Abstract
Background and Aims
Although hepatitis C (HCV) is associated with diabetes, few studies have examined pre-diabetes in this population. We aimed to evaluate factors associated with pre-diabetes in HCV-infected patients, including direct measurement of insulin action.
Methods
Ninety-seven non-cirrhotic, non-diabetic, HCV-infected patients underwent clinical evaluation and oral glucose tolerance testing (OGTT). Insulin sensitivity was measured directly by steady-state plasma glucose (SSPG) concentration during insulin suppression test. Early phase and total insulin secretion were determined using OGTT.
Results
Rates of pre-diabetes were: 21% impaired fasting glucose (IFG), 7% impaired glucose tolerance (IGT), and 9% combined IFG/IGT. 12% of Caucasians, 50% of African-Americans, and 70% of Latinos had pre-diabetes (p=0.002). Patient characteristics among the glucose metabolism categories were similar except those with combined IFG/IGT who had a higher BMI versus normal glucose tolerance (NGT) (30 vs. 26 kg/m2, p=0.007) and lower LDL versus NGT and IGT (74, 104, and 112 mg/dL, respectively, p≤0.01). On multivariable analysis, non-Caucasian race (OR 23.1, p=0.003), BMI (OR 3.4, p=0.02), and greater liver inflammation (OR 7.9, p=0.03) predicted IFG, whereas non-Caucasian race (OR 14.8, p=0.01) and SSPG (OR 1.1/per 10 units, p=0.01) predicted IGT. Early and total insulin secretion adjusted for the degree of insulin resistance were decreased in pre-diabetes compared to NGT (p=0.01 and p=0.02, respectively).
Conclusions
Pre-diabetes is highly prevalent among HCV-infected patients, and in some instances coincides with host responses to the virus. In most cases, however, factors that are associated with pre-diabetes in HCV-infected patients are similar to those observed in the non-HCV population.
Keywords: Chronic Hepatitis C Virus Infection, Insulin Resistance, Insulin Secretion, Impaired Glucose Tolerance, Liver Inflammation, Impaired Fasting Glucose
Hepatitis C virus (HCV) is a leading cause of chronic liver disease and hepatocellular carcinoma(1), and epidemiologic studies have shown a strong association between HCV and type II diabetes mellitus (DM).(2) The pre-diabetic states, impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), are exceedingly prevalent in the United States, with nearly 20% of the population suffering from IFG, 5% with IGT, and 10% with combined IFG/IGT.(3) Both IFG and IGT confer a moderately increased risk for cardiovascular disease(4), and the majority of individuals with these conditions will progress to overt diabetes. Appropriate screening and treatment of pre-diabetes is particularly relevant in the setting of HCV, as impaired glucose metabolism has been shown to promote liver fibrosis(5), decrease responsiveness to antiviral therapy(6), and lead to poor outcomes following liver transplantation.(7)
IFG and IGT are manifestations of early derangements in glucose homeostasis that precede diabetes. IFG and IGT are thought to represent pathophysiologically distinct entities, characterized by higher degrees of hepatic insulin resistance in the former and higher degrees of peripheral (skeletal muscle) insulin resistance in the latter.(8, 9) Given the adverse health outcomes of impaired glucose metabolism, it is important to understand the determinants of pre-diabetic states, including insulin resistance. To date, most studies evaluating insulin resistance in HCV have used surrogate estimates of insulin resistance.(6, 10) However, the correlation between surrogate estimates such as the homeostasis model assessment (HOMA-IR) and direct measurements of insulin resistance is impacted by ethnicity and obesity, and caution must be exercised in interpreting data based on these estimates in the HCV population.(11)
To date, no study has closely examined pre-diabetes within the HCV population using direct measurements of insulin action. We therefore aimed to evaluate the prevalence and factors associated with pre-diabetic states among patients with chronic HCV infection, including use of direct and dynamic measurements of insulin action.
PATIENTS AND METHODS
Study Subjects
Ninety-seven consecutive non-diabetic patients with chronic HCV infection (detectable HCV viral load) between ages 18–60 were recruited from San Francisco General Hospital (SFGH) from 2002–2009. Diabetics, based on a fasting plasma glucose concentration (FPG) ≥ 126 mg/dl (12) or a known history of diabetes, were excluded. Additional exclusion criteria included presence of HBV or HIV infection, liver disease other than HCV, clinical, histologic, or known diagnosis of cirrhosis, prior HCV treatment, and medical conditions influencing study participation. Subjects provided informed consent, and the study was approved by the UCSF Committee on Human Research.
Study Procedures
Subjects underwent a medical interview, physical examination, and fasting laboratory evaluation at screening. Liver biopsy was performed in 77 (79%) subjects, and histologic evaluation was performed by a pathologist blinded to the patient’s metabolic profile using the Ludwig-Batts scoring system.(13) Subjects were admitted to the UCSF Clinical and Translational Science Institute-Clinical Research Center (CRC) for study tests.
Assessment of Glucose Tolerance
A 75-g oral glucose tolerance test (OGTT) was performed at the CRC after an overnight 12-hour fast. OGTT results were used to classify subjects as normal glucose tolerance (NGT), IFG [FPG ≥100 mg/dl and <126 mg/dl], and IGT [FPG <100 mg/dl and 2-h plasma glucose concentration ≥140 mg/dl and <200 mg/dl] (12). Glucose and insulin response to oral glucose was measured by area under the curve of glucose (G-AUC) and insulin (I-AUC) using the trapezoidal method.
Measurement of Insulin Resistance
After another overnight 12-hour fast, subjects underwent the modified insulin suppression test (IST).(11, 14) During this test, new glucose production is inhibited, and similar plasma levels of exogenous insulin are reached in all patients. The steady-state plasma glucose (SSPG) concentration as the result of an identical glucose infusion rate in all patients is a direct measure of insulin mediated glucose uptake. Higher SSPG levels represent higher degrees of insulin resistance.
Measurement of Insulin Secretion
The early insulin secretory response to oral glucose was measured using the insulinogenic index as determined by the ratio of the increment of plasma insulin to that of plasma glucose at 30 minutes during OGTT.(15) The total insulin secretion during OGTT was reported as I-AUC divided by G-AUC.
Statistical Analyses
Descriptive analyses of the patient populations within each glucose metabolism category (NGT, IFG, IGT, and IFG/IGT) were summarized using mean±SD, median (range), and frequency. Viral and host factors were compared across the glucose metabolism categories using the Kruskal-Wallis test for continuous variables and chi-squared test (Fisher’s exact as appropriate) for categorical variables. Pairwise comparisons of variables within categories that were statistically significant were performed using the Mann-Whitney rank sum test for continuous variables and chi-squared test (Fisher’s exact as appropriate) for categorical variables. Multivariable stepwise forward selection logistic regression modeling was used to evaluate the host and viral predictors associated with IFG and IGT (isolated IGT or combined IFG/IGT) from an a priori compiled list. Statistical significance was assessed at a p-value of <0.05 (2-sided) in all models. All analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Rates of Pre-Diabetes Among Patients with Chronic HCV Infection
A total of 97 subjects were enrolled. 63% of study subjects were found to have NGT and 37% were diagnosed with pre-diabetes. Among all subjects, 21% had IFG, 7% had IGT, and 9% had combined IFG and IGT. The patient characteristics were similar among the different glucose metabolism categories with respect to age, sex, family history of diabetes, current alcohol consumption, and ALT levels (Table 1). In addition, HCV-related factors, including viral load, genotype, duration of infection, and liver histology (inflammation, fibrosis or steatosis) did not vary significantly among the different categories (Table 1).
Table 1.
Characteristics of Study Subjects with Different Categories of Glucose Tolerance
| Characteristic | All Patients (N=97) |
NGT (N=61) |
IFG (N=20) |
IGT (N=7) |
IFG/IGT (N=9) |
*P- Value |
|---|---|---|---|---|---|---|
| Age (mean±SD), years | 48±7 | 48±7 | 48±7 | 49±7 | 51±4 | 0.5 |
| Male Sex (N, (%)) | 70 (72) | 44 (72) | 16 (80) | 5 (71) | 5 (56) | 0.6 |
| Race/Ethnicity† (N, (%)) Caucasian African-American Hispanic/Latino Other |
43 (44) 20 (21) 27 (28) 7 (7) |
38 (62) 10 (16) 8 (13) 5 (8) |
3 (15) 5 (25) 10 (50) 2 (10) |
1 (14) 3 (43) 3 (43) -- |
1 (11) 2 (22) 6 (67) -- |
0.002 |
| BMI (mean±SD), kg/m2 | 27±5 | 26±4 | 28±5 | 28±3 | 30±4 | 0.01 |
| Current Alcohol Consumption (N, (%)) | 26 (27) | 17 (28) | 6 (30) | 2 (29) | 1 (11) | 0.7 |
| Diabetes Family History (N, (%)) | 43 (44) | 26 (43) | 11 (55) | 2 (29) | 4 (44) | 0.6 |
| HCV Viral Load (mean±SD), log10 IU/mL | 6±0.7 | 6±0.7 | 6±0.4 | 5±1.2 | 6±0.8 | 0.4 |
| HCV Genotype (N) Genotype 1 (N, (%)) Genotype 2 (N, (%)) Genotype 3 (N, (%)) |
95 65 (68) 16 (17) 14 (15) |
60 42 (70) 12 (20) 6 (10) |
20 13 (65) 3 (15) 4 (20) |
6 3 (50) 1 (17) 2 (33) |
9 7 (78) -- 2 (22) |
0.5 |
| Duration of HCV Infection (mean±SD), years | 26±10 | 26±10 | 27±9 | 28±10 | 26±8 | 1.0 |
| Liver Biopsy Findings (N) Inflammation Grade ≥ 2 (N, (%)) Fibrosis Score ≥ 2 (N, (%)) Steatosis (N, (%)) |
77 48 (62) 34 (44) 27 (35) |
52 29 (56) 20 (38) 15 (29) |
14 12 (86) 9 (64) 6 (43) |
5 2 (40) 1 (20) 2 (40) |
6 5 (83) 4 (67) 4 (67) |
0.1 0.1 0.3 |
| ALT (mean±SD), units/L | 93±85 | 97±88 | 76±52 | 57±19 | 127±134 | 0.4 |
| Ferritin (mean±SD), ng/mL | 172±152 | 174±166 | 155±87 | 130±99 | 228±197 | 0.7 |
| Total cholesterol (mean±SD), mg/dL | 172±40 | 175±38 | 169±54 | 179±22 | 157±26 | 0.4 |
| LDL (mean±SD), mg/dL | 101±34 | 104±28 | 99±49 | 112±16 | 74±29 | 0.01 |
| HDL (mean±SD), mg/dL | 50±13 | 50±12 | 54±12 | 46±9 | 48±21 | 0.4 |
| Triglycerides (mean±SD), mg/dL | 103±53 | 102±55 | 97±45 | 100±31 | 127±71 | 0.6 |
P values are for comparison of all four groups of glucose tolerance (NGT, IFG, IGT, and IFG/IGT) and P <0.05 (2-sided) is considered statistically significant.
By self-report.
BMI, body mass index; HCV, hepatitis C virus; ALT, alanine aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Statistically significant differences among the groups were detected with respect to race, BMI, and low-density lipoprotein (LDL) cholesterol levels. On pairwise comparison, subjects with combined IFG/IGT had a higher BMI than subjects with NGT (30 vs. 26 kg/m2, p=0.007). Subjects with combined IFG/IGT also had lower LDL cholesterol levels than subjects with NGT (74 vs 104 mg/dL, p=0.005) and isolated IGT (74 vs 112 mg/dL, p=0.01). However, there were no statistically significant differences in total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels among individuals with different degrees of glucose tolerance. Notably, the prevalence of pre-diabetes was high among African-Americans (50%) and Latinos (70%). Overall, in comparison to Caucasians, non-Caucasians had a significantly higher prevalence of pre-diabetes, defined as IFG, IGT, or combined IFG/IGT (57% vs. 12%, p<0.0001). In those with pre-diabetes, the proportion of patients with IFG or IGT (isolated or combined IFG/IGT) was equally distributed among each of the African-American and Latino racial/ethnic groups.
Glucose and Insulin Responses to Oral Glucose
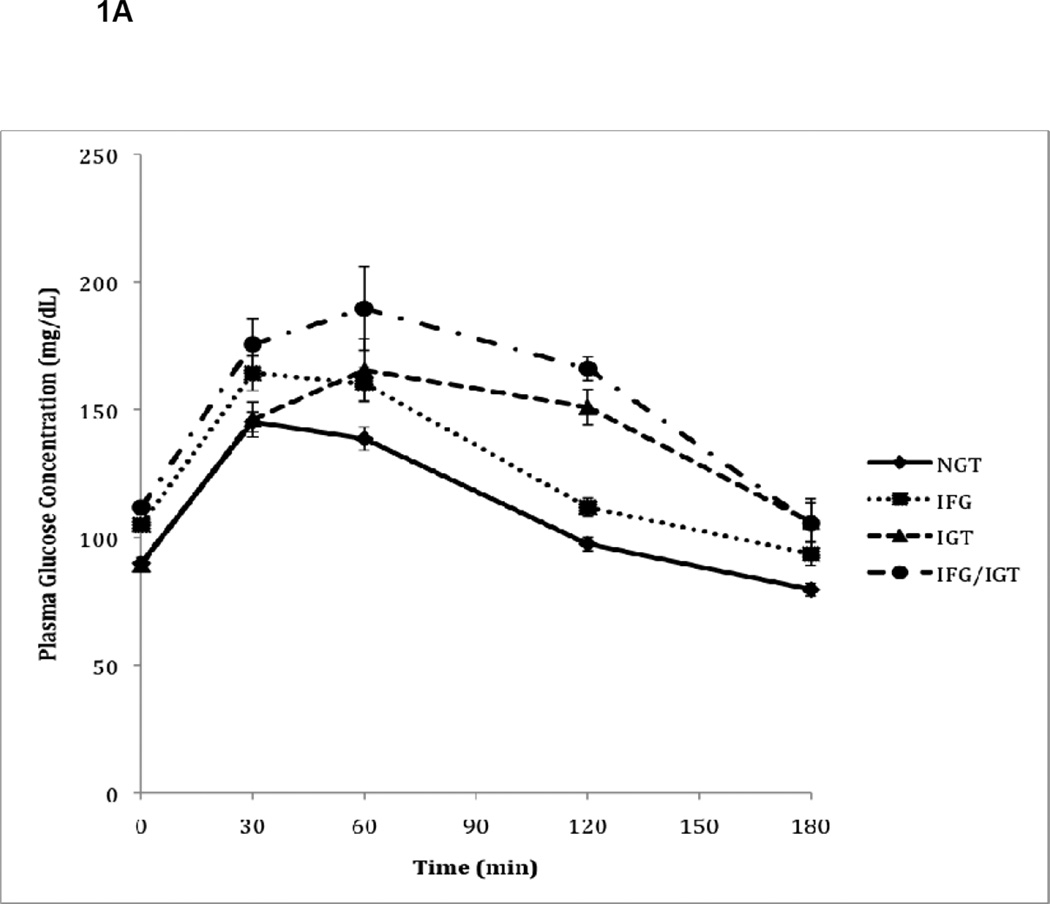
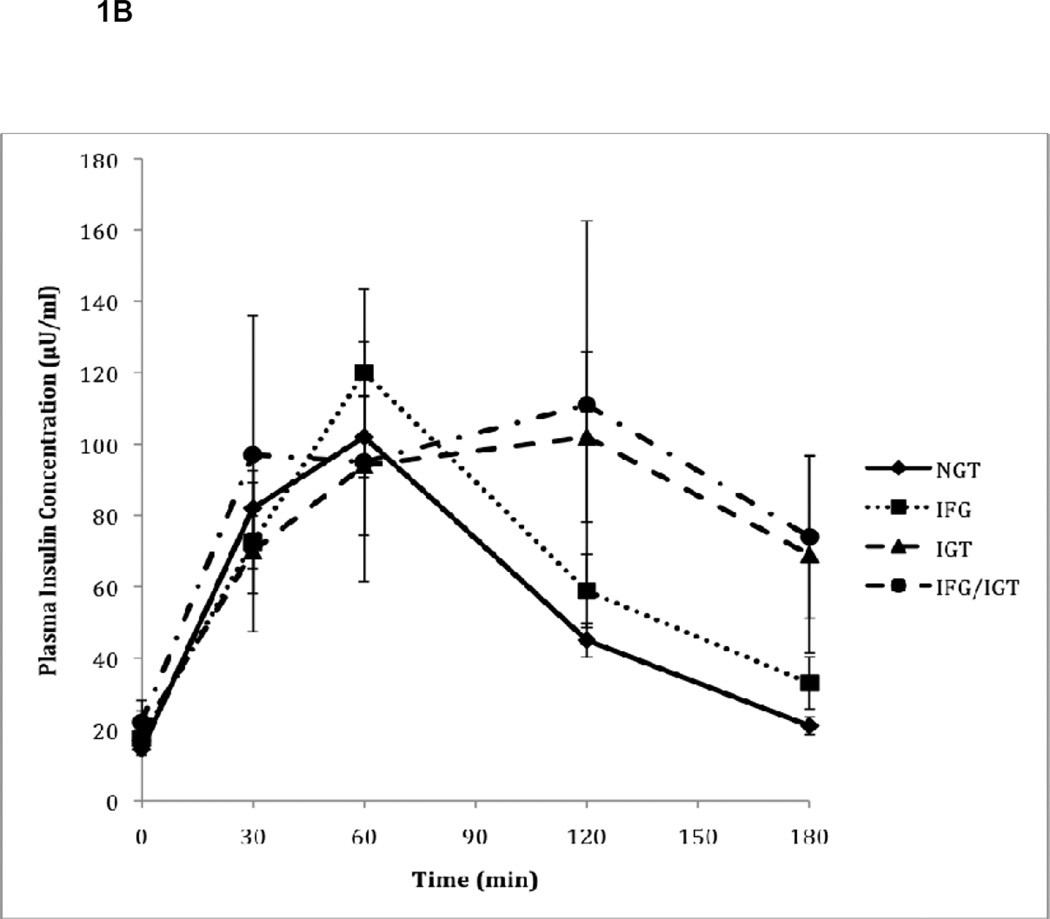
As expected, glycemic control measurements varied according to their classification among the four groups of glucose metabolism (Table 2). Patients with isolated IGT, by definition, had similar fasting glucose levels compared to NGT subjects, but during OGTT, their glucose levels continued to increase and remained elevated at the 120-minute timepoint (Figure 1A). Subjects with IFG had a higher fasting glucose than subjects with NGT (106 vs. 90 mg/dL, p<0.0001) and isolated IGT (106 vs. 89 mg/dL, p<0.0001). In addition, although subjects with IGT had similar fasting glucose levels to NGT, subjects with combined IFG/IGT had higher fasting glucose levels compared to NGT (112 vs 90 mg/dL, p<0.0001) and isolated IGT (112 vs 89 mg/dL, p<0.0001) subjects. Following the oral glucose load, the pattern of glucose response in IFG was similar to the NGT group and returned to normal at 120-minutes, although the glucose levels were higher at each timepoint. However, those with isolated IGT and combined IFG/IGT had glucose levels that remained persistently elevated during the OGTT and did not return to normal at the 120-minute timepoint. Fasting insulin levels, on the other hand, were higher in all pre-diabetic groups compared to NGT subjects, with the highest levels detected among subjects with combined IFG/IGT, though this finding did not reach statistical significance (Table 2). During OGTT, subjects with isolated IFG or IGT had lower plasma insulin concentrations at 30 minutes compared to NGT subjects despite comparable or higher plasma glucose concentrations (Figure 1B). However, the total glucose (G-AUC) and insulin (I-AUC) responses to oral glucose were higher in subjects with IFG, IGT, and combined IFG/IGT compared to subjects with NGT (p<0.0001 and p=0.04, respectively) (Table 2).
Table 2.
Insulin Sensitivity and Secretion in Study Subjects with NGT or Pre-Diabetes
| Characteristic | NGT (N=61) |
IFG (N=20) |
IGT (N=7) |
IFG/IGT (N=9) |
*P- Value |
|---|---|---|---|---|---|
| Fasting Glucose (mean±SD), mg/dL |
90±7 | 106±5 | 89±7 | 112±8 | <0.0001 |
| 2-Hour Glucose on OGTT (mean±SD), mg/dL |
97±21 | 112±16 | 151±18 | 166±14 | <0.0001 |
| Fasting Insulin (mean±SD), µIU/mL |
14±6 | 17±9 | 19±16 | 25±17 | 0.2 |
| SSPG (median (min-max)), mg/dL |
98 (39–298) | 111 (63–307) | 204 (57–275) | 206 (135–328) | 0.01 |
| SSPI (median (min-max)), microIU/mL |
70 (44–134) | 71(37–148) | 98 (54–165) | 80 (38–108) | 0.5 |
| G-AUC (mean±SD) | 336±44 | 387±27 | 423±39 | 473±67 | <0.0001 |
| I-AUC (mean±SD) | 167±97 | 212±139 | 248±135 | 274±123 | 0.04 |
|
†Early Insulin Secretory Response (mean±SD) [ΔI0–30/ΔG0–30] |
1.65±1.95 | 1.11±0.75 | 0.91±0.74 | 1.29±0.74 | 0.3 |
| Early Insulin Secretory Response adjusted for degree of insulin resistance (mean±SD) [ΔI0–30/∆G0–30] /SSPG |
0.015±0.013 | 0.009±0.009 | 0.006±0.004 | 0.006±0.004 | 0.01 |
| Total Insulin Secretion adjusted for degree of insulin resistance (mean±SD) [I-AUC/G-AUC]/SSPG |
0.005±0.003 | 0.004±0.002 | 0.004±0.001 | 0.003±0.001 | 0.02 |
P values are for all group comparisons and P <0.05 (2-sided) is considered statistically significant.
Insulinogenic index.
OGTT, oral glucose tolerance test; SSPG, steady-state plasma glucose; SSPI, steady-state plasma insulin; G-AUC, area under the curve of glucose response during OGTT; I-AUC, area under the curve of insulin response during OGTT.
Figure 1.
A. Plasma glucose responses to oral glucose during OGTT in subjects with normal glucose tolerance (NGT), impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and combined IFG/IGT. Values represent mean plasma glucose concentrations (± SE) measured during OGTT for N=97 patients.
B. Plasma insulin responses to oral glucose during OGTT in subjects with normal glucose tolerance (NGT), impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and combined IFG/IGT. Values represent mean plasma insulin concentrations (± SE) measured during OGTT for N=97 patients.
Insulin Resistance
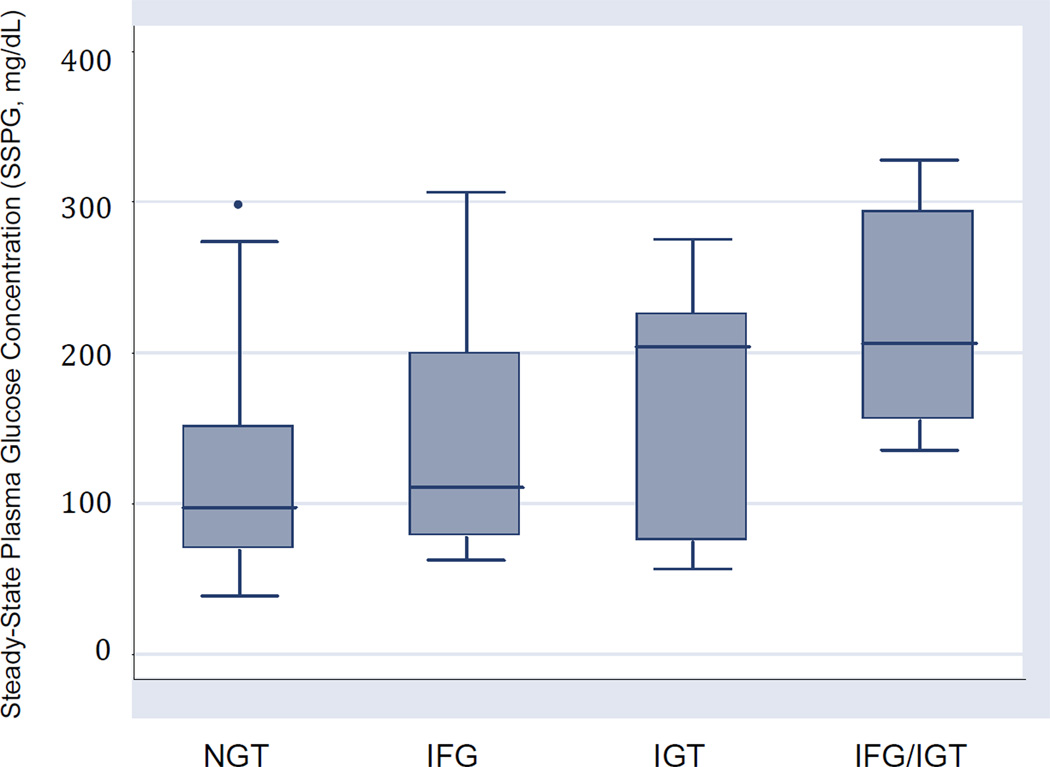
When comparing insulin resistance indices, there was a statistically significant difference in peripheral insulin resistance among the four groups as determined by the IST, with the median SSPG concentration being higher among the IGT groups (isolated IGT and combined IFG/IGT) compared to the NGT or IFG groups (Table 2 and Figure 2). The median SSPG concentrations however, were similar among the NGT and IFG groups. Of note, steady-state plasma insulin concentration (SSPI) during IST was similar between the four groups.
Figure 2. Insulin resistance (SSPG) by glucose metabolism categories.
Box plots illustrate median and range of SSPG concentrations for all four groups of subjects, with boundaries of the box representing upper and lower quartiles. • Outliers with values between 1.5 and 3 box lengths from the boundaries of the box.
Insulin Secretion
Although the overall insulin response to oral glucose (I-AUC) was increased in pre-diabetic states, OGTT results show that the early insulin secretory response, as determined by the insulinogenic index, was lower among subjects with pre-diabetes compared to subjects with NGT, suggesting impairment of the early insulin secretory response, although this did not reach statistical significance (Table 2). When adjusted for degree of insulin resistance, the early insulin secretory response was significantly lower in pre-diabetics compared to subjects with NGT (p=0.01). On pairwise comparison, compared to NGT, the impairment of early insulin secretory response was greater in subjects with IFG (0.015 vs 0.009, p=0.02), IGT (0.015 vs 0.006, p=0.03), and combined IFG/IGT (0.015 vs 0.006, p=0.05). In addition, total insulin secretion adjusted for the degree of insulin resistance was decreased in subjects with pre-diabetes compared to those with NGT (p=0.02). On pairwise comparison, the impairment of total insulin secretion was significant among subjects with combined IFG/IGT compared to subjects with NGT (0.003 vs 0.005, p=0.04).
Independent Predictors of IFG and IGT Among HCV Patients
On univariable analysis comparing IFG to NGT subjects, non-Caucasian race was significantly associated with IFG (OR 9.4, 95%CI 2.5–6.4, p=0.001). All non-Caucasian racial categories had a positive association with IFG, with Hispanic/Latinos having the highest OR when compared to Caucasians (African American OR 6.3, 95%CI 1.3–31.1, Hispanic/Latino OR 15.8, 95%CI 3.5–70.9, other races OR 5.1, 95%CI 0.7–38.1). On multivariable stepwise forward selection regression analysis comparing IFG to NGT subjects, non-Caucasian race (OR 23.1, 95% CI 2.8–187.0, p=0.003), increasing BMI (OR 3.4, 95% CI 1.2–9.8, p=0.02) and higher grades of inflammation on liver biopsy (OR 7.9, 95% CI 1.2–53.4, p=0.03) were independently associated with IFG, and adjusting for age, sex, or HCV genotype did not significantly affect these odds ratios (Table 3). In comparing IGT (with or without IFG) to NGT subjects, on univariable analysis, non-Caucasian race (OR 11.6, 95%CI 2.4–55.6, p=0.002), BMI (OR 2.7 per 5 units, 95%CI 1.3–5.5, p=0.005), and SSPG (OR 1.2 per 10 units, 95%CI 1.06–1.3, p=0.001) were associated with IGT. Among non-Caucasians, only African Americans and Hispanics/Latinos had IGT, and each of these racial groups were positively associated with IGT when compared to Caucasians (African American OR 9.5, 95%CI 1.6–56.4, Hispanic/Latino OR 21.4, 95%CI 3.9–118.3). On multivariable stepwise forward selection analysis comparing IGT (with or without IFG) to NGT subjects, non-Caucasian race (OR 14.8, 95% CI 1.7–127.3, p=0.01) and increasing SSPG concentrations (OR 1.1 per 10 units, 95% CI 1.02–1.2, p=0.01) were independent predictors of IGT, and adjusting for age, sex, BMI, or HCV genotype did not significantly alter the observed odds ratios (Table 3).
Table 3.
Multivariable Logistic Regression Assessment of Viral and Host Factors Associated with IFG and IGT
| IFG | ||
|---|---|---|
| Characteristic | Unadjusted Odds Ratio (95% CI) | *Adjusted Odds Ratio (95% CI) |
| Non-Caucasian Race** (vs. Caucasians) | 23.1 (2.8–187.0) | 24.7 (3.0–206.3) |
| Inflammation on Liver Biopsy (Grade ≥ 2 vs. < 2) | 7.9 (1.2–53.4) | 11.0 (1.4–87.0) |
| BMI (per 5 units), kg/m2 | 3.4 (1.2–9.8) | 3.1 (1.07–8.9) |
| IGT (Isolated IGT or IFG/IGT) | ||
| Characteristic | Unadjusted Odds Ratio (95% CI) | †Adjusted Odds Ratio (95% CI) |
| SSPG (per 10 units), mg/dL | 1.1 (1.02–1.2) | 1.1 (1.01–1.2) |
| Non-Caucasian Race** (vs. Caucasians) | 14.8 (1.7–127.3) | 19.7 (1.7–224.3) |
Adjusted for age, sex, and HCV genotype.
Adjusted for age, sex, BMI, and HCV genotype.
Each of the non-Caucasian racial categories had a positive association with pre-diabetes and these races were combined due to small sample size to improve the precision of the point estimates.
BMI, body mass index; HCV, hepatitis C virus; SSPG, steady-state plasma glucose.
DISCUSSION
In this study, we found high rates of pre-diabetes in our HCV population, similar to the prevalence observed in the general population. Non-Caucasian race predicted both IFG and IGT; BMI and liver inflammation predicted IFG; and insulin resistance was associated with IGT (IGT or combined IFG/IGT). In addition, both early phase and total insulin secretion (in relation to the degree of insulin resistance) were decreased in pre-diabetic states compared to individuals with NGT.
Studies in HCV-uninfected individuals have shown increasing age and BMI, male sex, Latino/Hispanic ethnicity, insulin resistance and dyslipidemia as risk factors for pre-diabetes.(3, 18, 19) Similarly, higher BMI, Latino ethnicity, and higher degrees of insulin resistance were associated with pre-diabetes in HCV. However, whereas African American race has not been associated with pre-diabetes(3), African Americans with HCV had high rates of pre-diabetes. Moreover, unlike prior studies of HCV-uninfected individuals, age and sex were not associated with pre-diabetes in HCV. These findings suggest that there may be a different phenotype of pre-diabetes in the HCV-infected population.
The precise nature of the complex interaction of host and viral factors that leads to the development of impaired glycemic control in patients with chronic HCV infection is poorly understood. Although inconsistent, several studies suggest that HCV alters glucose homeostasis by interfering with insulin signaling through mechanisms that may be genotype-specific and influenced by higher levels of viral replication.(5, 20) In addition, advanced stages of liver disease and steatosis have been associated with insulin resistance in HCV.(5, 21) In this study, viral factors, including HCV viral load, duration of infection, and genotype were not predictive of pre-diabetes. Furthermore, fibrosis and steatosis were not associated with pre-diabetes, possibly due to the fact that the majority of subjects had mild-to-moderate degrees of liver fibrosis and steatosis. However, the presence of liver inflammation was independently associated with IFG. HCV infection can directly induce insulin-signaling defects in the liver that lead to hepatic insulin resistance(22, 23), which is thought to be the principal metabolic abnormality in individuals with IFG.(16)
Different pre-diabetic states have unique insulin and glucose responses to oral glucose and whether these responses are altered within the context of HCV has not been previously assessed. In this study, the glucose and insulin responses during OGTT (Figure 1) mimicked those observed in the HCV-uninfected population.(16) Accordingly, greater degrees of peripheral insulin resistance were detected among HCV-infected subjects with IGT as compared with NGT and IFG subjects. Moreover, consistent with prior studies in the HCV-uninfected individuals, (25, 26) the early phase insulin response was impaired among those with IFG and IGT. Limited studies evaluating total insulin secretion adjusted for degree of insulin resistance have suggested that patients with IGT have a greater degree of impairment than patients with IFG.(25) In HCV, the most significant reduction in total insulin secretion was evident in the combined IFG/IGT group compared to subjects with NGT. However, overall, the abnormalities in insulin action and secretion characteristic of pre-diabetic states do not appear to be significantly altered by HCV infection.
Similar to other studies incorporating direct measurements of insulin resistance (25, 27), this study is limited by a small sample size. However, accurate assessment of peripheral insulin resistance by direct measurement using IST allows for adequate comparisons and performing this test would be impractical in a larger patient population. Since the prevalence of pre-diabetic states and their pathophysiology have been extensively studied in the general population with a similar mean age (48 vs 46 years) and BMI (27 vs 28 kg/m2) to this HCV cohort, a control group of HCV-negative subjects was not included.(3) However, this study has allowed for confirmation of prior findings or identification of distinguishing features associated with pre-diabetes within the context of HCV infection.
The pre-diabetic states are highly prevalent in the HCV population. Given its potential to disrupt insulin signaling pathways, HCV may accelerate progression of these intermediate states of glucose homeostasis. As such, early identification of pre-diabetes in the HCV population is needed to prevent the development of overt diabetes and its complications. Moreover, treatment of HCV infection may be indicated in patients with additional risk factors for diabetes. The findings of this study suggest that host factors play a more significant role than viral factors in the development of pre-diabetes among HCV-infected patients. However, HCV-induced liver inflammation was shown to be associated with IFG, and HCV infection may represent a significant risk factor for this condition. As such, aggressive HCV therapy in these individuals may be warranted. Furthermore, this study confirms that the abnormalities in insulin action characteristic of pre-diabetic states are preserved in the presence of chronic HCV infection. This finding supports future studies investigating the role of targeted pharmacologic treatment of pre-diabetes in HCV-infected patients.
Acknowledgements
Financial Support:
Declaration of funding interests: This work was supported by National Institute of Health Grant numbers R01 DK074673 (M.K.), UL1 RR024131 (NIH/NCRR UCSF-CTSI), P30 DK026743 (UCSF Liver Center), and the American Diabetes Foundation Grant number 1-08-CR-30 (M.K.).
Abbreviations
- HCV
hepatitis C virus
- OGTT
oral glucose tolerance test
- SSPG
steady-state plasma glucose determined by the insulin suppression test
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- NGT
normal glucose tolerance
- DM
type II diabetes mellitus
- HOMA-IR
homeostasis model assessment of insulin resistance
- FPG
fasting plasma glucose
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- G-AUC
area under the curve of glucose response following a 75g oral glucose load during oral glucose tolerance testing
- I-AUC
area under the curve of insulin response following a 75g oral glucose load during oral glucose tolerance testing
- IST
insulin suppression test
- SSPI
steady-state plasma insulin determined by the insulin suppression test
- ALT
alanine aminotransferase
- BMI
body mass index
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
Footnotes
Conflict of Interest: No conflicts of interest exist.
Authors declaration of personal interests: Authors have nothing to disclose.
References
- 1.NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002 Jun 10–12;19(3):1–46. [PubMed] [Google Scholar]
- 2.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000 Oct 17;133(8):592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 3.Karve A, Hayward RA. Prevalence, diagnosis, and treatment of impaired fasting glucose and impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care. 2010 Nov;33(11):2355–2359. doi: 10.2337/dc09-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004 Oct 25;164(19):2147–2155. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 5.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003 Dec;125(6):1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005 Mar;128(3):636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Khalili M, Lim JW, Bass N, Ascher NL, Roberts JP, Terrault NA. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl. 2004 Mar;10(3):349–355. doi: 10.1002/lt.20092. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007 Mar;30(3):753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care. 2009 Nov;32(Suppl 2):S194–S198. doi: 10.2337/dc09-S309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasso A, Malfatti F, De Leo P, Martines H, Fabris P, Toscanini F, et al. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha 2b plus ribavirin. J Hepatol. 2009 Dec;51(6):984–990. doi: 10.1016/j.jhep.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Lam KD, Bacchetti P, Abbasi F, Ayala CE, Loeb SM, Shah V, et al. Comparison of surrogate and direct measurement of insulin resistance in chronic hepatitis C virus infection: impact of obesity and ethnicity. Hepatology. 2010 Jul;52(1):38–46. doi: 10.1002/hep.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003 Jan;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 13.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995 Dec;19(12):1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin T, Deng A, Gonzales O, Aillaud M, Yee G, Lamendola C, et al. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural] 2008 Dec;51(12):2303–2308. doi: 10.1007/s00125-008-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994 Apr;11(3):286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006 May;29(5):1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007 Jun;30(6):1544–1548. doi: 10.2337/dc06-1331. [DOI] [PubMed] [Google Scholar]
- 18.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998 Apr;21(4):518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 19.Festa A, D'Agostino R, Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes. 2004 Jun;53(6):1549–1555. doi: 10.2337/diabetes.53.6.1549. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CS, Liu CJ, Liu CH, Wang CC, Chen CL, Lai MY, et al. High hepatitis C viral load is associated with insulin resistance in patients with chronic hepatitis C. Liver Int. 2008 Feb;28(2):271–277. doi: 10.1111/j.1478-3231.2007.01626.x. [DOI] [PubMed] [Google Scholar]
- 21.Fartoux L, Poujol-Robert A, Guechot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005 Jul;54(7):1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004 Nov;165(5):1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003 Dec;38(6):1384–1392. doi: 10.1016/j.hep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004 Mar;126(3):840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006 May;55(5):1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 26.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care. 2003 Mar;26(3):868–874. doi: 10.2337/diacare.26.3.868. [DOI] [PubMed] [Google Scholar]
- 27.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 1999 Nov;48(11):2197–2203. doi: 10.2337/diabetes.48.11.2197. [DOI] [PubMed] [Google Scholar]