Abstract
Background/Objectives
Despite the strong need for evidence-based diagnostics, there is disagreement about the cognitive tools that best predict incident Alzheimer's disease (AD) in nondemented elders. We investigated the independent and combined contributions to the risk of AD of three key domains of cognitive assessment: neuropsychological measurement, self reports, and informant reports.
Design
Longitudinal, community-based sample.
Setting
Einstein Aging Study.
Participants
Six hundred twenty-seven non-demented older adults aged 70 and above systematically recruited from the Bronx, NY.
Measurements
Comprehensive assessment included neurological exam, behavioral questions, and neuropsychological testing. AD diagnoses were based on DSM-IV criteria assigned at a multidisciplinary consensus case conference. The major statistical analyses utilized Cox proportional hazards models (with age as the time scale), adjusted for gender, education, and depressive symptoms.
Results
Forty-eight participants developed incident AD during a median of 3.3 years of follow-up. Self and informant reports of cognitive status as well as baseline scores on tests of episodic memory and psychomotor speed predicted the onset of AD. In models examining all the variables simultaneously, however, only the episodic memory tests and informant reports were associated with risk of AD. A likelihood ratio test confirmed the incremental effect of informant reports in addition to the neuropsychological test scores (P=0.035).
Conclusion
Informant ratings improved the prediction of AD conversion above and beyond objective memory impairment in non-demented elders. Combining these cognitive measures may provide a useful, empirical method for identifying individuals at high risk for future AD.
Keywords: Cognitive complaints, Subjective memory complaints, Informant reports, AD prediction, Neuropsychological tests
INTRODUCTION
Developing strategies for preventing Alzheimer's disease (AD) in at-risk older adults is an important clinical and research priority.1 Studying the nondemented elderly longitudinally and examining predictors of the onset of AD may help identify individuals at high risk. These individuals and their families can then be educated, counseled, and ultimately receive intervention, while those with low risk can be reassured. Early cognitive markers are essential to prediction models, and often include information derived from neuropsychological performance, self reports, and informant reports of cognitive functioning. In addition, blood and spinal fluid based biomarkers and neuroimaging findings have played an expanding role in predicting incident AD.1-2 Despite the need for evidence-based models for predicting AD, there is widespread disagreement regarding the independent and joint contribution of these various assessment domains and the optimal strategy for measurement of each domain. 3,4 One approach may be to screen with noninvasive cognitive tests, followed by biological assessments in those who screen positive.
For neuropsychological assessment, episodic memory is usually the first cognitive ability to decline during the preclinical onset of AD, the most common form of dementia.4 Methods of memory assessment differ widely, though measures of verbal episodic memory are most commonly employed, often including paragraph recall and word list-learning tasks.2,4 Declines in other cognitive abilities such as executive functioning, processing speed, attention, and semantic knowledge may contribute to early identification of AD,2,4 and such domains are typically assessed during neuropsychological evaluations, though their relative predictive diagnostic value has yet to be established.
Cognitive complaints are also used to predict AD.5-8 Cognitive complaints are very common in diverse elderly populations, and are sometimes associated with AD pathology in otherwise healthy individuals 5,9-11 However, the contribution of cognitive complaints to the risk of future AD requires additional study. In addition, there are complex interrelationships between memory complaints and affective symptoms, personality characteristics, and various medical and demographic factors.12-14 There is also wide variability in how complaints are assessed, with many researchers relying on a single, dichotomous question about memory problems or a small number of items with varied content and unknown measurement properties. Most studies have classified individuals as either having or not having subjective cognitive impairment without assessment of the degree of cognitive complaint or predictive value of specific complaint items.
Informant reports also have been used to diagnose or predict AD. For example, after finding that informant-reported memory loss distinguished nondemented from demented individuals and predicted future diagnosis of AD in normal elders (i.e., Clinical Dementia Rating=0), Carr and colleagues15 concluded that “informants may be identifying a population at risk for the development of DAT [mild dementia of the Alzheimer type], even before clinically evident dementia is detected by standard assessment.” (p. 1726) Storandt and colleagues also have proposed a detection method for preclinical AD (i.e., mild cognitive impairment, “MCI”) that relies on intraindividual change as reported by collateral sources rather than interindividual comparison of cognitive test scores.16 They argued that informant-based reports of intraindividual change help detect AD at an even earlier stage of the disease process than typical definitions of MCI. In a recent study of nondemented, community-dwelling elders, Slavin and colleagues found that informants were more accurate in endorsing a cognitive complaint when objective impairment was present.12 In addition, informant complaints were more highly correlated with neuropsychological performance than self complaints, though correlations tended to be weak and informant complaints were influenced by mood and personality variables.12
Early identification of individuals at high risk for AD represents an important goal, in part because understanding the biological and cognitive processes occurring at the prodromal stage may lead to novel therapeutic targets for disease prevention or treatment.1 While sophisticated methods used in research settings (e.g., neuroimaging, biomarkers) may prove valid in predicting AD, they are not currently used in practice and the high cost and invasive nature of the tests may make them ill suited for large-scale screening.17, 18 Although much research has been devoted to finding low-cost, valid indicators of early cerebral dysfunction and decline, the independent and combined contributions of objective and subjective cognitive data to the prediction of AD onset have yet to be determined. The current study investigated whether the addition of self and informant report data to cognitive test scores would enhance the ability to predict incident AD in a representative community sample of non-demented elders. Our findings may contribute to more reliable and valid assessments in everyday clinical settings and inform conceptualizations of the prodromal period preceding the onset of AD.
METHODS
Participants
Participants were a subset of individuals drawn from the Einstein Aging Study (EAS), a longitudinal community-based study of aging, of individuals 70 years and older residing in the Bronx, NY. Briefly, potential participants were recruited through systematic sampling from Medicare or voter registration lists for Bronx County (see Lipton et al., 2003 and Katz et al., 2012).19, 20 Exclusion criteria included severe audiovisual disturbances and being nonambulatory. Participants provided written informed consent according to protocols approved by the local IRB. Participants eligible for the current study: (1) were enrolled in the EAS between October 1993 and January 2011; (2) were free of AD at baseline, (3) had at least one year of follow-up data; and (4) had baseline neuropsychological and subjective report data. AD diagnosis was based on findings from a consensus case conference attended by a study neurologist, neuropsychologist, and social worker, applying the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text revision; DSM-IV) criteria and using the Clinical Dementia Rating Scale.
Measures
Neuropsychological and Mood Assessment
Neuropsychological tests included (see Katz et al. 20 for references): sum of words recalled over three free recall trials of the Free and Cued Selective Reminding Test (FR-FCSRT); immediate recall scores from the Logical Memory subtest of the Wechsler Memory Scale-Revised; Trail Making Test Parts A and B; Digit Symbol and Digit Span subtests of the Wechsler Adult Intelligence Scale-Revised (WAIS-R); F-A-S Letter Fluency Test; short form of the Boston Naming Test; and Category Fluency. We assessed depressive symptoms with the short form of the Geriatric Depression Scale (GDS), a 15-item self-report measure. In cases where GDS scores were not available (approximately 6% of participants), we instead used endorsement of “history of being treated for depression” on the EAS Baseline Medical History Form (unpublished).
Self and Informant Assessment of Cognitive Abilities
The EAS includes questions that assess self perception of cognitive abilities: 15 items from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) clinical history questionnaire, a yes/no rating scale of current functioning in several cognitive domains; 5 items from the Albert Einstein Health Self-Assessment Form (unpublished), which inquires about current memory problems and changes in memory compared to 1 and 10 years ago (dichotomized as impairment/no impairment); and 1 dichotomous item from the short form of GDS, which asks whether participants feel they have “more memory problems than most.” The EAS also includes questions that assess informant perceptions of participants’ cognitive abilities: 25 items from the CERAD (informant form), a yes/no rating scale of current functioning in several cognitive domains.
Overview of Analyses
Selecting Predictor Variables
We began with a process of data reduction given the large number of cognitive variables available. First, the self and informant report items with prevalence of less than 5% were excluded. Internal consistency among the items was evaluated using Cronbach alpha, and complaint scores were obtained by summing all consistent items. A small proportion of participants (less than 10% among non-prevalent AD participants with follow-up data) had partial self or informant report data. We calculated average scores among the available items for those who had information on 5 or more items on each of the self and informant measures. Next, the cognitive tests were selected by forward variable selection regression procedures.
Analyses
Baseline characteristics were then compared with descriptive statistics, for those with and without incident AD, applying non-parametric tests as appropriate. Cox proportional hazards models were used to examine self and informant report data and the neuropsychological tests with the incidence of AD, with age as the time scale. Using this approach, the hazard function can be directly interpreted as the age-specific incidence function and age is accounted for in the non-parametric term of the hazard function, providing a more flexible and effective control of age.21 Time to event was from age at baseline, which accounts for the left truncation occurring at entry to the study, to age at AD or to final study contact, whichever came first. Hazard ratios (HR) with 95% confidence intervals (CI) were reported. All models adjusted for gender, years of education, and depression. We first examined self and informant complaint scores separately and then simultaneously with neuropsychological tests. Finally, a likelihood ratio test examined whether the prediction of incident AD was improved by adding the subjective report data to the neuropsychological test data.
The proportional hazards assumptions of the Cox models were checked using methods based on scaled Schoenfeld residuals analytically and graphically, and were adequately met. Analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC), with Splus 8.0 (Insightful Corp., Seattle, WA) used for testing the proportional hazards assumption and generating survival plots.
RESULTS
Data Reduction and Demographics
For the subjective report data, 10 self report items and 11 informant report items were retained after excluding items with prevalence of less than 5%, and we calculated the average of these scores for each participant. We then multiplied by 100 so that self and informant scores corresponded to the percentage of reported complaints (see Table 1). For the forward selection procedure, we computed the adjusted chi-square statistics for each neuropsychological test variable not in the model. If a neuropsychological test was significant at the 0.05 level, it was added to the model. The process was repeated until none of the remaining variables met the entry level. FR-FCSRT (free recall), Logical Memory (story memory), and Digit Symbol (psychomotor speed) were the only tests selected by forward variable selection procedures and used in subsequent analyses (see Table 2).
Table 1.
Summary of Baseline Characteristics by Alzheimer's Disease Status at Follow-Up (Mean ± SD for all Except Percent for Female)
| Non-Demented | Incident AD | P Value | |
|---|---|---|---|
| N | 579 | 48 | |
| Age in years | 78.2 ± 5.4 | 81.9 ± 5.6 | <0.001 |
| Education in years | 13.9 ± 3.5 | 12.9 ± 3.6 | 0.031 |
| Female, % | 59.6 | 64.6 | 0.497 |
| GDS (max = 15) | 2.3 ± 2.2 | 3.0 ± 2.4 | 0.013 |
| FR-FCSRT (max = 48) | 31.3 ± 5.8 | 23.5 ± 6.7 | <.0001 |
| Digit Symbol (max = 93) | 41.9± 13.9 | 31.4 ± 10.6 | <.0001 |
| Logical Memory (max = 50) | 20.5± 7.0 | 15.3± 6.3 | <.0001 |
| Self Report (max = 100) | 20.8 ± 19.9 | 28.7 ± 24.4 | .0188 |
| Informant Report (max = 100) | 12.8 ± 19.4 | 27.5 ± 27.0 | <.0001 |
Note. AD = Alzheimer's disease; GDS = Geriatric Depression Scale, short form score; FR-FCSRT = Free and Cued Selective Reminding Test, free recall score; Digit Symbol = WAIS-R Digit Symbol subtest score; Logical Memory = WMS-R Logical Memory immediate memory score; Self report = average score across self report items multiplied by 100; Informant report = average score across informant report items multiplied by 100.
Table 2.
Hazard ratios (HR) with 95% CI for the effect of baseline predictors on incident AD
| Alzheimer's Disease | |||
|---|---|---|---|
| n = 48 | |||
| Hazard Ratio | 95% Confidence Interval | P Value | |
| FR-FCSRT | 3.25 | 2.24 - 4.69 | <.0001 |
| Digit Symbol | 1.49 | 0.97 - 2.23 | 0.065 |
| Logical Memory | 1.54 | 1.04 - 2.25 | 0.029 |
| Self Report | 0.97 | 0.71 - 1.32 | 0.857 |
| Informant Report | 1.33 | 1.02 - 1.74 | 0.039 |
Note. Effects corresponding to 1 SD unit worse performance (SDs for FR-FCSRT, Digit Symbol, Logical Memory, self report, and informant report, respectively, are 6.3, 14.0, 7.1, 20.0, and 20.0). FR-FCSRT = Free and Cued Selective Reminding Test, free recall score; Digit Symbol = WAIS-R Digit Symbol subtest score; Logical Memory=WMS-R Logical Memory immediate memory score; Self report = average score across self report items multiplied by 100; Informant report = average score across informant report items multiplied by 100. Models use age as the time scale and adjust for gender, education, and depressive symptoms.
As per study requirements, we excluded 67 participants with missing neuropsychological data and 479 participants with missing informant report data. As compared to the eligible sample of 627 participants, the sample without informant report data had slightly lower education (P = .02) and worse performance on Logical Memory (P =0.001), but was not significantly different in age (P =0.069), depression (P =0.421), gender (P =0.585), or FRFCSRT (P =0.657). Importantly, there was no significant difference in the risk of AD between participants with and without informant complaint information (P =0.258).
Of the 627 eligible participants, mean age at entry was 78.5 (± 5.5) years, mean level of education was 13.8 (±3.5) years, and percentage female was 60.0; 71.4% were Caucasian participants (Black 23.8%; Hispanic 2.9%). The majority (60%) of informants identified themselves as participants’ sons or daughters, 10% as spouses, 23% as friends, and 7% as “other.” Forty-eight participants developed AD during a median of 3.3 years of follow-up (range = 1 to 12 years). Baseline data, stratified by whether participants developed AD during follow-up, are summarized in Table 1. Participants who developed AD were older, had lower initial cognitive performance, more depressive symptoms (though mean scores were well below the cutoff associated with clinical depression), and more self and informant reports of cognitive difficulties at baseline compared with participants who did not develop AD during follow-up. Survival Analysis for Time to AD
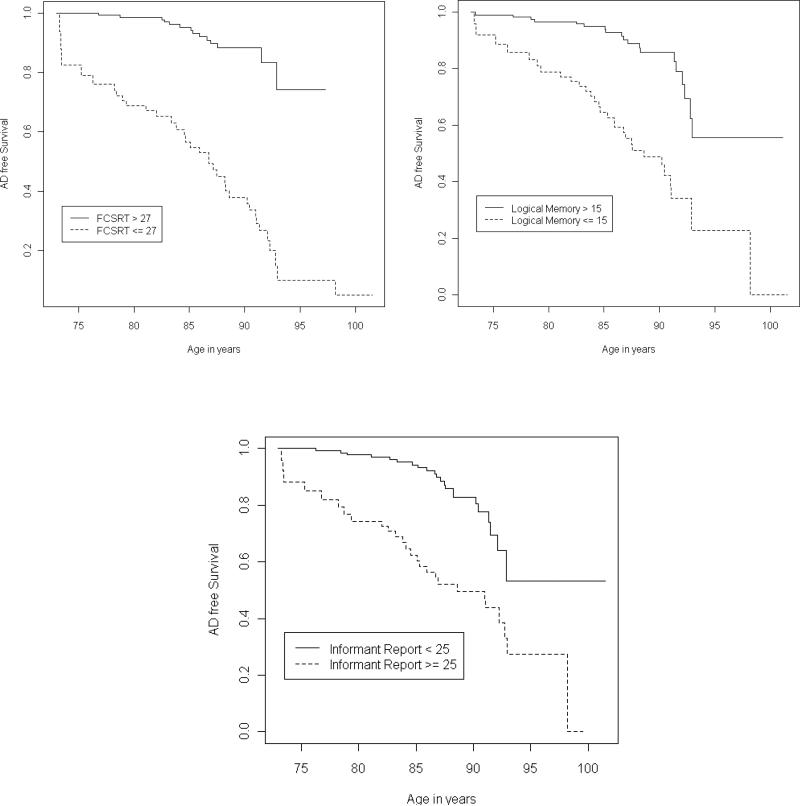
We report the effects corresponding to a 1 SD increase in self and informant report of cognitive difficulties, or 1 SD decrease in FR-FCSRT, Logical Memory, and Digit Symbol. When examined separately, adjusting for gender, education, and depression and using age as the time scale, worse performance on self report (HR = 1.105, 95% CI = 1.072-1.801, P=0.013) and informant report (HR = 1.223, 95% CI = 1.384-2.156, P<.0001) was associated with an elevated risk of AD. When all of the variables were examined simultaneously, however, the effect of self report diminished (HR = 0.97, 95% CI = 0.71-1.32, P =0.857) while the effect of informant report remained (HR = 1.33, 95% CI = 1.02-1.74, P =0.039); Table 2 presents hazard ratios of all baseline predictors on the risk of AD. For display purposes, each significant variable was also dichotomized to compare the lowest quartile to the rest (see survival curves in Figure 1).
Figure 1.
Kaplan-Meyer Curves, without adjustment, comparing the lowest quartile to the rest. FCSRT = Free and Cued Selective Reminding Test, free recall score; Logical Memory = WMSR Logical Memory immediate memory score; Informant Report = average score across informant report items multiplied by 100.
A likelihood ratio test examined whether adding informant report to the model improved the prediction of incident AD compared to using neuropsychological data alone, adjusting for gender, education, and depression. The result was significant (χ2 = 4.45, df = 1, P = 0.035).
DISCUSSION
We investigated the independent and combined contributions of three key modalities of cognitive assessment for the prediction of incident AD: neuropsychological performance, self reports, and informant reports. After controlling for gender, education, depressive symptoms, and age, results revealed that episodic memory tests (FR-FCSRT, Logical Memory), psychomotor speed (Digit Symbol), and self and informant reports of cognitive difficulties each contributed to the prediction of AD in non-demented older adults. When all of the cognitive variables were examined simultaneously, however, psychomotor speed and self reports were no longer significant. This latter finding is consistent with other observations that informant-based cognitive reports may be better predictors of objective performance than self reports and may facilitate identification of very early neurodegenerative decline.15-16, 22-23 Importantly, results confirmed the incremental effect of informant reports in addition to the most predictive neuropsychological variables.
The possibility that informant ratings may incrementally improve the prediction of AD conversion above and beyond objective cognitive assessment has implications for conceptualizations of intermediate diagnostic states such as MCI. For example, refining criteria for MCI3 to emphasize collateral report of cognitive decline may enhance the ability to detect individuals who will ultimately undergo diagnostic conversion. Findings also may inform enrollment of participants into clinical trials designed to examine conversion to AD or the effectiveness of interventions for those at risk. For example, one might derive probabilities of reaching the criterion threshold for AD in 1, 2, or 3 years from a logistic regression model solved using an individual's cognitive test and informant report scores. Knowing that an individual has a certain chance of developing AD over a given time period also would facilitate detection of the benefit of treatment that reduces the probability by a designated amount. Because it is more labor intensive to administer and interpret neuropsychological tests than questionnaires, informant report data could be used to identify individuals who warrant further evaluation.
Our results also indicated that greater degree of complaint (self and informant) was associated with higher risk of incident AD. This is consistent with Saykin and colleagues’ finding that reduced gray matter density in medial temporal and other regions was correlated with level of complaint (calculated as the percentage of items endorsed across eight self and informant cognitive report measures) in nondemented, nondepressed elders.11 Results are also consistent with recent work by Hohman and colleagues,24 which showed that overall levels of self reported complaints (across an average of 3 visits) predicted longitudinal declines in verbal memory performance in cognitively intact elders and were related to cross-sectional patterns of regional brain activity outside the normal network for memory tasks (i.e., increased activity in insular, lingual, and cerebellar regions in those with greater complaints despite comparable memory task performance). Findings such as these offer support for the validity of subjective cognitive complaints as markers of early changes in memory and brain activity and suggest that dichotomizing older adults as simply having or not having complaints is not the best approach.
Our study has certain strengths. We enrolled a systematically recruited sample of Bronx residents free of dementia at baseline and over age 70, reducing concerns about selection bias that arise in clinical samples. We statistically controlled for variables associated with both cognitive complaints and test performance such as gender and depressive symptoms. Notably, in addition to our clinical definition of dementia, we derived a psychometric diagnosis that was independent of the cognitive tests considered in the predictive models; analyses utilizing this definition resulted in a similar pattern of findings (available upon request). Given issues of criterion contamination and bias, a psychometric approach to dementia classification may be preferable, particularly in research settings, as it is less vulnerable to fluctuations in clinical decision making and may facilitate standardization of the diagnostic threshold across centers.
In addition, we employed a statistical approach to selection of the objective and subjective cognitive items for the predictive models. While our current focus was not to identify specific complaints items most predictive of future AD, it is worth noting that the 11 informant items dealt primarily with current memory difficulties, though some also related to general orientation and executive functioning such as judgment and the ability to operate household appliances. By contrast, the 10 self report items dealt with current memory ability and perceived memory decline over the past year. At present, there is no consensus regarding the optimal self and informant report instruments to identify and monitor individuals at high risk for AD. Though our current focus was not to develop “gold standard” subjective report instruments or to identify specific complaint items most predictive of future AD, these are important future directions.
The finding that FR-FCSRT was the neuropsychological test score most strongly associated with incident AD is consistent with other studies that have found this measure to have high discriminative and predictive validity for dementia, particularly AD.25-26 Through the provision of semantic cues that ensure effective registration of words, FR-FCSRT is thought to enhance the differentiation of age-associated and AD-associated memory deficits by isolating impairments in retrieval of stored information.25 Logical Memory, by contrast, assesses the ability to learn and retrieve short paragraphs (i.e., contextual verbal information). Although both tasks assess short-term, declarative verbal memory, FR-FCSRT provides an index of memory not confounded by difficulties in attention or aspects of executive functioning such as strategy use, which may account for its stronger association with incident AD. Digit Symbol was the only other neuropsychological test retained for the prediction analyses, indicating a strong association with incident AD, though its effect was diminished when all the variables were examined simultaneously. Performance on this task tends to correlate with numerous cognitive measures,27 making it a general marker for neurocognitive dysfunction with sensitivity to diffuse brain lesions in older adults including medial temporal atrophy and white matter hyperintensities and lacunae in MCI.28 Recent work has pointed to the value of processing speed and reaction time measures for predicting cognitive decline in nondemented elders, particularly those incorporating indices of intraindividual variability.29 As Digit Symbol is a brief test that is relatively independent of years of education,27 one could argue for its continued use in diverse aging populations and further investigation into its ability to enhance the prediction of incident AD when combined with other measures.
Some study limitations warrant mention. All participants had an informant willing to provide information about important aspects of their functioning. Findings may have differed had the sample included individuals lacking informant report scores. For example, compromised performance on executive function tests (a risk factor for dementia) may contribute to the prediction of AD in those who lack the social and functional support commonly associated with a highly involved significant other. Investigation of this possibility represents a future direction. In addition, informant report measures are not without their drawbacks. Though less subject to contamination by premorbid levels of intelligence and education than cognitive tests, scores may be influenced by noncognitive factors such as affective state of the patient or informant, quality of the relationship, tendency to deny or magnify problems, or misjudgment of the degree of cognitive difficulty.30 Finally, self reports should not be dismissed, as these data were predictive in some models and might be the only available or reliable subjective cognitive indicator for some individuals.
In conclusion, making judgments about which subgroup of older adults with cognitive concerns is most likely to succumb to AD and determining how to proceed in such cases represent important challenges for clinicians and researchers. The search for characteristics most likely to herald progressive cognitive decline has led to the use of varied objective and subjective cognitive instruments, many of which have unknown psychometric properties or clinical utility. Given limited time for screening, our results suggest that administration of an informant report questionnaire may serve as a valid initial warning sign, signaling the need for further evaluation. Furthermore, the combination of informant complaints and select cognitive tasks may represent complementary approaches, which provide better information than either alone, and which may represent the optimal screening approach for incident AD.
ACKNOWLEDGMENTS
This research was supported by National Institute on Aging Grants AG03949, AG039235, and a PSC CUNY grant.
Statistical Expertise: Wang. Initial manuscript preparation: Rabin. Critical revision of manuscript for important intellectual content: Rabin, Lipton, Katz, Wang, Derby, Buschke. All authors contributed to and have approved the final manuscript.
Sponsor's Role: None.
Footnotes
Author Contributions: Study concept and design: Rabin, Lipton, Katz, Derby. Data Acquisition: Katz. Data analysis and interpretation: Wang, Rabin, Lipton, Katz, Derby, Buschke.
Conflict of Interest: All authors declare that there are no financial, personal, or other potential conflicts of interest to report.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Khachaturian ZS, Petersen RC, Gauthier S, et al. A roadmap for the prevention of dementia: The inaugural Leon Thal Symposium. Alzheimers Dement. 2008;4:156–163. doi: 10.1016/j.jalz.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging and Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 4.Bondi MW, Jak AJ, Delano-Wood L, et al. Neuropsychological contributions to the early identification of Alzheimer's disease. Neuropsychol Rev. 2008;18:73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatr. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 8.Dufouil C, Fuhrer R, Alpérovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The Epidemiology of Vascular Aging Study. J Am Geriatr Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 9.Mosconi L, De Santi S, Brys M, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatr. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes LL, Schneider JJ, Boyle PA, et al. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18:701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 13.Dux MC, Woodard JL, Calamari JE, et al. The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. J Int Neuropsychol Soc. 2008;14:327–336. doi: 10.1017/S1355617708080363. [DOI] [PubMed] [Google Scholar]
- 14.Comijs HC, Deeg DJH, Dik MG, et al. Memory complaints: The association with psychoaffective and health problems and the role of personality characteristics: A 6-year follow-up study. J Affect Disord. 2002;72:157–164. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 15.DB, Gray S, Baty J, et al. The value of informant versus individual's complaints of memory impairment in early dementia. Neurology. 2010;55:1724–1726. doi: 10.1212/wnl.55.11.1724. [DOI] [PubMed] [Google Scholar]
- 16.Storandt M, Grant EA, Miller JP, et al. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 17.Forlenza OV, Chiu E. Mild cognitive impairment: A concept ready to move on? Curr Opin Psychiatr. 2008;21:529–532. doi: 10.1097/YCO.0b013e328316c2ab. [DOI] [PubMed] [Google Scholar]
- 18.Wright CF, Hall A, Matthews FE, et al. Biomarkers, dementia, and public health. Ann NY Acad Sci. 2009;1180:11–19. doi: 10.1111/j.1749-6632.2009.04942.x. [DOI] [PubMed] [Google Scholar]
- 19.Lipton RB, Katz MJ, Kulansky G, et al. Screening for dementia by telephone using the Memory Impairment Screen. J Am Geriatr Soc. 2003;51:1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 20.Katz MJ, Lipton RB, Hall CB, et al. Age and sex specific prevalence and incidence of mild cognitive impairment, dementia and Alzheimer's dementia in Blacks and Whites: A report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012 doi: 10.1097/WAD.0b013e31823dbcfc. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiebaut AC, Benichou J. Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23:3803–3820. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 22.Tierney MC, Szalai JP, Snow G, et al. The prediction of Alzheimer disease: The role of patient and informant perceptions of cognitive deficits. Arch Neurol. 1996;53:423–427. doi: 10.1001/archneur.1996.00550050053023. [DOI] [PubMed] [Google Scholar]
- 23.Frerichs RJ, Tuokko HA. Reliable change scores and their relation to perceived change in memory: Implications for the diagnosis of mild cognitive impairment. Arch Clin Neuropsychol. 2006;21:109–115. doi: 10.1016/j.acn.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Hohman TJ, Beason-Held LL, Lamar M, et al. Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychol. 2011;25:125–130. doi: 10.1037/a0020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grober E, Lipton RB, Hall C, et al. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 26.Sarazin M, Berr C, De Rotrou J, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: A longitudinal study. Neurology. 2007;69:1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 27.Hoyer WJ, Stawski RS, Wasylyshyn C, et al. Adult age and digit symbol substitution performance: A meta-analysis. Psychol Aging. 2004;19:211–214. doi: 10.1037/0882-7974.19.1.211. [DOI] [PubMed] [Google Scholar]
- 28.Van de Pol LA, Korf ES, van der Flier WM, et al. Magnetic resonance imaging predictors of cognition in mild cognitive impairment. Arch Neurol. 2007;64:1023–1028. doi: 10.1001/archneur.64.7.1023. [DOI] [PubMed] [Google Scholar]
- 29.Bielak AA, Hultsch DF, Strauss E, et al. Intraindividual variability is related to cognitive change in older adults: Evidence for within-person coupling. Psychol Aging. 2010;25:575–586. doi: 10.1037/a0019503. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon A, Mulligan R. Combining cognitive testing and informant report to increase accuracy in screening for dementia. Am J Psychiatry. 1998;155:1529–1535. doi: 10.1176/ajp.155.11.1529. [DOI] [PubMed] [Google Scholar]