Abstract
Background
Nocardia farcinica is a gram-positive, partially acid-fast, methenamine silver-positive aerobic actinomycete. Nocardia spp. are opportunistic pathogens, and N. farcinica is the least common species of clinical importance.
Methods
Review of the recent literature and description of a immunocompetent patient with no known risk factors who contracted fatal N. farcinica sepsis.
Results
Positive pre-mortem and post-mortem cultures from the lung and synovium correlated with acute bronchopneumonia and synovitis at autopsy. Colonies of filamentous bacteria, which were not apparent in conventional hematoxylin and eosin-stained sections, were observed with gram and methenamine silver stains, but acid-fast stains were negative. A literature review revealed that disseminated N. farcinica often is associated with an underlying malignant tumor or autoimmune disease (88% of patients). Chemotherapy or corticosteroid treatments are additional risk factors.
Conclusions
Trimethoprim–sulfamethoxazole typically is the first-line therapy for N. farcinica; treatment with amikacin and imipenem-cilastatin is used less often (7% of patients). Despite aggressive therapy, we observed that the death rate (39%) associated with N. farcinica in recent publications was eight percentage points higher than reported in a review from 2000.
Nocardiosis is a localized or disseminated infection caused by the soil-dwelling, weakly gram-positive aerobic actinomycete Nocardia [1]. The organism, characterized by filamentous branches measuring less than 1 micron thick, is not easily observed on conventional hematoxylin and eosin-stained sections, possibly because of its failure to form the “granules” characteristic of other actinomycetes. Nocardia is partially acid-fast by conventional Ziehl–Nielsen staining and is reactive with Gomori methenamine silver. The typical portal of entry for Nocardia is the respiratory tract with subsequent dissemination to distant organs [2]. Nocardia is considered an opportunistic pathogen [3,4] and is associated with compromised immune function (for example, solid organ or bone marrow transplant [5], long-term steroid use, connective tissue disease, or human immunodeficiency virus [HIV] infection), chronic obstructive pulmonary disease (COPD), alcoholism, cirrhosis, systemic vasculitis, ulcerative colitis, or renal failure) [6]. Nocardia asteroides typically is reported as the most frequent cause of nocardiosis in the United States [4–6]. Other clinically significant species are N. brasiliensis [7], N. farcinica, N. nova, N. pseudobrasiliens, and N. transvalensis. Nocardia brasiliensis is commonly associated with primary cutaneous infection following trauma in immunocompetent patients. N. farcinica is one of the least frequent clinically important species, with a reported prevalence of 5% in Switzerland and 6.7% in Crete [6, 8] and a modestly increased prevalence in Turkey [9]. It is found in a 2:1 ratio over other strains of Nocardia in Germany [10]. A recent report identified N. farcinica as a nosocomial pathogen that infected three patients in the same ward over a six-month period [11]. In the United States, 500–1,000 cases of nocardiosis are diagnosed each year [4], with N. farcinica constituting 19% of isolates [12]. The present report of fatal systemic nocardiosis concerns N. farcinica in an immunocompetent patient for whom the portal of entry was not established definitively. The diagnostic elements of, and recent literature on, this unusual infection are reviewed.
Case Report
A 78-year old male presented with a one-day history of right knee pain and swelling. The patient had received a steroid injection in the same knee one week before admission. Three years prior to admission, the patient had an unexplained illness consisting of one month of fever (100.3°F), a 19-pound weight loss, and an elevated white blood cell count (15,300/mm3). At that time, he also had an infected cyst in the posterior scalp, which was treated with cephalexin but was not cultured. The full extent of clinical evaluation at that time is unknown.
On admission, laboratory evaluation revealed hemoglobin 13.4 g/dL, hematocrit 40.1%, white blood cell count 23,300/mm3, and platelets 435,000/mm3. Pertinent chemistry findings were blood urea nitrogen 74 mg/dL, creatinine 2.6 mg/dL, and glucose 151 mg/dL. Cultures from six knee synovial fluid aspirations and peripheral blood grew N. farcinica susceptible to ciprofloxacin (minimum inhibitory concentration [MIC] 1 mcg/mL), linezolid (2 mcg/mL), amikacin (2 mcg/mL), and sulfamethoxazole (4 mcg/mL). A computed tomography (CT) scan revealed numerous subcentimeter non-calcific pulmonary nodules (Fig. 1). The patient was treated with intravenous trimethoprim–sulfamethoxazole (TMP-SMX). The patient's renal function improved with hydration. Eleven days after admission, the patient developed tachypnea and respiratory distress acutely and died.
FIG. 1.
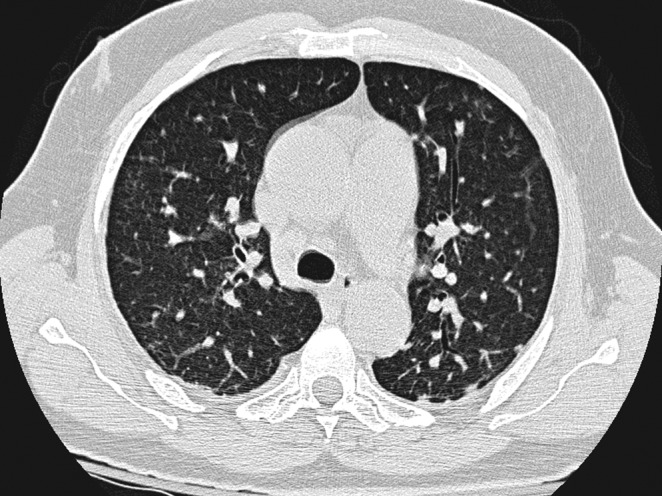
Chest CT scan demonstrates multiple subcentimeter nodules in both lungs.
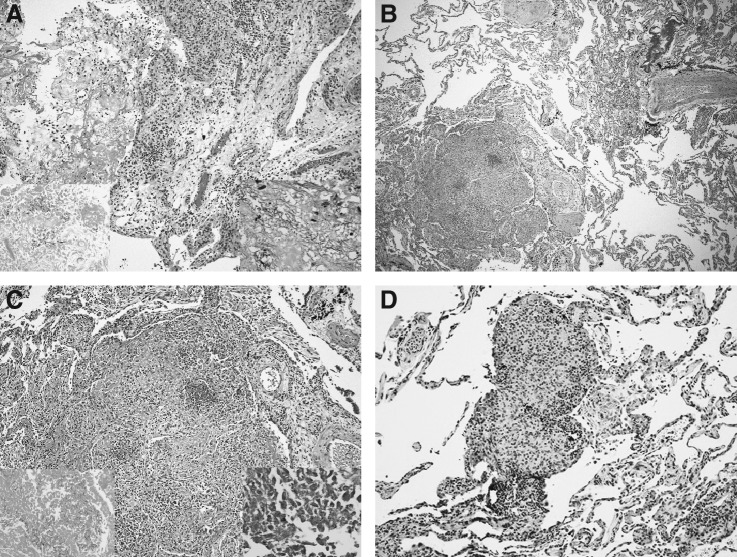
Autopsy findings included bilateral serosanguinous pleural effusions (200–300 mL) and copious turbid, greenish fluid in the right knee. Histologically, there was acute suppurative and chronic synovitis (Fig. 2A). In both lungs, extensive organizing, nodular, intra-alveolar pneumonia (Fig. 2B, C) with abscesses and associated miliary granulomas (Fig. 2D) were observed. Cultures of both lungs and the synovial fluid were positive for N. farcinica. Gram-positive, methenamine silver-positive, beaded, branching, filamentous bacteria were identified in both locations. Although acid-fast stains were negative, N. farcinica was identified by polymerase chain reaction amplification and sequencing of the bacterial rRNA.
FIG. 2.
Histologic views. (A) Acute fibrinous and chronic synovitis. No organisms are visible by hematoxylin and eosin stain. Insets show fibrinous exudates. Lower right: Gram stain demonstrating gram-positive thin, branching, filamentous forms. Lower left: Gomori methenamine silver (GMS) stain, similarly demonstrating organism morphology. (B) Lung nodule. Low-magnification view of lung with centrally necrotic nodule of organizing pneumonia, lower left. (C) Higher-magnification view of the nodule showing central necrotic focus and peripheral lymphoid infiltrate. Lower right: Gram stain of center of nodule demonstrating numerous gram-positive thin, branching, filamentous organisms. Lower left: GMS stain showing similar organisms. (D) Microscopic, non-necrotic granulomas were associated with nodular abscess.
Discussion
A relatively infrequent cause of nocardiosis, N. farcinica is a clinically aggressive infection, particularly in immunocompromised patients. For unknown reasons, small numbers of immunocompetent patients also are affected. In the last review of N. farcinica sepsis by Torres et al. in 2000, a retrospective analysis of 53 patients identified eight cases (15%) in which no predisposing factors for infection were discernible [13]. Of the eight patients, one presented with a brain abscess [14] and two had lung or kidney involvement or both [15,16]. Similarly, Beaman et al. reported that 15% of patients infected with Nocardia had no identifiable underlying condition [4]. In most cases, the pathogenesis of Nocardia infection is presumed to be via an airborne route from soil inhabited by latent forms. Following colonization of the respiratory tract, T lymphocyte-mediated cellular immunity is activated after phagocytosis of the organism [15,16]. The infection may remain localized or disseminate promptly. If the infection is localized, latency and subsequent reactivation may occur, resembling the pathogenesis of tuberculosis.
Our search of the literature for cases of N. farcinica published since the last review [14] demonstrates the continued rare occurrence of N. farcinica infection in immunocompetent patients [17]. Of the 67 cases reported since 2000, 59 (88%) patients suffered from a predisposing illness or had risk factors associated with diminished immunocompetence (Table 1). Of the eight immunocompetent patients in this cohort, six presented with brain abscesses and two with disseminated disease. Previous reports indicate that Nocardia spp. were responsible for 2%–20% of cerebral abscesses in immunocompromised patients [18,19], with a mortality rate of 30%–80% [14,20].
Table 1.
Clinical Characteristics of 67 Patients with Nocardia farcinica Infections since Last Review by Torres et al. [13]
| Age/sex | Underlying condition | Systemic steroids? | Prophylaxis with TMP-SMX | Disease | Therapy | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| 78/M | None | No | No | Lung, joint, blood | TMP-SMX (11 days) | Death | This study |
| 85/M | NHL, DM, chronic bronchitis | Yesa | No | Lung, blood | IV CTX (1 g qid)+IND (20 days) | Death | 14 |
| 67/M | Liver transplant | Yes | Yes | Lung | TMP-SMX (12 mos) | Survived | 48 |
| 28/M | Sarcoidosis | Yes | No | CNS | TMP-SMX+MER (15 days); TMP-SMX (6 mos) | Survived | 48 |
| 81/M | COPD | Yes | No | Lungb | AMK+AMX/CLAV (12 days) | Death: Related | 48 |
| 65/F | CLL, hemolytic anemia | No | Yes | Disseminated | TMP-SMX+MER (1.5 mos); TMP-SMX+AMK (4 mos); TMP-SMX+AMX/CLAV (6 mos) | Survived | 48 |
| 38/F | Transplantation of intestine (Gardner's syndrome) | Yes | No | Lung, liverc | TMP-SMX (800 mg/160 mg qd); CPX (200 mg q 12 h)+AMX/CLAV (2.2 g q 8 h)+CFZ+IMI | Death: Related | 8 |
| 77/M | COPD, invasive Aspergillus | No | No | Lung | TMP-SMX (3 mos) | Survived | 48 |
| 29/M | HIV | No | No | Cerebral | Surgery | Died | 49 |
| 30/F | HIV, MAC | No | No | Cerebral, pulmonary | TMP-SMX; MIN (6 weeks) | Died | 49 |
| 34/F | SLE, drug abuse | Yes | No | Brain abscess, subcutaneous leg abscess | Died | 50 | |
| 70/M | COPD, pneumoconiosis | Yes | No | Lung | TMP-SMX (10 mg/kg/d; 7 mos) | Death: Related | 51 |
| 62/M | Kidney transplant | NR | NR | Soft tissue/blood | TMP-SMX+CPX | Survived | 38 |
| 60/F | Kidney transplant | NR | NR | Soft tissue/blood | TMP-SMX | Survived | 38 |
| 51/M | Kidney transplant | NR | NR | Soft tissue/lung/brain | TMP-SMX+CTR | Survived | 38 |
| 50/F | Lymphoma | NR | NR | Blood | IMI | Survived | 38 |
| 58/F | COPD | NR | NR | Lung | TMP-SMX+IMI+AMK | Survived | 38 |
| 74/F | Glioma | Yes | No | Lung | MER (7 days); TMP-SMX (3 mos) | Survived | 48 |
| 47/F | Lung transplant | Yesd | No | Lung | TMP-SMX+CTR+IMI | Death | 20 |
| 46/M | Lung transplant | Yese | Yes | Lung | TMP-SMX+CTR | Death | 20 |
| 54/M | Alcoholism | No | No | Spine, CNS, psoas | Surgery, DXN+GEN | Survived | 52 |
| 2 mo/M | None | No | No | Disseminated lymphatic abscesses | CTX+CXN+MET; AMK (3 weeks); TMP-SMX (3 mos) | Survived | 24 |
| 37/M | HIV | No | No | Lung | TMP-SMX (7 mos) | Survived | 48 |
| Leg fracture/trauma | No | No | Brain abscesses | VAN+GEN+CTZ; TEI+CTZ, TMP-SMX; VAN+AMK; LIN+MIN | Survived | 53 | |
| 62/M | Evans syndrome | Yes | No | CNS | TMP-SMX+IMI (90 days) | Death: Not related | 48 |
| 68/M | COPD, DM2 | Yes | No | Septic arthritis of kneef | TMP-SMX (6 mos) | Survived | 54 |
| 60/M | Insulin-dependent DM2, systemic vasculitis with ESRD | Yes | No | Brain abscess | MER (2 g/d)+AMP (2 g/d) (7 days) | Death | 55 |
| 52/M | Non-small-cell lung cancer, radiation, chemotherapy | No | No | Bacteremia | IV CFZ 1 g tid; AMK 500 mg bid; MET (600 mg tid) (24 h) | Death | 56 |
| 58/M | Immunosuppression | Yes | No | Brain abscesses | CFZ+MER; LIN+MER | Survived | 57 |
| 75/M | Immunosuppression | Yes | No | Thyroid, psoas, spine, basal ganglia, lungs | TMP-SMX (320/1600 mg q 6 h; 2 days) | Death: Related | 34 |
| NR | Renal transplant, DM | Yes | No | Lung | CTR+AMK; IMI; TMP-SMX (6 mos) | Survived | 11 |
| NR | Bullous pemphigoid, DM | Yes | No | Pulmonary and cerebral | CTR+AMK; IMI; TMP-SMX, surgery (37 days) | Death | 11 |
| NR | None | No | No | Brain abscess | CTR+AMK; MER; TMP-SMX, surgery (6 mos) | Survived | 11 |
| NR | None | No | No | Brain abscess | CTR+AMK; TMP-SMX, surgery (6 mos) | Survived | 11 |
| NR | None | No | No | Brain abscess | CTR+AMK; MER; TMP-SMX, surgery (6 mos) | Survived | 11 |
| 75/M | Interstitial pneumonia | Yes | No | Lung | CFP; TMP-SMX (12 mos) | Survived | 7 |
| 28/M | Ulcerative colitis | Yes | No | Subcutaneous abscess in left popliteal space | Surgery, TMP-SMX | Survived | 7 |
| 72/F | BOOP, cirrhosis | Yes | No | Lung | IMI+ITC; TMP-SMX | Death | 7 |
| 68/M | ITP | Yes | No | Lung, brain abscesses, blood | AMP/SULB (1.5 g/day; TMP-SMX (160 mg/800 mg/day) | Survived | 58 |
| 12/M | Renal transplant | Yes | Yes | Brain abscesses | TEI+CTR; LIN 600 mg bid, MER (20 days); AMX/CLAV (PO) | Survived | 22 |
| 91/M | IPF | Yesg | No | Lung | IV TMP-SMX | Death | 41 |
| 76/F | COPD, vasculitis | Yesa | Yes | Lung | LVX+CTX; MER | Survived | 41 |
| 49/M | Renal transplant, alcoholism | Yes | No | Lung, brain | IMI (1 g tid)+TMP-SMX (320 mg/1600 mg qid); MOX | Survived | 44 |
| 42/M | DM, alcoholism | No | No | Brain abscess | CTR+MET+TMP-SMX | Survived | 36 |
| 26/F | SLE | Yesa | No | Subretinal abscess, lung abscess | Vitreous tap with injection of AMK; BAL; TMP-SMX IV (80 mg/400 mg/q 6 h); CPX PO (1 g qd) | Survived | 42 |
| 57/M | None | No | No | Brain abscess | Surgery, TMP-SMX+IMI; MOX+IMI; MOX (1 yr) | Survived | 59 |
| 62/F | SLE, COPD, DM | Yes | NR | Lung | Unknown | Death | 60 |
| 35/M | HIV, COPD | No | NR | Lung, disseminated | SUF | Death | 60 |
| 78/M | COPD | Yes | NR | Lung | SUF | Survived | 60 |
| 73/F | Hodgkin's disease | No | NR | Disseminated | TMP-SMX | Death | 60 |
| 46/M | HIV | No | NR | Lung, disseminated | TMP-SMX | Death | 60 |
| 65/F | COPD | No | NR | Lung | TMP-SMX | Death | 60 |
| 79/M | Rheumatoid arthritis | Yes | NR | Lung, disseminated | IMI; AMK | Death | 60 |
| 85/M | Lymphoma, COPD | Yes | NR | Lung, disseminated | CTR | Death | 60 |
| 68/M | Renal transplant, HCV | Yes | NR | Lung | SUF | Survived | 60 |
| NR | NHL | Noh | No | Lung, gluteal region, iliac fossa, kidney, cerebrum | TMP-SMX+IMI; AMK+TMP-SMX; TMP-SMX (630 days) | Survived | 33 |
| NR | Multiple myeloma | Noh | No | Lung | TMP-SMX 2×1920 mg IV (60 days) | Survived | 33 |
| NR | Polymyalgia rheumatica | Yes | No | Upper leg abscess | TMP-SMX 2×1920 mg IV (90 days) | Survived | 33 |
| 49/M | ITP | Yes | No | Lung | TMP-SMX (3 mos) | Death | 61 |
| 8/M | CF, ABPA | Yes | No | Lung | TMP-SMX 80/400 mg (62 days) | Survived | 23 |
| 73/M | Multiple myeloma | Yes | No | Disseminated subcutaneous nodules | TMP-SMX 800 mg (6 mos) | Survived | 37 |
| 65/F | HIV, COPD, vasculitis | Yes | No | Lungs | IV TMP-SMX+IMI (5 days) | Death | 62 |
| 43/M | None | No | No | Brain abscess | CTR; TMP-SMX+MOX | Survived | 63 |
| 64/M | Lung transplant | Noi | Yes | Lung | IMI+AMK; TMP-SMX IV | Survived | 25 |
| NR | Lung transplant | Yesd,g | Yes | Lung | TMP-SMX 160/800 mg PO bid) | Survived | 31 |
| 53/M | None | No | No | Brain abscess, lung | TMP-SMX (7 days); IMI; LIN; TMP-SMX (1 yr) | Survived | 19 |
| 71/M | Bladder cancer | Yes | No | Lung | IMI+AMK | Survived | 10 |
Plus cyclophosphamide.
Co-infection with cytomegalovirus and Pneumocystis jirovecii.
Co-infection with Pseudomonas aeruginosa.
Plus tacrolimus.
Plus cyclosporine.
Co-infection of lungs with P. aeruginosa, and Escherichia coli.
Plus azathioprine.
Patients were treated with chemotherapy and immunotherapy for cancer.
Microphenolate+tacrolimus immunosuppression.
ABPA=allergic bronchopulmonary aspergillosis; AMK=amikacin; AMP/SULB=ampicillin/sulbactam; AMX/CLAV=amoxicillin/clavulanic acid; BAL=bronchoalveolar lavage; BOOP=bronchiolitis obliterans organizing pneumonia; CF=cystic fibrosis; CFP=cefozopran; CFZ=ceftazidime; CLL=chronic lymphocytic leukemia; CPX=ciprofloxacin; COPD=chronic obstructive pulmonary disease; CTR=ceftriaxone; CTX=cefotaxime; CTZ=ceftazidine; CXN=cloxacillin; DXN=dicloxacillin; DM=diabetes mellitus; ESRD=end-stage renal disease; GEN=gentamicin; HCV=hepatitis C virus; HIV=human immunodeficiency virus; IMI=imipenem-cilastatin; IND=indomethacin; IPF=idiopathic pulmonary fibrosis; ITC=itraconazole; ITP=idiopathic thrombocytopenic purpura; IV=intravenous; LIN=linezolid; LVX=levofloxacin; MAC=Mycobacterium avium complex; MER=meropenem; MET=metronidazole; MIN=minocycline; MOX=moxifloxacin; NHL=non-Hodgkin lymphoma; NR=not reported; PO=orally; SLE=systemic lupus erythematosus; SUF=sulfadiazine; TEI=teicoplanin; TMP-SMX=trimethoprim–sulfamethoxazone; VAN=vancomycin.
In agreement with previously published reports [13], the ratio of male:female infection was 3:1. Although typically responsible for infections in adults, N. farcinica also infected a 12-year-old adolescent following renal transplant [21], an 8-year-old boy with cystic fibrosis [22], and an otherwise-healthy 2-month-old boy [23]. Our review of the literature demonstrated that 10.4% of patients receiving TMP-SMX prophylaxis became infected. Nocardia infection also is reported in 60% of lung transplant recipients [19,24]. Furthermore, most of the patients (61.2%) infected with N. farcinica were receiving systemic steroids or chemotherapy. Corticosteroid treatment inhibits the cytokine response and phagocytic killing of microbes by macrophages [25].
Table 2 summarizes the co-morbidity factors most commonly associated with infection for the cases in Table 1. Hui et al. [26] reported that 63% of pulmonary nocardiosis patients had underlying respiratory disorders. In particular, COPD was identified as a risk factor in 23% of patients with pulmonary nocardiosis [27]. Nocardia infection is reported in as many as 3% of transplant recipients [7] with an associated mortality rate ranging from 0% to 75% in lung transplant recipients [19,28]. Alcoholism (3.0%), hematologic malignancy (4.5%), HIV infection (7.5%), idiopathic thrombocytopenic purpura (ITP) (3.0%), systemic lupus erythematosus (SLE)(4.5%), neoplastic disease (7.5%), diabetes mellitus (9.0%), and vasculitis (4.5%) were co-morbid conditions identified in at least two patients.
Table 2.
Co-Morbid Conditions in 67 Cases of Nocardia farcinica Infectiona
| Predisposing factor | No. (%) of patients |
|---|---|
| Solid organ transplant recipient | 12 (17.9) |
| Chronic obstructive pulmonary disease | 9 (13.4) |
| Hematologic neoplasm | 3 ( 4.5) |
| Human immunodeficiency virus infection | 5 ( 7.5) |
| Idiopathic thrombocytopenic purpura | 2 ( 3.0) |
| Systemic lupus erythematosus | 3 ( 4.5) |
| Solid neoplasm | 5 ( 7.5) |
| Alcoholism | 2 ( 3.0) |
| Diabetes mellitus | 6 ( 9.0) |
| Vasculitis | 3 ( 4.5) |
| Immunosuppression (steroids or chemotherapy) | 41 (61.2) |
| Miscellaneousb | 17 (25.4) |
Some patients presented with more than one factor.
One case each of invasive aspergillosis, Mycobacterium avium complex, drug abuse, pneumoconiosis, Evans syndrome, bullous pemphigoid, interstitial pneumonia, ulcerative colitis, bronchiolitis obliterans organizing pneumonia, idiopathic pulmonary fibrosis, polymyalgia rheumatica, cystic fibrosis, allergic bronchopulmonary aspergillosis, cirrhosis, sarcoidosis, trauma, and chronic bronchitis.
Disseminated nocardiosis is associated with a mortality rate ranging from 7% to 85% in immunocompromised hosts [29]. Disseminated disease and bacteremia occurred in 37% of the cases reported since 2000 (Table 3). Assuming that patients with central nervous system lesions also had a lung infection that was unrecognized [13], 39 patients (58%) had disseminated disease. Soft tissue infection involving muscles or connective tissue, including subcutaneous abscesses, was present in 17.9% of the cases. Torres et al. [13] reported a mortality rate of 31% in cases diagnosed before 2000; the mortality rate in the cases reported since then was 39%.
Table 3.
Organ Involvement in 67 Patients with Nocardia farcinica Infection
| Organ or site | No. (%) of patients |
|---|---|
| Lung | 40 (59.7) |
| Brain | 22 (32.8) |
| Soft tissue | 12 (17.9) |
| Spine | 2 ( 3.0) |
| Kidneys | 1 ( 1.5) |
| Lymphatics | 1 ( 1.5) |
| Disseminateda | 25 (37.3) |
“Disseminated” includes bacteremia or more than one organ involved.
The diagnosis rests on the demonstration of organisms in tissue, cultures, or both. Histologically, organisms are difficult to recognize by hematoxylin and eosin stains. Also, as demonstrated here, acid-fast staining is variable and unreliable [1, 13]. Gram and methenamine silver (GMS) stains usually are positive, although gram staining may be weak. Cultures of Nocardia can take more than five days to grow [30]. Biochemical tests may be used for identification of a subset of Nocardia spp., but 16S rRNA gene sequencing or restriction analysis of amplified DNA (16S rRNA or hsp65 genes) allows rapid identification [31, 32]. This is significant, as it is important to distinguish N. farcinica from N. asteroides—the former is more resistant to antimicrobial agents and has a higher risk of dissemination [33]. The treatment for N. farcinica is complicated by its resistance to most β-lactam anti-microbials, tobramycin, and tetracyclines [12, 34]. The treatment of choice is TMP-SMX [35]. However, side effects such as skin reactions may necessitate alternative therapy [6,24,36]. In addition, as many as 50% of isolates demonstrate TMP-SMX resistance, emphasizing the need for antibiotic susceptibility testing of clinical isolates [13,37,38].
N. farcinica is susceptible to TMP-SMX, minocycline, linezolid, moxifloxacin, and amikacin and demonstrates variable susceptible to imipenem-cilastatin and ciprofloxacin [20, 39–43]. It is recommended that immunocompetent patients be treated for at least six months [44]. If the central nervous system is involved, 12 months of therapy is recommended [44]. Therapy for N. farcinica has become more aggressive, with increasing administration of multiple antimicrobials. In this review, 74.6% of patients (n=50) received TMP-SMX as part of their treatment. Carbapenems (n=25; 37.3%), amikacin (n=16; 23.9%), and ceftriaxone (n=12; 17.9%) also were used commonly (see Table 1). A previous review found TMP-SMX was administered in 54% of patients infected with N. farcinica, whereas amikacin with imipenem-cilastatin and amoxicillin/clavulanic acid were used in only 7% [13]. Nevertheless, the death rate was 31% with TMP-SMX and 38.8% with carbapenems and amikacin [13].
The patient described here was unusual in that he was immunocompetent. Although the primary origin of his infection is not documented, it is tempting to speculate that the prior febrile episode and infected cyst of three years earlier was his initial encounter with the organism. Subsequent reactivation with dissemination may have been prompted by unknown factors and perhaps facilitated by the local steroid injection. The distribution of his infection was pulmonary and musculoskeletal (limited to the right knee) with no radiologic or post-mortem evidence of central nervous system involvement. Despite therapy, the patient died from Nocardia sepsis, attesting to the virulence N. farcinica.
Acknowledgments
JMB is a trainee of the National Institutes of Health Medical Scientist Training Program (Grant GM07281) at the University of Chicago.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891–903. doi: 10.1093/clinids/22.6.891. [DOI] [PubMed] [Google Scholar]
- 2.Beaman BL. Beaman L. Nocardia species: Host–parasite relationships. Clin Microbiol Rev. 1994;7:213–264. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandell GL. Douglas RG. Bennett JE. Dolin R. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. New York: Elsevier/Churchill Livingstone; 2005. p. 3661. [Google Scholar]
- 4.Beaman BL. Burnside J. Edwards B. Causey W. Nocardial infections in the United States, 1972–1974. J Infect Dis. 1976;134:286–289. doi: 10.1093/infdis/134.3.286. [DOI] [PubMed] [Google Scholar]
- 5.Wiesmayr S. Stelzmueller I. Tabarelli W, et al. Nocardiosis following solid organ transplantation: A single-centre experience. Transplant Int. 2005;18:1048–1053. doi: 10.1111/j.1432-2277.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 6.Matulionyte R. Rohner P. Uckay I, et al. Secular trends of Nocardia infection over 15 years in a tertiary care hospital. J Clin Pathol. 2004;57:807–812. doi: 10.1136/jcp.2004.016923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peleg AY. Husain S. Qureshi ZA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: A matched case-control study. Clin Infect Dis. 2007;44:1307–1314. doi: 10.1086/514340. [DOI] [PubMed] [Google Scholar]
- 8.Maraki S. Scoulica E. Nioti E. Tselentis Y. Nocardial infection in Crete, Greece: Review of fifteen cases from 2003 to 2007. Scand J Infect Dis. 2009;41:122–127. doi: 10.1080/00365540802651905. [DOI] [PubMed] [Google Scholar]
- 9.Yildiz O. Alp E. Tokgoz B, et al. Nocardiosis in a teaching hospital in the Central Anatolia region of Turkey: Treatment and outcome. Clin Microbiol Infect. 2005;11:495–499. doi: 10.1111/j.1469-0691.2005.01145.x. [DOI] [PubMed] [Google Scholar]
- 10.Beaman BL. Boiron P. Beaman L, et al. Nocardia and nocardiosis. J Med Vet Mycol. 1992;30(Suppl 1):317–331. [PubMed] [Google Scholar]
- 11.Kachi S. Okazaki M. Takeda H, et al. Outbreak of Nocardia farcinica infection with the same pattern in randomly amplified polymorphic DNA analysis. J Hosp Infect. 2006;62:502–506. doi: 10.1016/j.jhin.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Wallace RJ., Jr Tsukamura M. Brown BA, et al. Cefotaxime-resistant Nocardia asteroides strains are isolates of the controversial species Nocardia farcinica. J Clin Microbiol. 1990;28:2726–2732. doi: 10.1128/jcm.28.12.2726-2732.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres OH. Domingo P. Pericas R, et al. Infection caused by Nocardia farcinica: Case report and review. Eur J Clin Microbiol Infect Dis. 2000;19:205–212. doi: 10.1007/s100960050460. [DOI] [PubMed] [Google Scholar]
- 14.Krone A. Schaal KP. Brawanski A. Schuknecht B. Nocardial cerebral abscess cured with imipenem/amikacin and enucleation. Neurosurg Rev. 1989;12:333–340. doi: 10.1007/BF01780852. [DOI] [PubMed] [Google Scholar]
- 15.Bergstrom R. Edebo L. Fors B. Tegner KB. Systemic Nocardia infection. Scand J Respir Dis. 1966;47:75–84. [PubMed] [Google Scholar]
- 16.Debieuvre D. Dalphin JC. Jacoulet P, et al. [Disseminated infection due to an unusual strain of Nocardia farcinica] (Fre) Rev Mal Respir. 1993;10:356–358. [PubMed] [Google Scholar]
- 17.Schiff TA. McNeil MM. Brown JM. Cutaneous Nocardia farcinica infection in a nonimmunocompromised patient: Case report and review. Clin Infect Dis. 1993;16:756–760. doi: 10.1093/clind/16.6.756. [DOI] [PubMed] [Google Scholar]
- 18.Iannotti CA. Hall GS. Procop GW, et al. Solitary Nocardia farcinica brain abscess in an immunocompetent adult mimicking metastatic brain tumor: Rapid diagnosis by pyrosequencing and successful treatment. Surg Neurol. 2009;72:74–79. doi: 10.1016/j.surneu.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Husain S. McCurry K. Dauber J, et al. Nocardia infection in lung transplant recipients. J Heart Lung Transplant. 2002;21:354–359. doi: 10.1016/s1053-2498(01)00394-1. [DOI] [PubMed] [Google Scholar]
- 20.Mamelak AN. Obana WG. Flaherty JF. Rosenblum ML. Nocardial brain abscess: Treatment strategies and factors influencing outcome. Neurosurgery. 1994;35:622–631. doi: 10.1227/00006123-199410000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Vigano SM. Edefonti A. Ferraresso M, et al. Successful medical treatment of multiple brain abscesses due to Nocardia farcinica in a paediatric renal transplant recipient. Pediatr Nephrol. 2005;20:1186–1188. doi: 10.1007/s00467-005-1978-6. [DOI] [PubMed] [Google Scholar]
- 22.Petersen BE. Jenkins SG. Yuan S, et al. Nocardia farcinica isolated from bronchoalveolar lavage fluid of a child with cystic fibrosis. Pediatr Infect Dis J. 2007;26:858–859. doi: 10.1097/INF.0b013e31805cdbff. [DOI] [PubMed] [Google Scholar]
- 23.Singh NP. Goyal R. Manchanda V. Gupta P. Disseminated nocardiosis in an immunocompetent child. Ann Trop Paediatr. 2003;23:75–78. doi: 10.1179/000349803125002904. [DOI] [PubMed] [Google Scholar]
- 24.Poonyagariyagorn HK. Gershman A. Avery R, et al. Challenges in the diagnosis and management of Nocardia infections in lung transplant recipients. Transpl Infect Dis. 2008;10:403–408. doi: 10.1111/j.1399-3062.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- 25.Davenpeck KL. Zagorski J. Schleimer RP. Bochner BS. Lipopolysaccharide-induced leukocyte rolling and adhesion in the rat mesenteric microcirculation: Regulation by glucocorticoids and role of cytokines. J Immunol. 1998;161:6861–6870. [PubMed] [Google Scholar]
- 26.Hui CH. Au VW. Rowland K, et al. Pulmonary nocardiosis re-visited: Experience of 35 patients at diagnosis. Respir Med. 2003;97:709–717. doi: 10.1053/rmed.2003.1505. [DOI] [PubMed] [Google Scholar]
- 27.Martinez Tomas R. Menendez Villanueva R. Reyes Calzada S, et al. Pulmonary nocardiosis: Risk factors and outcomes. Respirology. 2007;12:394–400. doi: 10.1111/j.1440-1843.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 28.Khan BA. Duncan M. Reynolds J. Wilkes DS. Nocardia infection in lung transplant recipients. Clin Transplant. 2008;22:562–566. doi: 10.1111/j.1399-0012.2008.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agterof MJ. van der Bruggen T. Tersmette M, et al. Nocardiosis: A case series and a mini review of clinical and microbiological features. Neth J Med. 2007;65:199–202. [PubMed] [Google Scholar]
- 30.Severo CB. Oliveira F de M. Cunha L, et al. Disseminated nocardiosis due to Nocardia farcinica: Diagnosis by thyroid abscess culture. Rev Inst Med Trop São Paulo. 2005;47:355–358. doi: 10.1590/s0036-46652005000600009. [DOI] [PubMed] [Google Scholar]
- 31.Brown-Elliott BA. Brown JM. Conville PS. Wallace RJ., Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259–282. doi: 10.1128/CMR.19.2.259-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatti KM. Shieh WJ. Phillips S, et al. Molecular diagnosis of Nocardia farcinica from a cerebral abscess. Hum Pathol. 2006;37:1117–1121. doi: 10.1016/j.humpath.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Angeles RM. Lasala RP. Fanning CV. Disseminated subcutaneous nocardiosis caused by Nocardia farcinica diagnosed by FNA biopsy and 16S ribosomal gene sequencing. Diagn Cytopathol. 2008;36:266–269. doi: 10.1002/dc.20804. [DOI] [PubMed] [Google Scholar]
- 34.Farina C. Boiron P. Ferrari I, et al. Report of human nocardiosis in Italy between 1993 and 1997. Eur J Epidemiol. 2001;17:1019–1022. doi: 10.1023/a:1020010826300. [DOI] [PubMed] [Google Scholar]
- 35.Lederman ER. Crum NF. A case series and focused review of nocardiosis: Clinical and microbiologic aspects. Medicine. 2004;83:300–313. doi: 10.1097/01.md.0000141100.30871.39. [DOI] [PubMed] [Google Scholar]
- 36.Roberts SA. Franklin JC. Mijch A. Spelman D. Nocardia infection in heart–lung transplant recipients at Alfred Hospital, Melbourne, Australia, 1989–1998. Clin Infect Dis. 2000;31:968–972. doi: 10.1086/318150. [DOI] [PubMed] [Google Scholar]
- 37.Hitti W. Wolff M. Two cases of multidrug-resistant Nocardia farcinica infection in immunosuppressed patients and implications for empiric therapy. Eur J Clin Microbiol Infect Dis. 2005;24:142–144. doi: 10.1007/s10096-005-1285-y. [DOI] [PubMed] [Google Scholar]
- 38.Dodds EM. Echandi LV. Puente SI. Kaufman S. Subretinal abscess due to Nocardia farcinica resistant to trimethoprim–sulfamethoxazole in a patient with systemic lupus erythematosus. Ocul Immunol Inflamm. 2006;14:249–251. doi: 10.1080/09273940600760514. [DOI] [PubMed] [Google Scholar]
- 39.Hansen G. Swanzy S. Gupta R, et al. In vitro activity of fluoroquinolones against clinical isolates of Nocardia identified by partial 16S rRNA sequencing. Eur J Clin Microbiol Infect Dis. 2008;27:115–120. doi: 10.1007/s10096-007-0413-2. [DOI] [PubMed] [Google Scholar]
- 40.Fihman V. Bercot B. Mateo J, et al. First successful treatment of Nocardia farcinica brain abscess with moxifloxacin. J Infect. 2006;52:e99–e102. doi: 10.1016/j.jinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Fleetwood IG. Embil JM. Ross IB. Nocardia asteroides cerebral abscess in immunocompetent hosts: Report of three cases and review of surgical recommendations. Surg Neurol. 2000;53:605–610. doi: 10.1016/s0090-3019(00)00242-1. [DOI] [PubMed] [Google Scholar]
- 42.Peters BR. Saubolle MA. Costantino JM. Disseminated and cerebral infection due to Nocardia farcinica: Diagnosis by blood culture and cure with antibiotics alone. Clin Infect Dis. 1996;23:1165–1167. doi: 10.1093/clinids/23.5.1165. [DOI] [PubMed] [Google Scholar]
- 43.Jodlowski TZ. Melnychuk I. Conry J. Linezolid for the treatment of Nocardia spp. infections. Ann Pharmacother. 2007;41:1694–1699. doi: 10.1345/aph.1K196. [DOI] [PubMed] [Google Scholar]
- 44.Minero MV. Marin M. Cercenado E, et al. Nocardiosis at the turn of the century. Medicine. 2009;88:250–261. doi: 10.1097/MD.0b013e3181afa1c8. [DOI] [PubMed] [Google Scholar]