Abstract
Fluorescent conjugated polymers have received a great deal of recent interest due to their ability to act as chemosensors to detect various chemical species in both environmental and biological systems with sensitivity and selectivity. Examples from the literature include polymer chemosensors that operate on either fluorescence “turn-on” or “turn-off” as mechanisms of sensor response. These responses can be related to either photoinduced electron transfer or electronic energy transfer mechanisms. Recently, a series of metal-containing polymers or metallopolymers have been explored by various research groups for their use as chemosensors. In many cases, these metallopolymers have been shown to be more sensitive and selective for specific chemical species. This review focuses on fluorescent conjugated polymers as chemosensors, with a specific concentration on recent advances in metallopolymer chemosensors.
Keywords: metallopolymer, conjugated polymer, chemosensors, fluorescent enhancement and quenching
1. Introduction
1.1 Fluorescent Chemosensors
The development of sensitive and selective sensors has been a primary focus for many scientists in recent years. A sensor is defined by the Oxford English Dictionary as “a device that detects or measures a physical property and records, indicates or otherwise responds to it”. A sensor achieves this goal by responding to an external stimulus and converting it into a signal which can be measured or recorded.
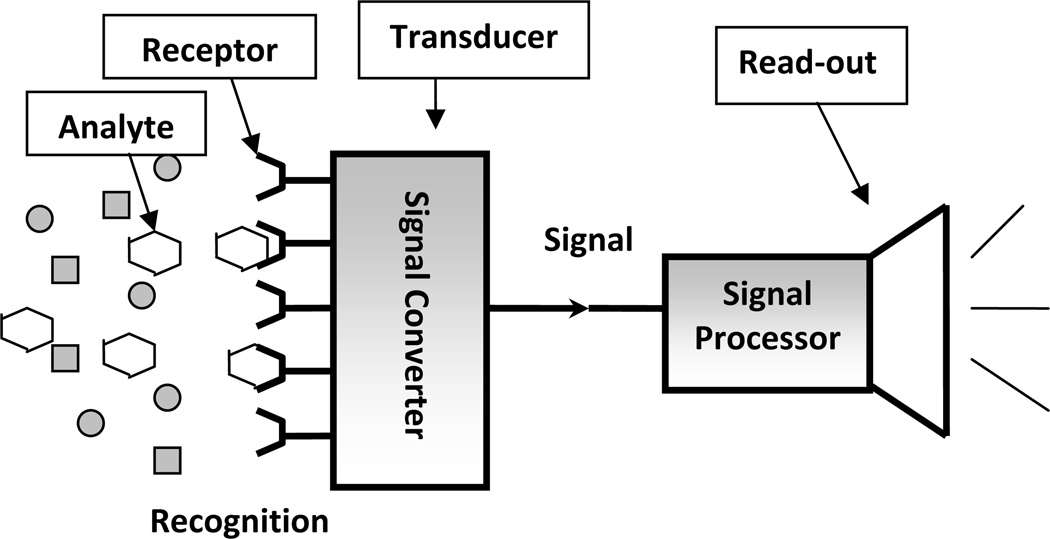
A sensor generally contains three components: a receptor, a signal transducer and a read-out mechanism (see Figure 1) [1]. The recognition element is known as the receptor which is the key component of the sensor device that interacts directly with the analyte. The receptor should be selective in having the ability to discriminate the stimulus of interest, avoiding interferences from the environment. The transducer component converts the input from the stimulus interaction with the receptor to another form, suitable for readout. The read-out domain is the part responsible for reporting the recognition event and processing the signal from the transducer.
Figure 1.
Schematic illustration of a sensor
A chemical sensor is a device that qualitatively or quantitatively detects the presence of specific chemical substances, a class of chemicals or a specific chemical reaction. Chemical sensors have been developed for cations, anions, neutral molecules, acids, vapors, volatile organics and many more [2–4]. A wide variety of transduction mechanisms exist including electrochemical (such as potentiometric, voltammetric), optical (such as fluorescent or chromophoric), thermal and so on. The ideal performance of a sensor device for real application is determined by the following factors [5]: selectivity, sensitivity, detection limit, reversibility, response and recovery time and lifetime.
Chemosensors based on fluorescence signal changes are commonly referred to as fluorescent chemosensors [6]. Fluorescent chemosensors are gaining increased attention due to their high sensitivity and ease of measurement [7–15]. Fluorescent chemosensors are usually made up of three components: a receptor, a fluorophore and a spacer moiety that links them together. These three components are not exactly the same as the three components shown in Figure 1. The read-out of a fluorescent sensor can be either a change in the fluorescence intensity, a shift in the emission wavelength, or a change in the fluorescence lifetime.
1.2 Mechanism of analyte detection
There are several mechanisms of fluorescence sensing, among them, photoinduced electron transfer (PET) and electronic energy transfer (EET) have been extensively studied and widely used in the design of small molecule sensors with fluorescence intensity changes as the signal read-out [7–9].
1.2.1 Photoinduced electron transfer - turn-on vs turn-off chemosensors
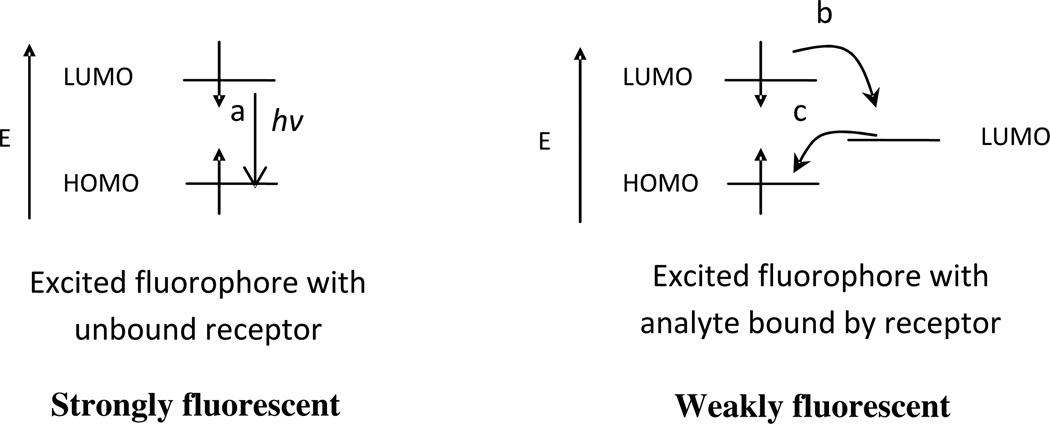
In a fluorescent conjugated polymer chemosensor system, the PET process can be classified into two categories, fluorescence “turn-off” or fluorescence “turn-on”. In these two categories, the receptors take part in the photophysical process either directly or indirectly, a role facilitated by the flexibility and conjugation between the polymer backbone and the receptor. In a fluorescence “turn-off” sensor, the fluorescence intensity decreases when the analyte becomes bound to the receptor and in this case the receptor plays an indirect role in the PET process. For indirect quenching to occur, the only known role of the receptor is to recognize the analytes and hold it close to the fluorophore. When this happens, the LUMO energy level of the receptor/analyte pair is between the HOMO and LUMO energy levels of the fluorophore, creating a non-radiative PET quenching pathway (Figure 2) which leads to dissipation of the excitation energy and the quenching of the fluorescence of the fluorophore [16].
Figure 2.
Orbital energy diagrams for fluorescent “turn-off” PET sensors before and after binding analyte: (a) fluorescence emission; (b) forward electron transfer; (c) backward electron transfer processes.
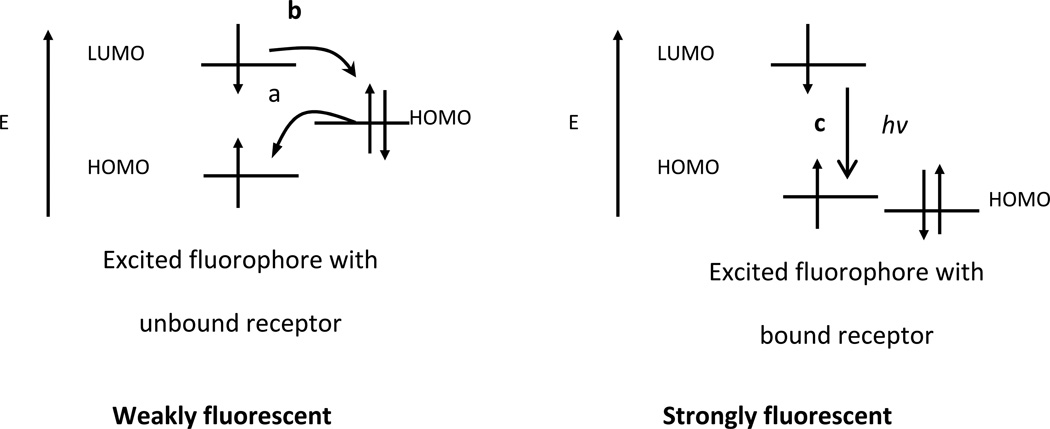
For fluorescence “turn-on” chemosensors, the receptors usually contain a relatively high energy non-bonding electron pair [17]. In the absence of analytes, this electron pair quenches the fluorescence of the chemosensor by rapid intra-molecular electron transfer from the receptor to the excited polymer backbone, as shown in Figure 3. When this electron pair coordinates to electron deficient analytes in solution, the energy of the HOMO of the receptor is lowered creating a direct fluorescence emission pathway to the ground state. This decreases the driving force for the PET process an0d can turn on the fluorescence of the chromophore. We are able to deduce this because the changes in absorption of the bound and unbound chemosensor are minor, suggesting that the energy gap of the chemosensor is unaffected by the binding event. The binding of the cation however, is usually accompanied by a large increase in fluorescence suggesting that the binding event created a new radiative pathway that was absent prior to binding[18].
Figure 3.
Orbital energy diagrams for fluorescence “turn-on” PET sensors before and after binding analyte: (a) forward electron transfer; (b) backward electron transfer; (c) fluorescence emission processes.
As demonstrated, there is a fundamental difference between the “turn-on” and “turn-off” mechanisms of the PET process. In the first case, the PET process occurs before binding the analyte, which consequently “turns-off” or stops the process. In the latter case, PET is created by the binding event. In both cases, the relative electronic potential of the HOMO and LUMO gap are critical in the design of a selective system.
1.2.2 Electronic Energy Transfer
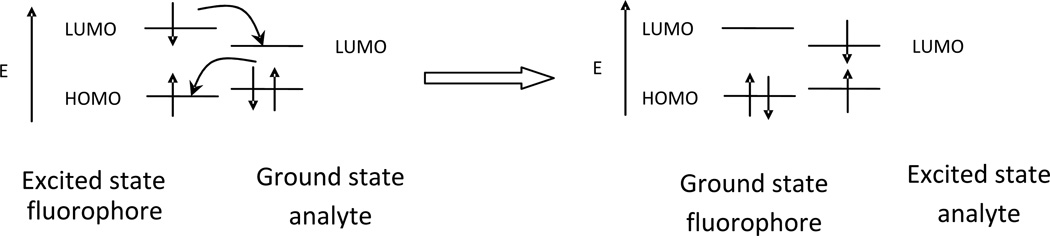
Electronic energy transfer (EET) is another mechanism that can be involved in fluorescence quenching. While all current examples of EET chemosensors are turn-off in nature, there are two kinds of EET mechanisms that should be considered: the through bond exchange (Dexter) energy transfer or the dipole-dipole coupling (Förster) energy transfer [19, 20]. In organic fluorophore systems, usually the Dexter energy transfer dominates as shown in Figure 4. In this case, the fluorophore returns to the ground state through a nonradiative decay. Dexter energy transfer requires close contact between the donor and the acceptor. This would involve direct orbital overlap. This type of fluorescence quenching requires matching in energy levels between the donor and acceptor orbitals.
Figure 4.
Orbital energy diagrams for double exchange transfer between the excited fluorophore to the analyte bound by receptor followed by analyte return to the ground state by nonradiative decay.
The Förster energy transfer mechanism involves the long range coupling of dipoles, allowing for an exchange of excitation energy through space, i.e. without a path of direct orbital overlap [19,20]. In this case, overlap of the emission and absorption spectrum is the key factor and there is no dependence on conjugation.
2. Fluorescent conjugated polymers as chemosensors
Small molecules have been extensively studied as fluorescence sensors in the past several decades [7–15]. The most commonly used fluorophores are aromatic compounds, especially anthracene [21]. Recently, conjugated polymer chemosensors have been used with great success in the past decade as fluorophores for detection of a range of analytes from biomolecules to explosives [22–27]. Swager and co-workers [22] first demonstrated that polyreceptor, conjugated polymer sensors, with their receptors connected in conjugation with each other, as molecular wires, have several advantages over small molecules for sensing applications. They were determined to be more sensitive which was linked to the efficient mobility of excitation energy between the receptors along the polymer backbone [22]. The polymer structure also provided for facile structural modification and good processibility.
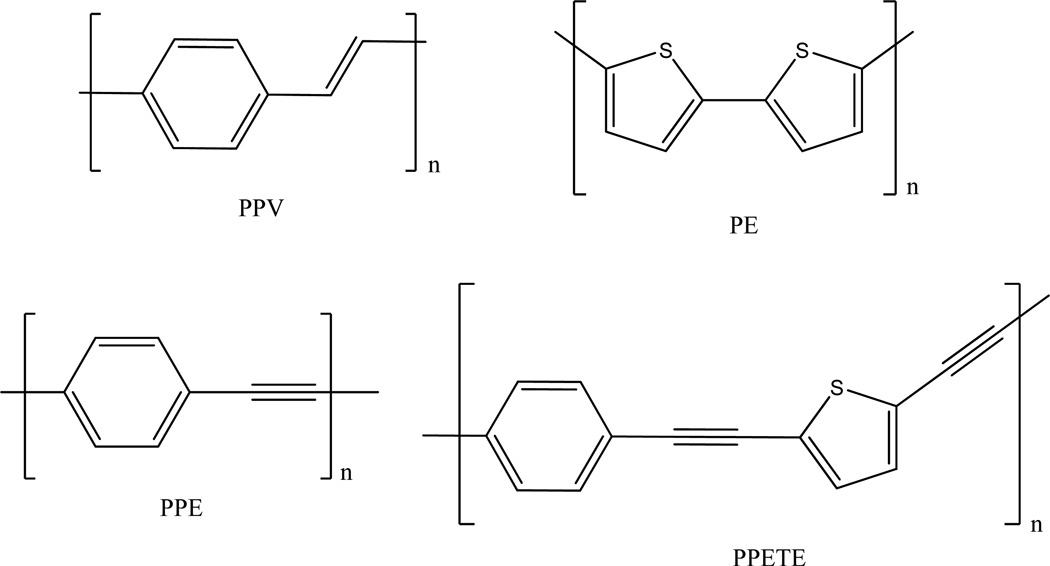
Numerous studies have been made on the properties and applications of conjugated polymers such as poly(p-phenylenevinylene) (PPV) [28–31], poly(p-phenyleneethylene) (PPE) [32–37] and polythiophene (PT) [38–40] (Figure 5). Several research groups, including our own, have investigated the structure-property relationships of poly [p-(phenyleneethynylene)-alt-(thienyleneethynylene) (PPETE) [12,41–43]. The PPETEs were found to be highly luminescent with good processibility as a material. They differ slightly from the PPE rigid-rod structure due to the inclusion of the five member thiophene ring, but still have a relatively high degree of π electron delocalization.
Figure 5.
Basic structures of several fluorescent conjugated polymers
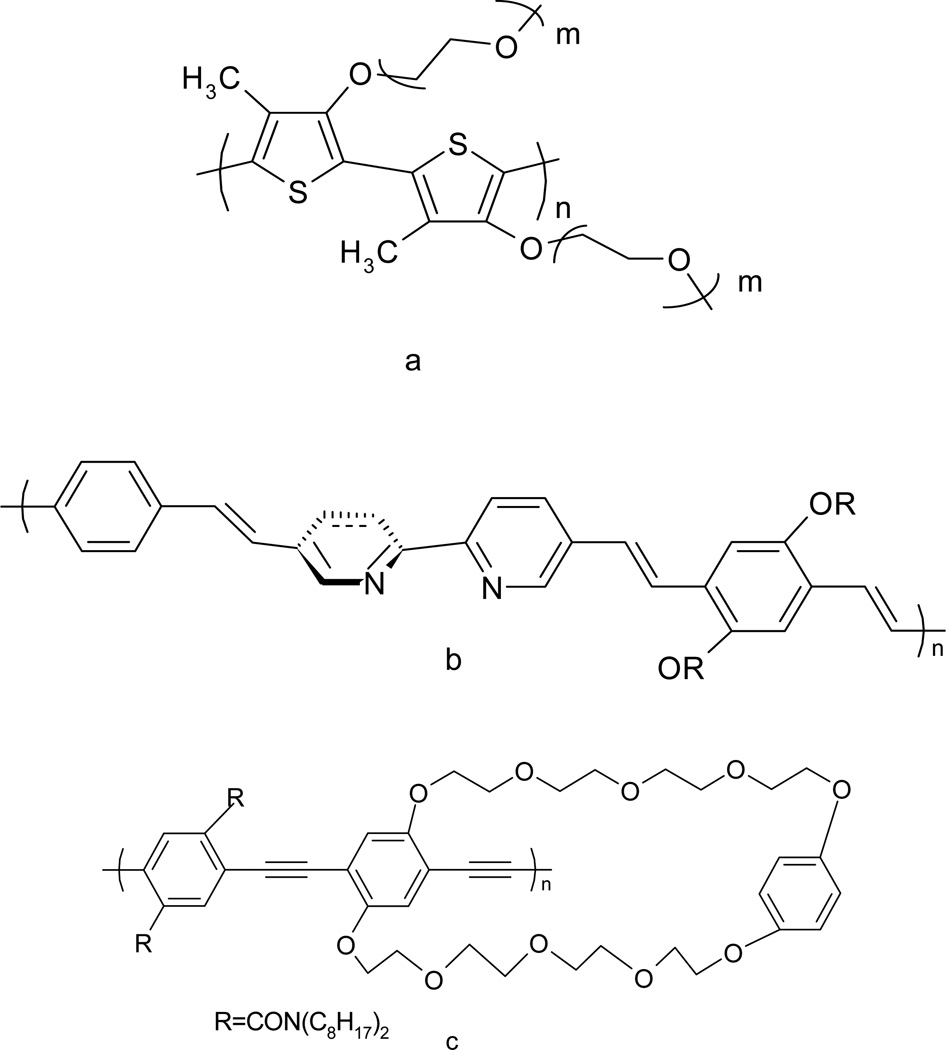
The combination of the sensitivity of fluorescence and the unique electron and energy transfer properties of conjugated polymers provides new opportunities for sensory system development. One type of fluorescent conjugated polymer sensor is based on the conformational change of the conjugated backbone driven by interaction with the analyte. For instance, the fluorescence of poly[3-oligo(oxyethylene)-4-methythiophene] (Figure 6a) is either enhanced or quenched in the presence of alkali metals, as the polymer changes conformation. In this case, both the intensity of fluorescence and the emission wavelength changed as a function of the concentration of alkali metal cations [44].
Figure 6.
Structures of several conjugated polymers as fluorescent sensors (a) poly[3-oligo(oxyethylene)-4-methythiophene] [44] (b)poly(bipyridyl-phenylenevinylene) [45] (c) para poly(phenyleneethynylene) [24]
Another early type of conjugated polymer sensor involved the introduction of molecular recognition units directly into the conjugation of the polymer backbone. An early example of this was the polymer synthesized by Wang and Wasielewski [45]. (Figure 6b) A bipyridyl group with a dihedral angle of 20° between two pyridine planes in the 2,2′-bipyridine system was introduced into the polymer backbone. It was forced into a planar configuration after chelation to certain transition metals such as Mn2+, Zn2+ and Pd2+. The result was an increased conjugation length. The conformational change was monitored by changes in both the UV-Vis and fluorescence spectroscopy.
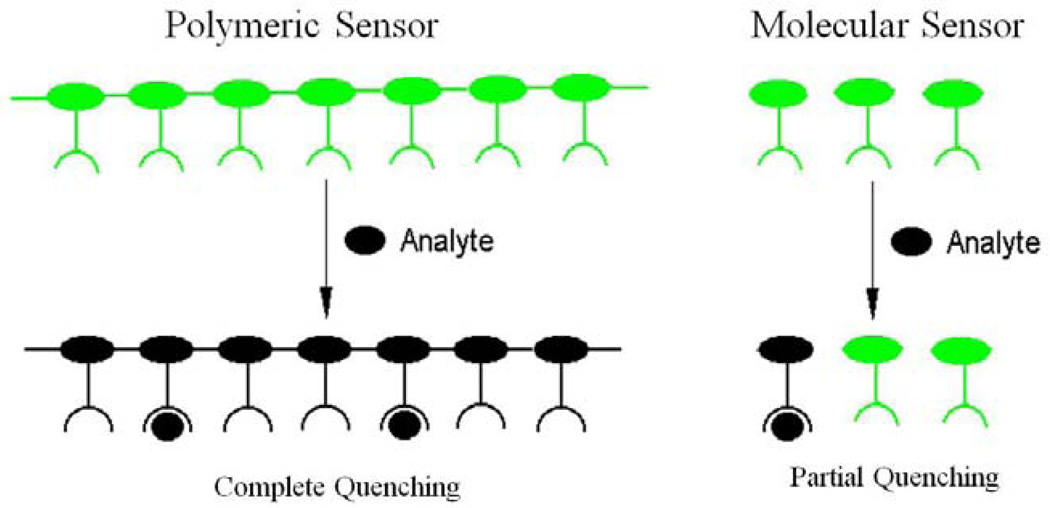
Polymer synthetic strategies offer many more options for sensor application. Another excellent approach is to assemble the receptor pendants onto the conjugated polymer backbone [22,23]. This concept was first advanced and demonstrated by Swager’s group [22,23]. They assembled cyclophane-based receptors onto a para-poly(phenyleneethynylene) backbone (Figure 6c). This fluorescent conjugated polymer showed a 65-fold fluorescence enhancement in sensitivity to paraquat, compared to a similar small molecule fluorescent chemosensor having one receptor. The enhancement in this polymer system was attributed to facile energy migration, along the fluorescent conjugated backbone, where the excitation energy gets quenched upon encountering the lower energy receptor bound site. In poly-receptor polymer systems, as illustrated in Figure 7, a single binding event affects the energy of the entire polymer producing a greater overall quenching effect or response relative to small molecule sensors [22,23].
Figure 7.
Schematic representation of sensory signals amplification in fluorescent conjugated polymers/ “molecular wires” a concept advanced by Swager et al [22-24].
3. Metallopolymers as chemosensors
Many recent reviews are available which discuss fluorescent conjugated polymers as chemosensors [14,46–47]. Metallopolymers are an exciting class of compounds which can be used in a variety of electronic and photonic applications [48]. This review specifically targets recent research that has been conducted on fluorescent metallopolymers for their applications as chemosensors for various chemical species [49–56].
3.1 Copper(II)metallopolymer chemosensors for iron cations
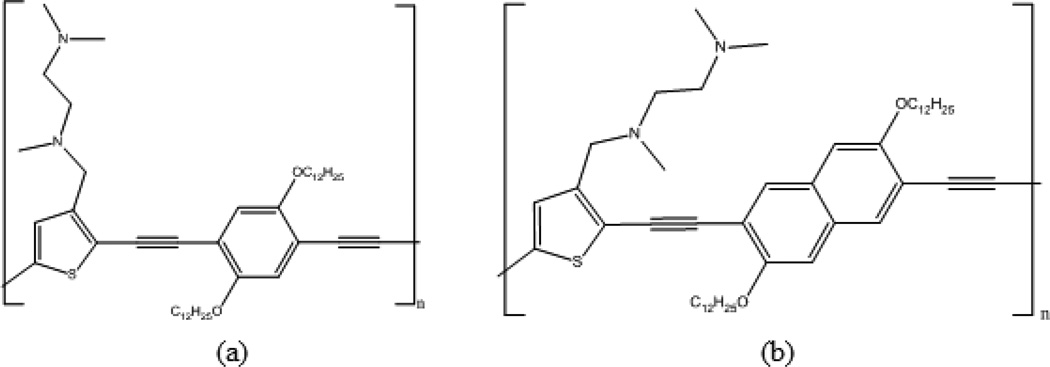
Inorganic/organic hybrid polymer chemosensors have been developed to selectively target key transition metal toxicants through a fluorescence “turn-on” response. Orginally, the polymer tmeda-PPETE, N,N,N’-trimethylethylene-diamino receptors loaded on the thiophenes of the PPETE backbone has been studied in our group [50,51]. (Figure 8a) The tmeda-PPETE polymer showed small fluorescence “turn-on” responses when most metal cations were added, but a significant quenching upon addition of Cu2+. Based on the Cu2+ quenching, an inorganic/organic hybrid system, polymer preloaded with Cu2+, was created in order to improve sensitivity by completely quenching the initial background fluorescence. Thus a fluorescence “turn-on” chemosensory system for cations was created based on a tmeda-PPETE/Cu2+ hybrid system. This hybrid system was shown to be highly selective for iron cations with fluorescence enhancements of 150-fold.
Figure 8.
Structures of (a) tmeda-PPETE and (b) tmeda-PNETE.
Recently, the role of conjugation in the polymer backbone has been explored. This work has involved increasing the conjugation of the backbone through replacement of the diethynylphenylene units of the PPETE system with diethynylnaphthalene units to create a poly [p-(naphthaleneethynylene)-alt-(thienyleneethynylene)] (PNETE) system [52]. Increasing π conjugation modifies the energy gap of the polymer backbone and could maximize the chemosensor’s sensitivity and selectivity. Like tmeda-PPETE, the newly synthesized tmeda-PNETE polymer (Figure 8b) was shown to have a “turn-on” response to most metals except Cu2+, which showed significant quenching upon addition.
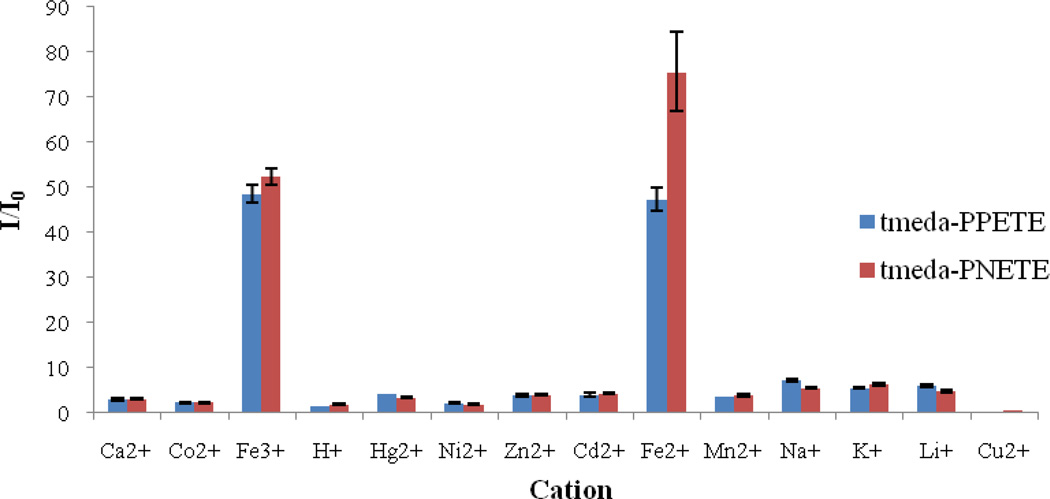
As a comparison study, polymer/Cu2+ hybrid sensory systems were created for both polymers. The hybrid systems were prepared from THF solutions of each polymer with a repeat unit concentration of 5µM. CuCl2 was added from an aqueous stock solution to reach a final concentration of 5µM and to achieve a 1:1 mole ratio of Cu2+ receptor. Each hybrid system was titrated with 13 different metal cations. During the titration, the emission maximum did not shift and the UV-vis spectra showed negligible changes, suggesting no significant change in the overall electronic structure of the polymer upon addition of metal cations. All cations were shown to increase fluorescence upon addition and both systems showed high selectivity for iron cations. (Figure 9) Interestingly, the tmeda-PNETE/Cu2+ hybrid sensor showed a greater response to Fe2+. These polymer systems may provide the basis to distinguish Fe2+ from Fe3+ in biological environments.
Figure 9.
Fluorescence response of tmeda-PPETE/Cu2+ or tmeda-PNETE to various 5µM cation concentrations. Concentration of polymer (with respect to the repeat unit) and Cu2+ were 5µM.
Results for both polymers showed there was no significant difference in fluorescence enhancement among any of these cations for the pure chemosensor polymers. In all cases, the fluorescence enhancement was significantly smaller for the pure polymer model compared to the polymer/Cu2+ systems. From these results, it was concluded that the high sensitivity and selectivity requires the polymer/Cu2+ hybrids. Both polymer/Cu2+ systems were highly selective toward iron cations in solution. Since this selectivity is not present for the parent tmeda-PPETE solutions, it must not be based simply on the association constant between the receptor ligand and the cation. This would typically be expected for a simple host–guest interaction, in this case between the Lewis acid metal ion and the Lewis base receptor. It is likely that the selectivity is related to the relative ability of the cations to replace the Cu2+ already in coordination with the receptor. Competitive binding has previously been observed in small molecule sensors and other host–guest interactions [57,58]. The relative coordination of metal cations can often be related to their Lewis acid–base properties. However, Fe2+ and other cations do not follow a consistent trend so more work is necessary to specifically identify the mode of action for the selectivity.
3.2 Metallopolymers for nitric oxide detection
3.2.1 Copper(II) metallopolymer for nitric oxide and nitrosyl detection
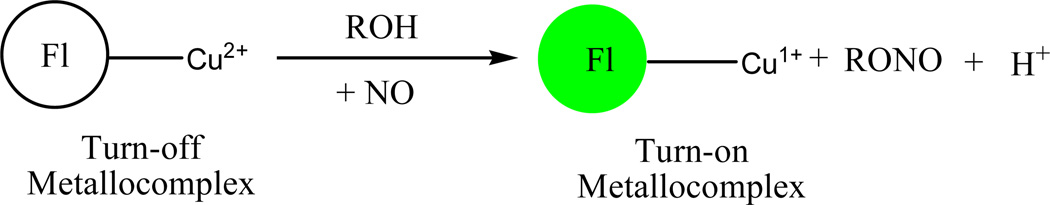
Lippard and co-workers explored a strategy whereby a nitric oxide (NO) analyte was used to reduce Cu (II) ion-based metallocomplexes of π-conjugated polymer (CP) fluorophores [53]. When reduction occurs in this system the fluorescence intensity of the complex is enhanced, as illustrated in Scheme 1 below. One particular fluorophore used in this sensing study consists of poly(p-phenyleneethynylene) building blocks with bipyridyl ligands embedded within the π-conjugated backbone. The function of the bipyridyl ligands is to act as the binding units for the copper (II) ions in the formation of the metallopolymer.
Scheme 1.
Strategy for Transition Metal-Based Fluorescent Detection of Nitric Oxide by Reductive Nitrosylation [53]
In another example, Lippard and co-workers showed that both nitric oxide (NO) and nitrosyl (HNO) were able to reduce the cupric-based metallopolymer [54]. The polymer used in this study differs from the first, in that, bithiophene replaces the bipyridyl units. Emission response studies of metallopolymer to similar concentrations of NO and HNO, in MeCN/H2O, resulted in an increase in integrated emission of the polymer. The sensitivity of the metallopolymer to HNO is however greater.
3.2.2 Cobalt(II) metallopolymer for nitric oxide detection
Holliday and co-workers synthesized solid-state based cobalt containing conducting metallopolymers sensors that responded to parts per million levels of NO in the gaseous phase [55]. This specific example uses the conducting property of conjugated polymers versus fluorescence for its sensing abilities. The metallopolymers are based on a 3,4-(ethylenedioxy)thiophene monomer unit. The authors prepared two identical metallopolymer films that were conditioned at different voltages. Their response against gaseous analytes such as O2, NO, NO2, CO and CO2 was measured and compared to a poly-EDOT film for control and comparison purposes. Tremendous selectivity of the metallopolymers towards nitric oxides was reported, the films showed only a response to NO and NO2. NO2 displayed an irreversible resistance change to the initial resistance that was large in magnitude. The authors attributed the magnitude and the irreversibility as a one-electron electron transfer between the NO2 analyte and the cobalt conducting metallopolymer leading to the formation of a Co3+/nitrite complex. Overall, the films displayed very good detection limits of NO and NO2 below 1 ppm.
3.3 Copper(II) metallopolymer for cyanide anion detection
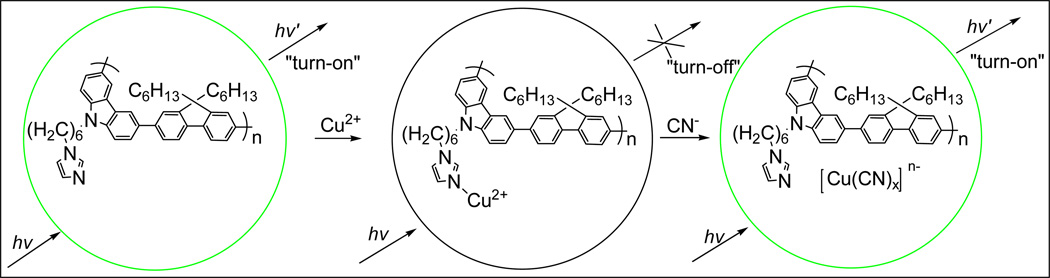
Li and co-workers developed a sensitive and selective chemosensor for cyanide (CN−) that was based on the formation of a stable cyanide complex [56]. In this study, various metal cations, Mn2+, Fe2+, Cd2+, Cr3+, Pb2+, Ag+, Zn2+, Fe3+, Ni2+, and Co2+, were found to quench the fluorescence of a imidazole-functionalized polyfluorene. However, addition of Cu2+ showed the most efficient quenching ability and completely quenched the initial fluorescence of the polymer. When cyanide anion was then added to the resulting polymer/Cu2+ hybrid, the fluorescence intensity was enhanced, and allowed for sensitive detection of cyanide down to 0.31 ppm. Overall, this polymer sensor system uses a “turn-off” approach for the detection of Cu2+ followed by an indirect “turn-on” approach utilizing the polymer/Cu2+ hybrid to detect cyanide with both sensitivity and selectivity, as shown in Figure 9.
4. Conclusion
Several new chemosensory systems based on metallopolymers have been explored by various research groups, including our own. These metallopolymers have been shown to be sensitive and selective for different chemical species including metal cations and inorganic anions present in biological and environmental systems. Specifically, most of these sensors are based on the successful combination of coordination between the receptor and transition metal and the molecular wire concept for conjugated polymers which has shown improved sensitivity and selectivity when compared to their pure organic polymer counterparts. The metallopolymer chemosensor examples described in this review were shown to be selective sensors for either iron cations, nitric oxide or cyanide anions. Further research in this field is essential in order to design the next generation of fluorescent conjugated polymer and metallopolymer sensors with the purpose of altering and improving the sensitivity and selectivity of chemosensors towards key toxicants.
Research Highlights.
Conjugated fluorescent polymers can be used as sensors for transition metal ions in solution.
Metallopolymers can be prepared which provide for better selectivity in chemosensor response.
Both “turn-on” and “turn-off” fluorescent chemosensors can be prepared by these methods.
Figure 10.
Polyfluorene chemosensor system for the detection of Cu2+ and CN− based on a fluorescence “turn-on” and “turn-off” response. [55]
Acknowledgements
This research was supported in part by a grant from the National Institute of Health (NIH grant 1R15ES10106-01) and the Research Foundation of the State University of New York. This research was also supported in part by a grant to SUNY Binghamton from the Howard Hughes Medical Institute through the Precollege and Undergraduate Science Education Program. The authors also thank Dr. Brendan Flynn and Dr. Jetty Duffy-Matzner for their useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fan LJ, Zhang Y, Murphy C, Angell S, Parker M, Flynn B, Jones WE., Jr Coord. Chem. Rev. 2009;253:410–422. [Google Scholar]
- 2.Lv F. Adv. Funct. Mater. 2011;21:845–850. [Google Scholar]
- 3.Lo K. New J. Chem. 2011;35:265–287. [Google Scholar]
- 4.Yang Y. Adv. Funct. Mater. 2010;20:2093–2097. doi: 10.1002/adfm.201000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng J, Swager TM. Poly(arylene ethynylene)s in Chemosensing and Biosensing. Adv. Polym. Sci. 2005;177:151–179. [Google Scholar]
- 6.Czarnik AW, editor. Fluorescent Chemosensors for Ion and Molecule Recognition. Washington, DC.: American Chemical Society; 1993. [Google Scholar]
- 7.de Silva AP, Gunaratne HON, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE. Chem. Rev. 1997;97:1515–1566. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Máñez R, Sancenón F. Chem. Rev. 2003;103:4419–4476. doi: 10.1021/cr010421e. [DOI] [PubMed] [Google Scholar]
- 9.Valeur B, Leray I. Coord. Chem. Rev. 2000;205:3–40. [Google Scholar]
- 10.de Silva AP, Fox DB, Huxley AJM, Moody TS. Coord. Chem. Rev. 2000;205:41–57. [Google Scholar]
- 11.Prodi L, Bolletta F, Montalti M, Zaccheroni N. Coord. Chem. Rev. 2000;205:59–83. [Google Scholar]
- 12.Duke RM, Veale EB, Pfeffer FM, Kruger PE, Gunnlaugsson T. Chem. Soc. Rev. 2010;39:3936–3953. doi: 10.1039/b910560n. [DOI] [PubMed] [Google Scholar]
- 13.Lodeiro C, Capelo JL, Mejuto JC, Oliveira E, Santos HM, Pedras B, Nunez C. Chem. Soc. Rev. 2010;38:2948–2976. doi: 10.1039/b819787n. [DOI] [PubMed] [Google Scholar]
- 14.Kim HN, Guo Z, Zhu W, Yoon J, Tian H. Chem. Soc. Rev. 2011;40:79–93. doi: 10.1039/c0cs00058b. [DOI] [PubMed] [Google Scholar]
- 15.Moragues ME, Martinez-Manez R, Sancenon F. Chem. Soc. Rev. 2011;40:2593–2643. doi: 10.1039/c0cs00015a. [DOI] [PubMed] [Google Scholar]
- 16.Fan LL. J. Fluoresc. 2009;19:555–559. doi: 10.1007/s10895-008-0444-9. [DOI] [PubMed] [Google Scholar]
- 17.Fan LJ, Jones WE., Jr J. Phys. Chem. B. 2006;110:7777. doi: 10.1021/jp056381q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rurack KA. J. Am. Chem. Soc. 2000;122:968–969. [Google Scholar]
- 19.Lakowicz JR. Principles of Fluorescence Spectroscopy. Springer Science + Business Media; 2006. [Google Scholar]
- 20.Turro NJ. Modern Molecular Photochemistry. Vol. 1. Sausalito, California: University Science Books; 1991. p. 628. [Google Scholar]
- 21.Czarnik AW. Acc. Chem. Res. 1994;27:302. [Google Scholar]
- 22.Swager T. Acc. Chem. Res. 1998;31:201. [Google Scholar]
- 23.McQuade D, Pullen A, Swager T. Chem. Rev. 2000;100:2537–2574. doi: 10.1021/cr9801014. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Q, Swager TM. J. Am. Chem. Soc. 1995;117:12593–12602. [Google Scholar]
- 25.Yang JS, Swager TM. J. Am. Chem. Soc. 1998;120:11864–11873. [Google Scholar]
- 26.Wosnick JH, Mello CM, Swager TM. J. Am. Chem. Soc. 2005;127:3400–3405. doi: 10.1021/ja043134b. [DOI] [PubMed] [Google Scholar]
- 27.Disney MD, Zheng J, Swager TM, Seeberger PH. J. Am. Chem. Soc. 2004;126:13343–13346. doi: 10.1021/ja047936i. [DOI] [PubMed] [Google Scholar]
- 28.Petrella A, Tamborra M, Curri ML, Cosma P, Striccoli M, Cozzoli PD, Agostiano A. J. Phys. Chem. B. 2005;109:1554–1562. doi: 10.1021/jp046597c. [DOI] [PubMed] [Google Scholar]
- 29.Dwight SJ, Gaylord BS, Hong JW, Bazan GC. J. Am. Chem. Soc. 2004;126:16850–16859. doi: 10.1021/ja0469737. [DOI] [PubMed] [Google Scholar]
- 30.Watt A, Thomsen E, Meredith P, Rubinsztein-Dunlop H. Chem. Commun. 2004:2334–2335. doi: 10.1039/b406060a. [DOI] [PubMed] [Google Scholar]
- 31.Tang H, Duan X, Feng X, Liu LWS, Li Y, Zhu D. Chem. Commun. 2009:641–643. doi: 10.1039/b817788k. [DOI] [PubMed] [Google Scholar]
- 32.Burroughes JJH. Nature (London) 1990;347:539–541. [Google Scholar]
- 33.Burn PPL. J. Am. Chem. Soc. 1993;115:10117–10124. [Google Scholar]
- 34.Kraft A, Grimsdale AC, Holmes AB. Angewandte Chemie-International Edition. 1998;37:402–428. doi: 10.1002/(SICI)1521-3773(19980302)37:4<402::AID-ANIE402>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Friend RH. Nature (London) 1999;397:121. [Google Scholar]
- 36.Heeger AJ. Angew. Chem. Int. Edit. 2001;40:2591. [Google Scholar]
- 37.Mahmoud MMA. J. Am. Chem. Soc. 2010;132:2633–2641. doi: 10.1021/ja907657j. [DOI] [PubMed] [Google Scholar]
- 38.Ho HA, Boissinot M, Bergeron MG, Corbeil G, Dore K, Boudreau D, Leclerc M. Angew. Chem. Int. Ed. 2002;41:1546–1548. doi: 10.1002/1521-3773(20020503)41:9<1548::aid-anie1548>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 39.Ho HA, Leclerc M. J. Am. Chem. Soc. 2004;126:1384–1387. doi: 10.1021/ja037289f. [DOI] [PubMed] [Google Scholar]
- 40.Choi JJ. Macromolecules. 2010;43:1964–1974. [Google Scholar]
- 41.Li JJ. Macromolecules. 1998;31:5740–5745. [Google Scholar]
- 42.Pang YY. J. Mater. Chem. 1998;8:1687–1690. [Google Scholar]
- 43.Murphy CB, Zhang Y, Troxler T, Ferry V, Martin JJ, Jones WE., Jr J. of Phys. Chem. B. 2004;108:1537–1543. [Google Scholar]
- 44.Lévesque II. Chem. Mater. 1996;8:2843–2849. [Google Scholar]
- 45.Wang B, Wasielewski MR. J. Am. Chem. Soc. 1997;119:12. [Google Scholar]
- 46.Thomas SW, III, Joly GD, Swager TM. Chem. Rev. 2007;107:1339–1386. doi: 10.1021/cr0501339. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez A, Salinas-Castillo A, Costa-Fernández JM, Pereiro R, Sanz-Medel A. Trac-Trend. Anal. Chem. 2011;30:1513–1525. [Google Scholar]
- 48.Ho CL, Wong WY. Coord. Chem. Rev. 2011;255:2469–2502. [Google Scholar]
- 49.He S, Iancono ST, Budy SM, Dennis AE, Smith DW, Jr, Smith RC. J. Mater. Chem. 2008;18:1970–1976. [Google Scholar]
- 50.Fan LJ, Jones WE., Jr J. Phys. Chem. B. 2006;110:7777. doi: 10.1021/jp056381q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan LJ, Zhang Y, Jones WE., Jr Macromolecules. 2005;38:2844. [Google Scholar]
- 52.Fegley M, Nguyen H, Alherech M, Angugo D, Dill S, Duffy-Matzner J, Jones WE., Jr Synthesis and photophysics of fluorescent conjugated polymers as chemosensors for iron cations. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 2011 submitted for publication. [Google Scholar]
- 53.Smith RC, Tennyson AG, Won AC, Lippard SJ. Inorg. Chem. 2006;45:9367–9373. doi: 10.1021/ic061099z. [DOI] [PubMed] [Google Scholar]
- 54.Tennyson AG, Do L, Smith RC, Lippard SJ. Polyhedron. 2007;26:4625–4630. [Google Scholar]
- 55.Holliday BJ, Standford TB, Swager TM. Chem. Mater. 2006;18:5649–5651. [Google Scholar]
- 56.Zhong’an Li, Lou X, Yu H, Zhen Li, Qin J. Macromolecules. 2008;41:7433–7439. [Google Scholar]
- 57.Wiskur S, Ait-Haddou H, Anslyn E, Lavigne J. Acc. Chem. Res. 2001;34:963. doi: 10.1021/ar9600796. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen BT, Anslyn EV. Coord. Chem. Rev. 2006;250:3118. [Google Scholar]