Summary
Jahid and colleagues have demonstrated that miR-23a promotes the transition from indolent to invasive colorectal cancer through inhibition of the MTSS1 tumor suppressor. This study reveals a novel role of miR-23a in the acceleration of colorectal cancer progression.
Keywords: Colorectal cancer, miR-23a, miR-27a, FBXW7, MTSS1
Human colorectal cancer (CRC) is a highly aggressive malignantdisease, which remains the fourth most common human cancer and the second leading cause of cancer-related mortality in the United States. It is estimated that approximately 103,170 new CRC cases will be diagnosed and 51,690 deaths will occur in 2012 (1). Despite recent therapeutic advances, patients are often diagnosed with CRC at late stages that have already metastasized either as micro or macro metastatic disease. Because metastasis is the leading cause of treatment failure and tumor recurrence, there is an urgent need for achieving earlier diagnosis and developing innovative treatment strategies for improving the overall treatment outcome of this deadly disease (1).
Although the molecular mechanisms for CRC metastasis are not fully elucidated, accumulating evidence suggests that microRNAs (miRNAs) could play a critical role. miRNAs bind to the 3′untranslated region (UTR) of target mRNAs, subsequently leading to target mRNA translational repression or degradation (2). Intriguingly, certain miRNAs with deregulated expression display anti-tumor activities in human malignances, whereas others exhibit oncogenic activities. Furthermore, unlike the “one mRNA one transcribed protein” theme, a single miRNA could regulate multiple mRNA targets, and one given gene could also be governed by several different miRNAs (2).
Recent studies have demonstrated that several miRNAs including miR-23a and miR-27a are involved in the development and progression of human cancers although details of the underlying molecular mechanisms remain unclear and may be context-dependent. Numerous studies have shown that expression of miR-23a is suppressed by c-Myc in prostate cancer and lymphoma cells and is down-regulated by the oncogenic PML-RARA (promyelocytic leukemia protein-retinoic acid receptor alpha) fusion protein in acute promyelocytic leukemia (3). In contrast, other reports have documented up-regulation of miR-23a in a variety of human cancers including bladder cancer, gastric cancer, glioblastoma, hepatocellular carcinoma, breast cancer, and pancreatic cancer (3). miR-27a has been shown to exert an oncogenic function in human tumorigenesis. For example, in breast cancer, miR-27a exhibits oncogenic activity through down-regulating zinc finger ZBTB10 protein, a putative SP (specificity proteins) repressor, leading to over-expression of SP and SP-dependent pro-survival and pro-angiogenic genes including survivin, VEGF, VEGFR1 (VEGF receptor 1) (4). Moreover, down-regulation of miR-27a decreases the percentage of breast cancer cells in the S phase. Consistent with this finding, down-regulation of miR-27a inhibited the proliferation of gastric cancer cells which was in part mediated through inhibiting Cyclin D1 and up-regulating p21 expression (5). Interestingly, miR-27a was also found to be involved in drug resistance through regulation of the expression of the drug transporter MDR1 (P-glycoprotein), a protein implicated in paclitaxel resistance in a variety of human cancers. However, the roles of miR-23a and miR-27a in the development and progression of CRC have not been mechanistically investigated.
In this issue of Cancer Discovery, Jahid and colleagues (6) revealed the novel roles of miR-23a and miR-27a in CRC progression. To determine whether these two miRNAs are deregulated in human CRC, they measured their expression in CRC clinical tissue samples at different malignant stages. They found that miR-27a displayed higher expression in all tumor tissues, whereas miR-23a expression was more restricted to invasive CRC. Consistent with this, the authors also showed that both miR-23a and miR-27a expression levels are increased in the mouse model with intestinal invasive adenocarcinomas (6). Since colon cancer stem cells (CCSC) have been characterized to play a critical role in the invasion and metastasis processes, this group performed LNA-microarray based miRNA profiling of CCSC. As expected, both miR-23a and miR-27a were highly expressed in CCSC as well as in the invasive non-CCSC CRC cell lines. Taken together, miR-23a and miR-27a are highly expressed in CRC cell lines and mouse intestinal tumor as well as in human CRC tumor tissue specimens.
In order to further determine the molecular mechanisms by which miR-27a exerts its oncogenic functions in CRC, the authors used several molecular approaches to reveal FBXW7 as a direct target of miR-27a (6). FBXW7 is a well-studied E3 ubiquitin ligase that is reported to target various oncogenic proteins for ubiquitination, including cyclin E, Notch, Mcl-1, c-Myc, and c-Jun (7) and is considered a tumor suppressor largely due to its negative regulation of the stability of these oncogenic proteins. Indeed, it has been demonstrated that FBXW7 is frequently inactivated by mutation, deletion or promoter hypermethylation in multiple neoplasms including colon cancer (7). In addition, FBXW7 mutation was found in 11% of colorectal cancer. Lerner et al. found that miR-27a suppresses FBXW7 expression during specific cell cycle phases, and promotes the degradation of proteins regulating G1 to S-phase transition, such as Cyclin E They also showed that over-expression of miR-27a induces DNA replication stress and causes Cyclin E dysregulation in part through inhibition of the FBXW7 expression (8). Moreover, Wang and colleagues also independently discovered that FBXW7 is a potential miR-27a target and that the regulation of FBXW7 substrates such as Cyclin E, c-Jun and Notch1 might account for the observed elevation of cell growth arising from specific inhibition of FBXW7 mediated by over-expressing miR-27a (9). Consistent with previous reports, Jahid et al. confirmed that the E3 ubiquitin ligase FBXW7 is a direct miR-27a target in CRC and showed that depletion of endogenous miR-27a leads to the down-regulation of oncogenic FBXW7 substrates such as Myc, Jun, and Notch (6). Taken together, miR-27a was demonstrated to function as an oncogene, which is in part mediated through regulating the expression of the FBXW7 tumor suppressor in CRC.
As for the physiological role of miR-27a, knockdown by LNA (lockednucleotide analog) or shRNA led to inhibited CCSC growth and suppressed clonogenecity in vitro. Conversely, miR-27a over-expression promoted cell growth and increased clonogenicity in CRC. Furthermore, miR-27a knockdown and significantly retarded in vivo tumor growth in a mouse xenograft model.
Interestingly, miR-23a knockdown had modest and minimal effect on cell proliferation and clonogenicity, respectively. Consistent with this notion, miR-23a knockdown did not cause a reduction in tumor volume in vivo either. Taken together, these results suggest that miR-27a, but not miR-23a, promotes cell proliferation in CRC both in vivo and in vitro. Having excluded a role for miR-23a in cell proliferation, the authors continued to further dissect a role for miR-23a in CCSC cell migration and invasion (6). To this end, one recent study has shown that miR-23a promotes colon cancer cell growth, invasion and metastasis through inhibiting MTSS (Metastasis suppressor) gene expression (10). Furthermore, the up-regulation of miR-23a expression was associated with an advanced clinical stage and the depth of invasion as well as lymph node metastasis, indicating that miR-23a could be a bio-marker for the prognosis of CRC. In line with this finding, Jahid et al. found that miR-23a knockdown inhibited the cell motility, cell migration and invasion (6). Moreover, they demonstrated that suppression of migration and invasion by miR-23a knockdown is primarily due to up-regulation of its target MTSS1 and downstream inhibition of SRC signaling pathway (6), indicating that miR-23a could play an important role in cell migration and invasion, leading to the development of invasive CRC.
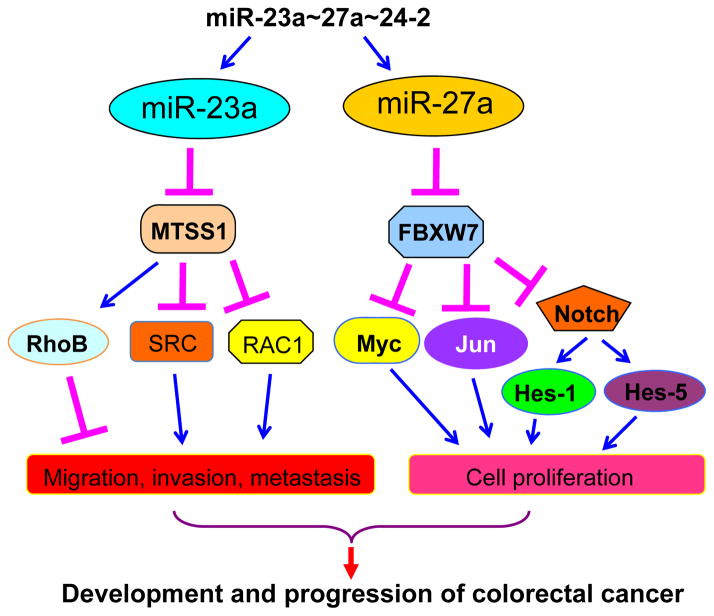
To further determine the roles of miR-23a and miR-27a in metastasis, Jahid and colleagues (6) performed an in vivo assay of metastasis by injection of cells into the tail vein of immunodeficient mice. They showed that both miR-23a and miR-27a knockdown led to fewer lung tumors. Moreover, knockdown of miR-23a or miR-27a increased overall survival of mice. Altogether, they obtained experimental evidence to support the notion that miR-23a primarily increases cell motility, while miR-27a mainly promotes cell proliferation (Figure 1).
Figure 1. The critical roles of miR-23a and miR-27a in CRC progression.
miR-23a primarily increases cell motility through down-regulation of its target MTSS1 and up-regulation of the SRC signaling pathway, while miR-27a promotes cell proliferation in part via inhibition of FBXW7, leading to the up-regulation of FBXW7 ubiquitin substrates including Myc, Jun, and Notch.
These interesting studies shed light on the roles of miR-23a and miR-27a in CRC progression; however several questions still need to be addressed in follow up studies. For example, why was miR-23a expression lower in the late stage of CRC patients? Why there is high expression of miR-27a target genes even through miR-27a levels do not decrease in CRC? Why did expression of cyclin E, a FBXW7 target, not change after miR-27a knockdown? Are there other additional miRNAs involved in CRC progression? Could miR-27a and miR-23a be biomarkers of prognosis for CRC, or only restricted to invasive CRC? Without a doubt, further studies will be ignited and are warranted to determine the physiological functions of miR-23a and miR-27a in the development and progression of CRC.
Although further investigation is required to fully answer the questions raised above, the results reported by Jahid et al. open a new avenue to target miR-23a and miR-27a for clinical benefits. Intriguingly, one recent study has shown that natural compound-derivatives could decrease the expression of miR-27a in CRC cells (11) and inhibit cell growth and induction of apoptosis, suggesting that miR-27a could be a useful therapeutic target for CRC. We anticipate that establishing oncogenic roles of miR-23a and miR-27a in CRC progression will thus open new research directions for clinical management of this deadly disease.
Acknowledgments
Some component of the work has been supported by grants from the National Cancer Institute, NIH (5R01CA131151, 1R01CA132794 and 1R01CA154321 to F.H.S), and from the National Institute of General Medicines, NIH (GM089763 and GM094777 to W.W.). W.W is an American Cancer Society Scholar. Z. W is supported by the NIH NRSA fellowship.
Footnotes
Disclosure of Potential Conflicts of Interest.
No potential conflicts of interests were disclosed.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nature reviews Cancer. 2011;11:849–64. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chhabra R, Dubey R, Saini N. Cooperative and individualistic functions of the microRNAs in the miR-23a~27a~24–2 cluster and its implication in human diseases. Molecular cancer. 2010;9:232. doi: 10.1186/1476-4598-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X, Yang L, Hu J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. J Exp Clin Cancer Res. 2011;30:55. doi: 10.1186/1756-9966-30-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahid SS, Edwards J, Dizon RA, Sikandar D, Gumus SS, HLipkin ZSM. miR-23a promotes the transition from indolent to invasive colorectal cancer. Cancer Discovery. 2012 doi: 10.1158/2159-8290.CD-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 8.Lerner M, Lundgren J, Akhoondi S, Jahn A, Ng HF, Moqadam FA, et al. miRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle. 2011:10. doi: 10.4161/cc.10.13.16248. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Li DC, Li ZF, Liu CX, Xiao YM, Zhang B, et al. Upregulation of miR-27a contributes to the malignant transformation of human bronchial epithelial cells induced by SV40 small T antigen. Oncogene. 2011;30:3875–86. doi: 10.1038/onc.2011.103. [DOI] [PubMed] [Google Scholar]
- 10.Tang HL, Deng M, Liao QJ, Zeng X, Zhou XT, Su Q. Expression and clinical significance of miR-23a and metastasis suppressor 1 in colon carcinoma. Zhonghua bing li xue za zhi Chinese journal of pathology. 2012;41:28–32. [PubMed] [Google Scholar]
- 11.Chintharlapalli S, Papineni S, Abdelrahim M, Abudayyeh A, Jutooru I, Chadalapaka G, et al. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18beta-olean-1,12-dien-30-oate in colon cancer cells. International journal of cancer Journal international du cancer. 2009;125:1965–74. doi: 10.1002/ijc.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]