (SD) | 6.4 (1.9) | 8.9 (3.1) | 8.7 (1.8) | 8.0 (2.5) | 0.64 (0.46 – 0.88)** | 0.007 |
| Psychotropic Medication Useb | 4 (1) | 21 (8) | 14 (5) | 38 (14) | 0.12 (0.02 – 0.68)* | 0.016 |
| Terminal Illness Acknowledgmentb | ||||||
| Present | 3 (1) | 47 (17) | 8 (3) | 56 (20) | 0.11 (0.02 – 0.59)** | 0.010 |
| Absent | 8 (3) | 21 (8) | 11 (4) | 123 (45) | ||
| Peaceful Awarenessb,c | ||||||
| Present | 2 (0.7) | 31 (11) | 5 (2) | 31 (11) | 0.14 (0.02 – 0.99)* | 0.048 |
| Absent | 9 (3) | 36 (13) | 13 (5) | 148 (54) | ||
Note.
p < 0.05.
p < 0.01.
p < 0.001.
Sample sizes vary due to missing data.
Binary outcomes indicating the presence or absence of characteristic or outcome.
Peaceful awareness coded as present when both terminal illness acknowledgment and peacefulness present; otherwise coded as absent.
Physical Symptom Burden
Closeness to death was significantly associated with patients' physical symptom burden, as reported in Table 5. Patients who were approaching death reported that they were not feeling well and had more physical symptoms that bothered them to a greater extent, β = −0.31, p < 0.0001.
Table 5.
Associations between Closeness to Death and Other Medical and Psychosocial Factors among Patients
| Dependent Variable | Unadjusted OR (95% CI) or Standardized β Coefficient | p value | Adjusted OR (95% CI)b | p value | ||
|---|---|---|---|---|---|---|
| Psychotropic Medication Usea | 0.96 | (0.86 – 1.08) | 0.499 | 1.02 | (0.90 – 1.15) | 0.783 |
| Physical Symptom Burdenc | −0.31*** | < 0.001 | ||||
| Terminal Illness Acknowledgmenta | 1.31*** | (1.17 – 1.46) | < 0.001 | 1.25*** | (1.12 – 1.40) | < 0.001 |
| Peacefulnessa,d | 1.03 | (0.93 – 1.15) | 0.587 | 1.04 | (0.93 – 1.17) | 0.488 |
| Peaceful Awarenessa,e | 1.25*** | (1.11 – 1.41) | < 0.001 | 1.21** | (1.06 – 1.37) | 0.004 |
| Existential Well-Beingc | 0.12* | 0.044 | 0.01 | 0.928 | ||
| Wish to Livea,f | 0.74** | (0.60 – 0.91) | 0.005 | 0.812 | (0.65 – 1.02) | 0.069 |
| Wish to Diea,f | 1.39*** | (1.16 – 1.67) | < 0.001 | 1.28 | (1.05 – 1.55) | 0.014 |
| Patient Grief about Illnessa | 0.89 | (0.76 – 1.04) | 0.137 | 0.94 | (0.79 – 1.11) | 0.444 |
Note.
p < 0.05.
p < 0.01.
p < 0.001.
Binary outcomes indicating the presence or absence of variable.
Logistic regression analyses adjusted for patients' physical symptom burden.
Linear regression analyses.
Peacefulness coded as present with frequency rating of experiencing a deep sense of inner peace or harmony on “most days” or more; absent with lower frequency.
Peaceful awareness coded as present when both terminal illness acknowledgment and peacefulness present; otherwise coded as absent.
“Strong” and “moderate” wish to live/die coded as present; “weak” and “no wish to live/die” coded as absent.
Terminal Illness Acknowledgment, Peacefulness, and Existential Distress
Proximity to death also significantly predicted TIA among patients, who were 1.3 times more likely to acknowledge their terminal health status when they were closer to the end of life (adjusted OR = 1.25, 95% CI: 1.12 – 1.40). Results are presented in Table 5. TIA was found among 65% (n = 30 / 46) of patients assessed within one month of dying, 60% (n = 22 / 37) assessed within two months, 28% (n = 11 / 39) assessed within three months, 58% (n = 15 / 26) assessed within four months, 53% (n = 9 / 17) assessed within five months, and 43% (n = 9 / 21) assessed within six months. Rates of TIA were highest in the last two months of life. Twenty-two percent (n = 22 / 101) of patients assessed more than six months away from their death acknowledged their terminal health status.
Similar to its association with TIA, closeness to death predicted peaceful awareness. Patients were 1.2 times more likely to be peacefully aware as death approached (adjusted OR = 1.21, 95% CI: 1.06 – 1.37). Physical symptom burden was associated with both TIA (r = 0.22, p < 0.0001) and peaceful awareness (r = 0.19, p = 0.002), and the relationship between closeness to death and these outcomes remained statistically significant even when controlling for patients' reported physical burden. However, as peacefulness alone was not significantly related to closeness to death (p > 0.10), the statistical significance of the test of the modest association between closeness to death and peaceful awareness may have been driven by the observed increases in TIA.
We found additionally support for the hypothesis that higher rates of peaceful awareness were due to increases in TIA rather than a growing sense of peacefulness as death approached in our examination of existential well-being. Decreases in existential well-being, suggesting existential distress, were associated with proximity to death in an unadjusted analysis, β = 0.12, p = 0.044. However, application of Baron and Kenny's [46] criteria for evaluating the presence of mediation demonstrated that physical symptom burden mediated the relationship between existential well-being and closeness to death in this patient sample. Physical symptom burden was positively associated with closeness to death (see above, p < 0.0001) and was negatively associated with existential well-being (β = −0.35, p < 0.0001). When physical symptom burden was controlled for in the regression model predicting existential well-being from closeness to death, physical symptom burden remained a strong predictor of existential well-being (β = −0.31, p < 0.0001), whereas closeness to death did not (β = 0.01, p = 0.928). Existential distress was greater among patients who felt physically worse, and was therefore higher as patients approached death because they were experiencing an increase in physical symptom burden.
Wish to Live, Wish to Die, and Patient Grief
Patients were more likely to report a wish to die as death approached even after adjusting for physical symptom burden (adjusted OR = 1.33, 95% CI: 1.05 – 1.55). In addition, closeness to death was associated with a decreased likelihood of the wish to live among patients (unadjusted OR = 1.36, 95% CI: 1.09 – 1.68), though this finding was only marginally significant when controlling for physical symptom burden (adjusted OR = 1.23, 95% CI: 0.98 – 1.54). The association between closeness to death and patient grief over losses related to their illness was nonsignificant (p > 0.10).
DISCUSSION
This cross-sectional study examined rates of mental disorders along different time points at the end of life to determine if they increased among advanced cancer patients as death approached. Research addressing the impact of proximity to death on the prevalence rates of psychiatric illness is limited. Given the likelihood of stressful circumstances such as multiple losses, uncertain future, and physical discomfort increasing as disease advances, we hypothesized that vulnerable patients would be at increased risk of developing Axis I psychiatric morbidity as death approached. Contrary to our hypotheses, the prevalence estimates of MDD, GAD, PTSD, or PD were not associated with closeness to death in the current sample. These findings begin to address outstanding questions about the course of depressive and anxiety disorders at the end of life, challenging the long-standing assumption that mental health problems are generally exacerbated.
Our results are similar to those of Kissane and colleagues [16], who found that psychiatric disorders were similarly prevalent among early stage and advanced breast cancer patients. To explain this finding, the authors suggested that patients with metastatic disease may not experience heightened rates of psychiatric morbidity despite the potential increase in perception of existential threat because they may adapt to initial existential distress, to related feelings of uncertainty, and to the management of symptoms and side effects that are common among more newly diagnosed patients [16]. However, in the current sample, we observed that patients closer to death reported increased existential distress without a parallel increase in rates of psychiatric disorders. The association between greater existential distress and proximity to death appeared to be driven by the heightened physical symptom burden reported by more imminently dying patients. This may argue against the idea proposed by Kissane et al. [16] that more advanced patients adapt to existential distress via better management of their physical symptoms and side effects; rather, physical symptoms may play a more direct role in inciting existential concerns.
Another explanation for our findings is that individuals experiencing more somatic symptoms were those who were assessed later in their disease course and, therefore more likely to perceive themselves to be closer to death, were more likely to be existentially distressed. Physical frailty and discomfort are reminders of one's physical existence that may result in increased dependence on others and a potential loss of dignity and, in turn, could disturb existential well-being. Although physical pain has been conceptually distinguished from “spiritual pain” [47], the role that these factors and their interaction play in the onset or exacerbation of Axis I disorders is not clear. A recent investigation found that physical symptom burden was not associated with anxiety and mood symptoms among advanced cancer patients [48]. In contrast, other studies have demonstrated a relationship between psychiatric illness (particularly depression) and physical symptoms [23, 24]. Wilson et al. [49] found that palliative care patients diagnosed by semi-structured interview with an anxiety or depressive disorder reported a greater number of moderate or severe physical symptoms, as well as existential concerns, than patients without a disorder. The discrepancies between these findings may be explained in part by differences in assessment techniques and predictive abilities of scales.
In addition to observing a relationship between increased existential distress and closeness to death, we found that rates of TIA and peaceful awareness were predicted by closeness to death as hypothesized. Consistent with prior examinations, we found that patients' awareness of their terminal health status increased at the end of life [26, 27]. Heightened awareness might have been due to patients facing a greater number of physical symptoms and feeling frailer as death approached. However, our finding that closeness to death predicted TIA even when controlling for physical symptom burden suggests that factors other than physical distress contributed to patients' acknowledgment that death was imminent. These factors may not be related to mental distress (e.g., social cues), because psychiatric morbidity in the current sample did not appear to increase monotonically (again, even when adjusting for physical symptom burden).
Other reports from the CwC have demonstrated that TIA was associated with elevated distress [50] and with increased rates of mental disorders among those patients who were aware, but not at peace (although this result did not reach statistical significance) [25]. However, TIA alone was also linked with positive outcomes, such as the development of advance care planning and advance directives [50]. In addition, when TIA was coupled with a sense of peacefulness, decreased psychological distress and greater quality of death has been observed among patients, in addition to improved bereavement outcomes among their caregivers [25]. The small number of “aware, but not peaceful” patients within each temporal bin prohibited us from examining changes in rates of mental disorders within this subgroup.
Our analyses also demonstrated that patients closer to dying in general were more likely to report a wish to die and were less likely to endorse a wish to live. This finding is particularly important given that patients are still at risk of committing suicide during advanced stages of illness despite increased physical debilitation [51]. However, the increase in rates of the wish to die and the related, though distinct, decrease in the prevalence of the wish to live observed could also have reflected a natural progression of death acceptance. The increases in TIA and peaceful awareness as death approached that we observed support this hypothesis, as do prior studies of patients at the end of life. Hinton [26] observed that acceptance increased in the last eight weeks of life and that awareness of dying was not associated with subjective depression ratings. In fact, Chochinov and colleagues [27] found that patients who did not acknowledge their prognosis in the final weeks of life were three times more likely to meet criteria for clinical depression. Many patients experience growing acceptance once they have acknowledged that they are dying; the complete absence of TIA when death is imminent, however, may be associated with psychopathology.
While a common clinical course of mental disorders among dying patients did not emerge in this study, we attempted to better characterize the subset of individuals who met criteria for a depressive and/or anxiety disorder within two months of dying. On average, these patients were younger and therefore as expected, had lower scores on the Charlson Comorbidity Index. Potentially warranting more clinical concern were the findings that membership in this group was associated with decreased use of psychotropic medication (1% of the total sample) and decreased TIA and peaceful awareness (1% and 0.7% of the total sample, respectively). Despite meeting criteria for an Axis I disorder, these patients were not being treated psychopharmacologically as frequently as patients farther away from death nor as frequently as patients who did not meet criteria for an Axis I disorder. There are several possible explanations for this, which are complicated by the fact that it is not clear when the onset of Axis I disorders occurred in these patients. These findings might reflect a lack of adherence to prescribed psychotropic medications. It may also be that these patients, who were generally younger and possibly less frail, did not garner the same attention from clinicians as older patients who appeared more overtly ill. This, in conjunction with the presentation of their psychiatric symptoms (e.g., withdrawal due to depression or frequent requests for reassurance or clinical attention due to anxiety), may have resulted in providers overlooking or under-treating their psychopathology.
Study Strengths and Limitations
Strengths of this study included the use of standardized clinical interviews for the assessment of mental disorders in an ethnically and geographically diverse sample. Kelly et al. [6] argued that assessment of psychiatric diagnoses among terminally ill patients with structured clinical interviews is particularly advantageous because it allows for adaptations to be made by the evaluating clinician as needed. An additional strength was the wide range of proximity to death represented in our sample. The variability in closeness to death available provided preliminary evidence that suggests that, in general, rates of depression and anxiety disorders do not increase at the end of life. Still, these findings do not undermine the importance of clinicians conducting frequent and comprehensive mental health assessments. In fact, a subset of individuals may be at heightened risk for developing existential distress that is distinct from psychopathology as it is conceptualized in our standardized diagnostic manuals.
This study was also strengthened by its application of screening for cognitive dysfunction. Evaluation of a lucid patient sample likely increased the validity of the diagnoses made by clinicians. At the time of this report, 8.4% (n = 94) of the 1115 patients screened for the CwC were excluded because of cognitive dysfunction, and 17.9% (n = 200) were excluded because they were too frail to participate. Exclusion of these individuals likely captured patients who were delirious or demented. However, an unfortunate consequence of the exclusion determination was its attendant limitations on further study of the excluded individuals, including a formal evaluation of the source or type of cognitive dysfunction. Given this, we cannot provide information about patients with delirium or dementia, a cognitively impaired population who comprised a substantial proportion of those cancer patients diagnosed with a mental disorder at the end of life in previous epidemiological studies [12, 14].
In addition, episodes of delirium can be brief, and thus the incidence of diagnosable depression and anxiety disorders that we found may have been lower than their true prevalence if individuals who would have met criteria for depression when not delirious were excluded because of cognitive impairment at the time they were screened. Given the prevalence of delirium increases as death approaches [52, 53], patients closer to death (i.e., sicker patients) may have been excluded. Despite this concern, examination of the distribution of patients who were assessed within the six months before dying revealed that the greatest number of patients were interviewed during their last month alive. Future studies should include assessment of other mental disorders, including delirium, dementia, and adjustment disorders.
Epidemiological data on psychiatric morbidity is limited, largely because of the methodological challenges inherent in end-of-life research, which targets patients coping with complex symptoms, scheduling limitations, shifting priorities, and their imminent death [54, 55]. Because accruing a sample large enough to detect significant trends is difficult, we used cross-sectional data rather than a longitudinal design. However, the cross-sectional design of the study is one of its primary limitations. While it would likely require substantial study resources and flexibility to conduct repeated assessments of this vulnerable population, questions about the course of mental illness at the end of life would best be addressed by longitudinal investigations. Future studies might conduct repeated longitudinal assessments of mental health and death acceptance, but the difficulties of recruitment and losing patients over time will continue to present a challenge to end of life researchers.
Other limitations include those noted in previous reports from the CwC study [see 22, 25], such as the potential presence of a selection bias for more mental and physically healthy participants given that those patients who refused to participate were more distressed than those who did participate. In addition, we used SCID-I screening questions that may have been insufficiently sensitive, possibly resulting in an underestimation of true mental disorder prevalence, and we assessed peacefulness with only a single item [see 22, 25].
Clinical Implications and Future Directions
This study did not find support for the common speculation that rates of depressive and anxiety disorders increase as patients approach death. The challenge clinicians often face distinguishing normative distress from psychopathology is only exacerbated at end of life, a time during which patients are expected to experienced some distress and in addition, suffer numerous overlapping physical symptoms [56]. As a strategy for meeting this challenge, Block [18] has recommended that clinicians maintain a low threshold for treating depression. Given the significant treatment implications, however, it is imperative to distinguish expected sadness and anxiety from clinical symptoms and to continue research to identify risk factors such as proximity to death despite the numerous challenges of conducting end of life research [54].
Future investigations should attempt to replicate findings from this cross-sectional study using a prospective, longitudinal design. Other factors to explore in future investigations include the course of mental disorders among different patient subgroups, such as various age cohorts, cancer types, and times since diagnosis. The post-death impact of the timing of onset of patient mental disorders close to death on surviving significant others might also be examined. Researchers should also identify and differentiate factors associated with existential distress and death acceptance so that interventions that help resolve the former and foster the latter might be further developed. Through a clearer understanding of psychological phenomena among patients with advanced cancer, it may be possible to improve the application of appropriate clinical approaches to optimize the quality of life of this vulnerable population at the end of life.
Acknowledgments
This research was supported in part by the following grants to Dr. Prigerson: MH63892 from the National Institute of Mental Health and CA 106370 from the National Cancer Institute; the Center for Psycho-Oncology and Palliative Care Research, Dana-Farber Cancer Institute. Support for Dr. Lichtenthal was provided in part by a National Cancer Institute training grant (T32 CA009461-23).
References
- 1.Kissane DW, Clarke DM, Street AF. Demoralization syndrome--a relevant psychiatric diagnosis for palliative care. J Palliat Care. 2001;17:12–21. [PubMed] [Google Scholar]
- 2.Ironside W. Conservation-withdrawal and action-engagement: on a theory of survivor behavior. Psychosomatic medicine. 1980;42:163–175. doi: 10.1097/00006842-198001001-00011. [DOI] [PubMed] [Google Scholar]
- 3.Engel GL, Schmale AH. Conservation-withdrawal: a primary regulatory process for organismic homeostasis. Ciba Foundation symposium. 1972;8:57–75. doi: 10.1002/9780470719916.ch5. [DOI] [PubMed] [Google Scholar]
- 4.Periyakoil VS, Kraemer HC, Noda A, Moos R, Hallenbeck J, Webster M, Yesavage JA. The development and initial validation of the Terminally Ill Grief or Depression Scale (TIGDS) Int J Methods Psychiatr Res. 2005;14:202–212. doi: 10.1002/mpr.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durkin I, Kearney M, O'Slorain L. Psychiatric disorder in a palliative care unit. Palliative medicine. 2003;17:212–218. doi: 10.1191/0269216303pm670oa. [DOI] [PubMed] [Google Scholar]
- 6.Kelly BJ, Pelusi D, Burnett PC, Varghese FT. The prevalence of psychiatric disorder and the wish to hasten death among terminally ill cancer patients. Palliative & supportive care. 2004;2:163–169. doi: 10.1017/s1478951504040222. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Williams M. Screening for depression in palliative care. The American journal of hospice & palliative care. 2001;18:79–80. doi: 10.1177/104990910101800202. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Williams M, Spiller J, Ward J. Which depression screening tools should be used in palliative care? Palliative medicine. 2003;17:40–43. doi: 10.1191/0269216303pm664oa. [DOI] [PubMed] [Google Scholar]
- 9.Pinder KL, Ramirez AJ, Black ME, Richards MA, Gregory WM, Rubens RD. Psychiatric disorder in patients with advanced breast cancer: prevalence and associated factors. Eur J Cancer. 1993;29A:524–527. doi: 10.1016/s0959-8049(05)80144-3. [DOI] [PubMed] [Google Scholar]
- 10.Bukberg J, Penman D, Holland JC. Depression in hospitalized cancer patients. Psychosomatic medicine. 1984;46:199–212. doi: 10.1097/00006842-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Massie MJ. Prevalence of depression in patients with cancer. Journal of the National Cancer Institute. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 12.Derogatis LR, Morrow GR, Fetting J, Penman D, Piasetsky S, Schmale AM, Henrichs M, Carnicke CL., Jr. The prevalence of psychiatric disorders among cancer patients. Jama. 1983;249:751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 13.Akechi T, Okamura H, Nishiwaki Y, Uchitomi Y. Psychiatric disorders and associated and predictive factors in patients with unresectable nonsmall cell lung carcinoma: a longitudinal study. Cancer. 2001;92:2609–2622. doi: 10.1002/1097-0142(20011115)92:10<2609::aid-cncr1614>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Akechi T, Okuyama T, Sugawara Y, Nakano T, Shima Y, Uchitomi Y. Major depression, adjustment disorders, and post-traumatic stress disorder in terminally ill cancer patients: associated and predictive factors. J Clin Oncol. 2004;22:1957–1965. doi: 10.1200/JCO.2004.08.149. [DOI] [PubMed] [Google Scholar]
- 15.Kugaya A, Akechi T, Okuyama T, Nakano T, Mikami I, Okamura H, Uchitomi Y. Prevalence, predictive factors, and screening for psychologic distress in patients with newly diagnosed head and neck cancer. Cancer. 2000;88:2817–2823. doi: 10.1002/1097-0142(20000615)88:12<2817::aid-cncr22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC. Psychiatric disorder in women with early stage and advanced breast cancer: a comparative analysis. The Australian and New Zealand journal of psychiatry. 2004;38:320–326. doi: 10.1080/j.1440-1614.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- 17.Grabsch B, Clarke DM, Love A, McKenzie DP, Snyder RD, Bloch S, Smith G, Kissane DW. Psychological morbidity and quality of life in women with advanced breast cancer: a cross-sectional survey. Palliative & supportive care. 2006;4:47–56. doi: 10.1017/s1478951506060068. [DOI] [PubMed] [Google Scholar]
- 18.Block SD. Assessing and managing depression in the terminally ill patient. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians - American Society of Internal Medicine. Ann Intern Med. 2000;132:209–218. doi: 10.7326/0003-4819-132-3-200002010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists' recognition of depression in their patients with cancer. J Clin Oncol. 1998;16:1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- 20.McDonald MV, Passik SD, Dugan W, Rosenfeld B, Theobald DE, Edgerton S. Nurses' recognition of depression in their patients with cancer. Oncol Nurs Forum. 1999;26:593–599. [PubMed] [Google Scholar]
- 21.Fulton C. The prevalence and detection of psychiatric morbidity in patients with metastatic breast cancer. European journal of cancer care. 1998;7:232–239. doi: 10.1046/j.1365-2354.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 22.Kadan-Lottick NS, Vanderwerker LC, Block SD, Zhang B, Prigerson HG. Psychiatric disorders and mental health service use in patients with advanced cancer: a report from the coping with cancer study. Cancer. 2005;104:2872–2881. doi: 10.1002/cncr.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd-Williams M, Dennis M, Taylor F. A prospective study to determine the association between physical symptoms and depression in patients with advanced cancer. Palliative medicine. 2004;18:558–563. doi: 10.1191/0269216304pm923oa. [DOI] [PubMed] [Google Scholar]
- 24.Chen ML, Chang HK. Physical symptom profiles of depressed and nondepressed patients with cancer. Palliative medicine. 2004;18:712–718. doi: 10.1191/0269216304pm950oa. [DOI] [PubMed] [Google Scholar]
- 25.Ray A, Block SD, Friedlander RJ, Zhang B, Maciejewski PK, Prigerson HG. Peaceful awareness in patients with advanced cancer. J Palliat Med. 2006;9:1359–1368. doi: 10.1089/jpm.2006.9.1359. [DOI] [PubMed] [Google Scholar]
- 26.Hinton J. The progress of awareness and acceptance of dying assessed in cancer patients and their caring relatives. Palliative medicine. 1999;13:19–35. doi: 10.1191/026921699672169546. [DOI] [PubMed] [Google Scholar]
- 27.Chochinov HM, Tataryn DJ, Wilson KG, Ennis M, Lander S. Prognostic awareness and the terminally ill. Psychosomatics. 2000;41:500–504. doi: 10.1176/appi.psy.41.6.500. [DOI] [PubMed] [Google Scholar]
- 28.Prigerson HG. Socialization to dying: social determinants of death acknowledgement and treatment among terminally ill geriatric patients. J Health Soc Behav. 1992;33:378–395. [PubMed] [Google Scholar]
- 29.Kiernan RJ, Mueller J, Langston JW, Van Dyke C. The Neurobehavioral Cognitive Status Examination: a brief but quantitative approach to cognitive assessment. Ann Intern Med. 1987;107:481–485. doi: 10.7326/0003-4819-107-4-481. [DOI] [PubMed] [Google Scholar]
- 30.Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Jr., Rounsaville B, et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Archives of general psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 31.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American journal of clinical oncology. 1982;5:649–655. [PubMed] [Google Scholar]
- 32.Karnofsky DA. Nitrogen mustards in the treatment of neoplastic disease. Advances in internal medicine. 1950;4:1–75. [PubMed] [Google Scholar]
- 33.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 36.Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliative medicine. 1997;11:3–20. doi: 10.1177/026921639701100102. [DOI] [PubMed] [Google Scholar]
- 37.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of general psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 38.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 39.Prigerson HG, Jacobs SC. Diagnostic criteria for traumatic grief: a rationale, consensus criteria, and preliminary empirical test. In: Stroebe MS, Hansson RO, Stroebe W, Schut H, editors. Handbook of Bereavement Research: Consequences, Coping and Care. American Psychological Association Press; Washington, DC: 2001. pp. 614–646. [Google Scholar]
- 40.Prigerson HG, Vanderwerker LC, Maciejewski PK. Prolonged Grief Disorder: Inclusion in DSM. In: Stroebe M, Hansson R, Schut H, Stroebe W, editors. Handbook of Bereavement Research and Practice: 21st Century Perspectives. American Psychological Association Press; Washington, DC: 2007. Chapter in press. [Google Scholar]
- 41.Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53:2243–2249. doi: 10.1002/cncr.1984.53.s10.2243. [DOI] [PubMed] [Google Scholar]
- 42.Vanderwerker LC, Laff RE, Kadan-Lottick NS, McColl S, Prigerson HG. Psychiatric disorders and mental health service use among caregivers of advanced cancer patients. J Clin Oncol. 2005;23:6899–6907. doi: 10.1200/JCO.2005.01.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Multidimensional Measurement of Religiousness/Spirituality for Use in Health Research: A Report of the Fetzer Institute / National Institute on Aging Working Group. 1999. [Google Scholar]
- 44.Steinhauser KE, Voils CI, Clipp EC, Bosworth HB, Christakis NA, Tulsky JA. “Are you at peace?”: one item to probe spiritual concerns at the end of life. Arch Intern Med. 2006;166:101–105. doi: 10.1001/archinte.166.1.101. [DOI] [PubMed] [Google Scholar]
- 45.Latham AE, Prigerson HG. Suicidality and bereavement: complicated grief as psychiatric disorder presenting greatest risk for suicidality. Suicide Life Threat Behav. 2004;34:350–362. doi: 10.1521/suli.34.4.350.53737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 47.Mako C, Galek K, Poppito SR. Spiritual pain among patients with advanced cancer in palliative care. Journal of palliative medicine. 2006;9:1106–1113. doi: 10.1089/jpm.2006.9.1106. [DOI] [PubMed] [Google Scholar]
- 48.Teunissen SC, de Graeff A, Voest EE, de Haes JC. Are anxiety and depressed mood related to physical symptom burden? A study in hospitalized advanced cancer patients. Palliative medicine. 2007;21:341–346. doi: 10.1177/0269216307079067. [DOI] [PubMed] [Google Scholar]
- 49.Wilson KG, Chochinov HM, Skirko MG, Allard P, Chary S, Gagnon PR, Macmillan K, De Luca M, O'Shea F, Kuhl D, Fainsinger RL, Clinch JJ. Depression and anxiety disorders in palliative cancer care. Journal of pain and symptom management. 2007;33:118–129. doi: 10.1016/j.jpainsymman.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 50.Ray A, Jackson VA, Block SD, Prigerson HG. Risks and benefits of terminal illness acknowledgment in advanced cancer patients. Cancer. 2007 Manuscript under review. [Google Scholar]
- 51.Bolund C. Suicide and cancer: II. Medical and care factors in suicide by cancer patients in Sweden. Journal of Psychosocial Oncology. 1985;3:17–30. [Google Scholar]
- 52.Pereira J, Hanson J, Bruera E. The frequency and clinical course of cognitive impairment in patients with terminal cancer. Cancer. 1997;79:835–842. [PubMed] [Google Scholar]
- 53.Breitbart W. Diagnosis and management of delirium in the terminally ill. In: Bruera E, Portenoy R, editors. Topics in Palliative Care. Oxford University Press; New York: 2001. pp. 303–321. [Google Scholar]
- 54.Kaasa S, Hjermstad MJ, Loge JH. Methodological and structural challenges in palliative care research: how have we fared in the last decades? Palliative medicine. 2006;20:727–734. doi: 10.1177/0269216306072620. [DOI] [PubMed] [Google Scholar]
- 55.Jordhoy MS, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliative medicine. 1999;13:299–310. doi: 10.1191/026921699668963873. [DOI] [PubMed] [Google Scholar]
- 56.Tremblay A, Breitbart W. Psychiatric dimensions of palliative care. Neurol Clin. 2001;19:949–967. doi: 10.1016/s0733-8619(05)70055-4. [DOI] [PubMed] [Google Scholar]