Abstract
The ability to flexibly break out of routine behaviors develops gradually and is essential for success in life. We discuss three key developmental transitions toward more flexible behavior. First, children develop an increasing ability to overcome habits by engaging cognitive control in response to environmental signals. Second, children shift from recruiting cognitive control reactively, as needed in the moment, to recruiting cognitive control proactively, in preparation for needing it. Third, children shift from relying on environmental signals for engaging cognitive control to becoming more self-directed. All three transitions can be understood in terms of the development of increasingly active and abstract goal representations in prefrontal cortex.
Keywords: cutive function, endogenous control, task-switching, perseveration
The world of the infant is not a “blooming, buzzing confusion” as once thought (James, 1890). Instead, infants rapidly detect regularities in their environments to bring order to what they see and hear (Romberg & Saffran, 2010), allowing them to quickly learn routines. However, this sensitivity to regularities comes at a price: Infants and children show striking limitations in their abilities to break out of habitual ways of thinking and behaving.
For example, after repeatedly watching a toy being hidden in one location, infants rapidly detect the regularity (of where the toy usually is) and learn to search there. Then, even after seeing the toy hidden in a different location, infants continue to search in the first location (Piaget, 1954). Similarly, children can rapidly learn a habit of sorting cards by one rule (e.g., color), such that they continue to follow this rule even after being instructed to switch to a new rule (e.g. shape; Zelazo, Frye, & Rapus, 1996).
Overcoming such perseveration constitutes an important aspect of development that is predictive of success in life (e.g. academic achievement, health, and income, Blair & Razza, 2007, Moffit et al., 2011). How do we progress from searching where we last found a toy, to flexibly breaking away to search in new locations, to flexibly breaking away from playing to put away toys when told? How do we then progress to flexibly moving between activities on our own, and to planning errands or a vacation? How do we achieve these fundamental aspects of what it means to grow up?
We and others have focused on the development of abstract goal representations actively maintained in working memory (Bunge & Zelazo, 2006; Morton & Munakata, 2002a). Abstract representations (e.g., for the goal of sorting cards by color) encompass individual examples (e.g., red, blue, orange), coding for shared features while generalizing over differences. Building upon the well-established role of prefrontal cortical regions in supporting such goal representations (Miller & Cohen, 2001; Figure 1), we have formulated a unified framework for understanding how these regions support executive functions like inhibitory control (Munakata et al., 2011) and their development (Figure 2). Here, we describe how the development of abstract goal representations can support three key transitions toward more flexible behavior.
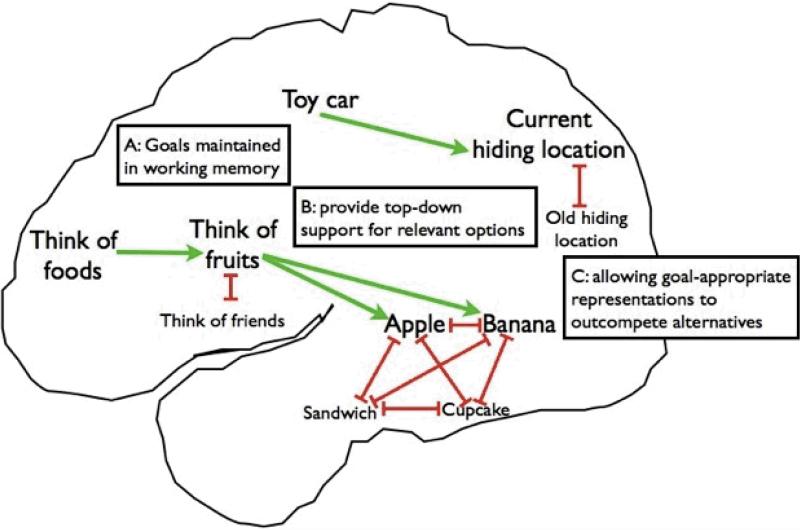
Figure 1.
A well-established characterization of prefrontal cortical regions in cognitive control, which we build upon in our framework for understanding key developmental transitions in cognitive control. Here we show two distinct pathways, one for finding a toy, the other for naming as many foods as possible in a brief period. A) Goal representations (e.g., “Get the toy car”) are maintained in working memory and supported by the sustained firing of neurons in prefrontal cortical regions. B) These goal representations provide top-down support, via excitatory connections from prefrontal cortex (green arrows), for relevant options represented in other brain regions, causing goal-appropriate representations (e.g., a toy's current hiding location) to become more strongly activated. C) Representations compete with one another via inhibitory connections (red t-bars), allowing the most active, goal-appropriate representations to win out over alternatives (e.g., the toy's previous hiding location) and thus guide behavior. In this way, goal representations can support flexible behaviors over habitual ones (as in searching for the toy car). Goal representations can also support selection among multiple competing options, as with the goal of “Think of foods,” which activates the sub-goal of “Think of fruits”; this sub-goal in turn provides top-down support for a more limited pool of food items, helping to resolve the competition and support a choice. By providing top-down support for relevant options in this way, such goal representations could also potentially interfere with more bottom-up learning of regularities in the world.
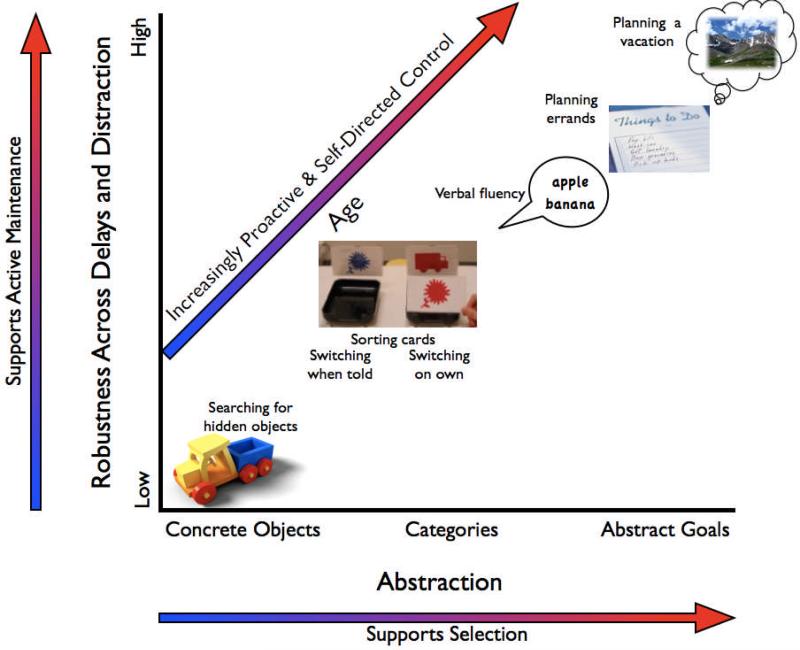
Figure 2.
Our framework focuses on the role of increasingly robust, abstract goal representations in three key developmental transitions in cognitive control. With development, children's goal representations become more abstract (from concrete objects to abstract goals, as shown on the x-axis) and more robust to distraction and delay (y-axis). Increasingly robust representations support active maintenance of goals in working memory, which provide top-down support for goal-relevant representations. Increasingly abstract representations support selection from among options, by providing top-down support for a limited pool of competitors. These increasingly robust, abstract goal representations: 1) support increasingly flexible, goal-appropriate behaviors over habitual ones in response to signals from the environment (e.g., searching for a toy in a new hiding location after seeing it hidden there, and later in development, sorting cards according to a new abstract rule when instructed), 2) allow such cognitive control to become less externally-driven and more self-directed (e.g., sorting cards according to a new, self-generated, abstract rule, and later in development, switching among more abstract, self-generated categories), and 3) allow such cognitive control to become less reactive and more proactive (e.g., maintaining abstract goals over a sufficiently long period and in the face of distraction to support planning errands or a vacation). Although robust representations (y-axis) could be viewed as most critical for proactive control, and abstract representations (x-axis) could be viewed as most critical for self-directed control, developments in both aspects of goal representation support increasingly proactive, self-directed control (as shown along the diagonal, and discussed in the Variability Across Tasks section). Thus, these developmental transitions can occur in concert. These developments likely reflect effects of both learning and brain maturation, within prefrontal cortical regions and in the development of large-scale, distributed networks of brain regions. For example, the formation of increasingly abstract goal representations may be supported by the maturation of anterior prefrontal cortex, and by the building of representations from the bottom-up, with the formation of more concrete representations providing the foundation for the formation of more abstract representations (Bunge & Zelazo, 2006). Similarly, the increasing ability to maintain representations over delays and distractions may be supported by both maturational changes in prefrontal cortical regions and associated networks for sustaining neuronal firing (Edin et al., 2007) and by learning processes that detect the utility of maintaining goal-relevant information (e.g., O'Reilly & Frank, 2006).
TRANSITION 1: FROM PERSEVERATING TO OVERCOMING HABITS WHEN DIRECTED
First, children become increasingly able to overcome habits in response to environmental signals. Within our framework, these developments are driven by improvements in the ability to maintain information relevant to goals. For example, infants gradually become better at maintaining concrete information, such as a toy's new hiding location, over delays and distractions. As children develop increasingly abstract representations, they can start maintaining information such as task rules. These actively-maintained representations provide top-down support for goal-relevant thoughts and behaviors (Figure 1). Such cognitive control allows children to respond flexibly to changes in their environment, rather than falling back on habits (Figure 2).
This framework has been implemented in biologically-based neural network models (e.g., Chatham, Yerys, & Munakata, 2012; Morton & Munakata, 2002a), which have simulated developing cognitive control and led to counterintuitive predictions. For example, children who perseverate in sorting cards according to an old rule (e.g., sorting a red truck based on color) nonetheless seem to know where the cards should go, correctly answering questions such as “Where do trucks go in the shape game?” (Zelazo, Frye, & Rapus, 1996). Our framework explains such dissociations in terms of the strength of required goal representations (e.g., to sort by shape). A weak representation suffices when no information is presented relevant to the old rule (as with questions that focus on the new rule). A stronger representation is needed to overcome conflict when presented with information relevant to the old rule (as with cards that include both color and shape information). We thus predicted that such dissociations should disappear when measures are equated for conflict (e.g., sorting a red truck, and answering “Where do red trucks go in the shape game?”). This prediction has been confirmed (Munakata & Yerys, 2001; see also Morton & Munakata, 2002b), challenging the characterization of children as showing “knowledge-action” dissociations, and highlighting the importance of goal representations in overcoming conflict.
Goal representations also provide top-down support to speed responses and support generalization to new situations. Strongly representing a new abstract rule such as shape (as flexibly-switching children are thought to do) should speed the processing of thoughts and behaviors related to shape, and allow this rule to be applied to novel shapes. Our framework thus leads to two additional predictions that have been confirmed. First, although all children can correctly answer simple questions about what they should be doing (e.g., “Where do trucks go in the shape game?”), children who flexibly switch between rules answer such questions faster than children who perseverate with old rules (Blackwell, Cepeda, & Munakata, 2009). Second, although children who perseverate seem dramatically stuck on old behaviors (e.g., sorting red and blue cards), they cannot generalize their behaviors to new stimuli (e.g., instead sorting orange cards randomly), whereas flexibly-switching children can (Kharitonova, Chien, Colunga, & Munakata, 2009).
TRANSITION 2: REACTIVE TO PROACTIVE CONTROL
Many developmental theories have assumed that children are like adults in the temporal dynamics of how they activate goal representations, just less skilled. For example, even infants have been viewed as proactively maintaining goal-relevant information (e.g., the location of a hidden toy) until it is needed (e.g., when they are allowed to search), just less robustly than adults (e.g., Diamond, 1991; Munakata, 1998). However, recent work suggests that children use a qualitatively different, reactive form of cognitive control, which is recruited on a more as-needed basis (Andrews-Hanna et al, 2011; Chatham, Frank, & Munakata, 2009). We interpret the developmental transition from reactive to proactive control in terms of children's increasing ability to maintain robust goal representations.
Striking evidence for this transition has been observed in the AX continuous performance task (AX-CPT), in which people must provide a target response to a frequent sequential pair of stimuli (“A” followed by “X”), and a nontarget response to all other pairs (Braver, Gray, & Burgess, 2007). Eight-year-olds proactively maintain the first stimulus in each pair and use it to prepare their response. In contrast, 3-year-olds show no sign of maintaining the first stimulus, instead retrieving it reactively, when needed in the face of an “X” stimulus. These contrasting approaches are evident in distinct profiles of errors, reaction times, and effort (Chatham et al., 2009). Eight-year-olds show relatively more difficulty than 3-year-olds when the “A” stimulus is followed by a “Y” stimulus–a case in which lack of preparation actually benefits performance, by not biasing children towards preparing an incorrect target response. Conversely, 3-year-olds show more difficulty than 8-year-olds when the first stimulus (a “B”) fully predicts the nontarget response–as though failing to adequately prepare a nontarget response in advance. Moreover, 3-year-olds exert more effort (indexed by pupil diameter, Beatty & Lucero-Wagner, 2002) after the second stimulus in a pair is presented, consistent with reactively engaging cognitive control then, while 8-year-olds exert more effort after the first stimulus is presented, consistent with proactively maintaining this information until needed (Chatham et al., 2009).
This qualitative shift in the temporal dynamics of control may arise from more gradual changes in the ability to robustly and actively maintain abstract information (Figure 2). Younger children may be unable to proactively prepare for even the predictable future, because they cannot maintain relevant information across delays or distractions. As their capacity for active maintenance increases across development, it increasingly becomes sufficient to support proactive control. Such developments may support additional aspects of future-oriented thinking, such as monitoring for goal-relevant cues (e.g., a store, when the goal is to buy milk), rather than relying on such cues to serve as reminders (Einstein et al. 2005).
TRANSITION 3: EXTERNALLY-DRIVEN TO SELF-DIRECTED CONTROL
While children become increasingly skilled at actively maintaining goals to support flexible behavior, their early successes often occur with exogenous (externally-driven) goals (e.g., stopping playing and putting away toys when told), and only later with endogenous (internally-generated) goals (e.g., turning off the TV and doing homework without prompting). For example, 4-year-olds can switch between two rules for sorting cards when an adult tells them the new rule (Zelazo et al., 1996). However, when children are simply asked to sort cards in “a new way”, without being told what rule to switch to, children younger than 7 perseverate on the old rule (e.g., Jacques & Zelazo, 2001; Smidts, Jacobs, & Anderson, 2004). Under even greater endogenous demands, performance improves through adolescence (e.g. Kave, Kigel, & Kochva, 2008). For example, the verbal fluency task requires generating as many items as possible from a category (e.g. foods) in one minute. Maximal performance requires clustering (producing words within semantic subcategories) and switching (shifting between subcategories). People must thus endogenously detect the need to switch (e.g. when they cannot think of more vegetables) and select what to switch to (e.g., fruits, meats, or desserts). Children often fail (e.g. naming five fruits and declaring there are no more foods).
Giving children some initial support in activating abstract representations helps them subsequently act in a self-directed manner. When children are provided with subcategories designed to induce the use of abstract representations (e.g. “vegetables are foods”) before completing verbal fluency, they endogenously switch among subcategories more than children given examples (e.g. “broccoli is a food”) (Snyder & Munakata, 2010). This benefit also extends to subcategories that were not provided.
The transition from exogenous to endogenous control can thus be understood partly in terms of the development of increasingly active, abstract goal representations (Figure 2). Abstract representations provide top-down support for relevant category members, making it easier to select a response (Figure 1). For example, instead of selecting from all category members (many competing responses), abstract representations can limit competing items to the small pool in the subcategory, or to the small pool of other subcategories when switching. Thus, whether planning errands or a vacation, actively maintaining abstract representations of our goals (e.g., going to the grocery store and the hardware store, as opposed to buying milk, nails, and bread) should guide the selection processes that are critical for self-directed behavior. As children develop increasingly abstract representations, these should help them to control and sequence their own behavior without strong environmental support.
CURRENT AND FUTURE DIRECTIONS
Many questions remain regarding these developmental transitions and their implications.
Variability Across Tasks
Children's abilities can appear quite different across tasks (“horizontal décalage,” in Piagetian terms), in ways that can shed light on processes supporting behavior and development (Munakata, 2001). For example, depending on the task, the shift from reactive to proactive control could seem complete by infancy (Diamond, 1985), 8 years (Chatham et al., 2009; Lorsbach & Reimer, 2010), or post-adolescence (Andrews-Hanna et al., 2011; Finn, Sheridan, Kam, Hinshaw, & D'Esposito, 2010). Similarly, shifts from externally-driven to self-directed control are observed from childhood through adolescence, across different tasks (Jacques & Zelazo, 2001; Kave et al., 2008; Siklos & Kerns, 2004). Even adults can find it easier to rely on reactive or externally-driven control (Braver et al., 2007; Forstmann, Brass, Kock, & Cramon, 2005).
Our framework suggests this variability may arise partly from the interdependence of active and abstract representations. Children may engage in self-directed control when they need to maintain abstract representations only briefly (e.g. naming foods in 1 minute). Self-directed control in other contexts (e.g., planning to buy these foods) requires later-developing abilities to maintain abstract representations over longer periods and across greater environmental distraction. Similarly, infants may have sufficiently robust active maintenance of concrete representations (e.g., a hidden toy) to support proactive control. However, proactive control in other contexts requires the robust maintenance of later-developing abstract representations (e.g. task rules). In addition, proactive control is energetically costly, due to its reliance on sustained firing of prefrontal neurons; it may thus be engaged more when it is clear what information should be maintained to succeed (Locke & Braver, 2008; e.g. a hidden toy's location may be more clearly predictive of success than arbitrary game rules).
Applications
Given the importance of executive functions, one might ask whether their development can be accelerated, or effective alternative approaches encouraged. Some aspects of executive function can be trained in children (Diamond & Lee, 2011). Our framework suggests that such training works in part by supporting the use of abstract goal representations. In this case, we would expect training to also improve children's proactive, self-directed cognitive control. Children's ability to shift from old habits can also be dramatically improved by scaffolding them to build up new habits (e.g., Brace, Morton, & Munakata, 2006). This approach taps children's reliable learning of regularities, but these new habits are unlikely to generalize well. Another possible strategy is encouraging reactive control before children can effectively employ proactive control (e.g., providing children with cues that encourage them to recall instructions when needed, instead of providing instructions that require children to plan in advance).
The consequences of such approaches are unclear, however. On the one hand, encouraging less mature forms of cognitive control could delay the development of more mature forms. On the other hand, using reactive control might support the learning of regularities that ultimately aid proactive control (e.g., learning what information should be maintained to succeed). Similarly, programs that improve executive functions may benefit children who are struggling; however, improvements in executive function may bring some cost in learning regularities in the world, given that top-down goals (e.g., to search where a toy was just hidden) can override the learning of regularities (e.g., regarding the toy's most frequent hiding location) (Thompson-Schill, Ramscar, & Chrysikou, 2009). For example, strongly-maintained goals can lead to a confirmation bias, where expectations about what should happen interfere with learning regularities about what actually happens (Doll, Hutchison, & Frank, 2011).
CONCLUSION
Every adult was once a perseverating child. Perseveration makes sense, to the extent that strong habits reflect our abilities to quickly learn regularities. But developing increasingly active and abstract goal representations that allow us to overcome habits also makes sense, allowing us to respond flexibly to new situations, and to engage cognitive control in preparation for needing it and based on our assessment of this need, rather than only in the moment or requiring signals from the environment. Understanding these fundamental developmental transitions should allow us to better understand children's struggles and potentially improve their outcomes.
Acknowledgements
The preparation of this article and the research described here were supported by grants from the National Institutes of Health (RO1 HD37163 and P50-MH079485). We thank Silvia Bunge, Akira Miyake, Roselinde Kaiser Henderson and members of the Cognitive Development Center for providing useful feedback on an earlier draft of this article, and Eden Davis for assistance with manuscript preparation.
Footnotes
Braver, T. S., Gray, J. R., & Burgess, G. C. (2007). See reference list.
The definitive introduction to the distinction between reactive and proactive control, and aging-related changes in their expression.
Diamond & Lee (2011). See reference list.
A highly accessible overview of the importance of executive functions in school and life, and the types of interventions that can aid their development.
Munakata, Y., Chatham, C. H., and Snyder, H. R. (in press). Mechanistic accounts of frontal lobe development. To appear in D.T. Stuss & R.T. Knight (Eds.), Principles of Frontal Lobe Function, 2nd edition.
A provocative chapter addressing the often-neglected question of how frontal lobe development leads to improvements in cognitive control and executive function more generally–that is, what unique characteristics allow prefrontal cortical regions (as opposed to other brain regions) to support developments in executive functions (as opposed to other cognitive functions).
Munakata et al. (2011). See reference list.
A concise review of how the unified framework presented in this article can explain how we inhibit a variety of unwanted thoughts, actions, and emotions, through the use of different types of active and abstract goal representations in distinct subregions of the prefrontal cortex.
Rougier, N.P., Noelle, D., Braver, T.S., Cohen, J.D. & O'Reilly, R.C. (2005).
Prefrontal Cortex and the Flexibility of Cognitive Control: Rules Without Symbols. Proceedings of the National Academy of Sciences, 102, 7338-7343.
An elegant demonstration of how the active maintenance properties of the prefrontal cortex can support the formation of abstract representations across development, and how such representations can support flexible behavior.
References
- Andrews-Hanna JR, Seghete KLM, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: Neural underpinnings and relation to self-report behaviors. PLoS ONE. 2011;6(6):1–14. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J, Lucero-Wagoner B. The pupillary system. In: Caccioppo J, Tassinary LG, Berntson G, editors. The handbook of psychophysiology. Cambridge University Press; Hillsdale, NJ: 2000. [Google Scholar]
- Blackwell K, Cepeda N, Munakata Y. When simple things are meaningful: Working memory strength predicts children's cognitive flexibility. Journal of Experimental Child Psychology. 2009;103:241–249. doi: 10.1016/j.jecp.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78(2):647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Brace JJ, Morton JB, Munakata Y. When actions speak louder than words: Improving children's flexibility in a card-sorting task. Psychological Science. 2006;17(8):665–669. doi: 10.1111/j.1467-9280.2006.01763.x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in working memory. Oxford University Press; Oxford: 2007. [Google Scholar]
- Bunge SA, Zelazo PD. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15(3):118–121. [Google Scholar]
- Chatham CH, Yerys BE, Munakata Y. Why won't you do what I want? The informative failures of children and models. Cognitive Development. 2012 doi: 10.1016/j.cogdev.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Frank MJ, Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):5529–5533. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Development. 1985;56(4):868–883. [PubMed] [Google Scholar]
- Diamond A. Neuropsychological insights into the meaning of object concept development. In: Carey S, Gelman R, editors. The epigenesis of mind: Essays on biology and knowledge. Lawrence Erlbaum Associates; Hillsdale, NJ: 1991. pp. 67–110. [Google Scholar]
- Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Hutchison KE, Frank MJ. Dopaminergic genes predict individual differences in susceptibility to confirmation bias. Journal of Neuroscience. 2011:6188–6198. doi: 10.1523/JNEUROSCI.6486-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin F, Macoveanu J, Olesen P, Tegner J, Klingberg T. Stronger synaptic connectivity as a mechanism behind development of working memory-related brain activity during childhood. Journal of Cognitive Neuroscience. 2007;19(5):750–760. doi: 10.1162/jocn.2007.19.5.750. doi: 10.1162/jocn.2007.19.5.750. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: factors determining monitoring versus spontaneous retrieval. J. Exp Psychol. Gen. 2005;134(3):327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CLH, Hinshaw S, D'Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. Journal of Neuroscience. 2010;30(33):11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann B, Brass M, Koch I, Cramon D. Internally generated and directly cued task sets: An investigation with fMRI. Neuropsychologia. 2005;43(6):943–952. doi: 10.1016/j.neuropsychologia.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O'Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1485):1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques S, Zelazo PD. The Flexible Item Selection Task (FIST): A measure of executive function in preschoolers. Developmental Neuropsychology. 2001;20(3):573–591. doi: 10.1207/S15326942DN2003_2. [DOI] [PubMed] [Google Scholar]
- James W. The principles of psychology. I. Holt; New York: 1890. [Google Scholar]
- Kave G, Kigel S, Kochva R. Switching and clustering in verbal fluency tasks throughout childhood. Journal of Clinical and Experimental Neuropsychology. 2008;30(3):349–359. doi: 10.1080/13803390701416197. [DOI] [PubMed] [Google Scholar]
- Kharitonova M, Chien S, Colunga E, Munakata Y. More than a matter of getting “unstuck”: Flexible thinkers use more abstract representations than perseverators. Developmental Science. 2009;12(4):662–669. doi: 10.1111/j.1467-7687.2008.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(1):99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Lorsbach TC, Reimer JF. Developmental differences in cognitive control: Goal representation and maintenance during a continuous performance task. Journal of Cognition and Development. 2010;11(2):185–216. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Houts R, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JB, Munakata Y. Active versus latent representations: A neural network model of perseveration, dissociation, and decalage. Developmental Psychobiology. 2002a doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Morton JB, Munakata Y. Are you listening? Exploring a developmental knowledge-action dissociation in a speech interpretation task. Developmental Science. 2002b;5(4):435–440. [Google Scholar]
- Munakata Y. Infant perseveration and implications for object permanence theories: A PDP model of the A B task. Developmental Science. 1998;1(2):161–184. [Google Scholar]
- Munakata Y. Graded representations in behavioral dissociations. Trends in Cognitive Sciences. 2001;5(7):309–315. doi: 10.1016/s1364-6613(00)01682-x. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O'Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011 doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Yerys BE. All together now: When dissociations between knowledge and action disappear. Psychological Science. 2001;12(4):335–337. doi: 10.1111/1467-9280.00361. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Frank MJ. Making Working Memory Work: A Computational Model of Learning in the Frontal Cortex and Basal Ganglia. Neural Computation. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Romberg AR, Saffran JR. Statistical learning and language acquisition. Wiley Interdisciplinary Reviews: Cognitive Science. 2010;1(6):906–914. doi: 10.1002/wcs.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The construction of reality in the child. Basic Books; New York: 1954. [Google Scholar]
- Siklos S, Kerns KA. Assessing multitasking in children with ADHD using a modified Six Elements Test. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists. 2004;19(3):347–361. doi: 10.1016/S0887-6177(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Smidts DP, Jacobs R, Anderson V. The Object Classification Task for Children (OCTC): A measure of concept generation and mental flexibility in early childhood. Developmental Neuropsychology. 2004;26(1):385–401. doi: 10.1207/s15326942dn2601_2. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Munakata Y. Becoming self-directed: Abstract representations support endogenous flexibility in children. Cognition. 2010;116(2):155–167. doi: 10.1016/j.cognition.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Ramscar M, Chrysikou EG. Cognition without control: When a little frontal lobe goes a long way. Current Directions in Psychological Science. 2009;18(5):259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cognitive Development. 1996;11(1):37–63. [Google Scholar]