Abstract
Objectives
Epidemiologic studies report that lack of adjuvant radiation (RT) after breast conserving surgery (BCS) is associated with higher short-term mortality. It is generally accepted that adjuvant RT decreases risk of breast cancer recurrence and thereby lowers long-term mortality; here, we explore reasons for its relationship to short-term mortality.
Materials and Methods
We studied 1,583 publically insured women who had BCS between 1998 and 2002 (mean 71.8 years, range 27-101), of whom 1,346 (85%) received RT. Multivariate analyses with Cox Proportional Hazards and Logistic Regression models included: age; race; comorbidity; insurance status; tumor size; number of nodes positive; hormone receptor status; receipt of radiation; adjuvant chemotherapy; preventive care - including mammography, Pap smear and primary care visits; and hospitalization.
Results
At a mean follow-up of 52.8 months, overall mortality was significantly lower in those who received RT (HR 0.45, p<0.0001) and higher with older age (HR 1.05, p<0.0001) and greater comorbidity (HR 1.16, p=0.0007). Local recurrence was less with receipt of optimal radiation (HR 0.47; p=0.03). Breast cancer event, as determined by a clinically logical algorithm to detect breast cancer recurrence and death, however, was not significantly associated with receipt of RT (OR 1.32, p=0.2).
Conclusion
These results imply that the higher short-term mortality in women not receiving RT after BCS is related to factors other than breast cancer recurrence.
Keywords: Breast cancer, breast conserving surgery, adjuvant radiation, local recurrence, metastases, survival, primary care, comorbidity, age
Introduction
Adjuvant radiation after breast conserving therapy (BCS) leads to lower rates of local and distant breast cancer recurrence and improved long-term survival.(1-4) In previous work, we found that use of adjuvant radiation after BCS was associated with lower six-year all cause (hazard ratio (HR) = 0.42, 95% confidence interval (CI) 0.21-0.85) and cancer specific mortality (HR = 0.22, 95% CI -0.09-0.57).(5) These results might suggest that assuring patients’ access to adjuvant RT after BCS would decrease disparities in outcomes by resulting in short term improvements in breast cancer outcomes. However, it is uncertain whether underuse of radiotherapy is directly linked to poorer short-term survival, or if the association is due to confounding or intervening factors, such as a general underuse of key medical and prevention health services among those with lower access or underlying poor prognosis.
To explore confounding factors in the association between receipt of adjuvant radiation and short-term mortality, we conducted a new study. We included markers of other preventive services in the model, since receipt of radiation services may be a proxy for use of medical services in general.(6, 7) We also included in the analysis a marker of cancer recurrence. For the purpose of this study, we developed and extensively tested an algorithm for detecting cancer recurrence using treatment and procedure claim codes, allowing us to explore the associations among breast cancer local treatment, local and distant breast cancer recurrence, use of preventive health services, and overall mortality.
Materials and Methods
This study was approved by the Institutional Review Boards of Pennsylvania State University, Wake Forest University School of Medicine, and Duke University Medical Center.
Study population. Among 18,859 patients from the North Carolina Central Cancer Registry (NC CCR) and insured by NC Medicaid and/or Medicare, 1,659 were diagnosed with first-time primary, non-metastatic, invasive breast cancer, had BCS between 1998 and 2002, were alive for two years after diagnosis, were not enrolled in a Medicare Health Maintenance Organization and were included (Figure 1). Patients insured by Medicaid only were included based on our previous work in this population and as an indicator of socioeconomic status. We included Medicaid and Medicare insured patients. Medicaid is a jointly funded federal-state program that covers low-income adults, their children, and people with certain disabilities, and is managed by the state government. Medicare is a federally funded and run health insurance program that provide health insurance coverage to people who are 65+ years old or have a disability or permanent kidney failure. Currently, there are 9 million people who quality for both Medicaid and Medicare, and are among the sickest and poorest individuals. Consistent to literature, we excluded patients who enrolled in some types of managed care program (e.g., HMOs) under Medicare and Medicaid because their medical claims data may be incomplete. Definition of variables. Multivariate analysis included: age at diagnosis; race (Caucasian, African-American, or other); comorbidity (Charlson Score); insurance (Medicaid, Medicare fee-for-service, or dual); SEER (Surveillance, Epidemiology and End Results Program) summary stage (from NC CCR (8): local stage included SEER stage 1 or 2; regional stage included SEER stage 3, 4, and 5); tumor size (from NC CCR); number of positive nodes (from NC CCR); hormone receptor status (from NC CCR; 2 cases where ER was borderline and PR negative and were coded as hormone receptor negative); receipt of adjuvant radiation during the first year; days on chemotherapy; trimester primary care visits in one year after surgery; Pap smears, mammography, and number of hospitalizations during the first 2 years after diagnosis. Primary care visits, Pap smears, and mammography were included as indicators of engagement in the healthcare system.(6, 9) Human epidermal growth factor receptor-2 (HER2) status was not included because it was not consistently available in the tumor registry database during the years studied.
Figure 1.
Eligibility criteria
1 The primary course of treatment must have been breast conserving surgery (BCS), as identified by NC CCR surgery summary variable, with no indication of a mastectomy occurring within 2 years after diagnosis.
2 Continuous enrollment in Medicare fee-for-service (no HMO during study period) or Medicaid (or both) was assessed for the first 2 years (24 months) after primary course surgery; the participant must have been enrolled for at least 80% of the time from surgery until the end of follow-up.
3 Cases in which staging was in-situ, distant were excluded.
4 Cases with inconsistent data (such as treatment and surgery occurring 30 days before diagnosis date) were excluded. Cases where there was evidence of multiple tumors or second primary tumor, according to the NC CCR, after diagnosis were excluded.
5 Primary tumor at diagnosis was required to be the first tumor recorded in the registry; this was done by examining the CCR tumor sequence number and comparing it to a second, unconsolidated data set.
Comorbidity
The weighted comorbidity score, derived from claims within 2 years after diagnosis, was adapted from the algorithm described in D’Hoore 1996.(10) ICD-9 codes were scanned for the first three digits to produce a composite score.
Radiation
Total number of radiation treatment days during the 12 month period from diagnosis date in the cancer registry was based on the following: days of post-surgery radiation services, as listed in Medicare and Medicaid claims; CPT (American Medical Association Current Procedural Terminology) 77247, 77340 (radiation treatment management in units of 5 sessions), 77417 (therapeutic radiologic port film in units of 5 sessions), and 77401 to 77416 (radiologic treatment days). Patients receiving >15 days of adjuvant radiation were deemed to have received radiation.(11) Cases lacking radiation services in claims were categorized as having received radiation if radiation therapy was recorded in the cancer registry, otherwise radiation treatment was assumed not to have occurred.
Chemotherapy
Use of chemotherapy during the first year was determined from chemotherapy service codes in claims data (ICD-9 codes V581, V662, V672, V5811, V5812; CPT/HCPCS codes 96400-96549; J8999,J9999; Q0083-Q0085, and Q0163-Q0181); CPT specific and NDC codes for 43 specific chemotherapy agents; and for NC Medicaid recipients, specific procedure codes, W8222, W8225. Patients who had more than one day of chemotherapy in claims within 1 year of diagnosis and those for whom the registry indicated that chemotherapy was given within one year after surgery, were defined as having received chemotherapy.
Quadrimester Primary Care visits
A binary variable for each quadrimester during the second year after surgery was created for primary care visits, using codes in Medicaid and Medicare for physician specialty, place of service, facility type, and CPT/HCPCS codes. If visits occurred at each quadrimester, then the variable was set to one from zero.
Pap smear visits
A binary variable for Pap smear within 2 years following the date of surgery was created, using the following CPT/HCPCS codes: G0101, G0123, P3000, G0143-45, G0147-48, 32252, 31449, 88164-67, 88147-48, 88150 - 54, 88147-48, 88142-43, 88141, 32252, 31449; and any of the following ICD-9 codes in the diagnostic fields, V7231, V7232, V7647, V7649, V1589, 79500, 79502, 79505, V762.
Mammography
A binary variable was for mammography within 2 years after date of surgery using CPT /HCPCS codes for diagnostic and screening mammography (76090, 76091, 76092, 76083, G0202, G0204, G0206 and ICD-9 V7611, V7612).
Total Hospitalizations
Hospitalization dates were extracted from available claims in the Medicare inpatient file and hospital/inpatient related claims in the Medicaid file (using claim type and category of service variables). Number of inpatient admissions during the first two years after surgery was inferred algorithmically by counting the indicators for hospitalization for each of 365 days starting from diagnosis and excluding all positive indicators for which any hospitalization was detected four days prior (suggesting the patient was already hospitalized).
Main Outcomes
Local Recurrence
Local recurrence was defined as either mastectomy or a secondary BCS (ICD-9-CM 198.81) more than two years from the date of BCS, based on methods previously reported in Surveillance, Epidemiology, and End Results-Medicare data.(12, 13)
Breast Cancer Event
A composite score was created that combined local or distant breast cancer recurrence and breast cancer death. Breast cancer event was defined by an algorithm to detect radiation, chemotherapy, or surgery codes occurring after restaging codes, provided the restaging codes occur 1 year or later after BCS surgery. Procedure codes for restaging events included any biopsy, X-ray, CT and MRI imaging (ICD-9 40.23, 40.3, 40.51, 85.1, 85.11, 85.12, 85.19, 85.91,54.21,50.11, or CPT/HCPCS G0231,G0253,10021-22,19000-01, 19020, 19100-03, 19290-91, 20220, 20225, 32405, 38500, 38525, 38740, 38745, 70551-54, 71550-52,71555, 72141-42,72146-49, 72156-59,72195-98, 73218-19,20-23,25, 73718-20, 73721-23, 73725, 74181-83, 76003, 76095-96, 76098, 76360, 76942, 77021, 78102-04,78195, 78201-02,78206,78215-16, 78300-20, 78800-02,78804,78811-16, 88104, 88106, 88108, 88160-61, 88170, 88172-73, 88305, 88307, 88329, 88331-32). Radiation was identified by the following codes: ICD-9 V58.0, V66.1, V67.1, 92.20-92.29, CPT 77261-63, 77247, 77280-77499, 77600-15, 77750-90. Chemotherapy codes are described above. Surgery codes included: for BCS, CPT 19162, 19160, 19120, 19125, 19126 or ICD-9 85.20-85.23, 85.25, or DRG 259,260; for mastectomy 19240, 19220, 19180, 19200 or ICD-9 85.41-48, 85.33-36, or DRG 257, 258. To assess the validity of our recurrence algorithm, we separately coded all cases (n=416) reported by one of the study hospital registrys serving a comprehensive cancer center and compared the classification of recurrence identified from the algorithm using claims,with the medical records for these cases based upon clinical notes. Cases in the validation sample were selected based upon having both diagnosis and treatment for the index cancer at the validation hospital site. The validation hospital registry tracked all patients for whom breast cancer was diagnosed. The date of recurrence was taken to be the date that a notation was made in the medical chart establishing recurrence as a medical diagnosis. Of the n=60 cases classified as recurrent by our algorithm, 53 were found to have medical record notes indicating recurrent disease or a second (new) primary. Thus the observed sensitivity of our algorithm was 88.3%. Among cases found to be not recurrent by our algorithm, 89.9% were found to have no mention or record in the medical chart of possible or diagnosed recurrence or a secondary (new) tumor. This validation process may have patients who developed breast cancer recurrence and were diagnosed and treated at a different hospital or medical center.
Breast cancer as cause of death was detected from the National Death Index (NDI) Plus system.
All-cause Mortality
Death date and cause of death were matched by name and social security number through the National Death Index (NDI) Plus system.
Statistical Methods
Multivariate logistic regression was used to determine predictors of local recurrence and of composite distant recurrence/breast cancer death. In a survival analysis, Cox Proportional Hazard models were used to determine the predictors of all cause mortality among all patients in the sample and among age groups (<70 years, 70 +) . We examined the Proportional Hazards assumption and nonlinearity of the continuous predictors. Lastly, Kaplan Meier survival curves were generated to graphically examine the survival trends in patient’s receiving/not receiving radiation.
Results
Of 1,583 women having BCS, 1,346 (85%) received adjuvant radiation and 237 (15%) did not. (Table 1) Mean age was 71.8 years, with range 27 to 101 years. Most patients were insured by Medicare fee-for-service only (79%). Mean comorbidity score was 1.71, with range 0 to 11. Ninteen percent received adjuvant chemotherapy. Mean follow-up was 52.8 months. The majority of the study population used preventive health care services; quadrimester primary care visits occurred in 73%, mammography in 65%, and Pap smears in 41%. Hospitalization rate was low (<1%). There were 54 (3%) local recurrences, 270 (17%) breast cancer events, and 149 (9%) total deaths.
Table 1.
Patient Characteristics and Distribution by Radiation Status, n = 1,583
| Study Population N = 1,583 |
Less than Optimal Radiation N=237 |
Optimal Radiation N=1,346 |
P Value | |
|---|---|---|---|---|
| Age at dx (years) | ||||
| <45 | 26 ( 2%) | 8 (3%) | 18 (1%) | < 0.0001 |
| 45 - 54 | 52 ( 3%) | 8 (3%) | 44 (3%) | |
| 55 - 64 | 133 ( 8%) | 18 (8%) | 115 (9%) | |
| 65 – 74 | 807 (51%) | 65 (27%) | 742 (55%) | |
| 75 + | 565 (36%) | 138 (58%) | 427 (32%) | |
| Mean(SD) | 71.79 (9.36) | 76.20 (12.56) | 71.02 (8.45) | <0.0001 |
| Race | ||||
| African American | 226 (14%) | 38 (16%) | 188 (14%) | 0.7030 |
| Caucasian | 1,337 (84%) | 196 (83%) | 1,141 (85%) | |
| Other | 20 (1%) | 3 (1%) | 17 ( 1%) | |
| Comorbidity (Charlson) | ||||
| Mean (SD) | 1.71 (1.87) | 2.26 (2.09) | 1.62 (1.81) | <0.0001 |
| Insurance status | ||||
| Dual | 285 (18%) | 65 (27%) | 220 (16%) | <0.0001 |
| Medicaid only | 42 ( 3%) | 10 (4%) | 32 ( 2%) | |
| Medicare only | 1256 (79%) | 162 (68%) | 1094 (81%) | |
| Stage | ||||
| Local | 1383 (87%) | 221 (93%) | 1162 (86%) | 0.0031 |
| Regional | 200 (13%) | 16 ( 7%) | 184 (14%) | |
| Tumor size, mm | ||||
| 0-10 | 449 (28%) | 63 (27%) | 386 (29%) | 0.0236 |
| 11-20 | 760 (48%) | 101 (43%) | 659 (49%) | |
| 21-50 | 348 (22%) | 70 (30%) | 278 (21%) | |
| 51+ | 26 ( 2%) | 3 (1%) | 23 ( 2%) | |
| Mean (SD) | 1.53 cm (1.57) | 1.61 (1.02) | 1.52 (1.65) | 0.2908 |
| Lymph nodes positive | ||||
| 0 | 1379 (87%) | 222 (94%) | 1157 (86%) | <0.0001 |
| 1 - 3 | 180 (11%) | 10 (4%) | 170 (13%) | |
| 4 - 9 | 21 (1%) | 5 (2%) | 16 (1%) | |
| 10 + | 3 (0%) | 0 (0%) | 3 (0%) | |
| Hormone receptor status |
1100 (69%) | 142 (60%) | 958 (71%) | 0.0019 |
| Positive | 156 (10%) | 28 (12%) | 128 (10%) | |
| Negative | 327 (21%) | 67 (28%) | 260 (19%) | |
| Not determined | ||||
| Radiation days | ||||
| None | 224(14%) | 224 (94%) | 0 | - |
| 1-15 | 13 ( 1%) | 13 (5%) | 0 | |
| > 15 * | 1346 (85%) | 0 (0%) | 1346 (100%) | |
| Chemotherapy | ||||
| Yes | 302 (19%) | 21 (9%) | 281 (21%) | <0.0001 |
| No | 1281 (81%) | 216 (91%) | 1065 (79%) | |
| Quadrimester Primary Care visits 2nd year after surgery |
1161 (73%) | 134 (56%) | 1027 (76%) | |
| Yes | 422 (27%) | 103 (43%) | 319 (24%) | <0.0001 |
| No | ||||
| Pap smear during first 2 years |
||||
| Yes | 643 (41%) | 49 (21%) | 594 (44%) | <0.0001 |
| No | 940 (59%) | 188 (79%) | 752 (56%) | |
| Mammography during first 2 years |
||||
| Yes | 1033 (65%) | 120 (51%) | 913 (68%) | <0.0001 |
| No | 550 (35%) | 117 (49%) | 433 (32%) | |
| Number of hospitalizations during first 2 years Mean (SD) |
0.63 (0.98) | 0.83 (1.16) | 0.60 (0.94) | 0.0028 |
| Follow-up until censoring/death (months) Mean (STD) |
52.83 (12.14) | 50.72 (12.77) | 53.21(11.99) | 0.0036 |
| Local Recurrence | ||||
| Yes | 54 (3%) | 16 (7%) | 38 (3%) | 0.0021 |
| No | 1529 (97%) | 221 (93%) | 1308 (97%) | |
| Breast Cancer Events | ||||
| Yes | 270 (17%) | 29 (12%) | 241 (18%) | 0.0324 |
| No | 1313 (83%) | 208 (88%) | 1105 (82%) | |
| Deaths from all causes | 149 (9%) | 58 (24%) | 91 (7%) | <0.0001 |
Note: Radiation receipt reported in the Cancer Registry only was included as > 15.
Univariate analysis comparing groups by radiation status is shown in Table 1. Women receiving radiation were younger (mean 71.0 years versus 76.2 years; p≤ 0.0001), had less comorbidity (mean score 1.62 versus 2.26; p<0.0001), were more likely to have received adjuvant chemotherapy (p<0.0001), and were more likely insured by Medicare only (versus dual or Medicaid only, p<0.0001). Their tumors were more likely regional stage (p=0.0031), hormone receptor positive (p<0.0019), and node positive (p<0.0001). There was a direct relationship with receipt of radiation and of preventive services, including primary care visits (76% vs 56%, p<0.0001), mammography (68% vs 51%, p<0.0001), and Pap smears (44% vs 21%, p<0.0001). Women receiving radiation had less days in the hospital (0.60 vs 0.83, p=0.002). Receipt of radiation was associated with fewer local recurrences (3% vs 7%, p=0.0021) and overall deaths (7% versus 24%; p<0.0001), but more breast cancer events (18% vs 12%, p=0.032).
Predictors of local recurrence and breast cancer events were examined in multivariate analyses (Table 2). Local recurrence was less likely in those receiving radiation (OR 0.47, p=0.03) and more likely with greater comorbidity (OR 1.18, p=0.03). In terms of breast cancer event, the risk was inversely associated with Medicare-only insurance coverage, as opposed to Medicaid or dual coverage (OR 0.63, p=0.01). Breast cancer events were more likely with longer follow-up (OR 1.02, p=0.008), and mammography (OR 1.55, p=0.002).
Table 2.
Logistic Regression Models of Local Recurrence and Breast Cancer Events
| Outcome: Relapse | ||||
|---|---|---|---|---|
| Local Recurrence OR (95% CI) |
P | Breast Cancer Event OR (95% CI) |
P | |
| Age at surgery | 1.01(0.98,1.04) | 0.4471 | 0.99(0.97,1.00) | 0.1342 |
| Race (white) | 1.20(0.54,2.67) | 0.6530 | 1.14(0.77,1.70) | 0.5178 |
| Medicare only | 0.69(0.34,1.42) | 0.3157 | 0.63(0.43,0.91) | 0.0146 |
| Optimal RT | 0.47(0.24,0.92) | 0.0286 | 1.32(0.85,2.06) | 0.2178 |
| Comorbidity index | 1.18(1.02,1.36) | 0.0255 | 1.01(0.94,1.10) | 0.7123 |
| (Log) tumor size | 1.12(0.72,1.76) | 0.6146 | 1.13(0.91,1.40) | 0.2687 |
| Lymph nodes positive | 1.14(0.99,1.31) | 0.0786 | 1.07(0.96,1.18) | 0.2107 |
| ER/PR negative | 1.61(0.75,3.49) | 0.2247 | 1.16(0.76,1.78) | 0.4853 |
| Chemotherapy | 1.24(0.60,2.55) | 0.5574 | 1.36(0.96,1.92) | 0.0830 |
| Length of follow-up | 1.02(1.00,1.04) | 0.0899 | 1.02(1.01,1.03) | 0.0026 |
| Primary Care Visits | 0.71(0.37,1.35) | 0.2929 | 1.25(0.89,1.75) | 0.1909 |
| Mammography | 0.98(0.54,1.79) | 0.9578 | 1.48(1.11,1.97) | 0.0076 |
| Pap Smear | 1.54(0.85,2.79) | 0.1525 | 1.09(0.82,1.44) | 0.5560 |
| # of hospitalizations | 1.15(0.90,1.46) | 0.2601 | 1.03(0.89,1.19) | 0.6851 |
Multivariate analyses were performed to explore factors associated with all-cause mortality, overall and by age younger or older than 70 years (Table 3). Overall, significant predicators of increased all-cause mortality included older age (HR 1.05, p<0.0001); higher comorbidity (HR 1.15, p=0.0007); larger tumor size (HR 1.58, p=0.0007), and more involved lymph nodes (HR 1.13, p=0.043). Receipt of radiation was significantly associated with lower mortality (HR = 0.45, p<0.0001), regardless of age. In women age 70 and younger, optimal radiation predicted lower risk (HR = 0.31, p =0.018) and node involvement predicted higher risk (HR 1.13, p=0.05). For women 70 years and older, higher mortality was predicted by older age (HR 1.07, p<0.0001), higher comorbidity (HR 1.07, p=0.0001), and larger tumor size (HR 1.64, p=0.0012), and lower mortality was predicted by receipt of RT (HR 0.52, p=0.005).
Table 3.
Cox Proportional Model Hazard Ratio for All Cause Mortality Stratified by Older Age Group (< 70 years vs. ≥ 70 years).
| Outcome: Deaths due to All Causes | ||||||
|---|---|---|---|---|---|---|
| < 70 years HR (95% CI) |
p | ≥ 70 years HR (95% CI) |
p | All HR (95% CI) |
p | |
| Age at surgery | 1.01(0.96,1.07) | 0.7188 | 1.07(1.03,1.10) | 0.0001 | 1.05(1.03,1.08) | <.0001 |
| Race (white) | 0.99(0.40,2.47) | 0.9792 | 0.89(0.53,1.50) | 0.6704 | 0.93(0.59,1.46) | 0.7574 |
| Medicare only | 1.31(0.49,3.51) | 0.5972 | 0.83(0.52,1.31) | 0.4200 | 0.85(0.57,1.28) | 0.4383 |
| Optimal RT | 0.31(0.12,0.82) | 0.0184 | 0.52(0.33,0.82) | 0.0046 | 0.45(0.30,0.66) | <.0001 |
| Comorbidity index | 1.20(1.00,1.45) | 0.0550 | 1.15(1.04,1.26) | 0.0051 | 1.16(1.06,1.26) | 0.0007 |
| (Log) tumor size | 1.13(0.65,1.99) | 0.6612 | 1.64(1.22,2.22) | 0.0012 | 1.58(1.21,2.06) | 0.0007 |
| Lymph nodes positive |
1.13(1.00,1.28) | 0.0497 | 1.13(0.92,1.40) | 0.2353 | 1.13(1.00,1.26) | 0.0426 |
| ER/PR negative | 1.41(0.55,3.63) | 0.4706 | 1.03(0.55,1.93) | 0.9286 | 1.18(0.71,1.98) | 0.5195 |
| Chemotherapy | 1.27(0.58,2.79) | 0.5536 | 0.69(0.35,1.38) | 0.2925 | 0.89(0.54,1.44) | 0.6271 |
| Primary Care Visits | 1.95(0.73,5.22) | 0.1844 | 0.72(0.47,1.10) | 0.1256 | 0.84(0.58,1.23) | 0.3797 |
| Mammography | 1.61(0.67,3.89) | 0.2867 | 1.13(0.76,1.68) | 0.5321 | 1.17(0.82,1.67) | .3732 |
| Pap Smear | 0.80(0.38,1.69) | 0.5628 | 0.78(0.48,1.26) | 0.3098 | 0.78(0.52,1.15) | 0.2116 |
| # of hospitalizations | 1.09(0.75,1.58) | 0.6461 | 1.15(0.99,1.34) | 0.0757 | 1.14(1.00,1.31) | 0.0589 |
We suspected that the association between radiation and short-term mortality varied by both age and comorbidity. We therefore explored the association by age group (<70 and 70+) and comorbidity level (score <5 versus 5+). The risk of short-term mortality was significantly lower with radiation therapy in both age groups: <70 years (HR 0.31, 95% CI 0.12, 0.82) and 70 and older (HR 0.45, 95% CI 0.30,0.66). For comorbidity strata, the association between short-term mortality and radiation was significant in the group with lesser comorbidity (HR 0.33, 95% CI 0.21, 0.50, Charlson Score < 5), but not for the group with high comorbidity (HR 1.89, 95% CI 0.71,4.99). We also explored the association of radiation and breast cancer event and found no significant associations with age and comorbidity strata.
Since the variable for breast cancer event is time dependent, it was not included in the Proportional Hazards Models for overall mortality (above). When the variable for breast cancer event is added to Proportional Hazard Model, it is a significant predictor (P < .0001) of higher mortality, but the relationship between radiation and all cause mortality remained.
Discussion
Lack of radiation predicted higher mortality among Medicare fee-for-service and Medicaid insured women with early stage breast cancer who had BCS, with 4.4 years of follow-up. In clinical trials, 15 to 20 years was necessary to realize a survival benefit attributable to adjuvant radiation after BCS, whereas other epidemiologic studies observed decrements in survival much earlier.(14-16) For instance, in Medicaid-insured patients, one-in-three of whom did not get radiation after BCS, omission of radiation was associated with higher 6-year mortality.(5, 17) We suspect that factors other than breast cancer contribute to the higher mortality. Despite the inclusion of multiple possible confounding factors in a multivariable model for both local control and overall survival, radiation therapy remained a significant beneficial factor in overall short-term mortality and local control. With regard to mortality, use of radiation therapy was significantly associated with lower hazard of death, but to a greater extent for women below the age of 70. In order to explore other factors associated with higher mortality in patients not receiving radiation, multivariate analyses included variables available from the NC CCR and claims that were potentially related to receipt of radiation and to survival. Higher mortality was significantly assoiated with older age, higher comorbidity, larger tumor size, and greater number of involved lymph nodes - factors not modifiable by radiation, but known risk factors for mortality. Receipt of radiation and lower comorbidity were also significantly associated with lower risk of local recurrence, but not with breast cancer events. Stratification by age led to similar findings.
Predictors of higher risk of breast cancer events, which included recurrence and disease-specific mortality, were Medicaid insurance coverage, longer length of follow-up, and receipt of mammography. Low socioeconomic status is a risk factor for higher breast cancer mortality (18-20), a phenomenon associated with less than standard therapy, black race, or more aggressive tumor subtype.(20-24)
Strengths of this study include the follow-up of over 4 years, the large administrative database with availability of tumor, patient, and management information from claims, and inclusion of an algorithm to detect not only local recurrence, but distant recurrence and cancer-specific mortality. The study adds to the literature, where there are reports of higher local recurrence rates with incomplete XRT (< 25 sessions)(25), but not large analyses that determine the effect of lack of radiation on breast cancer metastases.
The observational nature of the study, and the availability of claims data after but not before the breast cancer diagnoses, are weaknesses of the study. There are patient specific factors, such as social situation and functional status that are not available from an administrative database, but that may play a role in receipt of radiation and patient outcomes. The use of claims within 2 years after diagnosis to determine comorbidity, may overestimate comorbidity level by including diagnoses related to cancer treatment and not reflect prediagnosis comorbidity level. The majority of the subjects had hormone receptor positive breast cancer and may or may not have taken or adhered to adjuvant endocrine therapy, which may have affected outcomes; in this administrative database, however, information was not consistently available on endocrine therapy use so it could not be included in the analyses. The algorithm for local recurrence is subject to over-reporting, by including new primary breast cancers as local recurrences, or under-reporting, by missing local recurrences that occur with metastases and are not coded.(12, 13) To explore breast cancer specific outcomes, as opposed to overall mortality, we created a clinically intuitive algorithm, designed to detect breast cancer recurrence and death. To our knowledge, this is among the first studies using an algorithm to detect cancer recurrence from an administrative database. We found only one other small study, which described an algorithm using ICD9 diagnostic codes in Medicare claims to detect breast cancer recurrence.(26) Our study therefore adds to the literature by describing a clinically intuitive algorithm, employing procedures, procedure codes, and ICD9 codes to detect local and distant recurrence and breast cancer death from administrative data, and allowing the exploration of breast cancer recurrence, in addition to breast cancer and overall mortality.
Higher overall short-term mortality in women not receiving adjuvant radiation could not be attributed to breast cancer relapse in this study. We suspected that comorbidity would account for a significant amount of the increased mortality found in patients who did not get radiation, not only because higher comorbidity is associated with a higher risk of death, but because women who were more ill would be less likely to get radiation. In fact, comorbidity was a significant predictor of overall death, but only with a HR of 1.16 in multivariate analysis, and comorbidity was not significantly associated with breast cancer recurrence/death. When we stratified by comorbidity, we found that comorbidity was not a clear effect modifier. We suspect that either we may not have captured the actual level of illness or, since 40% of the patients had no comorbidity, we lacked the power to see an association.
Use of preventive and/or follow-up care was also explored, but no significant relationship was seen. In prior studies levels of comorbidity(27-29) and disability(30, 31) increase with age. We, therefore, expected that more preventive care among older patient with breast cancer might improve outcomes. Though the significance level found in this study is not significant, additional research in this area is warranted because, especially in older patients, the presence of comorbid diagnoses is more prevalent.
In conclusion, we find that women who do not receive adjuvant radiation after BCS are at higher risk of death, even within this relative short length of follow-up of just over 4 years. Higher level of comorbidity and older age were both independently associated with a greater risk of death. We suspect that factors related to co-morbidity, access to care, quality of care, socio economic status, and patient behavior - - or a combination thereof – are important and that interventions to improve delivery of care will lead to improved survival rates.
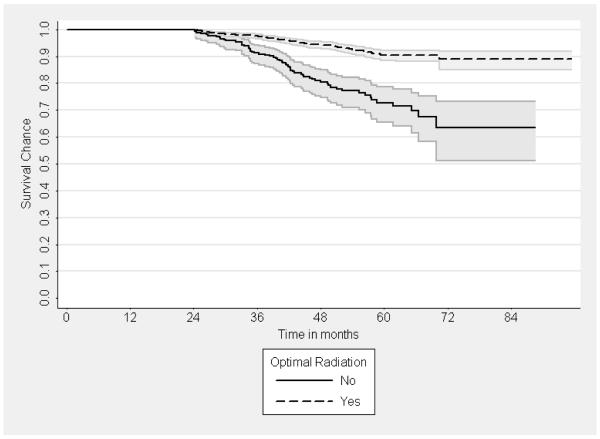
Figure 2.
Kaplan Meyer curves for overall death by receipt of radiation therapy after breast conserving surgery (in restricted sample).
Note: 95% Pointwise Confidence Bounds shown as colored areas. Log rank test p < .0001.
Acknowledgements
This work was supported by funding from the National Cancer Institute at the National Institutes of Health Grant No. R01-CA121317-3 to Roger Anderson, PhD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vallis KA, Tannock IF. Postoperative radiotherapy for breast cancer: Growing evidence for an impact on survival. Journal of the National Cancer Institute. 2004;96(2):88. doi: 10.1093/jnci/djh029. [DOI] [PubMed] [Google Scholar]
- 2.Carlson RW, Edge SB, Theriault RL. NCCN Breast Cancer Practice Guidelines Panel. Cancer Control. 2001;8(6 Suppl 2):54. [PubMed] [Google Scholar]
- 3.Lee-Feldstein A, Anton-Culver H, Feldstein PJ. Treatment differences and other prognostic factors related to breast cancer survival. Delivery systems and medical outcomes. JAMA. 1994 Apr 20;271(15):1163–8. [PubMed] [Google Scholar]
- 4.Lash TL, Silliman RA, Guadagnoli E, Mor V. The effect of less than definitive care on breast carcinoma recurrence and mortality. Cancer. 2000;89(8):1739. doi: 10.1002/1097-0142(20001015)89:8<1739::aid-cncr14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Foley KL, Kimmick G, Camacho F, Levine EA, Balkrishnan R, Anderson R. Survival disadvantage among Medicaid-insured breast cancer patients treated with breast conserving surgery without radiation therapy. Breast Cancer Res Treat. 2007 Jan;101(2):207–14. doi: 10.1007/s10549-006-9280-2. [DOI] [PubMed] [Google Scholar]
- 6.Snyder CF, Frick KD, Kantsiper ME, Peairs KS, Herbert RJ, Blackford AL, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009 Mar 1;27(7):1054–61. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21(8):1447. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CH, Adamo M, editors. SEER Program Coding and Staging Manual 2007. National Cancer Institute; Bethesda, MD: 2007. NIH Publication number 07-5581. [Google Scholar]
- 9.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003 Apr 15;21(8):1447–51. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 10.D’Hoore W, Bouckaert A, Telquin C. Practical considerations on the use of the Charlson Comorbidity Index with administrative data bases. J Clin Epidemiol. 1996;49(12):1429. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 11.Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast. 4th ed Lippincott, Williams, and WIlkins; Philadelphia: 2010. [Google Scholar]
- 12.Punglia RS, Saito AM, Neville BA, Earle CC, Weeks JC. Impact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysis. BMJ. 340:c845. doi: 10.1136/bmj.c845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006 May 17;98(10):681–90. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New England Journal of Medicine. 2002;347(16):1233. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 15.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 Dec 17;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 16.Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: Pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. Journal of the National Cancer Institute. 2004;96(2):115. doi: 10.1093/jnci/djh013. [DOI] [PubMed] [Google Scholar]
- 17.Kimmick G, Camacho F, Foley KL, Levine EA, Balkrishnan R, Anderson R. Racial Differences in Patterns of Care Among Medicaid-Enrolled Breast Cancer Patients. JOP. 2006;2(5):205. doi: 10.1200/jop.2006.2.5.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrijvers CT, Mackenbach JP, Lutz JM, Quinn MJ, Coleman MP. Deprivation and survival from breast cancer. Br J Cancer. 1995 Sep;72(3):738–43. doi: 10.1038/bjc.1995.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentil-Brevet J, Colonna M, Danzon A, Grosclaude P, Chaplain G, Velten M, et al. The influence of socio-economic and surveillance characteristics on breast cancer survival: a French population-based study. Br J Cancer. 2008 Jan 15;98(1):217–24. doi: 10.1038/sj.bjc.6604163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley CJ, Gardiner J, Given CW, Roberts C. Cancer, Medicaid enrollment, and survival disparities. Cancer. 2005 Apr 15;103(8):1712–8. doi: 10.1002/cncr.20954. [DOI] [PubMed] [Google Scholar]
- 21.Downing A, Prakash K, Gilthorpe MS, Mikeljevic JS, Forman D. Socioeconomic background in relation to stage at diagnosis, treatment and survival in women with breast cancer. Br J Cancer. 2007 Mar 12;96(5):836–40. doi: 10.1038/sj.bjc.6603622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalton SO, Ross L, During M, Carlsen K, Mortensen PB, Lynch J, et al. Influence of socioeconomic factors on survival after breast cancer--a nationwide cohort study of women diagnosed with breast cancer in Denmark 1983-1999. Int J Cancer. 2007 Dec 1;121(11):2524–31. doi: 10.1002/ijc.22979. [DOI] [PubMed] [Google Scholar]
- 23.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt) 2009 Jun;18(6):883–93. doi: 10.1089/jwh.2008.1127. [DOI] [PubMed] [Google Scholar]
- 24.Newman LA, Martin IK. Disparities in breast cancer. Curr Probl Cancer. 2007;31(3):134–56. doi: 10.1016/j.currproblcancer.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Srokowski TP, Fang S, Duan Z, Buchholz TA, Hortobagyi GN, Goodwin JS, et al. Completion of adjuvant radiation therapy among women with breast cancer. Cancer. 2008 Jul 1;113(1):22–9. doi: 10.1002/cncr.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamont EB, Herndon JE, 2nd, Weeks JC, Henderson IC, Earle CC, Schilsky RL, et al. Measuring disease-free survival and cancer relapse using Medicare claims from CALGB breast cancer trial participants (companion to 9344) J Natl Cancer Inst. 2006 Sep 20;98(18):1335–8. doi: 10.1093/jnci/djj363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fish EB, Chapman JA, Link MA. Competing causes of death for primary breast cancer. Ann Surg Oncol. 1998;5(4):368. doi: 10.1007/BF02303502. [DOI] [PubMed] [Google Scholar]
- 28.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994 Jan 15;120(2):104–10. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 29.Yancik R, Wesley MN, Ries LAG, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–92. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 30.Bild DE, Fitzpatrick A, Fried LP, Wong ND, Haan MN, Lyles M, et al. Age-related trends in cardiovascular morbidity and physical functioning in the elderly - the Cardiovascular Health Study. Journal of the American Geriatrics Society. 1993;41(10):1047. doi: 10.1111/j.1532-5415.1993.tb06451.x. [DOI] [PubMed] [Google Scholar]
- 31.Strawbridge WJ, Kaplan GA, Camacho T, Cohen RD. The dynamics of disability and functional change in an elderly cohort - results from the Alameda County Study. Journal of the American Geriatrics Society. 1992;40(8):799. doi: 10.1111/j.1532-5415.1992.tb01852.x. [DOI] [PubMed] [Google Scholar]