Abstract
Individuals with autism spectrum disorders (ASD) have documented deficits in face processing, face memory and abnormal activation of the neural circuitry that supports these functions. To examine speed of processing of faces in ASD, high density event-related brain potentials were recorded to images of faces, inverted faces and non-face objects from 32 high-functioning adults with ASD and controls. Participants were instructed to focus on a cross hair prior to stimulus onset; the cross-hair location directed the participant's eye gaze to the eye region at stimulus onset. Although the ASD group preformed more poorly on behavioral tests of face and object memory, both groups demonstrated similar ERP responses, characterized by greater (positive) P1 and (negative) N170 amplitude to faces vs houses. N170 speed of processing to faces did not differ between groups. However, only the control group demonstrated differential responses to upright vs inverted faces. For the ASD group, the differential response to inverted vs upright faces was associated with better performance on face memory and self-reported social skills. It is possible that the use of attention cues may facilitate face processing in high-functioning adults with ASD, suggesting that the underlying neural circuitry can be activated in adults with ASD under specific demands.
Keywords: event-related potential, P100, N170, autism, face processing
Autism is a developmental disorder characterized by impairments in social interaction and communication and a restricted range of activities (American Psychiatric Association, 1994). A number of researchers have proposed that these impairments likely relate to dysfunction of the brain system underlying social cognition (Brothers, 1990; Le Doux, 1994; Dawson, et al., 1998; Baron-Cohen et al., 1999, 2000) and specifically may be related to face processing impairments (Dawson et al., 2005; Schultz, 2005). There is increasing evidence that individuals with autism spectrum disorders have impairments in face processing and face recognition (Hauck et al., 1998; Klin et al., 1999; Teunisse and DeGelder, 1994; see Dawson et al., 2004; Jemel et al., 2006 for review).
Poor face discrimination and recognition abilities may stem from abnormal information processing strategies or from abnormal attention strategies. Children with autism have been shown to be better at recognizing individual facial features and partially obscured faces than typical children (Hobson et al., 1988; Tantam et al., 1989) and perform better when using the lower features of the face (Joseph and Tanaka, 2003). As well, children with autism fail to demonstrate a ‘face inversion effect’; that is, fail to show disrupted processing when a face is inverted similar to typically developing individuals (Langdell, 1978). It has been suggested that these findings reflect a failure to utilize configural processing strategies typically associated with face processing (Freire et al., 2000; Elgar and Campbell, 2001). Instead, individuals with autism may rely on feature-based processing or be biased toward examining the lower half of the face (Spezio et al., 2007).
Event-related potentials (ERPs) provide additional information about the temporal qualities of face processing. In adults, an ERP component called the N170 has been characterized as a ‘face’ sensitive component. The N170 is recorded over the posterior temporal region, is of greater negative amplitude and faster latency in the right than left hemisphere for faces, and peaks between 130 and 170 ms to face stimuli. Within the category of faces, the N170 is of greatest negative amplitude to eyes and inverted faces than upright faces, which in turn are more negative in amplitude than responses to noses or mouths; latency is faster to eyes and upright faces than inverted faces, noses and mouths (Bentin et al., 1996; Rossion et al., 1999; Itier and Taylor, 2002). These stimulus manipulations are thought to disrupt configural processing, and it has been suggested that deviations of the N170 may reflect this sensitivity. In contrast, Thierry and colleagues found that when showing pictures of faces and cars, it was not the category that evoked a more negative N170, but rather the within category variability in position, angle and size of the stimuli that resulted in amplitude modification (Bentin et al., 2007; Thierry et al., 2007). This suggests that the N170 may be sensitive to additional perceptual dimensions beyond those involved in configural processing.
Adults with autism spectrum disorder (ASD) also show disruptions in face processing measured via ERPs. McPartland et al. (2004) found that nine adolescents and adults with ASD had slower N170 responses to faces than objects. O’Connor et al. (2005) found that the group with Asperger's (ASP) group had slower P1 and N170 responses to facial expressions of emotions compared to controls; also had a reduced N170 amplitude group. O’Connor et al. (2005, 2007) interpreted these results as reflecting impaired holistic and configural processing of faces, potentially due to decreased attention to internal features or a failure of expertise processing.
Functional magnetic resonance imaging (fMRI) studies suggest that face processing abilities may be highly variable in individuals with ASD. While most typical individuals exhibit activation in the right fusiform gyrus when viewing faces, individuals with ASD have been shown to exhibit reduced fusiform activation and/or increased activation of object processing areas (Schultz et al., 2000; Pierce et al., 2001). However, when attention was explicitly directed at the eye region such as when a fixation point in the center of the face was used (Hadjikhani et al., 2004) or when participants self-directed attention to the eye region, while viewing the stimuli (Dalton et al., 2005), participants activated more normative neural sources (e.g. fusiform gyrus, amygdala).
The current study used high-density ERP recordings to measure face processing in high-functioning adults with ASD and age and full scale IQ-matched typical individuals. Participants viewed images of upright and inverted faces and upright and inverted houses. Attention was directed to the center of the stimuli by use of a cross hair that appeared prior to the onset of the stimuli. Based on previous reports, we predict that adults with ASD would demonstrate an impairment in temporal processing of faces characterized by longer latencies of the P1 and N170 ERP components. However, if prior reports of delayed processing were due to abnormal direction of attention, then use of the crosshair to direct attention may result in more normalized responses, especially at the P1. In addition, we examined whether ERP responses to faces (amplitude and latency) were correlated with behavioral measures assessing social cognition and face memory. We hypothesized that abnormal ERP responses would be correlated with more social impairments and poorer behavioral performance on face memory tests.
MATERIALS AND METHODS
Participants
Two groups of adults participated in the study: 39 individuals with ASD and 38 control adults. ASD participants had a current clinical diagnosis of ASD and met research diagnostic standards for ASD based on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999) and expert clinical diagnostic judgment based on DSM- IV criteria. In addition, all individuals met criteria for autism on the Autism Diagnostic Interview on the social and communication domains (ADI-R; Lord et al., 1994). Meeting full criteria on the ADI was not required because parents were not always available or were not always confident in their recollection of the onset of early symptoms. Exclusionary criteria for participants with ASD and controls included known genetic disorders, seizures, significant sensory or motor impairment, major physical abnormalities, serious head injury and use of anti-convulsant or barbiturate medications. Exclusionary criteria for control participants also included birth or developmental abnormalities, psychotropic medication usage and a first degree relative with ASD. The study was approved by the University of Washington Human Subjects Institutional Review Board and all subjects were consented to the procedures.
Additional autism and social assessments
For both groups, the Autism Quotient (AQ), Social Avoidance and Distress Scale (SADS), and the Social Competence Questionnaire (SCQ) were used to obtain participant self report of autism symptoms and social skills (N = 30 ASD; N = 31 controls). The AQ was developed to assess autism traits in those with average/normal intelligence (Baron-Cohen et al., 2001; Woodbury-Smith et al., 2005). The AQ assesses social skills, attention switching, attention to detail, communication and imagination. Higher scores reflect more autism traits. The SADS is a 28-item measure developed to assess anxiety in social situations (Watson and Friend, 1969). The SADS assesses two aspects of anxiety: one's experience of distress, discomfort, fear and anxiety, and the deliberate avoidance of social situations. Lower scores reflect less distress, discomfort, fear and anxiety during social situations. The SCQ is a 10-item self-report measure assessing social comfort (Sarason et al., 1985). Higher scores reflect greater social competence.
Face and object recognition memory
We used four tests to asses face and object memory. This battery included (i) the Wechsler Memory Scale – Third Edition, Faces Subtest (Wechsler, 1997a, b) to assess immediate and delayed recognition memory for faces; and (ii) the House Memory Test designed to assess immediate and delayed recognition memory for houses. The house memory test parallels the WMS face subtest in administration. Participants view 24 stimuli presented each for 2 s. To test recall, immediately and after a 30 min delay, the participant is presented with 48 stimuli and the participant indicated if the stimulus was one that he or she was asked to remember. (iii) The Woodcock Johnson Object Recognition Subtest (Woodcock and Johnson, 1989) was used to assess short term recognition of objects. (iv) The Benton Facial Recognition Test (Benton et al., 1994) was used to assess recognition of unknown faces. Fewer participants (ASD = 26; control = 19) had the Benton as it was added to the study at a later date.
EEG recording procedure
Of the initial sample of 39 individuals with ASD, 32 provided adequate artifact-free data (seven provided too few artifact-free trials). For the ASD sample, 13 participants met DSM-IV criteria for Autistic Disorder, three met criteria for Pervasive Developmental Disorder- Not Otherwise Specified and 16 met criteria for Asperger's Disorder. Of the initial sample of 38 control participants, 32 provided adequate artifact-free data (six provided too few artifact-free trials). Table 1 presents sample demographic and descriptive information for both groups.
Table 1.
Characteristics of participants with artifact free ERP data; mean, s.d. in parenthesis and range in brackets provided when applicable
| Group | N (Female) | Hand Right/Left | Age (years) | Full Scale IQ | Performance IQ | Verbal IQ | |
|---|---|---|---|---|---|---|---|
| ASD | 32 | 30/2 | 23.1 (6.9) | 111.3 (13.9) | 109.1 (15.2) | 110.8 (15.4) | |
| (2) | [18–44] | [86–136] | [83–139] | [79–140] | |||
| Control | 32 | 27/5 | 23.7 (6.7) | 110.0 (12.8) | 109.3 (14.9) | 108.4 (12.9) | |
| (3) | [18–43] | [83–139] | [78–136] | [84-132] | |||
| F (1,62) = 0.12 | F (1,62) = 0.15 | F (1,62) = 0.45 | F (1,62) = 0.003 | ||||
| P = ns | P = ns | P = ns | P = ns | ||||
Stimuli
Stimuli consisted of gray-scale digital images of faces and houses presented on a computer monitor with a gray background. All facial images were standardized so that the center of the eyes was presented at the center of the screen; visual angle for the faces was 11° (height) by 7.6° (width). The ethnicity of the images reflected regional demographics. All house pictures were taken from local neighborhoods, or from stimulus sets provided by N. Kanwisher and M. Eimer. All houses were symmetrical, where shown in the same point of reference, and were matched on perceptual size. The visual angle was 7.1° (height) by 7.1° (width).
Stimuli were presented randomly in four blocks composed of 58 trials of five different stimulus categories: upright faces (50), inverted faces (50), upright houses (50), inverted houses (50), and scrambled faces (32). To control for attention, participants were instructed to press a button to each scrambled face. Accuracy did not differ between the two groups; however, individuals with ASD (M = 422.8 ms, s.d. = 55.6) were significantly quicker to identify targets than the control group (M = 459.2 ms, s.d. = 79.2), F (1, 61) = 4.4, P < .05.
Data collection
EEG was recorded in an electrically shielded, sound-attenuated, darkened room. The participant was seated comfortably ∼75 cm from the stimulus monitor. A large, threefold screen obscured the back of the monitor and the back part of the room from the participant's view. A 128 channel Geodesic sensor net (EGI; Eugene OR) was dipped into potassium-chloride electrolyte solution, placed on the participant's head, and fitted according to the manufacturer's specifications. Impedances were kept below 40 kΩ. The amplification was set at 1000× and filtering was done through a 0.1 Hz high-pass filter and a 200 Hz elliptical low-pass filter. The recording rate was 500 Hz. The vertex electrode was used as a reference.
EEG was recorded continuously throughout each stimulus presentation trial, consisting of a 500 ms baseline containing a visual fixation cross in the middle of the screen, 300 ms stimulus presentation, and intertrial interval, which varied randomly between 1000 and 1300 ms.
Data editing and analysis
Trials were processed using NetStation 4.0 (EGI; Eugene, OR). First, data were low-pass filtered at 30 Hz. Second, artifact detection included: marking channels bad in each trial if the fast amplitude exceeded 100 μV, the differential average amplitude exceeded 50 μV, or the channel had zero variance; excluding trials with eye movement artifacts; marking trials bad if they contained more than 10 bad channels; and replacing electrodes (with spline interpolation) when >20% of trials for that electrode were contaminated by artifact. Third, data were averaged for each stimulus type and were re-referenced to an average reference. Fourth, electrodes at which the N170 could not be verified by visual inspection were not included.
Electrodes of interest were selected based on review of the literature and examination of grand averages and individual participant data. Four lead groups were selected: (i) posterior lateral left (leads 5/P9, 59/P7, 64 and 65), (ii) posterior medial left (leads 66/PO7, 70, 71 and 72/O1), (iii) posterior medial right (leads 77/O2, 84, 85/PO8 and 90) and (iv) posterior lateral right (leads 91, 92/P8, 96 and 97/P10). The time windows for ERP components were chosen based on visual inspection of the grand-average and data for individual participants. The P1 time window was 60–130 ms after stimulus presentation. The N170 was 120–180 ms after stimulus presentation and was visually verified as occurring within that window by two authors (K.M., S.W.).
For each component of interest, a diagnostic group (ASD/Control) by stimulus (face/house), by orientation (upright/inverted), by electrode hemisphere (right/left), by electrode region (posterior superior/posterior inferior) repeated measures analysis of variance was conducted to identify main effects. Planned comparisons for stimulus type based on McPartland et al. (2004) included face upright by house upright and face upright by face inverted. The Greenhouse-Geisser correction was employed and Fisher's Least Significant Differences was used for follow up tests. An α-level of P < 0.05 was used unless otherwise noted.
RESULTS
Autism and social assessments
As expected, individuals with ASD had higher AQ scores (M = 29.4, s.d. 7.0 range 16–44), higher SADS scores (M = 17.3, s.d. 7.8 range 3–27) and lower SCQ scores (M = 21.1, s.d. 6.3 range 10–34) than controls (AQ M = 11.9, s.d. 4.5 range 3–21; SADS M = 2.3, s.d. 3.6 range 0–13; SCQ M = 33.6, s.d. 4.3 range 21–40), F(1,60) > 90.7, P < 0.001. The Autism (n = 13) and Asperger's (n = 16) sub-groups did not differ on the ADOS [F(1,28) = 1.3, P = ns] or any of the questionnaires (AQ Fs < 1.2, Ps = ns) and were thus combined for future analyses.
Face and object recognition memory
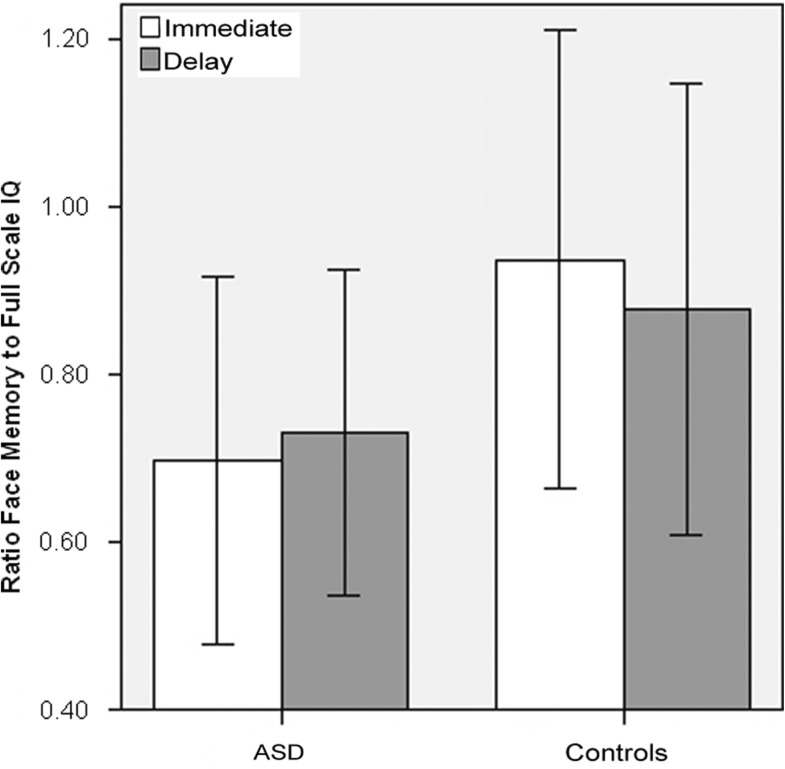
Individuals with ASD had lower scores than controls on the WMS Face Memory Subtest (Immediate and Delay), the House Memory Test (Immediate and Delay), the Woodcock Johnson Object Memory test and the Benton Face Recognition test; values provided in Table 2. Both groups had lower WMS scores than would be predicted given their Full Scale IQ. However, the ASD group showed a larger discrepancy (Figure 1).
Table 2.
Scores for the face and object behavioral tests
| Group | WMS Face Memory Imm | WMS Face Memory Delay | House Memory Imm | House Memory Delay | WJ Object Memory | Benton Face Recog |
|---|---|---|---|---|---|---|
| ASD | 7.7 (2.3) | 8.0 (2.0) | 7.7 (2.6) | 7.4 (2.1) | 20.0 (3.8) | 43.6 (3) |
| [2–12] | [3–12] | [3–13] | [3–13] | [10–27] | [36–49] | |
| Control | 10.2 (2.6) | 9.5 (2.6) | 9.3 (2.1) | 8.6 (1.4) | 22.7 (3.4) | 46.1 (3) |
| [5–18] | [5–17] | [5–15] | [5–12] | [15–29] | [42–52] | |
| F (1,62) = 16.2 | F (1,62) = 6.8 | F (1,61) = 6.7 | F (1,61) = 6.9 | F (1,59) = 8.5 | F (1,43) = 7.9 | |
| P < 0.01 | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.01 | P < 0.01 | |
Key: WMS = Wechsler Memory Scale; WJ = Woodcock Johnson; Imm = immediate; Recog = Recognition
Mean, s.d. in parenthesis, and range in brackets provided when applicable.
Fig. 1.
Ratio of WMS Face memory to Full Scale WAIS IQ. (e.g. a score of 1.0 would reflect a scaled score of 10 on the WMS Face Memory and a scaled score of 100 IQ). Error bars represent ± 1 s.d.
ERP analyses
Because the WMS Face Memory Subtest scores suggested that some controls had relative impairments in face processing, we performed all analyses first with the whole control group, and then only controls with WMS face memory scores ≥ 7 or ratio WMS: Full Scale IQ ≥ 0.7 (n = 24). The composition of the control group did not alter the results. Only results for the full control group will be presented. ERP waveforms are in Figure 2.
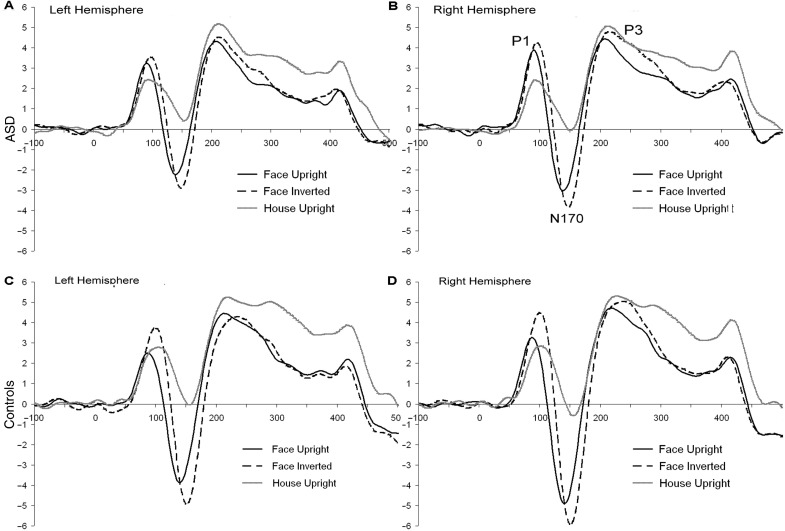
Fig. 2.
ERP responses to upright faces (black line), inverted faces (dotted line) and upright houses (gray line) by hemisphere (A/C left hemisphere; B/D right hemisphere) for individuals with ASD (A/B) and controls (C/D).
P1
For both groups, the P1 to faces compared to objects was more positive in amplitude [F(1,52) = 23.1, P < 0.001] and faster in latency [F(1,52) = 10.9, P < 0.01]. Inverted stimuli compared to upright stimuli were more positive in amplitude [F(1,52) = 5.8, P < 0.05] but slower in latency [F(1,52) = 18.7, P < 0.001]. Additionally, the amplitude was more positive in the lateral vs the medial leads [F(1,52) = 11.4, P = 0.001] and more positive in the right than left hemisphere [F(1,52) = 4.3, P < 0.05].
P1 amplitude, group differences
Both groups showed more positive P1 amplitude to faces than houses. In the control group [F(1,27) = 7.2, P = 0.01] (Figure 2C and D), but not the ASD group [F(1,25) = 0.9, P = ns] (Figure 2A and B), inverted stimuli had the greatest P1 amplitude and were significantly larger than upright stimuli. In summary, the control but not the ASD group demonstrated differential processing of upright and inverted faces.
P1 latency, group differences
No group differences were found.
N170
The N170 was more negative in amplitude [F(1,53) = 164.8, P < 0.001] and faster in latency [F(1,53)= 126.7, P < 0.001] to faces than houses. Inverted compared to upright stimuli were more negative [F(1,53) =50.2, P < 0.001] but slower in latency [F(1,53) = 193.7, P < 0.001]; see Figure 2D for example. As well, the amplitude was more negative [F(1,53) = 12.2, P = 0.001] but slower [F(1,53) = 4.9, P < 0.05] in the lateral vs the medial leads and more negative [F(1,53) = 10.6, P < 0.01] in the right than left hemisphere (Figure 2A vs B and Figure 2C vs D). Overall, the N170 response was most negative to inverted faces but fastest to upright faces.
N170 amplitude, group differences
Both groups demonstrated the pattern described above. However, there were subtle differences between the two groups (e.g. interactions between stimulus, orientation, region, hemisphere and group). In general, the control group had more negative amplitude to upright faces [F(1,63) = 3.5, P < 0.1], specifically at right medial leads [F(1,63) = 3.9, P = 0.05] (Figure 2D) and to inverted faces [F(1,63) = 4.3, P < 0.05], specifically at lateral leads [F(1,63) = 5.7, P < 0.05] than the ASD group (Figure 2B).
N170 latency group
Both groups showed faster response to faces than houses. Likewise, both groups showed faster responses to upright than inverted stimuli. Only the control group showed a faster response at the medial than lateral leads [control, F(1,28) = 14.7, P =0.001; ASD, F(1,25) = 0.006, P = ns]. There was an interaction between stimulus, orientation, region and group but there were no significant follow up effects [F(1,53) = 4.9, P < 0.05].
Slope from P1 to N170
To better understand the morphology of the waveform, we analyzed the change in slope between the P1 and N170. The slope calculation takes into consideration the peak to peak change in amplitude over the peak to peak change in latency. Using this slope value, the control group showed a steeper change in slope for the inverted compared to the upright stimuli than the ASD group [F(1,51) = 6.3, P < 0.05]. For the control group, the slope was larger to inverted faces than upright faces [F(1,27) = 19.1, P < 0.001].
Relation between behavioral tests and ERP
In order to reduce the number of comparisons conducted, we utilized three strategies. First, correlations were examined separately for the ASD and Control group. Second, we examined the relation between amplitude and latency of response to upright faces and face memory similar to McPartland et al. (2004). Third, as the primary difference between the ASD group and the Control group was in the differential processing of upright and inverted faces, we created ERP difference scores (inversion difference = face upright – face inverted) for P1 and N170 latency and amplitude and compared those to the social measures (ADI, ADOS, questionnaires, memory tests).
There were no relations between amplitude and latency of the N170 response to upright faces and face memory (immediate memory and amplitude r = −0.21, ns; delayed memory amplitude r = −0.13, immediate memory and latency r = −0.16, delayed memory and latency r = −0.04, all ns) for the ASD and Control group (immediate memory and amplitude r = −0.14, ns; delayed memory amplitude r = 0.14, immediate memory and latency r = −0.01, delayed memory and latency r = −0.00, all ns, respectively). Thus, our data do not replicate McPartland et al. (2004).
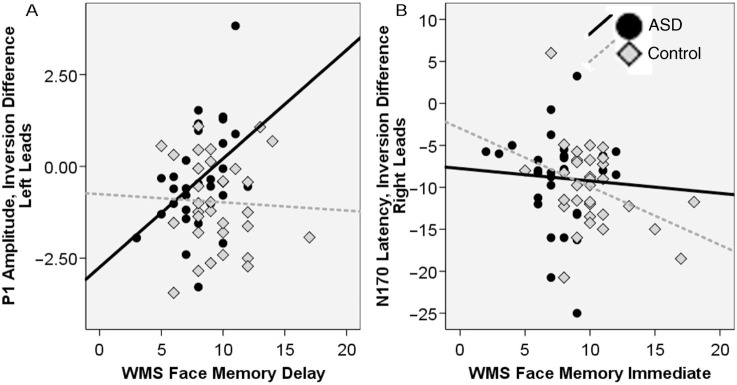
We next examined the inversion difference and its relation to face memory. For the P1, the response to inverted faces was more positive in amplitude than to upright faces; this would be represented by a negative value for the amplitude inversion difference. The amplitude inversion difference (in both the right and left hemisphere) was associated with performance on the WMS face memory delay score for the ASD group (right r = 0.51, left r = 0.43, Ps < 0.05) but not the Control group (right r = −0.11, left r = −0.05, all ns). As seen in Figure 3A, a more negative value was associated with worse memory performance. Thus, those individuals with ASD who demonstrated a greater amplitude inversion difference (inversion more positive than upright), had worse face memory.
Fig. 3.
Relation between ERP responses and face memory for the ASD group (black circles, black line) and the controls (gray diamonds, dotted line).
For the N170, the response to upright faces was faster than to inverted faces; this would be represented by a negative value for the latency inversion difference. The latency inversion difference for the N170 was related to face memory (immediate) for the controls (right leads r = −0.37, P < 0.05; left leads r = 0.03, P < ns) but not the ASD group (right r = −0.06, left r = −0.02, all ns). As seen in Figure 3B, a more negative value was associated with better face memory. Thus, those controls who demonstrated a greater latency inversion difference (upright faster than inverted), had better face memory.
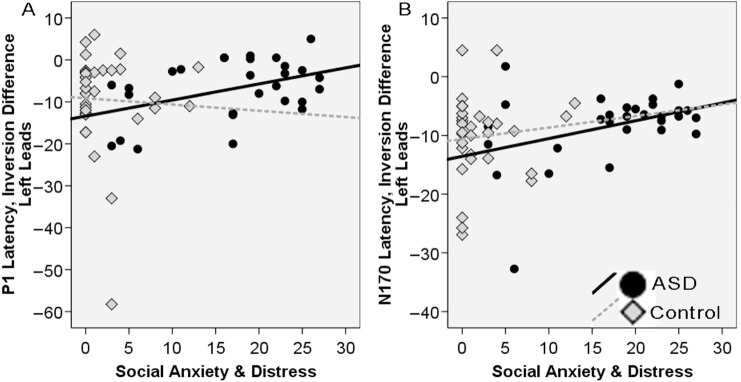
There were several relations between the inversion difference and self reported social skills in the ASD but not the Control group. For the P1 amplitude, a negative inversion value would reflect a more positive amplitude to inverted than upright; for P1 latency, a negative inversion value would reflect a faster response to upright than inverted. The SADS total score, SCQ total score and AQ social skill subscale for the ASD group were related to the P1 latency inversion difference at left leads (SADS r = 0.43, P < 0.05; SCQ r = −0.53, P < 0.01; AQ Social r = 0.45, P < 0.05); this relation was not present for the Control group (SADS r = 0.04, P = ns; SCQ r = 0.04, P = ns; AQ Social r = 0.21, P = ns). A more negative value, faster response to upright compared to inverted faces, was correlated with less social anxiety and distress (Figure 4A), more self reported social competence, and less social symptoms related to autism.
Fig. 4.
ERP inversion difference scores at left hemisphere leads for the (A) P1 and (B) N170, and responses to the social anxiety and distress scale for the ASD group (black circles, black line) and the controls (gray diamonds, dotted line).
Similarly, a negative latency inversion difference for the N170 at left leads, was related to better social skills in the ASD group (SADS r = 0.38, P < 0.05; SCQ r = −0.47, P < 0.05; AQ Social r = 0.37, P < 0.05) but not the Controls (SADS r = 0.10, SCQ r = 0.20, AQ Social r = −0.07, all ns). As seen in Figure 4B, the relation between the N170 inversion difference and the SADS is the same as in Figure 4A, which depicts the P1 relation. The SADS total score was also related to the N170 amplitude inversion difference at left leads for the ASD group (r = 0.41, P < 0.05) but not the control group (r = 0.02, P = ns). A greater amplitude inversion difference, that is a more negative response to upright compared to inverted faces, was related to less social anxiety and distress. In both groups, the AQ total score was not related to the inversion difference measures for the P1 and N170 for amplitude or latency. There was also no relation between the inversion difference and symptom domains (ADI, ADOS).
DISCUSSION AND CONCLUSION
This report compared a relatively large group of adults with high functioning ASD (N = 32) and typical adults (N = 32) matched on age, gender and full scale IQ using ERP responses to upright and inverted faces and upright and inverted houses. We found that early stage face processing did not significantly differ between groups for the main comparison between faces and houses, but did differ for the comparison between inverted faces compared to upright faces.
For the main comparison between response to faces and houses, both groups demonstrated an ERP response (at the P1 and N170) to faces that was greater in amplitude and faster in latency than to houses. In comparison to tasks used by McPartland et al. (2004) and O’Connor et al. (2005, 2007) examining ERP responses to faces in high functioning adolescents and adults, our study included a cross hair to direct attention to the true center of the head (bridge of the nose). Prior research using fMRI suggests that attention alters face processing circuits in individuals with ASD. Using fMRI, Dalton et al. (2005) found that fusiform activation was correlated with the amount of time spent looking at the eye region of the face. Hadjikhani et al. (2004) found that a cross hair positioned on the stimuli increased fusiform activity and resulted in no difference between ASD and controls. Similarly, in an eye tracking experiment using familiar faces and a cross hair located on the bridge of the nose, individuals with ASD (who were a sub sample of the participants in this study), demonstrated a greater percentage of fixations in the eye region compared to the mouth region, similar to the pattern demonstrated by typical controls (Sterling et al., manuscript submitted for publication). These results raise the possibility that minor manipulations in how stimuli are presented to individuals with ASD may impact the pattern of responses and may help to recruit face processing systems.
Of note, the ASD group failed to exhibit differential ERP responses to inverted faces compared to upright faces at the P1 and P1-N170 slope. A lack of sensitivity to inversion of face stimuli has been interpreted as reflecting a lack of configural or holistic processing for faces. Making a decision that a stimulus is a face may occur through first order processing of the basic structure of a face (two eyes, nose and mouth) (Maurer et al., 2002) and the P1 has been found to be sensitive to first order configural information in a face (Halit et al., 2000; Itier and Taylor, 2002; Boutsen et al., 2006; Macchi Cassia et al., 2006; Mercure et al.,, 2008). Facial inversion is also thought to disrupt second order relations necessary for configural processing, which might be represented by changes in the N170 (Halit et al., 2000; Boutsen et al., 2006; Macchia Cassia et al., 2006; Scott & Nelson, 2006; Mercure et al., 2008) and it has been suggested that the face inversion effect originates during the slope between the P1 and N170 peaks (Jacques and Rossion, 2007).
One interpretation of our results is that individuals with ASD were able to use first order relations when attention was initially directed toward the eye region of the face and this resulted in relatively more typical differentiation of faces and houses. Because our cross hair and faces were centered on the bridge of the nose, inversion of the face kept the eye region at the same spatial location and resulted in greater spatial change in the location of the nose and mouth, altering the resources needed to process the first order relations to the inverted face. A second interpretation is that the default face processing system in ASD is biased toward part based processing; a system that is biased toward part base processing would not show a disadvantage when stimuli were inverted (Teunisse and DeGelder, 2003; Jemel et al., 2006; Lahaie et al., 2006). Lastly, it has been proposed that the N170 is mediated by the eye region (Doi et al.,, 2007; Itier et al.,, 2007); thus directed attention to the eye region may have resulted in a more normative N170 in the ASD group.
The behavioral tasks also differentiated the two groups in our study. The ASD group had worse face and object memory scores compared to the Control group. The impairments on the WMS face memory subtest and the Benton face recognition test replicate previous reports suggesting impairments in face recognition in ASD. The results on the house memory test and the Woodcock Johnson Memory subtest, however, suggest that memory for complex objects was also impaired in the ASD sample. Given that our specific house stimuli were chosen to contain more within category similarity (e.g. symmetry, common configuration and orientation of parts) and the Woodcock Johnson test items require item selection within a category (e.g. memory for a specific flower within an array of flowers), it is possible that a higher degree of within category discrimination was necessary to make correct identification and rejection. A system biased toward parts would be impacted when stimuli had less perceptual variability.
The differential ERP responses to upright vs inverted faces was also associated with performance on face memory (Figure 3) and self-reported social skills (Figure 4). For face memory in the ASD group, a negative inversion amplitude value at the P1, that is a more positive response to inverted vs upright, was associated with worse memory. The ERP responses suggest that both groups showed a more positive P1 amplitude to faces than houses but only the control group showed a greater positive response to inverted stimuli than upright stimuli (Figure 3A; Table 3). Thus, it is unclear why this more normative pattern would be associated with worse face memory in individuals with ASD. More consistent with current theories of face processing, is the relation between speed of processing and face memory in the Control group, that is the relation depicted in Figure 3B showing the negative inversion latency value at the N170 (faster response to upright vs inverted), was associated with better face memory. These relations will need to be replicated in future reports.
Table 3.
Pattern of results for the Control and ASD groups at the P1 and N170
| Faces vs Houses |
Upright vs Inverted |
|||
|---|---|---|---|---|
| Controls | ASD | Controls | ASD | |
| Amplitude | ||||
| P1 amp | F > H | F > H | FU > FI | FI = FU |
| N170 amp | F < H | F < H | U < I | U < I |
| Slope | F > H | F > H | I > U | I = U |
| FI > FU | FI = FU | |||
| Latency | ||||
| P1 latency | F < H | F < H | U < I | U < I |
| N170 latency | F < H | F < H | U < I | U < I |
Key: F Face; H House; U Upright; I Inverted; FI Face Inverted; FU Face Upright; < more negative amplitude or faster than in latency; > more positive amplitude or greater change in slope; = equivalent.
Significant differences between groups are in bold.
The relation between the inversion value and self reported social skills in the ASD group suggest that more normative temporal responses, that is a faster response to upright vs inverted faces, is related to better social skills (Figure 4), less social anxiety and distress, greater social competence, and less autism social symptoms. As a caveat, the control group's scores on the self report questionnaire lacked variability and thus may have reduced the ability to detect a relation. It is possible that if we selected for a higher degree of lesser autism symptoms in our control group, that is a greater range social skills, we may have been better able to articulate this relation.
These results differ from an earlier report of N170 differences between adolescents and adults with ASD and control individuals (McPartland et al., 2004). Several reasons may account for this discrepancy. First, McPartland's et al.'s study included nine participants aged 15–42; whereas this report concerns 32 adults aged 18–44. Second, our participants had better face memory—75% of our sample scored in the normal range (above a 7) on the WMS immediate face memory subtest (24/32), whereas only a third (3/9) did so in the McPartland et al. study. Third, we used a more narrowly defined object category (symmetrical houses with similar features). Based on Thierry et al. (2007) the greater category variability in position, angle and size of furniture (McPartland et al., 2004) and cartoon images (O’Connor et al., 2007) may have resulted in greater amplitude modification. Fourth, our ERP tasks involved a button press to the target versus counting; the counting would have involved basic working memory and may have altered attention to the task. Fifth, McPartland et al. analyzed the semi medial electrode groups used in Halit et al.'s (2000) study of ERPs to faces in typical adults. We found that many of the more lateral outer electrodes in our data contained artifact due to poor electrode contact with the scalp, and thus did not include them.
There are two caveats about our sample. First, our sample may differ from previous reports due to ‘real-world’ external events. This study was conducted at a time of increased public awareness of the face processing impairment in ASD. This may have led to a bias in those individuals who participated in the study. Within the testing session, several ASD participants reported that they had been working at maintaining eye contact, and some expressed concern about attending to the tasks correctly. Second, it is also possible that those adults with ASD who are capable of participating in a multi-visit study and who choose to participate in a study of face processing, may be (historically) more socially motivated compared to other individuals with ASD. This combined with the use of two-dimensional faces that are socially non-demanding and the use of an external cue to begin each trial may have allowed individuals with ASD the perceptual and attentional support necessary to activate basic face processing circuits. However, because this sample included 32 individuals with ASD with an average ADOS social score of 8.2 (s.d. 2.6 range 4–14) and ADI social score of 17.6 (s.d. 5, range 11–29), we suggest that our sample represents the full spectrum of social skills within the disorder. Further, this sample is 2–3 times as large as that of other comparable adult studies (McPartland et al., 2004; O’Connor et al., 2005, 2007) and may better reflect the diversity of ASD profiles. In either case, basic training strategies that emphasize attention or configural processing may have profound impact on the activation of the face processing system.
Although there are a number of differences across reports, if face processing was a pervasive and encompassing deficit in ASD, we would expect the results to be more similar. Jemel et al. (2006) conclude that there is not strong empirical evidence for a deficit in ‘overall face recognition’ and that the versatility and abilities of face processing in persons with ASD have been underestimated’ (page 102). Our data support this conclusion and suggest that subtle manipulations in the structure of the experiment can provide necessary support for individuals with ASD to evoke relatively typical patterns of response. Defining the effects of each individual manipulation may provide important information about concurrent deficits in the disorder (e.g. category development, attention, memory).
Acknowledgments
We gratefully acknowledge the contributions of these funding sources, the Clinical and Statistical Cores of this project, and the individuals who participated in this study. This research was funded by a program project grant from the NIMH Studies to Advance Autism Research and Treatment (U54MH066399). The Murdock Trust provided funds for purchase of the system for recording electroencephalographic activity.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Baron-Cohen S, Ring H, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams S. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24:355–64. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Taylor MJ, Rousselet GA, et al. Controlling interstimulus perceptual variance does not abolish N170 face sensitivity. Nature Neuroscience. 2007;10:801–2. doi: 10.1038/nn0707-801. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher KD, Varney NR, Spreen O. Contributions to Neuropsychological Assessment. A Clinical Manual. 2nd. New York: Oxford University Press; 1994. [Google Scholar]
- Boutsen L, Humphreys GW, Praamstra P, Warbrick T. Comparing neural correlates of configural processing in faces and objects: an ERP study of the Thatcher illusion. Neuroimage. 2006;32:352–67. doi: 10.1016/j.neuroimage.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Brothers L. The neural basis of primate social communication. Motivation and Emotion. 1990;14:81–91. [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, Rinaldi J. Neuropsychological correlates of early symptoms of autism. Child Development. 1998;69:1277–85. [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science. 2004;7(3):340–359. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;26:403–24. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Doi H, Sawada R, Masataka N. The effects of eye and face inversion on the early stages of gaze direction perception–An ERP study. Brain Research. 2007;1183:83–90. doi: 10.1016/j.brainres.2007.08.073. [DOI] [PubMed] [Google Scholar]
- Elgar K, Campbell R. Annotation: the cognitive neuroscience of face recognition: Implications for developmental disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:705–17. doi: 10.1111/1469-7610.00767. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K, Symons L. The face-inversion effect as a deficit in the encoding of configural information: direct evidence. Perception. 2000;29:159–70. doi: 10.1068/p3012. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. NeuroImage. 2004;22:1141–50. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Modulation of event-related potentials by prototypical and atypical faces. Neuroreport. 2000;11(9):1871–5. doi: 10.1097/00001756-200006260-00014. [DOI] [PubMed] [Google Scholar]
- Hauck M, Fein D, Maltby N, Waterhouse L, Feinstein C. Memory for faces in children with autism. Clinical Neuropsychology. 1998;4:187–98. [Google Scholar]
- Hobson P, Ouston J, Lee A. What's in a face? The case of autism. British Journal of Psychology. 1988;79:441–53. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Alain C, Sedore K, McIntosh AR. Early Face Processing Specificity: It's in the Eyes! Journal of Cognitive Neuroscience. 2007;7(3):340–359. doi: 10.1162/jocn.2007.19.11.1815. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: a repetition study using ERPs. NeuroImage. 2002;15:353–72. doi: 10.1006/nimg.2001.0982. [DOI] [PubMed] [Google Scholar]
- Jacques C, Rossion B. Early electrophysiological responses to multiple face orientations correlate with individual discrimination performance in humans. Neuroimage. 2007;36(3):863–76. doi: 10.1016/j.neuroimage.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: face or artifact? Journal of Autism and Developmental Disorders. 2006;36:91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:529–42. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow S, de Bildt A, Cicchetti D, Cohen D, Volkmar F. A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Lahaie A, Mottron L, Arguin M, Berthiaume C, Jemel B, Saumier D. Face Perception in High-Functioning Autistic Adults: Evidence for Superior Processing of Face Parts, Not for a Configural Face-Processing Deficit. Neuropsychology. 2006;20(1):30–41. doi: 10.1037/0894-4105.20.1.30. [DOI] [PubMed] [Google Scholar]
- Langdell T. Recognition of faces: an approach to the study of autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1978;19:255–68. doi: 10.1111/j.1469-7610.1978.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Le Doux JE. Emotion, memory, and the brain. Scientific American. 1994;270:50–54. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview – Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Scale-WPS (ADOS-WPS) Los Angles, CA: Western Psychological Services; 1999. [Google Scholar]
- Macchi Cassia V, Kuefner D, Westerlund A, Nelson CA. Modulation of face-sensitive event-related potentials by canonical and distorted human faces: the role of vertical symmetry and up-down featural arrangement. Journal of Cognitive Neuroscience. 2006;18(8):1343–58. doi: 10.1162/jocn.2006.18.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Neuroscience. 2002;6:255–60. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related potentials reveal abnormalities in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45:1235–45. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Mercure E, Dick F, Johnson MH. Featural and configural face processing differentially modulate ERP components. Brain Research. 2008;1239:162–170. doi: 10.1016/j.brainres.2008.07.098. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Hamm JP, Kirk IJ. The neurophysiological correlates of face processing in adults and children with Asperger's syndrome. Brain and Cognition. 2005;59:82–95. doi: 10.1016/j.bandc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Hamm JP, Kirk IJ. Neurophysiological responses to face, facial regions and objects in adults with Asperger's syndrome: an ERP investigation. International Journal of Psychophysiology. 2007;63:283–93. doi: 10.1016/j.ijpsycho.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller R, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–73. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Rossion B, Delvenne J–F, Debatisse D, et al. Spatio-temporal localization of the face inversvion effect: an event-related potentials study. Biological Psychology. 1999;50:173–89. doi: 10.1016/s0301-0511(99)00013-7. [DOI] [PubMed] [Google Scholar]
- Sarason B, Sarason I, Hacker A, Basham R. Concomitants of social support: social skills, physical attractiveness, and gender. Journal of Personality and Social Psychology. 1985;49:469–80. [Google Scholar]
- Schultz R. Developmental deficits in social perception in autism: the role of the amygdale and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz R, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–40. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS E, Piven J. Abnormal use of facial information in high-functioning autism. Journal of Autism and Developmental Disorders. 2007;37:929–39. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children's ability to interpret faces: a research note. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1989;30:623–30. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Teunisse J, DeGelder B. Do autistics have a generalized face processing deficit? International Journal of Neuroscience. 1994;77:1–10. doi: 10.3109/00207459408986014. [DOI] [PubMed] [Google Scholar]
- Thierry G, Martin CD, Downing P, Pegna AJ. Controlling for interstimulus perceptual variance abolishes N170 face selectivity. Nature Neuroscience. 2007;10:505–11. doi: 10.1038/nn1864. [DOI] [PubMed] [Google Scholar]
- Watson D, Friend R. Measurement of social-evaluation anxiety. Journal of Consulting and Clinical Psychology. 1969;33:448–57. doi: 10.1037/h0027806. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition (WMS-III) San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Woodbury-Smith MR, Robinson J, Wheelwright S, Baron-Cohen S. Screening adults for Asperger syndrome using the AQ: a preliminary study of its diagnostic validity in clinical practice. Journal of Autism and Developmental Disorders. 2005;35:331–5. doi: 10.1007/s10803-005-3300-7. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-Johnson psycho-educational battery-revised (WJ-R) Itasca, IL: Riverside Publishing; 1989. [Google Scholar]