Abstract
Objectives
To assess the use of an electronic dose calculator to improve accuracy in the use of a complex Gentamicin prescription policy and assess turnaround time of blood sampling to dose delivery in an NHS hospital.
Design
Retrospective review of drug chart, case notes and hospital antibiotic database.
Setting
University Hospitals Bristol, UK
Participants
Patients receiving once daily intravenous gentamicin using the trust protocol, during the same time window for 3 consecutive years.
Main outcome measures
i) Accuracy of dose and frequency prescription of Gentamicin. ii) Time frame for measurement of serum Gentamicin levels.
Results
Following the introduction of the online calculator, prescribing errors in obese patients dropped from 43% to 20%, a similar level as in non-obese patients. Errors in frequency calculations dropped from 12.8% to 4%. On average, drug doses could be administered within 2.5 hours of a blood sample being taken.
Conclusions
Online tools can be used to improve prescribing for the complex dosing policies that will increasingly been required to tailor prescribing in obese patients. Serum gentamicin levels can be measured within a 2.5 hour time frame in the environment of an NHS hospital.
Introduction
The increasing prevalence of obesity has led some to question whether prescribing should be more tailored to the individual, such that patients receive dosages that will create plasma concentrations falling more reliably within therapeutic ranges. In the field of oncology we see that therapy can be successfully personalized through treatments matched to biomarkers expressed by an individual,1 and in cardiology various methods have been utilized to personalize antiplatelet therapy to allow individuals within the population to achieve an equally effective dosage.2
Despite personalized care according to body weight already being possible, the practicalities of prescribing routine drugs on an individualized basis on busy general surgical wards have hindered the introduction of these advances.
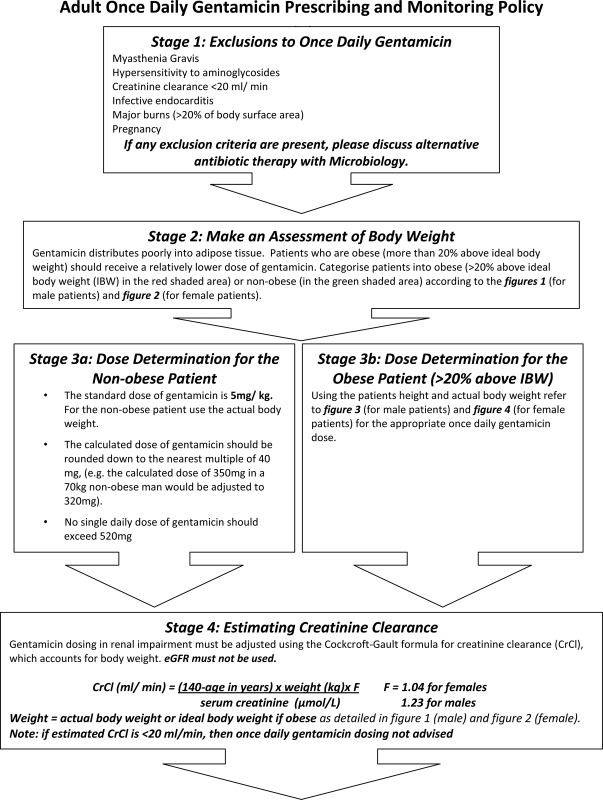
Gentamicin, owing to its potent ototoxic and nephrotoxic side effects, is a drug that has always required individualized dose calculation to some extent, with some trusts already having a dosing protocol in place to dose patients based on weight. However, gentamicin is a hydrophilic drug that will undergo differential distribution in the obese patient compared to patients with normal body mass index, leading to over-dosing in obese patients if prescribed according to actual body weight.3 Recently, Gentamicin has regained popularity as antibiotic protocols have been altered in an attempt to decrease the incidence of drug associated clostridium difficile infection. In 2008, a new prescription protocol was established at the Bristol Royal Infirmary, UK. Since obesity may affect 20–30% of patients,4 the opportunity was taken to introduce a new calculation policy that would more accurately dose patients to improve safety, especially in these obese patients. This required a series of step-wise calculations to be carried out to determine the patients ideal body weight, creatinine clearance and dosing schedule.
It was also unknown if hospital systems would be able to withstand the pressures imposed by a regime that is reliant on staff to take a blood sample at a specified time and transport it to pathology where it would be processed with sufficient speed to allow nursing staff to deliver subsequent doses without delay. The success of the policy was audited on three occasions, with changes made to facilitate the use of the protocol by doctors at each stage.
Two primary outcomes were identified for this study. Firstly, to assess the extent to which an electronic online calculation tool can help improve accuracy of drug prescription in obese patients and secondly to assess the time taken for systems within an NHS hospital to process gentamicin levels.
Methods
Three audit cycles were undertaken over the same time period each year and included all patients at University Hospitals Bristol, UK, that had gentamicin levels sent to pathology during the time period August to September. This time period corresponds to the period when new junior doctors start working at the trust. Inclusion criteria was any patient receiving once daily intravenous gentamicin using the trust protocol within the given time window.
Prior to the introduction of the online calculator, the patients' ideal body weight (IBW) was calculated, with the aid of reference tables (Appendix, Figures 1, 2), using the following formulae:
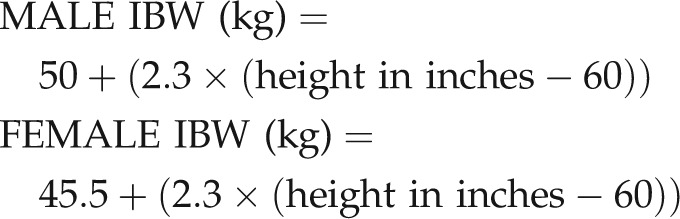 |
Patients were categorized as obese if their weight was greater than 20% above IBW.
Non-obese patients received a standard dose of 5 mg/kg rounded down to the nearest multiple of 40. In obese patients, a ‘Dose-determining weight’(DDW) was calculated using the formula:
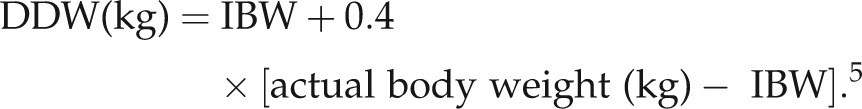 |
Gentamicin dose was then calculated by multiplying DDW(kg) by 5 mg, then rounding down to the nearest multiple of 40. Reference tables were made available to assist with this calculation (Appendix, Figure 3).
Gentamicin was dosed daily according to this protocol, except in patients with impaired renal function. To determine renal function, creatinine clearance (CrCl) was calculated using the Cockcroft Gault equation:6
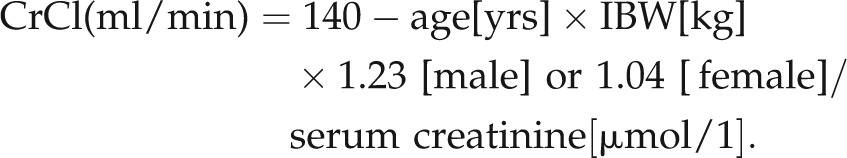 |
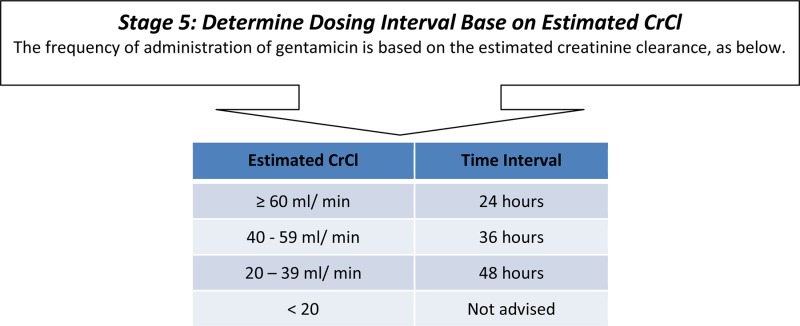
If CrCl was 40–59 µmol/l frequency reduced to 36 hourly and to 48 hourly in patients with CrCl 20–39 µmol/l. Patients with CrCl less than 20 were not suitable for Gentamicin.
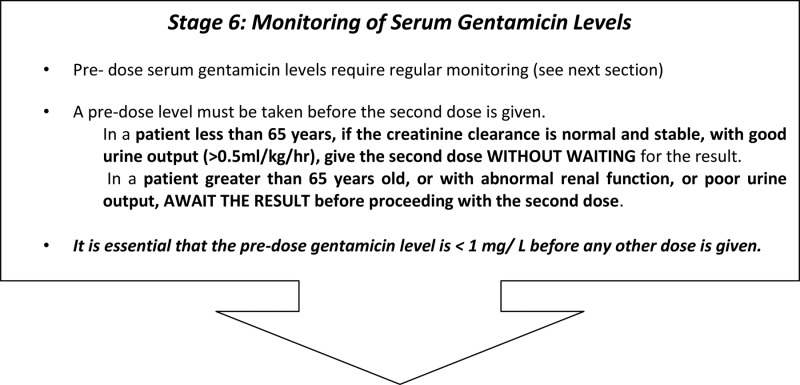
The protocol required gentamicin levels to be taken after the first dose in all patients. Subsequently, levels were required to be taken after every dose in patients over 65 or with poor renal function, but levels were not required to be checked in patients with normal creatinine clearance provided they were passing adequate volumes of urine.
Following the first audit cycle, an online calculator was produced and published on the hospital intranet. This calculator required the input of a patient's age, sex, weight and serum creatinine, in order to calculate dosage and frequency, and, following the second audit cycle, the prescription chart was altered to advertise this tool and remove dosing formulas.
Each drug dose was assessed retrospectively by one of the authors (JM,ARS,CT,SS,KS). Patients were excluded if there was a documented reason for the prescription not being dosed according to protocol e.g. given according to microbiology advice. Adults were included from all specialties.
The Bristol Royal Infirmary online antibiotic database which was set up to prior to the launch of the antibiotic policy. This recorded specimen processing activity including time of blood sampling, time of arrival in the laboratory, Gentamicin level result and time of level being available. Data for each serum level taken was extracted from this database by the authors (JM,ARS,CT,SS,KS).
On the prescription form it was the responsibility of doctors to calculate dose using patient weight and record creatinine clearance in order to calculate frequency. These calculations were often checked by pharmacists. It was the responsibility of nurses to record the timing of blood samples and record the dose given and the time given. Doctors bore overall responsibility for the correct use of the protocol.
The tests for statistical significance were performed using the SPSS 17.0 statistics program (SPSS Inc. Chicago, IL, USA).
Results
A total of 334 Gentamicin doses were administered during the 3 audit windows. The majority of these being delivered to surgical patients, as would be expected with the local prescribing guidelines. Population size was 31 patients in the 2008 audit (17 non-obese, 14 obese), 32 patients in the 2009 audit (20 non-obese, 12 obese) and 27 patients in the 2010 audit (17 non-obese, 10 obese). Overall, 40% of patients identified were obese. The median age of patients was 59 (inter-quartile range 40–72.5 yrs).
Over the 3 years, dosing accuracy in non-obese patients remained fairly consistent with between 71 and 88% of patients having all doses correct. In total, 22% of non-obese patients had a dose prescribed incorrectly.
In 2008, 43% of obese patients received all doses calculated according to protocol. In 2009, this figure was 42%. By 2010, this figure was 20% (Figure 1).
Figure 1.
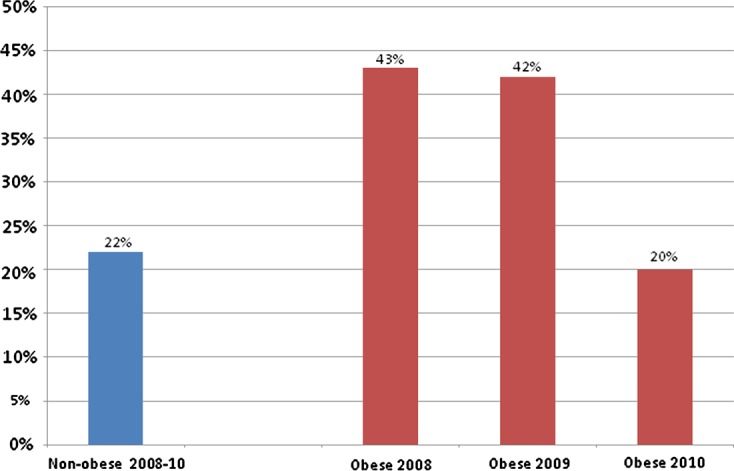
% of patients prescribed an incorrect dose according to protocol
According to the protocol, frequency of prescription can vary every dose as renal function varies. As such, the total number of prescriptions are reported rather than the number of patients with all frequencies correct. The accuracy of frequency prescription was difficult to assess due to occasional delays between prescription and dosing. Although the prescription recorded the planned time of the next dose and the time the previous dose was given, the time of the prescription was not always recorded and may differ from the dosing time. As such, up to 65% of frequency prescriptions could not be accurately assessed, although cases in which errors were present were easier to identify, primarily because most changes require extending the period between doses, leaving doses occurring early in spite of any delays occurring on the ward. In the first cycle 13 of 107 (12.2%) of prescriptions were at the incorrect frequency, 9 of which errors were in obese patients; in the second cycle this was 4 of 106 (3.8%) with 3 in obese patients; and 3 of 75 (4%) in the third cycle, 2 of which were obese. These results were significantly different between the 3 cycles (Pearson χ2 P = 0.017). (Figure 2)
Figure 2.
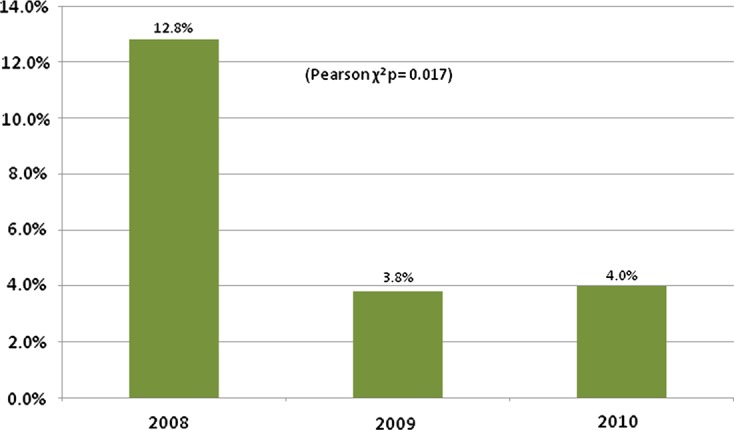
% of patients with an incorrectly calculated frequency according to protocol
Median time for the drug level samples to reach pathology in the 3 audits varied between 70 to 91 minutes, but these differences were not statistically significant (Kruskal-Wallis P = 0.274). As such, the overall median time is reported as 75.5 minutes (inter-quartile range: 76.5). Median time for the specimen to be processed by the laboratory increased from 61 and 58 minutes (inter-quartile ranges: 31.5 and 23.5) in the first 2 audit cycles to 70 minutes (inter-quartile range: 38.25) in the third cycle (P < 0.001).
For all doses that were delivered at the correct dose and frequency, all pre-dose levels were found to be below 1 mg/L.
Discussion
In our population of patients we found 40% to be obese and, as such, the tailored prescribing of gentamicin is necessary to avoid over dosing a large number of patients. However, in our first audit since individualized dosing was introduced, less than 60% of obese patients had all doses calculated correctly requiring the development of an online calculation tool.
Although the second audit occurred after the introduction of the online calculator, it was clear from reviewing prescription charts that the majority of errors were still resulting from doctors attempting to calculate the dosage using the formulas on the chart. Through further education of the doctors at induction and by altering the prescription chart to remove the calculation and highlight the online calculator, the frequency of correct calculation increased to 80%, similar to that seen in non-obese patients. The remaining errors appear to primarily be related to doctors estimating the dosage and then subsequent prescriptions copying the error. These errors may be prompted by attempts at dosing when patient variables remain unknown, and so the future focus of improvements in prescribing must include further education of both doctors and nursing staff to ensure that patient weight and renal function are known early during acute admissions.
A similar trend was seen with regards to frequency calculations. The frequency at which gentamicin is given should change as the patient's renal function varies according to the protocol. In the first audit cycle, errors occurred in 12.8% of obese patients during frequency prescribing. Following introduction of the calculator and education, this figure was improved in the two subsequent audits.
In order for gentamicin to be used safely it is important to measure the serum gentamicin levels. Pre-dose levels are taken 1 hour before the dose is due. Over the 3-year period, findings were that more than 50% of results were available to ward staff 2.5 hours after they took the blood sample. These results demonstrate that within this hospital it is possible to aim for all doses to be given within 2 hours of the prescription time. However, for other hospitals to adopt a similar protocol it would be important to consider the capacity of phlebotomy staff, nursing staff, portering staff and the pathology laboratory to ensure that the samples are able to be processed urgently enough to allow for timely dosing.
We found that when Gentamicin dose and frequency was calculated according to the protocol, there were no instances where the pre-dose level was above 1 mg/L, suggesting that this protocol provides safe dosing.
When used, doctors reported the online calculator to be very popular, reducing the time taken to use the protocol. Many doctors had previously worked in trusts where long calculations were required for intravenous gentamicin dosing. Some of these trusts reportedly used printed stickers or booklets to aid doctors with calculations, but no literature is available on this. Some doctors reported instances in other hospitals where no clear guidance existed on safe dosing. These doctors reported feeling more able to prescribe safely when using the calculator.
Conclusion
With the increasing incidence of obesity it is also important to identify a practical way of dosing gentamicin so that all patients are provided with therapeutic dosages and protected from the adverse effects of higher doses. Within the environment of an NHS hospital it has been possible to measure the majority of serum gentamicin levels within a 2.5 hour time frame. Such a turnaround time suggests that protocols that dose according to patient serum levels are feasible in an NHS hospital. This data also demonstrates that through the introduction of a simple online calculator, the accuracy of prescribing in obese patients could be improved to the same levels as in non-obese patients. With the predicted increase in the requirement for tailored prescribing in the future, technological support is vital in the busy clinical environment.
DECLARATIONS
Competing interests
None declared
Funding
None
Ethical approval
The study was registered with the trust audit committee and ethical approval was deemed not required
Guarantor
JM
Contributionship
JM & AS crafted the manuscript;
JM, CT & EJ designed the study;
JM,ARS,CT,SS & KS collected data and analysed results;
RL & EJ designed the original protocol and supervised the study
Acknowledgements
None
Reviewer
Mubarak Al Ameri
Appendix
UH Bristol Adult Once Daily Gentamicin Prescribing and Monitoring Policy
Gentamicin should be prescribed as an infusion in 100 ml sodium chloride 0.9% or glucose 5% over 30 minutes.
All patients on gentamicin should be on a fluid chart.
Bloods for gentamicin levels should be sent as urgent.
Please review all IV treatment after 48 hours, and no patient should be given gentamicin for greater than 7 days without discussion with Microbiology
Once Daily Gentamicin Dosing Monitoring and Interpretation of Levels
Monitoring
On the request form please state clearly the exact time when the last dose of gentamicin was given and when the sample was taken.
Take pre-dose gentamicin level up to 1 hour before the second dose is due. NB Random level monitoring is NOT recommended.
Collect blood in a clotted tube (yellow top).
Pre-dose levels of gentamicin should be less than 1 mg/L.
In a patient less than 65 years, if the creatinine clearance is normal and stable, with good urine output (>0.5 ml/kg/hr), give the second dose WITHOUT WAITING for the result. The result should be checked and interpreted before the third dose is due to be given.
In a patient greater than 65 years old, or with abnormal renal function, or poor urine output, AWAIT THE RESULT before proceeding with the second dose.
There is no need to measure peak (post dose) serum levels.
Subsequent Monitoring
For patients with normal and stable renal function check pre-dose gentamicin levels every 48 hours.
For patients with abnormal renal function a pre-dose serum gentamicin level is required before each dose is given.
In a patient less than 65 years, if the creatinine clearance is normal and stable, the patient has good urine output (>0.5 ml/kg/hr), and previous levels have been in range the next dose can be given without waiting for the result.
-
Renal function must be checked regularly – at least three times a week. If renal function deteriorates, more frequent monitoring may be needed, and the dosing interval may need to be increased or discontinuation of therapy may be required. Please discuss with Microbiology.
Please review all IV treatment after 48 hours, and no patient should be given gentamicin for greater than 7 days without discussion with Microbiology
Interpretation of levels
| RESULT | ACTION |
|---|---|
| Pre-dose level ≤1 mg/L | Continue current therapy. |
| If any pre-dose level is >1 mg/L then withhold next dose and check for reason why e.g. incorrect dosage or timing of sample. | |
| If timing was incorrect, recheck the level at the appropriate time. | |
| Pre-dose level HIGH | If the timing of the sample was correct then: |
| • The pre-dose gentamicin level must be 1 mg/L or less before a further dose is given. | |
| >1 mg/L | • Levels will need to be repeated at 12–24 hours depending on the original result. |
| • It may be necessary to increase the dosing interval. | |
| • Any treatment changes should be discussed with the Microbiologist or Pharmacist as continuing therapy may not be appropriate. |
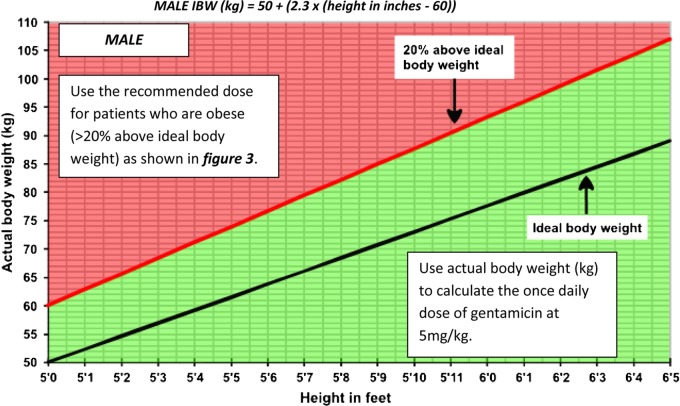
Figure 1.
Obesity categorization for MALE patients. If the patient falls in the red shaded area, then they are categorized as obese (>20% above IBW) – use figure 3 to determine the dose of gentamicin to be used. For those who fall in the green shaded area, use the actual body weight to calculate the dose of gentamicin, as in stage 3a. The IBW for males is calculated using:
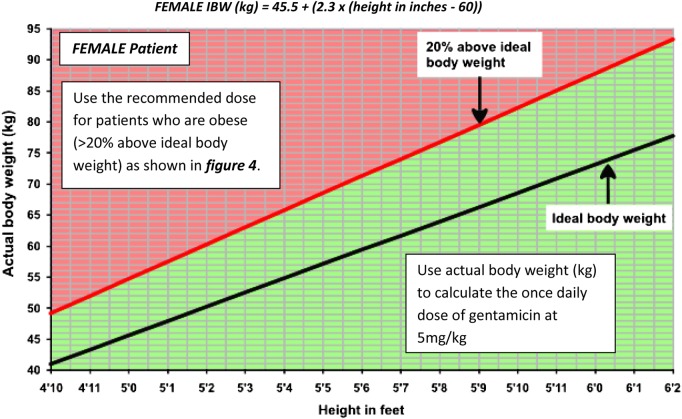
Figure 2.
Obesity categorization for FEMALE patients. If the patient falls in the red shaded area, then they are categorized as obese (>20% above IBW) – use figure 4 to determine the dose of gentamicin to be used. For those who fall in the green shaded area, use the actual body weight to calculate the dose of gentamicin, as in stage 3a. The IBW for females is calculated using:
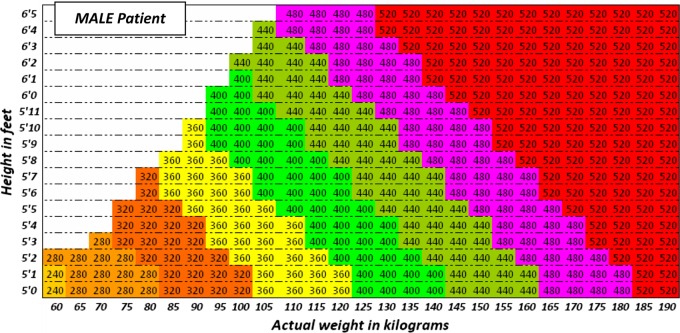
Figure 3.
Use the height and actual body weight to determine the once daily dose of gentamicin (in milligrams) for obese MALE patients
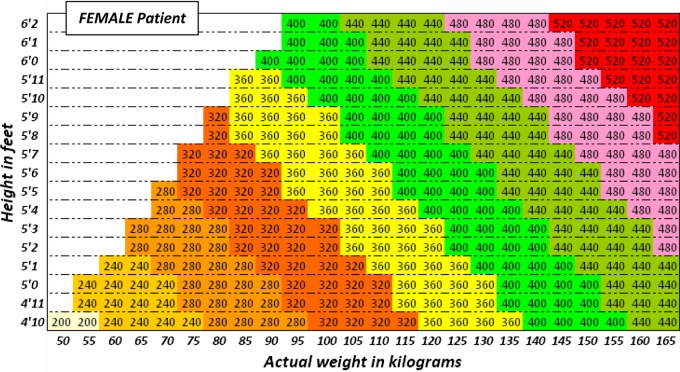
Figure 4.
Use the height and actual body weight to determine the once daily dose of gentamicin (in milligrams) for obese FEMALE patients
Recommended dose of once daily gentamicin (in milligrams) for obese individuals (more than 20% above ideal body weight) is derived by calculating the Ideal Body Weight (IBW) as described above, and then calculating the dose determining weight (DDW) (kg) = IBW + 0.4 × (actual body weight (kg) − IBW).
The dose of gentamicin is then calculated by multiplying the DDW (kg) by 5 mg, then rounding down to the nearest multiple of 40.
References
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41 [DOI] [PubMed] [Google Scholar]
- Devine BJ. Gentamicin pharmacokinetics. Drug Intell Clin Pharm 1974;8:650–5 [Google Scholar]
- Traynor AM, Nafziger AN, Bertino JS Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrobial Agents and Chemotherapy 1995; 39:545–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz R, Itokazu G, Rodvold K Antimicrobial Dosing in Obese Patients. Clinical Infectious Diseases 1997;25:112–8 [DOI] [PubMed] [Google Scholar]
References
- 1.Nathanson KL Using genetics and genomics strategies to personalize therapy for cancer: focus on melanoma. Biochem Pharmacol 2010;80:755–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonello L Personalised Antiplatelet Therapy – Whether to Choose Genotype or Phenotype. European Cardiology 2010;6:45–53 [Google Scholar]
- 3.Wurtz R, Itokazu G, Rodvold K Antimicrobial Dosing in Obese Patients. Clinical Infectious Diseases 1997;25:112–8 [DOI] [PubMed] [Google Scholar]
- 4.Zaninotto P, Wardle H, Stamatakis E, Mindell J, Head J Forecasting Obesity to 2010 (2006) http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_073033.pdf (last accessed 19 March 2011)
- 5.Traynor AM, Nafziger AN, Bertino JS. Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrobial Agents and Chemotherapy 1995;39:545–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockcroft DW, Gault MH Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41 [DOI] [PubMed] [Google Scholar]