Abstract
Brain mechanisms underlying explicit evaluation of emotion have been explored using different tasks including ‘stimulus-focused evaluation’, ‘evaluation of one's own emotion’ and ‘evaluation of others’ emotions’. Yet the extent to which similar brain mechanisms underlie different evaluation tasks is unclear. A meta-analysis of published neuroimaging studies of explicit emotional evaluation was conducted to examine common and distinct regions underlying these different evaluation tasks. This study revealed regions common to all three tasks: The amygdala and LPFC as common regions may be involved in emotion–cognition interactions, and the DMPFC may possibly play integrative roles in explicit emotional evaluation. Distinct regions were also identified: (i) the sensory cortex and VLPFC were specifically associated with ‘stimulus evaluation’, possibly involved in perceptual and conceptual processing; (ii) the insula and rACC were specifically associated with ‘evaluation of one's own emotion’, potentially associated with interoceptive and experiential processing; and (iii) the STS and TPJ were specifically associated with ‘evaluation of others’ emotions’, potentially reflecting their roles in TOM and empathy. These findings suggest that different types of explicit emotional evaluation may involve common and distinct networks and provide new insights on multiple mechanisms underlying explicit emotional evaluation.
Keywords: explicit evaluation, emotion, neuroimaging, meta-analysis
INTRODUCTION
This study examines brain mechanisms associated with different types of explicit emotional evaluation. We define explicit evaluation of emotion as the processes of how people interpret, evaluate and judge emotional meanings and value (either valence or discrete emotions) of objects (Cunningham et al., 2003; Barrett, 2005; Nielsen and Kaszniak, 2007). During explicit evaluation of emotion, people evaluate emotional value in conscious and deliberate manners and verbalize their responses. Explicit evaluation of emotion allows people to label their emotions, and to express or report current or past emotions, so that they can explain their emotions and share them with other people. However, due to limitations of an imaging environment, explicit emotional evaluation should be simplified and restricted; for example, subjects are asked to evaluate a specific emotional value (e.g. valence, happy and sad, or emotional intensity) under specific task instructions and respond by pressing a button in the scanner, a task termed ‘on-line’ in this article.
Neuroimaging studies have identified brain regions that are activated more by explicit than implicit/automatic emotional processing (for a review, see Phan et al., 2002; Hutcherson et al., 2005). However, results are inconsistent; for example, (i) increased activation (Liberzon et al., 2000; Gorno-Tempini et al., 2001; Lee et al., 2004; Vollm et al., 2006), decreased activation (e.g. Critchley et al., 2000; Hariri et al., 2000; Taylor, Phan, Decker et al., 2003), or no different activation in the amygdala (e.g. Hutcherson et al., 2005) in explicit emotional evaluation compared to control conditions; and (ii) increased activation in the medial prefrontal cortex (MPFC; Taylor et al., 2003) and in the lateral PFC (e.g. Gorno-Tempini et al., 2001; Lee et al., 2004), or both PFC regions (e.g. Hutcherson et al., 2005).
One potential reason for such discrepancies is that previous studies have used different explicit evaluation tasks, which may depend on diverse brain networks, to examine underlying brain mechanisms of explicit evaluation of emotion. The extent to which similar brain mechanisms underlie such different evaluation tasks is unclear. Perhaps there are central shared mechanisms for explicit evaluation of emotion regardless of evaluation tasks or distinct mechanisms depending on emotional evaluation tasks.
In particular, previous studies have used tasks that ask participants to explicitly evaluate (i) the emotionality of stimuli/situations (e.g. pushing a button for whether a picture or word is positive, negative, or neutral in tone; for example, Critchley et al., 2000; Hariri et al., 2000; Narumoto et al., 2000), (ii) one's own emotional states (e.g. ‘How do you feel?’ Gusnard et al., 2001; Ochsner et al., 2004a; Hutcherson et al., 2005) and (iii) other people's emotional states (e.g. ‘How does the person of this picture feel?’) (e.g. Mitchell et al., 2005; Jackson et al., 2006; Vollm et al., 2006). Answers to these questions may differ. For example, a picture of an evil-doer being apprehended may simultaneously yield a positive feeling for the observer, but an assertion that the evil-doer is feeling upset.
Two lines of evidence suggest the specific importance of these three domains. Developmental psychologists emphasize that emotional knowledge acquired by engaging with physical stimuli/situations, one's own body and other people is important for normal emotional development (Trevarthen and Aitken, 2001). Self-report studies also demonstrate that people describe specific situations, their own internal states and other people's internal states when they talk about emotion (Davitz, 1969; Stein and Levine, 1999; Barrett et al., 2001). Thus, the following sections will concentrate on these three domains.
Emotion–cognition interactions are hypothesized to be common to all three types of emotional evaluation (Frijda and Zeelenberg, 2001; Roseman and Smith, 2001; Scherer, 2001; Lewis, 2005). Thus, we hypothesized that neural substrates of interactions between cognition and emotion would be common to all three tasks. Such interactions, from emotional evaluation to emotion regulation to emotional interference with basic cognitive processes are subserved by several brain regions which were used as a priori structures in the present investigation. These included several prefrontal cortex (PFC) regions including the caudal anterior cingulate cortex (cACC), ventromedial, dorsomedial and dorsolateral prefrontal cortices (VMPFC, DMPFC, DLPFC), and limbic regions such as amygdala, thalamus, hippocampus and orbitofrontal cortex (OFC; see Table 1; for a review, see Lewis, 2005).
Table 1.
Summary of hypothesized brain regions associated with theoretical accounts
| Theoretical accounts | Possible brain networks (BA) |
|---|---|
| General mechanisms | Common brain networks |
| Emotion–cognition interactive processing | PFC regions (e.g. LPFC, MPFC) |
| Subcortical-Limbic regions (e.g. amygdala) | |
| Evaluative and regulatory processing | DMPFC (BA10/32) and PCC |
| Task-dependent mechanisms | Distinct brain networks |
| Stimulus-focused evaluation | |
| Perceptual processing | Sensory-related brain regions (e.g. FFA) |
| High-level of conceptual processing | VLPFC (BA47) |
| Evaluation of one's own emotion | |
| Interoceptive processing | Insula (BA13) and rACC (BA24/32) |
| Experiential processing | rACC (BA24/32) |
| Evaluation of other's emotions | |
| Inferential processing (Theory of Mind) | STS (BA22/39) and TPJ (BA39/40) |
| Empathetic processing (Perspective-taking) | STS and TPJ |
BA: Brodmann Area; LPFC: Lateral Prefronal Cortex (PFC); MPFC: Medial PFC; DMPFC: Dorsomedial PFC; PCC: Posterior Cingulate Cortex; FFA: Fusiform Face Area; VLPFC: Ventrolateral PFC; rACC: rostral Anterior Cingulate; STS: Superior Temporal Sulcus; TPJ: Temporo-Parietal Junction.
In addition, the three specific evaluation tasks may also each recruit distinct mechanisms.
For example, stimulus-focused evaluation may focus on physical features and meaning of stimuli/situations (e.g. Arnold, 1960; Roseman, 1984) and include interpretation of perceived information in terms of conceptual knowledge of stimuli in the representation and memory system (e.g. Leventhal and Scherer, 1987; Smith and Kirby, 2001). Perceptual processing may rely on modality of a stimulus or types of stimuli, indicating that this processing activates sensory cortex regions relevant to the modality such as visual stimuli (i.e. faces and words) and visual cortex (i.e. face fusiform area: FFA; for example, Adolphs, 2002a, b). Higher level of conceptual processing is associated with the ventrolateral PFC (VLPFC: BA47), a core brain region involved in explicit evaluation of a stimulus (Cunningham et al., 2004) and evaluation of object knowledge (e.g. Mitchell et al., 2002). Thus, this evidence suggests the sensory cortices and VLPFC (BA47) may play a key role in evaluating emotionality of stimuli/situations.
In contrast, evaluation of one's own emotion may involve inferring emotional experience from inner states and conscious thoughts about current and past experience. Evaluative processing of one's own emotion may thus include evaluation of bodily experience (interoception), conscious thoughts and a felt action tendency (for a review, see Lane, 2000; Lambie and Marcel, 2002). The insula is a key structure associated with subjective awareness of inner states (Craig, 2002, 2004) and has been implicated in subjective interoceptive and emotional states (Critchley et al., 2004). The rostral ACC (rACC) has also been implicated in the representation of conscious emotional experience (Lane, 2000; Lane and McRae, 2004; Barrett et al., 2007). Therefore, the insula and rACC are hypothesized to play specific roles in evaluating one's own emotional experience.
Evaluation of others’ emotions involves understanding others’ psychological properties including beliefs, intention and emotion. Theory of Mind (TOM) suggests that people have mental state concepts such as belief and intention, so they use this explicit knowledge or rules to infer others’ mental states (for a review, see Gallese and Goldman, 1998). Another theoretical account, simulation theory (ST), proposed that people put themselves ‘in the other person's shoes’ to infer other people's mental states (Gallese and Goldman, 1998; Goldman and Sripada, 2005). Similarly, empathy requires perspective taking and simulation of others’ emotions (e.g. Decety and Jackson, 2004). Neuroimaging studies have revealed brain regions associated with TOM and ST. The superior temporal sulcus (STS) is implicated in understanding of others’ intentionality (Frith and Frith, 1999; Gallagher and Frith, 2003) and the temporal poles and temporo-parietal junction (TPJ) are involved in reasoning about others’ mental states (Blakemore et al., 2004; Saxe, 2006). Thus, these brain regions may be particularly associated with evaluation of others’ emotions.
To summarize, well-documented theoretical and empirical evidence allows us to hypothesize brain networks underlying explicit emotional evaluation. Therefore, our meta-analytic study focused on mainly these hypothesized brain regions. Table 1 presents theoretical accounts and possible brain networks associated with general/shared and task-dependent mechanisms underlying explicit evaluation of emotion which served as our hypotheses.
To understand the extent to which such shared and distinct regions are associated with different types of emotional evaluation, we conducted a quantitative meta-analytic review of published neuroimaging studies. Neuroimaging studies were, therefore, categorized into three different tasks to evaluate emotion consciously including (i) evaluating the emotionality of a stimulus (stimulus-focused emotion), (ii) evaluating one's own emotion and (iii) evaluating others’ emotions. Overlapping brain regions among neuroimaging studies using different evaluation tasks were considered as shared/common brain mechanisms. Distinct regions among these studies were interpreted as task-dependent mechanisms.
METHODS
Studies were identified primarily by searching the PUBMED database. Keywords such as emotion, evaluation, cognition and imaging (fMRI and PET) were used to find neuroimaging studies related to emotion and evaluation, which yielded over 100 studies available until 2006. Another search was performed with more specific keywords such as identification, recognition, judgment, self-reports, experience and empathy. A final search reviewed reference lists in the identified papers was performed. The title and abstract of each study were checked to decide whether the study used online evaluation tasks to assess brain mechanisms of explicit emotional evaluation.
Papers were included if they met the following criteria: (i) They involved healthy participants; (ii) They used on-line tasks to evaluate emotions which subjects evaluated explicitly emotion in the scanner; (iii) They used trial-based emotional evaluation (e.g. evaluation per trial/stimulus) in either block or event-related designs; (iv) They collected imaging data from the whole brain and used either whole-brain analysis or both ROI-based and whole-brain analysis; (v) They reported standard Talairach (Talairach and Tournoux, 1988) or Montreal Neurologic Institute (MNI) coordinates. The 37 studies identified were assigned into three groups. Studies were categorized as stimulus-focused evaluation (STIM) if they used tasks to focus on evaluating emotionality of stimuli including explicit identification, judgment or labelling of emotional stimuli. Studies were categorized as evaluation of one's own emotion (SELF) if they used an evaluation task which required subjects to pay attention to their own emotional states. Studies were categorized as evaluation of others’ emotions (OTHER) if they used any tasks that give instructions about evaluating others’ emotion and pain or about judging empathy of other people.
Table 2 quantitatively summarizes methodological information including the sample size, evaluation tasks, types and modalities of stimuli, emotions and contrasts in the identified studies. Specific tasks of emotional evaluation were broadly assigned to four categories including ‘identification’, ‘labeling’, ‘matching’ and ‘intensity’ tasks. The identification task was defined as any tasks demanding subjects to assess stimuli, their own emotion or others’ emotions based on emotional valence (e.g. positive and negative), discrete emotions (e.g. happy, sad and fear) or social emotions (e.g. shame). The labelling task was involved in judging stimuli by two linguistic labels that were presented on the screen (Hariri et al., 2000). In the matching task, subjects matched sampled faces/words with one of two faces (Narumoto et al., 2000). The intensity task included any tasks if the subjects evaluated emotional intensity of stimuli, their own emotion (arousal) or others’ emotions. As summarized in Table 2, most studies have used either identification or intensity tasks, but the STIM more frequently used identification than intensity task, whereas two other groups used both tasks equally. The STIM group was more heterogeneous in stimulus modality including auditory and olfactory compared to the two other groups. In addition, the STIM and OTHER groups were more diverse in emotions such as discrete emotions and social emotions than the SELF group. Most studies contrasted explicit conditions with implicit conditions (e.g. passive viewing or gender task) whereas some studies contrasted emotion conditions with neutral conditions. Some studies also used both contrast methods.
Table 2.
List of neuroimaging studies in this meta-analytic study
| Imaging studies | Imaging Tech | N | Specific Task |
Stimulus |
Emotion |
Contrast |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (First author) | Vis |
Aud |
Olf/Tas | Dimension |
Pain | Soc | Discrete emotions |
|||||||||||||||||||||||||
| Iden | Label | Mat | Int | Fa | W | Pic | Film | Sent | W | Sent | Snd | Pos | Neg | Ar | H | S | D | F | Ang | Sur | Ex vs I | E vs N | ||||||||||
| STIM (n = 20) | ||||||||||||||||||||||||||||||||
| Nakamura | PET | 7 | o | o | o | o | o | |||||||||||||||||||||||||
| Hariri | fMRI | 16 | o | o | o | o | o | o | ||||||||||||||||||||||||
| Narumoto | fMRI | 8 | o | o | o | o | o | o | o | o | o | |||||||||||||||||||||
| Critchley | fMRI | 9 | o | o | o | o | o | |||||||||||||||||||||||||
| Gorno-Tempini | fMRI | 10 | o | o | o | o | o | |||||||||||||||||||||||||
| Lange | fMRI | 9 | o | o | o | o | ||||||||||||||||||||||||||
| Winston | fMRI | 12 | o | o | o | o | o | o | o | |||||||||||||||||||||||
| Paradiso | PET | 17 | o | o | o | o | o | |||||||||||||||||||||||||
| Liberzon | PET | 10 | o | o | o | o | o | |||||||||||||||||||||||||
| Taylor | PET | 10 | o | o | o | o | o | |||||||||||||||||||||||||
| Grimm | fMRI | 29 | o | o | o | o | o | |||||||||||||||||||||||||
| Tabert | fMRI | 9 | o | o | o | o | ||||||||||||||||||||||||||
| Maddock | fMRI | 8 | o | o | o | o | o | |||||||||||||||||||||||||
| Cunningham | fMRI | 24 | o | o | o | o | o | |||||||||||||||||||||||||
| Imaizumi | PET | 6 | o | o | o | o | o | o | o | |||||||||||||||||||||||
| Wildgruber | fMRI | 10 | o | o | o | o | ||||||||||||||||||||||||||
| Wildgruber | fMRI | 10 | o | o | o | o | o | o | o | o | ||||||||||||||||||||||
| Royet | PET | 12 | o | o | o | o | o | o | o | o | ||||||||||||||||||||||
| Royet | PET | 12 | o | o | o | o | o | o | ||||||||||||||||||||||||
| Royet | fMRI | 28 | o | o | o | o | o | |||||||||||||||||||||||||
| Subtotals | 12 | 1 | 1 | 7 | 7 | 3 | 5 | 0 | 0 | 1 | 2 | 1 | 3 | 8 | 11 | 1 | 0 | 0 | 6 | 3 | 5 | 5 | 5 | 3 | 16 | 7 | ||||||
| SELF (n = 9) | ||||||||||||||||||||||||||||||||
| Lane | PET | 10 | o | o | o | o | o | |||||||||||||||||||||||||
| Gusnard | fMRI | 24 | o | o | o | o | o | |||||||||||||||||||||||||
| Ochsnera | fMRI | 13 | o | o | o | o | o | |||||||||||||||||||||||||
| Lee | fMRI | 10 | o | o | o | o | o | |||||||||||||||||||||||||
| Phan | fMRI | 12 | o | o | o | o | o | |||||||||||||||||||||||||
| Garrett | fMRI | 9 | o | o | o | o | ||||||||||||||||||||||||||
| Jacksona | fMRI | 18 | o | o | o | o | ||||||||||||||||||||||||||
| Hutcherson | fMRI | 28 | o | o | o | o | o | |||||||||||||||||||||||||
| Rubya | PET | 10 | o | o | o | o | ||||||||||||||||||||||||||
| Subtotals | 5 | 0 | 0 | 4 | 0 | 0 | 7 | 1 | 1 | 0 | 0 | 0 | 0 | 5 | 6 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 7 | 2 | ||||||
| OTHER (n = 8) | ||||||||||||||||||||||||||||||||
| Mitchell | fMRI | 18 | o | o | o | o | ||||||||||||||||||||||||||
| Ochsnera | fMRI | 13 | o | o | o | o | o | |||||||||||||||||||||||||
| Jackson | fMRI | 15 | o | o | o | o | ||||||||||||||||||||||||||
| Jacksona | fMRI | 18 | o | o | o | o | ||||||||||||||||||||||||||
| Vollm | fMRI | 15 | o | o | o | o | ||||||||||||||||||||||||||
| Lawrence | fMRI | 12 | o | o | o | o | o | |||||||||||||||||||||||||
| Hynes | fMRI | 20 | o | o | o | o | ||||||||||||||||||||||||||
| Rubya | PET | 10 | o | o | o | o | ||||||||||||||||||||||||||
| Subtotals | 4 | 0 | 0 | 4 | 1 | 0 | 4 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 7 | 1 | ||||||
| Specific Task |
Stimulus Type |
Emotion |
Contrast |
|||||||||||||||||||||||||||||
| Vis |
Aud |
Olf/Tas | Dimension |
Pain | Soc | Discrete |
||||||||||||||||||||||||||
| Iden | Label | Mat | Int | Fa | W | Pic | Film | Sent | W | Sent | Snd | Pos | Neg | Ar | H | S | D | F | Ang | Sur | Ex vs I | E vs N | ||||||||||
| Totals | 21 | 1 | 1 | 15 | 8 | 3 | 16 | 2 | 3 | 1 | 2 | 1 | 3 | 15 | 18 | 1 | 3 | 4 | 8 | 4 | 5 | 5 | 6 | 3 | 30 | 10 | ||||||
Imaging Tech = Imaging Technique, Specific Task: Iden. = Identification Task, Label = Labelling Task, Mat = Matching Task, Int = Intensity Task, Stimulus: Vis = Visual, Aud = Auditory, Olf/Tas = Olfactory/Taste, Fa = Face, W = Word, Pic = Picture, Sent = Sentence, Snd = Sound, Emotion: Pos = Positive, Neg = Negative, Ar = Arousal, Soc = Social, H = Happy, S = Sad, D = Disgust, F = Fear, Ang = Anger, Sur = Surprise, Contrast: Ex = Explicit Condition, I = Implicit/other control (e.g., rest) Condition, E = Emotional Condition, N = Neutral Condition. (i) Lists of studies in STIM (Imaizumi et al., 1997; Nakamura et al., 1999; Paradiso et al., 1999; Critchley et al., 2000; Hariri et al., 2000; Liberzon et al., 2000; Narumoto et al., 2000; Royet et al., 2000; Gorno-Tempini et al., 2001; Royet et al., 2001; Tabert et al., 2001; Lange et al., 2003; Maddock et al., 2003; Royet et al., 2003; Taylor et al., 2003; Winston et al., 2003; Cunningham et al., 2004; Wildgruber et al., 2004; Wildgruber et al., 2005; Grimm et al., 2006); (ii) Lists of studies in SELF (Gusnard et al., 2001; Lee et al., 2004; Ochsner et al., 2004a; Phan et al., 2004; Ruby and Decety, 2004; Garrett and Maddock, 2006; Jackson et al., 2006; Lane et al., 1997; Hutcherson et al., 2005); (iii) Lists of studies in OTHER (Ochsner et al., 2004a; Ruby and Decety, 2004; Jackson et al., 2005; Mitchell et al., 2005; Hynes et al., 2006; Lawrence et al., 2006; Jackson et al., 2006; Vollm et al., 2006).
aThe studies that examine two evaluation tasks and directly compare their effects.
To determine whether hypothesized brain regions were activated in each study, reported coordinates were checked manually. The coordinates were recorded separately in the left and right side of a priori ROIs for this study (see Table 1). The ROIs were defined based on the Brodmann Areas (BA) and previous literature (e.g. Bush et al., 2000; Amodio and Frith, 2006). For example, rACC (BA24/32) and cACC (BA24) were defined based on the boundaries used in Bush and colleagues (2000). The coordinates in each study were checked for possible location on one of the ROIs. If several coordinates were reported in the same brain regions, one coordinate with the highest statistical values (e.g. z scores or t values) was selected as a representative to control the relative contributions by each study. Relative distance among these coordinates within the same regions was not considered to avoid some possible biases by a coder in picking the coordinates. Figure 1 shows all the regions of interests and coordinates (peaks) in these regions. Coordinates from neuroimaging studies that reported Montreal Neurological Institute (MNI) brain were transformed to Talairach coordinate system (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) and were double checked using the AFNI program (Analysis of Functional NeuroImages; Cox, 1996).
Fig. 1.
The boundaries of a priori ROIs in this study and activation peaks colour-coded based on three different groups of emotional evaluation.
A meta-analysis was performed using the Activation Likelihood Estimate (ALE) method (Turkeltaub et al., 2002), implemented in BrainMap (Laird et al., 2005) using the coordinates in regions listed in Table 1. The ALE values for each voxel were computed using a 3D Gaussian probability density function with FWHM = 10 mm, as recommended by Laird et al. The voxel-wise significance of the ALE values was determined by a permutation test using 5000 permutations (Laird et al., 2005). The images of P values for each voxel were corrected for multiple comparisons using false discovery rates (FDR), at P < 0.05 corrected. The final step was to identify clusters of voxels exceeding 100 mm3 in volume, as commonly used in the GingerALE software (http://brainmap.org).
Separate meta-analyses were conducted with the STIM, SELF and OTHER groups. Overlapping brain maps were drawn via conjunction analysis on the three maps derived from the separate meta-analyses. To identify distinct brain regions associated with individual evaluation tasks, meta-analytic contrast maps were created using a subtraction meta-analysis (Laird et al., 2005). The contrast maps showed regions in which the two groups of foci are significantly different. Conjunction maps between two contrast maps (e.g. SELF vs STIM and SELF vs OTHER) were also created to identify unique distinct regions of each specific group (e.g. SELF) that are commonly different from the two other groups (STIM and OTHER).
RESULTS
Common networks associated with explicit evaluation of emotion
To elucidate common brain regions associated with explicit emotional evaluation, we found brain regions associated with each emotional evaluation task separately and then identified overlapping area commonly activated by three different types of explicit emotional evaluation.
The results from the separate meta-analysis of the 141 coordinates identified in 20 studies (32 contrasts) of ‘STIM’ showed activation in several prefrontal regions (VLPFC [BA47], DMPFC [BA32], DLPFC [BA9] and IFG [BA46]), visual cortices (fusiform and ligual gyrus, BA19) and subcortical-limbic regions (PCC [BA31], thalamus, amygdala and amygdala extending to the parahippocampal gyrus; Table 3). The separate map was created using 61 coordinates in nine studies (14 contrasts) of SELF including rACC (BA32), DMPFC (BA10), insula (BA13), LPFC (BA45/47), cACC and amygdala (Table 4). The map from the separate meta-analysis of 59 coordinates in eight studies (12 contrasts) of OTHER group showed activation in the STS (BA39), TPJ (BA39), DMPFC (BA10), precuneus/PCC (BA31), LPFC (BA44/47), VLPFC (BA47) and amygdala (Table 5).
Table 3.
Reported foci resulting from meta-analysis in stimulus-focused evaluation (voxel-wise P < 0.05; FDR corrected)
| Region | Side | BA | x | y | z | Volume (mm3) | Maximum ALE Value |
|---|---|---|---|---|---|---|---|
| Cingulate gyrus | R | 32 | 2 | 28 | 30 | 6128 | 0.0170 |
| Inferior frontal gyrus | L | 47 | –28 | 26 | –10 | 5072 | 0.0090 |
| Inferior frontal gyrus | R | 47 | 44 | 28 | –10 | 4480 | 0.0111 |
| Amygdala | L | –24 | –6 | –14 | 2672 | 0.0150 | |
| Thalamus (ventral lateral nucleus) | L | –10 | –10 | 6 | 2264 | 0.0092 | |
| Amygdala extending to parahippocampal gyrus | R | 24 | –6 | –12 | 2224 | 0.0124 | |
| Inferior frontal gyrus | L | 9 | –48 | 10 | 28 | 1720 | 0.0115 |
| Posterior cingulate | L | 31 | –6 | –54 | 24 | 1696 | 0.0089 |
| Middle frontal gyrus | R | 9 | 50 | 20 | 28 | 840 | 0.0065 |
| Superior temporal gyrus | L | 22 | –62 | –44 | 14 | 816 | 0.0071 |
| Lingual gyrus | L | 19 | –16 | –44 | –2 | 624 | 0.0067 |
| Inferior occipital gyrus | R | 19 | 44 | –78 | –4 | 592 | 0.0076 |
| Lingual gyrus | R | 19 | 18 | –58 | –2 | 528 | 0.0064 |
| Middle frontal gyrus | R | 11 | 24 | 50 | –10 | 440 | 0.0057 |
| Medial frontal gyrus | L | 32 | –6 | 10 | 44 | 424 | 0.0073 |
| Posterior cingulate | R | 30 | 4 | –60 | 8 | 328 | 0.0059 |
| Lingual gyrus | L | 19 | –22 | –62 | –2 | 256 | 0.0060 |
| Fusiform gyrus | L | 19 | –46 | –66 | –14 | 200 | 0.0056 |
| Inferior frontal gyrus | R | 46 | 50 | 30 | 12 | 160 | 0.0051 |
| Anterior cingulate | L | 24 | –6 | 24 | –6 | 144 | 0.0054 |
| Medial frontal gyrus | R | 11 | 4 | 58 | –14 | 120 | 0.0047 |
Table 4.
Reported foci resulting from meta-analysis in evaluation of one's own emotion (voxelwise P < 0.05; FDR corrected)
| Region | Side | BA | x | y | z | Volume (mm3) | Maximum ALE |
|---|---|---|---|---|---|---|---|
| Medial frontal gyrus | L | 10 | –2 | 50 | 12 | 8312 | 0.0104 |
| Insula | L | 13 | –46 | 11 | 2 | 4560 | 0.0080 |
| Inferior frontal gyrus | R | 45 | 48 | 20 | 10 | 4120 | 0.0057 |
| Amygdala | L | –20 | –6 | –12 | 1184 | 0.0069 | |
| Cingulate gyrus | R | 32 | 8 | 14 | 40 | 1056 | 0.0071 |
| Thalamus (medial dorsal nucleus) | R | 4 | –18 | 12 | 888 | 0.0061 | |
| Cingulate gyrus | L | 24 | 0 | –2 | 34 | 520 | 0.0045 |
| Middle frontal gyrus | R | 47 | 48 | 36 | –8 | 480 | 0.0050 |
| Cingulate gyrus | L | 32 | –4 | 26 | 26 | 448 | 0.0043 |
| Thalamus (pulvinar) | R | 4 | –28 | 2 | 112 | 0.0040 | |
| Medial frontal gyrus | R | 8 | 4 | 48 | 42 | 104 | 0.0039 |
Table 5.
Reported foci resulting from meta-analysis in evaluation of others’ emotions (voxelwise P < 0.05; FDR corrected)
| Region | Side | BA | x | y | z | Volume (mm3) | Maximum ALE |
|---|---|---|---|---|---|---|---|
| Precuneus | L | 31 | –10 | –56 | 34 | 3312 | 0.0080 |
| Superior frontal gyrus | L | 9 | –8 | 54 | 28 | 3136 | 0.0081 |
| Medial frontal gyrus | R | 10 | 2 | 56 | 10 | 2080 | 0.0066 |
| Superior temporal gyrus | R | 39 | 48 | –52 | 24 | 1592 | 0.0078 |
| Medial frontal gyrus | R | 11 | 4 | 50 | –18 | 1560 | 0.0052 |
| Fusiform gyrus | L | 20 | –58 | –4 | –26 | 1496 | 0.0083 |
| Superior temporal gyrus | L | 39 | –50 | –60 | 18 | 1480 | 0.0069 |
| Amygdala extending to parahippocampal gyrus | L | –22 | –2 | –18 | 592 | 0.0051 | |
| Inferior frontal gyrus | L | 47 | –54 | 28 | –4 | 552 | 0.0062 |
| Cuneus | L | 18 | 0 | –78 | 14 | 504 | 0.0043 |
| Middle frontal gyrus | L | 46 | –46 | 18 | 24 | 120 | 0.0040 |
| Inferior frontal gyrus | L | 44 | –46 | 12 | 16 | 112 | 0.0042 |
In short, amygdala, LPFC and DMPFC seemed to activate commonly in all three conditions of explicit emotional evaluation although slightly different sizes and locations of activation were identified within these regions. Overlapping brain regions were created using three maps generated by separate meta-analyses. This confirmed that some parts of these brain regions overlapped across different evaluation conditions, providing evidence that some activation peaks in each group were located near the overlapping area. Thus, these overlapping areas represent higher probability of common involvement in different types of emotional evaluation. However, activation peaks in broad regions (e.g. LPFC) were more distributed and spatially distinguishable compared to peaks in small regions (e.g. amygdala), so activation peaks in broad regions may be separable. To understand whether similar numbers of peaks from each group were located in the regions (suggesting common functionality), Figure 2 displays overlapping regions (A) and individual peaks (B) in the DMPFC, left LPFC and left amygdala. Because observed peak distributions largely overlapped across tasks, the amygdala, LPFC and DMPFC were considered common brain networks involved in explicit emotional evaluation irrespective of the different types of explicit evaluation tasks.
Fig. 2.
Overlapping areas representing common brain networks underlying explicit evaluation of emotion. (A) The overlapping areas included the DMPFC, LPFC and amygdala. Different colours represent brain regions associated with different evaluation tasks (Blue: brain regions activated by stimulus-focused evaluation (STIM), Red: brain regions associated with evaluation of one's own emotion (SELF), Green: brain areas associated with evaluation of others’ emotions (OTHER), Yellow: Overlapping areas commonly associated with three tasks (OVERLAP) and Triangle: Overlapping areas between STIM and OTHER. (B) All reported peaks near the overlapping areas. (Abbreviations: DMPFC = Dorsomedial Prefrontal Cortex, LPFC = Lateral Prefrontal Cortex).
Distinct networks depending on different evaluation task
To examine which brain regions were specifically involved in individual evaluation tasks, three contrast meta-analytic maps (STIM vs SELF, STIM vs OTHER and SELF vs OTHER) were created using the same procedures, yielding brain regions which showed significant differences between two comparison conditions. Conjunction maps between two contrast maps represented distinct regions commonly more activated by one specific group than two other groups. Table 6 summarizes identified foci in each conjunction map showing significant differences between one group and two other groups.
Table 6.
Reported foci resulting from conjunction analyses (voxelwise P < 0.05; FDR corrected)
| Regions | Side | BA | x | y | z | Volume (mm3) | Maximum ALE |
|---|---|---|---|---|---|---|---|
| Conjunction map of stimulus-focused: ‘STIM’ greater than ‘SELF’ and ‘STIM’ greater than ‘OTHER’ | |||||||
| Cingulate gyrus | R | 32 | 2 | 30 | 30 | 2048 | 0.014 |
| Inferior frontal gyrus | R | 47 | 46 | 26 | –12 | 2000 | 0.009 |
| Amygdala | R | 24 | –6 | –12 | 1744 | 0.012 | |
| Amygdala | L | –26 | –6 | –14 | 656 | 0.009 | |
| Ventral lateral nucleus | L | –10 | –10 | 6 | 1160 | 0.009 | |
| Posterior cingulate gyrus | L | 31 | –6 | –54 | 22 | 1072 | 0.008 |
| Inferior frontal gyrus | L | 9 | –48 | 10 | 28 | 816 | 0.010 |
| Inferior frontal gyrus | L | 47 | –28 | 24 | –10 | 664 | 0.008 |
| Superior temporal gyrus | L | 22 | –62 | –44 | 14 | 656 | 0.007 |
| Parahippocampal gyrus | L | 19 | –16 | –44 | –4 | 368 | 0.006 |
| Lingual gyrus | R | 19 | 18 | –58 | –2 | 312 | 0.006 |
| Inferior frontal gyrus | R | 9 | 52 | 8 | 24 | 224 | 0.006 |
| Middle frontal gyrus | R | 11 | 24 | 50 | –10 | 192 | 0.006 |
| Medial frontal gyrus | L | 32 | –6 | 10 | 46 | 192 | 0.007 |
| Anterior cingulate gyrus | L | 32 | –18 | 40 | –10 | 176 | 0.006 |
| Posterior cingulate gyrus | R | 30 | 4 | –60 | 8 | 168 | 0.006 |
| Inferior frontal gyrus | L | 13 | –42 | 24 | 6 | 168 | 0.006 |
| Lingual gyrus | L | 19 | –22 | –62 | –2 | 136 | 0.006 |
| Inferior occipital gyrus | R | 19 | 44 | –78 | –4 | 112 | 0.006 |
| Conjunction map of one's own emotion: ‘SELF’ greater than ‘STIM’ and ‘SELF’ greater than ‘OTHER’ | |||||||
| Anterior gingulate gyrus | L | 24/32 | –2 | 46 | 8 | 296 | 0.007 |
| Cingulate gyrus | R | 32 | 8 | 14 | 40 | 216 | 0.007 |
| Insula extending to the Inferior Frontal gyrus | L | 13/47 | –50 | 14 | 0 | 208 | 0.006 |
| Conjunction map of others’ emotions: ‘OTHER’ greater than ‘STIM’ and ‘OTHER’ greater than ‘SELF’ | |||||||
| Superior temporal gyrus | R | 39 | 48 | –52 | 24 | 488 | 0.008 |
| Temporo-parietal junction | L | 39 | –50 | –60 | 18 | 256 | 0.007 |
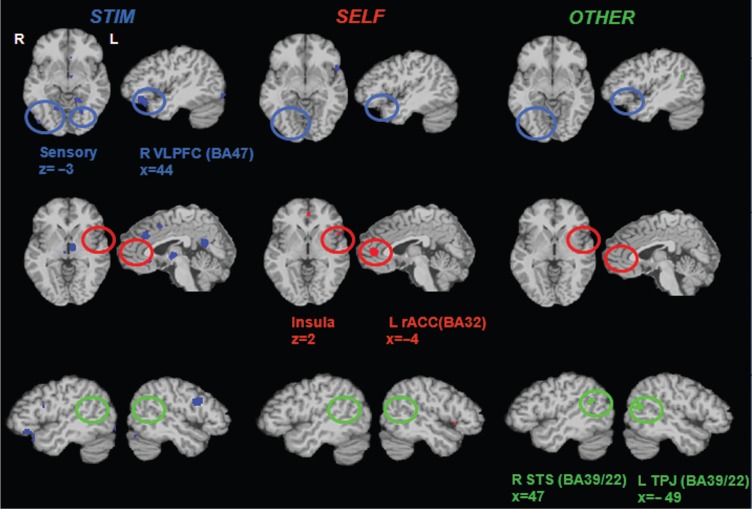
In the conjunction map of STIM vs SELF and STIM vs OTHER, studies of stimulus-focused evaluation showed more activation in prefrontal regions (e.g. DMPFC [BA32], VLPFC [BA47], DLPFC [BA9]), sensory regions (e.g. visual and temporal cortices) and subcortical regions (e.g. thalamus and parahippocampus gyrus) compared to both studies of evaluation of one's own emotion and of others’ emotions, suggesting that these regions are differentially involved in evaluation of emotional stimuli (Table 6). Figure 3 shows activation clusters in the right VLPFC (BA47) and visual cortex hypothesized regions specifically associated with evaluation of stimuli. Consistent with our hypothesis, sensory cortices and VLPFC (BA47) were revealed as distinct brain regions specifically associated with evaluation of emotional stimuli.
Fig. 3.
Contrast meta-maps derived from the comparisons among three different tasks to assess brain mechanisms associated with explicit evaluation of emotion (first column: STIM, second column: SELF and third column: OTHER). Explicit evaluation of emotion engages distinct brain networks depending on different evaluation tasks. First row shows the results in the sensory (visual) cortex and VLPFC. Activation in these regions was identified in only stimulus-focused evaluation condition. Second row displays the results in the insula and rACC. Activation in both regions was revealed in only evaluation of one's own emotion condition. Third row presents the findings in the STS and TPJ. Both regions were activated by only evaluation of others’ emotions condition. These results suggest that some brain regions are involved in specific types of emotional evaluation. (Abbreviations: L = left, R = right, VLPFC = ventrolateral prefrontal cortex, rACC = rostral anterior cingulate cortex, STS = superior temporal sulcus, TPJ = temporo-parietal junction).
The conjunction map of SELF vs STIM and SELF vs OTHER demonstrated that brain regions such as the rACC (BA24/32) and insula (BA13) near the IFG are more likely associated with evaluation of one's own emotion compared to other groups (Table 6). Identified clusters in the rACC and left insula extending to the IFG are displayed in Figure 3. Consistent with our hypotheses, the rACC and insula were specifically involved in evaluation of one's own emotional states.
Brain regions including the STS and TPJ were hypothesized to specifically be associated with evaluation of other people's emotions. The STS (BA39) and TPJ (BA39) were commonly activated by studies of evaluation of others’ emotions compared to studies of two other tasks (Table 6). Figure 3 presents activation clusters in the right STS and left TPJ. This result also supports the hypothesis that the STS and TPJ are involved in evaluation of others’ emotions via inferring emotional states in others.
Volume-wise differences for regions of interest
The ALE method provides relative peak concentration in a given region for each group which allows for the possibility that differences between tasks are apparent when two tasks activate different parts of the same ROI. To examine whether activation frequencies within the entire volume of each region of interest were significantly different across the three groups of studies, χ2 and Fisher's exact tests were conducted. Data were coded as 1 or 0 if the distinct regions were activated or not in each group, respectively. Figure 4 displays percentages of activation peaks in each group and χ2 results. Activation frequencies in the sensory, VLPFC, insula, rACC and STS were significantly different among three task groups, but activation frequencies in the TPJ were marginally different among groups. These χ2 results were consistent with ALE results. Follow-up 2 × 2 comparisons were conducted. Except for the comparison between STIM vs OTHER in the VLPFC, most 2 × 2 comparisons showed significant or marginally significant differences in activation frequencies.
Fig. 4.
Comparison of activation frequencies across evaluation task groups (STIM, SELF and OTHER). χ2 results in each region were displayed (Fisher's exact tests were reported in the parenthesis). Follow-up 2 × 2 comparisons were reported (x = non significant, + P < 0.10, *P < 0.05 and **P < 0.01).
Potential confounds
Finally, potential confounds were examined using a logistic regression analysis. One group of emotional evaluation was more diverse than the other groups in stimulus type (e.g. face, word, film and picture) and emotions (e.g. valence, discrete emotions, social emotions and pain). A logistic regression was performed to examine whether these factors would predict activation in the distinct regions. Each study was coded based on whether the evaluation task was performed according to valence, discrete emotions, social emotions and pain and further based on whether the task was performed with presentation of specific stimulus types such as face, word, film and picture. The emotion factor did not predict activation in the VLPFC (Wald χ2 = 0.05, P = 0.975), sensory (Wald χ2 = 0.51, P = 0.774), insula (Wald χ2 = 4.17, P = 0.124), rACC (Wald χ2 = 1.38, P = 0.50) except for the STS (Wald χ2 = 7.17, P < 0.05) and TPJ (Wald χ2 = 6.08, P < 0.05). Emotion predicted activation in the STS and TPJ because studies which showed activation in the STS and TPJ used particularly pain and social emotions. However, only three studies reported activation in the STS and TPJ, so this factor did not have sufficient variance to account for activation. Stimulus type did not significantly predict activation in any distinct regions (VLPFC: Wald χ2 = 2.99, P = 0.392; sensory: Wald χ2 = 7.24, P = 0.065; insula: Wald χ2 = 2.60, P = 0.785; rACC: Wald χ2 = 1.07, P = 0.785; STS: Wald χ2 = 4.51, P = 0.211; TPJ: Wald χ2 = 0.987, P = 0.804). Thus, these potential factors did not confound our results. However, one should interpret such findings with caution because this method to control for confound variables is useful when many studies reported activation in brain regions (Wager et al., 2007).
DISCUSSION
A meta-analysis was conducted to elucidate common and distinct brain networks associated with general and task-specific mechanisms of explicit emotional evaluation. Consistent with our hypotheses, results suggest that the amygdala, LPFC (BA47) and DMPFC (BA10/32) are involved in general mechanisms underlying the explicit evaluation of emotion, whereas some brain regions are involved in task-specific mechanisms associated with specific evaluation tasks. Task instructions for cognitive evaluation of emotional stimuli might recruit sensory and VLPFC (BA47) regions. Conscious evaluation of one's own emotion might activate the insula (BA13) and rACC (BA24), whereas evaluation of other people's emotions might be associated with activation in the STS (BA39) and TPJ (BA39).
General brain mechanisms of explicit emotional evaluation
We hypothesized that cognition–emotion interactions involving evaluative and regulatory processing, subserved by prefrontal and limbic regions, would be shared mechanisms associated with many types of explicit evaluation of emotion. In support of this idea, three hypothesized regions, the amygdala, LPFC and DMPFC were observed as common to all three emotional evaluation tasks.
The amygdala is involved in a broad range of emotional processing including perceptual processing (e.g. recognition of facial expressions; Adolphs, 2002a), encoding emotional arousal (e.g. Anderson et al., 2003) and evaluation of emotional valence (Paradiso et al., 1999; Liberzon et al., 2000). The amygdala appears to participate in fast and automatic processing of emotion (LeDoux, 1996; Adolphs, 2002b). The amygdala may thus be involved in the initial stages in the process of explicit emotional evaluation such as encoding of emotional information and initial emotional reactivity to emotional information.
The LPFC is associated with memory retrieval (e.g. Wagner et al., 2001; Bunge et al., 2004) and cognitive control including selective attention (Bishop et al., 2004; Cardillo et al., 2004; Bedwell et al., 2005). Similarly, the LPFC may guide selective attention to the given task of explicit emotional evaluation and may maintain the task instruction information to accomplish the desired consequences of conscious evaluation of emotion.
The DMPFC (BA10/32) is connected with other brain regions associated with emotional and cognitive processing (e.g. amygdala, LPFC and cACC), possibly reflecting a role in emotion–cognition integration (Paradiso et al., 1999; Taylor et al., 2003; Lieberman et al., 2007). Explicit emotional evaluation also includes evaluative processing depending on individuals’ internal value systems, associated with DMPFC activity (Cunningham et al., 2004). The DMPFC has also been implicated in emotion regulation that may occur after conscious evaluation of emotion (Ochsner et al., 2004b; Banks et al., 2007).
To summarize, the amygdala, LPFC and DMPFC (BA10/32) appear commonly associated with explicit emotional evaluation. Divergent experimental designs across studies may prevent identification of other overlapping regions. Potentially, the amygdala is involved in initial emotional processing of explicit evaluation, whereas the LPFC more specifically subserves cognitive aspects of evaluation. These regions could interact via the DMPFC, a convergent region involved in integrative and evaluative processing.
Task-dependent brain mechanisms of explicit emotional evaluation
We examined underlying brain mechanisms distinctively associated with three explicit emotional tasks that required stimulus-focused evaluation, and focusing on one's own emotion and other people's emotions.
Sensory cortex and VLPFC (BA47) were associated with evaluation of stimuli/situations in accordance with our hypothesis. Presumably, such stimulus-focused evaluation requires perceptual processing of physical features, yielding recruitment of sensory cortex (visual and temporal cortices) in the evaluation of emotional stimuli (Paradiso et al., 1999; Critchley et al., 2000; Gorno-Tempini et al., 2001; Taylor et al., 2003). The VLPFC (BA47) is associated with evaluative judgment (Cunningham et al., 2003), appraisal of object knowledge (Mitchell et al., 2002) and declarative knowledge about emotional stimuli (Schaefer et al., 2003), possibly responsible for higher-level of conceptual processing of emotional stimuli.
Also consistent with our hypothesis, the insula (BA13) and rACC were specifically associated with evaluation of one's own emotion. The insula has been associated with perception of inner state changes such as interoceptive processing, mediating conscious emotional experience (Craig, 2002; Critchley et al., 2004). The rACC (BA24/32) is also involved in subjective emotional experience (e.g. Lane, 2000).
Finally, consistent with our hypotheses, the STS and TPJ were involved in evaluation of other people's emotions. Both regions are associated with social affective judgment including simulation and empathetic processing as well as social cognitive judgment including understanding of and reasoning others’ intention and thoughts (Frith and Frith, 1999; Ochsner et al., 2004a; Ruby and Decety, 2004; Vollm et al., 2006).
Although we found distinct regions associated with three different tasks, it is possible that there are common mechanisms associated with both stimulus-focused evaluation and evaluation of others’ emotions using faces. Potentially, evaluating the emotionality (e.g. angry or sad) for face stimuli could make people evaluate emotional states of the subjects whose faces are being viewed. But it is also possible that this task is more dependent on perception and can be accomplished without inferring others’ emotional states. In contrast, explicitly demanding people evaluate emotional states of others by presenting only faces is likely to recruit mechanisms associated with TOM. Consistently, past research on empathy has attempted to distinguish evaluating emotions based on more perceptual cues (e.g. stimulus) from evaluating emotions based on inferences about others’ emotions (Eisenberg et al., 1997; Graham and Ickes, 1997). Our distinct regions also supported the idea that stimulus-focused evaluation is specifically associated with the visual cortex; however, evaluation of others’ emotions is specifically associated with the STS and TPJ, although the precuneus/PCC and DMPFC are overlapped between two tasks (Figure 2).
The ALE and χ2 results broadly agreed, but two-way χ2 and ALE were slightly different. It is possible that ALE and χ2 results are different because the ALE method provides relative peak concentration in a given region for each group whereas χ2 results provide absolute differences in activation frequencies across groups (Wager et al., 2007). Thus, a region-wide analysis using χ2 does not detect significant differences in activation frequencies among groups, whereas a finer spatial resolution analysis using ALE does detect different activation clusters if groups differ in the subregions of a single structure they activate. For example, χ2 did not show significant differences in the TPJ among groups, but ALE detected TPJ as a distinct region associated with evaluation of others’ emotions. As shown in Figure 5, peaks of OTHER group were placed on different subregions of the TPJ compared to peaks of STIM group. Therefore, activation frequencies in this region might not be significantly different among the groups whereas the cluster of subregions might be detected as a distinct region associated with evaluation of others’ emotions.
Fig. 5.
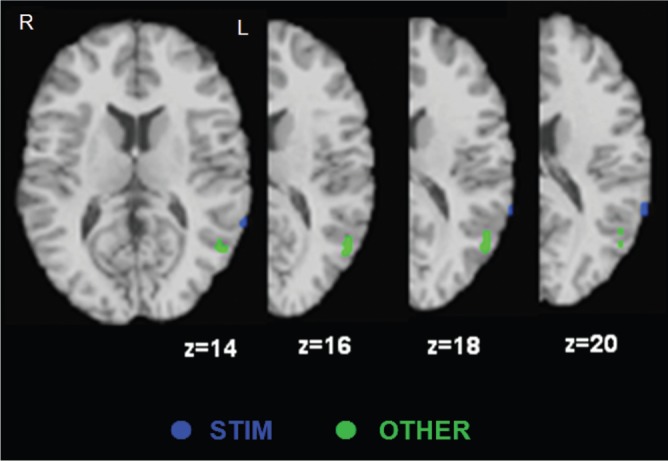
Peaks activated by STIM and OTHER in the TPJ. Peaks in two groups were distinguishable in the TPJ.
In summary, different tasks to assess underlying processing of explicit emotional evaluation recruited distinct brain networks suggested by theoretical accounts. Distinct brain networks may be specialized for specific emotional evaluation, so these networks are necessary to accomplish specific evaluation tasks.
Multiple processes in explicit evaluation of emotion
Findings from our meta-analysis supported the notion that explicit emotional evaluation is associated with shared mechanisms common to all types of emotional evaluation such as PFC–subcortical interactions, as well as different mechanisms specifically involved in different types of emotional evaluation. PFC–subcortical interactions are commonly acknowledged in imaging research on explicit emotional evaluation. Adding distinct brain mechanisms associated with specific tasks, in particular self vs other evaluation, sheds additional light on understanding the process of explicit emotional evaluation.
There are several reasons why the distinct mechanisms associated with evaluation of three objects should be considered in explicit emotional evaluation. First, as mentioned earlier, developmental psychologists suggest that engagements with people, other physical objects (e.g. stimuli and situations) and self are critical in normal emotional development (Trevarthen and Aitken, 2001). Abnormal development of one distinct mechanism can cause specific affective developmental disorders such as relationships between other-specific mechanisms and autistic spectrum disorder (ASD). Second, different types of explicit emotional evaluation can be used to detect affective disorders such as ASD, alexithymia and psychopathy (for a review, see Blair, 2008a, b; Decety and Moriguchi, 2007). Individuals with ASD exhibit problems with distinct mechanisms associated with evaluating others’ emotions whereas individuals with alexithymia exhibit abnormality in distinct mechanisms associated with evaluating one's own emotional states. Thus, distinct mechanisms associated with different types of evaluation contribute to the understanding of mechanisms in affective disorder.
Third, distinct mechanisms of different evaluation based on objects to be evaluated may be differentially associated with other emotional processes such as emotional regulation (Ochsner et al., 2004b). Consistent with our findings, Ochsner and colleague reported distinct functions in the VLPFC and VMPFC (rACC), associated with situation-focused and self-focused regulation, respectively. Specific distinct mechanisms may be associated with particular types of emotion regulation such as reappraisal and suppression. For example, reappraisal (e.g. reinterpreting emotional situations) may be more likely associated with stimulus-focused evaluation whereas suppression (e.g. inhibiting one's own emotional behaviour) may be more likely associated with self. Thus, distinct mechanisms of emotional evaluation contribute to the understanding of other emotional processing.
Possible interactive model
Both common and distinct networks associated with explicit evaluation of emotion were observed. Based on theoretical framework and these results, we suggest the interactive model in Figure 6, which depicts salient interactions between regions subserving common and more distinct functions for different types of explicit emotional evaluation. Of course, the model could include many more connections and reciprocal interactions; rather, our attempt here is to illustrate some of the most conceptually salient connections which tell a story suggested by the meta-analytic data and well-known functions in the regions.
Fig. 6.
Hypothetical interactive model of explicit emotional evaluation. Dotted lines represent hypothesized precedence based on the task design (i.e. task instructions are presented before stimuli and responses are generated after some amount of processing). The bi-directional arrow between common input and output regions reflects the extensive literature documenting functional and anatomical connectivity between these regions. Connections to regions associated with distinct functions are less well specified in the literature, and thus future research is necessary to establish directionality of relationships.
In our model and in all types of explicit emotional evaluation, the amygdala is hypothesized to be involved in encoding emotional information and initial responses to the information, and the LPFC is hypothesized to manage selective attention to guide a desired task performance. The DMPFC possibly plays a role in bridging between the common input regions (amygdala and LPFC; Lieberman et al., 2007). Integrative information in the DMPFC could further be fed back to the amygdala and LPFC subserving cognition–emotion interactions. Task-dependent mechanisms would be required to accomplish additional processing demanded by specific task instructions. For example, in the case of stimulus-focused evaluation, the sensory cortices and VLPFC would be recruited for perceptual and conceptual processing of stimuli. We assume that these task-dependent mechanisms would operate for specific processes related to particular task demand, possibly after early common mechanism but before late common mechanism.
Future research could test this hypothesized interactive model using different types of emotional evaluation tasks within the same fMRI design, controlling for experimental factors such as stimulus characteristics. Our model's dynamic interactions and directionality among hypothesized common and distinct regions could specifically be examined via analyses of functional and effective connectivity. Reciprocal connectivity between the amygdala, LPFC and DMPFC is predicted to show a good fit to the fMRI data independent of particular evaluation tasks. Modulation by specific evaluation tasks would be supported if (i) a specific task is associated with increased relationships between early common regions and specific task-dependent regions; and (ii) enhanced activity in the specific networks alters connectivity among the common networks, ideally by feed-forward signals to the DMPFC, which consequently interacting with amygdala and LPFC.
In general, the reciprocal and causal connectivity suggests that underlying mechanisms of explicit emotional evaluation can be better understood within more distributed neural networks. This concept is consistent with recent suggestions that broader and more distributed networks would be more appropriate for elucidating emotion–cognition interactive mechanisms (Taylor and Liberzon, 2007; Pessoa, 2008). This model also demonstrates one example of how subtle differences in task instructions could contribute to alteration of neural circuits associated with psychological mechanisms. Therefore, careful selection of task instructions is suggested.
Limitations and concluding remarks
There are several limitations of this review. We included coordinates in only hypothesized brain regions for this meta-analysis, so we did not examine how other brain regions are associated with different types of conscious emotional evaluation. There is also a difference in the number of studies in three assigned groups. The group of stimulus-focused evaluation included more studies (20 studies) than two other groups (nine and eight studies in evaluation of one's own emotion and others’ emotions, respectively). This may cause additional brain regions including the DLPFC and thalamus associated with stimulus-focused evaluation. The ALE technique does not account for the size and shape of activity in each study. Thus, differential roles among adjacent regions such as the insula and LPFC may not adequately be separated by this technique. In addition, nearly many of the examined tasks used stimuli for which the nominal interpretation of the stimulus would be consistent among any of the three tasks which were required. For example, a picture of a crying person would be evaluated as negative and might make the observer feel sad in addition to provoking an empathetic response. Thus, regions labelled as common may reflect processes that occurred regardless of the nominal task as a function of the stimulus, but are not actually common to these tasks. Experiments using stimuli that are evaluated differently from different perspectives (e.g. stimuli in which the observer would take pleasure in the subject's misfortune) could be helpful in this regard.
Despite these limitations, the proposed framework integrates evidence across a variety of theoretical accounts and neuroimaging studies of explicit emotional evaluation. We suggest that explicit evaluation of emotion is not a unitary process but instead multiple processes mediated by shared/common and distinct mechanisms. The review has important implications for the investigation of understanding mechanisms underlying explicit evaluation of emotion. Given the different brain responses associated with different tasks, instructions for emotional evaluation tasks should be specific in terms of particular research interests in specific mechanisms underlying explicit evaluation. This framework may also have clinical implications for understanding mechanisms of affective disorders such as alexithymia and autism. These disorders are often considered to reflect general deficits in emotional evaluation. Potentially, by carefully assessing evaluation of different domains (e.g. self vs other) pockets of preserved competence could be identified.
Acknowledgments
We thank Walter Schneider, Julie Fiez and William Klein, for comments on previous versions of this manuscript. This work was supported by N00014-05-1-0881 and MH074807.
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002a;12:169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002b;1:21–61. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews in Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Arnold MB. Emotion and Personality: Vol.1 Psychological Aspects. |. New York: Columbia University Press; 1960. [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Feeling is perceiving. In: Barrett LF, Niedenthal PM, Winkielman P, editors. Emotion and Consciousness. New York: The Guilford Press; 2005. pp. 255–84. [Google Scholar]
- Barrett LF, Gross J, Christensen TC, Benvenuto M. Knowing what you are feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition & Emotion. 2001;15:713–24. [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Reviews in Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell JS, Horner MD, Yamanaka K, et al. Functional neuroanatomy of subcomponent cognitive processes involved in verbal working memory. International Journal of Neuroscience. 2005;115:1017–32. doi: 10.1080/00207450590901530. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of Royal Society of London B Biological Sciences. 2008a;363:2557–65. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. The Quarterly Journal of Experimental Psychology (Colchester) 2008b;61:157–70. doi: 10.1080/17470210701508855. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Winston J, Frith U. Social cognitive neuroscience: where are we heading? Trends in Cognitive Sciences. 2004;8:216–22. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain and Cognition. 2004;56:141–52. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cardillo ER, Aydelott J, Matthews PM, Devlin JT. Left inferior prefrontal cortex activity reflects inhibitory rather than facilitatory priming. Journal of Cognitive Neuroscience. 2004;16:1552–61. doi: 10.1162/0898929042568523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends in Cognitive Sciences. 2004;8:239–41. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Gatenby JC, Gore JC, Banaji MR. Neural components of social evaluation. Journal of Personality and Social Psychology. 2003;85:639–49. doi: 10.1037/0022-3514.85.4.639. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16:1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Davitz JR. The Language of Emotion. London: Academic Press; 1969. [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Moriguchi Y. The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. Biopsychosoc Medicine. 2007;1:22. doi: 10.1186/1751-0759-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Murphy BC, Shepard S. The development of empathic accuracy. In: Ickes W, editor. Empathic Accuracy. New York: The Gilford Press; 1997. pp. 73–116. [Google Scholar]
- Frijda NH, Zeelenberg M. Appraisal: What is the dependent? In: Davidson R J, Ekman P, Scherer KR, editors. Appraisal Process in Emotion. New York: Oxford; 2001. [Google Scholar]
- Frith CD, Frith U. Interacting minds-a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of theory of mind. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Maddock RJ. Separating subjective emotion from the perception of emotion-inducing stimuli: an fMRI study. Neuroimage. 2006;33:263–74. doi: 10.1016/j.neuroimage.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Goldman AI, Sripada CS. Simulationist models of face-based emotion recognition. Cognition. 2005;94:193–213. doi: 10.1016/j.cognition.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, et al. Explicit and incidental facial expression processing: an fMRI study. NeuroImage. 2001;14:465–73. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Graham T, Ickes W. When women's intuition isn't greater than men's. In: Ickes W, editor. Empathic Accuracy. New York: The Guilford Press; 1997. pp. 117–143. [Google Scholar]
- Grimm S, Schmidt CF, Bermpohl F, et al. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex-an fMRI study. NeuroImage. 2006;30:325–40. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of National Academy of Sciences of the USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44:374–83. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ. Attention and emotion: does rating emotion alter neural responses to amusing and sad films? Neuroimage. 2005;27:656–68. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Imaizumi S, Mori K, Kiritani S, et al. Vocal identification of speaker and emotion activates different brain regions. Neuroreport. 1997;8:2809–12. doi: 10.1097/00001756-199708180-00031. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie JA, Marcel AJ. Consciousness and the varieties of emotion experience: a theoretical framework. Psychological Review. 2002;109:219–59. doi: 10.1037/0033-295x.109.2.219. [DOI] [PubMed] [Google Scholar]
- Lane RD. Neural correlates of conscious emotional experience. In: Lane RD, Nadel L, editors. Cognitive Neuroscience of Emotion. New York: Oxford; 2000. pp. 345–70. [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8(18):3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K. Neural substrates of conscious emotional experience: a cognitive neuroscientific perspective. In: Beauregard M, editor. Consciousness, Emotional Self-Regulation, and the Brain. Amsterdam: Benjamins; 2004. pp. 87–122. [Google Scholar]
- Lange K, Williams LM, Young AW, et al. Task instructions modulate neural responses to fearful facial expressions. Biological Psychiatry. 2003;53:226–32. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Shaw P, Giampietro VP, Surguladze S, Brammer MJ, David AS. The role of shared representations in social perception and empathy: an fMRI study. NeuroImage. 2006;29:1174–83. doi: 10.1016/j.neuroimage.2005.09.001. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The Emotional Brain. New York: Simon & Schuster; 1996. [Google Scholar]
- Lee GP, Meador KJ, Loring DW, et al. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cognitive & Behavioral Neurology. 2004;17:9–17. doi: 10.1097/00146965-200403000-00002. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Scherer KR. The relationship of emotion to cognition: a functional approach to a semantic controversy. Cognition & Emotion. 1987;1:3–28. [Google Scholar]
- Lewis MD. Bridging emotion theory and neurobiology through dynamic systems modeling. Behavioral and Brain Sciences. 2005;28:168–245. doi: 10.1017/s0140525x0500004x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S. Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology. 2000;23:508–16. doi: 10.1016/S0893-133X(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18(5):421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MP, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proceedings of National Academy of Sciences of the U S A. 2002;99:15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Ito K, et al. Activation of the right inferior frontal cortex during assessment of facial emotion. Journal of Neurophysiology. 1999;82:1610–4. doi: 10.1152/jn.1999.82.3.1610. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Yamada H, Iidaka T, et al. Brain regions involved in verbal or non-verbal aspects of facial emotion recognition. NeuroReport. 2000;11:2571–6. doi: 10.1097/00001756-200008030-00044. [DOI] [PubMed] [Google Scholar]
- Nielsen L, Kaszniak AW. Conceptual, theoretical, and methodological issues in inferring subjective emotion experience: recommendations for researchers. In: Coan J A, Allen JJB, editors. Handbook of Emotion: Elicitation and Assessment. New York: Oxford University Press; 2007. pp. 361–78. [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004a;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004b;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Paradiso SP, Johnson DL, Andreasen NC, et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. American Journal of Psychiatry. 1999;156:1618–29. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9:148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage. 2004;21:768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Roseman IJ. Cognitive determinants of emotion: A structural theory. In: Shaver P, editor. Review of Personality and Social Psychology: Vol 5. Emotions, Relationships, and Health. Beverly Hills, CA: Sage; 1984. pp. 11–36. [Google Scholar]
- Roseman IJ, Smith CA. Appraisal theory: overview, assumptions, varieties, controversies. In: Davidson RJ, Ekman P, Scherer K R, editors. Appraisal Process in Emotion. New York: Oxford; 2001. pp. 3–19. [Google Scholar]
- Royet JP, Hudry J, Zald DH, et al. Functional neuroanatomy of different olfactory judgments. NeuroImage. 2001;13:506–19. doi: 10.1006/nimg.2000.0704. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. NeuroImage. 2003;20:713–28. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. Journal of Neuroscience. 2000;20:7752–9. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Saxe R. Four brain regions for theory of mind? In: Cacioppo JT, Visser PS, Pickett CL, editors. Social Neuroscience: People Thinking About Thinking People. Cambridge, MA: The MIT Press; 2006. pp. 83–101. [Google Scholar]
- Schaefer A, Collette F, Philippot P, et al. Neural correlates of ‘hot’ and ‘cold’ emotional processing: a multilevel approach to the functional anatomy of emotion. NeuroImage. 2003;18:938–49. doi: 10.1016/s1053-8119(03)00009-0. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Appraisal considered as a process of multilevel sequential checking. In: Davidson RJ, Ekman P, Scherer KR, editors. Appraisal Process in Emotion. New York: Oxford; 2001. pp. 92–120. [Google Scholar]
- Smith CA, Kirby LD. Toward delivering on the promise of appraisal theory. In: Davidson RJ, Ekman P, Scherer KR, editors. Appraisal Process in Emotion. New York: Oxford; 2001. pp. 121–140. [Google Scholar]
- Stein NL, Levine LJ. The early emergence of emotional understanding and appraisal: Implications for theories of development. In: Dalgleish T, Power MJ, editors. Handbook of Cognition and Emotion. New York: John Wiley & Sons Ltd; 1999. pp. 384–408. [Google Scholar]
- Tabert MH, Borod JC, Tang CY, et al. Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: an fMRI study. Neuropsychologia. 2001;39:556–73. doi: 10.1016/s0028-3932(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Science. 2007;11:413–8. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage. 2003;18:650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Trevarthen C, Aitken KJ. Infant intersubjectivity: research, theory, and clinical applications. Journal of Child Psychology and Psychiatry. 2001;42:3–48. [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Taylor ANW, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29:90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Social Cognitive and Affective Neuroscience. 2007;2:150–8. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–38. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Hertrich I, Riecker A, et al. Distinct frontal regions subserve evaluation of linguistic and emotional aspects of speech intonation. Cerebral Cortex. 2004;14:1384–9. doi: 10.1093/cercor/bhh099. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Riecker A, Hertrich I, et al. Identification of emotional intonation evaluated by fMRI. Neuroimage. 2005;24:1233–41. doi: 10.1016/j.neuroimage.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. NeuroImage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]