Abstract
Terminal airspaces of the lung, alveoli, are sites of gas exchange which are sensitive to disrupted fluid balance. The alveolar epithelium is a heterogeneous monolayer of cells interconnected by tight junctions at sites of cell-cell contact. Paracellular permeability depends on claudin-family tight junction proteins. Of over a dozen alveolar claudins, cldn-3, cldn-4 and cldn-18 are the most highly expressed; other prominent alveolar claudins include cldn-5 and cldn-7. Cldn-3 is primarily expressed by type II alveolar epithelial cells whereas cldn-4 and cldn-18 are expressed throughout the alveolar epithelium. Lung diseases associated with pulmonary edema, such as alcoholic lung syndrome and acute lung injury affect alveolar claudin expression which is frequently associated with impaired fluid clearance due to increased alveolar leak. However, recent studies have identified a role for increased cldn-4 in protecting alveolar barrier function following injury. Thus, alveolar claudins are dynamically regulated, tailoring lung barrier function to control the air-liquid interface.
Keywords: tight junction, acute lung injury, acute respiratory distress syndrome, alcoholic lung disease, sepsis
Introduction
Gas exchange between the lung airspace and the circulatory system is necessary to support respiration in mammals. In order for gas exchange to occur, the lung must maintain a highly specialized barrier between the atmosphere and fluid filled tissues. The airspace is not completely dry, rather, it is covered by a highly regulated thin layer of fluid known as the air-liquid interface. Lung epithelia maintain the air-liquid interface by providing both a physical barrier to prevent leakage into airspaces and active transport of excess fluid 1, 2. Clinically, during acute respiratory distress syndrome (ARDS), failure of the lung epithelial barrier leads to airspace flooding significantly decreasing the efficiency of gas exchange that exacerbates the severity of acute lung injury 3, 4. The terminal airspaces of the lung, known as alveoli, provide the physical barrier to paracellular fluid permeability.
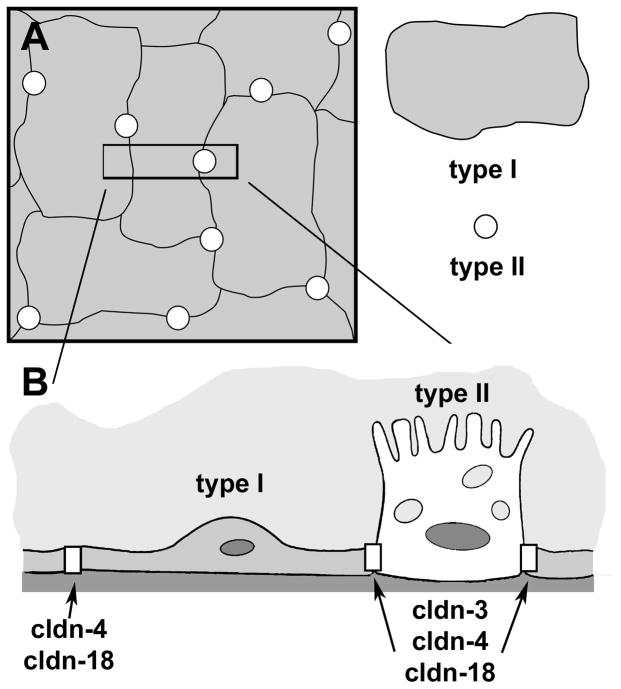
The alveolar epithelium is heterogeneous and consists of two different cell types, type I and type II alveolar epithelial cells (Fig. 1). Type I cells are a squamous epithelium which cover over 90% of the alveolar surface area.5 These large thin cells are the primary site of gas exchange between the airspaces and pulmonary capillary vasculature. Type II cells are interspersed throughout the alveoli and serve several functions. Most critically, type II cells produce pulmonary surfactant, lowering surface tension of the air-liquid interface to maintain open airspaces. Type II cells can also differentiate into type I cells in response to injury. As shown in Figure 1, the vast majority of alveolar intercellular junctions are between adjacent type I cells, however, there are also heterocellular interfaces between type I and type II cells.
Figure 1. Alveolar epithelial cells.
A. En face view of alveolar epithelium, showing the relative size and number of type I and type II cells. B. Cross section of the area delineated by the box in A. Shown are type I-type I and type I-type II cell junctions which have distinct claudin composition. The major difference is the presence of high levels of cldn-3 at type I-type II cell tight junctions.
There are several types of intercellular junctions between alveolar epithelial cells. These include tight junctions, adherens junctions, gap junctions and desmosomes, each serving a distinct function.6–10 Tight junctions are the most critical determinant of epithelial barrier function although evidence suggests that other junctions, particularly adherens junctions, contribute to barrier function by regulating tight junction assembly.9, 11, 12
Tight junctions are a network of interwoven strands forming a ring around the lateral plasma membrane. Claudins, a family of tetraspan transmembrane proteins, form the structural and functional basis for control of tight junction permeability.13–17 The extracellular domains of claudins interact with extracellular domains of claudins on adjoining cells to form a barrier that restricts ion, solute and liquid trafficking between cells via the paracellular pathway.18–21 There are over two dozen mammalian claudins that have tissue specific patterns of expression that determine tight junction permeability.
Claudins require additional protein components in order to be assembled into tight junctions. They are directly tethered to the actin cytoskeleton via cytosolic scaffold proteins that interact primarily with the C-terminal domain.22, 23 ZO-1 and ZO-2, are the best characterized scaffold proteins and have been shown to promote and regulate claudin incorporation into tight junctions.24, 25 Tight junctions are also controlled by other transmembrane proteins. For example, the tetraspan transmembrane protein occludin interacts with claudins to regulate tight junction assemblyand barrier function 19, 26. However, occludin is not an absolute requirement for a high resistance barrier and acts as a pro-apopotic signal when junctions are disrupted, suggesting an important role for occludin in cell signaling.27, 28 Immunoglobulin-fold transmembrane proteins, including Junction Adhesion Molecule A (JAM-A), also regulate claudin expression and tight junction permeability.29 Although claudins are one part of the multiprotein complex required to form tight junctions, they nonetheless function as the primary structural component that controls paracellular permeability and the tight junction barrier.
Claudin expression by the Alveolus
At least 14 different claudins are expressed at the mRNA and protein level by alveolar epithelial cells.17, 30 The predominant claudins expressed by the alveolar epithelium are cldn-3, cldn-4, and cldn-18.31 However, other claudins expressed by alveolar epithelium can also influence alveolar barrier function, e.g. cldn-5 and cldn-7 which are associated with decreased and increased alveolar barrier function, respectively (see below) 32, 33. Claudin expression is not uniform throughout the alveolus, instead, type II and type I alveolar epithelial cells have distinct patterns of claudin expression 31 (Table 1). The most prominent difference is that type II cells express over 17 fold more cldn-3 than type I cells. By contrast, cldn-4 expression by type II and type I cells is comparable at baseline, although cldn-4 is upregulated during acute lung injury (see below). Type II and type I cells also express comparable levels of cldn-18, which is specifically expressed by alveolar epithelium and is absent from the upper airways. There are two cldn-18 splice variants, cldn-18.1 found primarily in the lung and cldn-18.2 expressed in the stomach 34, 35.
Table 1.
Alveolar epithelial claudin expression
| Human Fetal77, 78 | Human Adult42, 69, 79 | Rat Type II31, 32, 65 | Rat Type I31–33, 65 | Mouse Type II44, 80 | |
|---|---|---|---|---|---|
| Claudin-3 | RP* | P | RP# | RP# | P |
| Claudin-4 | RP | P | RP | RP | P |
| Claudin-18 | RP | P | RP | RP | P |
| Claudin-5 | RP | P | RP | RP | P |
| Claudin-7 | RP | RP | RP | P | |
| Claudin-10b | R | R | R | P | |
| Claudin-12 | R | RP | |||
| Claudin-15 | R | RP | |||
| Claudin-19 | R | R | |||
R = mRNA expression detected; P = protein expression detected
Rat type II cells express ~17-fold more cldn-3 than type I cells, cldn-4 and cldn-18 expression is comparable.31, 32, 65
Other claudin mRNAs expressed by rat alveolar epithelial cells: cldn-9, cldn-11, cldn-20, cldn-22, and cldn-23 (Ref 31).
Resistance to Triton X-100 extraction is a commonly used biochemical assay that correlates with the incorporation of transmembrane proteins into junctional complexes.36–39 Using this approach, cldn-18 is significantly more insoluble (~75 % insoluble) as compared with cldn-3 (~40% insoluble) or cldn-4 (~30% insoluble).40 Although the basis for enhanced cldn-18 resistance to detergent extraction is not known at present, the C-terminal domain of cldn-18 is roughly twice as large as that of cldn-3 or cldn-4, which could provide a more effective template for scaffold proteins to bind and crosslink cldn-18 to the cytoskeleton. Whether this is the case remains to be determined, however, this would suggest that association of cldn-18 with cortical actin is an important contributor to alveolar barrier function.31, 41 Consistent with this, pro-inflammatory hormones decrease cldn-18 expression and assembly into alveolar epithelial tight junctions in vitro which correlates with decreased barrier function42 (Table 2). Moreover, cldn-18 expression is decreased in murine models of sepsis using cecal ligation and puncture and bleomycin induced lung injury, which is expected to compromise alveolar barrier function.43, 44
Table 2.
Changes to alveolar epithelial claudin expression in disease
| Alcoholic lung syndrome59 | Ventilator induced lung injury65 | Inflammation42 | Sepsis-induced ARDS43 | Pulmonary Fibrosis44 | |
|---|---|---|---|---|---|
| Claudin-3 | decreased | unchanged | unchanged | unchanged | decreased |
| Claudin-4 | unchanged | increased | unchanged | decreased | decreased |
| Claudin-18 | decreased | unchanged | decreased | decreased | decreased |
| Claudin-5 | increased | unchanged | unchanged | decreased | |
| Claudin-7 | decreased | unchanged | decreased | ||
Alcoholic lung syndrome impairs alveolar barrier function
Chronic alcohol abuse is a clinically significant risk factor for the development of ARDS.45–47 A key root cause of this effect is that prolonged ethanol ingestion induces a significant oxidant load driving the alveolar epithelium to produce transforming growth factor beta (TGF-β) which has a deleterious effect on alveolar barrier function48 and primes the lung for an amplified response to acute lung injury.49, 50 Production of TGF-β in response to alcohol further exacerbates oxidant stress by inhibiting glutathione transport into the airspaces, impairing the anti-oxidant capacity of the lung.51, 52
In an otherwise healthy alcoholic, ion channels (e.g. amiloride sensitive sodium channels) can compensate for compromised barrier function and maintain a proper air-liquid interface.1, 53 However, because the alcoholic lung is already under a significant oxidant burden,17 it is highly susceptible to the effects of a so-called “second hit”, such as direct trauma or inflammation due to sepsis. As a result of a second hit, alveolar barrier function in the alcoholic lung is further compromised overwhelming mechanisms of fluid clearance that are already near capacity.
In addition to affecting oxidant load, TGF-β has a direct influence on alveolar epithelial function by promoting epithelial-to-mesenchyme transition (EMT).54–56 EMT induced by TGF-β directly affects tight junctions through increased expression of transcription factors such as snail which repress cell polarity and claudin expression.57, 58 In fact, chronic alcohol ingestion decreases expression of several claudins, including cldn-1, cldn-7, and cldn-18 59. Alcohol also decreases expression of other alveolar tight junction proteins, including occludin and ZO-1, which are likely to contribute to a leaky lung phenotype 60. In addition to the changes to tight junction protein expression, tight junction formation is also impaired in response to chronic alcohol exposure (Fig. 2). The overall decrease in ZO-1 expression in the alcoholic lung is likely to contribute to decreased tight junction formation, since a decrease in the scaffold impairs the ability of claudins to stably incorporate into tight junctions 25.
Figure 2. Alcohol impairs assembly of claudins into tight junctions.
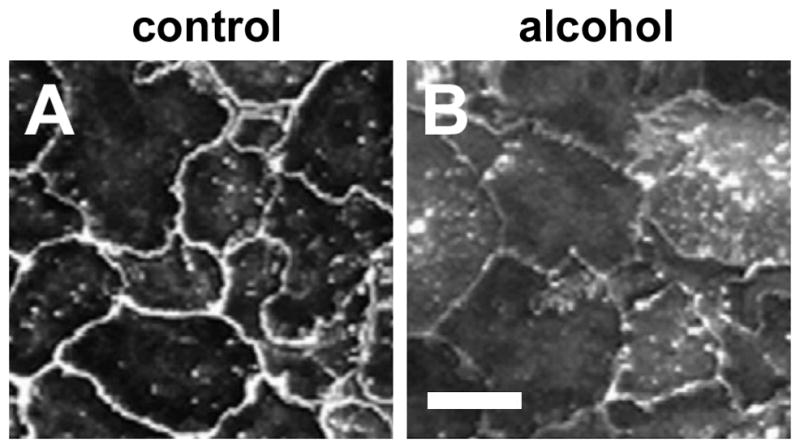
Model type I alveolar epithelial monolayers were derived from primary cells isolated from either control (A) or alcohol fed (B) rats which were cultured for 6 days and then immunolabeled for cldn-7. In contrast with control alveolar epithelial cells, where cldn-7 prominently localized to sites of cell-cell contact (A), cells isolated from alcohol fed rats had impaired claudin assembly (B) which correlated with impaired barrier function. Bar = 10 microns. Adapted from Fernandez, et. al. 59.
In parallel to alcohol-induced decreases alveolar epithelial cell claudin expression, alcohol surprisingly increases cldn-5 expression 59. The effect of alcohol on cldn-5 requires an as yet unknown post-translational mechanism of regulation, since alveolar cldn-5 mRNA remains unchanged by alcohol exposure 59. Several studies have correlated increased cldn-5 with increased lung epithelial paracellular permeability 32, 61, 62, suggesting that increased cldn-5 expression is a critical aspect of impaired barrier function in the alcoholic lung. Consistent with this potential mechanism, we have found that transducing normal alveolar epithelial cells with YFP-cldn-5 decreases barrier function in vitro (C.E. Overgaard and M. Koval, unpublished results). How cldn-5 could decrease alveolar epithelial barrier function is not known at present. Nonetheless, the ability of cldn-5 to reduce alveolar barrier function is likely to be tissue specific, since cldn-5 is necessary for maintaining the blood brain barrier and overexpression of cldn-5 by low resistance epithelia can increase barrier function 63, 64. Defining roles for cldn-5 in the pathology of alcoholic lung disease will require understanding how cldn-5 interacts with other alveolar epithelial claudins and tight junction proteins.
Cldn-4 expression correlates with increased alveolar fluid clearance
A role for cldn-4 in response to acute lung injury was first implicated in studies where cldn-4 was found to be acutely upregulated by mice in response to ventilator-induced lung injury (VILI) 65. The increase in cldn-4 correlated with decreased severity of injury and was specific, as other claudins, including cldn-3 and cldn-18, were unchanged in response to VILI 65. Interestingly, alveolar epithelial cldn-4 expression is downregulated in sepsis that is likely to increase the severity of lung injury 43. Moreover, cultured alveolar epithelial cells show considerable variation in endogenous cldn-4 expression, even within the same monolayer. This suggests that cldn-4 expression is more sensitive to cell phenotype or microenvironment as opposed to other claudins that are more uniformly expressed and regulated, such as cldn-18.32, 66
A functional role for cldn-4 in lung fluid clearance in vivo was confirmed using a peptide fragment derived from Clostridium perfringens enterotoxin (CPE), which binds to cldn-3 and cldn-4 with high affinity.67, 68 Intratracheal instillation of a CPE peptide decreased lung cldn-4 content and rendered the lungs more sensitive to VILI.65 Recently, cldn-4 was found to be associated with increased alveolar fluid clearance rates in ex vivo perfused human donor lungs.69 The extent of lung injury was inversely correlated with cldn-4 expression, supporting a clinically relevant role for cldn-4 in protecting the lung from damage along with improved fluid clearance.
Differential effects of cldn-3 and cldn-4 on alveolar barrier function
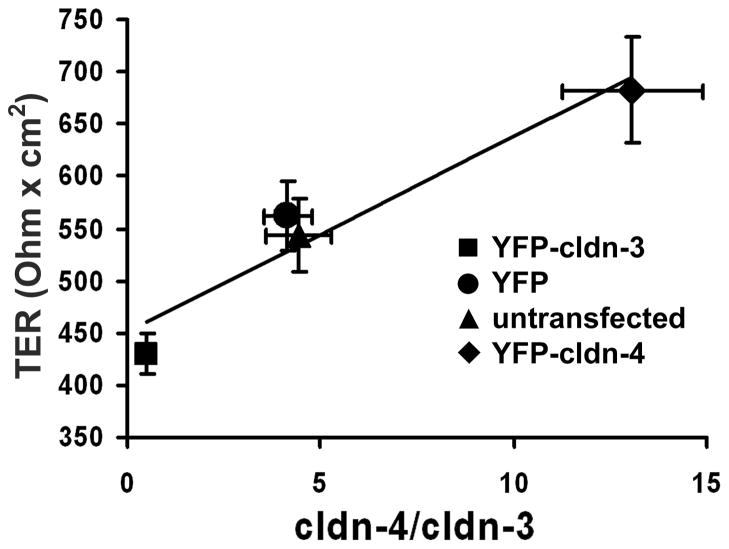
Cldn-3 and Cldn-4 are the most closely related by amino acid homology.62 However, since cldn-3 and cldn-4 differ dramatically in their pattern of expression in the alveolus, this suggests that these two claudins serve different roles in alveolar barrier function. To directly test this, alveolar epithelial cells cultured on permeable supports were transduced to specifically increase expression of YFP-tagged versions of either cldn-3 or cldn-4.40 Expression of YFP-cldn-3 or YFP-cldn-4 has no effect on the expression or localization of other claudins (Fig. 3). However, YPF-cldn-3 and YFP-cldn-4 have differential effects on alveolar epithelial barrier function (Fig. 4). Alveolar epithelial cells transduced with YFP-cldn-4 increased trans epithelial resistance (TER) by nearly 50%, providing direct evidence that increased cldn-4 improves alveolar barrier function 40. By contrast, cells transduced with YFP-cldn-3 show a decrease in TER from ~550 Ohm × cm2 to ~400 Ohm × cm2 (Fig. 4). Thus, cldn-3 and cldn-4 have differential effects on alveolar epithelial barrier function.
Figure 3. Increasing cldn-3 or cldn-4 expression by alveolar epithelium does not affect tight junction morphology.
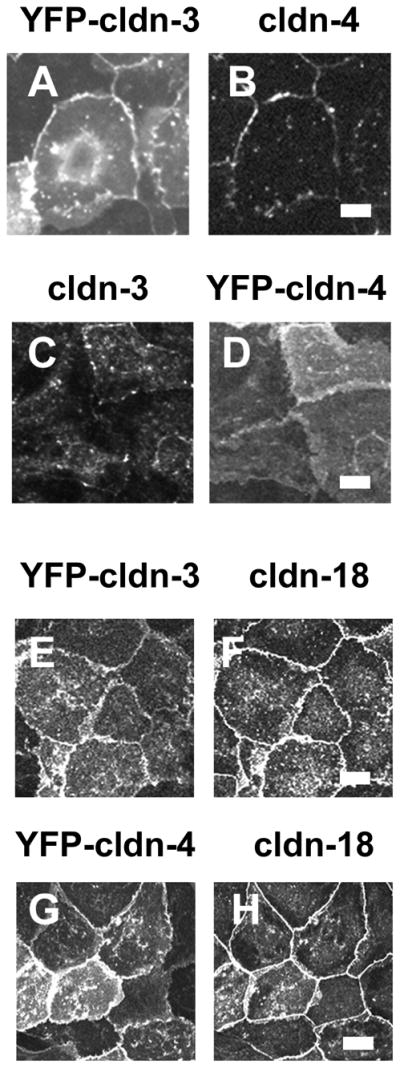
Model type I alveolar epithelial cells transduced with YFP-cldn-3 (A, B, E, F) or YFP-cldn-4 (C, D, G, H) were fixed and immunostained for cldn-4 (B), cldn-3 (C) or cldn-18 (F, H). YFP-cldn-3 and YFP-cldn-4 localized to the plasma membrane at sites of cell-cell contact. E–H. Increasing expression of either cldn-3 or cldn-4 had little effect on cldn-18 localization (F, H). Bar, 10 micron. Adapted from Mitchell, et. al. 40.
Figure 4. Differential effect of increasing cldn-3 or cldn-4 on alveolar epithelial cell barrier function.
Model type I alveolar epithelial cells transduced with YFP-cldn-3 (■), YFP-cldn-4 (◆), YFP-control virus (●) or untransfected controls (▲) were assessed for the effect of altering claudin expression on barrier function, as determined using transepithelial resistance (TER; Ohm × cm2) (y axis). The expression ratio cldn-4/cldn-3 was determined by immunoblot (x axis) demonstrating that there was a linear relationship between cldn-4/cldn-3 ratio and TER (r2 = 0.93). Cells expressing increased cldn-3 had significantly lower TER than either control cells or cells expressing increased cldn-4 (P < 0.05). Increasing cldn-4 also significantly increased barrier function (P < 0.05). Adapted from Mitchell, et. al. 40.
The context of claudin expression influences their function, since claudin-claudin interactions alter paracellular permeability 70. For instance, increased cldn-3 augments barrier function of a low resistance clone of Madin Darby Canine Kidney (MDCK) epithelial cells from ~50 Ohm × cm2 to ~100 Ohm × cm2, most likely by interacting with cldn-2 which acts as a pore forming claudin 71. This contrasts with the observation that cldn3 decreases alveolar epithelial barrier function 40. In this light, it is interesting that cldn-3 is capable of a broad range of heterotypic interactions with other claudins, as opposed to cldn-4, which appears to be restricted to homotypic interactions with cldn-4 61, 62. Whether cldn-3 and cldn-4 have fundamentally different roles in directing tight junction assembly and whether this affects paracellular permeability remains to be determined. However, given that cldn-3 is mainly localized to type II-type I cell interfaces in the alveolus (Fig. 1), it seems likely that type II-type I tight junctions differ from type I-type I cell junctions in paracellular permeability. Determining whether this is the case will require novel methods to measure alveolar tight junction permeability in situ.
Conclusions and perspectives
The strong correlation between cldn-4 and improved lung fluid clearance makes this an appealing therapeutic target for the prevention of ARDS by improving fluid clearance from airspaces. This is particularly appealing for prevention of lung injury during sepsis, where cldn-4 is downregulated 43. In fact, cldn-4 transcription is under the control of the grainyhead-like 2 (Grhl2) transcription factor which is activated during development in several epithelia, including lung 72, 73. Whether Grhl2 is expressed by the adult lung and whether it is activated endogenously in response to injury or through a pharmacologically activated pathway remain unknown at present. It is also unclear whether Grhl2 can overcome the effects of transcription factors that are activated in EMT that suppress claudin expression, including snail, slug and twist 74–76. This could be a critical issue when attempting to target cldn-4 in alcoholic lung disease, where TGFβ promotes EMT in the alveolus.
Even if cldn-4 is upregulated, is this sufficient to improve fluid clearance when expression of other claudins is impaired? For example, in the context of decreased cldn-18, which is the major alveolar epithelial claudin, increasing cldn-4 may not augment the alveolar barrier enough to maintain a proper air-liquid balance. Moreover, in the case of alcoholic lung disease, increased cldn-5 may antagonize cldn-4. This could be due to a direct effect on cldn-4, if cldn-5 heteromerically influences cldn-4 assembly or function. Alternatively, cldn-5 may compete with cldn-4, and other claudins, for interaction with scaffold proteins. Cldn-5 may also recruit specific subclasses of scaffold proteins to tight junctions that could influence assemble as well. In these models, differential affinity for scaffold proteins by claudins dictate the priority, or stability, of claudin integration into tight junction strands through competition for binding to scaffold proteins. In essence, claudin composition could control recruitment of scaffold proteins to tight junctions, the converse of models where scaffold proteins control claudin incorporation junctional strands 25. If this is the case, then steady state tight junction composition is dictated by coordinated bi-directional interplay between claudins and scaffold proteins.
Acknowledgments
This work was supported by Emory Alcohol and Lung Biology Center/National Institutes of Health (NIH) grants P50-AA013757 (M.K.), R01-HL083120 (M.K.), AA-013528 (to C.E.O. and L.A.M.) and by the Emory University Research Committee (M.K.).
Bibliography
- 1.Eaton DC, et al. The Contribution of Epithelial Sodium Channels to Alveolar Function in Health and Disease. Annual review of physiology. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- 2.Van Driessche W, et al. Interrelations/cross talk between transcellular transport function and paracellular tight junctional properties in lung epithelial and endothelial barriers. American journal of physiology. 2007;293:L520–524. doi: 10.1152/ajplung.00218.2007. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England journal of medicine. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Crapo JD, et al. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- 6.Bartels H. The air-blood barrier in the human lung. A freeze-fracture study. Cell and tissue research. 1979;198:269–285. doi: 10.1007/BF00232010. [DOI] [PubMed] [Google Scholar]
- 7.Koval M. Sharing signals: connecting lung epithelial cells with gap junction channels. American journal of physiology. 2002;283:L875–893. doi: 10.1152/ajplung.00078.2002. [DOI] [PubMed] [Google Scholar]
- 8.Boitano S, et al. Cell-cell interactions in regulating lung function. American journal of physiology. 2004;287:L455–459. doi: 10.1152/ajplung.00172.2004. [DOI] [PubMed] [Google Scholar]
- 9.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 10.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 11.Capaldo CT, Macara IG. Depletion of E-Cadherin Disrupts Establishment but Not Maintenance of Cell Junctions in MDCK Epithelial Cells. Mol Biol Cell. 2006 doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. The American journal of pathology. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amasheh S, et al. Tight junction proteins as channel formers and barrier builders. Annals of the New York Academy of Sciences. 2009;1165:211–219. doi: 10.1111/j.1749-6632.2009.04439.x. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause G, et al. Structure and function of claudins. Biochimica et biophysica acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Overgaard CE, et al. Claudins: Control of Barrier Function and Regulation in Response to Oxidant Stress. Antioxidants & redox signaling. 2011;15:1179–1193. doi: 10.1089/ars.2011.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piehl C, et al. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell Mol Life Sci. 2010;67:2131–2140. doi: 10.1007/s00018-010-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mrsny RJ, et al. A key claudin extracellular loop domain is critical for epithelial barrier integrity. The American journal of pathology. 2008;172:905–915. doi: 10.2353/ajpath.2008.070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piontek J, et al. Formation of tight junction: determinants of homophilic interaction between classic claudins. Faseb J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 21.Van Itallie CM, et al. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol. 2006;291:F1288–1299. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 22.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruewer M, et al. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol. 2004;287:C327–335. doi: 10.1152/ajpcell.00087.2004. [DOI] [PubMed] [Google Scholar]
- 24.Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell. 2012;23:577–590. doi: 10.1091/mbc.E11-09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umeda K, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 26.Raleigh DR, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. The Journal of cell biology. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu AS, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 28.Beeman N, Webb PG, Baumgartner HK. Occludin is required for apoptosis when claudin-claudin interactions are disrupted. Cell death & disease. 2012;3:e273. doi: 10.1038/cddis.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laukoetter MG, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. The Journal of experimental medicine. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soini Y. Claudins in lung diseases. Respiratory research. 2011;12:70. doi: 10.1186/1465-9921-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lafemina MJ, et al. Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells. American journal of physiology. 2010;299:L724–734. doi: 10.1152/ajplung.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, et al. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29:62–70. doi: 10.1165/rcmb.2002-0180OC. [DOI] [PubMed] [Google Scholar]
- 33.Chen SP, et al. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol. 2005;98:322–328. doi: 10.1152/japplphysiol.00681.2004. [DOI] [PubMed] [Google Scholar]
- 34.Niimi T, et al. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Molecular and cellular biology. 2001;21:7380–7390. doi: 10.1128/MCB.21.21.7380-7390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi D, et al. Deficiency of Claudin-18 Causes Paracellular H(+) Leakage, Up-regulation of Interleukin-1beta, and Atrophic Gastritis in Mice. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 36.Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. The Journal of membrane biology. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- 37.Nunbhakdi-Craig V, et al. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. The Journal of cell biology. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. The Journal of cell biology. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell LA, et al. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. American journal of physiology. 2011;301:L40–49. doi: 10.1152/ajplung.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koval M. Keratinocyte growth factor improves alveolar barrier function: keeping claudins in line. American journal of physiology. 2010;299:L721–723. doi: 10.1152/ajplung.00365.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang X, et al. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. The Journal of biological chemistry. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen TS, Gray Lawrence G, Margulies SS. Cultured alveolar epithelial cells from septic rats mimic in vivo septic lung. PloS one. 2010;5:e11322. doi: 10.1371/journal.pone.0011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohta H, et al. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. American journal of physiology. 2011 doi: 10.1152/ajplung.00349.2010. [DOI] [PubMed] [Google Scholar]
- 45.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. American journal of physiology. 2007;292:L813–823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- 46.Moss M, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Critical care medicine. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 47.Moss M, et al. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. American journal of respiratory and critical care medicine. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 48.Bechara RI, et al. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. American journal of respiratory and critical care medicine. 2004;170:188–194. doi: 10.1164/rccm.200304-478OC. [DOI] [PubMed] [Google Scholar]
- 49.Munger JS, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 50.Pittet JF, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arsalane K, et al. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17:599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- 52.Jardine H, et al. Molecular mechanism of transforming growth factor (TGF)-beta1-induced glutathione depletion in alveolar epithelial cells. Involvement of AP-1/ARE and Fra-1. The Journal of biological chemistry. 2002;277:21158–21166. doi: 10.1074/jbc.M112145200. [DOI] [PubMed] [Google Scholar]
- 53.Pelaez A, et al. Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. American journal of physiology. 2004;286:L106–111. doi: 10.1152/ajplung.00148.2003. [DOI] [PubMed] [Google Scholar]
- 54.Kasai H, et al. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respiratory research. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim KK, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. American journal of physiology. 2007;293:L525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 57.Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JM, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. The Journal of cell biology. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez AL, et al. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol (Fayetteville, NY. 2007;41:371–379. doi: 10.1016/j.alcohol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan X, et al. Chronic alcohol ingestion exacerbates lung epithelial barrier dysfunction in HIV-1 transgenic rats. Alcoholism, clinical and experimental research. 2011;35:1866–1875. doi: 10.1111/j.1530-0277.2011.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coyne CB, et al. Role of claudin interactions in airway tight junctional permeability. American journal of physiology. 2003;285:L1166–1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 62.Daugherty BL, et al. Regulation of heterotypic claudin compatibility. The Journal of biological chemistry. 2007;282:30005–30013. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 63.Amasheh S, et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell and tissue research. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 64.Nitta T, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. The Journal of cell biology. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wray C, et al. Claudin 4 augments alveolar epithelial barrier function and is induced in acute lung injury. American journal of physiology. 2009;297:L219–227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koval M, et al. Extracellular Matrix Influences Alveolar Epithelial Claudin Expression and Barrier Function. Am J Respir Cell Mol Biol. 2010;42:172–180. doi: 10.1165/rcmb.2008-0270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veshnyakova A, et al. Mechanism of Clostridium perfringens enterotoxin interaction with claudin-3/-4 suggests structural modifications of the toxin to target specific claudins. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M111.312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitchell LA, Koval M. Specificity of Interaction between Clostridium perfringens Enterotoxin and Claudin-Family Tight Junction Proteins. Toxins. 2010;2:1595–1611. doi: 10.3390/toxins2071595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rokkam D, et al. Claudin-4 Levels Are Associated with Intact Alveolar Fluid Clearance in Human Lungs. The American journal of pathology. 2011;179:1081–1087. doi: 10.1016/j.ajpath.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 71.Milatz S, et al. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochimica et biophysica acta. 2010 doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Werth M, et al. The transcription factor grainyhead-like 2 regulates the molecular composition of the epithelial apical junctional complex. Development. 2010;137:3835–3845. doi: 10.1242/dev.055483. [DOI] [PubMed] [Google Scholar]
- 73.Auden A, et al. Spatial and temporal expression of the Grainyhead-like transcription factor family during murine development. Gene Expr Patterns. 2006;6:964–970. doi: 10.1016/j.modgep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Carrozzino F, et al. Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins. Am J Physiol Cell Physiol. 2005;289:C1002–1014. doi: 10.1152/ajpcell.00175.2005. [DOI] [PubMed] [Google Scholar]
- 75.Taube JH, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinez-Estrada OM, et al. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. The Biochemical journal. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daugherty BL, et al. Developmental regulation of claudin localization by fetal alveolar epithelial cells. American journal of physiology. 2004;287:L1266–1273. doi: 10.1152/ajplung.00423.2003. [DOI] [PubMed] [Google Scholar]
- 78.Kaarteenaho R, et al. Divergent expression of claudin -1, -3, -4, -5 and -7 in developing human lung. Respiratory research. 2010;11:59. doi: 10.1186/1465-9921-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaarteenaho-Wiik R, Soini Y. Claudin -1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem. 2009;57:187–195. doi: 10.1369/jhc.2008.951566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzon E, Cuzzocrea S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respiratory research. 2007;8:75. doi: 10.1186/1465-9921-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]