Abstract
It has been argued that power activates a general tendency to approach whereas powerlessness activates a tendency to inhibit. The assumption is that elevated power involves reward-rich environments, freedom and, as a consequence, triggers an approach-related motivational orientation and attention to rewards. In contrast, reduced power is associated with increased threat, punishment and social constraint and thereby activates inhibition-related motivation. Moreover, approach motivation has been found to be associated with increased relative left-sided frontal brain activity, while withdrawal motivation has been associated with increased right sided activations. We measured EEG activity while subjects engaged in a task priming either high or low social power. Results show that high social power is indeed associated with greater left-frontal brain activity compared to low social power, providing the first neural evidence for the theory that high power is associated with approach-related motivation. We propose a framework accounting for differences in both approach motivation and goal-directed behaviour associated with different levels of power.
Keywords: power, EEG, asymmetry, approach, inhibition
INTRODUCTION
Having and being able to exercise power is of prominent importance to our status and our social position compared to that of others (Flynn et al., 2006). On the one hand, high status guarantees power; on the other hand, power can be a means to achieve a higher status in our social environment. This status with its associated power has a profound impact on virtually every aspect of our lives. Being high or low in power determines whether we have easy access to important resources and whether we can achieve our goals without interference from others (who may have their own goals that differ from ours).
Power has been defined as an individual's relative capacity to modify others’ states by providing or withholding resources or administering punishments (Keltner et al., 2003). While these resources can be both material and social, in many conceptualizations of power the capacity to influence others is of primary importance. This type of power has been called social power because it is derived from ones relationship to others (Fiske, 1993). Therefore, Galinsky and colleagues defined power as the ability to control resources, own and others’, without social interference (Galinsky et al., 2003). Having access to many resources without interference from others suggest that people with power can behave in a much more unconstrained manner compared to people lacking power. Indeed, in their integrative review of the effects of social power, Keltner and colleagues (2003) propose that high power is associated with approach behaviour, while low power is related to inhibitory behaviour.
Perspectives on approach and inhibition behaviour have been shaped to a large extent by the theory postulated by Gray (1987) that proposes two interacting motivational systems: the behavioural approach system (BAS) and the behavioural inhibition system (BIS). According to Gray, the BIS is sensitive to signals of punishment and inhibits behaviour that may lead to aversive or harmful outcomes. In contrast, the BAS is proposed to be sensitive to positive signals of reward. Although research has largely focussed on individual (trait) differences in approach and inhibition (e.g. Carver and White, 1994; Boksem et al., 2006), Keltner and colleagues (2003) proposed that power influences the relative balance between approach and inhibition. Their theory holds that high power activates approach-related processes, while low power activates inhibitory processes. This, they propose, has two major reasons.
First, power is by definition related to controlling important resources. Therefore, powerful people more often than not find themselves in environments offering many potential rewards, both of a material and a social nature, making it easier for powerful people to approach these rewards. Second, powerful people are less dependent on others to acquire these resources compared to less powerful people, which is why powerful people experience less constraints and interference from others, making it easier for them to act in ways that enable them to reach their goals.
For complementary reasons, less powerful people are more inclined to inhibit approach behaviour. These people lack access to material and social resources and experience more social threat and punishments. They are more sensitive to the limitations imposed upon them by people higher in power and are therefore less able to attain their goals. The environment of people lacking power is characterized by a high degree of threat and potential punishment, limited access to resources, and social constraints. Therefore, these people are more inclined to inhibit approach behaviour.
In the psychophysiological literature, approach and inhibition have been related to different neural systems that are associated with asymmetries in frontal cortical activity as measured using electroencephalography (EEG; Sutton and Davidson, 1997). Approach, a promotion focus, and approach-related positive affect have been related to greater left-sided frontal cortical activation (Tomarken et al., 1992; Sutton and Davidson, 1997; Amodio et al., 2004), while avoidance-related negative affect and a prevention focus have been associated with greater right-sided, or possibly reduced left-sided, frontal activation (Henriques and Davidson, 1990; Amodio et al., 2004).
So far, the literature linking social power to approach behaviour and the literature linking approach behaviour to its neural correlates have not been integrated. This is unfortunate, not only because finding the suggested relationship between power and frontal asymmetry would support the power-approach theory proposed by Keltner and colleagues (2003), but also because the neural correlates of power may provide new insights in the origins and functionality of power differences between individuals. The present research aims to rectify this omission in the literature.
Here, we operationalized power by using a widely used power prime (Galinsky et al., 2003, 2006), in which power is made accessible by asking subjects to either write about an experience in their lives in which they had power over others (high power prime), or to write about an experience in which others had power over them (low power prime). While subjects were engaged in this priming task, we recorded their EEG. If high power is indeed related to approach, increased left frontal activity should be observed in comparison to situations characterized by low power.
METHODS
Participants and task
Thirty-six right-handed undergraduate students from Tilburg University [average age = 20 years (s.d. = 1.5); 15 males] participated for extra course credit. Subjects completed a writing task, adapted from Galinsky and colleagues (2003) that served to prime high or low power. Participants primed with high power (n = 18) wrote about ‘a particular situation in which you had power over another individual or individuals’. Participants primed with low power (n = 18) wrote about ‘a particular situation in which someone else had power over you’. Subjects were instructed to think of as many details about this situation such as what exactly happened, how they felt at that moment, and write them down on the sheet of paper with 17 blank lines provided. While participants were working on this task, their EEG was recorded.
EEG acquisition and analysis
EEG was recorded from 43 sites using active Ag–AgCl electrodes (Biosemi ActiveTwo, Amsterdam, Netherlands) mounted in an elastic cap. Horizontal EOGs were recorded from two electrodes placed at the outer canthi of both eyes. Vertical EOGs were recorded from electrodes on the infraorbital and supraorbital regions of the right eye placed in line with the pupil. The EEG and EOG signals were sampled at a rate of 256 Hz, and offline rereferenced to an averaged mastoid reference.
All EEG analyses were performed using the Brain Vision Analyser software (Brain Products). The data was resampled at 100 Hz and further filtered with a 0.53 Hz high-pass filter and a 40 Hz low-pass filter both with a slope of 48 dB/oct. Artefacts were rejected and eye movement artefacts were corrected, using the Gratton et al. (1983) method. The time period in which subjects were working on the writing task was segmented into 50% overlapping, 5.12 s segments. After artefact detection and ocular correction as described above, the data was submitted to a fast Fourier transform (FFT), using a 100% Hanning window. Using this window results in complete attenuation of the jump discontinuity effect caused by performing FFT on segmented EEG data, while using a 50% overlap ensures that data at the edge of one segment (where it is dampened the full 100%) is not attenuated at all in the next segment, thus minimizing data loss due to this attenuation of data near the edges of the segments. To remove segment to segment differences in total EEG power, FFT data was normalized in the 0.5–20 Hz range for every channel. Following this, segments were averaged using only the first 50 segments recorded. This was done to arrive at an equal number of segments in the average for all subjects and to make certain that subjects were engaged in the writing task at every time segment analysed. Averaged segments were then log-transformed to normalize the distributions.
Because alpha power (activity in the 8–12 Hz frequency range) is inversely related to cortical activity (Laufs et al., 2003), averaged spectral power within the alpha frequency range was calculated for every electrode, and used for statistical analyses. To obtain a measure of left–right asymmetry in frontal brain activation, asymmetry scores were calculated for an array of three homologous frontal electrode pairs (AF3, AF4, F3, F4, F5, F6) by subtracting the spectral power value for the left side from the right side (e.g. F4 – F3). This was also done to control for individual differences from non-neural sources such as skull thickness (Tomarken et al., 1992; Pivik et al., 1993). For alpha power, positive asymmetry scores reflect greater left-sided neural activity. To be able to show that effects are specific for frontal sites, we also analysed asymmetry data from three homologous posterior electrode pairs (C5, C6, CP5, CP6, P3, P4).
RESULTS
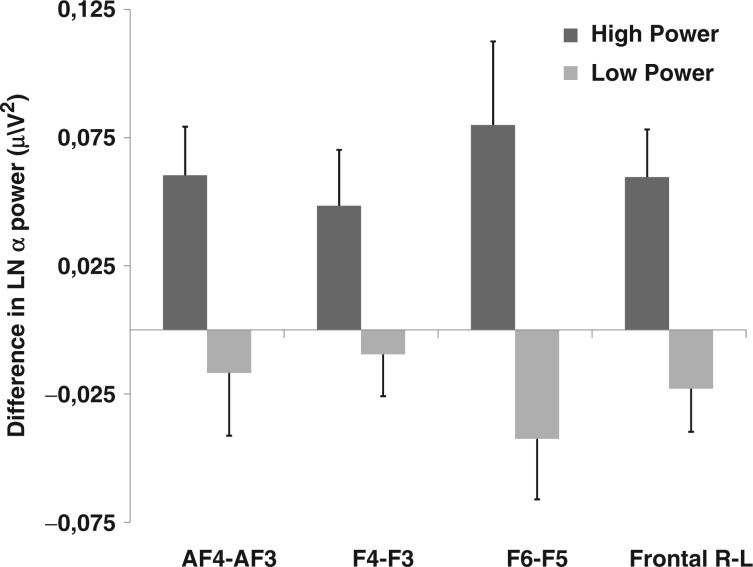
Differences in frontal left–right asymmetry of cortical activation were examined for subjects primed with high or low power. We predicted that priming high power would result in greater left-sided frontal brain activation, consistent with the theory by Keltner and colleagues (2003) that power activates approach-related tendencies, which in turn have been related to greater left-frontal cortical activation (Sutton and Davidson, 1997).Our prediction was confirmed by the pattern of activation from the homologous frontal electrode pairs under consideration. Figure 1 presents the difference in average alpha power between right and left electrodes, recorded when subjects worked on the high or low power prime. Greater left-sided (compared to right-sided) neural activity was observed for all frontal electrode pairs (2.13 < t(34) < 3.06, P < 0.05; Table 1). Combining the separate electrodes into arrays over the left- and the right-frontal hemisphere, respectively, clearly shows a greater left-frontal activation for the high power condition compared to the low power condition, t(34) = 3.30, P < 0.001. As can be observed in Figure 2, this lateralization is not perfectly symmetrical, stressing the importance of using an aggregate measure of left–right differences by pooling electrode pairs like we did. Moreover, this effect was specific for frontal electrode pairs: no differences in alpha power were observed between left and right posterior electrode sites, t(34) < 1.36, n.s.
Fig. 1.
Difference in average alpha power (in μV2) between right and left electrodes, recorded when subjects worked on the high or low power prime. Greater left-sided (compared to right-sided) neural activity was observed for all the frontal electrode pairs. Combining the separate frontal electrodes into arrays over the left and the right hemisphere, respectively, clearly shows a greater left frontal activation for the high power condition compared to the low power condition.
Table 1.
T-statistics for cortical asymmetries
| Electrode pair | t-value | |
|---|---|---|
| AF4-AF3 | 2.48a | |
| F4-F3 | 2.13a | |
| F6-F5 | 3.06b | |
| C6-C5 | 1.36 | |
| CP6-CP5 | 1.07 | |
| P4-P3 | 0.98 | |
| Frontal right vs Left | 3.30b |
Note: N = 36.
aP < 0.05; bp < 0.005.
Fig. 2.
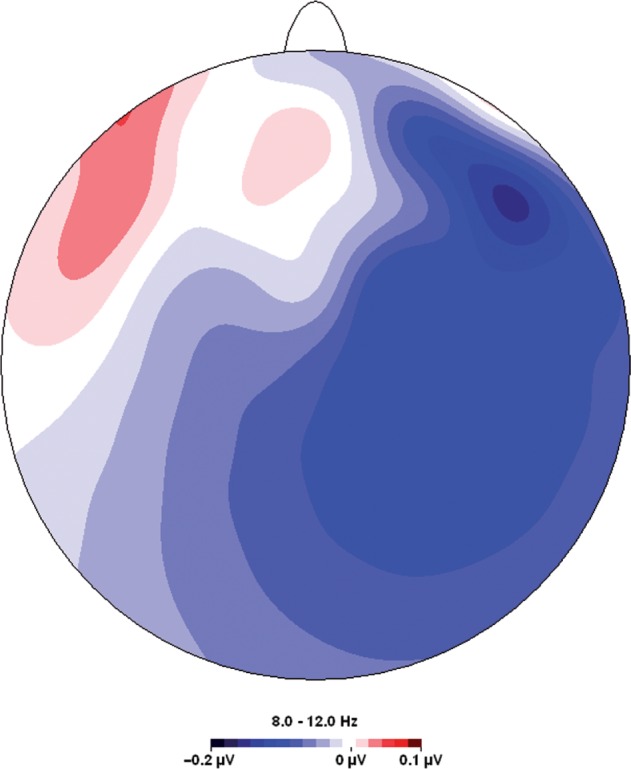
Topographical map of cortical activation (in μV2) on frontal electrode positions in high vs low power conditions. Positive values indicate relative activation in the high power condition, while negative values indicate relative deactivations.
DISCUSSION
In their review of the literature, Keltner and colleagues (2003) proposed that elevated power, involving reward-rich environments, would trigger approach-related behaviour. Reduced power, in contrast, would be associated with inhibition-related and constrained behaviour. Subsequently, it was shown by Galinsky and colleagues (2003) that priming subjects with high power indeed lead these subjects to take more direct action.
The study presented here provides the first evidence that the experience of power directly activates the motivational systems in the brain that regulate approach behaviour. Compared to subjects primed with low power, subjects primed with high power showed a greater suppression of alpha activity over left-frontal cortical areas, compared to right frontal areas, indicating that high power is associated with increased left-frontal brain activity (power in the EEG alpha band is inversely related to brain activity). Because enhanced left-frontal activity has been associated with approach behaviour (e.g. Sutton and Davidson, 1997), these findings provide direct support for the premise that high power is associated with approach motivation.
Importantly, left-frontal brain activity has been related specifically to approach motivation and not to positive affect, which may also be associated with high power (Keltner et al., 2003). Although past research does seem to indicate that positive emotions are related to left-frontal activity (e.g. Davidson et al., 1990; Tomarken et al., 1992), more recent work by Harmon-Jones and co-workers suggests that these findings resulted from confounds between approach motivation and positive emotional valence (Harmon-Jones, 2003; see also Harmon-Jones and Allen, 1998). This research shows that anger, a state involving negative feelings and outcomes (e.g. Lazarus, 1991), but also approach motivation (e.g. Berkowitz, 1999), is associated with left-frontal brain activity (e.g. Harmon-Jones and Allen, 1998), indicating that motivational direction and not emotional valence is related to frontal asymmetry (Harmon-Jones, 2003). In addition, Harmon-Jones and co-workers (2008) recently showed that positive affect does not increase relative left-frontal activation, while approach motivation does. These findings are in clear support of our interpretation that increased left-frontal brain activation is associated with approach motivation. This is not to say that high power is not associated with positive affect, but that our findings specifically reflect that power is associated with approach motivation.
In addition to facilitating approach, high power has also been suggested to specifically facilitate behaviour that is directed at achieving personal goals. High power individuals have been shown to have a greater capacity for maintenance of self-set goals and are better able to keep these goals at the focus of their attention, while low power individuals are more guided by situational constraints and have difficulties inhibiting goal-irrelevant information (Overbeck and Park, 2006; Guinote, 2007). A key brain area in goal-directed behaviour is the dorsolateral prefrontal cortex (dlPFC). This area of the brain is thought to maintain the representation of goals, as well as the means to achieve them (Miller, 2000). Davidson and colleagues (Davidson and Irwin, 1999) suggest that the left dlPFC (and other prefrontal areas) are involved in Gray's BAS and are specifically implicated in approach behaviour, while the right dlPFC is proposed to be an important component of the BIS and is related to withdrawal behaviour. In turn, this differential activation of left and right PFC is thought to underlie findings of frontal EEG asymmetry. Supporting this interpretation, a meta-analysis of PET and fMRI studies of human emotion indicated that greater left-sided frontal activity was observed for approach emotions (i.e. happiness and anger; Murphy et al., 2003), while an EEG source localization study confirmed that activity in left dlPFC was associated with a stronger bias to response to reward-related cues (Pizzagalli et al., 2005).
However, our findings appear to contradict earlier studies reporting that powerful people tend to have a more global attentional focus, which has been proposed to make them more inclined to use heuristics in decision-making and to stereotype those below them (Fiske, 1993; Smith and Trope, 2006). Because a global attentional focus has been associated with increased right hemisphere activity (Fink et al., 1996; Derryberry and Reed, 1998), this seems to be at odds with the present findings of enhanced left-frontal activity in powerful subjects. Indeed, Smith and Trope (2006) have argued that high power may be related to enhanced right hemisphere activation. This paradox may be resolved by observing that approach motivation has been related to left-frontal activity specifically, while a global attentional focus has been related to more right posterior activation. Indeed, an affective state characterized by both arousal and positive valence (such as high power), has been proposed to be associated with greater left- than right-frontal activity, but also with enhanced right posterior (parietotemporal) activity (Heller, 1993).
Thusfar, findings on the behavioural correlates of high power, such as enhanced approach motivation (Keltner et al., 2003) and more efficient goal-directed behaviour (Smith et al., 2008), have been difficult to capture in a single (neural) model. We propose that differences in power may be related to differential activation of two separate neural control (or attention) pathways that project from limbic areas in the brain to the PFC (Tucker and Williamson, 1984; Corbetta and Shulman, 2002). A mediodorsal pathway projects bilaterally to the dlPFC and is involved in planning, goal-directed behaviour and applying top-down control over selection of stimuli from the environment. A right lateralized ventrolateral pathway projects to the orbitofrontal cortex and ventral PFC and is more sensitive to external cues and is specialized in detecting salient unexpected events in the environment. Importantly, the ‘dorsal’ control system is considered to be proactive in that it is engaged when behaviour follows a predetermined action plan, while the ‘ventral’ system is considered to be reactive, interrupting dorsal goal-directed behaviour when events in the environment call for a change of plans.
We suggest that powerful people may rely more on the proactive dorsal control system, stimulating approach and goal-directed behaviour, while the behaviour of powerless people depends more on the right-lateralized, reactive ventral system, which down-regulates approach and is sensitive to salient external events, leaving powerless people less able to inhibit distracting information from the environment. This would make adaptive sense: being relatively unconstrained, powerful people are in a position to act in accordance with predetermined plans, while powerless people continuously have to monitor their unpredictable environment for unexpected changes, perhaps caused by more powerful people. Therefore, low power most likely does not impair executive control, but rather activates a more reactive mode of behavioural control that is actually more adaptive for those low in power. Applying this proactive/reactive model of behavioural control to the concept of social power would integrate several separate lines of research on the motivational, behavioural and neural determinants of social power. In addition, it provides a framework for guiding future research on the neural and behavioural correlates of power.
REFERENCES
- Amodio D.M., Shah J.Y., Sigelman J., Brazy P.C., Harmon-Jones E. Implicit regulatory focus associated with asymmetrical frontal cortical activity. Journal of Experimental Social Psychology. 2004;40:225–32. [Google Scholar]
- Berkowitz L. Anger. In: Dalgleish T., Power M.J., editors. Handbook of Cognition and Emotion. Chichester, England: John Wiley and Sons; 1999. pp. 411–28. [Google Scholar]
- Boksem M.A.S., Tops M., Wester A.E., Meijman T.F., Lorist M.M. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Carver C.S., White T.L. Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment - the bis bas scales. Journal of Personality and Social Psychology. 1994;67:319–33. [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Ekman P., Saron C.D., Senulis J.A., Friesen W.V. Approach-withdrawl and cerebral asymmetry: Emotional expression and brain physiology I. Journal of Personality and Social Psychology. 1990;58:330–41. [PubMed] [Google Scholar]
- Davidson R.J., Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Derryberry D., Reed M.A. Anxiety and attentional focussing: trait, state and hemispheric influences. Personality and Individual Differences. 1998;25:745–61. [Google Scholar]
- Fink G.R., Halligan P.W., Marchall J.C., Frith C.D., Frackowiak R.S.J., Dolan R.J. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–8. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Fiske S.T. Controlling other people – the impact of power on stereotyping. American Psychologist. 1993;48:621–8. doi: 10.1037//0003-066x.48.6.621. [DOI] [PubMed] [Google Scholar]
- Flynn F.J., Reagans R.E., Amanatullah E.T., Ames D.R. Helping one's way to the top: Self-monitors achieve status by helping others and knowing who helps whom. Journal of Personality and Social Psychology. 2006;91:1123–37. doi: 10.1037/0022-3514.91.6.1123. [DOI] [PubMed] [Google Scholar]
- Galinsky A.D., Gruenfeld D.H., Magee J.C. From power to action. Journal of Personality and Social Psychology. 2003;85:453–66. doi: 10.1037/0022-3514.85.3.453. [DOI] [PubMed] [Google Scholar]
- Galinsky A.D., Magee J.C., Inesi M.E., Gruenfeld D.H. Power and perspectives not taken. Psychological Science. 2006;17:1068–74. doi: 10.1111/j.1467-9280.2006.01824.x. [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gray J.A. The neuropsychology of emotion and personality. In: Stahl S.M., Iverson S.D., Guinote E.C., editors. Cognitive neurochemistry. Oxford: Oxford University Press; 1987. pp. 171–90. [Google Scholar]
- Guinote A. Power and goal persuit. Personality and Social Psychology Bulletin. 2007;33:1076–1087. doi: 10.1177/0146167207301011. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Allen J.J.B. Anger and prefrontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74:1310–6. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40:838–48. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Harmon-Jones C., Fearn M., Sigelman J.D., Johnson P. Left frontal activation and spreading of alternatives: Test of the action-based model of dissonance. Journal of Personality and Social Psychology. 2008;94:1–15. doi: 10.1037/0022-3514.94.1.1. [DOI] [PubMed] [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7:476–89. [Google Scholar]
- Henriques J. B., Davidson R. J. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Keltner D., Gruenfeld D.H., Anderson C. Power, approach, and inhibition. Psychological Review. 2003;110:265–84. doi: 10.1037/0033-295x.110.2.265. [DOI] [PubMed] [Google Scholar]
- Laufs H., Kleinschmidt A., Beyerle A, et al. EEG-correlated fMRI of human alpha activity. Neuroimage. 2003;19:1463–76. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Lazarus R.S. Emotion and Adaptation. New York: Oxford University Press; 1991. [Google Scholar]
- Miller E.K. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Murphy F.C., Nimmo-Smith I., Lawrence A.D. Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective and Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Overbeck J.R., Park B. Powerful perceivers, powerless objects: Flexibility of powerholders’ social attention. Organizational Behavior and Human Decision Processes. 2006;99:227–43. [Google Scholar]
- Pivik R.T., Broughton R.J., Coppola R., Davidson R.J., Fox N., Nuwer M.R. Guidelines for the recording and quantitative-analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–58. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Sherwood R.J., Henriques J.B., Davidson R.J. Frontal brain asymmetry and reward responsiveness a source localization study. Psychological Science. 2005;16:805–13. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Smith P.K., Trope Y. You focus on the forest when you’re in charge of the trees: power priming and abstract information processing. Journal of Personality and Social Psychology. 2006;90:578–96. doi: 10.1037/0022-3514.90.4.578. [DOI] [PubMed] [Google Scholar]
- Smith P.K., Jostmann N.B., Galinsky A.D., van Dijk W.W. Lacking power impairs executive functions. Psychological Science. 2008;19:441–7. doi: 10.1111/j.1467-9280.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- Sutton S.K., Davidson R.J. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–10. [Google Scholar]
- Tomarken A.J., Davidson R.J., Wheeler R.E., Doss R.C. Individual-differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62:676–87. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Tucker D.M., Williamson P.A. Asymmetric neural control systems in human self-regulation. Psychological Review. 1984;91:185–215. [PubMed] [Google Scholar]