Abstract
Regulation of blood glucose concentrations requires an adequate number of β-cells that respond appropriately to blood glucose levels. β-Cell mass cannot yet be measured in humans in vivo, necessitating autopsy studies, although both pre- and postmorbid changes may confound this approach. Autopsy studies report deficits in β-cell mass ranging from 0 to 65% in type 2 diabetes (T2DM), and ~70–100% in type 1 diabetes (T1DM), and, when evaluated, increased β-cell apoptosis in both T1DM and T2DM. A deficit of β-cell mass of ~50% in animal studies leads to impaired insulin secretion (when evaluated directly in the portal vein) and induction of insulin resistance. We postulate three phases for diabetes progression. Phase 1: selective β-cell cytotoxicity (autoimmune in T1DM, unknown in T2DM) leading to impaired β-cell function and gradual loss of β-cell mass through apoptosis. Phase 2: decompensation of glucose control when the pattern of portal vein insulin secretion is sufficiently impaired to cause hepatic insulin resistance. Phase 3: adverse consequences of glucose toxicity accelerate β-cell dysfunction and insulin resistance. The relative contribution of β-cell loss versus β-cell dysfunction to diabetes onset remains an area of controversy. However, because cytotoxicity sufficient to induce β-cell apoptosis predictably disturbs β-cell function, it is naïve to attempt to distinguish the relative contributions of these linked processes to diabetes onset.
Keywords: β-cell apoptosis, β-cell mass, pulsatile insulin secretion, type 2 diabetes, type 1 diabetes
Introduction
Diabetes mellitus is defined by hyperglycaemia. In health, blood glucose levels are maintained within a narrow range, primarily by the actions of the hormone insulin. Insulin is released by pancreatic β-cells at an appropriate rate in response to circulating glucose concentrations, the response being modulated by other factors including other circulating nutrients, islet innervation and incretin hormones [1]. Insulin maintains glucose concentrations by constraining the rate of hepatic glucose release to match the rate of glucose clearance [2].
Therefore, regulation of blood glucose concentrations requires an adequate number of pancreatic β-cells that respond appropriately to blood glucose concentrations. The collective β-cell numbers are often referred to as the β-cell mass, and the appropriate release of insulin by pancreatic β-cells to circulating glucose as β-cell function, the terms that will be used hereafter in this review.
β-Cell Mass by Autopsy
Because there is as yet no sensitive or specific measure of β-cell mass in vivo, there are limited data available on β-cell mass in humans. The data that are available have been derived primarily from autopsy studies [3,4–15]. Autopsy studies to quantify β-cell mass have major limitations, which are listed below. The pancreas is one of the first organs to undergo autolysis [16]. There may be changes in β-cell mass as a consequence of the final illness leading to death, particularly if this is prolonged [17,18]. The clinical information available in relation to autopsies is often limited and unreliable. As many as 40% of patients in hospital with type 2 diabetes (T2DM) are undiagnosed [19]. The extent of the pancreatic dissection at autopsy is usually limited to the pancreatic tail, so that whole pancreas is often not available. When pancreas is available, the reliability of measured pancreatic weight is questionable given the variable amount of interlobular pancreatic fat [20]. There is no means to retrospectively measure insulin sensitivity or insulin secretion to relate to the measured b-cell mass in different individuals. There is no realistic means to obtain pancreas at autopsy from individuals during the presymptomatic phase of declining β-cell function in either type 1 diabetes (T1DM) or T2DM. These limitations not-withstanding, the available data on β-cell mass in diabetes by autopsy are reasonably consistent [3–5,9–13,15,21]
β-Cell Mass in Non-Diabetic Humans
Measured β-cell mass in non-diabetic humans via autopsy studies shows a wide range between individuals, with a mean of approximately 0.8 g [22]. β-Cell mass grows rapidly in childhood, primarily through the mechanism of β-cell replication so that the adult β-cell mass is almost accomplished by 5 years of age [22]. Perhaps surprisingly there is not an obvious secondary phase of growth of β-cell mass in humans during the rapid period of somatic growth in adolescence, perhaps providing an explanation for the peak incidence of both T1DM and T2DM in this age range [23,24]. The wide range in β-cell mass in adults becomes apparent after the childhood growth phase, implying that the rate of growth of β-cell mass during this period has a major impact on the ultimate β-cell mass in adults. In mice, β-cell mass is in part determined by the number of embryonic progenitor cells [25].
It is plausible that the wide range of β-cell mass in adult humans depends in part on the islet progenitor cell pool and the status of the embryo during the period of rapid expansion of differentiated endocrine cells during fetal development. Also, because a number of genes known to regulate pancreatic β-cell cycle have been linked to T2DM in genome-wide association scans [26], it is plausible that part of the variance in β-cell mass in adult humans may be genetically determined.
β-Cell Mass in T1DM
T1DM is believed to be caused by an autoimmune mediated decline in β-cell function and mass, leading eventually to insulin deficiency [27]. Prospective studies of high-risk individuals have shown a progressive decline in β-cell function over many years preceding the onset of hyperglycaemia [28]. At present there is no way of determining to what extent this loss of β-cell function is due to loss of β-cell mass and/or is exclusively due to loss of β-cell function. Indeed, given the fact that cytotoxicity sufficient to induce β-cell apoptosis will predictably impair cell function, it is naïve to attempt to separate these two highly dependent processes. Transient independence from a need for insulin therapy (the ‘honeymoon period’) in many patients shortly after induction of insulin therapy implies a major component of impaired β-cell function superimposed on β-cell loss [29].
Likewise studies of insulin secretion in adults with recent-onset T1DM have revealed residual insulin secretion that was estimated to be approximately 40% of normal [30]. Autopsy studies of β-cell mass in people with recent-onset T1DM have reported a β-cell mass of approximately 10% of normal [9–13,15] (table 1). These studies have usually been carried out in patients who died of diabetic ketoacidosis, and so have a potential systematic bias in favour of cases with a lower β-cell mass at diabetes onset.
Table 1.
Summary of autopsy studies that examined β-cell mass in patients with recent-onset T1DM
| References | N (M/F) | Duration of diabetes |
Reported decrease in β-cell mass in recent-onset T1DM |
|---|---|---|---|
| Gepts 1965 [12] | 12/10 T1DM, 26 ND | 3–180 days | ~90–100% decrease in β-cell numbers per section area vs. ND controls |
| Gepts and De Mey 1978 [13] | 7/9 T1DM | 3–90 days | ~90–100% decrease in β-cell numbers per section area vs. ND control |
| Kloppel et al. 1984 [9] | 1 T1DM, 4 ND | ~7 days | ~80% decrease in β-cell volume vs. ND controls |
| Lernmark et al. 1995 [10] | 1/1 T1DM, 9 ND | 0–4 weeks | ~80% decrease in β-cell islet volume density vs. ND controls |
| Butler et al. 2007 [15] | 2/5 T1DM, 6/3 ND | 0–3 years | ~90% decrease in β-cell area vs. ND controls |
Abbreviations: T1DM, type 1 diabetes; ND, non-diabetic.
The rate of decline in β-cell function (and probably mass) is greater in children with T1DM than adults [31,32], and eventually in both groups there remains minimal insulin secretion or β-cell mass (approximately 1% or less of normal) [3 6,8,33,34]. The decline in both β-cell mass and β-cell function in T1DM (presumably in consequence of cytotoxic T cells) serves to emphasize the interrelated nature of β-cell dysfunction and loss.
β-Cell Mass in T2DM
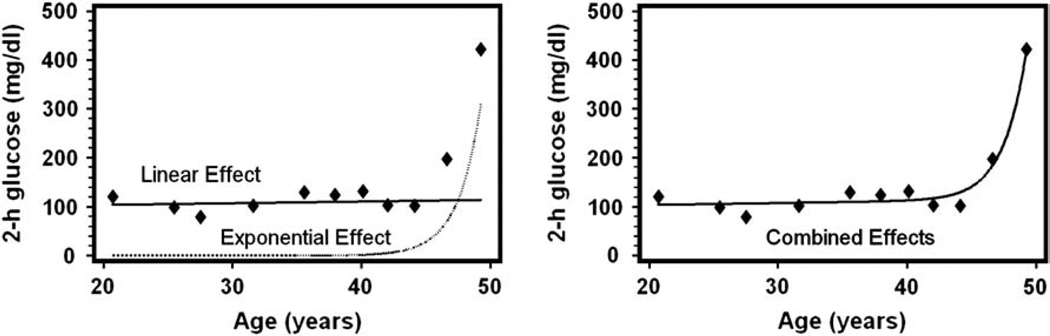
The onset of T2DM is poorly defined. It has been estimated that most patients with T2DM are not diagnosed until ~10 or more years after disease onset [35]. As in T1DM, prospective studies of T2DM indicate a progressive decline in β-cell function preceding relatively abrupt diabetes onset (figure 1) [36–38]. However there is no means to establish to what extent, if at all, this decline in β-cell function is due to impaired β-cell mass or simply due to declining function.
Fig. 1.
Blood glucose values over the years prior to diabetes onset in prospective study. Glucose effects model. Copyright ©2007 American Diabetes Association. From Ref. [37]. Reprinted with permission from The American Diabetes Association.
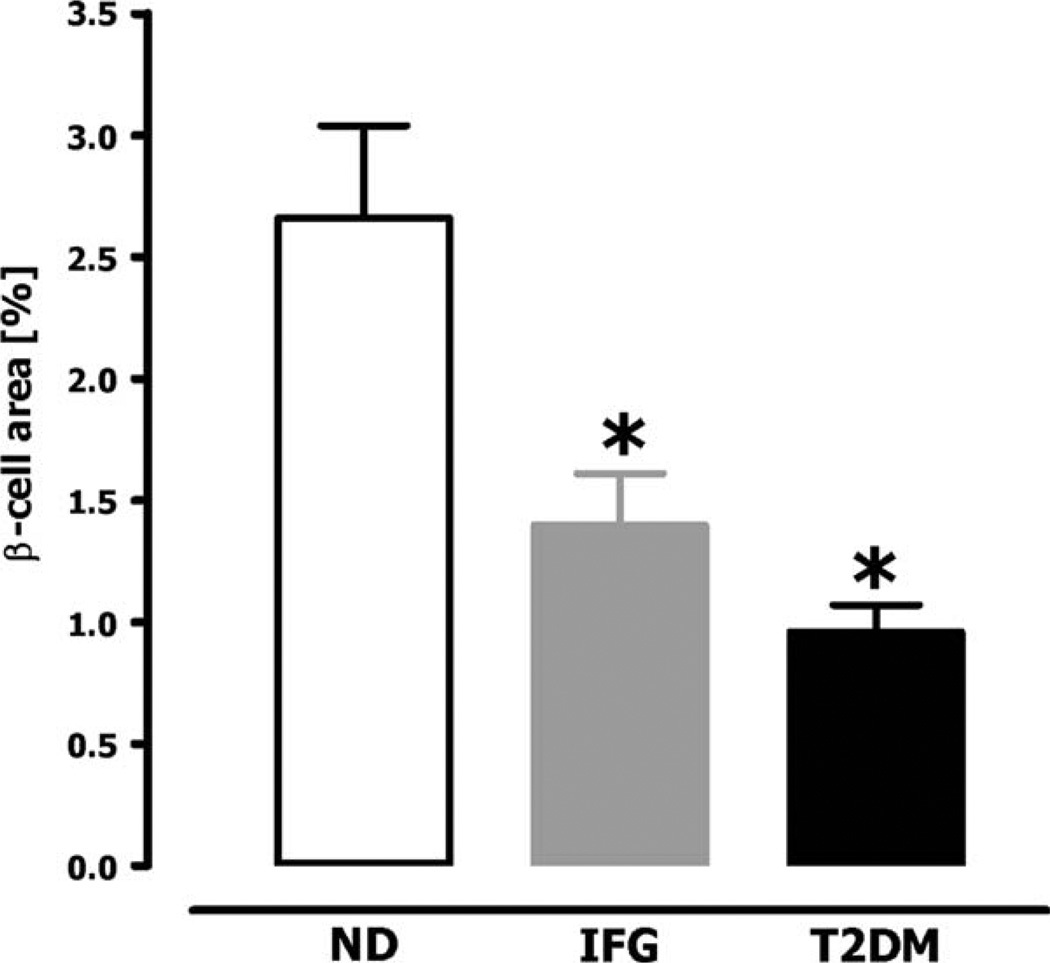
Autopsy studies of patients with T2DM have revealed a β-cell mass of ~0–65% compared to body mass index-matched controls (figure 2, table 2) [3–5,7,21]. There is also increased β-cell apoptosis compared to controls [4,39], implying that the loss of β-cell mass is likely progressive unless there is concurrently increased β-cell formation. Given the wide range of β-cell mass in non-diabetic humans, the possibility exists that vulnerability to T2DM is based in part upon the β-cell mass accomplished as an adult. In the face of insulin resistance, those individuals with the lowest β-cell mass would have the highest requirement per β-cell for proinsulin and proislet amyloid polypeptide synthesis and processing.
Fig. 2.
Fractional β-cell volume in obese humans [non-diabetic (ND), impaired fasting glucose (IFG) and type 2 diabetes (T2DM)]. Copyright © 2003 American Diabetes Association. From Ref. [4]. Reprinted with permission from American Diabetes Association.
Table 2.
Summary of autopsy studies comparing β-cell mass in patients with T2DM vs. BMI-matched controls
| References | N (M/F) | BMI | Reported decrease in β-cell mass in T2DM |
|---|---|---|---|
| Rahier et al. 1983 [7] | 4/4 lean T2DM ; 3/5 lean ND | Unknown | No significant difference between T2DM vs. ND |
| Kloppel et al. 1985 [3] | 1/5 obese T2DM; 3/1 obese ND; 7/1 lean T2DM; 4/3 lean ND | 29 ± 2 (obese T2DM); 30 ± 1 (obese ND); 21 ± 1 (lean T2DM); 20 ± 2 (lean ND) | ~50% decrease in β-cell volume in obese T2DM vs. obese ND~60% decrease in β-cell volume in lean T2DM vs. lean ND |
| Sakuraba et al. 2002 [21] | 10/4 lean T2DM; 10/5 lean ND | 21 ± 3 (lean T2DM); 21 ± 3 (lean ND) | ~30% decrease in β-cell mass vs. ND controls |
| Yoon et al. 2003 [5] | 15/10 lean T2DM; 10/9 lean ND | 22 ± 4 (lean T2DM); 23 ± 3 (lean ND) | ~50% decrease in β-cell volume in patients with BMI < 25 |
| Butler et al. 2003 [4] | 17/21 obese T2DM; 9/10 obese IFG; 15/16 obese ND; 7/9 lean T2DM; 7/10 lean ND | 38 ± 1 (obese T2DM); 37 ± 2 (obese IFG); 36 ± 1 (obese ND); 22 ± 1 (lean T2DM); 23 ± 1 (lean ND) | ~50% decrease in β-cell volume in obese IFG vs. obese ND; ~65% decrease in β-cell volume in obese T2DM vs. obese ND; ~40% decrease in β-cell volume in lean T2DM vs. lean ND |
Abbreviations: IFG, impaired fasting glucose; T2DM, type 2 diabetes; ND, non-diabetic; BMI, body mass index.
Because β-cell apoptosis in T2DM is characterized by endoplasmic reticulum stress [40,41], a high demand state per β-cell is a plausible precipitating factor in subjects prone to developing T2DM. Moreover, in the face of the unfolded protein response proinsulin synthesis would be constrained [42], possibly contributing to impaired insulin secretion. Additionally, once diabetes ensues, hyperglycaemia per se also likely contributes to both loss of β-cell mass and function [43]. Factors that have been implicated as mediators of this include oxygen free radical toxicity [44]. It has been postulated that excess availability of glucose to the cell leads to increased mitochondrial reactive oxygen species formation which is associated with decreased insulin and PDX-1 mRNA expression, impaired glucose-stimulated insulin secretion and presumably β-cell apoptosis, as recently reviewed in detail [45].
Although there is no way to measure β-cell mass in individuals prior to developing diabetes, obese individuals with impaired fasting glucose (IFG) had an ~50% deficit in β-cell fractional area compared to obese non-diabetics (figure 2) [4]. One explanation for this is that these individuals had IFG because of a low β-cell mass. Alternatively they may have been developing T2DM with a 50% deficit in β-cell mass reflecting a point of inflection between sufficient and insufficient β-cell mass in the face of obesity [46]. It is of interest that people who had a 50% pancreatectomy when acting as living hemi-pancreas donors had a relatively high risk of subsequently developing diabetes by the current blood glucose classification of diabetes [47–49]. On the other hand, most surgical studies on post-partial pancreatectomy suggest that a much higher resection percentage is required to cause diabetes, although follow-up in these studies appears to be short-term for the most part [50].
Animal Studies
Until it is possible to quantify β-cell mass in vivo, establishing its role at the onset of T2DM is not possible. For now, it is necessary to seek some guidance from animal studies. However, caution is required when extrapolating findings in animal studies to humans.
Most animal studies in relation to diabetes have been carried out in mice and rats, usually less than one year of age [51–54]. However, in contrast to humans, rodents have a high capacity for pancreas regeneration after partial pancreatectomy and a higher β-cell turnover as adults than in humans or large animals [55–57]. Other studies have been carried out in non-human primates [58], dogs [59–62] and pigs [63, 64]. Given the very high capacity for β-cell formation in young animals (and humans) any intervention seeking to decrease β-cells to find ‘the threshold’ for diabetes onset should ideally performed after the growth phase for β-cell mass is completed.
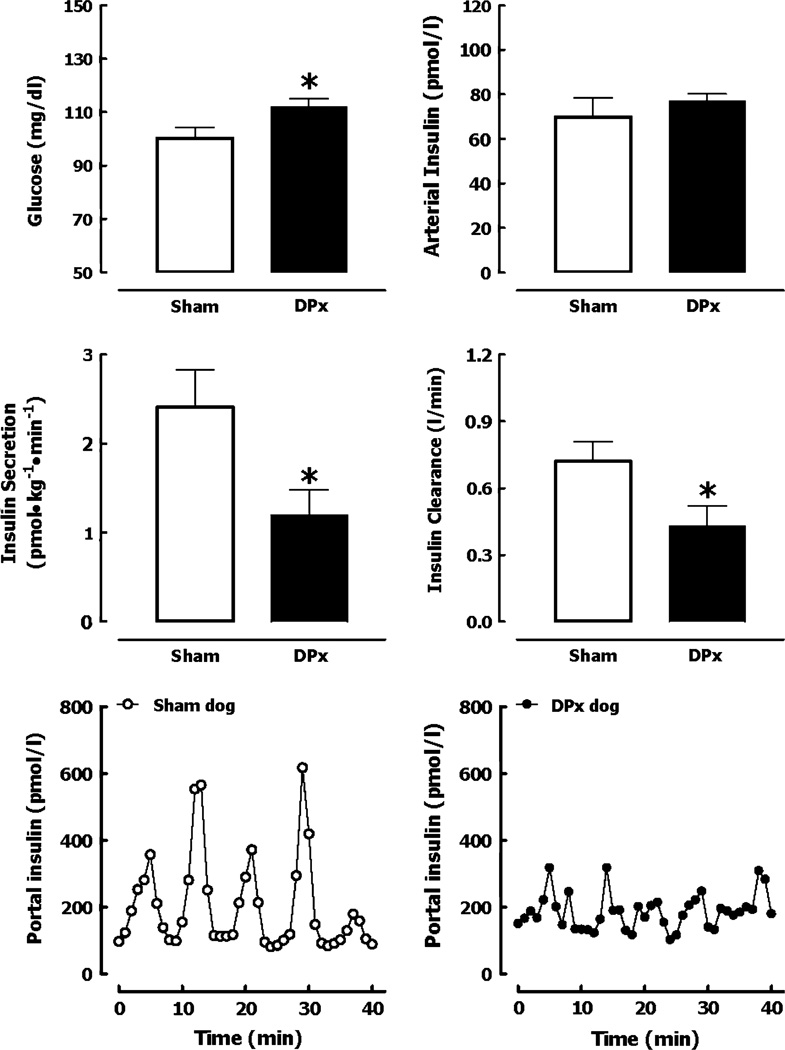
A number of studies meet this criteria [59–63,65] (table 3). With an approximately 50% pancreatectomy in dogs, in the short-term, animals show IFG and glucose intolerance and then develop diabetes during the subsequent year (figure 3) [61,62]. In the short-term they had impaired glucose-mediated insulin secretion (by meal or infusion) [59–62]. Because most insulin is secreted in discrete secretory bursts at four-minute intervals, not surprisingly these were also decreased in the fasting state and in particular after glucose stimulation [62]. However because first pass hepatic insulin extraction is directly proportional to the amplitude of portal vein insulin pulses [62,66,67], systemic insulin concentrations did not reflect this marked secretory defect, cautioning that reliance on systemic insulin concentrations to evaluate insulin secretion likely underestimates early defects. Unexpectedly partial pancreas resection in dogs led to insulin resistance [62]. A comparable decrease in β-cell mass (50%) in rats transgenic for human islet amyloid polypeptide (HIP rats) also leads to hepatic insulin resistance and IFG [68].
Table 3.
Summary of large animal models of reduced β-cell mass and their corresponding metabolic phenotype
| Reference | Animal (age) | Extent of β-cell mass decrease |
Metabolic phenotype |
|---|---|---|---|
| Matveyenko et al. 2006 [62] | Dog (1–3 years old) | ~50% (pancreatectomy) |
|
| Larsen et al. 2003 [64] | Gottengen pig (~1 year old) | ~66% (NIA + STZ) |
|
| Kjems et al. 2001 [63] | Gottengen pig (1–3 years old) | ~60% (Aloxan) |
|
| Stagner et al. 1991 [61] | Dog (1 year old) | ~50% ventral lobe (pancreatectomy) |
|
| Gotoh et al. 1989 [59] | Dog (adult >1 year old) | ~35–65% (pancreatectomy) |
|
| Marincola et al. 1984 [60] | Dog (adult >1 year old) | ~80% (pancreatectomy) |
|
Abbreviations: IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IVGTT, intravenous glucose tolerance test; NIA, nicotinamide; STZ, streptozotocin.
Fig. 3.
Fasting glucose and arterial insulin concentrations in sham-operated dogs and in dogs that underwent 50% pancreatectomy (DPx) (top panels). Corresponding deconvolved insulin secretion rate and insulin clearance rate (middle panels), Nand corresponding representative profiles of portal vein insulin concentrations (lower panels) in sham and 50% pancreatectomized dogs (DPx). Copyright © 2003 American Diabetes Association. From Ref. [62]. Reprinted with permission from The American Diabetes Association.
One possible explanation for this finding is that the impaired pulsatile delivery of insulin to the liver leads to hepatic insulin resistance. Preliminary studies (Matveyenko and Butler, unpublished) support this hypothesis. Prior studies delivering pulsatile insulin into the systemic circulation suggested that insulin pulses are more efficacious than non-pulsatile insulin, but these pulses were too small to realistically recapitulate portal insulin delivery [69–72]. If the postulate that abnormal pulsatile insulin delivery to the liver leads to hepatic insulin resistance is correct, once a threshold-low β-cell mass is reached, attenuation of insulin secretory bursts delivered to the liver would further exacerbate insulin deficiency and the resulting positive cycle between increasing insulin demand and declining β-cell function would be expected to lead to a relatively rapid loss of glycaemic control (figure 1). This is consistent with long-term prospective studies of people at risk of T2DM, which illustrate that blood glucose values do decompensate relatively rapidly (figure 1) [37,38].
Summary
Maintenance of blood glucose concentrations within a narrow range despite wide fluctuations in the rate of glucose entry (for example meals) and clearance (for example exercise) requires a complex system of regulation. Primacy in this regard falls to regulated insulin release in response to glucose levels, which in turn constrains hepatic glucose release and enhances glucose clearance [2].
By definition in diabetes this regulation fails. In both T1DM and T2DM there is a deficit in β-cell mass [3–6,9–13,15,21], impaired glucose-mediated insulin secretion [30,36], and insulin resistance [73]. The relative contribution and order that these changes develop are a matter of controversy. In view of the heterogeneous nature of both T1DM and T2DM, it is likely that their relative contributions and timing differ widely between individuals. Until a sensitive and specific method is developed to quantify β-cell mass in vivo, it is not possible to know the loss of β-cell mass at disease onset. Because loss of mass through cellular attrition surely involves damaged and therefore dysfunctional cells, from a pragmatic point of view it is reasonable to predict that loss of β-cell function precedes loss of mass.
Finally, we predict here that the point of inflection of blood glucose levels is in part due to hepatic insulin resistance developing as a consequence of the abnormal delivery pattern of insulin (attenuated insulin pulses) to the hepatic sinusoid. Further studies are required to test this hypothesis.
Acknowledgements
We greatly acknowledge funding from the National Institute of Health (DK 59579, DK 61539) and the Larry Hillblom Foundation to P.C.B. A.V.M. is supported by the National Institute of Health Ruth L. Kirschstein National Research Service Award. We acknowledge the support and excellent suggestions of our colleagues at the Larry Hillblom Islet Research Center at UCLA, Dr. Anil Bhushan, Dr. Alexandra Butler, Dr. Tatyana Gurlo and Dr. Leena Haataja. Figure 1 was kindly provided by Clinton C. Mason from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- 1.Meier JJ, Butler PC. Insulin secretion. In: DeGroot LJJJ, editor. Endocrinology. 5 edn. Philadelphia: Elsevier Saunders; 2005. pp. 961–973. [Google Scholar]
- 2.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 3.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Yoon KH, Ko SH, Cho JH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 6.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained b-cell apoptosis in patients with longstanding type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 7.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 8.Pipeleers D, Ling Z. Pancreatic b-cells in insulin-dependent diabetes. Diabetes Metab Rev. 1992;8:209–227. doi: 10.1002/dmr.5610080303. [DOI] [PubMed] [Google Scholar]
- 9.Kloppel G, Drenck CR, Oberholzer M, Heitz PU. Morphometric evidence for a striking B-cell reduction at the clinical onset of type 1 diabetes. Virchows Arch A Pathol Anat Histopathol. 1984;403:441–452. doi: 10.1007/BF00737292. [DOI] [PubMed] [Google Scholar]
- 10.Lernmark A, Kloppel G, Stenger D, et al. Heterogeneity of islet pathology in two infants with recent onset diabetes mellitus. Virchows Arch. 1995;425:631–640. doi: 10.1007/BF00199353. [DOI] [PubMed] [Google Scholar]
- 11.Junker K, Egeberg J, Kromann H, Nerup J. An autopsy study of the islets of Langerhans in acute-onset juvenile diabetes mellitus. Acta Pathol Microbiol Scand A. 1977;85:699–706. doi: 10.1111/j.1699-0463.1977.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 12.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 13.Gepts W, De Mey J. Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes. 1978;27(Suppl. 1):251–261. doi: 10.2337/diab.27.1.s251. [DOI] [PubMed] [Google Scholar]
- 14.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 15.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased b-cell apoptosis but no increased b-cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50:2323–2331. doi: 10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Hayashi T, Saitoh Y, Ohta K, Itoh H. Postmortem autolysis in the pancreas: multivariate statistical study. The influence of clinicopathological conditions. Pancreas. 1990;5:91–94. doi: 10.1097/00006676-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Fogar P, Pasquali C, Basso D, et al. Diabetes mellitus in pancreatic cancer follow-up. Anticancer Res. 1994;14:2827–2830. [PubMed] [Google Scholar]
- 18.Chari ST, Zapiach M, Yadav D, Rizza RA. Beta-cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology. 2005;5:229–233. doi: 10.1159/000085276. [DOI] [PubMed] [Google Scholar]
- 19.Levetan CS, Passaro M, Jablonski K, Kass M, Ratner RE. Unrecognized diabetes among hospitalized patients. Diabetes Care. 1998;21:246–249. doi: 10.2337/diacare.21.2.246. [DOI] [PubMed] [Google Scholar]
- 20.Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20:933–942. doi: 10.1002/ca.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 22.Meier JJ, Butler AE, Saisho, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of Type II diabetes in American Indian children. Diabetologia. 1998;41:904–910. doi: 10.1007/s001250051006. [DOI] [PubMed] [Google Scholar]
- 24.Pundziute-Lycka A, Dahlquist G, Nystrom L, et al. The incidence of Type I diabetes has not increased but shifted to a younger age at diagnosis in the 0–34 years group in Sweden 1983–1998. Diabetologia. 2002;45:783–791. doi: 10.1007/s00125-002-0845-2. [DOI] [PubMed] [Google Scholar]
- 25.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 26.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler AG, Ziegler R, Vardi P, Jackson RA, Soeldner JS, Eisenbarth GS. Life-table analysis of progression to diabetes of anti-insulin autoantibody-positive relatives of individuals with type I diabetes. Diabetes. 1989;38:1320–1325. doi: 10.2337/diab.38.10.1320. [DOI] [PubMed] [Google Scholar]
- 29.Heinze E, Thon A. Honeymoon period in insulin-dependent diabetes mellitus. Pediatrician. 1983;12:208–212. [PubMed] [Google Scholar]
- 30.Sherry NA, Tsai EB, Herold KC. Natural history of beta-cell function in type 1 diabetes. Diabetes. 2005;54(Suppl. 2):S32–S39. doi: 10.2337/diabetes.54.suppl_2.s32. [DOI] [PubMed] [Google Scholar]
- 31.Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M. A comparison of childhood and adult type I diabetes mellitus. N Engl J Med. 1989;320:881–886. doi: 10.1056/NEJM198904063201401. [DOI] [PubMed] [Google Scholar]
- 32.Bonfanti R, Bazzigaluppi E, Calori G, et al. Parameters associated with residual insulin secretion during the first year of disease in children and adolescents with Type 1 diabetes mellitus. Diabet Med. 1998;15:844–850. doi: 10.1002/(SICI)1096-9136(199810)15:10<844::AID-DIA679>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Snorgaard O, Lassen LH, Binder C. Homogeneity in pattern of decline of beta-cell function in IDDM. Prospective study of 204 consecutive cases followed for 7.4 yr. Diabetes Care. 1992;15:1009–1013. doi: 10.2337/diacare.15.8.1009. [DOI] [PubMed] [Google Scholar]
- 34.Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes. 2004;53:426–433. doi: 10.2337/diabetes.53.2.426. [DOI] [PubMed] [Google Scholar]
- 35.Harris MI. Epidemiologic studies on the pathogenesis of non-insulin-dependent diabetes mellitus (NIDDM) Clin Invest Med. 1995;18:231–239. [PubMed] [Google Scholar]
- 36.Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19:491–503. doi: 10.1210/edrv.19.4.0338. [DOI] [PubMed] [Google Scholar]
- 37.Mason CC, Hanson RL, Knowler WC. Progression to type 2 diabetes characterized by moderate then rapid glucose increases. Diabetes. 2007;56:2054–2061. doi: 10.2337/db07-0053. [DOI] [PubMed] [Google Scholar]
- 38.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes. 2006;55:1074–1079. doi: 10.2337/diabetes.55.04.06.db05-1109. [DOI] [PubMed] [Google Scholar]
- 39.Marchetti P, Del Guerra S, Marselli L, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab. 2004;89:5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- 40.Huang CJ, Lin CY, Haataja L, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 41.Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to b-cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 42.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 43.Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest. 1992;90:320–325. doi: 10.1172/JCI115865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and {beta}-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29:717–718. doi: 10.2337/diacare.29.03.06.dc05-1538. [DOI] [PubMed] [Google Scholar]
- 47.Robertson RP, Lanz KJ, Sutherland DE, Seaquist ER. Relationship between diabetes and obesity 9 to 18 years after hemipancreatectomy and transplantation in donors and recipients. Transplantation. 2002;73:736–741. doi: 10.1097/00007890-200203150-00013. [DOI] [PubMed] [Google Scholar]
- 48.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and b-cell function in healthy human donors. J Clin Invest. 1992;89:1761–1766. doi: 10.1172/JCI115779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kendall DM, Sutherland DE, Najarian JS, Goetz FC, Robertson RP. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. N Engl J Med. 1990;322:898–903. doi: 10.1056/NEJM199003293221305. [DOI] [PubMed] [Google Scholar]
- 50.Slezak LA, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25:452–460. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 51.Portha B, Giroix MH, Serradas P, et al. beta-cell function and viability in the spontaneously diabetic GK rat: information from the GK/Par colony. Diabetes. 2001;50(Suppl. 1):S89–S93. doi: 10.2337/diabetes.50.2007.s89. [DOI] [PubMed] [Google Scholar]
- 52.Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shafrir E, Ziv E, Mosthaf L. Nutritionally induced insulin resistance and receptor defect leading to beta-cell failure in animal models. Ann N Y Acad Sci. 1999;892:223–246. doi: 10.1111/j.1749-6632.1999.tb07798.x. [DOI] [PubMed] [Google Scholar]
- 54.Tomita T, Doull V, Pollock HG, Krizsan D. Pancreatic islets of obese hyperglycemic mice (ob/ob) Pancreas. 1992;7:367–375. doi: 10.1097/00006676-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Lee HC, Bonner-Weir S, Weir GC, Leahy JL. Compensatory adaption to partial pancreatectomy in the rat. Endocrinology. 1989;124:1571–1575. doi: 10.1210/endo-124-3-1571. [DOI] [PubMed] [Google Scholar]
- 56.Peshavaria M, Larmie BL, Lausier J, et al. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes. 2006;55:3289–3298. doi: 10.2337/db06-0017. [DOI] [PubMed] [Google Scholar]
- 57.Pearson KW, Scott D, Torrance B. Effects of partial surgical pancreatectomy in rats. I. Pancreatic regeneration. Gastroenterology. 1977;72:469–473. [PubMed] [Google Scholar]
- 58.Goodner CJ, Koerker DJ, Weigle DS, McCulloch DK. Decreased insulin- and glucagon-pulse amplitude accompanying beta-cell deficiency induced by streptozocin in baboons. Diabetes. 1989;38:925–931. doi: 10.2337/diab.38.7.925. [DOI] [PubMed] [Google Scholar]
- 59.Gotoh M, Monden M, Okamura J, Mori T, Shima K. Insulin and glucagon secretion after pancreatectomies. Correlation of secretion and hormonal contents of remaining pancreas. Diabetes. 1989;38:861–867. doi: 10.2337/diab.38.7.861. [DOI] [PubMed] [Google Scholar]
- 60.Marincola F, Cobb LF, Horaguchi A, Maeda M, Merrell R. Accommodation to a reduced islet cell mass in dogs. Am J Physiol. 1984;247:E456–E461. doi: 10.1152/ajpendo.1984.247.4.E456. [DOI] [PubMed] [Google Scholar]
- 61.Stagner JI, Samols E. Deterioration of islet beta-cell function after hemipancreatectomy in dogs. Diabetes. 1991;40:1472–1479. doi: 10.2337/diab.40.11.1472. [DOI] [PubMed] [Google Scholar]
- 62.Matveyenko AV, Veldhuis JD, Butler PC. Mechanisms of impaired fasting glucose and glucose intolerance induced by ~50% pancreatectomy. Diabetes. 2006;55:2347–2356. doi: 10.2337/db06-0345. [DOI] [PubMed] [Google Scholar]
- 63.Kjems LL, Kirby BM, Welsh EM, et al. Decrease in beta-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes. 2001;50:2001–2012. doi: 10.2337/diabetes.50.9.2001. [DOI] [PubMed] [Google Scholar]
- 64.Larsen MO, Rolin B, Wilken M, Carr RD, Gotfredsen CF. Measurements of insulin secretory capacity and glucose tolerance to predict pancreatic beta-cell mass in vivo in the nicotinamide/streptozotocin Gottingen minipig, a model of moderate insulin deficiency and diabetes. Diabetes. 2003;52:118–123. doi: 10.2337/diabetes.52.1.118. [DOI] [PubMed] [Google Scholar]
- 65.Larsen MO, Gotfredsen CF, Wilken M, Carr RD, Porksen N, Rolin B. Loss of beta-cell mass leads to a reduction of pulse mass with normal periodicity, regularity and entrainment of pulsatile insulin secretion in Gottingen minipigs. Diabetologia. 2003;46:195–202. doi: 10.1007/s00125-002-1011-6. [DOI] [PubMed] [Google Scholar]
- 66.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 67.Porksen N, Munn SR, Steers JL, Veldhuis JD, Butler PC. Effects of somatostatin on pulsatile insulin secretion: elective inhibition of insulin burst mass. Am J Physiol. 1996;270:E1043–E1049. doi: 10.1152/ajpendo.1996.270.6.E1043. [DOI] [PubMed] [Google Scholar]
- 68.Matveyenko AV, Butler PC. Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55:2106–2114. doi: 10.2337/db05-1672. [DOI] [PubMed] [Google Scholar]
- 69.Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes. 1983;32:617–621. doi: 10.2337/diab.32.7.617. [DOI] [PubMed] [Google Scholar]
- 70.Schmitz O, Arnfred J, Nielsen OH, Beck-Nielsen H, Orskov H. Glucose uptake and pulsatile insulin infusion: euglycaemic clamp and [3-3H]glucose studies in healthy subjects. Acta Endocrinol (Copenh) 1986;113:559–563. doi: 10.1530/acta.0.1130559. [DOI] [PubMed] [Google Scholar]
- 71.Ward GM, Walters JM, Aitken PM, Best JD, Alford FP. Effects of prolonged pulsatile hyperinsulinemia in humans. Enhancement of insulin sensitivity. Diabetes. 1990;39:501–507. doi: 10.2337/diab.39.4.501. [DOI] [PubMed] [Google Scholar]
- 72.Paolisso G, Sgambato S, Gentile S, et al. Advantageous metabolic effects of pulsatile insulin delivery in non-insulin-dependent diabetic patients. J Clin Endocrinol Metab. 1988;67:1005–1010. doi: 10.1210/jcem-67-5-1005. [DOI] [PubMed] [Google Scholar]
- 73.DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982;23:313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]